Abstract
The bradykinin B2 receptor (BDKRB2) plays a key role in the inflammation process of osteoarthritis. Nitric oxide has also long been considered to be a catabolic factor that contributes to inflammatory response and the osteoarthritis disease pathology. Several studies have reported that the BDKRB2 +9/−9 bp polymorphisms are associated with transcription of the receptor. However, the roles of BDKRB2 polymorphisms in inflammation in osteoarthritis remain unclear. This study enrolled 156 subjects with primary knee osteoarthritis and 58 healthy volunteers. BDKRB2 polymorphisms were genotyped, and the mRNA and protein levels of BDKRB2 in synovial tissues from osteoarthritis patients were measured by quantitative real-time polymerase chain reaction and western blot analysis, respectively. Nitric oxide production in serum from patients with osteoarthritis was measured using a nitric oxide assay kit. We found that the mean BDKRB2 mRNA levels were significantly higher in Kallgren-Lawrence grade-4 osteoarthritis patients than patients with lower grade osteoarthritis. The +9/−9 bp polymorphisms significantly affected the BDKRB2 mRNA and protein expression levels in synovial tissues from osteoarthritis subjects. Osteoarthritis patients with +9/−9 and −9/−9 genotypes had higher BDKRB2 expression levels in synovial tissue and nitric oxide production in serum. Moreover, positive correlation was found between the BDKRB2 levels in synovial tissue and nitric oxide production. Compared with health controls, significant increases of nitric oxide production in osteoarthritis were detected which were associated with increasing severity of osteoarthritis. Multiple linear regression analysis (adjusted for gender and age) showed serum nitric oxide level was positively associated with BDKRB2 polymorphism and Kallgren-Lawrence grade and was inversely associated with obesity. Our findings showed that the BDKRB2 +9/−9 bp polymorphisms affected the gene expression and nitric oxide production, which were associated with radiographic severity of osteoarthritis, suggesting that the BDKRB2 +9/−9 bp polymorphisms may act as a genetic modulator of osteoarthritis, and play an essential role in inflammatory process in osteoarthritis.
Keywords: Bradykinin B2 Receptor (BDKRB2), osteoarthritis (OA), gene polymorphisms, inflammation
Introduction
Osteoarthritis (OA), one of the most common forms of arthritis, is known to affect millions of people around the world. OA is characterized by chronic degeneration of joint structures in the body including the synovial membrane, cartilage, and bone tissues.1,2 The classic characteristic of this disease is the degradation of cartilage in the joint space leading to swollen, creaky joints. Epidemiological studies detail the many risk factors that predispose humans to OA; however, the events that lead to the initiation of the disease have not been identified. It has been reported that aging, trauma, hormonal, and mechanical factors all have the ability to contribute to both the onset and progression of OA.3–5 In addition to the classical predisposing factors listed here, several studies have demonstrated that gene polymorphisms may be related to the pathogenesis of OA.6–8
Currently, with limited diagnostic approaches, the major treatment of OA is to control the inflammation while managing the patient’s pain. The administration of Bradykinin B2 receptor (BDKRB2) antagonists, which reportedly produce enduring analgesic effects in patients afflicted with OA of the knee, signifies the potential key role played by bradykinins in the progression of the disease.9,10 Bradykinins are inflammatory mediators belonging to a family of oligopeptides that result from the enzymatic action of kallikreins proteolytically cleaving kininogens and are vasodilators known to be produced by the synovium.11,12 These newly produced bradykinins contribute to both the initiation and maintenance of inflammation, leading to the production of pain, advancement of the highly catabolic state, subsequent chondrocyte apoptosis, and the progressive degeneration of articular cartilage. Most of these actions are mediated by the receptor BDKRB2.13 BDKRB2 mediates the majority of the inflammatory events induced by bradykinin and is extensively dispersed among most tissues of the body, including the tissues of the joints commonly affected by OA.14 The BDKRB2 is considered a stronger mediator of vasodilatation and inflammation through increased production and release of nitric oxide (NO). Previous studies indicated that increased concentration of nitrite (an NO metabolite) in serum and synovial fluid samples of patients with rheumatioid arthritis and OA and suggested a role for NO as an inflammatory mediator in rheumatic diseases.15
The transcription of BDKRB2 is influenced by genetic polymorphisms of this receptor. Several studies reported that the BDKRB2 insertion of 9 bp (+9) and deletion of 9 bp (−9) polymorphisms were associated with the transcriptional activity of the receptor; the +9 but not the −9 polymorphism of BDKRB2 was associated with lower gene transcription.16,17 Genetic polymorphisms of the BDKRB2 are reportedly associated with a succession of pathological conditions including diabetes mellitus,18 pulmonary artery pressure,19 and increased left ventricular mass-associated hypertension.20 Our previous study first showed the association between the BDKRB2 polymorphisms (+9/−9 and −58T/C) and the susceptibility and severity of OA.21
Despite the role of BDKRB2 in inflammation and the association of its polymorphisms and OA being documented, the roles of BDKRB2 polymorphisms in synovial inflammation remain unclear. Given the known association of the BDKRB2 with OA, its association with NO production, and the link between NO production and inflammation, we sought to investigate the association between the genetic polymorphisms of BDKRB2 and the expression of the receptor and NO production, which may be involved in inflammation reaction in OA.
