Abstract
Colorectal cancer is the most common malignancy of the gastrointestinal tract. Surgical treatment combined with radiotherapy is the main treatment course for colorectal cancer; nevertheless, radio-resistance is commonly encountered during the treatment course and seriously influences the therapeutic efficacy. We tested the hypothesis that the CXCL12/CXCR4 axis is closely related to radiotherapy sensitivity in colorectal cancer cells. Here, we found that the decrease in cell viability and the increase in cell death induced by radiotherapy were attenuated by CXCL12 treatment, and the inhibition of CXCR4 promoted colorectal cancer cells to be more sensitive to radiotherapy. We also examined the critical roles of CXCL12/CXCR4 in cell survival and found that radiotherapy induced Bax expression and facilitated the activity of caspase-3 and caspase-9, which were reversed by CXCL12 treatment. Cell apoptosis was enhanced by the inhibition of CXCR4 under radiotherapy conditions. Furthermore, treatment with CXCL12 resulted in an increased expression of survivin, and the inhibitory roles of CXCL12 in radiotherapy-induced apoptosis were mitigated by survivin knockdown. These results indicate that CXCL12/CXCR4 protects colorectal cancer cells against radiotherapy via survivin, implying an important underlying mechanism of resistance to radiotherapy during colorectal cancer therapy.
Keywords: CXCL12, apoptosis, colorectal cancer, radio-resistance, survivin
Introduction
Colorectal cancer (CRC), whose 5-year survival rate is less than 30%, is the most common malignancy of the gastrointestinal tract.1 Currently, the principle and standard treatment for advanced colorectal cancer is surgical resection combined with radiotherapy (RT) and chemotherapeutic therapy.2,3 However, the frequent existence of resistance to radiotherapy in colorectal cancer cells during radiotherapy, which significantly affects and impairs the therapeutic effect, causes cancer cells to avoid death. Thus, it is necessary to study the critical factors involved in regulating the sensitivity of colorectal cancer cells to radiotherapy to improve the clinical therapeutic outcomes.
Stromal cell-derived factor 1 (CXCL12, also known as SDF-1) is an inducible chemokine that belongs to the human chemokine superfamily.4 CXCR4 is the most common chemokine receptor of CXCL12 in various types of cells, and the biological roles of CXCL12 in cell growth, differentiation, and angiogenesis are accomplished via binding to its receptor (CXCR4).5,6 It has been reported that elevated expression of CXCR4 is correlated with a significant reduction in median overall survival in renal and cervical cancer.7,8 The expression of CXCR4 is also frequently upregulated in the primary tumors of CRC patients.9,10 Moreover, recent studies have indicated that the CXCL12-CXCR4 pathway is closely related to the initiation and progression of some tumors, and inhibiting the CXCL12-CXCR4 pathway is being considered as a way to sensitize tumors towards anticancer therapies.11–13 However, the roles of CXCL12/CXCR4 in the radiotherapy sensitivity of colorectal cancer cells and the corresponding molecular mechanisms remain unclear.
Apoptosis is a major pathway of cell death, and an increase in cell survival largely results from the inhibition of cellular apoptosis. The overexpression of anti-apoptotic molecules in cancer cells is an important mechanism underlying tumor radio-resistance and recurrence. Survivin, belonging to the inhibitor of apoptosis (IAP) family, is a widely expressed anti-apoptotic protein; it participates in regulating the development and progression of many tumors because of its capacity to facilitate cell survival, promote cell cycle progression, and enhance aggressive tumor behavior.14,15 Previous studies have indicated that the increased level of survivin is closely related with cancer progression and radio-resistance in some cancers, and the inhibition of survivin is considered to be a candidate target for cancer molecular therapeutics.16,17 However, it is still necessary to explore the precise role of survivin in radiotherapy treatment and the regulatory mechanisms of survivin in colorectal cancer cells.
The purpose of our research is to determine whether the CXCL12/CXCR4 axis is closely associated with the sensitivity of radiotherapy in colorectal cancer cells and elucidate the corresponding molecular mechanisms. In this study, our results have shown that radiotherapy-inhibited cell growth is mitigated by CXCL12 treatment, whereas the inhibition of CXCR4 promotes colorectal cancer cell sensitivity to radiotherapy. Moreover, the activation of the CXCL12/CXCR4 pathway leads to the radio-resistance of colorectal cancers cells by upregulating the expression of survivin. These findings provide a novel strategy and potential target for improving the therapeutic efficiency of radiotherapy in colorectal cancer.
