Abstract
Cirrhotic patients with refractory ascites (RA) can be treated with repeated large volume paracentesis (LVP), with the insertion of a transjugular intrahepatic portosystemic shunt (TIPS) or with liver transplantation. However, side effects and complications of these therapeutic options, as well as organ shortage, warrant the development of novel treatments.
The automated low-flow ascites pump (alfapump®) is a subcutaneously-implanted novel battery-driven device that pumps ascitic fluid from the peritoneal cavity into the urinary bladder. Ascites can therefore be aspirated in a time- and volume-controlled mode and evacuated by urination.
Here we review the currently available data about patient selection, efficacy and safety of the alfapump and provide recommendations for the management of patients treated with this new method.
Keywords: albumin, cirrhosis, decompensation, diuretics, spontaneous bacterial peritonitis
Introduction
Refractory ascites (RA) is a serious complication of liver cirrhosis associated with poor prognosis.1,2 Ascites is considered refractory when no longer manageable with a low salt diet combined with medications including aldosterone antagonists and loop diuretics despite maximal dosing or due to inability of further increasing diuretic therapy because of a deterioration of the kidney function, hyponatremia, or hyperkalaemia. The development of RA represents a critical point in the natural history of patients with cirrhosis, because it is associated with a significantly worse outcome compared with patients without RA.1
In the case of RA, standard treatment consists of repeat paracentesis. However, paracentesis only provides temporary relief of symptoms and may be required as often as weekly. Although paracentesis-related complications including bleeding, infections and the post-paracentesis circulatory dysfunction occur relatively rarely,3,4 they may significantly affect not only the prognosis, but also the quality of life of patients with RA. Besides frequent medical consultations and procedures, further deterioration of the general conditions of these patients may be the consequence of malnutrition and sarcopenia. In fact, lack of appetite, early satiety, reduced food intake due to an increase abdominal volume and pressure, as well as systemic inflammation and chronic catabolism are commonly occurring problems in patients with decompensated ascites.5,6 Moreover, the need for repeated paracenteses is associated with a relevant burden of health-related costs.7
Until recently, the only alternative treatment option for patients with RA has been the placement of a transjugular intrahepatic portosystemic shunt (TIPS). Results from several randomized trials comparing TIPS with repeat paracentesis8–12 showed that patients treated with TIPS had a significant reduction in the need for paracentesis compared with the standard treatment group. In addition, a meta-analysis provided data demonstrating that besides a better control of ascites, treatment with TIPS was also associated with a better survival.13 On the other hand, compared with the paracentesis group, treatment with TIPS was accompanied by more frequent and severe episodes of hepatic encephalopathy.13 Although not specifically addressed in the randomized trials, better survival in patients with TIPS might be due to their improved nutritional conditions after this procedure.14 Moreover, in a recently published randomized trial, the proportion of patients with cirrhosis and recurrent ascites who were transplantation-free for 1 year, was significantly higher in patients treated with TIPS than in patients given repeated large volume paracentesis (LVP) and albumin.15
However, not all patients suffering from RA can be treated with TIPS. Patients with prior episodes of hepatic encephalopathy, high Model for End-Stage Liver Disease (MELD) score, age >65 years, platelets <125 g/l, glomerular filtration rate (GFR) < 90 ml/min or low haemoglobin might be at risk for complications after TIPS.16–18 Liver transplantation also represents a valuable treatment option for cirrhotic patients with RA and remains the ultimate and only curative treatment. However, only a minority of such patients will receive a liver graft due to organ shortage, advanced age or contraindications.
Therefore, alternative treatment options for RA are urgently needed.
Development of the alfapump
Medical devices to treat RA have been investigated for more than four decades. In 1974, Leveen and colleagues proposed a peritoneovenous shunt to drain the ascites fluid into the venous system.19 Similar shunts were subsequently developed, but their clinical use has been almost completely abandoned mainly due to infections and obstructions of the device, venous thrombosis and disseminated intravascular coagulation.20 In 1998, Rozenblit and colleagues proposed the first mechanical device that was designed to actively transport ascites from the peritoneal cavity into the urinary bladder.21 However, none of these systems has found its way to a broader clinical applicability mainly due to technical issues.