Materials and methods
Patients
From February 2013 to November 2014, 156 patients with primary OA of the knee and 58 healthy volunteers were recruited for the study. The research was approved by the ethics review committee of Jinling hospital, and written informed consent was obtained from all participants of the study. Diagnosis of subjects with knee OA was made based on the American College of Rheumatology criteria22 and the severity of OA was evaluated by the Kallgren-Lawrence (KL) grade. Patients diagnosed with other types of knee diseases, other than OA, were excluded from the patient group. And the control group had no history, signs, or symptoms of arthritis or joint disease such as joint pain, swelling, tenderness, or restricted movement. Demographic data and information on all the established risk factors including age, sex, body mass index (BMI), smoking status, knee sensitivity, and regular exercise were recorded for both groups. Obesity was defined as BMI > 30 kg/m2.
BDKRB2 genotyping
DNA was extracted from peripheral whole blood samples that were collected using a Qiagen DNA Isolation Kit (Qiagen, Valencia, CA, USA). Two polymorphic loci (+9/−9, rs5810761 and C −58 T, rs1799722) of the BDKRB2 gene were examined according to the previously described methods.21 Allele and genotype frequencies between the OA patients and the control subjects were compared using chi-square analysis. When observed or expected values included a cell with a value less than 5, Fisher’s exact test was used. In all cases, significance was accepted at P < 0.05.
Quantitative real-time polymerase chain reaction
Synovial tissue specimens were obtained from 156 OA patients during joint replacement surgery. None were obtained from controls due to ethical concerns. To compare expression levels of the different genetic variants of BDKRB2 in OA patients, RNA was extracted from the synovial tissues and used to synthesize cDNA using the Super Script First-Strand cDNA synthesis kit (Invitrogen, Grand Island, NY, USA). Gene expression was determined by quantitative real-time polymerase chain reaction (PCR) using Prime Time Mini qPCR Assays (Integrated DNA Technologies, IA, USA). PCR reactions were performed in an ABI PRISM 7900HT Sequence Detection System (Applied Biosystems) according to the manufacturer’s instructions. Quantitative expression of the BDKRB2 gene was measured relative to the average expression of the housekeeping genes HPRT1, GAPDH, and 18S. For each cDNA sample, three pipetting replicates were performed on all samples (18 S (n = 3), GAPDH (n = 3), HPRT1 (n = 3), and BDKRB2 (n = 3)). An average of three housekeeping gene Ct value (3 × 3) was used as the control to compare to BDKRB2. Relative expression of BDKRB2 and its variants compared with the housekeeping genes was analyzed using the 2−ΔCt method. Kruskal-Wallis tests were performed to assess whether there was a statistical difference between wild type BDKRB2 expression relative to the +9/−9 and −58C/T polymorphisms. All primer and probe sequences can be found in Table 1.
Table 1.
The PrimeTime Mini qPCR Assays for quantitative PCR analysis of BDKRB2
| Gene | Primer sequence (5′ – 3′) | Probe (5′ – 3′) |
|---|---|---|
| 18S | CGAATGGCTCATTAAATCAGTTATGG | TCCTTTGGTCGCTCGCTCCTCTCCC |
| TATTAGCTCTAGAATTACCACAGTTATCC | ||
| GAPDH | GGCCATCCACAGTCTTCTG | ATGACCACAGTCCATGCCATCACT |
| CAGCCTCAAGATCATCAGCAA | ||
| HPRT1 | TGCTGAGGATTTGGAAAGGG | AGGACTGAACGTCTTGCTCGAGATG |
| ACAGAGGGCTACAATGTGATG | ||
| BDKRB2 | GTGCTGCGGAACAACGAGAT | AGAAGTTCAAGGAGATCCAGACA |
| TGAATAGCAGCAGCACAACCA |
BDKRB2: bradykinin B2; PCR: polymerase chain reaction.
Western blot analysis
Proteins extracted from the synovial tissue were subject to sodium dodecyl sulfate polyacrylamide gel electrophoresis, and the separated proteins were electro-transferred onto polyvinylidene fluoride membranes (Millipore, Bedford, MA, USA). Nonspecific proteins on the membranes were blocked in PBS with 0.1% Tween-20 containing 5% nonfat milk for 1 h and then incubated with the rabbit anti-human BDKRB2 primary antibody (Sigma-Aldrich, St. Louis, MO, USA). The membranes were then incubated with the appropriate horseradish peroxidase-conjugated secondary antibodies (GE Healthcare, Buckinghamshire, UK). Immunoreactive proteins were visualized with the enhanced chemiluminescence (ECL) reagent (Amersham Pharmacia, Piscataway, NJ, USA).
