Abstract
Ineffective skin wound healing is a significant source of morbidity and mortality. Roughly 6.5 million Americans experience chronically open wounds and the cost of treating these wounds numbers in the billions of dollars annually. In contrast, robust wound healing can lead to the development of either hypertrophic scarring or keloidosis, both of which can cause discomfort and can be cosmetically undesirable. Appropriate wound healing requires the interplay of a variety of factors, including the skin, the local microenvironment, the immune system, and the external environment. When these interactions are perturbed, wounds can be a nidus for infection, which can cause them to remain open an extended period of time, or can scar excessively. Interleukin-2, a cytokine that directs T-cell expansion and phenotypic development, appears to play an important role in wound healing. The best-studied role for Interleukin-2 is in influencing T-cell development. However, other cell types, including fibroblasts, the skin cells responsible for closing wounds, express the Interleukin-2 receptor, and therefore may respond to Interleukin-2. Studies have shown that treatment with Interleukin-2 can improve the strength of healed skin, which implicates Interleukin-2 in the wound healing process. Furthermore, diseases that involve impaired wound healing, such as diabetes and systemic lupus erythematosus, have been linked to deficiencies in Interleukin-2 or defects Interleukin-2-receptor signaling. The focus of this review is to summarize the current understanding of the role of Interleukin-2 in wound healing, to highlight diseases in which Interleukin-2 and its receptor may contribute to impaired wound healing, and to assess Interleukin-2-modulating approaches as potential therapies to improve wound healing.
Keywords: Interleukin-2, wound healing, immunotherapy, therapeutic targets, cutaneous diseases, cytokines
Introduction
Wound healing impairment can be problematic for patients and frustrating for physicians. Wounds, which can result from injuries or surgery, can lead to substantial morbidity and even mortality when they fail to heal. The occurrence of chronic wounds, which affected upwards of 6.5 million Americans as of 2009, is increasing in the United States due to the increasing age of the population and prevalence of comorbidities.1–3 Recent reports concerning commercial opportunities in medicine indicate that spending on chronic wound care exceeds a billion dollars annually. On the other end of the spectrum, scar formation due to excess extracellular matrix (ECM) deposition and overzealous cell proliferation is also undesirable.4 About 100 million people worldwide develop scars following both trauma and elective surgery. In surgery patients, 40–70% experience a specific type of scarring, hypertrophic scarring, which is both cosmetically undesirable and can impair function, depending on location and severity. Patients are frequently unsatisfied with the appearance of their surgical wounds both during and after the healing process and these wounds can have a significant impact on their quality of life.5,6 Ideally, wounds should close rapidly to prevent infections but exhibit minimal scaring for the best possible functional and cosmetic result. However, it is unclear exactly what mediates the balance of appropriate wound healing verses excessive scarring.
Overview of wound healing
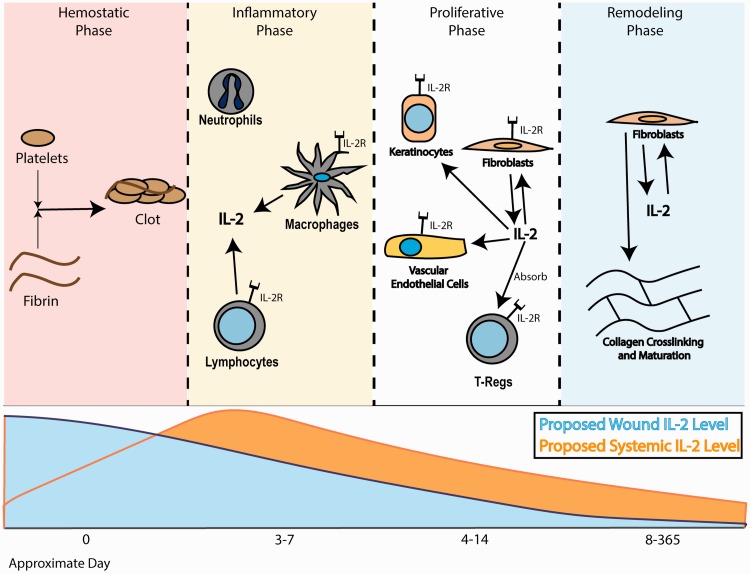
Wound healing requires orchestration of immune and skin cells at the site of injury to facilitate repair. The process of wound healing can be considered to have multiple phases: the hemostatic phase, the inflammatory phase, the proliferative phase, and the remodeling phase (Figure 1).7–10 These phases overlap; there is no abrupt separation between the phases. However, these designations are useful for understanding the overall wound healing process. During the hemostatic phase, a fibrin and platelet plug forms to stop blood loss at the injury site. Platelets and resident immune cells then secrete a variety of factors to attract neutrophils to the wound site.11 There, neutrophils defend the wound against pathogens, initiate the repair process, and attract other cells, such as macrophages, more neutrophils, and lymphocytes to the area.
