Abstract
Tiotropium is now delivered via two different inhaler devices: the original Handihaler 18 μg once daily, which uses a powder formulation; and the newer Respimat Soft Mist Inhaler (SMI) 5 μg once daily. It has been questioned whether the two devices can be assumed to have the same safety profile, although the TIOSPIR trial showed that tiotropium when administered via Respimat SMI 5 μg is not less safe than Handihaler 18 μg. Therefore, we have carried out a safety evaluation of tiotropium Handihaler 18 µg versus tiotropium Respimat SMI 5 µg and 2.5 µg, via systematic review and network meta-analysis of the currently available clinical evidence. The results of our meta-analysis with an extremely large number of patients analysed demonstrate that the safety profile of tiotropium HandiHaler is generally superior to that of tiotropium Respimat SMI, although no statistical difference was detected between these two devices. However, the SUCRA analysis favoured tiotropium Respimat SMI with regards to serious adverse events (AEs). We do not believe that using Respimat SMI rather that HandiHaler exposes patients to higher risks of real AEs. Rather, we believe that there may be a different cardiovascular (CV) response to muscarinic receptors blockage in individual patients. Therefore, it will be essential to make all possible efforts to proactively identify patients at increased risk of CV AEs when treated with tiotropium or another antimuscarinic drug.
Keywords: tiotropium, respimat SMI, handihaler, safety, COPD, meta-analysis
Introduction
There is well-built evidence indicating that tiotropium bromide is important in the maintenance treatment of chronic obstructive pulmonary disease (COPD) [Matera et al. 2014]. In fact, several large controlled trials have allowed documenting that this long-acting antimuscarinic agent not only improves lung function and reduces dyspnoea and rescue medication use in patients with COPD, but also impacts positively on health-related quality of life and reduces the risk of exacerbations, including those that require hospitalization [Keating, 2012; Karner et al. 2012].
However, concerns have been raised about the possible associations of tiotropium with cardiovascular (CV) morbidity and mortality [Singh et al. 2008], although a lot of data that have been generated since the publication of the first concerns were reassuring on the CV safety of tiotropium in COPD patients [Cazzola et al. 2010]. In particular, Celli and colleagues [Celli et al. 2010] revised 30 trials lasting at least 4 weeks, in which overall 10,846 patients received tiotropium, and documented a significant reduction in the risk of a major or even fatal CV event in the tiotropium group compared with the placebo group. Furthermore, a post hoc analysis of all-cause mortality and serious cardiac adverse events (AEs) in patients who suffered from cardiac arrhythmia, myocardial infarction (MI) or cardiac failure during the Understanding Potential Long-term Impacts on Function with Tiotropium (UPLIFT) study and completed the study, documented that tiotropium did not increase the risk of a major or even fatal CV event, following the occurrence of a cardiac event [Tashkin et al. 2015].
Tiotropium is now delivered via two different inhaler devices: the original Handihaler 18 μg once daily, which uses a powder formulation, and the newer Respimat Soft Mist Inhaler (SMI) 5 μg once daily. Respimat SMI delivers a higher fine-particle dose and allows higher drug deposition in the lung compared with aerosols produced by HandiHaler [Cazzola and Rogliani, 2015]. Remarkably, tiotropium HandiHaler 18 μg and Respimat SMI 5 μg have similar pharmacokinetic profiles. A recent extensive comparative pharmacokinetic and bronchodilator efficacy study in patients with COPD demonstrated a lower exposure but similar bronchodilator efficacy of once-daily tiotropium Respimat SMI 5 µg compared with tiotropium HandiHaler 18 µg [Hohlfeld et al. 2014].
Nonetheless, it has been questioned whether the two devices can be assumed to have the same safety profile [Cates, 2011]. In fact, Singh and colleagues [Singh et al. 2011] reported a 46% relative increase in risk of mortality from any cause in patients using the mist inhaler compared with placebo [relative risk 1.46, 95% confidence interval (CI) 1.01–2.10]. Furthermore, a Cochrane review, which used the Peto method for pooled estimation of odds ratio, suggested that tiotropium Respimat but not tiotropium HandiHaler significantly increases the risk of mortality [Karner et al. 2012]. Another direct treatment comparison meta-analysis of randomized controlled trials (RCTs) confirmed that tiotropium Respimat SMI increases the risk of death compared with tiotropium HandiHaler [Dong et al. 2013]. Although the massive Tiotropium Safety and Performance in Respimat (TIOSPIR) trial showed that tiotropium when administered via Respimat 5 μg is not less safe than Handihaler 18 μg [Wise et al. 2013], a large real-life study showed that use of tiotropium Respimat SMI was associated with an almost 30% increase of mortality compared with HandiHaler and the association was the strongest for CV/cerebrovascular death [Verhamme et al. 2013]. Therefore, it has been suggested that the administration of tiotropium via Respimat SMI should be avoided in patients with pre-existing CV comorbidities [Mathioudakis et al. 2014] and, more recently, also chronic kidney disease because of the renal excretion of tiotropium [Mathioudakis et al. 2015].
Therefore, in view of the patent dichotomy between what documented by the TIOSPIR study and the results of initial meta-analyses and the real-life study, we have carried out a safety evaluation of tiotropium Handihaler 18 µg versus tiotropium Respimat SMI 5 µg and 2.5 µg, via systematic review and network meta-analysis of the currently available clinical evidences.
Meta-analysis
Methods
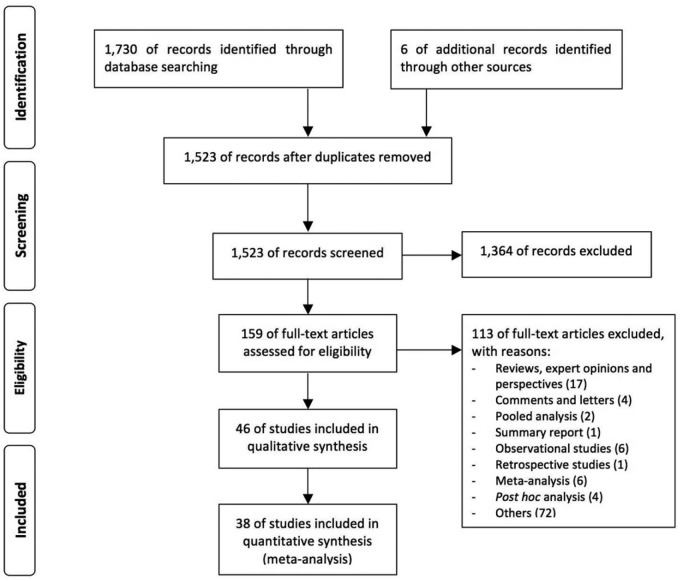
A network meta-analysis was performed in agreement with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement (Figure 1) [Moher et al. 2009].
Figure 1.
PRISMA flow diagram for the identification of studies included in the network meta-analysis concerning the safety profile of tiotropium Handihaler 18 µg versus tiotropium Respimat SMI 5 µg and 2.5 µg in COPD patients.
Data sources and searches
Published and unpublished RCTs were searched in PubMed and Google Scholar (there is now agreement that for quick clinical searches, Google Scholar returns twice as many relevant articles as PubMed and provides greater access to free full-text articles [Shariff et al. 2013]) through June 2016, and citations of a previous published pooled-analyses was examined to identify further pertinent studies, if any [Halpin et al. 2015]. The terms “tiotropium” AND “Handihaler” AND/OR “Respimat” were searched.
Study selection
RCTs lasting at least 2 weeks and reporting the safety of tiotropium administered in COPD patients via Handihaler 18 µg or Respimat 5 µg and 2.5 µg, compared with inhaler containing matching placebo, were selected. Studies that have directly compared Handihaler 18 µg versus Respimat 5 µg and 2.5 µg have been also selected.