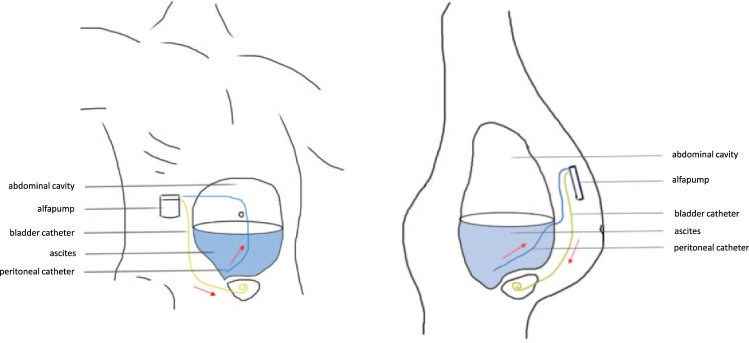
The recent development of the automated low-flow ascites pump (alfapump® system, Sequana Medical AG, Zurich, Switzerland) has specifically addressed these problems. The alfapump system is composed of a subcutaneously-implanted battery-powered pump. This is connected to a catheter placed in the abdominal cavity that aspirates ascitic fluid and transports it through a second subcutaneous catheter into the bladder (Figure 1). Ascites is then eliminated from the patient through normal urination. The alfapump is provided with internal sensors for the monitoring of the pressures in the peritoneal cavity and in the bladder in order to prevent pump operation when there is no ascites or when the urinary bladder becomes full.
Figure 1.
Schematic representation of the alfapump® system. Ascitic fluid is aspirated through a catheter placed into the abdominal cavity (blue) and further transported into the urinary bladder through a subcutaneous catheter (yellow). The battery-driven pump is implanted in the right middle quadrant of the abdomen and is recharged through the skin (see text for further details).
The alfapump is an automated system that can be programmed by the treating physician according to the patient’s needs. In contrast with LVP, in which several litres of ascites are evacuated from the abdominal cavity in a short period of time with simultaneous intravenous administration of albumin, the alfapump works in cycles of small volumes (generally 5–10 ml) that are pumped every 5–10 min into the urinary bladder, without the obligatory administration of albumin (Figure 2). Moreover, the pump is usually programmed to transport fluid only during the day and is deactivated during sleep for the patient’s comfort. The daily ascites volume that is removed usually ranges between 500 ml and 2.5 l.
Figure 2.
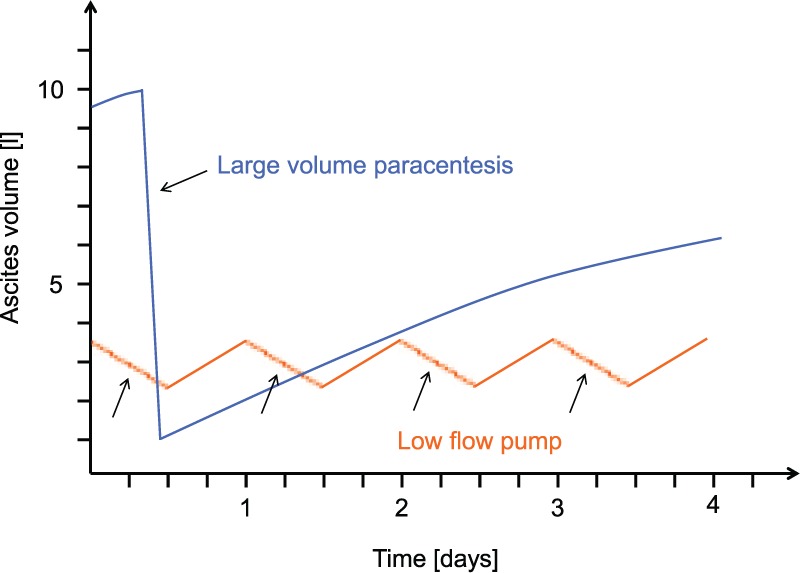
Changes of ascites volume in the peritoneal cavity in patients undergoing LVP (blue line) and in patients treated with the alfapump® system (orange line). With LVP, several litres of ascites are evacuated over a short period of time until the liquid accumulates again in the abdominal cavity, while with the alfapump system small amounts of ascites generally varying between 800 ml and 2 l per day are pumped into the bladder and are eliminated by urination during the day, whereas the pump is inactive overnight.
LVP, large volume paracentesis.
Charging of the pump battery is performed by means of a user-friendly charging device (Smart Charger, Sequana Medical AG, Zürich, Switzerland) by transcutaneous induction. The Smart Charger is placed over the area of the pump twice per day for a duration of not more than 20 min. During this time, data reflecting pump function parameters are automatically transmitted from the pump to the charger, including information about the volume transported, and pressures in the bladder and abdominal cavity. These data monitor pump function and may provide early warning to the physician regarding possible technical issues with the pump gears, catheters, or programming. This information is forwarded to a central databank and communicated, if needed, to the treating physician, who can then decide to intervene and contact the patient if appropriate.
