Abstract
Objective:
A systematic review was conducted to examine the efficacy, tolerability, and acceptability of asenapine compared with other antipsychotics in the treatment of psychotic disorders.
Methods:
Four databases, 8 trial registries, and conference presentations were searched for randomized clinical trials of asenapine versus any comparator for the treatment of any psychotic illness. Primary outcome measures were changes in the Positive and Negative Syndrome Scale (PANSS) total score and the incidence of withdrawal due to adverse effects.
Results:
Eight randomized clinical trials, encompassing 3765 patients, that compared asenapine with placebo (n = 5) and olanzapine (n = 3) were included. No differences were found between asenapine and olanzapine in terms of changes to PANSS total or PANSS negative subscale scores. Patients taking asenapine were more likely to experience worsening schizophrenia and/or psychosis than were those taking olanzapine. No differences were found between asenapine and olanzapine in rates of discontinuation due to adverse drug reactions or lack of efficacy, but those taking asenapine had higher rates of withdrawal for any reason than those taking olanzapine. Asenapine caused less clinically significant weight gain or increases in triglycerides than olanzapine and was more likely to cause extrapyramidal symptoms than olanzapine. In comparison to placebo, either no difference or superiority was demonstrated in favour of asenapine on all efficacy measures.
Conclusion:
The current evidence is limited, as asenapine has been compared only with placebo or olanzapine. In the randomized clinical trials analysed, asenapine was similar or superior to placebo and similar or inferior to olanzapine on most efficacy outcomes. While asenapine demonstrated fewer adverse metabolic outcomes than olanzapine, rates of extrapyramidal symptom–related adverse effects were higher.
Keywords: randomized clinical trials, psychosis, schizophrenia, schizoaffective disorder, asenapine, asenapine maleate, Sycrest, Saphris
Abstract
Objectif:
Une revue systématique a été menée pour examiner l’efficacité, la tolérabilité et l’acceptabilité de l’asénapine comparativement à d’autres antipsychotiques dans le traitement des troubles psychotiques.
Méthodes:
Quatre bases de données, 8 registres d’essais et des présentations à des congrès ont étés recherchés pour y repérer des essais cliniques randomisés d’asénapine contre tout comparateur pour le traitement des maladies psychotiques. Les principales mesures des résultats étaient les changements du score total à l’Échelle des symptômes positifs et négatifs (PANSS) et l’incidence de la cessation en raison d’effets indésirables.
Résultats:
Huit essais cliniques randomisés (ECR) englobant 3 765 patients et comparant l’asénapine avec un placebo (n = 5) et l’olanzapine (n = 3) ont été inclus. Il n’y avait aucune différence entre l’asénapine et l’olanzapine en ce qui concerne les changements de total à la PANSS ou des scores à la sous-échelle négative de la PANSS. Les patients prenant l’asénapine étaient plus susceptibles de subir une aggravation de la schizophrénie et/ou de la psychose que ceux qui prenaient l’olanzapine. Il n’y avait pas de différence entre l’asénapine et l’olanzapine pour ce qui est des taux de cessation attribuables aux effets indésirables ou au manque d’efficacité, mais ceux qui prenaient l’asénapine avaient des taux plus élevés de cessation pour n’importe quelle raison que ceux qui prenaient l’olanzapine. L’asénapine causait moins de prises de poids ou d’augmentations des triglycérides cliniquement significatives que l’olanzapine et était plus susceptible de causer des symptômes extrapyramidaux (SEP) que l’olanzapine. En comparaison avec le placebo, aucune différence ni supériorité n’a été démontrée en faveur de l’asénapine à toutes les mesures d’efficacité.
Conclusion:
Les données probantes actuelles sont limitées puisque l’asénapine n’a été comparée qu’avec un placebo ou l’olanzapine. Dans les ECR analysés, l’asénapine était semblable ou supérieure au placebo et semblable ou inférieure à l’olanzapine à la plupart des résultats d’efficacité. Même si l’asénapine démontrait moins de résultats métaboliques indésirables que l’olanzapine, les taux d’effets indésirables liés aux SEP étaient plus élevés.
Affecting approximately 1% of the population, schizophrenia is associated with significant social and occupational dysfunction.1 Various antipsychotic agents have been developed since the 1950s that have radically improved treatment of the positive symptoms of this disorder.2 However, negative and cognitive symptoms are more difficult to treat and respond poorly to currently available treatment options.2
While several new antipsychotics (such as asenapine, lurasidone, and iloperidone) have been developed in recent years, data are lacking that compare the efficacy and safety of these agents with established therapies in the treatment of psychotic disorders, making the decision to use the new agents difficult.
Asenapine (ASP) is the only antipsychotic available on the Canadian market for sublingual use, allowing for a more rapid onset of action than antipsychotics that are absorbed enterally.3,4 This could allow for a faster de-escalation of acute psychosis and improve adherence with medication in patients who, for a variety of reasons, may be unwilling to swallow medications.5 ASP is also of interest due to its broad range of receptor affinities, possibly enhancing its efficacy against negative and cognitive symptoms of schizophrenia.6
Available reviews on this topic are limited in several ways. Szegedi et al7 conducted a meta-analysis of placebo-controlled trials of 6 weeks’ duration and a multiple treatment meta-analysis to inferentially compare the relative efficacy of ASP to other antipsychotics, although direct comparisons may or may not have been performed.8 This meta-analysis did not study the long-term safety and efficacy of ASP, and a direct comparison between ASP and other agents was not provided. Instead, the investigators incorporated their results for ASP into a previously published review by Leucht et al.9 in order to compare its efficacy with that of other available antipsychotics.7 A systematic review and meta-analysis by DeHert et al.10 compared ASP, iloperidone, lurasidone, and paliperidone for metabolic adverse effects only. Leucht et al.11 conducted a multiple-treatments meta-analysis including both direct and indirect comparisons of 15 different antipsychotics in patients requiring acute treatment. This review excluded the patients with primarily negative symptoms of schizophrenia, patients with concurrent medical illnesses, patients with treatment refractory disease, and those who were clinically stable.11
The current review was conducted to examine the efficacy, tolerability, and acceptability of ASP in comparison to other currently available antipsychotics in the short- and long-term treatment of both acute and clinically stable psychotic disorders, in order to identify patient populations most likely to benefit from this medication.
Methods
Protocol and Registration
Methods of this review were specified in advance, and the protocol was registered with PROSPERO (CRD42014008970).
Search Strategy
Medline, EMBASE, PsychInfo, the Cochrane Central Register of Controlled Trials, and the Cochrane Schizophrenia Group Trials Registry were searched (2013 Oct 13) using the terms asenapine, asenapine maleate, Saphris®, and Sycrest®. No limits were placed on this search, and there were no language restrictions. Eight clinical trial registries and electronically available conference materials from the American Psychiatric Association, World Psychiatric Association, and Canadian Psychiatric Association (2009-2013) were searched for unpublished literature. Clinical experts, and the manufacturer of ASP, Lundbeck Canada, were also contacted to request unpublished trials. Reference lists of included and excluded articles were hand searched for randomized clinical trials matching the inclusion criteria.
Eligibility Criteria
Randomized clinical trials, regardless of publication status or language, were eligible for inclusion. We included patients of any age or disease severity (meeting criteria for the diagnosis of acute or chronic schizophrenia, psychosis, schizoaffective disorder, schizophreniform disorder, and other psychotic disorders as defined by the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition [DSM-IV], DSM-IV Text Revision [DSM-IV-TR], and/or ICD-10 Classification of Mental and Behavioural Disorders). Relevant interventions were ASP (any dosage form and dosing regimen) compared with any typical or atypical antipsychotic (any dosage form or dosing regimen) or placebo (PLC). The primary efficacy outcome measure was a change from baseline in the Positive and Negative Syndrome Scale (PANSS) total score. The primary safety outcome was the rate of discontinuation due to adverse effects. A list of secondary outcome measures can be found in Table 1.