Data extraction and quality assessment
Two reviewers independently checked the relevant RCTs found from literature, and any difference in opinion about eligibility was resolved by consensus.
Data from included studies were extracted and checked for study characteristics and duration, number of enrolled patients, doses of tiotropium, disease characteristics, and AEs. The Jadad score, with a scale of 1–5 (score of 5 being the highest), was used to assess the quality of the RCTs concerning the likelihood of bias related with randomization, double blinding, withdrawals and dropouts [Calzetta et al. 2016a and 2016b].
The effect of study quality was examined by excluding trials with a Jadad score <3. The risk of publication bias was assessed by Egger’s test [Rogliani et al. 2016].
Data synthesis and analysis
The endpoint of this network meta-analysis was to compare the safety profile of tiotropium with regard of HandiHaler and Respimat inhalers by analysing the occurrence of AEs, serious adverse events (SAEs) and risk of death in COPD patients.
The network meta-analysis was performed by using a full Bayesian evidence network (chains: 4; initial values scaling: 2.5; tuning iterations: 20,000; simulation iterations: 50,000; tuning interval: 10), the convergence diagnostics for consistency and inconsistency was assessed by using the Brooks–Gelman–Rubin method [Calzetta et al. 2016 a and 2016b]. Results of network meta-analysis have been expressed as relative effect and 95% credible level (CrI). Due to the complex evidence network, the inconsistency of evidence has been assessed by inconsistency factor (IF), indicating whether one of the treatment has a different effect when it is compared with the others [Mavridis et al. 2015]. The probability that each intervention arm was the most effective was calculated by counting the proportion of iterations of the chain in which each intervention arm had the highest mean difference, and the surface under the cumulative ranking curve (SUCRA), representing the summary of these probabilities, was also calculated [Calzetta et al. 2016a and 2016b]. The SUCRA is 100% when a treatment is certain to be the best, and 0% when a treatment is certain to be the worst [Calzetta et al. 2016a and 2016b].
The optimal information size (OIS) was calculated as previously reported [Rogliani et al. 2016], and the statistical significance was assessed forp < 0.05. Evidence of asymmetry from Egger’s test was considered to be significant for p < 0.1, and the graphical representation of 90% confidence bands have been presented [Calzetta et al. 2016a and 2016b]. GeMTC [Van Valkenhoef et al. 2012] was used for performing the network meta-analysis, and GraphPad Prism (CA, USA) software to graph the data.
Results
Study characteristics and OIS
Results obtained from 43,286 COPD patients (tiotropium HandiHaler 18 µg n = 16,016, tiotropium Respimat 5 µg n = 9,750, tiotropium Respimat 2.5 µg n = 5,889, matching placebo n = 11,631) were selected from 38 published and unpublished studies including 44 RCTs [Casaburi et al. 2002, 2005; Donohue et al. 2002; Brusasco et al. 2003; Calverley et al. 2003; Celli et al. 2003; Mcnicholas et al. 2004; O’Donnell et al. 2004; Covelli et al. 2005; Maltais et al. 2005; Niewoehner et al. 2005; Beeh et al. 2006; Dusser et al. 2006; Verkindre et al. 2006; Caillaud et al. 2007; Chan et al. 2007, Freeman et al. 2007; Garcia, 2007; Powrie et al. 2007; Ambrosino et al. 2008; Criner et al. 2008; Johansson et al. 2008; Magnussen et al. 2008; Moita et al. 2008; Tashkin et al. 2008; Tonnel et al. 2008; Voshaar et al. 2008; Bateman et al. 2010a, b; Ichinose et al. 2010; Sciurba et al. 2011; Fuhr et al. 2012; Abrahams et al. 2013; Cooper et al. 2013; Wise et al. 2013; Troosters et al. 2014; Beeh et al. 2015; Singh et al. 2015; Bouloukaki et al. 2016], between 2002 and 2016 (Figure 2).
Figure 2.
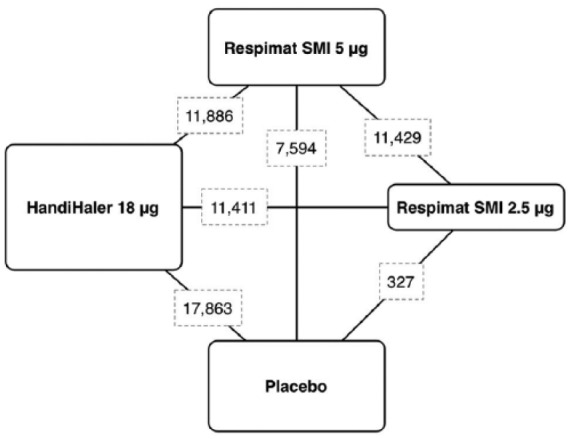
Diagram displaying the network of four arms involved in the Bayesian analysis. The links between nodes indicate the direct comparisons between pairs of treatments. The numbers shown along the link lines indicate the number of COPD patients comparing pairs of treatments head-to-head.
The relevant patient demographics, study characteristics, and Jadad score have been summarized in Table 1. The period of treatment ranged from 2 to 208 weeks, and two studies were assessed as having a Jadad score <3 [Garcia, 2007; Bouloukaki et al. 2016].
Table 1.
Patient demographics, baseline and study characteristics.