The costs of the alfapump include the current price of the device (EUR22,500), the operating room charges and the expenses of a short hospital admission and they should be balanced against the costs for infrastructure, personnel and albumin of repeated LVP.
Surgical procedure
Patient selection, probably the most crucial step prior to any surgery, is particularly important when planning the implantation of an alfapump. With the majority of patients with RA suffering from advanced liver disease, surgical risks may potentially outweigh the benefits of the pump, making detailed and objective preoperative patient information essential. Furthermore, judgement of patient compliance remains of outmost importance. A noncompliant patient is less likely to seek timely medical care in the case of pump dysfunction, potentially aggravating the underlying problem, making surgical reintervention a more complex procedure. In our experience, determining which patients are best suited for surgery is based on the combined judgement of an experienced hepatologist and a surgeon with knowledge of liver disease, ideally in a hospital with a liver transplant programme. While this is not an absolute prerequisite, it does help to identify potentially serious postoperative problems, allowing for a quick reintervention or adaptation of the medical therapy.
Although previous abdominal surgery does not exclude pump implantation, a patient with known severe abdominal adhesions and loculated ascites should not be considered for alfapump system therapy. While chylous ascites does not pose a problem, patients with fibrinous ascites are more likely to suffer from a clogged peritoneal catheter. Should this occur, more often than not, a local revision of the peritoneal catheter usually suffices to solve the problem. While not seen as a contraindication, patient selection is also crucial in individuals with recurrent spontaneous bacterial peritonitis or severe urinary tract infection and pump implant should always be postponed in the setting of an acute infection. Men with known prostate hyperplasia and outlet obstruction should be seen by a urologist prior to pump implantation. Untreated severe urinary tract obstruction is an absolute contraindication for pump implantation as the increased urinary volume may result in post-renal kidney failure. Local skin infections (bacterial or fungal) should be appropriately treated before implantation. Previous major abdominal surgery represents a relative contraindication due to the possibility of important adhesions. However, in such cases the alfapump implantation can be performed under laparoscopic control.
Data about combining umbilical hernia repair and alfapump implantation are very limited. In an elective setting (reducible hernia, not symptomatic) combined surgery may not be strictly indicated, because in most cases the decrease of ascites has a positive effect on hernia size and symptoms.
During the surgical procedure, the bladder should be filled retrograde with sufficient methylene blue-coloured saline solution to be easily palpable above the symphysis. Correct subcutaneous positioning of the pump in the right (or left) upper quadrant is crucial and needs to be adapted taking into account that most patients do not have a lot of subcutaneous fat. The pump pocket should be as small as possible to prevent pump migration, ascites accumulation and possible tension on the two catheters. The peritoneal catheter entry point into the abdomen (chosen a few cm supra-umbilica) needs to be sufficiently tightly sutured to prevent leakage of ascitic fluid. Previous hernia repair or other forms of midline surgery are not a contraindication. Peritoneal catheter placement can be carried out under direct vision, provided the abdomen is otherwise free of extensive adhesions.
In our experience, it is important to ensure that the abdomen remains as free from any tense ascites as possible in the immediate post-operative period to allow for healing of the incisions. This can be accomplished by draining the abdomen of ascites during placement of the alfapump system (with the simultaneous administration of 10 g albumin/l of ascites evacuated), followed by activation of the alfapump in the operating room. In view of poor wound healing in this population, leaving the skin sutures in place for a sufficiently long time (i.e. up to 3 weeks), helps prevent local wound dehiscence, particularly in the setting of ascitic leakage.
Results from clinical studies
After a period of several years of nonclinical development, the first alfapump systems were implanted in the framework of a feasibility study from 2008 to 2009 in the Czech Republic (unpublished data). From 2010 until 2013, safety and performance of this medical device were assessed in 40 patients in the PIONEER study.22 Furthermore, hemodynamic and renal effects are being investigated in a clinical trial in Barcelona, Spain [ClinicalTrials.gov identifier: NCT01438970]. In the ongoing Post-Marketing Surveillance Registry (PMSR) study, results of 100 real life patients treated with the alfapump are being collected and interim results have been published so far in abstract form.23
The first randomized controlled trial (RCT) was carried out from 2012 to 2015. In this study, standard therapy with repeat paracentesis has been compared with the alfapump treatment in 49 patients.24 Since patients with RA undergoing alfapump implantation are frequently in poor nutritional condition,25 an ancillary study was designed to assess the effect of the alfapump on nutritional parameters.