Materials and methods
Reagents
The Bax polyclonal antibody (1:500), the Survivin monoclonal antibody (1:500), and the beta-actin monoclonal antibody (1:5000) were all obtained from Santa Cruz Biotechnology. Caspase-3 and caspase-9 activity assay kits and the cytotoxicity assay kit were purchased from the Beyotime Institute of Biotechnology. CXCL12 was got from Peprotech.
Cell lines preparation and culture
HCT116 cells were acquired from ATCC (Rockville, Maryland, USA). We routinely cultured the cells at 37℃ in DMEM medium supplemented with 10% fetal bovine serum in a humidified atmosphere with 5% CO2.
IR treatment
IR treatment was conducted using an irradiator with a cobalt Co60 source (Co-V, Theratron 780; MDS Nordion, Ottawa, Ontario, Canada) at a dose rate of 1 Gy/min.
siRNA design and transfections
Survivin small interfering RNAs (siSurvivin) and non-targeted control siRNA (sicontrol) were purchased from Shanghai GenePharma Co., Ltd (Shanghai, China). HCT116 cells were transfected with siSurvivin or sicontrol. Briefly, we seeded HCT116 cells on a plate and cultured them until 30–50% confluency. Then, we diluted 2.0 µg of siRNAs and 10.0 µL of X-treme with serum-free Opti-MEM-1 media. After softly mixing them, the mixture was allowed to stand for 20 min at 25℃. After that, the mixture was added to the plates.
Cell viability assessment
The assessment of cell viability was determined by the CCK-8 reagent (Dojindo, Japan). HCT116 cells were digested by trypsin and seeded onto a 96-well plate. The density of each well was 2 × 103 cells. After starvation for 24 h, 100 µL of culture media with the indicated treatments were added into cells. After 48 h incubation, we then added 10 µL of the CCK-8 reagent into each well and kept them at 37℃ for 2 h. After that, a microplate reader at 450 nm was used to measure the absorbance.
LDH assay
A cytotoxicity detection kit was utilized to detect the release of LDH. Briefly, after the indicated treatment, we added 120 µL of supernatant into a new 96-well plate and added 60 µL of LDH detection solution from the kit into each well. After 30 min of incubation at 25℃ in the dark, a microplate reader at 490 nm was used to measure the absorbance.
Caspase-3 activity assay
A caspase-3 activity assay kit was utilized to detect its activity. Briefly, cells were solubilized with 100 µL of lysis buffer from the kit for 15 min on ice. Then, 50 µg of protein from the lysates was moved into a new tube, and the reaction buffer with Ac-DEVD-pNA, prepared in accordance with the manufacturer instructions, was added to the tube. After incubation for 4 h at 37℃, its absorbance at 405 nm was measured by a microplate reader.
Caspase-9 activity assay
The procedure and steps were similar to the measurement of caspase-3 activity, but the chromogenic caspase substrate of caspase-9, Ac-LEHD-pNA, was used.
Western blotting
After washing with PBS, 1 × 106 cells were treated with RIPA buffer for 20 min on ice. The lysates were centrifuged at 12,000 g for 10 min at 4℃. Protein concentration was evaluated by the Bradford protein assay. A total of 50 µg of protein from each group was then loaded into 12% SDS-polyacrylamide gels. After electrophoresis, the proteins were transferred from the gels to a PVDF membrane. The membrane was then incubated in 3% BSA for 1 h and then allowed to react with the primary antibodies overnight at 4℃, followed by reaction with the secondary antibody for 1 hour at 25℃ after washing with PBS containing 0.1% TWEEN-20. The membrane was then treated with enhanced chemiluminescence reagents.
Statistical analyses
Data were represented as the mean values ± SEM, and Student's t-test or one-way ANOVA followed by Dunnett’s test, where appropriate, was utilized for evaluating statistical significance. P < 0.05 was considered to be statistically significant.