Measurements of NO production by NO derivatives nitrate and nitrite
Blood samples obtained were collected in EDTA-Eppendorf tubes and resulting serum, after centrifugation at 2500 rpm for 10 min at 4℃, was preserved in a −70℃ until analysis. NO production was indirectly measured by NO derivatives nitrate and nitrite using a NO assay kit (Beyotime Biotechnology, Shanghai, China) according to the manufacturer’s protocol. Briefly, serum samples (50 µl) were added to the 96 well plates, followed by color development with Griess reagents, the absorbance was determined at 540 nm with a luminometer plate reader.
Statistical analyses
All analyses were performed using SPSS software (Statistical Package for the Social Sciences, version 16.0, SPSS Inc., Chicago, IL, USA). Correlation data were analyzed using Pearson correlation test. Multiple regression analysis was carried out in order to validate correlations found and the importance grade of one factor into another with adjustment for gender and age. Statistical significance was evaluated using the two-tailed Mann–Whitney U-test, Kruskal-Wallis tests, or the Student’s t-test for nonparametric and normally distributed data, respectively, and P values lower than 0.05 were considered to be significant.
Results
Quantitative expression of BDKRB2 in synovial tissue stratified by genotype at the BDKRB2 polymorphisms
Our previous study showed the association between the BDKRB2 polymorphisms (+9/−9 and −58T/C) and the susceptibility and severity of OA.21 To assess the effect that the two BDKRB2 polymorphisms has on BDKRB2 expression in OA, the BDKRB2 expression in synovial tissue specimens from OA patients were detected by quantitative RT-PCR and Western blot. Details regarding the patients can be found in Table 2. Osteoarthritic patients exhibited considerably higher BMI (more obesity) than the control individuals (P = 0.001), while other demographic and clinical characteristics (Table 2) showed no profound differences between the two groups. The other characteristics include sex, age, smoking status, or history of work involving heavy labor among the OA patients and the controls.
Table 2.
Demographic and clinical characteristics of all subjects in the study
| Variables | Cases (n = 156) | Control (n = 58) | P |
|---|---|---|---|
| Age (years), mean ± SD | 60.8 ± 7.2 | 60.4 ± 6.1 | ns |
| Female, n (%) | 85 (54.5%) | 32 (55.2%) | ns |
| Obesity, n (%) | 86 (55.1%) | 24 (41.4%) | 0.001a |
| Smoker, n (%) | 64 (41.0%) | 23 (39.7%) | ns |
| History of heavy labor work, n (%) | 44 (28.2%) | 17 (29.3%) | ns |
| Family history of OA, n (%) | 20 (12.8%) | 8 (13.8%) | ns |
| KL grade | |||
| ≤3 | 96 | – | |
| >3 | 60 | – |
OA: osteoarthritis; KL: Kallgren-Lawrence; ns: not significant.
Statistically significant (vs. control).
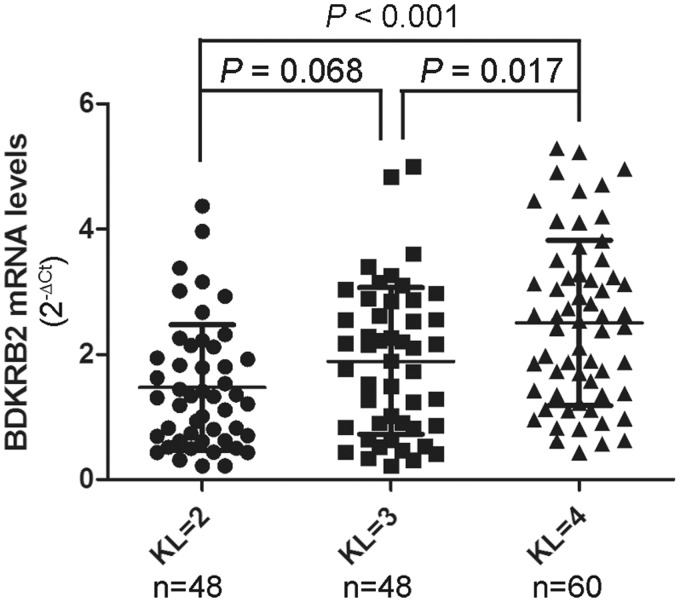
First, we analyzed the relationship between the BDKRB2 mRNA level in synovial tissue and the radiographic severity of OA. The mean BDKRB2 mRNA levels were significantly higher in KL grade-4 OA patients (2.5 ± 1.3) than KL grade-2 OA patients (1.5 ± 1.0, P < 0.001) or KL grade-3 OA patients (1.9 ± 1.2, P = 0.017) (Figure 1).
Figure 1.
