Abstract
Background:
Few studies have compared early gastric cancer (EGC) outcomes according to sex and age.
Methods:
We retrospectively reviewed 2085 patients who underwent curative gastrectomy for EGC between 1989 and 2000. Prognosis and risk factors for nodal involvement were evaluated according to sex and age.
Results:
Male sex and age were independent prognostic factors for overall survival (OS) but not relapse-free survival (RFS). In young (⩽55 years) patients, there were no significant differences in RFS and OS between men and women. However, older (>55 years) men had a poorer OS and older women had a poorer RFS. Young female patients had a higher proportion of gastric cancer-related death than young male patients. Female sex was an independent risk factor for nodal involvement in younger patients.
Conclusions:
Young women with EGC should be more intensively treated and monitored than other patient groups and should not be treated by endoscopic resection.
Keywords: age, early gastric cancer, prognosis, sex
Introduction
Gastric cancer (GC) is the leading cause of cancer-related death worldwide [Ferlay et al. 2010]. The World Health Organization (WHO) classification of tumors and the Japanese Society of Gastroenterological Endoscopy defines early gastric cancer (EGC) by invasion that is confined to either the mucosa or the submucosa, irrespective of lymph node metastasis [Japanese Gastric Cancer Association, 2011a]. In North-Eastern Asia, EGC represents over 50% of all new GC cases [Fujii et al. 1999; Kim et al. 2006]. The survival of patients with EGC exceeds 90% in Japan and in some western countries [Adachi et al. 1997; Oliveira et al. 1998; Kubota et al. 2000].
Histopathologic type, tumor size, and depth of invasion have been recognized as predictors of lymph node metastasis [Folli et al. 2001; Popiela et al. 2002; Kim et al. 2014] and prognostic factors for GC [Noda et al. 1980; Ribeiro et al. 1981; Adachi et al. 2000]. According to the treatment guidelines of the Japanese Gastric Cancer Association, differentiated EGCs of ⩽2 cm in size with no ulceration and confined to the mucosal layer are indicators for endoscopic treatment [Japanese Gastric Cancer Association, 2011b]. Recently, Kim and colleagues reported that sex was a predictor for lymph node metastasis and that the histologic subtype profile varied according to the male-to-female ratio and mean age [Kim et al. 2014]. Because we were unable to find any previous reports on the impact of sex or age on the outcomes of EGC, we evaluated this in our current study.
Methods
We retrospectively evaluated 2085 nonmetastatic patients who underwent curative gastrectomy for EGC between 1989 and 2000 at Asan Medical Center, Seoul, Korea. All patients in our study received intensive lymphadenectomy (above D1 plus) according to the treatment guidelines of the Japanese Gastric Cancer Association [Japanese Gastric Cancer Association, 2011b]. Macroscopic (endoscopic) findings were analyzed in accordance with the Japanese Classification of Gastric Cancer [Japanese Gastric Cancer Association, 2011]. Gastric adenocarcinomas were classified into the following histopathologic types according to the WHO classification [Hamilton and Aaltonen, 2000]: papillary adenocarcinoma, tubular adenocarcinoma, mucinous adenocarcinoma, and signet ring cell carcinoma (SRC). Tubular adenocarcinoma was further classified as well differentiated (WD-TUB), moderately differentiated (MD-TUB), or poorly differentiated (PD-TUB) using the American Joint Committee on Cancer (AJCC) seventh edition TNM staging [Edge et al. 2010]. We reviewed the numeric data, including lymph node metastasis and patient prognosis, and examined the correlation between sex and age. Relapse-free survival (RFS) was defined as time from tumor resection to the earliest of the following outcomes: disease recurrence, last follow up without evidence of disease, or death without evidence of disease. Overall survival (OS) was defined as time from resection until death from any cause or last contact.
Numeric data were expressed as mean with standard deviation and analyzed using Student t tests. Risk factors were analyzed using the chi-squared test (univariate analysis) or a logistic regression model (multivariate analysis). Survival data were analyzed using the Kaplan–Meier method with the log-rank test (univariate analysis) or Cox proportional hazards regression (multivariate analysis). All statistical data were analyzed using SPSS 21.0 (SPSS Inc., Chicago, IL). A p value of 0.05 was considered statistically significant. This study received institutional review board approval (protocol number 2012-0032).
This study received IRB approval (protocol number; 2012-0032). Informed consent was exempted by the IRB.
Results
General clinicopathologic characteristics were summarized in Table 1. All patients underwent curative resection with lymph node dissection. Of the 2085 patients evaluated in this study, 1369 (65.7%) were men and 716 (34.3%) were women. Male patients tended to be older and female patients tended to have a larger tumor size. A larger proportion of female patients had PD-TUB and SRC. In addition, female patients had more lymph node metastases than male patients and cancer stages were higher in the women subjects.
Table 1.