Results
Cell growth depressed by radiotherapy is mitigated by CXCL12 treatment in colorectal cancers cells
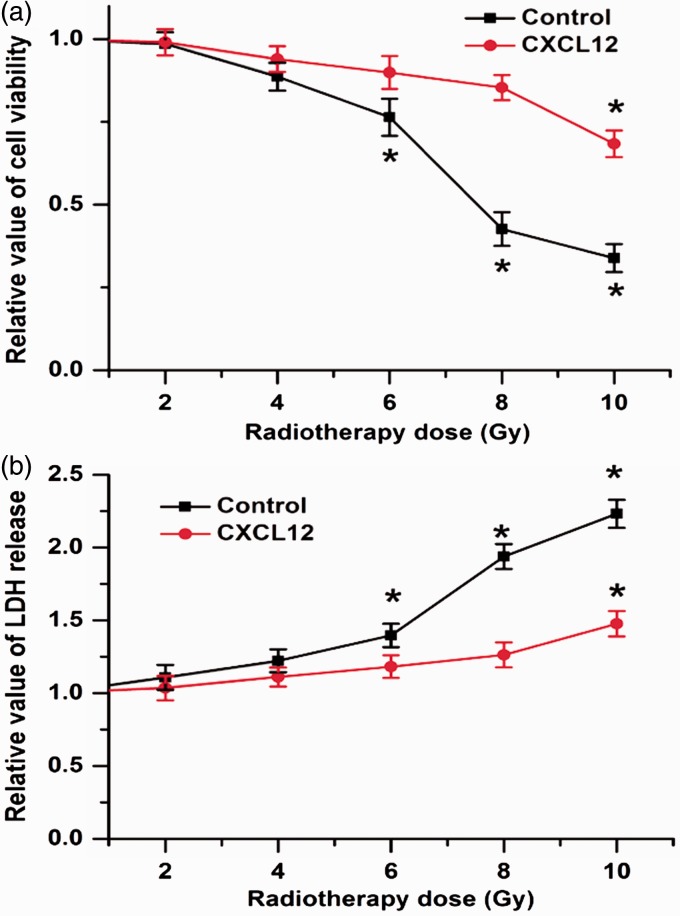
The frequent existence of radio-resistance significantly impairs the therapeutic effects of cancers. To determine whether the CXCL12/CXCR4 pathway is associated with the resistance to radiotherapy in colorectal cancers cells, we first treated HCT116 with exogenous CXCL12 and exposed them to radiotherapy. The CCK-8 results showed that radiotherapy reduced cell viability in a dose-dependent manner, and 8 Gy radiotherapy obviously decreased cell viability in the HCT116 cells. However, the decrease in cell viability by radiotherapy was reversed by CXCL12 treatment (Figure 1(a)). An LDH assay was used to detect cell death, and we found that the release of LDH was potentiated by 8 Gy radiotherapy, whereas CXCL12 treatment mitigated the effects of radiotherapy on LDH release (Figure 1(b)). These results indicate that CXCL12 treatment increases colorectal cancer cell survival after radiotherapy.
Figure 1.
CXCL12 treatment promotes cellular survival in cells exposed to radiation. (a) CCK-8 results showed that 8 Gy radiotherapy significantly decreased cell viability in colorectal cancers cells, which was attenuated by CXCL12 treatment. (b) 8 Gy radiotherapy led to the increased release of LDH, whereas CXCL12 treatment mitigated the effects of radiotherapy on LDH release. Data are expressed as the mean ± SEM (*p < 0.05 compared with 0 Gy). (A color version of this figure is available in the online journal.)
