Abstract
Objective:
The objective of this study was to develop a noninvasive diagnostic test for nonalcoholic hepatic steatosis in patients with chronic hepatitis B (CHB) by using routinely available clinical markers.
Methods:
A retrospective study of patients with CHB, with or without hepatic steatosis (fatty change) who were diagnosed with controlled attenuation parameter (CAP) measured by transient elastography were included. Patient information was analyzed on lifestyle; laboratory tests, including serum lipid levels; blood pressure; blood uric acid; and medical history of type 2 diabetes mellitus (T2DM).
Results:
A total of 1312 patients were included in the study; 618 patients had confirmed hepatic steatosis. The CAP levels were significantly correlated with patient height (p < 0.001), weight (p < 0.001), waistline measurement (p < 0.001), hipline measurement (p < 0.001), and diastolic blood pressure (DBP) (p < 0.001). Multivariate logistic regression analysis resulted in the development of an equation for the diagnostic of simple steatosis: the fatty liver (FL) test. The area under the receiver operating characteristic (AUROC) curve of the FL test was 0.79 (p < 0.001) in the training group and 0.82 in the validation group. When the FL test was >−0.425, the sensitivity, specificity, positive likelihood ratio (LR) and negative LR were 74.72%, 72.12%, 2.68, and 0.35 respectively. The average FL test result was −0.54 ± 1.26 in patients with CHB without hypertension, and 0.42 ± 1.35, 1.12 ± 1.65, and 1.98 ± 1.22 in patients with hypertension grade 1, 2, and 3, respectively (p < 0.001).
Conclusion:
This study has demonstrated a noninvasive test for hepatic steatosis in patients with CHB.
Keywords: blood pressure, chronic hepatitis B, controlled attenuation parameter, hepatic steatosis, hepatitis B virus, nonalcoholic fatty liver disease
Introduction
Hepatitis B virus (HBV) infection is a serious global public health problem. It is estimated that 2 billion people worldwide have been infected with HBV, and 240 million people are chronically infected with HBV. People who are chronically infected with HBV are at an increased risk of developing hepatic cirrhosis, hepatic decompensation, and hepatocellular carcinoma [Sarin et al. 2016]. The prevalence of non-alcoholic fatty liver disease (NAFLD) has increased recently. NAFLD is a disease that comprises a wide spectrum of liver damage, including simple steatosis, steatohepatitis, fibrosis and cirrhosis. NAFLD is now the most common liver disorder in Western countries, affecting between 17% and 46% of adults in 2016 [EASL-EASD-EASO, 2016]. Patients with NAFLD have an increased mortality from both liver-related and non-liver-related disease when compared with the general population [Levene and Goldin, 2012]. There are patients who have concurrent chronic hepatitis B (CHB) virus infection and NAFLD, which can cause a clinical dilemma regarding different clinical management for these two conditions. Therefore, the early diagnosis and appropriate management of hepatic steatosis in patients with CHB is important. However, at present, studies on the clinical characteristics and diagnosis of NAFLD in patients with CHB remain uncommon.
Liver biopsy is still regarded as the ‘gold standard’ for assessing hepatic steatosis. However, the invasive nature of the process of liver biopsy brings about a risk of complications in 0.5% of cases, and mortality of about 0.05% [Lok and McMahon, 2009; Peng et al. 2016]. It is important to develop methods for quantifying hepatic steatosis in a noninvasive way.
Liver ultrasound (US) is the most commonly used noninvasive imaging diagnostic tool to assess hepatic steatosis. A recent study has demonstrated that hepatic ultrasound has a high sensitivity and specificity for evaluating hepatic steatosis when hepatic fat content is >20% [Dasarathy et al. 2009]. For those patients with liver steatosis <20%, hepatic ultrasound may not be effective for clinical use. The controlled attenuation parameter (CAP) measured by transient elastography was recently introduced as a promising noninvasive quantitative test for measuring hepatic steatosis [de Ledinghen et al. 2014; Sasso et al. 2010, 2012]. CAP can be used to assess hepatic steatosis when hepatic fat content is at least 5% [Masaki et al. 2013]. Recent studies have confirmed that CAP is well correlated with the grade of hepatic steatosis when confirmed by histological grading, as well as hepatic steatosis assessed by hepatic ultrasound [de Ledinghen et al. 2014; Sasso et al. 2010]. However, due to the practical difficulty of performing the transient elastography assessment, and the lack of skilled operators, CAP measured by transient elastography has not been widely implemented clinically. A simple, economical, and effective diagnostic model would be valued, especially in low-income countries.
Therefore, the purpose of this study is to develop a noninvasive diagnostic test for nonalcoholic hepatic steatosis in patients with CHB by using routinely available clinical markers. Demographic and clinical parameters were compared in patients with CHB (control group) and in patients with concomitant CHB and hepatic steatosis (steatosis group).
Methods
Patients studied and clinical parameters
From June 2013 to January 2016, a retrospective study of data from 1312 patients with CHB from the First Affiliated Hospital, Xiamen University, were included and analyzed. The Institutional Review Board of First Affiliated Hospital, Xiamen University approved the study. Each enrolled patient provided informed consent.