Clinicopathologic characteristics of all patients.
| Characteristics | Number (n = 2085) |
Percentage (%) | Mean ± SD |
|---|---|---|---|
| Gender | |||
| Male | 1369 | 66.7 | |
| Female | 716 | 34.3 | |
| Age, years | 2085 | 100 | 54.8 ± 11.5 |
| Location of tumor | |||
| Lower third | 1270 | 60.9 | |
| Middle third | 631 | 30.3 | |
| Upper third | 184 | 8.8 | |
| Tumor size (mm) | 2085 | 100 | 30.5 ± 19.1 |
| Retrieved lymph node | 2085 | 100 | 25.0 ± 12.7 |
| Gastrectomy | |||
| Subtotal | 1850 | 88.7 | |
| Total | 235 | 11.3 | |
| Depth of invasion | |||
| Mucosa | 1033 | 49.5 | |
| Submucosa | 1052 | 50.5 | |
| Macroscopic finding | |||
| Superficial | 1752 | 84.0 | |
| Protruded | 119 | 5.7 | |
| Excavated | 214 | 10.3 | |
| Histopathologic type | |||
| Papillary adenocarcinoma | 8 | 0.4 | |
| Tubular adenocarcinoma | 1705 | 81.8 | |
| Well differentiated | 480 | 23.0 | |
| Moderately differentiated | 574 | 27.5 | |
| Poorly differentiated | 651 | 31.2 | |
| Signet ring cell carcinoma | 345 | 16.5 | |
| Mucinous adenocarcinoma | 26 | 1.2 | |
| Lymph node metastasis | |||
| No | 1829 | 87.7 | |
| Yes | 256 | 12.3 | |
| Tumor recurrence | |||
| No | 1990 | 95.4 | |
| Yes | 95 | 4.6 | |
| Stage | |||
| I | 1829 | 87.7 | |
| II | 156 | 7.5 | |
| III | 75 | 3.6 | |
| IV | 25 | 1.2 | |
| Adjuvant chemotherapy | |||
| No | 1963 | 94.1 | |
| Yes | 122 | 5.9 |
SD, standard deviation.
Evaluation of prognostic factors
Male sex, older age, lymph node metastasis, deeper tumor invasion, and histologic subtypes were found to be independent prognostic factors for OS using the Cox proportional hazard model (Table 2) However, lymph node metastasis was the only prognostic factor for RFS in this model (Table 2).
Table 2.
Multivariate analysis of factors influencing survival using a cox proportional hazards model.
| Characteristics | OS |
RFS | ||
|---|---|---|---|---|
| Hazards ratio (95% CI) |
p value | |||
| Sex | ||||
| Male | 1 | 1 | ||
| Female | 0.65 (0.53–0.80) | <0.05 | 0.93 (0.62–1.44) | NS |
| Age, years | ||||
| ⩽50 | 1 | 1 | ||
| >50 | 0.55 (0.38–0.79) | <0.05 | 1.29 (0.84–1.98) | NS |
| Tumor size | ||||
| ⩽3 cm | 1 | 1 | ||
| >3 cm | 1.13 (1.42–2.37) | NS | 0.83 (0.53–1.27) | NS |
| Lymphovascular invasion | ||||
| No | 1 | 1 | ||
| Yes | 1.17 (0.87–1.57) | NS | 1.62 (0.95–2.77) | NS |
| Lymph node metastasis | ||||
| No | 1 | 1 | ||
| Yes | 1.84 (1.42–2.37) | <0.05 | 6.25 (3.94–10.01) | <0.05 |
| Depth of invasion | ||||
| Mucosa | 1 | 1 | ||
| Submucosa | 1.28 (0.04–1.57) | <0.05 | 1.67 (0.98–1.27) | NS |
| Histology | ||||
| WD-TUB | 1 | 1 | ||
| MD-TUB | 0.74 (0.58–0.95) | <0.05 | 0.59 (0.32–1.10) | NS |
| PD-TUB | 0.88 (0.63–1.12) | NS | 0.69 (0.38–1.24) | NS |
| SRC | 0.55 (0.38–0.79) | <0.05 | 0.72 (0.34–1.54 | NS |
CI, confidence interval; MD-TUB, moderately differentiated tubular adenocarcinoma; NS, nonspecific; PD-TUB, poorly differentiated tubular adenocarcinoma; SRC, signet ring cell carcinoma; WD-TUB, well-differentiated tubular adenocarcinoma.
Evaluation of prognostic factors according to sex and age
We further found that prognostic factors differed according to sex and age (Tables 3 and 4). Age, lymph node metastasis, depth of invasion, and histologic subtype were prognostic factors in men (Table 3) but depth of invasion and histologic subtype did not influence prognosis in women. Tumor size and lymph node metastasis were prognostic factors in younger (⩽55 years) patients (Table 4). However, sex, lymph node metastasis, depth of invasion, and histologic type were prognostic factors in older (>55 years) patients. Of these factors, lymph node metastasis had the largest odds ratio (Tables 3 and 4).