Association between BDKRB2 mRNA levels in synovial tissue and radiographic severity of OA. The BDKRB2 mRNA levels in synovial tissues from patients with OA according to Kellgren-Lawrence (KL) grade. n = the number of patients studied for each group. The horizontal lines in each plot represent the mean and the standard deviation (SD) of the mean. P-values were calculated using a two-tailed Mann–Whitney U-test
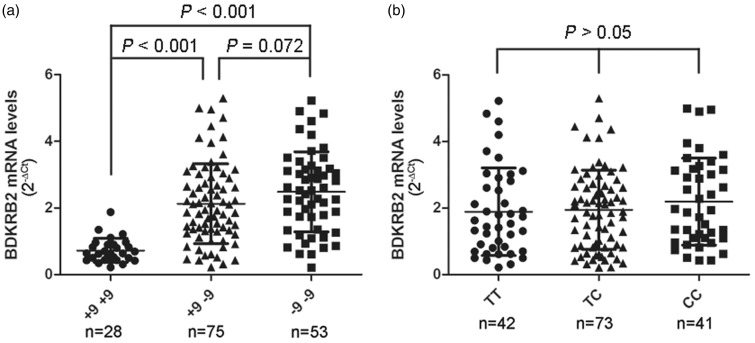
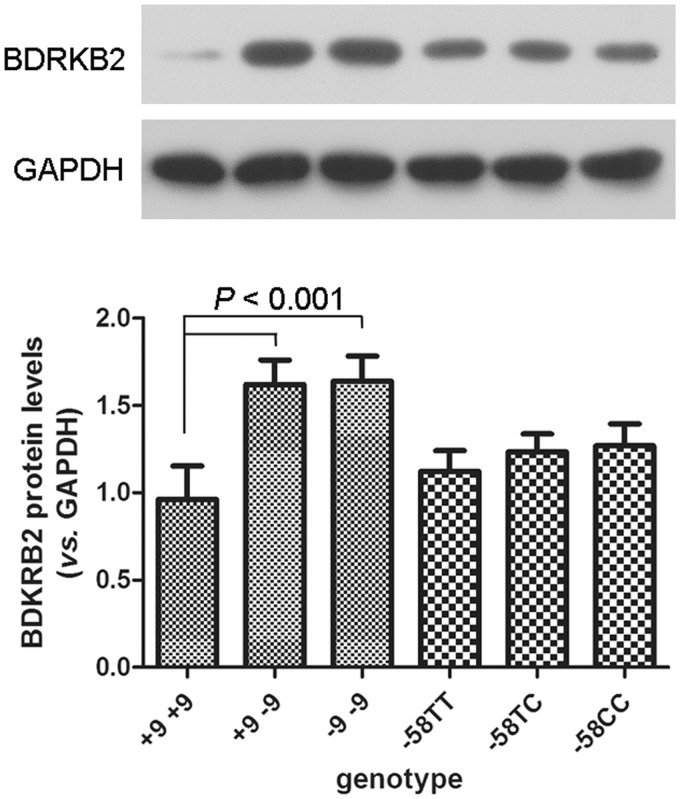
The BDKRB2 mRNA levels in synovial tissue were studied according to the genotypes of the BDKRB2 gene, and it emerged that the BDKRB2 mRNA levels in synovial tissue were significantly lower in +9/+9 carriers than in +9/−9 and −9/−9 carriers (Figure 2(a), 0.7 ± 0.4 vs. 2.1 ± 1.2 and 2.5 ± 1.2; both P < 0.001), while the genetic polymorphisms of −58T/C did not influence the BDKRB2 mRNA level in synovial tissue (Figure 2(b), P > 0.05). Western blot analysis also exhibited similar BDKRB2 protein levels in synovial tissue from the −58T/T, −58T/C, and −58C/C genotype carriers (Figure 3) and higher BDKRB2 expression levels in synovial tissue from +9/−9 and −9/−9 genotypes.
Figure 2.
Columnar scatter plot of the quantitative expression of BDKRB2 in synovial tissue from patients with OA by genotype at the (a) +9/−9 and (b) −58T/C polymorphisms. n = the number of patients studied for each group. The horizontal lines in each plot represent the mean and the standard deviation (SD) of the mean. P-values were calculated using a two-tailed Mann–Whitney U-test
Figure 3.