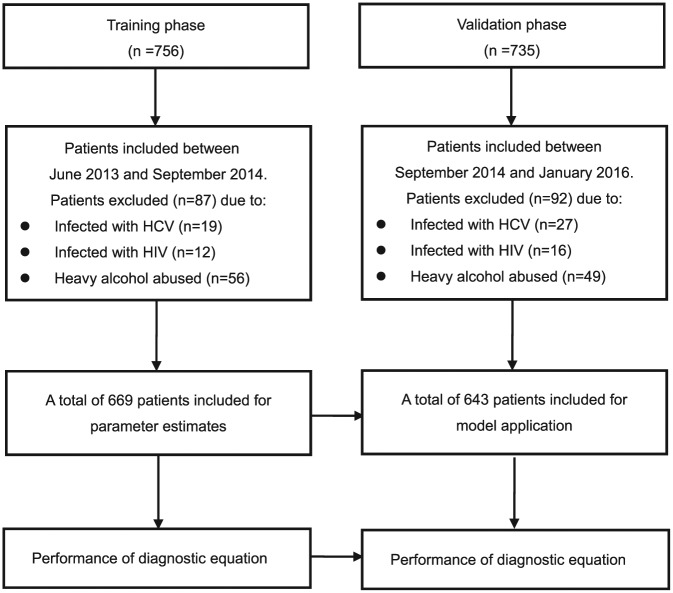
The clinical data obtained were allocated to two phases, in sequential chronological order (Figure 1). CHB was defined as the presence of seropositivity for HBV surface antigen (HBsAg) for more than 6 months with persistent or repetitive alanine aminotransferase (ALT) elevation [Sarin et al. 2016].
Figure 1.
Flow chart of the two phases in the study.
HCV, hepatitis C virus; HIV, human immunodeficiency virus.
The 1312 patients included in the study were diagnosed with chronic active HBV infection and with documented persistent or repetitive elevation of ALT (113.83 ± 82.79 U/L). The mean serum HBV DNA viral load was 7.19 ± 1.61 log10 IU/ml. Among the patients studied, 844 of 1312 patients (64.33%) were under antiviral treatment (331 patients received entecavir treatment; 143 received telbivudine; 171 received adefovir; 86 received lamivudine treatment; and 113 received combination treatment) at the time of the end of the study.
According to the histological scoring system designed by the Nonalcoholic Steatohepatitis Clinical Research Network of United States, simple steatosis was defined as hepatic steatosis ⩾ 5% of hepatocytes [Kleiner et al. 2005]. The CHB patients included in the study were diagnosed with simple hepatic steatosis when they had fatty liver (FL) with a CAP value >232.5 dB/m, according to the previously reported recommendations [Masaki et al. 2013].
In the patients studied, metabolic syndrome was defined as the cluster of any three of the following five clinical features: impaired fasting glucose or type 2 diabetes mellitus (T2DM); hypertriglyceridemia; low high-density lipoprotein-cholesterol; increased waist circumference; and high blood pressure. Hypertension was defined, according the 2013 European Society of Hypertension/European Society of Cardiology guidelines for the management of arterial hypertension, as an office sitting systolic blood pressure (SBP) of ⩾140 mmHg and/or office diastolic blood pressure (DBP) ⩾ 90 mmHg, measured by a mercury sphygmomanometer [Mancia et al. 2014]. According to current guidelines, for the diagnosis of hypertension, patients were divided into the following four groups. The normal group: SBP <139 mmHg and/or DBP <89 mmHg; Grade 1 hypertension group: SBP 140–159 mmHg and/or DBP 90–99 mmHg; Grade 2 hypertension group: SBP 160–179 mmHg and/or DBP 100–109 mmHg; Grade 3 hypertension group: SBP ⩾180 mmHg and/or DBP ⩾110 mmHg.
Patients identified with medical evidence of coinfection with hepatitis C or hepatitis D virus or HIV, and heavy alcohol abuse (>30 g/day) were excluded from the study [EASL, 2012].
CAP measured by transient elastography
The CAP value was assessed using transient elastography (Echosens, Paris, France) by a professionally trained technician according to the manufacturer’s handbook [Castera et al. 2010]. Briefly, the operation was performed on the right lobe of the liver through intercostal spaces on subjects lying in the decubitus position with the right arm in the abduction. Ultrasonic attenuation was measured at 3.5 MHz using signals assessed by transient elastography. The median CAP value was expressed in units of the decibel per meter (dB/m). A CAP value was considered reliable with both interrogative range/median <0.3 and success rate >60%.
At the time of transient elastography, patient information on lifestyle, including alcohol consumption and smoking, was collected and analyzed. Laboratory tests, including levels of serum lipids, and blood uric acid were assessed, according to standard procedures and the results performed within 14 days of transient elastography, by automated techniques, were also collected. HBV serological markers were determined using a commercially available radioimmunoassay (ARCHITECT i2000SR, Abbott Laboratories). Serum HBV DNA levels were measured using real-time polymerase chain reaction quantification. Blood pressure was measured twice at rest in a sitting position, spaced 2–5 min apart, and the mean of two values was used in the analysis. The medical history of T2DM, demographic data including age, height, weight, waistline, and hipline were measured and recorded.
Statistical analysis
The measurement units were expressed as mean ± SD for normally distributed data and median (range) for data showing non-normal distribution. Categorical data was expressed as a percentage. Paired t-test and Pearson correlation analysis were used to compare the differences, when appropriate. The area under the receiver operating characteristic (AUROC) curve and logistic regression were calculated. Sensitivity, specificity, and likelihood ratio (LR) were applied. All analyses were performed using SPSS (version 13.0) with an alpha level of 0.05.