Inhibition of CXCR4 by AMD3100 promotes colorectal cancer cell sensitivity to radiotherapy
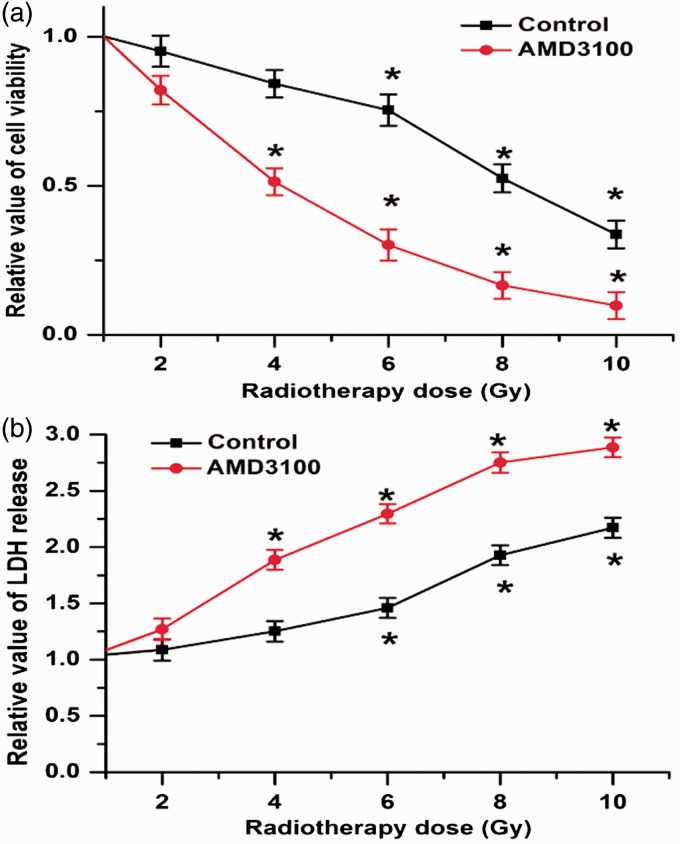
CXCR4 is the most important receptor of CXCL12. Subsequently, we utilized an inhibitor of CXCR4 (AMD3100) to examine the roles of CXCR4 during radiotherapy. We found that HCT116 cell viability was reduced by a minimum dose of 8 Gy of radiation, with a concomitant increase in LDH release. However, after inhibiting CXCR4 with AMD3100, the decrease in cell survival and the increase in cell death were elicited at a lower dose of 4 Gy of radiation, and even more cells were killed by the radiotherapy compared to the groups without treatment with AMD3100 (Figure 2(a) and 2(b)). These results indicate that the inhibition of CXCR4 promotes colorectal cancer cell sensitivity to radiotherapy.
Figure 2.
Inhibition of CXCR4 promotes colorectal cancer cell sensitivity to radiotherapy. (a) Cell viability is reduced by at least 8 Gy of radiotherapy, whereas treatment with AMD3100 in colorectal cancers cells led to a decrease in cell viability even with a lower radiotherapy dose of 4 Gy. (b) Inhibition of CXCR4 promoted colorectal cancer cell sensitivity to radiotherapy and more cells were killed by radiotherapy with AMD3100 co-treatment. Data are expressed as the mean ± SEM (*p < 0.05 compared with 0 Gy). (A color version of this figure is available in the online journal.)
The apoptosis induced by radiotherapy is depressed in colorectal cancer cells treated with CXCL12
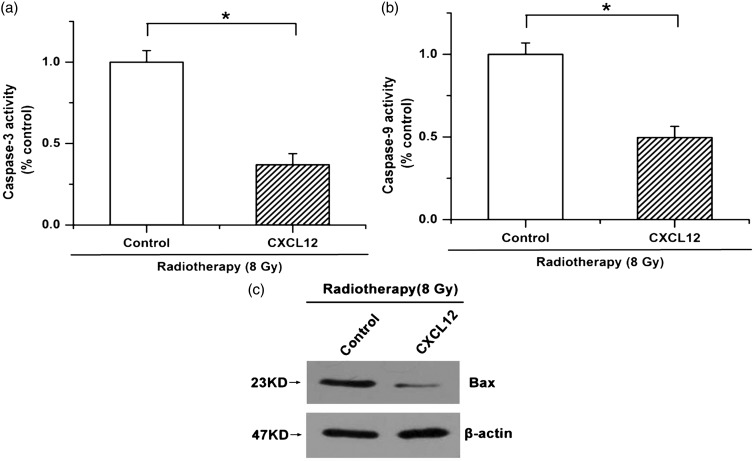
It is well known that the suppression of tumor progression and the death of cancer cells caused by radiotherapy largely result from an increase in cellular apoptosis; we thus examined whether the CXCL12/CXCR4 pathway participated in regulating cell apoptosis from radiotherapy. Our results showed that 8 Gy radiotherapy led to increased caspase-3 activity, whereas treatment with exogenous CXCL12 repressed the activation of caspase-3 induced by radiotherapy (Figure 3(a)). Moreover, radiotherapy led to an increase in caspase-9 activity and induced the expression of Bax, which was attenuated by CXCL12 treatment (Figure 3(b) and (c)). These results indicate that radiotherapy-facilitated cellular apoptosis is antagonized by CXCL12 treatment in colorectal cancer cells.
Figure 3.