Table 1.
Secondary and post hoc outcomes.
| Secondary efficacy outcomes |
|
| Secondary tolerability outcomes |
|
| Secondary acceptability outcomes |
|
| Post hoc outcomes |
|
PANSS = Positive and Negative Syndrome Scale.
Data Extraction
Data extraction was performed in duplicate by 2 authors (C.O. and S.D.). When trials used both mixed model of repeated measures and last observation carried forward to impute missing data, the former was used; if only last observation carried forward was used for missing data imputation, this was used as the data source. For trials in which patients were assigned to 5-mg BID or 10-mg BID arms, the 10-mg BID arm was selected for primary comparison, as this is the maximum recommended dose of ASP for the indications in question. For trials that included more than 2 arms, only data from the arms for which the trial was powered were extracted for this review (to avoid unit-of-analysis errors).12
Statistical Analysis
A random effects model was used to combine outcomes, using Review Manager 5.2.13 Odds ratios with 95% confidence intervals were calculated for dichotomous outcomes, and weighted mean difference was used to report continuous outcomes. Results were subdivided based on the comparator. We used the I2 statistic in Review Manager to assess heterogeneity, as described in the Cochrane Handbook.14
Risk of Bias Assessment
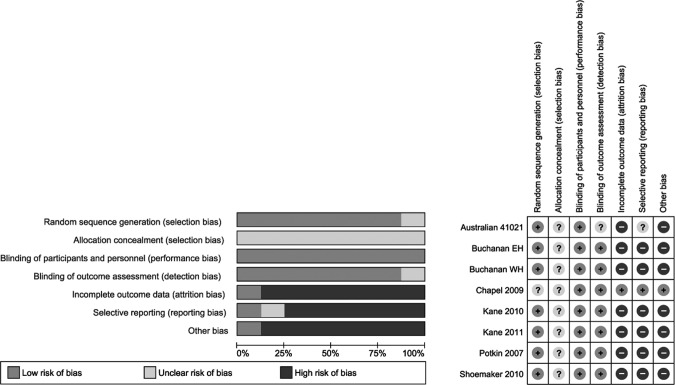
The risk of bias from the included trials was assessed using the Cochrane risk of bias assessment tool.14 All trials were randomized and double-blinded, but methods of randomization and allocation concealment were not mentioned. Additional sources of bias include the fact that all included articles were written in English and were sponsored by the manufacturers of ASP. Reporting bias was also possible as all trials reported frequent adverse reactions, omitting rare but potentially serious adverse effects. In addition, very few of the trials obtained through the initial search were available in published form, and very few of the unpublished trials were obtained from either the manufacturer or the authors. Publication bias may be present, and we speculate that some negative trials may be missing from our review. The decision was made by the authors not to include a funnel plot as it is very difficult to show true asymmetry when fewer than 10 articles are included.15 There was also significant attrition in all studies, as shown by high withdrawal rates. The majority of the authors were current employees or representatives of the manufacturer of ASP. See Figure 1 for more details.
Figure 1.
Cochrane risk of bias assessment.
Results
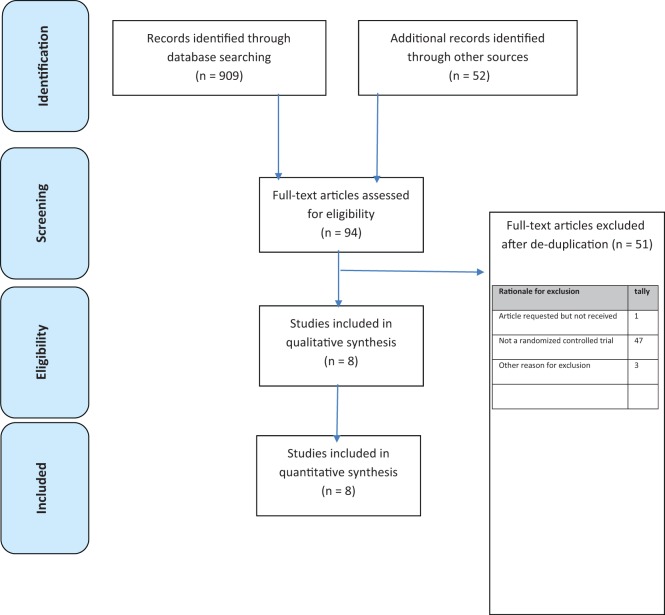
The search yielded 742 citations, of which 8 articles (3765 patients) met inclusion criteria.16–23 See the flow diagram in Figure 2 for additional details regarding trial selection and Table 2 for characteristics of the included studies. Table 3 lists the excluded trials and reasons for exclusion. Of the 8 trials included, 5 were placebo-controlled and 3 compared ASP with olanzapine (OZP). No unpublished studies were obtained from either the manufacturer or the expert authors. Data for the unpublished study 41021 were obtained through the FDA website and an Australian government document. All patients included had a diagnosis of either DSM-IV schizophrenia or schizoaffective disorder.
Figure 2.
Study selection.
Table 2.
Characteristics of included trials.
| Identifier | Duration | Participants and methods | Psychotic illnesses included | Interventions | Number randomized in each group | Primary efficacy outcome |
|---|---|---|---|---|---|---|
| Trial 41021 | 6 weeks | Adultsa Randomized, blinded, and placebo-controlled | DSM-IV acute schizophrenia | ASP 5 mg BID ASP 5 mg BID × 1 day and then 10 mg BID OZP 10 mg daily × 7 days and then 15 mg daily PLC | ASP 5 mg: 102 ASP 10 mg: 96 OZP: 95 PLC: 93 | Change from baseline in PANSS total score |
| Buchanan 2012 (EH)b | 26 weeks | ≥18 years old and stable on current therapy Randomized, double-blinded | DSM-IV schizophrenia (clinically stable disease) | ASP 5-10 mg BID OZP 5-20 mg daily | ASP: 241 OZP: 240 | Change in NSA-16 total score |
| Buchanan 2012 (WH)b | 26 weeks | ≥18 years old and stable on current treatment Randomized, double-blinded | DSM-IV schizophrenia (clinically stable disease) | ASP 5-10 mg BID OZP 5-20 mg daily | ASP: 244 OZP: 224 | Change in NSA-16 total score |
| Chapel 2009 | 16 days | ≥18 years olda Randomized, double-blinded, placebo-controlled, dose-escalating trial | DSM-IV schizophrenia or schizoaffective disorder (clinically stable disease) | ASP 5 mg BID × 10 days and then 10 mg BID × 6 days ASP 15 mg BID × 10 days and then 20 mg BID × 6 days QTE 375 mg BID × 16 days PLC × 16 days | ASP 5/10 mg: 38 ASP 15/20: 38 QTE: 37 PLC: 35 | Time-matched change from baseline in QTc with ASP vs. PLC |
| Kane 2010 | 6 weeks | ≥18 years olda Randomized, double-blinded, placebo-controlled trial | DSM-IV schizophrenia with acute exacerbation | ASP 5 mg BID ASP 10 mg BID HDL 4 mg BID PLC | ASP 5 mg: 114 ASP 10 mg: 106 HDL: 115 PLC: 123 | Change from baseline in PANSS total score |
| Kane 2011 | 26 weeks | ≥18 years old, stable on current treatment Randomized, double-blinded, placebo-controlled trial | DSM-IV schizophrenia (clinically stable disease) | ASP: 5-10 mg BID PLC | ASP: 194 PLC: 192 | Time to relapse or impending relapse |
| Potkin 2007 | 6 weeks | ≥18 years old with acute decompensation either on or off antipsychotics Randomized, double-blinded, double-dummy, placebo-controlled trial | DSM-IV acute schizophrenia | ASP: 1 mg BID on day 1, 2 mg BID on day 2, 3 mg BID on day 3, 4 mg BID on day 4, and then 5 mg BID onwards RIS: 1 mg BID on day 1, 2 mg BID on day 2, and then 3 mg BID onwards PLC | ASP: 60 RIS: 60 PLC: 62 | Change from baseline in PANSS total score |
| Schoemaker 2010 | 52 weeks | ≥18 years old inpatients or outpatients either on treatment or treatment naïve Randomized, double-blinded, double-dummy trial | DSM-IV schizophrenia or schizoaffective disorder (unstable disease) | ASP: 5 mg BID × 1 week and then 5 mg BID or 10 mg BID OZP: 10 mg daily × 1 week and then 10 or 20 mg daily | ASP: 908 OZP: 311 | Change from baseline in PANSS total score |
ASP = asenapine; BID = twice daily; DSM-IV = Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; HDL = haloperidol; NSA-16 = Negative Symptoms Assessment–16; OZP = olanzapine; PANSS = Positive and Negative Syndrome Scale; PLC = placebo; QTE = quetiapine; RIS = risperidone.
aIt is unclear from the trial whether patients were on or off antipsychotics at the time of study entry.
bThis paper included results from 2 core trials, one performed in the Eastern hemisphere (EH) (including 15 countries in Europe, South Africa, and Australia) and the other in the Western hemisphere (WH) (including 5 countries in North and South America).