Table 3.
Multivariate analysis of factors influencing survival using a cox proportional hazards model.
| Characteristics | Male |
Female |
||
|---|---|---|---|---|
| Hazards ratio | p value | Hazards ratio | p value | |
| Age | ||||
| ⩽55 | 1 | 1 | ||
| >55 | 4.46 (3.95–5.87) | <0.05 | 2.9 (1.53–3.45) | <0.05 |
| Tumor size | ||||
| ⩽3 cm | 1 | 1 | ||
| >3 cm | 1.15 (0.92–1.44) | NS | 1.15 (0.80–1.65) | NS |
| Lymphovascular invasion | ||||
| No | 1 | 1 | ||
| Yes | 1.39 (0.99–1.99) | NS | 0.72 (0.35–1.32) | NS |
| Lymph node metastasis | ||||
| No | 1 | 1 | ||
| Yes | 1.34 (0.96–1.84) | NS | 3.33 (2.14–5.04) | <0.05 |
| Depth of invasion | ||||
| Mucosa | 1 | 1 | ||
| Submucosa | 1.35 (1.06–1.71) | <0.05 | 1.11 (0.78–1.72) | NS |
| Histology | ||||
| WD-TUB | 1 | 1 | ||
| MD-TUB | 0.73 (0.53–0.92) | <0.05 | 0.95 (0.54–1.69) | NS |
| PD-TUB | 0.83 (0.63–1.10) | NS | 1.01 (0.61–1.68) | NS |
| SRC | 0.50 (0.39–0.83) | <0.05 | 0.69 (0.37–3.45) | NS |
NS, nonspecific; MD-TUB, moderately differentiated tubular adenocarcinoma; PD-TUB, poorly differentiated tubular adenocarcinoma; SRC, signet ring cell carcinoma; WD-TUB, well-differentiated tubular adenocarcinoma.
Table 4.
Multivariate analysis of factors influencing survival using a cox proportional hazards model.
| Characteristics | ⩽ 55 years |
> 55 years |
||
|---|---|---|---|---|
| Hazards ratio | p value | Hazards ratio | p value | |
| Sex | ||||
| Male | 1 | 1 | ||
| Female | 1.03 (0.68–1.55) | NS | 0.54 (0.42–0.69) | <0.05 |
| Tumor size | ||||
| ⩽3 cm | 1 | 1 | ||
| >3 cm | 1.55 (1.03–2.28) | <0.05 | 1.02 (10.7–1.27) | NS |
| Lymphovascular invasion | ||||
| No | 1 | 1 | ||
| Yes | 1.12 (0.62–2.02) | NS | 1.17 (0.83–1.65) | NS |
| Lymph node metastasis | ||||
| No | 1 | 1 | ||
| Yes | 2.97 (1.86–4.75) | <0.05 | 1.45 (1.07–1.98) | <0.05 |
| Depth of invasion | ||||
| Mucosa | 1 | 1 | ||
| Submucosa | 1.43 (0.93–2.21) | NS | 1.26 (1.00–1.59) | NS |
| Histology | ||||
| WD-TUB | 1 | 1 | ||
| MD-TUB | 0.64 (0.34–1.20) | NS | 0.76 (0.58–0.99) | <0.05 |
| PD-TUB | 0.71 (0.40–1.27 | NS | 0.93 (0.71–1.22) | NS |
| SRC | 0.63 (0.32–1.55) | NS | 0.54 (0.42–0.69) | <0.05 |
NS, nonspecific; MD-TUB, moderately differentiated tubular adenocarcinoma; PD-TUB, poorly differentiated tubular adenocarcinoma; SRC, signet ring cell carcinoma; WD-TUB, well-differentiated tubular adenocarcinoma.