Figure 1.
Flow chart describing key players at various times in wound healing. (A color version of this figure is available in the online journal.)
Recruitment of these immune cells, including macrophages and various lymphocyte subsets, occurs as part of the inflammatory phase.7,8 Upon arrival at the wound site, macrophages phagocytose debris, further protect the wound from pathogens, and initiate the production of extracellular matrix (ECM). Additionally, macrophages have been shown to contribute to the recruitment of other cells, including immune and skin cell subtypes, to the wound site.8,10,11 The role of lymphocytes in wound healing is less well-defined. However, it appears that B lymphocytes, T-helper cells (THC), and cytotoxic T lymphocytes (CTL) protect wounds from viruses. Additionally, lymphocytes, like macrophages, contribute to the production of cytokines that attract other immune cells and create the wound healing environment. Overall, cells of the inflammatory phase protect the wound from pathogen invasion and initiate the healing process by recruiting the cells that participate in repair.
Following the inflammatory phase is the proliferative phase, in which a layer of skin cells called keratinocytes cover the wound. Other epidermal cells migrate over these keratinocytes and proliferate to create the multiple skin layers.7 Additionally, the proliferative phase is when angiogenesis begins and production of the extracellular matrix (ECM) primarily occurs. Vascularization and ECM production are orchestrated by macrophages, keratinocytes, fibroblasts, and likely other cell types.8 Thus, the structures that will comprise the more permanent wound closure begin to appear during the proliferative phase.
Finally, during the remodeling phase, the collagen of the ECM matures, increasing the strength of the closed skin.7,9 Throughout this phase, which lasts for about a year after the initial injury, the quality of the wound changes and begins to resemble uninjured skin.8 The remodeling process appears to be primarily mediated by fibroblasts at the wound site, but other cells, such as macrophages, may also play a role. Additionally, it is possible that cytokines and growth factors in the systemic circulation affect the behavior of cells and ECM fibers at the wound site during the remodeling phase, impacting the quality of the healed wound. Thus, the wound healing process is very complex and involves the interplay of multiple factors, both locally at the wound and systemically in the body. These factors together determine the quality and rate of wound healing.
A variety of physiologic and pathologic processes have the potential to delay wound healing by impacting both the local environment and the body as a whole. These factors include older age, obesity, diabetes, tobacco smoking, and vascular diseases.2,12 Wound infections, which occur following roughly 0.3% of surgeries and can also complicate non-surgical injuries, also delay wound healing.2,13,14 Wounds can reopen following a surgical closure, a process called dehiscence, which often occurs due to an infection of the wound. Thus, a myriad of factors including infection, debris at the wound site, and patient comorbidities, can lead to a delay in wound healing.