Table 3.
Trials excluded from the review with reasons for exclusion.
| Study | Rationale for exclusion |
|---|---|
| Agarkar S, Anthony D, Ferrando S. Risk of akathisia associated with atypical antipsychotics [letter to the editor]. J Neuropsychiatry Clin Neurosci. 2013;25(1):E46-E47. | Not an RCT |
| Angersbach D. A new antipsychotic drug in development: asenapine for acute treatment of schizophrenia. Psychopharmakotherapie. 2008;15(2):97-98. | Not an RCT |
| Awad AG, Voruganti LN. The impact of newer atypical antipsychotics on patient-reported outcomes in schizophrenia. CNS Drugs. 2013;27(8):625-636. | Not an RCT |
| Beach SR, Celano CM, Noseworthy PA, et al. QTc prolongation, torsades de pointes and psychotropic medications. Psychosomatics. 2013;54(1):1-13. | Not an RCT |
| Berger A, Kushkuley S, Sanders K, et al. Clinical outcomes and economic costs of second generation antipsychotics in patients with chronic schizophrenia or schizoaffective disorders [conference abstract]. Value Health. 2011;14(3):A190. | Not an RCT |
| Bou Khalil R. Atypical antipsychotic drugs, schizophrenia, and metabolic syndrome in non-Euro-American societies. Clin Neuropharmacol. 2012;35(3):141-147. | Not an RCT |
| Catts SV. The place of asenapine in the treatment of schizophrenia and bipolar disorder. Med Today. 2012;13(7):59-61. | Not an RCT |
| Cazorla P, Mackle M, Zhao J, et al. Safety and tolerability of switching to asenapine from other antipsychotic agents in stable patients with persistent negative symptoms. Neuropsychiatr Dis Treat. 2012;8:247-257. | Not an RCT |
| Cazorla P, Mackle M, Zhao J, et al. Safety and tolerability of switching to asenapine from other antipsychotic agents: pooled results from two randomized multicenter trials in stable patients with persistent negative symptoms in schizophrenia. Neuropsychiatr Dis Treat. 2012;8:247-257. | Not an RCT |
| Cazorla P, Zhoa J, Szegedi A. Incidence, onset and duration of treatment emergent somnolence with asenapine in patients with schizophrenia or bipolar disorder. J Pharm Pract. 2011;24(2):254-255. | Not an RCT |
| Cetin M. Asenapine: a novel hope in the treatment of schizophrenia and manic and mixed episodes of bipolar 1 disorder. Bull Clin Psychopharmacol. 2013;23(1):99-106. | Not an RCT |
| Citrome L. A review of the pharmacology, efficacy and tolerability of recently approved and upcoming oral antipsychotics: an evidence based medicine approach. CNS Drugs. 2013;27(11):879-911. | Not an RCT |
| Citrome L. A systematic review of meta-analyses of the efficacy of oral atypical antipsychotics for the treatment of adult patients with schizophrenia. Expert Opin Pharmacother. 2012;13(11):1545-1573. | Not an RCT |
| Citrome L. Asenapine for schizophrenia and bipolar disorder: a review of the efficacy and safety profile for this newly approved sublingually absorbed second generation antipsychotic. Int J Clin Pract. 2009;63(12):1762-1784. | Not an RCT |
| De Hert M, Dobbelawere M, Sheridan EM, et al. Body weight and metabolic adverse effects of asenapine, iloperidone, lurasidone and paliperidone in the treatment of schizophrenia and bipolar disorder: a systematic review and exploratory meta-analysis. CNS Drugs. 2012;26(9):733-759. | Not an RCT |
| De Hert M, Dobbelaere M, Sheridan EM, et al. Metabolic and endocrine adverse effects of second generation antipsychotics in children and adolescents: a systematic review of randomized, placebo controlled trials and guidelines for clinical practice. Psiquiatria Biologica. 2011;18(3):89-104. | Not an RCT |
| De Hert M, Guiraud-Diawara A, Marre C. Comparison of metabolic syndrome incidence among schizophrenia patients treated with asenapine versus olanzapine. Eur Psychiatry. 2013;28 | Not an RCT |
| Dubovsky SL, Frobose C, Phiri P, et al. Asenapine: short term tolerability, safety and pharmacokinetic profile in older patients with psychosis. Int J Geriatr Psychiatry. 2012;27(5):472-482. | Not an RCT |
| Friberg LE, De Greef R, Kerbusch T, et al. Modeling and simulation of the time course of asenapine exposure response and dropout patterns in acute schizophrenia. Clin Pharmacol Ther. 2009;86(1):84-91. | Not an RCT |
| Gao K, Mackle M, Cazorla P, et al. Comparison of somnolence associated with asenapine, olanzapine, risperidone and haloperidol relative to placebo in patients with schizophrenia or bipolar disorder. Neuropsychiatr Dis Treat. 2013;9:1145-1157. | Not an RCT |
| Ivanov I, Charney A. Treating pediatric patients with antipsychotic drugs: balancing benefits and safety. Mount Sinai J Med. 2008;75(3):276-286. | Not an RCT |
| Janicak PG, Rado JT. Recent second generation antipsychotics implications for treating elderly patients with schizophrenia. Psychopharm Review. 2012;47(8):57-64. | Not an RCT |
| Janicak PG, Rado JT. Asenapine: a review of the data. Psychopharm Review. 2009;44(12):89-94. | Not an RCT |
| Jarskog LF. Advantages and limitations of newer antipsychotics: evidence from large comparative trials. Clin Schizophr Relat Psychoses. 2012;6(3):113-114. | Not an RCT |
| Johnsen E, Kroken RA. Drug treatment developments in schizophrenia and bipolar mania: latest evidence and clinical usefulness. Ther Adv Chronic Dis. 2012;3(6):287-300. | Not an RCT |
| Kirson NY, Weiden PJ, Yermakov S, et al. Efficacy and effectiveness of depot versus oral antipsychotics in schizophrenia: synthesizing results across different research designs. J Clin Psychiatry. 2013;74(6):568-575. | Not an RCT |
| Kumar A, Narayan M, Raja H, et al. Asenapine versus typical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2012;(11):CD010230. | Not an RCT |
| La Torre A, Conca A, Duffy D, et al. Sexual dysfunction related to psychotropic drugs: a critical review part II: antipsychotics. Pharmacopsychiatry. 2013;46(6):201-208. | Not an RCT |
| Lachaine J, Beauchemin C, Mathurin K, et al. Cost effectiveness of asenapine in the treatment of schizophrenia in Canada [conference abstract]. Value Health. 2012;15(4):A88. | Not an RCT |
| Leucht S, Cipriani A, Spineli, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple treatments meta-analysis. Lancet. 2013;382(9896):951-962. | Not an RCT |
| Marder SR. Newer antipsychotics and the differences between clinical experiences and clinical trials. CNS Spectrums. 2007;12(11):812-815. | Not an RCT |
| Mcdonagh M, Peterson K, Carson S, et al. Atypical antipsychotic drugs: drug class review. Oregon Health and Sciences University, July 2010. | Not an RCT |
| Minassian A, Young JW. Evaluation of the clinical efficacy of asenapine in schizophrenia. Expert Opin Pharmacother. 2010;11(12):2107-2115. | Not an RCT |