| Study and year | Study identifier | Study characteristics | Duration of study (weeks) | Number of analysed patients | Treatments and devices | Patients characteristics | Jadad score |
|---|---|---|---|---|---|---|---|
| Casaburi et al. [2002] | 205.114-115-117-128 | Randomized, double blind, placebo controlled | 52 | 921 | Tiotropium HandiHaler 18 μg; placebo HandiHaler | COPD patients ⩾40 years; FEV1 ⩽65% predicted; FEV1/FVC <70% | 5 |
| Freeman et al. [2007] | NCT00274079 | Multicentre, randomized, double blind, placebo controlled, parallel group | 12 | 374 | Tiotropium HandiHaler 18 μg; placebo HandiHaler | COPD patients ⩾40 years; FEV1 ⩾30% and ⩽65% predicted; FEV1/FVC <70% | 5 |
| Ichinose et al. [2010] | NCT02331940 | Randomized, double blind, double dummy, two-way crossover | 4 | 294 | Tiotropium Respimat SMI 5 μg; tiotropium HandiHaler 18 μg | COPD patients ⩾40 years; FEV1 ⩽80% predicted; FEV1/FVC ⩽70% | 5 |
| Niewoehner et al. [2005] | NCT00274547 | Parallel group, randomized, double blind, placebo controlled | 24 | 929 | Tiotropium HandiHaler 18 μg; placebo HandiHaler | COPD patients ⩾40 years; FEV1 ⩽60% predicted; FEV1/FVC ⩽70% | 5 |
| Abrahams et al. [2013] | NCT00528996 | Multiple dose, multicentre, multinational, randomized, double blind, parallel group | 24 | 856 | Tiotropium Respimat SMI 5 μg; placebo Respimat SMI | COPD patients ⩾40 years; FEV1 <80% predicted; FEV1/FVC <70% | 4 |
| Ambrosino et al. [2008] | NCT00157235 | Multicentre, randomized, double blind, placebo controlled, parallel group | 25 | 234 | Tiotropium HandiHaler 18 μg; placebo HandiHaler | COPD patients ⩾40 years; FEV1 ⩽60% predicted; FEV1/FVC ⩽70% | 4 |
| Bateman et al. [2010a] | NCT00387088 | Randomized, double blind, parallel group | 48 | 3,917 | Tiotropium Respimat SMI 5 μg; placebo Respimat SMI | COPD patients ⩾40 years; FEV1 ⩽80% predicted; FEV1/FVC <70% | 4 |
| Beeh et al. [2006] | NCT00274573 | Randomized, double blind, placebo controlled | 12 | 1,643 | Tiotropium HandiHaler 18 μg; placebo HandiHaler | COPD patients ⩾40 years; FEV1 ⩽70% predicted; FEV1/FVC ⩽70% | 4 |
| Brusasco et al. [2003] | 205.130 | Randomized, double blind, double dummy, parallel group | 24 | 802 | Tiotropium HandiHaler 18 μg; placebo HandiHaler | COPD patients ⩾40 years; FEV1 ⩽65% predicted; FEV1/FVC <70% | 4 |
| Calverley et al. [2003] | 205.123 | Multicentre, randomized, double blind, double dummy, parallel group | 6 | 121 | Tiotropium HandiHaler 18 μg; placebo HandiHaler | COPD patients ⩾40 years; FEV1 ⩾25% and ⩽65% predicted; FEV1/FVC <70% | 4 |
| Casaburi et al. [2005] | NCT00274521 | Multicentre, single country, randomized, double blind, parallel group | 25 | 108 | Tiotropium HandiHaler 18 μg; placebo HandiHaler | COPD patients ⩾40 years; FEV1 ⩽60% predicted; FEV1/FVC ⩽70% | 4 |
| Chan et al. [2007] | NCT00277264 | Multicentre, randomized, double blind, placebo controlled, parallel group | 48 | 913 | Tiotropium HandiHaler 18 μg; placebo HandiHaler | COPD patients ⩾40 years; FEV1 ⩽65% predicted; FEV1/FVC ⩽70% | 4 |
| Cooper et al. [2013] | NCT00525512 | Randomized, placebo controlled, double blind, parallel group | 96 | 519 | Tiotropium HandiHaler 18 μg; placebo HandiHaler | COPD patients ⩾40 years; FEV1 ⩽65% predicted; FEV1/FVC ⩽70% | 4 |
| Criner et al. [2008] | NCT00106821 | Randomized, double blind, placebo controlled, parallel group | 8 | 166 | Tiotropium HandiHaler 18 μg; placebo HandiHaler | COPD patients ⩾40 years; FEV1 ⩽65% predicted; FEV1/FVC ⩽70% | 4 |
| Donohue et al. [2002] | NA | Placebo controlled, multicentre, multinational, randomized, parallel group | 24 | 410 | Tiotropium HandiHaler 18 μg; placebo HandiHaler | COPD patients ⩾40 years; FEV1 ⩽60% predicted; FEV1/FVC <70% | 4 |
| Dusser et al. [2006] | NCT00274014 | Randomized, double blind, parallel group | 52 | 1,010 | Tiotropium HandiHaler 18 μg; placebo HandiHaler | COPD patients ⩾40 years; FEV1 ⩾30% and ⩽65% predicted; FEV1/FVC ⩽70% | 4 |
| Fuhr et al. [2012] | NCT00868231 | Two centre, double blind, placebo and active controlled crossover | 2 | 54 | Tiotropium HandiHaler 18 μg; placebo HandiHaler | COPD patients ⩾40 years; FEV1 ⩾30% and <80% predicted; FEV1/FVC <70% | 4 |
| Sciurba et al. [2011] | NCT00523991 | Randomized, double blind, placebo controlled, multicentre | 24 | 456 | Tiotropium HandiHaler 18 μg; placebo HandiHaler | COPD patients 40–80 years; FEV1 ⩾50% and <80% predicted; FEV1/FVC ⩽70% | 4 |
| Tashkin et al. [2008] | NCT00144339 | Randomized, double blind, placebo controlled, parallel group | 208 | 5,992 | Tiotropium HandiHaler 18 μg; placebo HandiHaler | COPD patients ⩾40 years; FEV1 ⩽70% predicted; FEV1/FVC ⩽70% | 4 |
| Tonnel et al. [2008] | NCT00274053 | Randomized, double blind, placebo controlled, multicentre, parallel group | 36 | 554 | Tiotropium HandiHaler 18 μg; placebo HandiHaler | COPD patients ⩾40 years; FEV1 ⩾20% and ⩽70% predicted; FEV1/FVC ⩽70% | 4 |
| Troosters et al. [2014] | NCT00523991 | Randomised, parallel group, double blind placebo controlled, multicentre | 24 | 457 | Tiotropium HandiHaler 18 μg; placebo HandiHaler | COPD patients 40-80 years; FEV1 ⩾50% and <80% predicted; FEV1/FVC <70% | 4 |
| Verkindre et al. [2005] | 205.215 | Multicentre, randomized, double blind, parallel group | 12 | 100 | Tiotropium HandiHaler 18 μg; placebo HandiHaler | COPD patients ⩾40 years; FEV1⩽50% predicted; FEV1/FVC ⩽70% | 4 |
| Voshaar et al. [2008] | NCT00239473; NCT00240435 | Two identical, randomized, double blind, double dummy, placebo and activecontrolled, parallel group | 12 | 361 | Tiotropium Respimat SMI 5 μg; placebo Respimat SMI | COPD patients ⩾40 years; FEV1 ⩽60% predicted; FEV1/FVC <70% | 4 |
| Wise et al. [2013] | NCT01126437 | Randomized, double blind, parallel group study | 119 | 17,116 | Tiotropium Respimat SMI 5 μg and 2.5 μg; tiotropium HandiHaler 18 μg HandiHaler | COPD patients ⩾40 years; FEV1 ⩽70% predicted; FEV1/FVC ⩽70% | 4 |