The BDKRB2 protein levels in synovial tissue from different genotype carriers by western blot analysis. The above panel shows a representative western blot of BDKRB2 in synovial tissue from different genotype carriers. The lower panel shows densitometric analysis of the BDKRB2/GAPDH ratio. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. The data are presented as mean ± SD. P-values were calculated using a Student’s t test
NO production in serum samples from OA patients stratified by genotype at the BDKRB2 +9/−9 bp polymorphisms
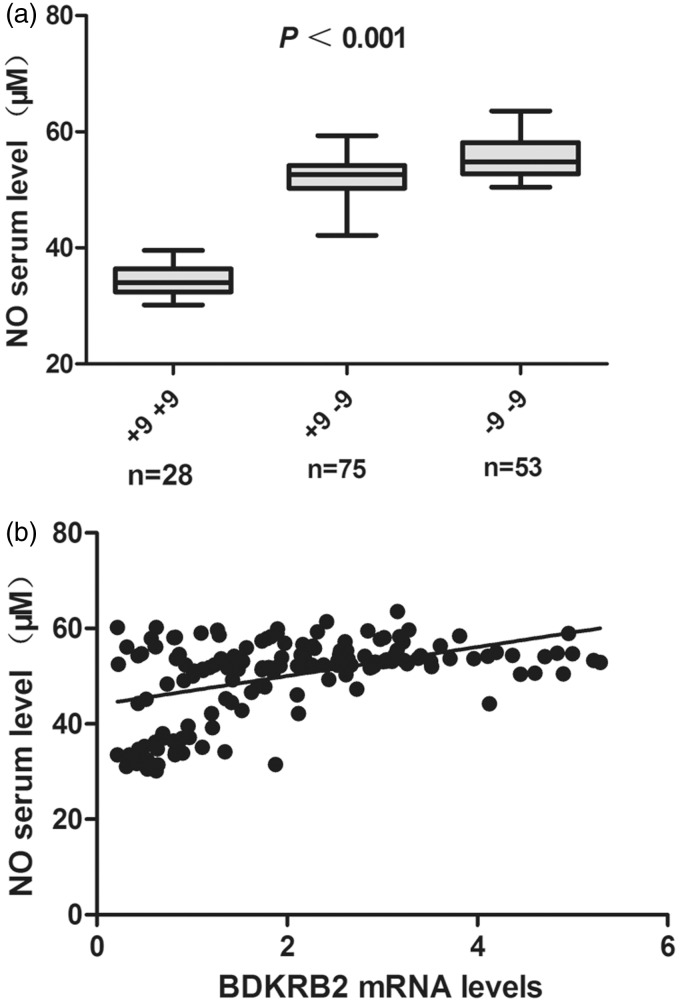
In order to identify whether the BDKRB2 +9/−9 bp polymorphisms had an impact on NO production in OA, NO production in serum samples of OA was measured by NO derivatives nitrate and nitrite. Our results showed that the NO levels in serum were significantly higher in +9/−9 and −9/−9 carriers compared with the +9/+9 carriers (Figure 4(a)). Positive correlation was found between the BDKRB2 levels in synovial tissue and NO production (Figure 4(b), Pearson correlation coefficient 0.458, P < 0.001).
Figure 4.
Effect of the BDKRB2 +9/−9 bp polymorphisms on the NO production in serum samples of OA patients. (a) Significant increased NO production in serum was found in +9/−9 and −9/−9 carriers compared with the +9/+9 carriers (P < 0.001, Kruskal-Wallis test). (b) Positive correlation was found between the BDKRB2 levels in synovial tissue and NO production (Pearson correlation coefficient 0.519, P < 0.001)
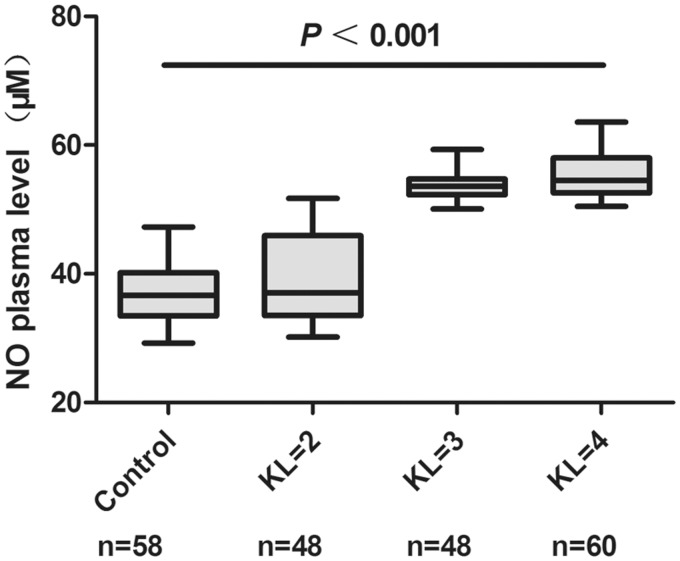
The association between the NO production in serum samples and the radiographic severity of OA were also analyzed. Compared with health controls, significant increases of NO production in OA were detected which were associated with increasing severity of OA (Figure 5).
Figure 5.
Correlation between NO production levels in serum and radiographic severity of OA. The NO production in serum from patients with OA according to Kellgren-Lawrence (KL) grade. n = the number of patients studied for each group. P-values were calculated using a Kruskal-Wallis test
Variables identified in stepwise multiple linear regression (MLR) analysis as significant independent predictors of serum NO level, and subsequently used in MLR analyses, were BDKRB2 polymorphism, obesity, KL grade, gender, and age. The final model for the dependent variable serum NO level is shown in Table 3 and indicates that BDKRB2 polymorphism, obesity, and OA score had independent associations with serum NO level. In the overall OA patients, the associations between serum NO level and BDKRB2 polymorphism, obesity, and KL grade were 0.806 (P < 0.001), −0.188 (P = 0.009), and 0.772 (P < 0.001), respectively. On adjusted MLR analysis (adjusted for gender and age), serum NO level was positively associated with BDKRB2 polymorphism and KL grade (β = 0.804; 95% CI, 8.37 to 10.60; and β = 0.259; 95% CI, 0.74 to 4.45) and was inversely associated with obesity (β = −0.132; 95% CI, 3.75 to −0.65).
Table 3.