In the PIONEER study,22 the results from 40 patients treated with an alfapump over a period of 6 months were analysed. After the implantation of the pump, the number of LVPs dropped from 3.4 to 0.2 per month (p < 0.001). About one third (n = 13) of the pump systems had to be explanted, most often due to infection, followed by catheter dislodgement or consecutive withdrawal of consent by the patient.
During the study period, eight patients (20%) died, three due to sepsis, two due to progressive liver disease, two for renal failure and one of undetermined cause. After the implantation of the pump, the MELD score and Child–Pugh score did not change significantly. However, there was a significant and continuous decrease of serum albumin over the period of 6 months and a significantly lower international normalized ratio (INR) at 6 months; the former probably reflecting the stopped regular substitution of albumin, the latter an improved nutrition in the remaining 14 patients, most likely representing a selection of well-performing patients. Kidney dysfunction was observed in 11 (27%) patients. Use of nonsteroidal anti-inflammatory drugs was identified as possible cause in four of these patients. With the exception of one acute renal failure and consequent death due to hepatorenal syndrome, all episodes of kidney dysfunction were reversible. Mean creatinine levels increased from 106 µmol/l to 123 µmol/l and 127 µmol/l at 1 and 3 months post-implant, respectively. Lower creatinine levels at 6 months post-implant might again reflect the selection of well-performing patients (14/40) rather than an improved kidney function over time.
Patients were divided into a cohort I, including the first 21 patients, and a cohort II, in which patients were managed according to some changes recommended by the data safety monitoring board. These included routine antibiotic prophylaxis with norfloxacin, strict avoidance of nonsteroidal anti-inflammatory drugs and the intravenous administration of albumin if ascites was aspirated during the surgical intervention. With these modifications, the total number of infections as well as episodes of systemic inflammatory response syndrome (SIRS) could be significantly reduced in the second cohort. Furthermore, bladder catheter dislocation did not occur any more in the second cohort (compared with 24% in the first cohort) after the catheter was anchored to the suprapubic aponeurosis and its length was increased.
The PMSR study was an observational cohort study in patients in four European countries (Germany, Switzerland, UK and Spain). In this study, 100 patients with an implanted alfapump for the treatment of RA have been included and a total period of observation of 24 months is currently ongoing. So far, results from the first 56 patients with a follow up of at least 12 months have been published in abstract form.26 Currently, 5.4% of patients reached the 24-month observation period, with a further 5.4% still on treatment. The proportion of these patients, who received a liver transplant was 16.1% while 41.1% died during this period of time. Of the 17 patients (30.4%) who were withdrawn from the study, 7 died after pump withdrawal. The pump was explanted in 17 patients due to an adverse event, in 9 patients as a consequence of liver transplant and in 1 patient, because the pump was not needed any more due to improvement of RA.
Paracentesis frequency dropped from 2.9 per month in the last 3 months prior to the implantation to 0.3 per month after the implantation. A reintervention was required in 39.3% of patients after the initial implantation. Serum albumin decreased over time (mean drop of 3.2 g/l at 6 months), whereas creatinine rose (mean increase of 47.3 µmol/l at 6 months). During the observation period, 30 patients deceased (including 7 patients with prior pump explantation). Progression of the liver disease was the most often observed cause of death (50.3%), followed by infection (10.2%) and renal failure (3.4%).
In the RCT comparing LVP with the alfapump system 49 patients were included [24 in the alfapump (AP) group, 25 in the LVP group]. The primary endpoint was time to first LVP. One month after implantation, the probability for LVP was significantly higher in the LVP group than in the AP group (0.75 versus 0.13, p < 0.0001). The median number of paracenteses per month was also significantly lower in the AP group (0.2 versus 1.4). In this preliminary analysis, there was no difference in overall survival, serum albumin or creatinine between the groups at 6 months. Acute kidney injury (AKI) was observed in 19 patients in the AP group (18 stage 1, one stage 2) and in 12 patients of the LVP group (9 stage 1, three stage 2). In patients with AP, most AKIs were observed within 7 days of implant and were of transient nature. Of all patients with AKI, 75% fully recovered in the AP group but only 30% in the LVP group. The effect of the alfapump on nutrition has been investigated in a sub-study of the RCT (n = 16 patients). At 3 months, patients in the alfapump group showed a significantly better nutritional status compared with the standard of care group, measured as a change in mid-arm muscle circumference and handgrip force.
The rate of infection was similar in both groups. Gastrointestinal haemorrhage was observed in four patients in the AP group compared with none in the LVP group. AKI occurred in five patients in the AP group (eight events) and in two patients in the LVP group (two events). Serious adverse device events were observed in five patients (three blockages of peritoneal catheter and bladder catheter, each). These events required a reintervention for revision.