| Bateman et al. [2010b] | NCT00168844; NCT00168831 | Two identical, multicentre, multinational, randomized, double blind, parallel group | 48 | 1,323 | Tiotropium Respimat SMI 5 μg; placebo Respimat SMI | COPD patients ⩾40 years; FEV1 ⩽60% predicted; FEV1/FVC <70% | 3 |
| Beeh et al. [2015] | NCT01559116 | Double blind, placebo controlled, multicentre, incomplete crossover study | 6 | 413 | Tiotropium Respimat SMI 5 μg and 2.5 μg; placebo Respimat SMI | COPD patients ⩾40 years; FEV1 <80% predicted; FEV1/FVC <70% | 3 |
| Caillaud et al. [2007] | 205.127 | Multicentre, randomised, double blind within device, parallel group, active and placebo controlled, dose ranging | 3 | 125 | Tiotropium Respimat SMI 5 μg and 2.5 μg; tiotropium HandiHaler 18 μg; placebo Respimat SMI; placebo HandiHaler | COPD patients ⩾40 years; FEV1 ⩾30% and ⩽65% predicted; FEV1/FVC <70% | 3 |
| Celli et al. [2003] | 205.218 | Randomized, double blind, placebo controlled, parallel group | 4 | 81 | Tiotropium HandiHaler 18 μg; placebo HandiHaler | COPD patients ⩾40 years; FEV1 ⩾30% and ⩽65% predicted; FRC ⩾120% | 3 |
| Covelli et al. [2005] | NCT00239460 | Randomized, double blind, placebo controlled, parallel group | 12 | 178 | Tiotropium HandiHaler 18 μg; placebo HandiHaler | COPD patients ⩾40 years; FEV1 ⩽60% predicted; FEV1/FVC <70% | 3 |
| Johansson et al. [2008] | NCT00144196 | Randomized, double blind, parallel group, multicentre | 12 | 224 | Tiotropium HandiHaler 18 μg; placebo HandiHaler | COPD patients ⩾40 years; FEV1 ⩾60% predicted; FEV1/FVC ⩽70% | 3 |
| Magnussen et al. [2008] | NCT00152984 | Multicentre, multinational, prospective, randomized, placebo controlled, double blind | 12 | 472 | Tiotropium HandiHaler 18 μg; placebo HandiHaler | COPD patients ⩾40 years; FEV1 ⩽80% predicted; FEV1/FVC ⩽70% | 3 |
| Maltais et al. [2005] | NCT00274508 | Randomized, double blind, placebo controlled, parallel group | 6 | 261 | Tiotropium HandiHaler 18 μg; placebo HandiHaler | COPD patients 40–75 years; FEV1 ⩽65% predicted; FRC ⩾120% | 3 |
| Moita et al. [2008] | NCT00239408 | Randomized, double blind, parallel group, placebo controlled | 12 | 311 | Tiotropium HandiHaler 18 μg; placebo HandiHaler | COPD patients ⩾40 years; FEV1 ⩽70% predicted; FEV1/FVC ⩽70% | 3 |
| O’Donnell et al. [2004] | 205.131 | Multicentre, randomized, placebo controlled, parallel group | 6 | 187 | Tiotropium HandiHaler 18 μg; placebo HandiHaler | COPD patients 40–70 years; FEV1 ⩽65% predicted; FRC ⩾120% | 3 |
| Powrie et al. [2007] | NCT00405236 | Single centre, double blind, randomized, placebo controlled | 52 | 142 | Tiotropium HandiHaler 18 μg; placebo HandiHaler | COPD patients ⩾40 years; FEV1 <80% predicted; FEV1/FVC ⩽70% | 3 |
| Singh et al. [2015] | NCT01964352; NCT02006732 | Two replicate, multinational, double-blind, parallel group, placebo controlled | 12 | 812 | Tiotropium Respimat SMI 5 μg; placebo Respimat SMI | COPD patients ⩾40 years; FEV1 ⩾30% and <80% predicted; FEV1/FVC <70% | 3 |
| Bouloukaki et al. [2016] | NCT02331940 | Prospective, randomized, open label, parallel group | 24 | 200 | Tiotropium Respimat SMI 5 μg; tiotropium HandiHaler 18 μg | COPD patients ⩾40 years; FEV1 ⩾50% and <80% predicted; FEV1/FVC ⩽70% | 2 |
| Garcia [2007] | NCT00144326 | Randomized, double blind, placebo controlled | 12 | 250 | Tiotropium HandiHaler 18 μg; placebo HandiHaler | COPD patients ⩾40 years; FEV1 ⩽60% predicted; FEV1/FVC ⩽70% | 2 |
COPD, chronic obstructive pulmonary disease; FEV1, force expiratory volume in 1 second; FRC, functional residual capacity; FVC, forced vital capacity.
The number of COPD patients from the selected RCTs permitted to carry out a meta-analysis with a reasonable OIS to ensure a very good (probability of observing 20% overestimation for τ2 = 0.25: <5% at true relative risk reduction 10%) low risk of observing an overestimated intervention effect due to random errors in scenarios where the control group risk was low (1–5%).
Safety profile of tiotropium Handihaler versus tiotropium Respimat
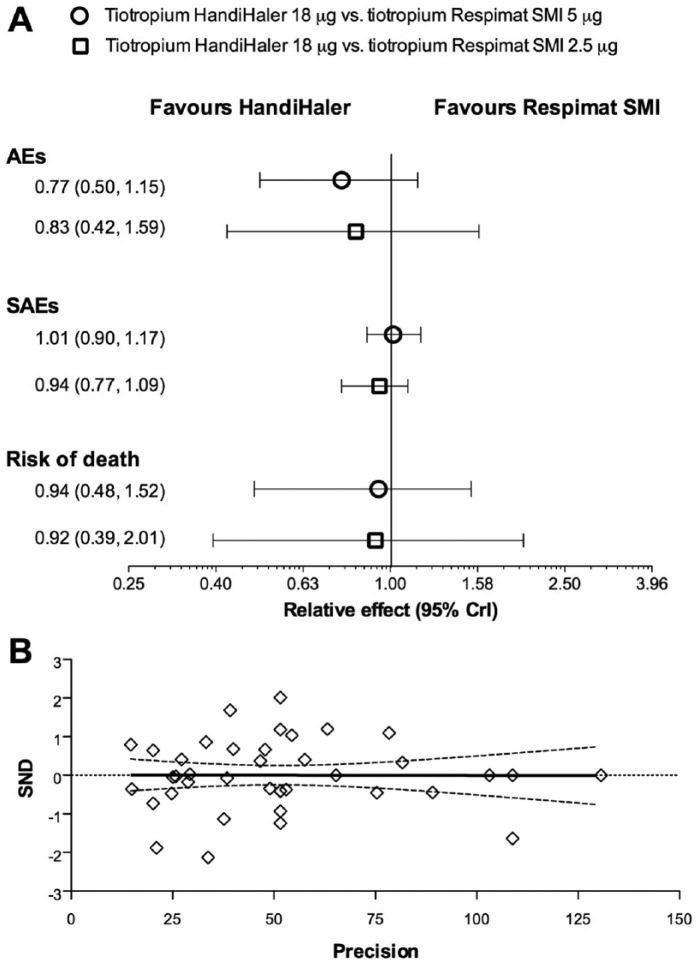
The network meta-analysis did not indicate any significant difference (p > 0.05) between the safety profile of tiotropium HandiHaler 18 µg and tiotropium Respimat 5 µg or 2.5 µg. However, the resulting relative effects were overall in favour of tiotropium HandiHaler than Respimat, with regard of AEs, SAEs and risk of death (Figure 3A). These results have been also confirmed by the subset analysis carried out by excluding the RTCs with Jadad score <3 (p > 0.05 versus network meta-analysis including all of the RCTs).
Figure 3.
Overall Forest plot of the impact of tiotropium Handihaler 18 µg versus tiotropium Respimat SMI 5 µg and 2.5 µg on AEs, SAEs and risk of death (A, data expressed as relative effect and 95% CrI). Publication bias assessment via Egger’s test (B). AEs, adverse events; SAEs, serious AEs; SND, standard normal deviate.
The analysis of inconsistency indicated that no discrepancy exists between direct and indirect evidences (AEs IF 0.01, 95% CrI −0.93 to 0.87; p > 0.05; SAEs IF 0.01, 95% CrI −0.38 to 0.81, p > 0.05; risk of death IF 0.03, 95% CrI −2.65 to 1.94. p > 0.05). The Egger’s test did not find any asymmetry (p > 0.1), suggesting that no publication bias was present in this network meta-analysis (Figure 3B).
Tiotropium HandiHaler 18 µg showed highest probability of being the best therapy with regard of AEs and risk of death (66% and 30%, respectively), as confirmed by SUCRA (87% and 61%, respectively), whereas tiotropium Respimat SMI 5 µg had the highest probability of being the best therapy with regard of SAEs (Table 2). In fact, the incidence of the most frequently reported CV SAEs such as cardiac failure, MI, and fibrillation was greater in patients receiving tiotropium HandiHaler (Table 3).
Table 2.