In contrast to delayed closure, wound healing can also be excessively robust, leading to scarring. In scars, excess ECM is laid down, leading to a closure with different qualities from normal skin. Scar types include atrophic scars, hypertrophic scars, and keloids, among others.4,6,15 Hypertrophic scars typically form immediately after injury or surgery and regress over time. Risk factors for hypertrophic scars include wound infections, wounds that cross joints or other high-tension skin, and genetics. Keloids, on the other hand, can begin growing long after the inciting injury. Risk factors for keloids include certain ethnicities, family history, and certain histocompatibility or human leukocyte antigen (HLA) types. Incidence of keloids seems to decrease with older age and especially following menopause. All types of scars have the potential to be itchy or painful, to be cosmetically undesirable, and to cause patient distress. The limited current strategies to address undesirable scars include corticosteroid injections, pressure treatments for keloids, laser treatment, cryotherapy, radiation, or in severe cases surgical intervention. However, these strategies are not universally effective and a better understanding of wound healing may provide novel treatment targets for excess scarring.
As stated previously, the immune system plays an active role in wound closure and the balance between proper wound healing and excess scarring.7,10 The innate immune system, which reacts non-specifically to protect the body, is clearly implicated in the wound healing process. Innate immune factors involved in wound healing include neutrophils, macrophages, and a vast array of cytokines produced both at the wound site and throughout the body.8 The adaptive immune system, which promotes specific responses to antigens, also appears to play a role in wound closure.16,17 Wound healing involves antigen presentation by macrophages and various lymphocyte subsets.8 The finding that polymorphisms in the HLA genes, which are involved in antigen presentation by the adaptive immune system, are associated with the risk of forming keloid scars further implicates the adaptive immune system in wound healing.4 Furthermore, keratinocytes and possibly other skin cells are capable of presenting antigens to the adaptive immune system.18 The integration of immune response in the context of wound healing is therefore quite complex and involves both innate and adaptive responses. More specifically, interleukins (ILs), which are known to regulate adaptive immune cells, may be involved in wound healing. One specific IL, IL-2, has attracted recent interest as a potential modulator of the wound healing process.
Interleukin-2 signaling
IL-2, a cytokine with a complex signaling cascade, has been extensively reviewed.19–22 This cytokine is secreted in its active form by many cells that participate in wound healing including THCs, CTLs, macrophages, and keratinocytes.8,23–25 The IL-2 receptor (IL-2R) subunits, which are described later in the review, are found principally on immune cells but are also present on the surfaces of various skin cell subtypes, including keratinocytes and fibroblasts.19,21,26,27 The behavior of cells in response to IL-2 signaling varies significantly by cell type depending up on the level and type of IL-2R that the cell has on its surface, the external cytokine environment, and the other intracellular signaling molecules present.22
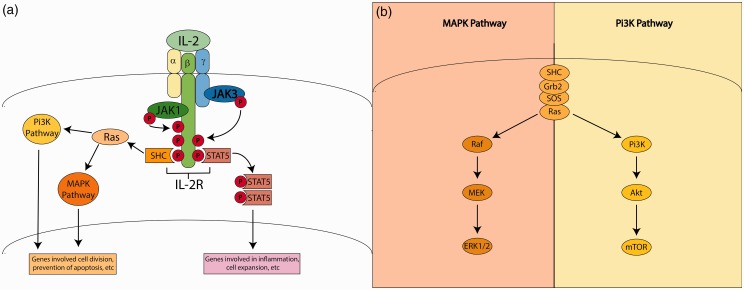