Discussion
The alfapump system represents a major technical advancement in the control of ascites and has the potential to significantly improve the management of patients with RA. In this review, we summarize the advantages, drawbacks and future challenges based on results from the clinical experience accumulated so far.
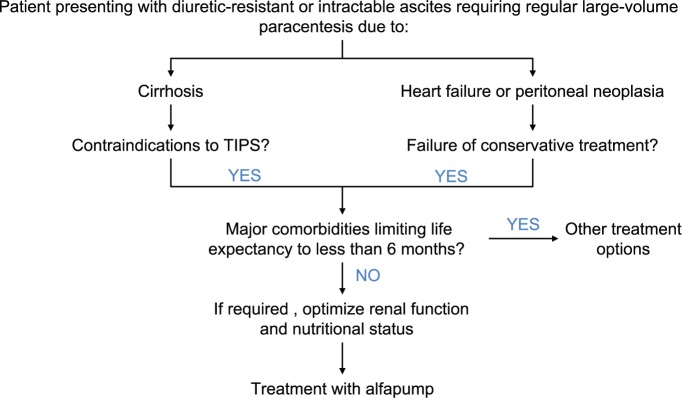
Patient selection and proof of indication before implantation represent a crucial step that determines the success of this procedure (Table 1). Prior to the implantation of the alfapump, the refractory nature of ascites should be confirmed for at least 1 month. If hyponatremia is not less than 130 mm, make sure that the patient is following a low salt diet. Further potential regarding optimization of diuretic treatment with the combination of an aldosterone antagonist and a loop diuretic should be excluded. In all patients, treatment with TIPS should be evaluated as an alternative treatment option in patients without hepatic encephalopathy, with bilirubin <50 µm and platelets >75 G/l.27 Moreover, major cardiac, pulmonary or neoplastic co-morbidities with an expected survival of <6 months should be considered as relative contraindications (Figure 3), as well as extreme obesity due to technical and surgical considerations. Further studies will provide additional data to better define which patients are best suited for treatment with an alfapump.
Table 1.
Recommendations for the management of patients before and after the implantation of the automated low-flow ascites pump.
| Before implantation | At implantation | After implantation |
|---|---|---|
| Confirm RA and assess patients for suitability | Ensure abdomen is flat and not under tension | Continue nutritional support |
| Optimize low salt diet and diuretic therapy with aldosterone antagonist and loop diuretic | Turn pump on in OR at volumes not less than 800–1000 ml/day | Adjust pump volume according to the clinical evolution |
| Evaluate TIPS as an equivalent option | Administer albumin 10 g/l ascites removed in the OR | Administer long-term antibiotic prophylaxis consistent with recommendation for prevention of SBP |
| Assess nutritional status and start nutritional support therapy | Check renal function and electrolytes every second week for the first 2 months | |
| Exclude or treat urinary outflow tract obstruction in male patients | Evaluate the use of terlipressin in case of perioperative deterioration of kidney function | |
| Remove sutures not before 14 days after implant, usually after 21 days | Consider administration of albumin as needed according to clinical guidelines (treatment of spontaneous bacterial peritonitis or hepatorenal syndrome) |
OR, operating room; RA, refractory ascites; SBP, spontaneous bacterial peritonitis; TIPS, transjugular intrahepatic portosystemic shunt.
Figure 3.
Flow chart describing therapy options in patients with diuretic resistant or intractable ascites and selection criteria for the implantation of an alfapump.
In patients with RA, malnutrition is a common finding. However, criteria used for the diagnosis of malnutrition in other diseases are difficult to apply in patients with decompensated ascites due to the undulating body weight and therefore also body mass index. What can be assessed in these patients is a deficit of caloric and protein intake. In addition, deficiencies of vitamins and micronutrients (e.g. vitamin D3 and zinc) should be addressed. Due to regular substitution of albumin, blood albumin levels are usually not helpful to adequately assess the preserved synthetic liver function unless very low. As a substitute parameter for albumin, pre-albumin can be measured. We highly recommend a thorough assessment regarding sarcopenia. Especially in obese patients, sarcopenia can be overlooked easily. Advanced sarcopenia can be identified by characteristic temporal atrophy, decreased psoas parameter in computerized tomography scans or reduced hand grip force.28
We are also aware of alfapump system implantation in select patients with severe right-sided heart failure and persistent congestion with RA. In these few cases, the pump has functioned as intended, successfully moving ascites from the peritoneum to the bladder. As in ascites due to cirrhosis, efficacy can be limited by patient nonadherence to treatment regimens and dietary advice. Although information on efficacy and safety in this population is limited, it is reasonable to assume that the pump is able to move ascitic fluid in the same way as in ascites due to cirrhosis.