| Potkin SG. Optimising short and long term schizophrenia treatment [conference abstract]. Eur Neuropsychopharmacol. 2012;22:S446. | Not an RCT |
| Potkin SG, Phiri P, Zhoa J, et al. A pooled analysis of the effects of asenapine on the persistent negative symptoms of schizophrenia. Neuropsychopharmacology. 2010;35:S213. | Not an RCT |
| Preda A, Faziola L. Asenapine versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2010;(12):CD008902. | Not an RCT |
| Prohn M, De Greef R, Chapel S, et al. Population pharmacokinetics of asenapine in patients with schizophrenia or bipolar disorder [conference abstract]. Eur Neuropsychopharmacol. 2009;19:S542-S543. | Not an RCT |
| Scarff JR, Casey DA. Newer oral atypical antipsychotic agents: a review. P T. 2011;26(12):832-841. | Not an RCT |
| Schimmelmann BG, Schmidt SJ, Carbon M, et al. Treatment of adolescents with early onset schizophrenia spectrum disorders: in search of a rational, evidence-informed approach. Curr Opin Psychiatry. 2013;26(2):219-230. | Not an RCT |
| Shoemaker J, Naber D, Vrijland P, et al. Long term assessment of asenapine vs olanzapine in patients with schizophrenia or schizoaffective disorder: erratum. Pharmacopsychiatry. 2011;44(7):343. | Not an RCT |
| Shoemaker J, Stet L, Vrijland P, et al. Long term efficacy and safety of asenapine or olanzapine in patients with schizophrenia or schizoaffective disorder: an extension study. Pharmacopsychiatry. 2012;45(5):196-203. | Not an RCT |
| Shulman M, Njoku IJ, Manu P. Thrombotic complications of treatment with antipsychotic drugs. Minerva Med. 2013;104(2):175-184. | Not an RCT |
| Stoner SC, Pace HA. Asenapine: a clinical review of a second generation antipsychotics. Clin Ther. 2012;34(5):1023-1040. | Not an RCT |
| Szegedi A, Verweij P, Van Duijnhoven, et al. Meta-analyses of the efficacy of asenapine for acute schizophrenia: comparisons with placebo and other antipsychotics. J Clin Psychiatry. 2012;73(12):1533-1540. | Not an RCT |
| Tarazi FI. The preclinical profile of asenapine: clinical relevance for the treatment of schizophrenia and bipolar mania. Expert Opin Drug Discov. 2013;8(1):93-103. | Not an RCT |
| Timpe EM, Chopra RA. Asenapine: a novel atypical antipsychotic agent for schizophrenia and bipolar disorder. J Pharm Technol. 2010;26(6):352-361. | Not an RCT |
| Zhao J, Cazorla P, Schoemaker J, et al. Weight change and metabolic effects of asenapine in placebo or olanzapine controlled studies. Eur Psychiatry. 2011;26. | Not an RCT |
| Buchanan RW, Panagides J, Zhao J, et al. Asenapine versus olanzapine in people with persistent negative symptoms of schizophrenia. J Clin Psychopharmacol. 2012;32(1):36-45. | Duplicate |
| Citrome L. A systematic review of meta-analyses of the efficacy of oral atypical antipsychotics for the treatment of adult patients with schizophrenia. Expert Opin Pharmacother. 2012;13(11):1545-1573. | Duplicate |
| Cazorla P, Zhoa J, Szegedi A. Incidence, onset and duration of treatment emergent somnolence with asenapine in patients with schizophrenia or bipolar disorder. J Pharm Pract. 2011;24(2):254-255. | Duplicate |
| De Hert M, Dobbelawere M, Sheridan EM, et al. Body weight and metabolic adverse effects of asenapine, iloperidone, lurasidone and paliperidone in the treatment of schizophrenia and bipolar disorder: a systematic review and exploratory meta-analysis. CNS Drugs. 2012;26(9):733-759. | Duplicate |
| Dubovsky SL, Frobose C, Phiri P, et al. Asenapine: short term tolerability, safety and pharmacokinetic profile in older patients with psychosis. Eur Neuropsychopharmacol. 2010;20:S489-S490. | Duplicate |
| Dubovsky SL, Frobose C, Phiri P, et al. Asenapine: short term tolerability, safety and pharmacokinetic profile in older patients with psychosis [conference abstract]. ECNP Congress, Amsterdam, the Netherlands, 2010 Aug 28–Sep 1. | Duplicate |
| Dubovsky SL, Frobose C, Phiri P, et al. Asenapine: short term tolerability, safety and pharmacokinetic profile in older patients with psychosis. Schizophr Res. 2010;117(2-3):263-264. | Duplicate |
| Dubovsky SL, Frobose C, Phiri P, et al. Asenapine: short term tolerability, safety and pharmacokinetic profile in older patients with psychosis. Schizophr Res. 2010;117(2-3):263-264. | Duplicate |
| Kane JM, Cohen M, Zhao J, et al. Efficacy and safety of asenapine in a placebo and haloperidol-controlled trial in patients with acute exacerbation of schizophrenia. J Clin Psychopharmacol. 2010;30(2):106-115. | Duplicate |
| Kane JM, Mackle M, Snowadami L, et al. A randomized placebo controlled trial of asenapine for the prevention of relapse of schizophrenia after long-term treatment. J Clin Psychiatry. 2011;72(3):349-355. | Duplicate |
| Kane JM, Cohen M, Zhao J, et al. Efficacy and safety of asenapine in a placebo and haloperidol-controlled trial in patients with acute exacerbation of schizophrenia. J Clin Psychopharmacol. 2010;30(2):106-115. | Duplicate |
| Kane JM, Cohen M, Zhao J, et al. Efficacy and safety of asenapine in a placebo and haloperidol-controlled trial in patients with acute exacerbation of schizophrenia. J Clin Psychopharmacol. 2010;30(2):106-115. | Duplicate |
| Kane JM, Mackle M, Snowadami L, et al. Double blind, placebo controlled trial of asenapine in prevention of relapse after long term treatment of schizophrenia. Int J Neuropsychopharmacol. 2010;13:223. | Duplicate |
| Kane JM, Mackle M, Snowadami L, et al. A randomized placebo controlled trial of asenapine for the prevention of relapse of schizophrenia after long-term treatment. J Clin Psychiatry. 2011;72(3):349-355. | Duplicate |
| Kirson NY, Weiden PJ, Yermakov S, et al. Efficacy and effectiveness of depot versus oral antipsychotics in schizophrenia: synthesizing results across different research designs. J Clin Psychiatry. 2013;74(6):568-575. | Duplicate |
| Landbloom R, Zhao J, Cazorla P, et al. Weight change and metabolic effects of asenpine in placebo or olanzapine controlled studies. Bipolar Disord. 2011;13:65. | Duplicate |
| Mackle M, Snowadami L, Zhao J, et al. Double blind placebo controlled trial of asenapine in prevention of relapse after long-term treatment of schizophrenia [conference abstract]. Eur Neuropsychopharmacol. 2009;19:S543. | Duplicate |
| Cazorla P, Mackle M, Zhao J, et al. Safety and tolerability of switching to asenapine from other antipsychotic agents in stable patients with persistent negative symptoms. Neuropsychiatr Dis Treat. 2012;8:247-257. | Duplicate |