Multiple linear regression for the dependent variable serum NO level in OA patients
| Independent variables | Variable estimate ± SEE | t value | P |
|---|---|---|---|
| (Intercept) | 28.27 ± 1.56 | 18.12 | <0.001 |
| BDKRB2 polymorphism | 6.754 ± 1.10 | 0.02 | <0.001 |
| Obesity | −1.83 ± 0.77 | 1.46 | 0.019 |
| KL grade | 2.59 ± 0.94 | 6.13 | 0.006 |
| Gender | 0.01 ± 0.78 | −2.37 | 0.985 |
| Age | 0.08 ± 0.05 | 2.76 | 0.147 |
BDKRB2: bradykinin B2; OA: osteoarthritis; KL: Kallgren-Lawrence. Multiple R2 = 0.682, Adjusted R2 = 0.676.
Discussion
In recent years, numerous studies have proven the biological significance of bradykinin in the regulation of various physiological conditions such as inflammation, vascular permeability, hypotension, edema, smooth muscle contraction, and glucose homeostasis.12,23,24 It has been accepted that nearly all the physiologically significant effects of bradykinin are exerted by activation of the B2 receptor (BDKRB2).25 In this study, we investigated the role of BDKRB2 gene polymorphisms in determining gene expression and NO production in serum samples of OA. Our results showed the BDKRB2 +9/−9 bp polymorphisms are associated with increased BDKRB2 gene expression and NO production in OA.
NO was considered a catabolic factor that contributes to the development of OA by mediating the expression of proinflammatory cytokines, inducing chondrocyte apoptosis, and inhibiting the synthesis of collagen and proteoglycans.26,27 Previous studies have demonstrated an increase in serum and synovial fluid nitrate/nitrite in rheumatic disease including OA.15,28,29 In our research, a significant increase of NO production in OA compared with health controls was also found, which was associated with increasing severity of OA. Although high levels of NO production are often thought to promote inflammatory responses, the precise role of NO in inflammation is still controversial. Recent studies suggest that NO and its redox derivatives may also have protective effects on chondrocyte.30 Further research may help to elucidate a potential role for NO in the inflammatory process of OA.
Bradykinin (BK) is a vasodilator and inflammatory nonapeptide which is generated in OA synovium. Via activation of BK receptors, BK promotes vasodilatation by stimulating the release of NO from the vascular endothelium. Expression of BDKRB2 has been observed on the synovial lining cells, fibroblasts, and endothelial lining cells of blood vessels from OA patients.14,31 BDKRB2 is involved in the initiation and maintenance of inflammation, producing pain, and activating synoviocytes and chondrocytes: the main cells involved in the homeostasis of synovial fluid and cartilage, respectively.13,32 BDKRB2 signaling leading to NO production via eNOS has been well studied.33 Given the known association of the BDKRB2 with OA and its association with NO production, we found positive correlation between the BDKRB2 levels in synovial tissue and NO production in serum from patients with OA. Then, the mechanism of BDKRB2-induced NO production in OA is unclear but appears to be related to inducible NO pathway.
The human BDKRB2 gene is mapped to chromosome 14q32.34 A number of polymorphic loci in the BDKRB2 gene, including a 9-bp insertion/deletion in the first exon of the gene (+9/−9 respectively, rs5810761) and a C to T transition in the promoter region (−58T/C, rs1799722), have been identified.35 Previous studies demonstrated that the BDKRB2 +9/−9 bp polymorphisms are associated with transcription of the gene.16,17 In this study, we investigated the role of BDKRB2 +9/−9 and −58C/T polymorphisms in determining BDKRB2 expression. Our results showed that the 9 bp deletion (−9) in the gene encoding BDKRB2 is associated with increased expression of BDKRB2 mRNA and higher protein levels in synovial tissue. The polymorphism −58T/C did not influence the BDKRB2 expression level. Moreover, the mean BDKRB2 mRNA levels were significantly higher in KL grade-4 OA patients as compared to grade-2 and grade-3 patients, further indicating a correlation between higher levels of BDKRB2 mRNA and disease severity. In addition, we have investigated whether the BDKRB2 +9/−9 bp polymorphisms had an impact on NO production in OA, our results showed that the NO levels in serum samples of OA in serum were significantly higher in +9/−9 and −9/−9 carriers compared with the +9/+9 carriers. MLR analysis confirmed that serum NO level was positively associated with BDKRB2 polymorphism and KL grade. Our data may suggest that the increased transcription of the B2 receptor presented by −9 allele carriers is associated with higher bradykinin activity, resulting in an increase in NO production, which may be related to cartilage degradation and inflammation in OA.
There are some limitations in our study. First, a large proportion of obese patients were included in this study. A considerably significant higher BMI was found among OA patients in our study, insinuating that obesity could be important risk factor for OA not only as a mechanical factor but also due to the related increase in cytokines, which helps with OA development. Alternatively, there might have been enrollment bias in the recruitment of primary OA patients into this study. Second, despite the BDKRB2 expression was associated with NO production, further functional analyses need to be performed to disclose the mechanism under which the BDKRB2 gene polymorphisms affect the inflammation and severity of OA.