In patients with confirmed malnutrition and or sarcopenia, we strongly recommend pre-implant nutritional support, ideally with protein enriched oral drinks or, if this is not possible, with nasogastric tube feeding, and to substitute vitamin and micronutrient deficiencies. As a general rule, nutritional therapy is continued after the implant of the alfapump until deficits are corrected together with clinical improvement or liver transplantation.
The fluid management of patients in the perioperative phase regarding albumin and volume replacement and pump programming remains challenging. We recommend the administration of albumin at a rate of 10 g/l ascites aspirated from the abdominal cavity during surgery. At the same time, we program the pump in the OR (operating room or theater) at an initial volume of 800–1000 ml/day to ensure the abdomen does not accumulate ascites and we gradually adjust it according to the needs of the patient. If fluid accumulates early on, one can increase the programming of the pump to remove a maximum of 4 l/day.
In case of ascites leakage through the abdominal surgical suture, good control of ascites is important to decrease the intra-abdominal pressure and therefore to facilitate wound healing.
During the early postoperative period some patients show a temporary deterioration of kidney function and proactive administration of albumin may help prevent these events. In some cases, the use of terlipressin, administered as a continuous perfusion at a rate ranging from 2–8 mg/24 h, has been associated with a significant decrease in serum creatinine (unpublished data). So far however, data confirming the effect of these treatments are not available.
Diuretic treatment is continued in patients with an alfapump in situ, as long as it is not contraindicated due to a deterioration of kidney function or due to an imbalance in serum electrolytes. In patients with increasing serum creatinine levels, diuretic therapy is stopped and the daily ascites volume of the pump is increased according to the body weight of the patient. We recommend checking kidney function parameters every second week during the first 2–3 months after the implantation of the alfapump system, and as needed later on.
Based on the experience reported in the PIONEER study, long-term antibiotic prophylaxis is recommended for all patients with an alfapump system in place. Norfloxacin (400 mg/day) is the antibiotic of choice; however, other antibiotics such as ciprofloxacin or amoxicillin-clavulanic acid may be considered in special situations. Broad spectrum antibiotics are not recommended for prophylactic treatment due to the selection of difficult-to-treat strains of bacteria. In case of suspected ascites infection, polymorphic neutrophil count should be performed on peritoneal fluid and the same criteria for antibiotic treatment should be used as for spontaneous bacterial peritonitis.
Pump or catheter dysfunctions are usually detected by the analysis of the technical data that is transferred on a daily basis to the central database, before they become clinically evident. Patients can then be contacted by their physicians to double check whether action is needed.
The most frequently observed complication in patients with an alfapump is obstruction of the peritoneal catheter. Catheters may block due to aspiration of parts of the omentum, as well as due to fibrinous particles that may be present in the ascitic fluid. In case of mechanical inflow obstruction, the catheter may need a surgical revision in which it can be cleaned or exchanged. In case of blocking of the pump gears for the same reasons, the device automatically activates a so-called shake mode, in which the gears rotate forwards and backwards with increased torque in order to free the pump from the debris. In addition, adaptation of the pump volume, pressure parameters, and signal thresholds may be needed to reach proper pump function. A list of possible side effects and complications after the implant of the alfapump and suggestions of possible solutions is presented in Table 2.
Table 2.
Incidence and management of possible side effects/complications after the implantation of the alfapump.
| Side effects/complication | Early (first 2–4 weeks) | Late (months) | Management |
|---|---|---|---|
| Impaired kidney function, hepatorenal syndrome | ++ | + | Perioperative volume substitution with albumin and saline, terlipressin |
| Infection of pump pocket | + | (+) | Antibiotics, pump explantation |
| Infection general | (+) | (+) | Prophylaxis with norfloxacin (400 mg/d) |
| Ascites leakage through skin sutures | + | – | Increase pumped volume, re-suture if needed |
| Clogged peritoneal tube | – | ++ | Wait a few days, if not resolved surgical revision or paracentesis |
| Dislocation/disconnection of peritoneal or bladder tube | rare | rare | Surgical revision |
| Ascites leakage into the pump pocket | (+) | + | Prevent by minimizing the pump pocket at implantation |
| Obstructive urinary tract problem in male patients | – | (+) | Prevention: urologic assessment pre implant |
| Clogged pump | – | + | Adjust pump pressure parameters while in shake mode. Pump replacement might be required |
Conclusion
So far, no data about a direct comparison of the alfapump with TIPS implantation are available. The AGUA RCT comparing the need for paracentesis and safety in patients with either implantation of an alfapump or placement of a TIPS [ClinicalTrials.gov identifier: NCT02612519] will provide data to answer this important question. This study will also provide more information regarding patient selection criteria and the identification of patient groups that may benefit most from treatment with an alfapump.