Probability of best therapy and SUCRA values.
| Treatment | Probability of being the best therapy (%) | SUCRA value (%) |
|---|---|---|
| AEs | ||
| Tiotropium HandiHaler 18 µg | 66 | 87 |
| Tiotropium Respimat SMI 5 µg | 6 | 45 |
| Tiotropium Respimat SMI 2.5 µg | 28 | 56 |
| SAEs | ||
| Tiotropium HandiHaler 18 µg | 14 | 55 |
| Tiotropium Respimat SMI 5 µg | 38 | 65 |
| Tiotropium Respimat SMI 2.5 µg | 4 | 11 |
| Risk of death | ||
| Tiotropium HandiHaler 18 µg | 30 | 61 |
| Tiotropium Respimat SMI 5 µg | 21 | 45 |
| Tiotropium Respimat SMI 2.5 µg | 23 | 40 |
AE, adverse event; SAE, serious adverse event; SUCRA, surface under the cumulative ranking curve.
Table 3.
Cardiovascular serious adverse events available by study results posted in the ClinicalTrials.gov repository database.
| Tiotropium HandiHaler 18 μg (n = 8,911) |
Tiotropium Respimat SMI 5 μg (n = 8,871) |
Tiotropium Respimat SMI 2.5 μg (n = 5,861) |
|
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Cardiac failure (including acute, chronic, congestive, tamponade) | 171 (1.88) | 83 (0.94) | 72 (1.23) |
| Myocardial infarction (including acute) | 118 (1.30) | 77 (0.87) | 74 (1.26) |
| Fibrillation (including atrial, flutter, ventricular) | 110 (1.21) | 49 (0.55) | 54 (0.92) |
| Angina (including pectoris, unstable) | 82 (0.90) | 55 (0.62) | 30 (0.51) |
| Tachycardia (including, atrial, sinus, supraventricular, ventricular) | 54 (0.59) | 37 (0.42) | 29 (0.49) |
| Aneurysm (including aortic, peripheral, rupture) | 40 (0.44) | 28 (0.32) | 22 (0.38) |
| Hypertension (including accelerated, crisis) | 38 (0.42) | 12 (0.14) | 13 (0.22) |
| Cardiac arrest | 38 (0.42) | 18 (0.20) | 12 (0.20) |
| Conduction disorders (including atrioventricular block, block complete, first degree block, bundle branch block left and right) | 35 (0.38) | 13 (0.5) | 8 (0.14) |
| Acute coronary syndrome | 34 (0.37) | 23 (0.26) | 12 (0.20) |
| Aortic disorders (including dissection, occlusion, rupture, stenosis, thrombosis) | 29 (0.32) | 8 (0.09) | 8 (0.14) |
| Arteriosclerosis (including coronary, obliterans) | 27 (0.30) | 12 (0.14) | 13 (0.22) |
| Cardiac disorders (including cardiomegaly, cardiomiopathy) | 25 (0.27) | 9 (0.10) | 1 (0.02) |
| Bradycardia (including sinus) | 23 (0.25) | 15 (0.17) | 9 (0.15) |
| Cardiovascular insufficiency | 17 (0.19) | 9 (0.10) | 12 (0.20) |
| Adams-Stokes syndrome | 16 (0.18) | 3 (0.03) | 5 (0.09) |
| Arteritis | 16 (0.18) | 7 (0.08) | 5 (009) |
| Varicose vein (including bleeding) | 15 (0.16) | 4 (0.05) | 4 (0.07) |
| Cardiac asthma | 12 (0.13) | 2 (0.02) | 5 (0.09) |
| Circulatory collapse | 12 (0.13) | 6 (0.07) | 3 (0.05) |
| Cor pulmonale (including acute, chronic) | 10 (0.11) | 5 (0.06) | 6 (0.10) |
| Coronary artery diseases (including embolism, insufficiency, occlusion, stenosis) | 10 (0.11) | 8 (0.09) | 7 (0.12) |
| Vein thrombosis | 9 (0.10) | 4 (0.05) | 4 (0.07) |
| Diastolic dysfunction | 8 (0.09) | 7 (0.08) | 5 (0.09) |
| Embolism | 6 (0.07) | 1 (0.01) | 0 (0.00) |
| Extrasystoles (including supraventricular, ventricular) | 4 (0.04) | 4 (0.05) | 5 (0.09) |
| Extremity necrosis | 4 (0.04) | 2 (0.02) | 1 (0.02) |
| Arterial disorders (including haemorrhage, insufficiency, occlusive disease, stenosis, thrombosis) | 4 (0.04) | 0 (0.00) | 0 (0.00) |
| Haematoma | 4 (0.04) | 5 (0.06) | 3 (0.05) |
| Haemorrhage | 4 (0.04) | 2 (0.02) | 5 (0.09) |
| Hypertensive cardiomyopathy | 4 (0.04) | 3 (0.03) | 4 (0.07) |
| Hypotension (including orthostatic) | 3 (0.03) | 0 (0.00) | 2 (0.03) |
| Shock (including cardiogenic, hypovolaemic, haemorragic) | 2 (0.02) | 0 (0.00) | 0 (0.00) |
| Artery stenosis (including iliac, peripheral, subclavian) | 2 (0.02) | 2 (0.02) | 4 (0.07) |
| Intermittent claudication | 2 (0.02) | 0 (0.00) | 0 (0.00) |
| Ischaemia (including ischaemic cardiomyopathy, myocardial and peripheral ischaemia) | 2 (0.02) | 2 (0.02) | 1 (0.02) |
| Ventricular disorders (including dysfunction, failure) | 2 (0.02) | 0 (0.00) | 0 (0.00) |
| Leriche syndrome | 1 (0.01) | 0 (0.00) | 0 (0.00) |
| Lymphoedema | 1 (0.01) | 1 (0.01) | 0 (0.00) |
| Valve diseases (including mixed, incompetence, stenosis) | 1 (0.01) | 0 (0.00) | 3 (0.05) |
| Palpitations | 1 (0.01) | 0 (0.00) | 1 (0.02) |
| Pericardial disorders (including effusion, pericarditis) | 1 (0.01) | 1 (0.01) | 1 (0.02) |
| Peripheral vascular disorder | 1 (0.01) | 0 (0.00) | 1 (0.02) |
| Post thrombotic syndrome | 1 (0.01) | 1 (0.01) | 0 (0.00) |
| Arrhythmia (including supraventricular, sinus arrhythmia, sick sinus syndrome, supraventricular, tachyarrhythmia, ventricular) | 1 (0.01) | 0 (0.00) | 0 (0.00) |
| Steal syndrome | 1 (0.01) | 0 (0.00) | 0 (0.00) |
| Vascular shunt | 1 (0.01) | 1 (0.01) | 0 (0.00) |
| Vasculitis | 0 (0.00) | 0 (0.00) | 1 (0.02) |
| Vasodilatation | 0 (0.00) | 1 (0.01) | 1 (0.02) |
| Venous insufficiency | 0 (0.00) | 0 (0.00) | 1 (0.02) |
Discussion
In recent years, several reviews and pooled safety analysis, probably not entirely independent because they include authors who are employees of the drug company that manufactures and markets tiotropium Respimat SMI and HandiHaler and therefore with a potential conflict of interest, indicate that tiotropium, given via either HandiHaler or Respimat SMI, does not increase the overall risks of AEs, SAEs, fatal AEs, or CV events [Halpin et al. 2015]. Furthermore, two post hoc analyses of TIOSPIR study have respectively demonstrated that tiotropium Respimat SMI and HandiHaler have similar safety and efficacy profiles in patients who are naïve to anticholinergic therapy [Wise et al. 2015] and it is safe to switch patients from tiotropium HandiHaler to tiotropium Respimat SMI also because the efficacy is maintained over the switch [Dahl et al. 2015].
The results of this independent network meta-analysis demonstrate that the safety profile of tiotropium HandiHaler is generally superior to that of tiotropium Respimat SMI, although no statistical difference was detected between these two devices.