Evaluation of survival according to sex and age
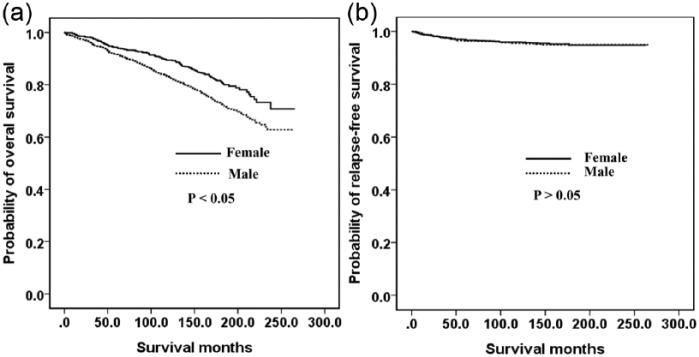
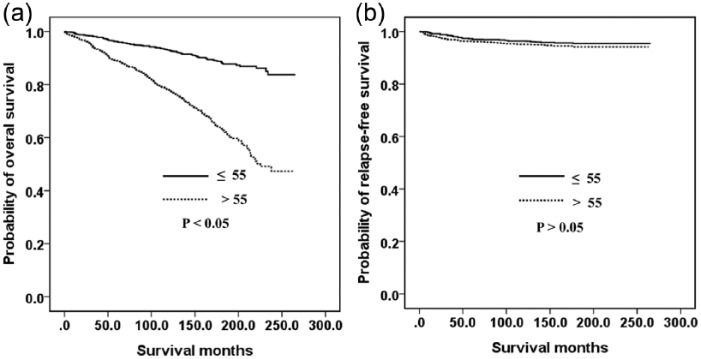
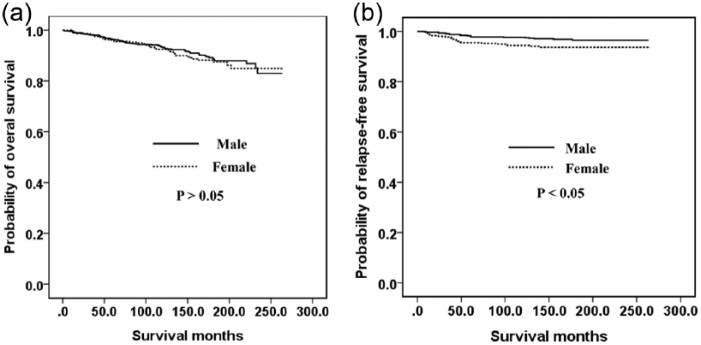
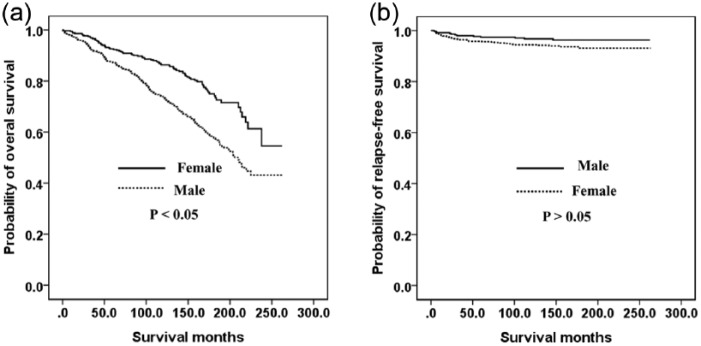
Figure 1 shows the relationship between the OS (A) and RFS (B) outcomes and the sex of the GC patient. Men had a poorer OS (p < 0.05) but there was no significant difference between the RFS of men and women (p > 0.05). Figure 2 indicates the association between the OS (A) and RFS (B) and age in our GC cohort. Older (>55 years) patients had a poorer OS (p < 0.05) but there was no significant difference between found in the RFS between younger (⩽55 years) and older (>55 years) patients (p > 0.05). The OS was also similar between younger men and younger women (Figure 3A). However, younger women had a poorer RFS (Figure 3B) and older men had poorer OS (Figure 4A, p < 0.05). The RFS rate was similar between older men and older women (Figure 4B, p > 0.05).
Figure 1.
Kaplan–Meier survival curves according to sex: (a) overall survival; (b) relapse-free survival.
Figure 2.
Kaplan–Meier curves according to according to age: (a) overall survival; (b) relapse-free survival.
Figure 3.
Kaplan–Meier survival curves according to sex in younger patients (⩽55 years): (a) overall survival; (b) relapse-free survival.
Figure 4.
Kaplan–Meier survival curves according to sex in older patients (>55 years): (a) overall survival; (b) relapse-free survival.
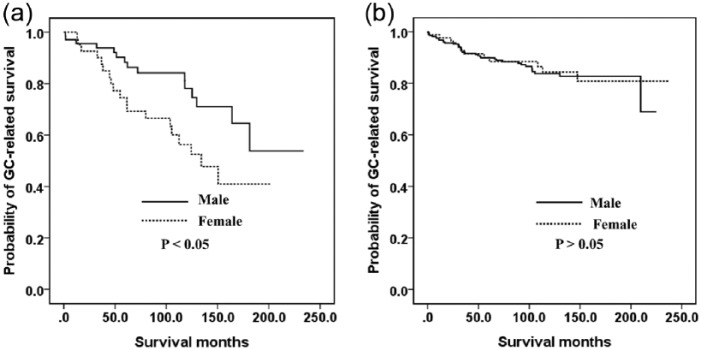
We additionally evaluated the causes of death in our GC series. During the study period, 350 of the male patients in our cohort died: 53 (15.1%) due to GC progression, 249 (71.3%) from a GC-unrelated cause, and 47 (13.4%) of an unknown cause. In the case of the female patients during the study period, 128 died in total: 31 (24.2%) of GC progression, 81 (63.3%) of a GC-unrelated cause, and 16 (12.5%) from an unknown cause. Younger female patients had a higher proportion of GC-related deaths than younger male patients (Figure 5A). However, there was a similar proportion of GC-related deaths among older male and older female patients (Figure 5B, p > 0.05).