Results
Baseline demographics and clinical characteristics
A total of 1312 patients with CHB infection were included in the study. Of these patients, 618 CHB patients were diagnosed with simple hepatic steatosis with a CAP value >232.5 dB/m and 694 CHB patients without hepatic steatosis. The height, weight, waistline, and hipline measurements of the steatosis group were significantly different from those of the control group: height: 166.31 ± 7.65 versus 164.91 ± 8.96 cm, p = 0.003; weight: 70.23 ± 12.31 versus 59.53 ± 9.63 kg p < 0.001; waistline measurement: 86.45 ± 9.51 versus 75.95 ± 9.45 cm, p < 0.001; hipline measurement: 94.35 ± 7.26 versus 89.37 ± 6.19 cm, p < 0.001). Blood pressure in the steatosis group was significantly higher than in the control group: SBP: 129.54 ± 13.72 versus 124.41 ± 12.74 mmHg; DBP: 83.39 ± 9.74 versus 79.09 ± 8.89 mmHg, p < 0.001. The mean CAP value in the steatosis group was 281.36 ± 36.59 dB/m, higher than in the control group with 191.09 ± 30.91 dB/m (p < 0.001), as shown in Table 1.
Table 1.
Demographics and clinical characteristics of the two groups.
| Characteristic | Control group | Steatosis group | p value |
|---|---|---|---|
| Sample size | 694 | 618 | – |
| Gender, male (%) | 478 (68.9) | 463 (74.9) | 0.015 |
| Age, year | 41.71 ± 12.38 | 43.19 ± 11.89 | 0.026 |
| Height, cm | 164.91 ± 8.96 | 166.31 ± 7.65 | 0.003 |
| Weight, kg | 59.53 ± 9.63 | 70.23 ± 12.31 | <0.001 |
| Waistline, cm | 75.95 ± 9.45 | 86.45 ± 9.51 | <0.001 |
| Hipline, cm | 89.37 ± 6.19 | 94.35 ± 7.26 | <0.001 |
| SBP, mmHg | 124.41 ± 12.74 | 129.54 ± 13.72 | <0.001 |
| DBP, mmHg | 79.09 ± 8.89 | 83.39 ± 9.74 | <0.001 |
| CAP value | 191.09 ± 30.91 | 281.36 ± 36.59 | <0.001 |
| CAP value in subgroup | |||
| Hyperlipidemia | <0.001 | ||
| Positive | 193.55 ± 29.21 | 289.32 ± 40.34 | |
| Negative | 190.81 ± 31.18 | 278.59 ± 35.02 | |
| Diabetes | <0.001 | ||
| Positive | 194.00 ± 28.53 | 279.18 ± 37.65 | |
| Negative | 190.89 ± 31.15 | 281.46 ± 36.37 | |
| Hypertension | <0.001 | ||
| Positive | 192.64 ± 29.18 | 285.29 ± 36.82 | |
| Negative | 190.78 ± 31.25 | 279.54 ± 36.36 | |
| Hyperuricemia | <0.001 | ||
| Positive | 192.88 ± 30.89 | 287.12 ± 39.11 | |
| Negative | 190.84 ± 30.97 | 279.27 ± 35.52 | |
| Alcohol consumption | <0.001 | ||
| Positive | 193.00 ± 28.75 | 284.22 ± 36.66 | |
| Negative | 190.73 ± 31.42 | 279.94 ± 36.41 |
CAP, controlled attenuation parameter; DBP, diastolic blood pressure; SBP, systolic blood pressure.
Analysis of demographic characteristics as a predictor of simple hepatic steatosis
The CAP levels of 1312 patients were significantly correlated with height (r = 0.107, p < 0.001), weight (r = 0.515, p < 0.001), waistline measurement (r = 0.530, p < 0.001), hipline measurement (r = 0.392, p < 0.001) and blood pressure (SBP: r = 0.218, p < 0.001; DBP: r = 0.253, p < 0.001).
A study of the relationships between the demographic characteristics and hepatic steatosis in the training group who were included between June 2013 and September 2014 (n = 669) used a multivariate logistic regression analysis designed to aid in the prediction of hepatic steatosis >5% (Table 2). The gender (odds ratio = 2.209, p = 0.001), weight (odds ratio = 1.057, p < 0.001), waistline measurement (odds ratio = 1.071, p < 0.001) and DBP (odds ratio = 1.023, p = 0.019) were predictors for diagnosis of simple hepatic steatosis. Therefore, an equation was established for the diagnostic of simple steatosis: the FL test = −12.367 + 0.023*DBP + 0.792(*2 if female) + 0.055*weight + 0.069*waistline.
Table 2.
Relationships between demographic characteristics and NAFLD.
| Variables | Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| Gender | 0.699 | 0.493–0.992 | 0.045 | 2.209 | 1.378–3.541 | 0.001 |
| Age | 1.008 | 0.995–1.021 | 0.224 | |||
| Height | 1.023 | 1.003–1.044 | 0.023 | |||
| Weight | 1.091 | 1.073–1.110 | <0.001 | 1.057 | 1.027–1.087 | <0.001 |
| Waistline | 1.110 | 1.089–1.132 | <0.001 | 1.071 | 1.041–1.102 | <0.001 |
| Hipline | 1.154 | 1.120–1.188 | <0.001 | |||
| SBP | 1.024 | 1.012–1.036 | <0.001 | |||
| DBP | 1.035 | 1.019–1.051 | <0.001 | 1.023 | 1.004–1.042 | 0.019 |
DBP, diastolic blood pressure; NAFLD, non-alcoholic fatty liver disease; OR, odds ratio; SBP, systolic blood pressure.