Radiotherapy-induced cellular apoptosis is mitigated by CXCL12 treatment in colorectal cancer cells. (a) Radiotherapy-enhanced caspase-3 activation was attenuated by treatment with exogenous CXCL12. (b) Increased activity of caspase-9 induced by radiotherapy was attenuated by CXCL12 treatment. (c) CXCL12 depressed radiotherapy-increased Bax expression. Data are expressed as the mean ± SEM (*p < 0.05)
Radiotherapy-mediated apoptosis of colorectal cancer cell is strengthened by an inhibitor of CXCR4
We utilized AMD3100 to block the physiological functions of the CXCL12/CXCR4 pathway. We observed that the apoptotic changes caused by radiotherapy, including the activation of caspase-3 and caspase-9 and the increased protein expression of Bax, were enhanced by the inhibitor of CXCR4, indicating that radiotherapy-induced cell apoptosis and death were strengthened after the inhibition of the CXCL12/CXCR4 pathway (Figure 4(a) to (c)). These results imply that suppressing the CXCL12/CXCR4 pathway renders colorectal cancer cells more sensitive to radiotherapy.
Figure 4.
Inhibition of CXCR4 strengthens radiotherapy-induced cellular apoptosis in colorectal cancer cells. (a and b): Activation of caspase-3 and capase-9 caused by radiotherapy was significantly enhanced by the inhibitor of CXCR4. C: Inhibition of CXCR4 with AMD3100 induced the expression of Bax in cells exposed to radiation. Data are expressed as the mean ± SEM (*p < 0.05)
Activation of the CXCL12/CXCR4 pathway leads to the radio-resistance of colorectal cancer cells through upregulating the expression of survivin
Survivin, which is overexpressed in most cancer tissues, regulates the onset and development of cancer via acting as an inhibitor of apoptosis, and caspase-mediated apoptosis is significantly inhibited by the increased expression of survivin. Given our data that CXCL12/CXCR4 renders colorectal cancers cells resistant to radiotherapy by antagonizing cell apoptosis, we examined whether there is a linkage between CXCL12/CXCR4 and survivin. Our results showed that the expression of survivin was upregulated by treatment with exogenous CXCL12, whereas the inhibition of CXCR4 repressed the expression of survivin in HCT116 cells (Figure 5(a) and 5(b)). Survivin siRNA was utilized to knockdown the expression of survivin (Figure 5(c)). We found that the protective roles of CXCL12 in cell survival were attenuated by survivin siRNA under conditions of radiotherapy treatment (Figure 5(d)). Similar results were acquired when we measured cell apoptosis. We also found that the CXCL12-mediated attenuation of caspase-3 and caspase-9 activity induced by radiotherapy was mitigated by the knockdown of survivin (Figure 5(e) and (f)). The results indicate that the radio-resistance triggered by CXCL12 treatment is mediated by survivin in colorectal cancer cells.
Figure 5.
Radio-resistance triggered by the activation of the CXCL12/CXCR4 pathway is mediated by survivin in colorectal cancers cells. (a and b) Expression of survivin was up-regulated by exogenous CXCL12, whereas the inhibition of CXCR4 repressed the expression of survivin in colorectal cancers cells. (c) Western blot was utilized to measure the efficiency of survivin siRNA. (d) The CXCL12-mediated increase in cell viability during radiotherapy was weakened by survivin siRNA. (e and f) CXCL12-inhibited activation of caspase-3 and capase-9 was mitigated by the knockdown of survivin. Data are expressed as the mean ± SEM (*p < 0.05)
Discussion
Colorectal cancer is the most common malignant tumor of the gastrointestinal tract. Surgical treatment combined with radiotherapy is one of the most important modalities for the treatment of tumors, but radio-resistance, which commonly occurs during the course of treatment, seriously affects the therapeutic efficacy of the treatment and the prognosis of the patients with colorectal cancer. Unfortunately, the mechanisms behind radio-resistance in colorectal cancer are still largely unknown. Thus, it is necessary to explore the critical factors and related signaling pathways that regulate the sensitivity to radiotherapy in colorectal cancer cells. The present study demonstrates that the activation of the CXCL12/CXCR4 pathway renders colorectal cancers cell less sensitive to radiotherapy via up-regulating survivin expression.