| Cazorla P, Mackle M, Zhao J, et al. Safety and tolerability of switching to asenapine from other antipsychotic agents in stable patients with persistent negative symptoms. J Pharm Pract. 2012;25(2):266. | Duplicate |
| Potkin SG, Cohen M, Panagides J. Efficacy and tolerability of asenapine in acute schizophrenia: a placebo and risperidone controlled trial. J Clin Psychiatry. 2007;68:1492-1500. | Duplicate |
| Potkin SG, Phiri P, Szegedi A, et al. Long-term effects of asenapine or olanzapine in patients with persistent negative symptoms of schizophrenia: a pooled analysis. Schizo Res. 2013;150(2-3):442-449. | Duplicate |
| Potkin SG, Phiri P, Zhao J, et al. A pooled analysis of the effects of asenapine on the persistent negative symptoms of schizophrenia. Eur Psychiatry. 2011;26. | Duplicate |
| Potkin S, Phiri P, Zhao J, et al. A pooled analysis of the effects of asenapine on the persistent negative symptoms of schizophrenia [conference abstract]. Neuropsychopharmacology. 2010;35:S213. | Duplicate |
| Shoemaker J, Naber D, Vrijland P, et al. Long term assessment of asenapine vs olanzapine in patients with schizophrenia or schizoaffective disorder. Pharmacopsychiatry. 2010;43(4):138-146. | Duplicate |
| Shoemaker J, Naber D, Vrijland P, et al. Long term assessment of asenapine vs olanzapine in patients with schizophrenia or schizoaffective disorder. Pharmacopsychiatry. 2010;43(4):138-146. | Duplicate |
| Shoemaker J, Naber D, Vrijland P, et al. Long term assessment of asenapine vs olanzapine in patients with schizophrenia or schizoaffective disorder: erratum. Pharmacopsychiatry. 2011;44(7):343 | Duplicate |
| Shoemaker J, Stet L, Vrijland P, et al. Safety and efficacy of long-term asenapine versus olanzapine in schizophrenia or schizoaffective disorder patients. Eur Psychiatry. 2010;25. | Duplicate |
| Shoemaker J, Stet L, Vrijland P, et al. Long term efficacy and safety of asenapine or olanzapine in patients with schizophrenia or schizoaffective disorder: an extension study. Pharmacopsychiatry. 2012;45(5):196-203. | Duplicate |
| Shoemaker J, Stet L, Vrijland P, et al. Long term efficacy and safety of asenapine or olanzapine in patients with schizophrenia or schizoaffective disorder. J Pharm Pract. 2010;23(2):162 | Duplicate |
| Dubovsky SL, Frobose C, Phiri P, et al. Asenapine: short term tolerability, safety and pharmacokinetic profile in older patients with psychosis. Int J Geriatr Psychiatry. 2012;27(5):472-482. | Duplicate |
| Szegedi A, Verweij P, Van Duijnhoven, et al. Meta-analyses of the efficacy of asenapine for acute schizophrenia: comparisons with placebo and other antipsychotics. J Clin Psychiatry. 2012;73(12):1533-1540. | Duplicate |
| Szegedi A, Verweij P, Van Duijnhoven, et al. Meta-analyses of the efficacy of asenapine for acute schizophrenia: comparisons with placebo and other antipsychotics. J Clin Psychiatry. 2012;73(12):1533-1540. | Duplicate |
| Szegedi A, Verweij P, Van Duijnhoven, et al. Meta-analyses of the efficacy of asenapine for acute schizophrenia: comparisons with placebo and other antipsychotics. J Clin Psychiatry. 2012;73(12):1533-1540. | Duplicate |
| Szegedi A, cazorla P, Zhao J. Incidence, onset and duration of treatment-emergent somnolence with asenapine in adult patients with schizophrenia or bipolar disorder [conference abstract]. Eur Neuropsychopharmacol. 2011;21:S502-S503. | Duplicate |
| Zhao J, Cazorla P, Schoemaker J, et al. Weight change and metabolic effects of asenapine in placebo or olanzapine controlled studies [conference abstract]. Neuropsychopharmacology. 2010;35:S322-S323. | Duplicate |
| Meltzer H, Cohen M, Snowadami, L, et al. Long term safety and maintenance of effect of asenapine in patients with acute exacerbation of schizophrenia [conference abstract]. Eur Neuropsychopharmacol. 2009;19:S536-S537. | Full text not provided by author |
| Harrigan EP, Miceli JJ, Anziano R, et al. A randomized evaluation of the effects of six antipsychotic agents on QTc, in the absence and presence of metabolic inhibition. J Clin Psychopharmacol. 2004;24(1):62-69. | Asenapine was not included |
| Alexander W. New Atypical agents and virtual reality with drug therapy. American Psychiatric Association. P T. 2008;33(6):364-367. | Wrong diagnosis studied |
| Boyda HN, Procyshyn RM, Pang CC, et al. Metabolic side effects of the novel second generation antipsychotic drugs asenapine and iloperidone: a comparison with olanzapine. PLoS One. 2013;8(1):e53459. | Not performed in human subjects |
RCT = randomized clinical trial. Full text screening was performed in duplicate by C.O. and S.D.
Efficacy Outcomes
PANSS
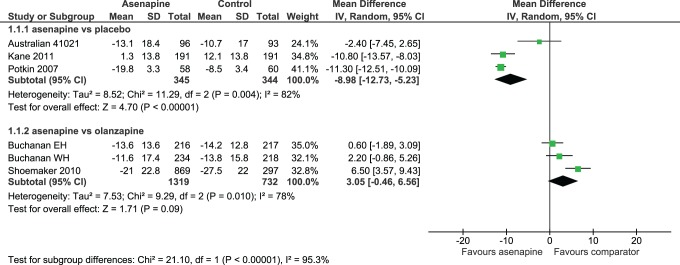
Patients were considered to be PANSS responders if they achieved a ≥30% decrease in their PANSS total score from baseline at the end of the study. Results favoured ASP over PLC in terms of both change in PANSS total score (OR –8.98; 95% CI, –12.73 to –5.23) (Figure 3) and percentage of PANSS responders (OR 1.88; 95% CI, 1.24 to 2.84), No differences were found between ASP and PLC in terms of changes to the 3 PANSS subscale scores. No differences were noted between ASP and OZP in terms of changes to PANSS total (Figure 3), nor were there any differences between ASP and OZP in changes to PANSS negative subscale scores. In addition, ASP was found to be inferior to OZP regarding changes to PANSS positive and PANSS psychopathology subscale scores, with differences of 0.94 (95% CI, 0.46 to 1.42) and 1.38 (95% CI, 0.51 to 2.25), respectively. Both the ASP vs. PLC and the ASP vs. OZP comparisons demonstrated considerable heterogeneity (82% and 78%, respectively) for the outcome change in PANSS total score.
Figure 3.
Changes in PANSS total score.
Clinical Global Impression (CGI)
Schoemaker et al.20 and Kane et al.17 considered CGI improvement scale (CGI-I) responders to be those with a CGI-I score of 1 (very much improved) or 2 (much improved) at study end. ASP was superior to PLC on improvement in CGI severity scale (CGI-S) scores, while no difference was observed between ASP and OZP in this outcome. There was no difference in the rates of CGI-I responders between those randomized to ASP and those randomized to PLC. ASP was inferior to OZP in this outcome (OR 0.66; 95% CI, 0.52 to 0.84).
Worsening schizophrenia and/or psychosis
Rates of worsening schizophrenia and/or psychosis did not differ between ASP and PLC. However, compared with those randomized to OZP, more patients randomized to ASP experienced this outcome (OR 1.38; 95% CI, 1.05 to 1.82). Refer to Table 4 for a summary of efficacy results.
Table 4.