Figure 5.
Kaplan–Meier survival curves for gastric cancer-related survival: (a) in younger patients (⩽55 years); (b) in older patients (>55 years).
Risk factors for lymph node metastasis in GC patients according to sex and age
In our present study, we found that lymph node metastasis was the most important prognostic factor (Table 2). We further found that female sex, larger tumor size, deeper tumor invasion, and lymphovascular invasion were independent risk factors for lymph node metastasis in a logistic regression model (Table 5). We evaluated risk factors according to sex (Table 6) and found that all categories, except histologic types, were risk factors for lymph node metastasis in men. In women, however, tumor size was excluded and histologic type was added to the risk factors. Female sex, large tumor size, lymphovascular invasion, submucosal cancer, and PD-TUB were identified as independent risk factors for lymph node metastasis in younger patients (Table 7). However, sex was not a risk factor in older patients.
Table 5.
Analysis of lymph node metastasis using the chi-square test and a logistic regression model.
| Characteristics | Univariate |
Multivariate |
||
|---|---|---|---|---|
| Number (%) | p value | Odds ratio (95% CI) |
p value | |
| Sex | <0.05 | |||
| Male (n = 1,369) | 606 | |||
| Female (n = 716) | 110 | 1.44 (1.07–1.94) | <0.05 | |
| Tumor size | <0.05 | |||
| ⩽3 cm (n = 1,039) | 111 | |||
| >3 cm (n = 779) | 145 | 1.71 (1.28–2.30) | <0.05 | |
| Lymphovascular invasion | <0.05 | |||
| No (n = 1,887) | 176 | |||
| Yes (n = 198) | 80 | 3.79 (2.29–5.42) | <0.05 | |
| Depth of invasion | <0.05 | |||
| Mucosa (n = 1,033) | 38 | |||
| Submucosa (n = 1,052) | 218 | 4.43 (3.04–6.47) | <0.05 | |
| Histology | <0.05 | |||
| WD-TUB (n = 480) | 25 | |||
| MD-TUB (n = 574) | 74 | 1.38 (0.83–2.29) | NS | |
| PD-TUB (n = 651) | 118 | 2.20 (1.37–3.54) | <0.05 | |
| SRC (n = 345) | 31 | 1.46 (0.85–2.06) | NS | |
CI, confidence interval; NS, nonspecific; MD-TUB, moderately differentiated tubular adenocarcinoma; PD-TUB, poorly differentiated tubular adenocarcinoma; SRC, signet ring cell carcinoma; WD-TUB, well-differentiated tubular adenocarcinoma.
Table 6.
Analysis of lymph node metastasis according to sex using a logistic regression model.
| Characteristics | Male |
Female |
||
|---|---|---|---|---|
| Odds ratio (95% CI) |
p value | Odds ratio (95% CI) |
p value | |
| Tumor size | ||||
| ⩽3 cm | 1 | 1 | ||
| >3 cm | 2.07 (1.41–3.02) | <0.05 | 1.34 (0.85–2.11) | NS |
| Lymphovascular invasion | ||||
| No | 1 | 1 | ||
| Yes | 4.10 (2.62–6.40) | <0.05 | 3.42 (1.86–6.27) | <0.05 |
| Depth of invasion | ||||
| Mucosa | 1 | 1 | ||
| Submucosa | 4.48 (2.67–7.52) | <0.05 | 4.51 (2.58–7.86) | <0.05 |
| Histology | ||||
| WD-TUB | 1 | 1 | ||
| MD-TUB | 1.31 (0.72–2.39) | NS | 1.14 (0.59–3.79) | NS |
| PD-TUB | 1.69 (0.93–6.05) | NS | 3.19 (1.37–7.45) | <0.05 |
| SRC | 1.58 (0.75–3.59) | NS | 1.58 (0.61–4.11) | NS |
CI, confidence interval; NS, nonspecific; MD-TUB, moderately differentiated tubular adenocarcinoma; PD-TUB, poorly differentiated tubular adenocarcinoma; SRC, signet ring cell carcinoma; WD-TUB, well-differentiated tubular adenocarcinoma.
Table 7.
Analysis of lymph node metastasis using a logistic regression model according to age.