It has been reported that the interaction of CXCL12 with CXCR4 leads to crucial signaling events not only in normal tissue but also in cancer cells.18,19 In colorectal cancers, the expression of CXCR4 is upregulated in the cancerous tissues. Moreover, the high expression of CXCR4 is correlated with a significant reduction in median overall survival.20–22 Accumulating evidence has indicated that the CXCL12/CXCR4 pathway participates in regulating the development of colorectal cancer. Nevertheless, the effects of CXCL12 and CXCR4 on cellular survival in colorectal cancer cells exposed to radiation are uncertain. In our study, exogenous CXCL12 attenuates the decrease in cell viability and the increase in cell death caused by radiotherapy. In contrast, more cells were killed by radiotherapy after inhibiting the CXCL12/CXCR4 pathway with AMD3100 (a competitive inhibitor of CXCR4). These results indicate that activation of the CXCL12/CXCR4 axis renders colorectal cancers cell less sensitive to radiotherapy, and inhibition of the CXCL12/CXCR4 pathway can promote colorectal cancer cell sensitivity to radiotherapy and augment the therapeutic effects of radiotherapy in colorectal cancer.
Apoptosis plays a key role in regulating cell growth, and radiotherapy induces cell death and inhibits cell growth partly through promoting cellular apoptosis.23 To emphasize the essential role of the CXCL12/CXCR4 axis in the radio-resistance of colorectal cancer cells, we examined whether the pathway regulated radiotherapy-induced apoptosis. Mitochondria-dependent apoptosis is an important subtype of cellular apoptosis, which is initiated by a decrease in the mitochondria membrane potential. Bax, localized on the outer membrane of mitochondria, participates in regulating the change of mitochondria membrane potential.24 The increased expression of Bax is associated with a decrease in mitochondria membrane potential, and caspase-9 is its downstream effecter molecule. The activation of caspase-9 subsequently increases the activity of caspase-3 (the executor of cell apoptosis).25–27 In the present study, our results showed that radiotherapy facilitated the expression of Bax and led to the increased activity of caspase-9 and caspase-3, whereas these pro-apoptotic changes induced by radiotherapy were attenuated by treatment with exogenous CXCL12. Moreover, CXCR4 inhibition enhanced cellular apoptosis induced by radiation exposure. These results show that the activation of the CXCL12/CXCR4 pathway inhibits cell apoptosis and promotes cell survival during radiotherapy.
Survivin, an important anti-apoptotic protein, belongs to the inhibitors of apoptosis protein (IAP) family.28 Previous studies have shown that the increased expression of survivin can significantly protect against p53 and caspase-mediated apoptosis.29,30 In consideration of the inhibitory effects of CXCL12/CXCR4 on radiotherapy-induced cellular apoptosis, we determined whether there is a linkage between CXCL12/CXCR4 and survivin. We found that the activation of the CXCL12/CXCR4 pathway resulted in increased levels of survivin, and the inhibition of this pathway had the opposite effects. Moreover, the knockdown of survivin mitigated the inhibitory roles of CXCL12 in cellular apoptosis. All of these results imply that the radio-resistance in colorectal cancer cells triggered by the activation of the CXCL12/CXCR4 pathway is, at least in part, mediated by survivin.
In conclusion, this study indicates that activation of the CXCL12/CXCR4 pathway inhibits cellular apoptosis and promotes cell survival via upregulating the expression of survivin in cells exposed to radiation and that blocking the CXCL12/CXCR4 pathway makes colorectal cancer cells more sensitive to radiotherapy. These findings suggest an important underlying mechanism for modulating the sensitivity to radiotherapy in colorectal cancer cells, which may provide a novel strategy and potential target for improving the therapeutic efficiency of radiotherapy in colorectal cancer.
Authors’ contributions
DW, CJ and ZC conceived and designed the experiments; DW, YZ, DL, MZ, XM, JG, YH, WL and JH performed the experiments and analyzed the results; CJ, ZZ and ZC prepared the submission together.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
This work was supported by grants from the China Postdoctoral Science Foundation, China (grant no. 2012M520769 to Dawei Wang), the Hei Long Jiang Postdoctoral Foundation, China (grant no. LBH-Z12201 to Dawei Wang), the First Affiliated Hospital of Harbin Medical University Research Foundation (grant no. 2013B02 to Dawei Wang), the Heilongjiang Province Department of Education Science and Technology Research fund project (grant no. 12541814 to Zhuoxin Cheng), the Natural Science Foundation of Heilongjiang Province (grant no. H201373 to Zhuoxin Cheng), and the Natural Science Foundation of Heilongjiang Province (grant no. QC2013C101 to Jie Hou).