In order to confirm the diagnostic feasibility of this equation, we used the AUROC curve to examine whether the FL test could be used as an indicator for the diagnosis of simple hepatic steatosis. The AUROC was 0.79 (95% CI: 0.758–0.827, p < 0.001), higher than the other predictors. When the FL test was >−0.425, the sensitivity, specificity, +LR, and –LR for predicting patients with simple steatosis were 74.72%, 72.12%, 2.68, and 0.35 respectively, as shown in Figure 2.
Figure 2.
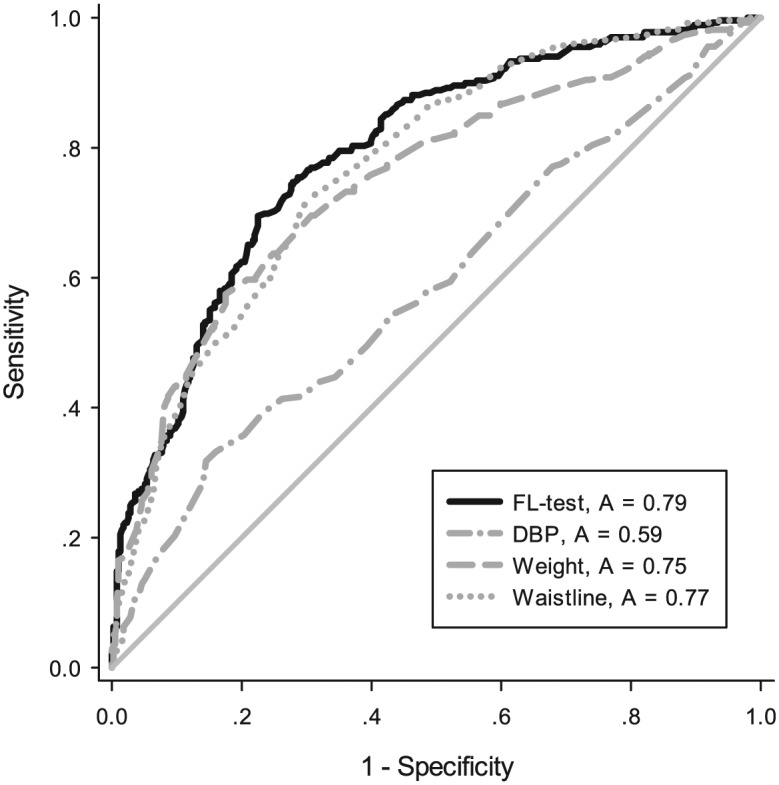
ROC curve when using FL test and other characteristics for diagnosis NAFLD. We used AUROC to examine the use of the FL test as an indicator for diagnosis of NAFLD. The AUROC was 0.79 (95% CI: 0.758–0.827, p < 0.001), higher than the other predictors. When the FL test was >–0.425, the sensitivity, specificity, +LR, and –LR to predict patients with NAFLD were 74.72%, 72.12%, 2.68, and 0.35 respectively.
AUROC, area under the receiver operating characteristic; FL, fatty liver; LR, likelihood ratio; NAFLD, nonalcoholic fatty liver disease; ROC, receiver operating characteristic.
To validate the FL test in the diagnosis of simple steatosis in patients with CHB, the parameters estimated from the training group were used to predict the probability of being diagnosed with simple steatosis for the independent validation group (n = 643). Similarly, the predicted probability was used to construct the ROC curve. The AUROC was 0.82 (95% CI: 0.788–0.853, p < 0.001), higher than the other demographics characteristics, as shown in Figure 3.
Figure 3.
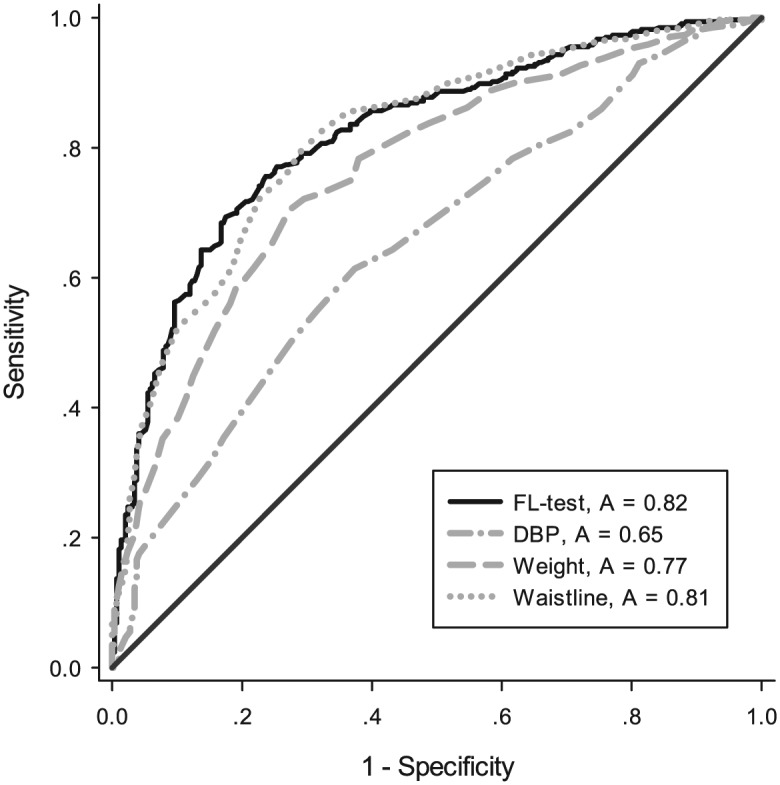
ROC curve to validate FL test in diagnosis of NAFLD. We used AUROC to validate the use of the FL test as an indicator for diagnosis of NAFLD. The AUROC was 0.820 (95% CI: 0.788–0.853, p < 0.001), higher than the other predictors.