| Characteristics | ⩽55 years |
>55 years |
||
|---|---|---|---|---|
| Odds ratio (95% CI) |
p value | Odds ratio (95% CI) |
p value | |
| Sex | ||||
| Male | 1 | <0.05 | 1 | |
| Female | 2.27 (1.42–3.30) | 0.98 (0.64–1.52) | NS | |
| Tumor size | ||||
| ⩽3 cm | 1 | <0.05 | 1 | |
| >3 cm | 1.92 (1.27–2.90) | 1.61 (1.06–2.43) | <0.05 | |
| Lymphovascular invasion | ||||
| No | 1 | <0.05 | 1 | |
| Yes | 4.01 (2.35–6.86) | 3.66 (2.24–5.98) | <0.05 | |
| Depth of invasion | ||||
| Mucosa | 1 | <0.05 | 1 | |
| Submucosa | 2.17 (1.42–3.30)1 | 5.12 (2.85–9.17) | <0.05 | |
| Histology | ||||
| WD-TUB | 1 | NS | 1 | |
| MD-TUB | 2.70 (0.99–7.32) | <0.05 | 0.95 (0.51–1.77) | NS |
| PD-TUB | 2.71 (1.02–7.14) | NS | 2.17 (1.22–3.85) | <0.05 |
| SRC | 2.99 (0.83–6.64) | 0.86 (0.22–2.29) | NS | |
CI, confidence interval; NS, nonspecific; MD-TUB, moderately differentiated tubular adenocarcinoma; PD-TUB, poorly differentiated tubular adenocarcinoma; SRC, signet ring cell carcinoma; WD-TUB, well-differentiated tubular adenocarcinoma.
Discussion
As has been well established previously [Kitamura et al. 1997; Katai et al. 2000; Roviello et al. 2006; Kim et al. 2014], lymph node metastasis was the most important risk factor for survival outcomes in our current study. However, it was not determined to be a risk factor for survival among the female GC patients in our analysis. The survival of patients with EGC confined to the mucosa is usually better than that of patients with EGC confined to the submucosa [Folli et al. 1995; Pertl et al. 1999; Saragoni et al. 2000; Popiela et al. 2002]. In contrast, some authors have reported that depth of infiltration does not influence long-term outcome in patients with EGC [Baba et al. 1995; Jentschura et al. 1997; Tsujitani et al. 1999; Piso et al. 2001]. Popiela and colleagues [Popiela et al. 2002] showed that age was an independent prognostic factor for EGC, which has not been consistently reported by others [Baba et al. 1995; Folli et al. 1995; Everett and Axon, 1997]. These discrepancies could depend on whether OS or disease-related survival is analyzed. In our current study, age was not found to be an independent risk factor for OS but was for RFS. However, there have been some conflicting results regarding other prognostic factors, and undifferentiated, diffuse, and larger tumors have been associated with poor survival outcomes [Hioki et al. 1990; Inoue et al. 1991; Baba et al. 1995; Everett and Axon, 1997; Jentschura et al. 1997; Ishigami et al. 1999; Pertl et al. 1999; Saragoni et al. 2000]. We found in our present analysis that the prognostic factors differed according to the sex and age of the GC patients. It is well known that older men have a poorer OS than older women because they generally have more comorbidities than similarly aged women [Lim et al. 2014; Lee et al. 2016]. In our present study, the men indeed had a poorer OS than the women. However, we found no statistically significant difference between the RFS of the men and women in our GC cohort.
The prime consideration for EGC treatment is whether the patient has a lymph node metastasis. EGC with lymph node metastasis, or a probability of lymph node metastasis, should not be treated using endoscopic resection. Hence, many studies attempted to predict a nodal involvement for EGC and reported that the presence of a nodal involvement is related to submucosal invasion, tumor size, poor differentiation, and lymphatic invasion [Maehara et al. 1992; Folli et al. 1995; Seto et al. 1997; Hochwald et al. 1999; Saragoni et al. 2000]. The Japanese Gastric Cancer Association thus recommended that endoscopic resection be indicated as the standard treatment for the following tumor type: a differentiated adenocarcinoma without ulcerative findings, with a depth of invasion clinically diagnosed as T1a and a diameter of ⩽2 cm [Japanese Gastric Cancer Association, 2011]. In our current study, we found that the risk of nodal metastasis for EGC differed according to sex and age. Female sex was identified as an independent risk factor for lymph node metastasis. In addition, an age younger than 55 years was found to be a risk factor for lymph node metastasis in women (odds ratio, 2.27). We think the reasons young females could be a prognostic factor in GC are as follows; first, females had larger tumors and a higher proportion in disuse type, PD-TUB and SRC than males (p < 0.05). Second, females had a lower proportion in WD-TUB or MD-TUB. Likewise, in younger patients, females had larger tumors and a higher proportion in diffuse type, PD-TUB and SRC than males (p < 0.05). In addition, females had a lower proportion in WD-TUB or MD-TUB in younger patients (p < 0.05). The odds ratio of this group was higher than that of the tumor size or depth of invasion categories. We contend therefore that women younger than 55 years with EGC would not be indicated for endoscopic resection.
There are some limitations of this study. First, this is a retrospective analysis, and we evaluated data from electronic medical records (EMRs). Second, we did not evaluate data for chemotherapy because the aim of this study was to confirm that sex and age influence survival and lymph node metastasis, and that sex and age could be categories of EMR/ESD treatment for EGC (T1) patients at the time of diagnosis. Third, we adopted two systems; treatment guidelines and macroscopic (endoscopic) findings were classified according to the JGCA guidelines [Japanese Gastric Cancer Association, 2011] because there are no macroscopic (endoscopic) classifications and treatment guidelines for EGC in the AJCC TNM system. Others, including pathologic factors, were classified according to AJCC TNM 7th edition or WHO.