Remarkably, the SUCRA analysis favoured tiotropium Respimat SMI with regards to SAEs. In fact, the incidence of the most frequently reported CV SAEs such as cardiac failure, MI, and fibrillation was greater in patients receiving tiotropium via HandiHaler. However, the results obtained by the SUCRA analysis should be interpreted with caution, because the relative effect estimate for SAEs was mainly centred between tiotropium HandiHaler 18 µg and tiotropium Respimat SMI 5 µg.
In any case, despite the large CrI values, the risk of death was always smaller for tiotropium HandiHaler than tiotropium Respimat SMI.
As expected, the extremely large number of patients analysed in this network meta-analysis has completely abolished any publication bias, regardless of the quality of the RCTs included in the analysis.
To the best of the authors’ knowledge, this is the first network meta-analysis aimed to investigate the safety profile of tiotropium Handihaler versus tiotropium Respimat SMI. Indeed, this study represents the natural step-forward from a recent pooled analysis [Halpin et al. 2015] that, inexplicably, did not include the data from RCTs in which the direct comparison between tiotropium Handihaler and tiotropium Respimat was performed, such as the studies of Bouloukaki and colleagues [Bouloukaki et al. 2016], Ichinose and colleagues [Ichinose et al. 2010], and Wise and colleagues [Wise et al. 2013], the latter being the largest RCT with >17,000 COPD patients treated with tiotropium for 2.3 years.
The trend towards a better safety profile of tiotropium HandiHaler compared with tiotropium Respimat SMI is difficult to be explained, given the repeated documentation of a systemic exposure for the two devices within the margins of equivalence [van Noord et al. 2009; Ichinose et al. 2010; Hohlfeld et al. 2014]. These pharmacokinetic data do not support the hypothesis proposed by Singh and colleagues [Singh et al. 2011] that the Respimat SMI results in earlier systemic exposure to, and higher plasma concentrations of, tiotropium after dosing increasing the risk of anticholinergic CV effects (arrhythmia). In any case, a study that analysed all data from the tiotropium clinical trial database involving Holter-ECG monitoring in patients with COPD did not show any clinically relevant differences between Respimat SMI and HandiHaler with respect to changes in heart rate or in the proportion of patients experiencing supraventricular or ventricular premature beats while on tiotropium [Hohlfeld et al. 2015].
The unexpected finding of our meta-analysis is the evidence that the incidence of the most frequently reported CV SAEs such as cardiac failure, MI, and fibrillation was greater in patients receiving tiotropium HandiHaler. In any case, it is important to highlight that we found a low absolute risk of CV AEs with both devices (Table 3).
It is obvious, at this point, to wonder whether the possible occurrence of AEs is linked to a particular genetic predisposition never investigated until now (modification of Regulator of G-protein signalling 6 (RGS6) [Patanè, 2015]) rather than to a specific device, emphasizing the need for further studies in a real-world setting to identify high-risk patients that may benefit from ECG surveillance.
In any case, it is now documented that M3 muscarinic receptor overexpression reduces the incidence of arrhythmias and mortality after myocardial ischemia-reperfusion by protecting the myocardium from ischemia at least in mice [Liu et al. 2011]. The protective mechanism of this receptor is rather complex. It regulates heart rate and cardiac repolarization, modulates inotropic effects, elicits cytoprotection against ischaemic injuries of myocardium, and regulates cell-to-cell communication [Wang et al. 2007]. Intriguingly, the expression of M3 muscarinic receptors appears to be increased in patients with atrial fibrillation, atrial dilatation, congestive heart failure, ventricular myocardial ischemia, and cardiac hypertrophy [Patanè, 2014]. Is it possible that changes in this overexpression can induce different responses to the blockade of muscarinic receptors operated by antimuscarinic drugs? In fact, all of the antimuscarinic drugs can cause more or less serious CV AEs [Sing et al. 2008; Matera et al. 2014].
We do not believe that using Respimat SMI rather that HandiHaler exposes patients to higher risks of real AEs. Rather, we believe that there may be a different CV response to muscarinic receptors blockage in individual patients. Therefore, it will be essential to make all possible efforts to proactively identify patients at increased risk of CV AEs when treated with tiotropium or another antimuscarinic drug.
In any case, we cannot forget this is a potentially dangerous occurrence, and health care providers need to be advised before incorporating antimuscarinic drugs in the management of COPD.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
Mario Cazzola, Department of Systems Medicine, University of Rome Tor Vergata, Via Montpellier 1, 00133 Rome, Italy.
Luigino Calzetta, Department of Systems Medicine, University of Rome Tor Vergata, Rome, Italy.
Paola Rogliani, Department of Systems Medicine, University of Rome Tor Vergata, Rome, Italy.
Maria Gabriella Matera, Department of Experimental Medicine, Second University of Naples, Naples, Italy.
References
- Abrahams R., Moroni-Zentgraf P., Ramsdell J., Schmidt H., Joseph E., Karpel J. (2013) Safety and efficacy of the once-daily anticholinergic BEA2180 compared with tiotropium in patients with COPD. Respir Med 107: 854–862. [DOI] [PubMed] [Google Scholar]
- Ambrosino N., Foglio K., Balzano G., Paggiaro P., Lessi P., Kesten S., et al. (2008) Tiotropium and exercise training in COPD patients: effects on dyspnea and exercise tolerance. Int J Chron Obstruct Pulmon Dis 3: 771–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman E., Singh D., Smith D., Disse B., Towse L., Massey D., et al. (2010a) Efficacy and safety of tiotropium Respimat SMI in COPD in two 1-year randomised studies. Int J Chron Obstruct Pulmon Dis 5: 197–208. [PMC free article] [PubMed] [Google Scholar]
- Bateman E., Tashkin D., Siafakas N., Dahl R., Towse L., Massey D., et al. (2010b) A one-year trial of tiotropium Respimat plus usual therapy in COPD patients. Respir Med 104: 1460–1472. [DOI] [PubMed] [Google Scholar]
- Beeh K., Beier J., Buhl R., Stark-Lorenzen P., Gerken F., Metzdorf N., et al. (2006) Efficacy of tiotropium bromide (Spiriva) in patients with chronic-obstructive pulmonary disease (COPD) of different severities. Pneumologie 60: 341–346. [DOI] [PubMed] [Google Scholar]
- Beeh K., Westerman J., Kirsten A., Hebert J., Gronke L., Hamilton A., et al. (2015) The 24-H lung-function profile of once-daily tiotropium and olodaterol fixed-dose combination in chronic obstructive pulmonary disease. Pulm Pharmacol Ther 32: 53–59. [DOI] [PubMed] [Google Scholar]
- Bouloukaki I., Tzanakis N., Mermigkis C., Giannadaki K., Moniaki V., Mauroudi E., et al. (2016) Tiotropium Respimat Soft Mist inhaler versus Handihaler to improve sleeping oxygen saturation and sleep quality in COPD. Sleep Breath 20: 605–612. [DOI] [PubMed] [Google Scholar]