AUROC, area under the receiver operating characteristic; FL, fatty liver; NAFLD, nonalcoholic fatty liver disease; ROC, receiver operating characteristic.
The relationship of the demographics characteristics and the FL test
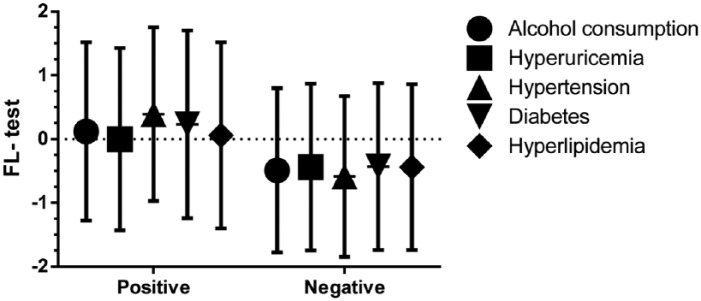
To verify the reliability of the FL test, we analyzed the demographic characteristics and lifestyle of patients against the score from the FL test. The Pearson correlation showed that the CAP value (r = 0.576, p < 0.001; Figure 4), SBP (r = 0.385, p < 0.001), DBP (r = 0.402, p < 0.001), height (r = 0.236, p < 0.001), weight (r = 0.881, p < 0.001), waistline measurement (r = 0.939, p < 0.001), and hipline measurement (r = 0.774, p < 0.001) were well correlated with the FL test. In addition, there were 219 patients (16.7%) with a diagnosis of metabolic syndrome among the CHB patients; the FL test value was 0.54 ± 1.47 versus -0.54 ± 1.24 (p < 0.001). The FL test value was also significantly different in patients of a different subgroup. The FL test value was 0.06 ± 1.46 in patients with hyperlipidemia, which was significantly higher than that for other patients with −0.44 ± 1.30 (p < 0.001). Similar trends were observed in patients with type 2 diabetes versus non-diabetics (0.23 ± 1.47 versus −0.43 ± 1.31, p < 0.001), hypertension versus non-hypertension (0.39 ± 1.36 versus −0.59 ± 1.26, p < 0.001), hyperuricemia versus non-hyperuricemia (−0.001 ± 1.43 versus −0.44 ± 1.31, p < 0.001) and alcohol consumption positive versus no alcohol consumption (0.12 ± 1.40 versus −0.49 ± 1.29, p < 0.001), as shown in Figure 5.
Figure 4.
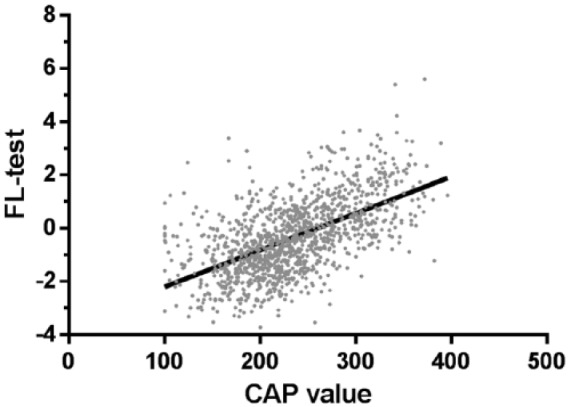
FL test positive correlation with CAP value. The Pearson’s correlation coefficient in the FL test and CAP value was r = 0.58 (p < 0.001).
CAP, controlled attenuation parameter; FL, fatty liver.
Figure 5.
FL test of CHB patients in different subgroups. **p < 0.01. FL test value was 0.06 ± 1.46 in patients with hyperlipidemia, significantly higher than in the other patients with −0.44 ± 1.30 (p < 0.001). Similar trends were observed in patients with diabetes versus non-diabetes (0.23 ± 1.47 versus −0.43 ± 1.31, p < 0.001), hypertension versus non-hypertension (0.39 ± 1.36 versus −0.59 ± 1.26, p < 0.001), hyperuricemia versus non-hyperuricemia (−0.001 ± 1.43 versus −0.44 ± 1.31, p < 0.001) and alcohol consumption positive versus negative (0.12 ± 1.40 versus −0.49 ± 1.29, p < 0.001).
CHB, chronic hepatitis B; FL, fatty liver.
When we grouped the patients with an FL test >−0.425, the number of patients with FL test >−0.425 diagnosed with hyperlipidemia, hypertension, diabetes, hyperuricemia, or alcohol consumption was significantly greater than the corresponding patients with an FL test ⩽ −0.425, as shown in Table 3.
Table 3.