The alfapump is an innovative treatment option for patients with RA and has shown excellent efficacy so far in the reduction in the need for LVP in clinical trials including the real world PMSR. To date, it is not clear, whether the alfapump has a significant survival benefit in patients with RA. However, quality of life may be improved due to a significantly decreased need for paracentesis and the avoidance of tense ascites. This novel treatment option cannot be regarded as the standard of care yet and further research is required to better define the role of the alfapump in the management of RA. Hence, pump implantation should be restricted for the moment to tertiary referral centres. The alfapump system can also be used as a bridging therapy for patients listed for liver transplant. Complications, such as infections and catheter obstruction, may occur and require treatment. Data for other indications (i.e. malignant ascites or pleural effusion), is scarce and no conclusion can be drawn regarding use of the alfapump in these patient populations at this time.
Acknowledgments
The authors are very thankful to Stefanie Mühlemann for the management of data of the PMSR study. Special thanks also to Dr Neil Inhaber for critically reviewing the manuscript.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: Guido Stirnimann received speaker fees from Sequana Medical (Zürich, Switzerland). Andrea De Gottardi received speaker fees and a research grant from Sequana Medical. Vanessa Banz and Federico Storni have no conflict of interest to declare.
Contributor Information
Guido Stirnimann, Hepatology, Clinic of Visceral Surgery and Medicine, Inselspital, Bern, Switzerland Department of Clinical Research, University of Bern, Switzerland.
Vanessa Banz, Visceral Surgery, Clinic of Visceral Surgery and Medicine, Inselspital, Bern, Switzerland Department of Clinical Research, University of Bern, Switzerland.
Federico Storni, Visceral Surgery, Clinic of Visceral Surgery and Medicine, Inselspital, Bern, Switzerland Department of Clinical Research, University of Bern, Switzerland.
Andrea De Gottardi, Hepatology, Clinic of Visceral Surgery and Medicine, Inselspital, Department of Clinical Research, University of Bern, 3010 Bern, Switzerland.
References
- 1. Ginès P, Cárdenas A, Arroyo V, et al. Management of cirrhosis and ascites. N Engl J Med 2004; 350: 1646–1654. [DOI] [PubMed] [Google Scholar]
- 2. Hernández-Gea V, Aracil C, Colomo A, et al. Development of ascites in compensated cirrhosis with severe portal hypertension treated with β-blockers. Am J Gastroenterol 2012; 107: 418–427. [DOI] [PubMed] [Google Scholar]
- 3. De Gottardi A, Thévenot T, Spahr L, et al. Risk of complications after abdominal paracentesis in cirrhotic patients: a prospective study. Clin Gastroenterol Hepatol 2009; 7: 906–909. [DOI] [PubMed] [Google Scholar]
- 4. Ginès A, Fernández-Esparrach G, Monescillo A, et al. Randomized trial comparing albumin, dextran 70, and polygeline in cirrhotic patients with ascites treated by paracentesis. Gastroenterology 1996; 111: 1002–1010. [DOI] [PubMed] [Google Scholar]
- 5. Kim HY, Jang JW. Sarcopenia in the prognosis of cirrhosis: going beyond the MELD score. World J Gastroenterol 2015; 21: 7637–7647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Norman K, Pirlich M. Gastrointestinal tract in liver disease: which organ is sick? Curr Opin Clin Nutr Metab Care 2008; 11: 613–619. [DOI] [PubMed] [Google Scholar]
- 7. Fagan KJ, Zhao EY, Horsfall LU, et al. Burden of decompensated cirrhosis and ascites on hospital services in a tertiary care facility: time for change? Intern Med J 2014; 44: 865–872. [DOI] [PubMed] [Google Scholar]
- 8. Rössle M, Ochs A, Gülberg V, et al. A comparison of paracentesis and transjugular intrahepatic portosystemic shunting in patients with ascites. N Engl J Med 2000; 342:1701–1707. [DOI] [PubMed] [Google Scholar]
- 9. Salerno F, Merli M, Riggio O, et al. Randomized controlled study of TIPS versus paracentesis plus albumin in cirrhosis with severe ascites. Hepatol Baltim Md 2004; 40: 629–635. [DOI] [PubMed] [Google Scholar]