In conclusion, our data showed that the BDKRB2 +9/−9 bp polymorphisms affected the gene expression and NO production, which were associated with radiographic severity of OA, suggesting that the BDKRB2 +9/−9 bp polymorphisms may act as a genetic modulator of OA, and play an essential role in inflammatory process in OA.
Authors contributions: SC, L Zhang, L Zhou, and JZ participated in the design, interpretation of the studies, and analysis of the data; RX, YT, and YZ conducted the experiments; SC wrote the manuscript; L Zhou and JZ review of the manuscript. SC and L Zhang equally contributed to this paper.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The study was supported by the grant from the National Natural Science Foundation of China (81301582).
References
- 1.Bonnet CS, Walsh DA. Osteoarthritis, angiogenesis and inflammation. Rheumatology (Oxford) 2005; 44: 7–16. [DOI] [PubMed] [Google Scholar]
- 2.Hunter DJ, Wilson DR. Imaging the role of biomechanics in osteoarthritis. Rheum Dis Clin North Am 2009; 35: 465–83. [DOI] [PubMed] [Google Scholar]
- 3.Bae JY, Park KS, Seon JK, Kwak DS, Jeon I, Song EK. Biomechanical analysis of the effects of medial meniscectomy on degenerative osteoarthritis. Med Biol Eng Comput 2012; 50: 53–60. [DOI] [PubMed] [Google Scholar]
- 4.Esser S, Bailey A. Effects of exercise and physical activity on knee osteoarthritis. Curr Pain Headache Rep 2011; 15: 423–30. [DOI] [PubMed] [Google Scholar]
- 5.Schenker ML, Mauck RL, Ahn J, Mehta S. Pathogenesis and prevention of posttraumatic osteoarthritis after intra-articular fracture. J Am Acad Orthop Surg 2014; 22: 20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magana JJ, Munoz B, Borgonio-Cuadra VM, Razo-Estrada C, Gonzalez-Huerta C, Cortes-Gonzalez S, Albores A, Miranda-Duarte A. The association of single nucleotide polymorphisms in the calcitonin gene with primary osteoarthritis of the knee in Mexican mestizo population. Rheumatol Int 2013; 33: 2483–91. [DOI] [PubMed] [Google Scholar]
- 7.Valdes AM, Loughlin J, Oene MV, Chapman K, Surdulescu GL, Doherty M, Spector TD. Sex and ethnic differences in the association of ASPN, CALM1, COL2A1, COMP, and FRZB with genetic susceptibility to osteoarthritis of the knee. Arthritis Rheum 2007; 56: 137–46. [DOI] [PubMed] [Google Scholar]
- 8.Zhang W, Doherty M. How important are genetic factors in osteoarthritis? Contributions from family studies. J Rheumatol 2005; 32: 1139–42. [PubMed] [Google Scholar]
- 9.Bellucci F, Cucchi P, Catalani C, Giuliani S, Meini S, Maggi CA. Novel effects mediated by bradykinin and pharmacological characterization of bradykinin B2 receptor antagonism in human synovial fibroblasts. Br J Pharmacol 2009; 158: 1996–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warde N. Osteoarthritis: local antagonism of endothelin-1 and bradykinin receptors improves OA pain and joint morphology in rats. Nat Rev Rheumatol 2011; 7: 375–375. [DOI] [PubMed] [Google Scholar]
- 11.Bouillet L, Boccon-Gibod I, Massot C. Bradykinin mediated angioedema. Rev Med Interne 2011; 32: 225–31. [DOI] [PubMed] [Google Scholar]
- 12.Regoli D. Pharmacology of bradykinin and related kinins. Adv Exp Med Biol 1983; 156: 569–84. [PubMed] [Google Scholar]
- 13.Meini S, Maggi CA. Knee osteoarthritis: a role for bradykinin? Inflamm Res 2008; 57: 351–61. [DOI] [PubMed] [Google Scholar]
- 14.Cassim B, Naidoo S, Ramsaroop R, Bhoola KD. Immunolocalization of bradykinin receptors on human synovial tissue. Immunopharmacology 1997; 36: 121–5. [DOI] [PubMed] [Google Scholar]
- 15.Farrell AJ, Blake DR, Palmer RM, Moncada S. Increased concentrations of nitrite in synovial fluid and serum samples suggest increased nitric oxide synthesis in rheumatic diseases. Ann Rheum Dis 1992; 51: 1219–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braun A, Kammerer S, Maier E, Bohme E, Roscher AA. Polymorphisms in the gene for the human B2-bradykinin receptor. New tools in assessing a genetic risk for bradykinin-associated diseases. Immunopharmacology 1996; 33: 32–5. [DOI] [PubMed] [Google Scholar]
- 17.Lung CC, Chan EK, Zuraw BL. Analysis of an exon 1 polymorphism of the B2 bradykinin receptor gene and its transcript in normal subjects and patients with C1 inhibitor deficiency. J Allergy Clin Immunol 1997; 99: 134–146. [DOI] [PubMed] [Google Scholar]