References
- 1.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin 2014; 64: 104–17. [DOI] [PubMed] [Google Scholar]
- 2.Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, Karstens JH, Liersch T, Schmidberger H, Raab R. German Rectal Cancer Study Group: preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004; 351: 1731–40. [DOI] [PubMed] [Google Scholar]
- 3.Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, Becker H, Raab HR, Villanueva MT, Witzigmann H, Wittekind C, Beissbarth T, Rödel C. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 2012; 30: 1926–33. [DOI] [PubMed] [Google Scholar]
- 4.Chu CY, Cha ST, Lin WC, Lu PH, Tan CT, Chang CC, Lin BR, Jee SH, Kuo ML. Stromal cell-derived factor-1alpha (SDF-1alpha/CXCL12)-enhanced angiogenesis of human basal cell carcinoma cells involves ERK1/2-NF-kappaB/interleukin-6 pathway. Carcinogenesis 2009; 30: 205–13. [DOI] [PubMed] [Google Scholar]
- 5.Noda M, Omatsu Y, Sugiyama T, Oishi S, Fujii N, Nagasawa T. CXCL12-CXCR4 chemokine signaling is essential for NK-cell development in adult mice. Blood 2011; 117: 451–8. [DOI] [PubMed] [Google Scholar]
- 6.Dillenburg-Pilla P, Patel V, Mikelis CM, Zárate-Bladés CR, Doçi CL, Amornphimoltham P, Wang Z, Martin D, Leelahavanichkul K, Dorsam RT, Masedunskas A, Weigert R, Molinolo AA, Gutkind JS. SDF-1/CXCL12 induces directional cell migration and spontaneous metastasis via a CXCR4/Gαi/mTORC1 axis. FASEB J 2015; 29: 1056–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D'Alterio C, Consales C, Polimeno M, Franco R, Cindolo L, Portella L, Cioffi M, Calemma R, Marra L, Claudio L, Perdonà S, Pignata S, Facchini G, Cartenì G, Longo N, Pucci L, Ottaiano A, Costantini S, Castello G, Scala S. Concomitant CXCR4 and CXCR7 expression predicts poor prognosis in renal cancer. Curr Cancer Drug Targets 2010; 10: 772–81. [DOI] [PubMed] [Google Scholar]
- 8.Schrevel M, Karim R, ter Haar NT, van der Burg SH, Trimbos JB, Fleuren GJ, Gorter A, Jordanova ES. CXCR7 expression is associated with disease-free and diseasespecific survival in cervical cancer patients. Br J Cancer 2012; 106: 1520–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim J, Takeuchi H, Lam ST, Turner RR, Wang HJ, Kuo C, Foshag L, Bilchik AJ, Hoon DS. Chemokine receptor CXCR4 expression in colorectal cancer patients increases the risk for recurrence and for poor survival. J Clin Oncol 2005; 23: 2744–53. [DOI] [PubMed] [Google Scholar]
- 10.Schimanski CC, Schwald S, Simiantonaki N, Jayasinghe C, Gönner U, Wilsberg V, Junginger T, Berger MR, Galle PR, Moehler M. Effect of chemokine receptors CXCR4 and CCR7 on the metastatic behavior of human colorectal cancer. Clin Cancer Res 2005; 11: 1743–50. [DOI] [PubMed] [Google Scholar]
- 11.Katsumoto K, Kume S. The role of CXCL12-CXCR4 signaling pathway in pancreatic development. Theranostics 2013; 3: 11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomez-Casal R, Epperly MW, Wang H, Proia DA, Greenberger JS, Levina V. Radioresistant human lung adenocarcinoma cells that survived multiple fractions of ionizing radiotherapy are sensitive to HSP90 inhibition. Oncotarget 2015; 6: 44306–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feys L, Descamps B, Vanhove C, Vral A, Veldeman L, Vermeulen S, De Wagter C, Bracke M, De Wever O. Radiotherapy-induced lung damage promotes breast cancer lung-metastasis through CXCR4 signaling. Oncotarget 2015; 6: 26615–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sah NK, Khan Z, Khan GJ, Bisen PS. Structural, functional and therapeutic biology of survivin. Cancer Lett 2006; 244: 164–71. [DOI] [PubMed] [Google Scholar]