- 10. Sanyal AJ, Genning C, Reddy KR, et al. The North American study for the treatment of refractory ascites. Gastroenterology 2003; 124: 634–641. [DOI] [PubMed] [Google Scholar]
- 11. Ginès P, Uriz J, Calahorra B, et al. Transjugular intrahepatic portosystemic shunting versus paracentesis plus albumin for refractory ascites in cirrhosis. Gastroenterology 2002; 123: 1839–1847. [DOI] [PubMed] [Google Scholar]
- 12. Narahara Y, Kanazawa H, Fukuda T, et al. Transjugular intrahepatic portosystemic shunt versus paracentesis plus albumin in patients with refractory ascites who have good hepatic and renal function: a prospective randomized trial.J Gastroenterol 2011; 46: 78–85. [DOI] [PubMed] [Google Scholar]
- 13. Salerno F, Cammà C, Enea M, et al. Transjugular intrahepatic portosystemic shunt for refractory ascites: a meta-analysis of individual patient data. Gastroenterology 2007; 133:825–834. [DOI] [PubMed] [Google Scholar]
- 14. Allard JP, Chau J, Sandokji K, et al. Effects of ascites resolution after successful TIPS on nutrition in cirrhotic patients with refractory ascites. Am J Gastroenterol 2001; 96: 2442–2447. [DOI] [PubMed] [Google Scholar]
- 15. Bureau C, Thabut D, Oberti F, et al. Transjugular intrahepatic portosystemic shunts with covered stents increase transplant-free survival of patients with cirrhosis and recurrent ascites. Gastroenterology. Epub ahead of print 20 September 2016. DOI: 10.1053/j.gastro.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 16. Luca A, Miraglia R, Maruzzelli L, et al. Early liver failure after transjugular intrahepatic portosystemic shunt in patients with cirrhosis with model for end-stage liver disease score of 12 or less: incidence, outcome, and prognostic factors. Radiology 2016; 280: 622–629. [DOI] [PubMed] [Google Scholar]
- 17. Bureau C, Thabut D, Oberti F, et al. TIPS with PTFE-covered stent improves liver transplant free survival in patients with cirrhosis and recurrent ascites: results of a multicentre randomized trial. Hepatology 2015; 62: 347A. [Google Scholar]
- 18. Hamel B, Guillaud O, Roman S, et al. Prognostic factors in patients with refractory ascites treated by transjugular intrahepatic porto-systemic shunt: from the liver to the kidney. Dig Liver Dis 2014; 46: 1001–1007. [DOI] [PubMed] [Google Scholar]
- 19. Leveen HH, Christoudias G, Ip M, et al. Peritoneo-venous shunting for ascites. Ann Surg 1974; 180: 580–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martin LG. Percutaneous placement and management of the Denver shunt for portal hypertensive ascites. AJR Am J Roentgenol 2012; 199: W449–W453. [DOI] [PubMed] [Google Scholar]
- 21. Rozenblit GN, Del Guercio LR, Rundback JH, et al. Peritoneal-urinary drainage for treatment of refractory ascites: a pilot study. J Vasc Interv Radiol 1998; 9: 998–1005. [DOI] [PubMed] [Google Scholar]
- 22. Bellot P, Welker M-W, Soriano G, et al. Automated low flow pump system for the treatment of refractory ascites: a multi-center safety and efficacy study. J Hepatol 2013; 58: 922–927. [DOI] [PubMed] [Google Scholar]
- 23. De Gottardi A, Banz V, Storni F, et al. P523 safety and efficacy of an automated low flow ascites (alfa) pump in cirrhotic patients with refractory ascites. J Hepatol 2014; 60: S244. [Google Scholar]
- 24. Adebayo D, Bureau C, Valla D, et al. P1319: alfapump® system versus large volume paracentesis in the treatment of refractory ascites. A multicenter randomised controlled study.J Hepatol 2015; 62: S849–S850. [Google Scholar]
- 25. Stirnimann J, Banz V, Storni F, et al. P520 nutritional deficiencies in cirrhotic patients with an alfapump®. J Hepatol 2014; 60: S243. [Google Scholar]
- 26. Stirnimann G, Berg T, Spahr L, et al. P0136: alfapump for the treatment of refractory ascites in cirrhotic patients. J Hepatol 2015; 62: S352. [Google Scholar]
- 27. Bureau C, Métivier S, D’Amico M, et al. Serum bilirubin and platelet count: a simple predictive model for survival in patients with refractory ascites treated by TIPS. J Hepatol 2011; 54: 901–907. [DOI] [PubMed] [Google Scholar]
- 28. Dasarathy S, Merli M. Sarcopenia from mechanism to diagnosis and treatment in liver disease. J Hepatol 2016; 65: 1232–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]