Proportion of patients in different subgroups with FL test cut-off value.
| Variable | FL Test score |
p value | |
|---|---|---|---|
| >−0.425 | ⩽−0.425 | ||
| Blood lipid | <0.001 | ||
| Hyperlipidemia | 132 (61.4) | 83 (38.6) | |
| Normal | 511 (47.8) | 559 (52.2) | |
| Blood pressure | <0.001 | ||
| Hypertension | 217 (72.1) | 84 (27.9) | |
| Normal | 427 (43.3) | 560 (56.7) | |
| Diabetes mellitus | <0.001 | ||
| Positive | 96 (69.1) | 43 (30.9) | |
| Negative | 548 (47.7) | 600 (52.3) | |
| Blood uric acid | <0.001 | ||
| Hyperuricemia | 148 (60.9) | 95 (39.1) | |
| Normal | 496 (47.5) | 548 (52.5) | |
| Alcohol consumption | <0.001 | ||
| Positive | 176 (63.8) | 100 (36.2) | |
| Negative | 467 (46.2) | 543 (53.8) | |
FL, fatty liver.
Additionally, we found that the FL test could effectively distinguish between the different grades of hypertension (Figure 6). The average FL test was −0.54 ± 1.26 in patients without hypertension, and 0.42 ± 1.35, 1.12 ± 1.65, and 1.98 ± 1.22 in patients with hypertension Grades 1, 2, and 3, respectively (p < 0.001).
Figure 6.
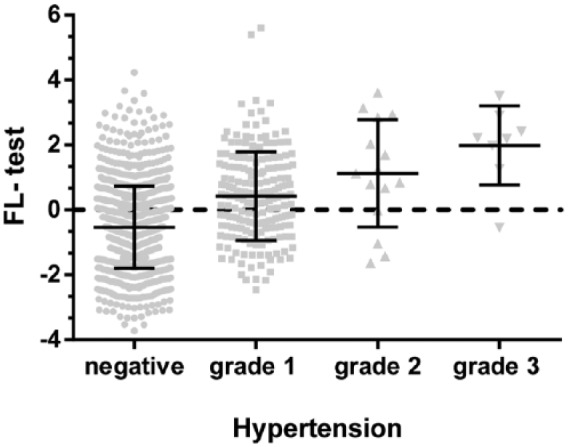
FL test can effectively distinguish between the different grades of hypertension. The average FL test was −0.54 ± 1.26 among patients without hypertension, and 0.42 ± 1.35, 1.12 ± 1.65, and 1.98 ± 1.22 among patients with hypertension grades 1, 2, and 3 (p < 0.001).
FL, fatty liver.
Discussion
The results of this study show that the FL test, a noninvasive diagnostic model, based on the CAP measured by transient elastography, could predict hepatic steatosis in patients with CHB. The FL could be developed as a diagnostic test of simple steatosis in CHB patients who refuse liver biopsy or in whom liver biopsy is contraindicated. The study findings support the argument that the FL test could be important for clinical practitioners in countries with limited medical resources and funding.
There are now many reports on the prevalence of the coexistence of NAFLD with CHB. In a study in China, 891 CHB patients were compared to 922 healthy people, and the prevalence of NAFLD in CHB patients was 13.5% compared with 28.3% in the control group [Wong et al. 2012]. Another study, reported by Bondini and colleagues [Bondini et al. 2007] showed that NAFLD was observed in 19% of patients with CHB, with a similar finding reported by Thomopoulos and colleagues [Thomopoulos et al. 2006].
In this study, we found that 618 patients were diagnosed with simple steatosis, from 1312 patients with CHB. The cut-off value for the CAP measurement was set at 232.5 with a sensitivity value of 87.0% and a specificity value of 77.2% [Masaki et al. 2013]. The prevalence of NAFLD in this study was similar to that in the general population as reported by the clinical practice guidelines for the management of NAFLD [EASL-EASD-EASO, 2016]. Perhaps, unlike hepatitis C virus (HCV) infection, the CHB virus infection did not have a significant impact on hepatic steatosis. For most CHB patients, the presence of NAFLD may be caused by an unhealthy lifestyle. However, larger studies are required to investigate this association further.
Current guidelines recommend that the treatment of patients with CHB should commence if they have persistently elevated ALT levels, at more than twice the upper limit of normal [Sarin et al. 2016]. However, an abnormal ALT level is often observed in patients with hepatic steatosis; the raised ALT level from HBV-infected patients who also have hepatic steatosis may not be caused by HBV infection alone. A marker such as serum HBV DNA level may not reflect antiviral response [Cai et al. 2014]. Similarly, inappropriate antiviral treatment with HBV associated with non-alcohol steatohepatitis (NASH) brings the risk of HBV drug resistance and increases the economic burden of treatment, which may also influence the treatment adherence of CHB patients [Peng et al. 2015]. However, lack of antiviral treatment in patients with CHB because the physicians mistakenly believe that elevated ALT was due to NASH carries the risk of progressive liver fibrosis and risk of hepatocellular carcinoma. Therefore, a noninvasive diagnostic model for hepatic steatosis, such as the FL test would be of value as an assessment for patients infected with HBV, especially before the antiviral treatment.
A study by Jin and colleagues [Jin et al. 2012], found that, in patients with CHB, the presence of FL was an independent risk factor for clinical outcome following entecavir treatment. Another study, reported by Ates and colleagues [Ates et al. 2011], of 84 patients with CHB who received interferon treatment, the sustained virological response showed no difference between patients who had hepatic steatosis and those who did not, although there was a trend towards a poorer response in the hepatic steatosis patients. In 2012, Shi and colleagues [Shi et al. 2012] observed a similar result, and reported that there was no significant difference in HBeAg seroconversion and virological response rate in patients with or without NAFLD.