- 18.de Oliveira Alvim R, Santos PC, Nascimento RM, Coelho GL, Mill JG, Krieger JE, Pereira AC. BDKRB2 +9/-9 polymorphism is associated with higher risk for diabetes mellitus in the Brazilian general population. Exp Diabetes Res 2012; 2012: 480251–480251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olson TP, Frantz RP, Turner ST, Bailey KR, Wood CM, Johnson BD. Gene Variant of the Bradykinin B2 Receptor Influences Pulmonary Arterial Pressures in Heart Failure Patients. Clin Med Circ Respirat Pulm Med 2009; 2009: 9–17. [PMC free article] [PubMed] [Google Scholar]
- 20.Brull D, Dhamrait S, Myerson S, Erdmann J, Woods D, World M, Pennell D, Humphries S, Regitz-Zagrosek V, Montgomery H. Bradykinin B2BKR receptor polymorphism and left-ventricular growth response. Lancet 2001; 358: 1155–6. [DOI] [PubMed] [Google Scholar]
- 21.Chen S, Zhou Y, Li J, Shan LQ, Fan QY. The effect of bradykinin B2 receptor polymorphisms on the susceptibility and severity of osteoarthritis in a Chinese cohort. J Biomed Biotechnol 2012; 2012: 597637–597637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, Christy W, Cooke TD, Greenwald R, Hochberg M, Howell D, Kaplan D, Koopman W, Longley S, Mankin H, Mcshane DJ, Medsger T, Meenan R, Mikkelsen W, Moskowitz R, Murphy W, Rothschild B, Segal M, Sokoloff L, Wolfe F. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum 1986; 29: 1039–49. [DOI] [PubMed] [Google Scholar]
- 23.Barros CC, Haro A, Russo FJ, Schadock I, Almeida SS, Reis FC, Moraes MR, Haidar A, Hirata AE, Mori M, Bacurau RF, Wurtele M, Bader M, Pesquero JB, Araujo RC. Bradykinin inhibits hepatic gluconeogenesis in obese mice. Lab Invest 2012; 92: 1419–27. [DOI] [PubMed] [Google Scholar]
- 24.Rocha ESM, Beraldo WT, Rosenfeld G. Bradykinin, a hypotensive and smooth muscle stimulating factor released from plasma globulin by snake venoms and by trypsin. Am J Physiol 1949; 156: 261–73. [DOI] [PubMed] [Google Scholar]
- 25.Duka I, Shenouda S, Johns C, Kintsurashvili E, Gavras I, Gavras H. Role of the B(2) receptor of bradykinin in insulin sensitivity. Hypertension 2001; 38: 1355–60. [DOI] [PubMed] [Google Scholar]
- 26.Abramson SB. Nitric oxide in inflammation and pain associated with osteoarthritis. Arthritis Res Ther 2008; 10: S2–S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abramson SB. Osteoarthritis and nitric oxide. Osteoarthritis Cartilage 2008; 16: S15–20. [DOI] [PubMed] [Google Scholar]
- 28.Ersoy Y, Ozerol E, Baysal O, Temel I, MacWalter RS, Meral U, Altay ZE. Serum nitrate and nitrite levels in patients with rheumatoid arthritis, ankylosing spondylitis, and osteoarthritis. Ann Rheum Dis 2002; 61: 76–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pham TN, Rahman P, Tobin YM, Khraishi MM, Hamilton SF, Alderdice C, Richardson VJ. Elevated serum nitric oxide levels in patients with inflammatory arthritis associated with co-expression of inducible nitric oxide synthase and protein kinase C-eta in peripheral blood monocyte-derived macrophages. J Rheumatol 2003; 30: 2529–34. [PubMed] [Google Scholar]
- 30.Abu El Maaty MA, Hanafi RS, El-Badawy S, Gad MZ. Interplay of vitamin D and nitric oxide in post-menopausal knee osteoarthritis. Aging Clin Exp Res 2014; 26: 363–368. [DOI] [PubMed] [Google Scholar]
- 31.Meini S, Cucchi P, Catalani C, Bellucci F, Giuliani S, Maggi CA. Bradykinin and B(2) receptor antagonism in rat and human articular chondrocytes. Br J Pharmacol 2011; 162: 611–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cambridge H, Brain SD. Kinin B2 and B1 receptor-mediated vasoactive effects in rabbit synovium. Peptides 1998; 19: 569–76. [DOI] [PubMed] [Google Scholar]
- 33.Kuhr F, Lowry J, Zhang Y, Brovkovych V, Skidgel RA. Differential regulation of inducible and endothelial nitric oxide synthase by kinin B1 and B2 receptors. Neuropeptides 2010; 44: 145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma JX, Wang DZ, Ward DC, Chen L, Dessai T, Chao J, Chao L. Structure and chromosomal localization of the gene (BDKRB2) encoding human bradykinin B2 receptor. Genomics 1994; 23: 362–9. [DOI] [PubMed] [Google Scholar]
- 35.Powell SJ, Slynn G, Thomas C, Hopkins B, Briggs I, Graham A. Human bradykinin B2 receptor: nucleotide sequence analysis and assignment to chromosome 14. Genomics 1993; 15: 435–8. [DOI] [PubMed] [Google Scholar]