- Brusasco V., Hodder R., Miravitlles M., Korducki L., Towse L., Kesten S. (2003) Health outcomes following treatment for six months with once daily tiotropium compared with twice daily salmeterol in patients with COPD. Thorax 58: 399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caillaud D., Le Merre C., Martinat Y., Aguilaniu B., Pavia D. (2007) A dose-ranging study of tiotropium delivered via Respimat Soft Mist inhaler or Handihaler in COPD patients. Int J Chron Obstruct Pulmon Dis 2: 559–565. [PMC free article] [PubMed] [Google Scholar]
- Calverley P., Lee A., Towse L., Van Noord J., Witek T., Kelsen S. (2003) Effect of tiotropium bromide on circadian variation in airflow limitation in chronic obstructive pulmonary disease. Thorax 58: 855–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calzetta L., Rogliani P., Matera M., Cazzola M. (2016a) A systematic review with meta-analysis of dual bronchodilation with LAMA/LABA for the treatment of stable COPD. Chest 149: 1181–1196. [DOI] [PubMed] [Google Scholar]
- Calzetta L., Rogliani P., Ora J., Puxeddu E., Cazzola M., Matera M.G. (2016b) Laba/Lama combination in COPD: a meta-analysis on the duration of treatment. Eur Respir Rev [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casaburi R., Kukafka D., Cooper C., Witek T., Jr., Kesten S. (2005) Improvement in exercise tolerance with the combination of tiotropium and pulmonary rehabilitation in patients with COPD. Chest 127: 809–817. [DOI] [PubMed] [Google Scholar]
- Casaburi R., Mahler D., Jones P., Wanner A., San P., Zuwallack R., et al. (2002) A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J 19: 217–224. [DOI] [PubMed] [Google Scholar]
- Cates C. (2011) Safety of tiotropium. Indirect evidence suggests the Respimat inhaler is riskier than the Handihaler. BMJ 342: d2970. [DOI] [PubMed] [Google Scholar]
- Cazzola M., Calzetta L., Matera M. (2010) The cardiovascular risk of tiotropium: is it real? Expert Opin Drug Saf 9: 783–792. [DOI] [PubMed] [Google Scholar]
- Cazzola M., Rogliani P. (2015) Inhaled medication: which device for which patient? ERS Monogr 69: 213–223 [Google Scholar]
- Celli B., Decramer M., Leimer I., Vogel U., Kesten S., Tashkin D. (2010) Cardiovascular safety of tiotropium in patients with COPD. Chest 137: 20–30. [DOI] [PubMed] [Google Scholar]
- Celli B., Zuwallack R., Wang S., Kesten S. (2003) Improvement in resting inspiratory capacity and hyperinflation with tiotropium in COPD patients with increased static lung volumes. Chest 124: 1743–1748. [DOI] [PubMed] [Google Scholar]
- Chan C., Maltais F., Sigouin C., Haddon J., Ford G., Group S. (2007) A randomised controlled trial to assess the efficacy of tiotropium in Canadian patients with chronic obstructive pulmonary disease. Can Respir J 14: 465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper C., Celli B., Jardim J., Wise R., Legg D., Guo J., et al. (2013) Treadmill endurance during 2-year treatment with tiotropium in patients with COPD: a randomised trial. Chest 144: 490–497. [DOI] [PubMed] [Google Scholar]
- Covelli H., Bhattacharya S., Cassino C., Conoscenti C., Kesten S. (2005) Absence of electrocardiographic findings and improved function with once-daily tiotropium in patients with chronic obstructive pulmonary disease. Pharmacotherapy 25: 1708–1718. [DOI] [PubMed] [Google Scholar]
- Criner G., Sharafkhaneh A., Player R., Conoscenti C., Johnson P., Keyser M., et al. (2008) Efficacy of tiotropium inhalation powder in african-american patients with chronic obstructive pulmonary disease. COPD 5: 35–41. [DOI] [PubMed] [Google Scholar]
- Dahl R., Calverley P., Anzueto A., Metzdorf N., Fowler A., Mueller A., et al. (2015) Safety and efficacy of tiotropium in patients switching from HandiHaler to Respimat in the TIOSPIR trial. BMJ Open 5: e009015. doi: 10.1136/bmjopen-2015-009015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y., Lin H., Shau W., Wu Y., Chang C., Lai M. (2013) Comparative safety of inhaled medications in patients with chronic obstructive pulmonary disease: systematic review and mixed treatment comparison meta-analysis of randomized controlled trials. Thorax 68: 48–56. [DOI] [PubMed] [Google Scholar]
- Donohue J., Van Noord J., Bateman E., Langley S., Lee A., Witek T., Jr., et al. (2002) A 6-month, placebo-controlled study comparing lung function and health status changes in COPD patients treated with tiotropium or salmeterol. Chest 122: 47–55. [DOI] [PubMed] [Google Scholar]
- Dusser D., Bravo M., Iacono P. (2006) The effect of tiotropium on exacerbations and airflow in patients with COPD. Eur Respir J 27: 547–555. [DOI] [PubMed] [Google Scholar]
- Freeman D., Lee A., Price D. (2007) Efficacy and safety of tiotropium in COPD patients in primary care: the SPiRiva Usual CarE (SPRUCE) study. Respir Res 8: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhr R., Magnussen H., Sarem K., Llovera A., Kirsten A., Falques M., et al. (2012) Efficacy of aclidinium bromide 400 μg twice daily compared with placebo and tiotropium in patients with moderate to severe COPD. Chest 141: 745–752. [DOI] [PubMed] [Google Scholar]
- Garcia R. (2007) A randomised, double-blind, placebo-controlled, 12 weeks trial to evaluate the effect of tiotropium inhalation capsules on the magnitude of exercise, measured using an accelerometer, in patients with chronic obstructive pulmonary disease (COPD) Boehringer Ingelheim Trial Results 2007. Available from: http://trials.boehringer-ingelheim.com/content/dam/internet/opu/clinicaltrial/com_EN/results/205/205.269.pdf (accessed 30 June 2016).
- Halpin D., Dahl R., Hallmann C., Mueller A., Tashkin D. (2015) Tiotropium HandiHaler® and Respimat® in COPD: a pooled safety analysis. Int J Chron Obstruct Pulmon Dis 10: 239–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohlfeld J., Furtwaengler A., Könen-Bergmann M., Wallenstein G., Walter B., Bateman E. (2015) Cardiac safety of tiotropium in patients with COPD: a combined analysis of Holter-ECG data from four randomized clinical trials. Int J Clin Pract 69: 72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohlfeld J., Sharma A., van Noord J., Cornelissen P., Derom E., Towse L., et al. (2014) Pharmacokinetics and pharmacodynamics of tiotropium solution and tiotropium powder in chronic obstructive pulmonary disease. J Clin Pharmacol 54: 405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose M., Fujimoto T., Fukuchi Y. (2010) Tiotropium 5microg via Respimat and 18microg via Handihaler; efficacy and safety in Japanese COPD patients. Respir Med 104: 228–236. [DOI] [PubMed] [Google Scholar]
- Karner C., Chong J., Poole P. (2012) Tiotropium versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 7: CD009285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating G. (2012) Tiotropium bromide inhalation powder: a review of its use in the management of chronic obstructive pulmonary disease. Drugs 72: 273–300. [DOI] [PubMed] [Google Scholar]
- Johansson G., Lindberg A., Romberg K., Nordstrom L., Gerken F., Roquet A. (2008) Bronchodilator efficacy of tiotropium in patients with mild to moderate COPD. Prim Care Respir J 17: 169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Sun L., Pan Z., Bai Y., Wang N., Zhao J., et al. (2011) Overexpression of M3 muscarinic receptor is a novel strategy for preventing sudden cardiac death in transgenic mice. Mol Med 17: 1179–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnussen H., Bugnas B., Van Noord J., Schmidt P., Gerken F., Kesten S. (2008) Improvements with tiotropium in COPD patients with concomitant asthma. Respir Med 102: 50–56. [DOI] [PubMed] [Google Scholar]