Studies have reported that high baseline ALT levels result in improved clinical outcomes after antiviral treatment [Cai et al. 2016; Liaw et al. 2009]. Whether CHB associated with NASH is also associated with increased ALT levels at baseline, and is an indicator of better clinical outcome, is as yet unknown. Liver steatosis has been shown to be associated with an unfavorable response to treatment with interferon and ribavirin in patients with HCV [Poynard et al. 2003]. For patients with CHB, diet and lifestyle changes may be recommended.
NAFLD had been shown to be associated with insulin resistance in the liver, muscle, and adipose tissues [Gaggini et al. 2013], and with metabolic syndrome, [Alberti et al. 2005], which is supported by the findings of this study. Cardiovascular disease is a common cause of death in patients with NAFLD [Targher et al. 2010], which raised the possibility that the FL test from our study could be developed as a method for risk stratification for patients with CHB who may develop cardiovascular disease.
It is important to mention that previous studies have described the use of the FL index and the FL algorithm as predictors of hepatic steatosis, based on liver ultrasound [Bedogni et al. 2006; Lin et al. 2011]. When the cut-off value of the FL index was set as ⩾50, the sensitivity was 70%, and the specificity was 80% [Bedogni et al. 2006; Lin et al. 2011]. However, these previous studies were undertaken in the general population, and patients infected with HBV were excluded [Bedogni et al. 2006; Lin et al. 2011]. The FL index has not been investigated in patients with CHB. Liver ultrasound has a limited sensitivity and specificity for the evaluation of hepatic steatosis when hepatic fat content is >20% [Dasarathy et al. 2009]. For those patients with hepatic steatosis <20%, liver ultrasound may not be effective, whereas CAP can be used to assess hepatic steatosis when the hepatic fat content is 5% or more [Masaki et al. 2013].
Although this preliminary study is one of the first to evaluate the FL test in patients with CHB, it has limitations. The study was retrospective in a single center, performed by clinicians from the same center. The small study size and the potential for study bias could be overcome in future studies with the use of multiple centers, possibly in several regions and countries. The FL test could also be validated using patients who have also had liver biopsies and a histological quantification of hepatic fat.
In conclusion, this study has demonstrated a noninvasive test for hepatic steatosis in patients with CHB infection. The findings of this study have supported the potential use for CAP value in the diagnosis of hepatic steatosis, as a sensitive and specific noninvasive test. However, it is important to stress that the findings of this preliminary study should be taken further and supported by larger, controlled, multicenter studies in patients with NAFLD with and without CHB virus infection.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Hongjie Ou, Department of infectious diseases, First Affiliated Hospital of Xiamen University, Fujian Province, China.
Shaohang Cai, Department of Pathology, Sun Yat-sen University Cancer Center, Guangzhou, China Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, China.
Ying Liu, The First people’s hospital of Shunde, Guangdong Province, China.
Muye Xia, Department of Infectious Diseases and Hepatology Unit, Nanfang Hospital, Southern Medical University, Guangdong Province, China.
Jie Peng, Department of Infectious Diseases and Hepatology Unit, Nanfang Hospital, Southern Medical University, Guangdong Province, China.
References
- Alberti A., Vario A., Ferrari A., Pistis R. (2005) Review article: chronic hepatitis C–natural history and cofactors. Aliment Pharmacol Ther 22(Suppl. 2): 74–78. [DOI] [PubMed] [Google Scholar]
- Ates F., Yalniz M., Alan S. (2011) Impact of liver steatosis on response to pegylated interferon therapy in patients with chronic hepatitis B. World J Gastroenterol 17: 4517–4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedogni G., Bellentani S., Miglioli L., Masutti F., Passalacqua M., Castiglione A., et al. (2006) The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol 6: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondini S., Kallman J., Wheeler A., Prakash S., Gramlich T., Jondle D., et al. (2007) Impact of non-alcoholic fatty liver disease on chronic hepatitis B. Liver Int 27: 607–611. [DOI] [PubMed] [Google Scholar]
- Cai S., Lv F., Zhang Y., Jiang Y., Peng J. (2014) Dynamic comparison between Daan real-time PCR and Cobas TaqMan for quantification of HBV DNA levels in patients with CHB. BMC Infect Dis 14: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai S., Yu T., Jiang Y., Zhang Y., Lv F., Peng J. (2016) Comparison of entecavir monotherapy and de novo lamivudine and adefovir combination therapy in HBeAg-positive chronic hepatitis B with high viral load: 48-week result. Clin Exp Med 16: 429–436. [DOI] [PubMed] [Google Scholar]
- Castera L., Foucher J., Bernard P., Carvalho F., Allaix D., Merrouche W., et al. (2010) Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology 51: 828–835. [DOI] [PubMed] [Google Scholar]