IL-2 signaling occurs via a receptor with three subunits, IL-2R -α, -β, and -γ. The subunits of the IL-2R have different affinities for and responses to IL-2. IL-2Rβ and IL-2Rγ are able to bind other cytokines, but IL-2Rα appears be specific for IL-2 binding.26 Each IL-2R subunit is low-affinity alone. However, when dimerized, the IL-2Rβ-IL-2Rγ complex achieves a higher affinity. The highest affinity is achieved when all three subunits (α, β, and γ) are together.19,21,26 IL-2Rs signal via Janus tyrosine kinases (JAK) and signal transducer and activator of transcription (STAT) pathways primarily (Figure 2(a)). Following IL-2 binding to its receptor, it appears that JAK1 is recruited to IL-2Rβ, while IL-Rγ recruits JAK3.22 The recruitment of JAK1 appears to depends upon JAK3 recruitment.28 These JAKs then phosphorylate domains of the IL-2Rβ, which goes on to recruit and phosphorylate STATs, principally STAT5a and STAT5b, but also STAT1 and STAT3. The phosphorylated STATs dimerize and this dimer enters the nucleus where it can activate a variety of genes depending on the cell type and other signals present.22 In addition to STATs, other signaling molecules, proteins from the src homologous and collagen (SHC) family, are also recruited to the IL-2Rβ cytoplasmic tail following its phosphorylation by the JAKs. The SHC proteins then go on to activate the phosphatidylinositol-3-kinase (Pi3K) and mitogen activated protein kinase (MAPK) pathways.19,21,26 In both the MAPK pathway and the Pi3K pathways, phosphorylation of SHC causes SHC to bind to a complex of several proteins, including Ras (Figure 2(b)).28,29 This complex contributes to rapidly accelerated fibrosarcoma (RAF) activation, which activates mitogen activated protein kinase (MEK); MEK goes on to activate MAPK, which is also known as extracellular regulated-signal kinase (ERK). The ERKs act as transcription factors for a variety of mediators of cell proliferation. In the Pi3K pathway, Ras activation activates Pi3K, which goes on to activate Akt, which activates mTOR.30 There are a myriad of subtypes of many of the participants in both the MAPK and Pi3K pathways. Additionally, many steps of both these cascades are regulated by both feedback mechanisms from within the pathway and signaling events in other pathways. The MAPK and Pi3K pathways are both associated with cell proliferation and likely contribute to the expansion of effector T-cells in response to IL-2.22,28–30 Thus, it is clear that IL-2 signaling is complicated, dependent upon cell type, extensively regulated, and likely contributes to cell expansion in some cell types.
Figure 2.
IL-2 receptor signaling. (a) Binding of IL-2 to its receptor initiates signaling via the JAK-STAT, MAPK, and Pi3K pathways. (b) The MAPK and Pi3K pathways in more detail. (A color version of this figure is available in the online journal.)
Complex regulation determines the degree to which each of IL-2’s downstream pathways contribute to its action in each separate cell type leading to the variability in cell behavior in response to IL-2 signaling. For example, FoxP3 levels are upregulated in T-regulatorys (T-regs), but not other T-cells, in response to IL-2R signaling.22 This effect in T-regs is due to transforming growth factor (TGF)-β cooperation; it has been shown that FoxP3 is upregulated in response to STAT5 signaling and that this effect requires SMADs, which are downstream of TGF-β.31,32 Thus, regulation of IL-2’s downstream signals involve multiple pathways. IL-2 downstream signaling has also been shown to be indirectly downregulated by protein tyrosine phosphatase non-receptor type II (PTPN2), which appears to decrease STAT5 phosphorylation and activity in response to IL-2R engagement.33 Thus, numerous signaling pathways cooperate with the IL-2R to affect cell fate, proliferation, differentiation, and behavior.
In addition to serving as a cell surface receptor, soluble IL-2R (sIL-2R) α can also be released from cells, primarily THCs. It is unclear whether surface IL-2Rα is released from cell surfaces by proteases or whether it is produced in a secreted form via alternative splicing or altered transcription.21,34 Both of these mechanisms may contribute to the production of sIL-2Rα. While sIL-2Rα has low affinity for IL-2, it is capable of regulating immune function, although the exact mechanism of this action is not well-understood.35–37 More study is needed to demonstrate the role of sIL-2Rα in regulating IL-2 signaling but this molecule may serve as a therapeutic target once its role is better understood.