In conclusion, male sex and age are independent prognostic factors for OS but not for RFS in GC patients. In younger patients (⩽55 years), there is no significant difference between the RFS and OS outcome of men and women with GC. However, older men have a poorer OS and older women (>55 years) have a poorer RFS. In addition, younger female GC patients have a higher proportion of GC-related deaths than younger male patients. We found from our current analysis that female sex is an independent risk factor for nodal involvement in younger GC patients. Hence, young women with EGC should be more intensively treated and monitored than other patient groups with GC and should not be treated by endoscopic resection.
Footnotes
Ethical Standards: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent or substitute for it was obtained from all patients for being included in the study.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Do Dam Suh, Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
Seong Tae Oh, Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
Jeong Hwan Yook, Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
Byung-Sik Kim, Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
Beom Su Kim, Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, 88, Olympic-ro 43-gil, Songpa-Gu, Seoul 05505, Korea.
References
- Adachi Y., Mori M., Maehara Y., Kitano S., Sugimachi K. (1997) Prognostic factors of node-negative gastric carcinoma: univariate and multivariate analyses. J Am Coll Surg 184: 373–377. [PubMed] [Google Scholar]
- Adachi Y., Yasuda K., Inomata M., Sato K., Shiraishi N., Kitano S. (2000) Pathology and prognosis of gastric carcinoma: well versus poorly differentiated type. Cancer 89: 1418–1424. [PubMed] [Google Scholar]
- Baba H., Maehara Y., Takeuchi H., Inutsuka S., Okuyama T., Adachi Y., et al. (1995) Effect of lymph node dissection on the prognosis in patients with node-negative early gastric cancer. Surgery 117: 165–169. [DOI] [PubMed] [Google Scholar]
- Edge S., Byrd D., Compton C., Fritz A., Greene F., Trotti A. (2010) AJCC Cancer Staging Manual. New York, NY: Springer [Google Scholar]
- Everett S., Axon A. (1997) Early gastric cancer in Europe. Gut 41: 142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay J., Shin H., Bray F., Forman D., Mathers C., Parkin D. (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127: 2893–2917. [DOI] [PubMed] [Google Scholar]
- Folli S., Dente M., Dell’Amore D., Gaudio M., Nanni O., Saragoni L., et al. (1995) Early gastric cancer: prognostic factors in 223 patients. Br J Surg 82: 952–956. [DOI] [PubMed] [Google Scholar]
- Folli S., Morgagni P., Roviello F., De Manzoni G., Marrelli D., Saragoni L., et al. (2001) Risk factors for lymph node metastases and their prognostic significance in early gastric cancer (EGC) for the Italian Research Group for Gastric Cancer (IRGGC). Jpn J Clin Oncol 31: 495–499. [DOI] [PubMed] [Google Scholar]
- Fujii M., Sasaki J., Nakajima T. (1999) State of the art in the treatment of gastric cancer: from the 71st Japanese Gastric Cancer Congress. Gastric Cancer 2: 151–157. [DOI] [PubMed] [Google Scholar]
- Hamilton S., Aaltonen L. (2000) Pathology and genetics of tumours of the digestive system. In: Kleihues P., Sobin L. (eds) World Health Organization Classification of Tumours. Lyon: IARC Press. [Google Scholar]
- Hioki K., Nakane Y., Yamamoto M. (1990) Surgical strategy for early gastric cancer. Br J Surg 77: 1330–1334. [DOI] [PubMed] [Google Scholar]
- Hochwald S., Brennan M., Klimstra D., Kim S., Karpeh M. (1999) Is lymphadenectomy necessary for early gastric cancer? Ann Surg Oncol 6: 664–670. [DOI] [PubMed] [Google Scholar]
- Inoue K., Tobe T., Kan N., Nio Y., Sakai M., Takeuchi E., et al. (1991) Problems in the definition and treatment of early gastric cancer. Br J Surg 78: 818–821. [DOI] [PubMed] [Google Scholar]
- Ishigami S., Natsugoe S., Hokita S., Tokushige M., Saihara T., Watanabe T., et al. (1999) Carcinomatous lymphatic invasion in early gastric cancer invading into the submucosa. Ann Surg Oncol 6: 286–289. [DOI] [PubMed] [Google Scholar]
- Japanese Gastric Cancer Association. (2011a) Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 14: 101–112. [DOI] [PubMed] [Google Scholar]
- Japanese Gastric Cancer Association. (2011b) Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 14: 113–123. [DOI] [PubMed] [Google Scholar]