- Dasarathy S., Dasarathy J., Khiyami A., Joseph R., Lopez R., McCullough A. (2009) Validity of real time ultrasound in the diagnosis of hepatic steatosis: a prospective study. J Hepatol 51: 1061–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ledinghen V., Vergniol J., Capdepont M., Chermak F., Hiriart J., Cassinotto C., et al. (2014) Controlled attenuation parameter (CAP) for the diagnosis of steatosis: a prospective study of 5323 examinations. J Hepatol 60: 1026–1031. [DOI] [PubMed] [Google Scholar]
- EASL (2012) EASL clinical practical guidelines: management of alcoholic liver disease. J Hepatol 57: 399–420. [DOI] [PubMed] [Google Scholar]
- EASL-EASD-EASO (2016) EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 64: 1388–1402. [DOI] [PubMed] [Google Scholar]
- Gaggini M., Morelli M., Buzzigoli E., DeFronzo R., Bugianesi E., Gastaldelli A. (2013) Non-alcoholic fatty liver disease (NAFLD) and its connection with insulin resistance, dyslipidemia, atherosclerosis and coronary heart disease. Nutrients 5: 1544–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X., Chen Y., Yang Y., Li Y., Zheng L., Xu C. (2012) Association between hepatic steatosis and entecavir treatment failure in Chinese patients with chronic hepatitis B. Plos One 7: e34198. doi: 10.1371/journal.pone.0034198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiner D., Brunt E., Van Natta M., Behling C., Contos M., Cummings O., et al. (2005) Design and validation of a histological scoring system for non-alcoholic fatty liver disease. Hepatology 41: 1313–1321. [DOI] [PubMed] [Google Scholar]
- Levene A., Goldin R. (2012) The epidemiology, pathogenesis and histopathology of fatty liver disease. Histopathology 61: 141–152. [DOI] [PubMed] [Google Scholar]
- Liaw Y., Gane E., Leung N., Zeuzem S., Wang Y., Lai C., et al. (2009) 2-Year GLOBE trial results: telbivudine is superior to lamivudine in patients with chronic hepatitis B. Gastroenterology 136: 486–495. [DOI] [PubMed] [Google Scholar]
- Lin Y., Chou S., Huang P., Chiou H. (2011) Risk factors and predictors of non-alcoholic fatty liver disease in Taiwan. Ann Hepatol 10: 125–132. [PubMed] [Google Scholar]
- Lok A., McMahon B. (2009) Chronic hepatitis B: update 2009. Hepatology 50: 661–662. [DOI] [PubMed] [Google Scholar]
- Mancia G., Fagard R., Narkiewicz K., Redon J., Zanchetti A., Böhm M., et al. (2014) 2013 ESH/ESC Practice guidelines for the management of arterial hypertension. Blood Press 23: 3–16. [DOI] [PubMed] [Google Scholar]
- Masaki K., Takaki S., Hyogo H., Kobayashi T., Fukuhara T., Naeshiro N., et al. (2013) Utility of controlled attenuation parameter measurement for assessing liver steatosis in Japanese patients with chronic liver diseases. Hepatol Res 43: 1182–1189. [DOI] [PubMed] [Google Scholar]
- Peng J., Cai S., Yu T., Chen Y., Zhu Y., Sun J. (2016) Aspartate aminotransferase to platelet ratio index - a reliable predictor of therapeutic efficacy and improvement of Ishak score in chronic hepatitis B patients treated with nucleoside analogues. Scand J Clin Lab Invest 76: 133–142. [DOI] [PubMed] [Google Scholar]
- Peng J., Yin J., Cai S., Yu T., Zhong C. (2015) Factors associated with adherence to nucleos(t)ide analogs in chronic hepatitis B patients: results from a 1-year follow-up study. Patient Prefer Adherence 9: 41–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poynard T., Ratziu V., McHutchison J., Manns M., Goodman Z., Zeuzem S., et al. (2003) Effect of treatment with peginterferon or interferon alfa-2b and ribavirin on steatosis in patients infected with hepatitis C. Hepatology 38: 75–85. [DOI] [PubMed] [Google Scholar]
- Sarin S., Kumar M., Lau G., Abbas Z., Chan H., Chen C., et al. (2016) Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int 10: 1–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasso M., Beaugrand M., de Ledinghen V., Douvin C., Marcellin P., Poupon R., et al. (2010) Controlled attenuation parameter (CAP): a novel VCTE™ guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol 36: 1825–1835. [DOI] [PubMed] [Google Scholar]
- Sasso M., Miette V., Sandrin L., Beaugrand M. (2012) The controlled attenuation parameter (CAP): a novel tool for the noninvasive evaluation of steatosis using fibroscan. Clin Res Hepatol Gastroenterol 36: 13–20. [DOI] [PubMed] [Google Scholar]
- Shi J., Lu L., Qian J., Ang J., Xun Y., Guo J., et al. (2012) Impact of liver steatosis on antiviral effects of pegylated interferon-alpha in patients with chronic hepatitis B. Zhonghua Gan Zang Bing Za Zhi 20: 285–288. [DOI] [PubMed] [Google Scholar]
- Targher G., Day C., Bonora E. (2010) Risk of cardiovascular disease in patients with non-alcoholic fatty liver disease. N Engl J Med 363: 1341–1350. [DOI] [PubMed] [Google Scholar]
- Thomopoulos K., Arvaniti V., Tsamantas A., Dimitropoulou D., Gogos C., Siagris D., et al. (2006) Prevalence of liver steatosis in patients with chronic hepatitis B: a study of associated factors and of relationship with fibrosis. Eur J Gastroenterol Hepatol 18: 233–237. [DOI] [PubMed] [Google Scholar]
- Wong V., Wong G., Chu W., Chim A., Ong A., Yeung D., et al. (2012) Hepatitis B virus infection and fatty liver in the general population. J Hepatol 56: 533–540. [DOI] [PubMed] [Google Scholar]