Interleukin-2 in immune cell development
As stated previously, IL-2 interacts with other cytokines to influence immune cell development and activation and these interactions between may impact wound healing.19,24,27,38 At wound sites, IL-2 is known to activate macrophages and natural killer (NK) cells, and to promote the proliferation of B- and T-lymphocytes.8 Interestingly, IL-2 has been shown to promote the development and activation of both THC subtypes, TH1 and TH2, depending upon the other cytokines present.24,39,40 TH1 cells form when IL-12 and interferon (IFN)-γ are present in addition to IL-2, while TH2 cells form when IL-4 is present in addition to IL-2.31 TH1 cells produce cytokines, including IFN-γ and are important in autoimmune diseases.24 TH2 cells, which also produce numerous cytokines including IL-4, IL-5, and IL-10, mediate defense against helminths and the pathogenesis of allergies.24 IL-2 also appears to play a role in THC activation. THCs only express IL-2Rβ and IL-2Rγ constitutively, but when they are stimulated by antigen, they produce IL-2 and express IL-2Rα.41 Thus, autocrine and paracrine signaling by IL-2 appears to promote further IL-2 signaling in THCs following antigen stimulation.41 IL-2 also influences the development of memory subsets of both CTL and THCs, which are important for responding to previously encountered antigens.24,27,40 As discussed previously, T-reg cells, which are important for dampening the immune response and promoting tolerance of self-tissues, also require IL-2 for development.24,41 Interestingly, T-regs appear to compete with other immune cells for IL-2.41 Thus, prevention of IL-2 signaling may be a mechanism by which T-regs decrease the activity of other immune cells, notably antigen-stimulated THCs.41 Additionally, γδ T-cells, a less well-studied lymphocyte subtype found in the skin, also respond to IL-2 in a variety of contexts and, given their location in the body, may play a role in cutaneous wound healing.16,42–44 Thus, numerous cells of the immune system respond to IL-2, implicating this cytokine in processes mediated by the immune system, such as wound healing.
Clinical uses of IL-2
Because IL-2 may be a good therapeutic target in wound healing, it is important to consider what IL-2-related treatments are currently available and the therapeutic and adverse effects of these treatments. IL-2-based products are currently used to treat several types of cancer, including renal cell carcinoma and melanoma.45–47 Unfortunately, when administered systemically at high doses, IL-2 has a narrow therapeutic window, making it difficult to employ as a treatment except when the benefits outweigh its significant risk profile.45–48 However, it is effective when it is injected intralesionally to treat a melanoma metastasis, indicating that local use is possible.49 Local treatment appears well-tolerated so this strategy might help prevent the toxicity associated with systemic administration of IL-2. Furthermore, if IL-2 is shown to improve wound healing, it could be added to wound dressings, adhesives, and sutures, which would provide options for modes of delivery that could be tailored to skin delivery and to the needs of each individual wound patient.
Interleukin-2 in wounds
IL-2 is produced throughout the body and by multiple cells types found at wounds. Thus, it has the potential to act both globally and locally at the wound site. The timing and location of IL-2 production and signaling may be critically important to its impact on wound healing. Elucidating the temporal and spatial contribution of IL-2 to wound healing will both allow characterization of the complex wound healing process and promote the development of wound-specific therapeutics targeting this pathway.
Systemic effects of IL-2 on wound healing
It is not just signals originating within the wound site that impact wound healing, signals from elsewhere in the body can impact wound healing in response to the initiation of the wound. One example of this is the pain and stress caused by the injury. Stress leads to increased glucocorticoid hormones levels, which are known to impact the immune system.50 Systemic signals have also been shown to contribute to the attraction of the immune and skin cells required for wound closure.8 Additionally, systemic IL-2 may also act as a growth factor or could impact growth factor secretion.51 Finally, IL-2 levels in the body in general have the potential to impact the local wound environment. Thus, more understanding of the levels and impact of systemic IL-2 during wound healing is likely important in understanding the role of IL-2 in this process.