- Jentschura D., Heubner C., Manegold B., Rumstadt B., Winkler M., Trede M. (1997) Surgery for early gastric cancer: a European one-center experience. World J Surg 21: 845–849. [DOI] [PubMed] [Google Scholar]
- Katai H., Yoshimura K., Maruyama K., Sasako M., Sano T. (2000) Evaluation of the New International Union against Cancer TNM staging for gastric carcinoma. Cancer 88: 1796–1800. [PubMed] [Google Scholar]
- Kim B., Oh S., Yook J., Kim B. (2014) Signet ring cell type and other histologic types: differing clinical course and prognosis in T1 gastric cancer. Surgery 155: 1030–1035. [DOI] [PubMed] [Google Scholar]
- Kim C., Lee S., Yang D. (2006) What is the prognosis for early gastric cancer with pN stage 2 or 3 at the time of pre-operation and operation. J Korean Gastric Cancer Assoc 6: 114–119. [Google Scholar]
- Kitamura K., Yamaguchi T., Taniguchi H., Hagiwara A., Sawai K., Takahashi T. (1997) Analysis of lymph node metastasis in early gastric cancer: rationale of limited surgery. J Surg Oncol 64: 42–47. [DOI] [PubMed] [Google Scholar]
- Kubota H., Kotoh T., Masunaga R., Dhar D., Shibakita M., Tachibana M., et al. (2000) Impact of screening survey of gastric cancer on clinicopathological features and survival: retrospective study at a single institution. Surgery 128: 41–47. [DOI] [PubMed] [Google Scholar]
- Lee J., Kim H., Kim Y., Hong J., Alshomimi S., An J., et al. (2016). Clinical significance of the prognostic nutritional index for predicting short- and long-term surgical outcomes after gastrectomy: a retrospective analysis of 7781 gastric cancer patients. Medicine (Baltimore) 95: e3539. doi: 10.1097/MD.0000000000003539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J., Lee D., Shin C., Kim N., Park Y., Jung H., et al. (2014) Clinicopathological features and surgical safety of gastric cancer in elderly patients. J Korean Med Sci 29: 1639–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehara Y., Orita H., Okuyama T., Moriguchi S., Tsujitani S., Korenaga D., et al. (1992) Predictors of lymph node metastasis in early gastric cancer. Br J Surg 79: 245–247. [DOI] [PubMed] [Google Scholar]
- Noda S., Soejima K., Inokuchi K. (1980) Clinicopathological analysis of the intestinal type and diffuse type of gastric carcinoma. Jpn J Surg 10: 277–283. [DOI] [PubMed] [Google Scholar]
- Oliveira F., Ferrao H., Furtado E., Batista H., Conceicao L. (1998) Early gastric cancer: report of 58 cases. Gastric Cancer 1: 51–56. [DOI] [PubMed] [Google Scholar]
- Pertl A., Jagoditsch M., Jatzko G., Denk H., Stettner H. (1999) Long-term results of early gastric cancer accomplished in a European institution by Japanese-type radical resection. Gastric Cancer 2: 115–121. [DOI] [PubMed] [Google Scholar]
- Piso P., Werner U., Benten D., Bektas H., Meuer U., Klempnauer J. (2001) Early gastric cancer–excellent prognosis after curative resection in 87 patients irrespective of submucosal infiltration, lymph-node metastases or tumor size. Langenbecks Arch Surg 386: 26–30. [DOI] [PubMed] [Google Scholar]
- Popiela T., Kulig J., Kolodziejczyk P., Sierzega M. (2002) Long-term results of surgery for early gastric cancer. Br J Surg 89: 1035–1042. [DOI] [PubMed] [Google Scholar]
- Ribeiro M., Sarmento J., Sobrinho Simoes M., Bastos J. (1981) Prognostic significance of Lauren and Ming classifications and other pathologic parameters in gastric carcinoma. Cancer 47: 780–784. [DOI] [PubMed] [Google Scholar]
- Roviello F., Rossi S., Marrelli D., Pedrazzani C., Corso G., Vindigni C., et al. (2006) Number of lymph node metastases and its prognostic significance in early gastric cancer: a multicenter Italian study. J Surg Oncol 94: 275–280; discussion 274. [DOI] [PubMed] [Google Scholar]
- Saragoni L., Gaudio M., Morgagni P., Folli S., Vio A., Scarpi E., et al. (2000) The role of growth patterns, according to Kodama’s classification, and lymph node status, as important prognostic factors in early gastric cancer: analysis of 412 cases. Gastric Cancer 3: 134–140. [DOI] [PubMed] [Google Scholar]
- Seto Y., Nagawa H., Muto T. (1997) Impact of lymph node metastasis on survival with early gastric cancer. World J Surg 21: 186–190. [DOI] [PubMed] [Google Scholar]
- Tsujitani S., Oka S., Saito H., Kondo A., Ikeguchi M., Maeta M., et al. (1999) Less invasive surgery for early gastric cancer based on the low probability of lymph node metastasis. Surgery 125: 148–154. [PubMed] [Google Scholar]