Summary table of efficacy outcomes.
| Outcome | ASP vs. PLC | ASP vs. OZP | ASP 10 mg BID vs. 5 mg BID |
|---|---|---|---|
| Changes to PANSS total | Favours ASP (greater reduction with ASP) WMD: –8.98 (95% CI, –12.73 to –5.23) | No difference WMD: 3.05 (95% CI, –0.46 to 6.56) | |
| Changes to PANSS negative | No difference WMD: –0.1 (95% CI, –1.25 to 1.05) | No difference WMD: –0.23 (95% CI, –1.02 to 0.55) | |
| Changes to PANSS Marder negative | Favours ASP (greater reduction with ASP) WMD: –1.54 (95% CI, –2.25 to –0.84) | No difference WMD: 0.22 (95% CI, –1.14 to 1.57) | No difference WMD: 0 (95% CI, –1.38 to 1.38) |
| Worsening schizophrenia | No difference OR 0.7 (95% CI, 0.22 to 2.26) | Favours OZP (lower incidence with OZP) OR 1.38 (95% CI, 1.05 to 1.82) | No difference OR 3.3 (95% CI, 0.9 to 12.7) |
| Changes to CGI-S | Favours ASP (greater reduction with ASP) WMD: –0.46 (95% CI, –0.78 to –0.14) | No difference WMD: 0.22 (95% CI, 0.00 to 0.44) | No difference WMD: 0.1 (95% CI, –0.18 to 0.38) |
| Rates of CGI-I responders | No difference WMD: 1.54 (95% CI, 0.90 to 2.64) | Favours OZP WMD: 0.66 (95% CI, 0.52 to 0.84) |
ASP = asenapine; BID = twice daily; CGI-I = Clinical Global Impression–improvement scale; CGI-S = Clinical Global Impression–severity scale; OR = odds ratio; OZP = olanzapine; PANSS = Positive and Negative Syndrome Scale; PLC = placebo; WMD = weighted mean difference.
Withdrawals
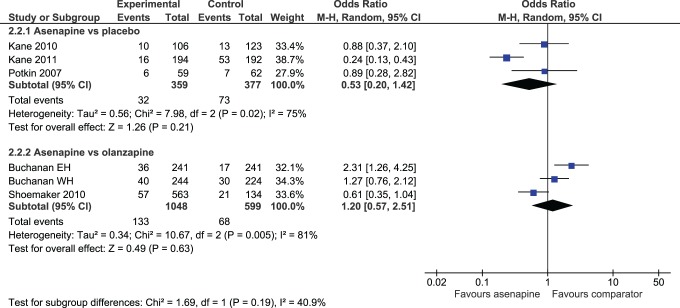
Rates of either withdrawal for any reason or withdrawal due to adverse effects were no different between patients receiving ASP and those receiving PLC (Figure 4). ASP-treated patients had lower rates of withdrawal due to lack of efficacy than those taking PLC (OR 0.37; 95% CI, 0.20 to 0.69). When ASP was compared with OZP, no difference was shown for rates of discontinuation due to adverse drug reactions (ADRs) (Figure 4) or lack of efficacy, but ASP had higher rates of overall withdrawal (OR 2.06; 95% CI, 1.71 to 2.49).
Figure 4.
Rates of withdrawal due to adverse effects.
Safety and Tolerability Outcomes
Adverse effects
No differences were found between ASP and either PLC or OZP in rates of “any ADR.” Higher rates of serious ADRs were demonstrated in those receiving ASP compared with OZP (OR 1.84; 95% CI, 1.36 to 2.47). Trials did not specify which serious adverse effects occurred.
Effect on sleep
Rates of insomnia were comparable between ASP and PLC, whereas patients randomized to ASP were more likely to report this adverse effect than those randomized to OZP (OR 1.46; 95% CI, 1.13 to 1.90). Rates of sedation were similar between ASP and both comparators.
Metabolic adverse effects
The incidence of clinically significant weight gain was greater with ASP compared with PLC (OR 3.58; 95% CI, 1.13-11.31). Those taking ASP were less likely to have reported this outcome than those randomized to OZP (OR 0.35; 95% CI, 0.27 to 0.44). Changes from baseline in triglyceride values were similar between ASP and PLC. Based on results from 1 trial, patients receiving ASP had less change in triglyceride than OZP-treated patients (OR –0.45; 95% CI, –0.71 to –0.19). No significant differences were found between ASP and either comparator in changes in fasting blood glucose or new-onset National Cholesterol Education Program metabolic syndrome.16
Extrapyramidal symptoms (EPS)
No differences were found between ASP and PLC in reported rates of EPS. Patients randomized to ASP were more likely to experience EPS and akathisia than were patients taking OZP: OR 2.06 (95% CI, 1.38 to 3.06) and OR 2.27 (95% CI, 1.45 to 3.55), respectively. No differences were noted between groups in changes to any of the EPS rating scales: Simpson Angus scale, Abnormal Involuntary Movement Scale, Extrapyramidal Symptoms Rating Scale, or Barnes Akathisia Rating Scale.
Patient Acceptance and Quality of Life Outcomes
Very few trials measured patient-reported outcomes, such as improvements in quality of life and subjective well-being while receiving neuroleptics. Schoemaker et al.20 reported both patients’ and investigators’ subjective impressions of improvement of the patients’ disease condition in comparison to when they were taking previous neuroleptics. Those taking ASP were less likely to be considered “much improved” by the investigator than those receiving OZP (OR 0.65; 95% CI, 0.45 to 0.93).
Refer to Table 5 for a summary of additional clinically relevant results.
Table 5.
Summary of clinically important outcomes.
| Outcome | No. of trials reporting the outcome | Total no. of patients in trials | Effects | Odds ratio (95% CI) or mean difference (95% CI) |
|---|---|---|---|---|
| Asenapine vs. placebo | ||||
| Changes to PANSS total | 3 | 689 | Favours ASP (greater reduction with ASP) | –8.98 (–12.73 to –5.23) |
| Changes to CGI-S | 2 | 609 | Favours ASP (greater reduction with ASP) | –0.46 (–0.78 to –0.14) |
| Changes to PANSS negative | 1 | 189 | No difference | –0.1 (–1.25 to 1.05) |
| Changes to PANSS Marder negative | 2 | 609 | Favours ASP (greater reduction with ASP) | –1.54 (–2.25 to –0.84) |
| Incidence of discontinuation due to adverse effects | 3 | 736 | No difference | 0.53 (0.20 to 1.42) |
| Incidence of all adverse effects | 3 | 736 | No difference | 0.91 (0.60 to 1.39) |
| Incidence of serious adverse effects | 3 | 736 | No difference | 0.75 (0.24 to 2.30) |
| Clinically significant increases in body weight (≥7% of baseline) | 3 | 736 | Favours PLC (lower incidence with PLC) | 3.58 (1.13 to 11.31) |
| Incidence of reported akathisia | 2 | 615 | No difference | 2.62 (0.87 to 7.87) |
| Incidence of patients reporting any EPS symptoms | 2 | 615 | No difference | 1.21 (0.40 to 3.67) |
| Incidence of sedation | 1 | 229 | No difference | 1.42 (0.42 to 4.78) |
| Incidence of insomnia | 3 | 736 | No difference | 0.91 (0.40 to 2.06) |
| Worsening schizophrenia | 3 | 736 | No difference | 0.7 (0.22 to 2.26) |
| Agitation | 3 | 736 | Favours ASP (lower incidence with ASP) | 0.54 (0.3 to 0.97) |
| Asenapine vs. olanzapine | ||||
| Changes to PANSS total | 4 | 2242 | No difference | 3.05 (–0.46 to 6.56) |
| Changes to CGI-S | 3 | 2051 | No difference | 0.22 (0.00 to 0.44) |
| Changes to PANSS negative | 3 | 1076 | No difference | –0.23 (–1.02 to 0.55) |
| Changes to PANSS Marder negative | 3 | 2051 | No difference | 0.22 (–1.14 to 1.57) |
| Incidence of discontinuation due to adverse effects | 3 | 1647 | No difference | 1.20 (0.57 to 2.51) |
| Incidence of all adverse effects | 3 | 2148 | No difference | 1.05 (0.78 to 1.40) |
| Incidence of serious adverse effects | 3 | 2168 | Favours OZP (lower incidence with OZP) | 1.84 (1.36 to 2.47) |
| Clinically significant increases in body weight (≥7% of baseline) | 3 | 2168 | Favours ASP (lower incidence with ASP) | 0.35 (0.27 to 0.44) |
| Incidence of reported akathisia | 3 | 2168 | Favours OZP (lower incidence with OZP) | 2.27 (1.45 to 3.55) |
| Incidence of patients reporting any EPS symptoms | 3 | 2168 | Favours OZP (lower incidence with OZP) | 2.06 (1.38 to 3.06) |
| Incidence of sedation | 3 | 2168 | No difference | 0.82 (0.59 to 1.16) |
| Incidence of insomnia | 3 | 2168 | Favours OZP (lower incidence with OZP) | 1.46 (1.13 to 1.90) |
| Worsening schizophrenia | 3 | 2168 | Favours OZP (lower incidence with OZP) | 1.38 (1.05 to 1.82) |
| Agitation | 2 | 949 | No difference | 2.7 (0.82 to 8.95) |
| Asenapine 10 mg vs. asenapine 5 mg | ||||
| Changes to PANSS total | ND | ND | ND | ND |
| Changes to CGI-S | 1 | 214 | No difference | 0.1 (–0.18 to 0.38) |
| Changes to PANSS negative | ND | ND | ND | ND |
| Changes to PANSS Marder negative | 1 | 214 | No difference | 0 (–1.38 to 1.38) |
| Incidence of discontinuation due to adverse effects | 1 | 217 | No difference | 2.2 (0.7 to 6.7) |
| Incidence of all adverse effects | 1 | 217 | No difference | 1.7 (0.92 to 3) |