Wounds have been shown to alter systemic IL-2 levels. One study of adult burn patients demonstrated that blood IL-2 levels are increased on both day 1 and day 5 following burn injury and that the levels of IL-2 correlate positively with the percentage of the body burned.52 Another study of burns in children demonstrates that IL-2 levels at the wound site are lower than they are in the peripheral blood, which serves as evidence for IL-2 acting systemically rather than locally.53 Alternatively, these data may indicate that at some points during the healing process, low levels of IL-2 are favorable.53 The high levels of IL-2 in the blood and high white blood cell counts may also mean that the role of IL-2 in wound healing involves systemic immune activation, rather than local immune cell activation at a wound. Thus, more research is needed to fully understand the timing and locations of IL-2’s contribution to wound healing.
To further understand global and local effectors of wound healing, one group has focused their study of healing following long bone fractures on the spatial and temporal expression of cytokines.54,55 This group examined IL-2 signaling within the muscle and blood vessels at the fracture site, 1 cm away from the fracture site, and in the opposite, unbroken limb at 0, 6, 24, and 168 h after the fracture. The data comparing IL-2 levels and the phosphorylation states of downstream signaling molecules are consistent with the possibility that systemic IL-2 contributes to wound healing. While this study did not involve skin wounds directly, some of its findings are relevant to cutaneous wound healing, especially given the spatial and temporal characterization of the behavior of IL-2 and several downstream signaling pathways.
Consistent with the studies of burn patients, the IL-2 level in the vessel was lower at the fracture site than in the opposing limb 6 h after fracture, which may indicate high systemic IL-2 signaling at this time point.54 Additionally, the IL-2 level in the muscle was higher at the fracture site than 1 cm away from it immediately following the fracture, where it could be playing a role in the initiation of healing. To further clarify the timing and location of IL-2 signaling, this group went on to perform a further study, which included not only IL-2 levels but also phosphorylation states of some downstream signaling pathways for the IL-2Rs. Immediately following fracture, phosphorylation of both STAT3 and ERK1/2 was higher in the muscle of the opposing limb than at the fracture site or 1 cm away from the fracture, indicating higher activity of these factors. These effects could be mediated by IL-2 signaling via the MAPK pathways. Another potential downstream mediator of IL-2 signaling, Akt, which signals in the Pi3K pathway, also showed increased phosphorylation at distant sites early in wound healing.55 These consistent patterns of MAPK and Pi3K signaling implicate IL-2, which signals via both pathways.22 These patterns may be consistent with a decrease in IL-2 signaling at the site of injury immediately or a relative increase in IL-2 signaling systemically that is not experienced at the wound site. These studies could hint that systemic IL-2 signaling helps motivate cells, especially immune cells, to migrate to wounds or could contribute to growth factor production. Thus, systemic production of IL-2 may be very important in orchestrating the wound healing process.
Several other wound healing studies also hint that systemic IL-2 might play a role in determining the quality of wound healing. In a rat model of wound healing, high doses of intraperitoneal IL-2 increased wound breaking strength as tested by a constant speed tensometer.56 The IL-2-treated wounds had higher levels of hydroxyproline, which is indicative of collagen crosslinking and may elude to increased ECM deposition or scarring in response to IL-2.57 The authors of this study interpret these results to mean that lymphocyte activation by IL-2, rather than IL-2 action on skin cells, mediates increased strength following IL-2 treatment, although this study does not directly test that hypothesis. However, this study does hint that the role of IL-2 in altering the course of wound healing may involve systemic actions. In order to successfully improve wound healing using IL-2-dependent mechanisms, the systemic role of IL-2 in wound healing would therefore need to be clarified.
Local effects of IL-2 on wound healing
Local IL-2 production may also alter the course of wound healing. As stated before, sources of IL-2 in wounds include macrophages, lymphocytes, and keratinocytes.8,10,11 These cells, as well as others found in wounds, are also capable of responding to IL-2.8,25,51 Understanding how IL-2 interacts with cells within the wound environment will help clarify its potential roles within the wound site.