- Maltais F., Hamilton A., Marciniuk D., Hernandez P., Sciurba F., Richter K., et al. (2005) Improvements in symptom-limited exercise performance over 8 h with once-daily tiotropium in patients with COPD. Chest 128: 1168–1178. [DOI] [PubMed] [Google Scholar]
- Matera M., Rogliani P., Cazzola M. (2014) Muscarinic receptor antagonists for the treatment of chronic obstructive pulmonary disease. Expert Opin Pharmacother 15: 961–977. [DOI] [PubMed] [Google Scholar]
- Mathioudakis A., Chatzimavridou-Grigoriadou V., Evangelopoulou E., Mathioudakis G., Siafakas N. (2014) Comparative mortality risk of tiotropium administered via handihaler or respimat in COPD patients: are they equivalent? Pulm Pharmacol Ther 28: 91–97. [DOI] [PubMed] [Google Scholar]
- Mathioudakis A., Mastoris I., Chatzimavridou-Grigoriadou V., Mathioudakis G. (2015) The risk of tachyarrhythmias in patients with moderate-to-severe chronic kidney disease receiving tiotropium bromide. Int J Cardiol 197: 105–106. [DOI] [PubMed] [Google Scholar]
- Mavridis D., Giannatsi M., Cipriani A., Salanti G. (2015) A primer on network meta-analysis with emphasis on mental health. Evid Based Ment Health 18: 40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcnicholas W., Calverley P., Lee A., Edwards J.; Tiotropium Sleep Study in COPD Investigators. (2004) Long-acting inhaled anticholinergic therapy improves sleeping oxygen saturation in COPD. Eur Respir J 23: 825–831. [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D. (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6: e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moita J., Barbara C., Cardoso J., Costa R., Sousa M., Ruiz J., et al. (2008) Tiotropium improves FEV1 in patients with COPD irrespective of smoking status. Pulm Pharmacol Ther 21: 146–151. [DOI] [PubMed] [Google Scholar]
- Niewoehner D., Rice K., Cote C., Paulson D., Cooper J., Jr., Korducki L., et al. (2005) Prevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once-daily inhaled anticholinergic bronchodilator: a randomised trial. Ann Intern Med 143: 317–326. [DOI] [PubMed] [Google Scholar]
- O’Donnell D., Fluge T., Gerken F., Hamilton A., Webb K., Aguilaniu B., et al. (2004) Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J 23: 832–840. [DOI] [PubMed] [Google Scholar]
- Patanè S. (2014) M3 muscarinic acetylcholine receptor in cardiology and oncology. Int J Cardiol 177: 646–649. [DOI] [PubMed] [Google Scholar]
- Patanè S. (2015) Regulator of G-protein signaling 6 (RGS6) in cardiology and oncology. Int J Cardiol 187: 99–102. [DOI] [PubMed] [Google Scholar]
- Powrie D., Wilkinson T., Donaldson G., Jones P., Scrine K., Viel K., et al. (2007) Effect of tiotropium on sputum and serum inflammatory markers and exacerbations in COPD. Eur Respir J 30: 472–478. [DOI] [PubMed] [Google Scholar]
- Rogliani P., Calzetta L., Cazzola M., Matera M. (2016) Drug safety evaluation of roflumilast for the treatment of COPD: a meta-analysis. Expert Opin Drug Saf 11: 733–744. [DOI] [PubMed] [Google Scholar]
- Sciurba F., Siafakas N., Troosters T. (2011) The efficacy of safety of tiotropium Handihaler®, 18 Ug, once daily plus prn salbutamol versus placebo plus prn salbutamolin COPD subjects naïve to maintenance therapy. American Thoracic Society International Conference, 13–18 May 2011 Denver, Co.: Abstract. [Google Scholar]
- Shariff S., Bejaimal S., Sontrop J., Iansavichus A., Haynes R., Weir M., et al. (2013) Retrieving clinical evidence: a comparison of Pubmed and Google Scholar for quick clinical searches. J Med Internet Res 15: e164. doi: 10.2196/jmir.2624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh D., Ferguson G., Bolitschek J., Gronke L., Hallmann C., Bennett N., et al. (2015) Tiotropium + olodaterol shows clinically meaningful improvements in quality of life. Respir Med 109: 1312–1319. [DOI] [PubMed] [Google Scholar]
- Singh S., Loke Y., Enright P., Furberg C. (2011) Mortality associated with tiotropium mist inhaler in patients with chronic obstructive pulmonary disease: systematic review and meta-analysis of randomised controlled trials. BMJ 342: d3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., Loke Y., Furberg C. (2008) Inhaled anticholinergics and risk of major adverse cardiovascular events in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA 300: 1439–1450. [DOI] [PubMed] [Google Scholar]
- Tashkin D., Celli B., Senn S., Burkhart D., Kesten S., Menjoge S., et al. (2008) A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 359: 1543–1554. [DOI] [PubMed] [Google Scholar]
- Tashkin D., Leimer I., Metzdorf N., Decramer M. (2015) Cardiac safety of tiotropium in patients with cardiac events: a retrospective analysis of the UPLIFT® trial. Respir Res 16: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonnel A., Perez T., Grosbois J., Verkindre C., Bravo M., Brun M., et al. (2008) Effect of tiotropium on health-related quality of life as a primary efficacy endpoint in COPD. Int J Chron Obstruct Pulmon Dis 3: 301–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troosters T., Sciurba F., Decramer M., Siafakas N., Klioze S., Sutradhar S., et al. (2014) Tiotropium in patients with moderate COPD naive to maintenance therapy: a randomised placebo-controlled trial. NPJ Prim Care Respir Med 24: 14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Noord J.A., Cornelissen P.J., Aumann J.L., Platz J., Mueller A., Fogarty C. (2009) The efficacy of tiotropium administered via Respimat Soft Mist Inhaler or HandiHaler in COPD patients. Respir Med 103: 22–29. [DOI] [PubMed] [Google Scholar]
- Van Valkenhoef G., Lu G., De Brock B., Hillege H., Ades A., Welton N. (2012) Automating network meta-analysis. Res Synth Methods 3: 285–299. [DOI] [PubMed] [Google Scholar]
- Verhamme K., Afonso A., Romio S., Stricker B., Brusselle G., Sturkenboom M. (2013) Use of tiotropium Respimat Soft Mist inhaler versus HandiHaler and mortality in patients with COPD. Eur Respir J 42: 606–615. [DOI] [PubMed] [Google Scholar]
- Verkindre C., Bart F., Aguilaniu B., Fortin F., Guerin J., Le Merre C., et al. (2006) The effect of tiotropium on hyperinflation and exercise capacity in chronic obstructive pulmonary disease. Respiration 73: 420–427. [DOI] [PubMed] [Google Scholar]
- Voshaar T., Lapidus R., Maleki-Yazdi R., Timmer W., Rubin E., Lowe L., et al. (2008) A randomised study of tiotropium Respimat Soft Mist inhaler vs. Ipratropium pMDI in COPD. Respir Med 102: 32–41. [DOI] [PubMed] [Google Scholar]
- Wang H., Lu Y., Wang Z. (2007) Function of cardiac M3 receptors. Auton Autacoid Pharmacol 27: 1–11. [DOI] [PubMed] [Google Scholar]
- Wise R., Anzueto A., Cotton D., Dahl R., Devins T., Disse B., et al. (2013) Tiotropium Respimat inhaler and the risk of death in COPD. N Engl J Med 369: 1491–1501. [DOI] [PubMed] [Google Scholar]
- Wise R., Calverley P., Dahl R., Dusser D., Metzdorf N., Müller A., et al. (2015) Safety and efficacy of tiotropium Respimat versus HandiHaler in patients naive to treatment with inhaled anticholinergics: a post hoc analysis of the TIOSPIR trial. NPJ Prim Care Respir Med 25: 15067. [DOI] [PMC free article] [PubMed] [Google Scholar]