| Incidence of serious adverse effects | 1 | 217 | No difference | 1.38 (0.49 to 3.84) |
| Clinically significant increases in body weight (≥7% of baseline) | 1 | 217 | No difference | 0.7 (0.19 to 2.5) |
| Incidence of reported akathisia | 1 | 217 | No difference | 2.3 (0.85 to 6.4) |
| Incidence of patients reporting any EPS symptoms | 1 | 217 | No difference | 1.2 (0.6 to 2.5) |
| Incidence of sedation | 1 | 217 | No difference | 0.9 (0.31 to 2.58) |
| Incidence of insomnia | 1 | 217 | No difference | 0.9 (0.45 to 1.7) |
| Worsening schizophrenia | 1 | 217 | No difference | 3.3 (0.9 to 12.7) |
| Agitation | 1 | 217 | No difference | 0.71 (0.3 to 1.95) |
ASP, asenapine; CGI-S, Clinical Global Impression–severity scale; EPS, extrapyramidal symptoms; ND, no data; OZP, olanzapine; PANSS, Positive and Negative Syndrome Scale; PLC, placebo.
Additional Analyses
Two post hoc subgroup analyses were conducted, one comparing ASP 5-mg BID and 10-mg BID dosing and the other including only long-term trials (those ≥26 weeks long).
ASP 5 mg BID vs. 10 mg BID
No statistically significant differences were found for efficacy, rates of withdrawal, or tolerability outcomes between the 2 doses.
Long-term trials
Four trials were included in this analysis.16,18,20 Trials with PLC comparator showed comparable results when limited to long-term trials. All trials with OZP comparator were long-term trials, so there was no change to those results.
Discussion
This review demonstrates that the current evidence for the use of ASP in psychotic disorders is limited. A total of 8 trials were included in this review: 5 were placebo-controlled and 3 compared ASP with OZP.
The results demonstrate that ASP 10 mg BID was superior to PLC on several efficacy outcomes but was not superior to OZP. ASP’s purported enhanced activity against negative and cognitive symptoms of schizophrenia was not confirmed by the results of this review. Nor was enhanced adherence with ASP demonstrated. In addition, patients taking ASP were more likely to withdraw from clinical trials, suggesting that adherence to ASP in the clinical setting may be inferior to OZP.
Second-generation antipsychotics have a documented adverse impact on the patient’s metabolic profile.2 Results from this review favoured ASP over OZP in this regard, which suggests that ASP may be advantageous in patients at higher risk of cardiovascular morbidity and mortality.
ASP demonstrated a similar propensity towards sedation as both PLC and OZP, a surprising finding considering the well-known sedative effects of OZP. A lack of power to detect a difference in rates of sedation may be at play, reducing our ability to detect a significant difference when results were combined for this meta-analysis.
Rates of EPS were lower with OZP than ASP and comparable between ASP and PLC. The authors believe that these findings may be due to the number and duration of trials included in this review. Some forms of EPS may occur months to years after the initiation of an antipsychotic. All trials comparing ASP and OZP were long-term trials, while only 1 placebo-controlled trial was a long-term trial. It is possible that with time, a significant difference favouring PLC would have been detected for this adverse effect; however, the majority of the placebo-controlled studies included were likely too short in duration to reveal a significant difference in this outcome.
No conclusions can be drawn about the impact of oral hypoesthesia, a common adverse effect reported with ASP,5 since only 1 placebo-controlled trial reported on this outcome.17
The subgroup analysis comparing ASP 5 mg BID and 10 mg BID demonstrated no differences in efficacy, withdrawals, or tolerability outcomes between the 2 doses. Although not powered for this comparison, these findings suggest that the efficacy of ASP is not dose-dependent and that dose escalation is not warranted.
The strengths of this review include the fact that it was conducted in accordance with PRISMA (Table 6) and Cochrane guidelines and was registered a priori.14,24 The breadth of the search strategy ensured that all relevant articles matching the inclusion criteria were found. All study selection and data extraction were done in duplicate, thus minimizing the risk of bias.25
Table 6.
PRISMA checklist.
| Section/topic | # | Checklist item | Reported on page # |
|---|---|---|---|
| Title | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both. | 1 |
| Abstract | |||
| Structured summary | 2 | Provide a structured summary including, as applicable, background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number. | 2 |
| Introduction | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known. | 5 |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS). | 7 |
| Methods | |||
| Protocol and registration | 5 | Indicate whether a review protocol exists and whether and where it can be accessed (e.g., Web address); if available, provide registration information including registration number. | 6 |
| Eligibility criteria | 6 | Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving rationale. | 7 |
| Information sources | 7 | Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched. | 6 |
| Search | 8 | Present full electronic search strategy for at least 1 database, including any limits used, such that it could be repeated. | 6 |
| Study selection | 9 | State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis). | 7 |
| Data collection process | 10 | Describe method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators. | 7 |
| Data items | 11 | List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and simplifications made. | Table 1 |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level) and how this information is to be used in any data synthesis. | 8 |
| Summary measures | 13 | State the principal summary measures (e.g., risk ratio, difference in means). | 8 |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g., I2) for each meta-analysis. | 8 |
There are some limitations of this review. All included trials had strict inclusion and exclusion criteria such as the exclusion of those with concurrent psychiatric illnesses, including substance abuse, which may limit generalizability of the results to a broad patient population. All patients were included in this meta-analysis regardless of whether patients were treated as inpatient or outpatient and whether they were in florid psychosis or had stable disease. This may explain the heterogeneity observed in this review, such as in the change in PANSS total score with an I2 of 95.3%. Trials handled missing data inconsistently, resulting in the combination of results obtained from either mixed model of repeated measures or last observation carried forward in the meta-analysis. This contributes further to the heterogeneity of results. Thirteen potentially eligible trials were unobtainable from authors and/or the manufacturer of ASP despite repeated requests. This large source of unknown data could have a significant effect on the outcomes reported in this review.
Conclusions
Insufficient evidence is available to make recommendations on preference of ASP over other antipsychotic medications in the treatment of psychotic disorders. Results from the included trials suggest that ASP is either comparable to or inferior to OZP for the majority of efficacy, tolerability, and acceptability outcomes. ASP demonstrated a safer metabolic profile than OZP with a trend towards less sedation; ASP was more likely than OZP to lead to EPS. With this in mind, ASP may be a treatment choice for those who cannot tolerate the sedative effects of OZP, such as drivers and night shift workers, as well as those who are at high risk of metabolic adverse effects.