- 15.Guha M, Altieri DC. Survivin as a global target of intrinsic tumor suppression networks. Cell Cycle 2009; 8: 2708–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan Z, Tiwari RP, Khan N, Prasad GB, Bisen PS. Induction of apoptosis and sensitization of head and neck squamous carcinoma cells to cisplatin by targeting survivin gene expression. Curr Gene Ther 2012; 12: 444–53. [DOI] [PubMed] [Google Scholar]
- 17.Salz W, Eisenberg D, Plescia J, Garlick DS, Weiss RM, Wu XR, Sun TT, Altieri DC. A survivin gene signature predicts aggressive tumor behavior. Cancer Res 2005; 65: 3531–4. [DOI] [PubMed] [Google Scholar]
- 18.Kucia M, Reca R, Miekus K, Wanzeck J, Wojakowski W, Janowska-Wieczorek A, Ratajczak J, Ratajczak MZ. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis. Stem Cells 2005; 23: 879–94. [DOI] [PubMed] [Google Scholar]
- 19.Burger JA, Peled A. CXCR4 antagonists: targeting the microenvironment in leukemia and other cancers. Leukemia 2009; 23: 43–52. [DOI] [PubMed] [Google Scholar]
- 20.Zeelenberg IS, Ruuls-Van Stalle L, Roos E. The chemokine receptor CXCR4 is required for outgrowth of colon carcinoma micro metastases. Cancer Res 2003; 63: 3833–9. [PubMed] [Google Scholar]
- 21.Kim J, Takeuchi H, Lam ST, Turner RR, Wang HJ, Kuo C, Foshag L, Bilchik AJ, Hoon DS. Chemokine receptor CXCR4 expression in colorectal cancer patients increases the risk for recurrence and for poor survival. J Clin Oncol 2005; 23: 2744–53. [DOI] [PubMed] [Google Scholar]
- 22.Schimanski CC, Schwald S, Simiantonaki N, Jayasinghe C, Gönner U, Wilsberg V, Junginger T, Berger MR, Galle PR, Moehler M. Effect of chemokine receptors CXCR4 and CCR7 on the metastatic behavior of human colorectal cancer. Clin Cancer Res 2005; 11: 1743–50. [DOI] [PubMed] [Google Scholar]
- 23.Kuo PL, Chen CY, Hsu YL. Isoobtusilactone A induces cell cycle arrest and apoptosis through reactive oxygen species/apoptosis signal-regulating kinase 1 signaling pathway in human breast cancer cells. Cancer Res 2007; 67: 7406–20. [DOI] [PubMed] [Google Scholar]
- 24.Zeng L, Li T, Xu DC, Liu J, Mao G, Cui MZ, Fu X, Xu X. Death receptor 6 induces apoptosis not through type I or type II pathways, but via a unique mitochondria-dependent pathway by interacting with Bax protein. J Biol Chem 2012; 287: 29125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacRedmond R, Singhera GK, Dorscheid DR. Erythropoietin inhibits respiratory epithelial cell apoptosis in a model of acute lung injury. Eur Respir J 2009; 33: 1403–14. [DOI] [PubMed] [Google Scholar]
- 26.D'Sa-Eipper C, Leonard JR, Putcha G, Zheng TS, Flavell RA, Rakic P, Kuida K, Roth KA. DNA damage-induced neural precursor cell apoptosis requires p53 and caspase 9 but neither Bax nor caspase 3. Development 2001; 128: 137–46. [DOI] [PubMed] [Google Scholar]
- 27.Müller M, Grunewald J, Olgart Höglund C, Dahlén B, Eklund A, Stridh H. Altered apoptosis in bronchoalveolar lavage lymphocytes after allergen exposure of atopic asthmatic subjects. Eur Respir J 2006; 28: 513–22. [DOI] [PubMed] [Google Scholar]
- 28.Sah NK, Khan Z, Khan GJ, Bisen PS. Structural, functional and therapeutic biology of survivin. Cancer Lett 2006; 244: 164–71. [DOI] [PubMed] [Google Scholar]
- 29.Li F, Ambrosini G, Chu EY, Plescia J, Tognin S, Marchisio PC, Altieri DC. Control of apoptosis and mitotic spindle checkpoint by survivin. Nature 1998; 396: 580–4. [DOI] [PubMed] [Google Scholar]
- 30.Salz W, Eisenberg D, Plescia J, Garlick DS, Weiss RM, Wu XR, Sun TT, Altieri DC. A survivin gene signature predicts aggressive tumor behavior. Cancer Res 2005; 65: 3531–4. [DOI] [PubMed] [Google Scholar]