The role of T-cells and other lymphocytes at the wound site is a newly appreciated phenomenon that may be a mechanism by which IL-2 may impact wounds. One study has shown that treatment with activating antibodies to Cluster of Differentiation 3 (CD3), a surface receptor that stimulates T-cell activation, along with fibroblast growth factor (FGF)-1 or -2, factors known to be important for wound healing and angiogenesis, appear to promote T-cell IL-2 production.58 Because T-cells are present and activated at wound sites and wound sites contain growth factors, including FGFs, this response to combined IL-2 and FGF may represent a mechanism by which T-cells produce IL-2 in wounds. This locally produced IL-2 could impact the rate and quality of the closure by promoting immune and skin cell proliferation and differentiation at the wound site. These findings may also indicate that there is a feed-forward mechanism by which FGF in wounds increases IL-2 production by immune cells and that both of these signaling molecules act as growth factors at the wound site.51 These results could mean that IL-2 treatment would increase scar formation through local action but that this type of treatment might be appropriate in patients in whom wound healing is delayed. Thus, more study of the response to IL-2 locally at the wound site is needed to understand how to best utilize it therapeutically.
In addition to immune cells, skin cells at the wound site also appear capable of directly responding to IL-2, which may alter their behavior and the behavior of cells around them. For example, IL-2 signaling may contribute directly to the activity of fibroblasts in wound sites. Several studies have shown that fibroblasts express IL-2R, specifically the β and γ subunits that are capable of signaling.59,60 One of these studies, concerning fibroblast-like cells isolated from human joint fluid, demonstrated that IL-2 treatment could induce production of pro-inflammatory cytokines by these cells. Another study demonstrated that fibroblast signaling through the IL-2Rγ-JAK3 pathway led to increased production of monocyte chemoattractant factors, including monocyte chemoattractant protein-1 (MCP-1) and intracellular adhesion molecule-1 (ICAM-1).59 These observations may mean that local IL-2 signaling in skin cells at a wound may promote the recruitment and activation of immune cells at wound sites. IL-2 appears to act as a growth signal in fibroblasts in a mechanism involving autophagy, or the digestion and reallocation of the materials of a cell’s organelles.61 Ultimately, all of these mechanisms by which IL-2 impacts fibroblasts may contribute to the local impact of IL-2 within wounds.
In addition to receiving signals from exogenous IL-2, studies have shown that human skin fibroblasts can produce IL-2 in some contexts. This IL-2 may act locally at the wound site. One study demonstrated IL-2 production by fibroblasts upon high-dose exposure to advanced glycation end products (AGEs), which are sugar-conjugated proteins that occur in diabetes.62 Blocking either AGE receptors or TGF-β signaling led to decreased IL-2 production. The authors interpret this to mean that IL-2 production may be involved in scar formation. Fibroblast secretion of IL-2 may thus be pathologic and lead to poor wound healing or could also be a compensatory response to a lack of IL-2 signal usually provided by other sources. Because fibroblasts can be induced to make IL-2 by AGEs, they may also be able to produce IL-2 in other contexts, which could have implications for the rate and quality of wound healing. Thus, this phenomenon of IL-2 production by fibroblast warrants more study.