ASP should also be considered an option in patients who will be able to follow the prescribed administration instructions.
There is a need for well-designed, randomized clinical trials that are adequately powered to compare the efficacy and acceptability of ASP with active comparators other than OZP. The outcome measures that are clinically relevant and merit further study include quality of life, cost, adherence with medication, and length of hospitalization. The comparisons of different doses of ASP may be beneficial in order to determine whether dosing flexibility affects treatment efficacy and safety.
Acknowledgments
We thank our clinical librarian, Sandra McKeown, for helping to establish a search strategy and library technician, Marshall Lemon, for help with article acquisition. We also thank our psychiatry pharmacists, Boris Tong, Bradley Linton, and Sharon Lawrence, for their clinical expertise during this project.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Our project was funded by London Health Sciences Centre, Pharmacy Department.
References
- 1. Public Health Agency of Canada. Schizophrenia [Internet]. 2012. [cited 2013 Sep 7] http://www.phac-aspc.gc.ca/publicat/miic-mmac/chap_3-eng.php
- 2. Crismon L, Argo TR, Buckley PF. Chapter 76. Schizophrenia In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM, editors. Pharmacotherapy: a pathophysiologic approach. 8th ed [Internet]. 2011 [cited 2013 Sep 7]. Available from: http://www.accesspharmacy.com/content.aspx?aID=7987911
- 3. Merck. Saphris: drug product monograph [Internet]. In: e-CPS Ottawa (ON): Canadian Pharmacist Association; 2011. [cited 2013 Sep 7]. Available from: https://www.e-therapeutics.ca/cps.select.preliminaryFilter.action?simplePreliminaryFilter=asenapine+maleate# [Google Scholar]
- 4. Lilly. Zyprexa [Internet] In: e-CPS Ottawa (ON): Canadian Pharmacist Association; 2013. [cited 2013 Sep 7]. Available from: https://www.e-therapeutics.ca/cps.select.preliminaryFilter.action?simplePreliminaryFilter=olanzapine [Google Scholar]
- 5. Health Canada, Drug Product Database. Saphris drug product monograph Kirkland (QC): Merck Canada Inc; 2011. [Google Scholar]
- 6. Rado J, Janicak P. Pharmacological and clinical profile of recently approved second-generation antipsychotics implications for treatment of schizophrenia in older patients. Drugs Aging. 2013;29:783–791. [DOI] [PubMed] [Google Scholar]
- 7. Szegedi A, Verweij P, Van Duijnhoven W, Mackle M, Cazorla P, Fennema H. Meta analyses of the efficacy of asenapine for acute schizophrenia: comparisons with placebo and other antipsychotics. J Clin Psychiatry. 2012;73(12):1533–1540. [DOI] [PubMed] [Google Scholar]
- 8. Mills E, Thorlund K, Loannidis J. Research methods and reporting: demystifying trial networks and network meta analysis [Internet]. BMJ 2013. [cited 2014 Nov 20]. Available from: http://www.bmj.com/content/346/bmj.f2914 [DOI] [PubMed]
- 9. Leucht S, Komossa K, Rummel-Kluge C, et al. A meta-analysis of head-to-head comparisons of second-generation antipsychotics in the treatment of schizophrenia. Am J Psychiatry. 2009;166(2):152–163. [DOI] [PubMed] [Google Scholar]
- 10. De Hert M, Yu W, Detraux J, Sweera K, Van Winkel R, Correll C. Body weight and metabolic adverse effects of asenapine, iloperidone, lurasidone and paliperidone in the treatment of schizophrenia and bipolar disorder a systematic review and exploratory meta-analysis. CNS Drugs. 2012;26(9):733–759. [DOI] [PubMed] [Google Scholar]
- 11. Leucht S, Cipriani A, Spineli L, Mavridis D, Crey D. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382:951–962. [DOI] [PubMed] [Google Scholar]
- 12. Higgins JPT, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. Section 16.5.4: Studies with more than two intervention groups In: Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions (Version 5.1.0) [Internet]. The Cochrane Collaboration [cited 2014 May 15]. Available from: http://handbook.cochrane.org/
- 13. Sterne JAC, Egger M, Moher D. Chapter 10: Addressing reporting biases. Section 10.4.4.1: Comparing fixed and random effects estimates. In: Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions (Version 5.1.0) [Internet]. The Cochrane Collaboration [cited 2015 Jun 6]. Available from: http://handbook.cochrane.org/
- 14. Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions [Internet]. Version 5.1.0 [updated March 2011]. London (UK): The Cochrane Collaboration; 2011. [cited 2014 Mar 20]. Available from: http://handbook.cochrane.org/ [Google Scholar]
- 15. Higgins JPT, Deeks JJ. Chapter 10: Addressing reporting biases. Section 10.4.3.1: Recommendations on testing for funnel plot asymmetry. In: Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions (Version 5.1.0) [Internet]. The Cochrane Collaboration [cited 2016 Jun 20]. Available from: http://handbook.cochrane.org/
- 16. Buchanan R, Panagides J, Zhao J, Phiri P, Hollande W, Ha X. Asenapine versus olanzapine in people with persistent negative symptoms of schizophrenia. J Clin Psychopharmacol. 2012;321(1):36–45. [DOI] [PubMed] [Google Scholar]
- 17. Kane J, Cohen M, Zhao J, Alphs L, Panagides J. Efficacy and safety of asenapine in a placebo- and haloperidol-controlled trial in patients with acute exacerbation of schizophrenia. J Clin Psychopharmacol. 2010;30(2):106–115. [DOI] [PubMed] [Google Scholar]
- 18. Kane J, Mackle M, Snow-Adami L, Zhao J, Szegedi A, Panagides J. A randomized placebo-controlled trial of asenapine for the prevention of relapse of schizophrenia after long-term treatment. J Clin Psychiatry. 2011;72(3):349–355. [DOI] [PubMed] [Google Scholar]
- 19. Potkin S, Cohen M, Panagides J. Efficacy and tolerability of asenapine in acute schizophrenia: a placebo- and risperidone-controlled trial. J Clin Psychiatry. 2007;68(10):1492–1500. [DOI] [PubMed] [Google Scholar]
- 20. Schoemaker J, Naber D, Vrijland P, Panagides J, Emsley R. Long-term assessment of asenapine versus olanzapine in patients with schizophrenia or schizoaffective disorder. Pharmacopsychiatry. 2010;43:138–146. [DOI] [PubMed] [Google Scholar]
- 21. Chapel S, Hutmacher M, Haig G, Bockbrader H, Greef R, Preskorn S. Exposure-response analysis in patients with schizophrenia to assess the effects of asenapine on QTc prolongation. J Clin Psychiatry. 2009;49:1297–1308. [DOI] [PubMed] [Google Scholar]
- 22. Laughren T. Department of Health and Human Services Public Health Service Food and Drug Administration, Center for Drug Evaluation and Research. Recommendation for approval action for asenapine sublingual tablets for the acute treatment of schizophrenia and for the acute treatment of mania or mixed episodes in bipolar 1 disorder 2009 (22-117) [Internet] [cited 2014 Apr 10]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022117s000_MedR_P1.pdf
- 23. Australian Government Department of Health and Ageing, Therapeutic Goods Administration. Australian public assessment report for asenapine [Internet]. 2011 [cited 2014 Apr 10]. Available from: http://www.tga.gov.au/auspar/auspar-asenapine
- 24. Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement [Internet]. PLoS Med. 2009;6(6):e1000097 [cited 2013 Dec]. Available from: http://www.prisma-statement.org/index.htm [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Higgins JPT, Deeks JJ, editors. Chapter 7: Selecting studies and collecting data. Section 7.2.4: Implementation of the selection process. In: Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions (Version 5.1.0) [Internet]. The Cochrane Collaboration [cited 2015 Jun 4]. Available from: http://handbook.cochrane.org/