At the wound site, IL-2 may also contribute to the secretion of and reaction to growth-promoting factors and cytokines by a variety of cell types, both immune and skin. IL-2 promotes the release of IFN-γ and the development of IFN-γ-producing TH1 cells, which then leads to IL-1 production, which may promote wound healing.24 Additionally, IL-2 appears to synergize with IFN-α to promote local endothelial cell growth and angiogenesis, which is necessary for revascularization of a wound site.51 Combined treatment with IL-2 and IFN-α increases endothelial cell proliferation and IFN-α alone increases expression of and signaling via IL-2R. Thus, there may be crosstalk between IL-2 and IFN-α, such that IFN-α increases the capacity for a response to IL-2 and IL-2 promotes endothelial cell growth. IL-2 and IFN-α together also increase release of FGF, which likely contributes to the increased endothelial cell growth. Blocking FGF decreases cell proliferation in response to IL-2 and IFN-α, thereby demonstrating that IFN-α and IL-2 synergy may be FGF-mediated. These results are consistent with the previously discussed study that hinted at a feed-forward mechanism by which IL-2 and FGFs synergize to promote growth.58 Growth promotion by IL-2 via FGF, and possibly other growth factors, may also be applicable to other cell types, including skin cells. Thus, IL-2 may be involved in the growth of both skin and blood vessel cells to close wounds.
Finally, IL-2 inhibition at the wound site may also be important to adequate wound healing, possibly by contributing to the resolution of inflammation.8 Wound exudates collected 10 days after wounding contain a specific inhibitor of THC proliferation that can be incompletely overcome by treating with IL-2.63 In contrast, cultured fibroblast cells proliferate in response to these same wound extracts. This study does not identify the inhibitor of IL-2-mediated THC proliferation, demonstrate the timing or source of the inhibitor, or determine the exact role of the inhibitor in wound healing. However, it is possible that the IL-2 inhibitor helps resolve inflammation, promote T-reg development by favoring a low level of IL-2, or slow IL-2-mediated collagen fiber crosslinking without preventing cell proliferation, thus improving the quality of wound closure.56,64,65 The discovery of this inhibitor lends evidence that IL-2, either directly or indirectly, promotes immune activation of early, but may be toxic if it is not inhibited later in the wound healing process. Alternatively, this inhibitor could be preventing overly robust cell growth or ECM deposition or maturation, processes which IL-2 may influence.8 Thus, more research is needed to determine the exact role of IL-2 and the timing and location of its action in wound healing before IL-2-related treatments can be designed.
IL-2 in diseases involving wound healing
A number of pathological processes involve IL-2, many of which occur as components of autoimmune diseases, highlighting the role of IL-2 in immune cell development and regulation.64 Interestingly, several diseases that involve IL-2 signaling alterations are diseases that also involve skin or tissue damage. Herein, we will review the pathologies of systemic lupus erythematosus (SLE), sarcoidosis, diabetes mellitus (DM), and myocardial infarction (MI) and the involvement of IL-2 in each case.
Systemic lupus erythematosus
SLE is an autoimmune disease of unclear etiology which damages multiple organ systems, notably the skin.66,67 SLE patients also have numerous immunologic abnormalities, including altered cytokine profiles and the production of antibodies targeting self-antigens, known as autoantibodies, within the body.68 These immune alterations may impact skin repair, supported by the fact that wound healing is impaired in SLE patients. A study of SLE patients undergoing hip arthroplasty demonstrated delayed wound healing in a subset of the patients that was not correlated with the corticosteroids being used to treat their SLE.69 However, observational studies present problems when used to study wound healing, given the variety of quality, depth, and etiology of wounds that patients experience. To control for this heterogeneity, researchers have performed wound healing studies in mouse models of SLE. Studies in SLE-model mice have yielded variable results, with studies reporting increased, decreased, or normal healing rates and quality compared to wild-type mice.70,71 Interestingly, in studies that have demonstrated improved wound healing in this model, wounds were located on the cartilaginous ear, which may explain the differences in healing.72 Overall, mouse models of SLE provide an avenue to explore causality between SLE, IL-2, and defective wound healing but more study is needed to understand what aspects of wound healing are affected in these models.
IL-2 levels in both SLE patients and mouse models of SLE appear to be below that of healthy controls, which may be involved in the pathogenesis of the skin damage and wound healing problems observed in SLE.71,73 It has been shown that IL-2 mRNA expression is decreased in skin biopsies from SLE patients in both healthy and lesioned skin compared with controls, which may implicate IL-2 in the cutaneous manifestations of SLE.74 Moreover, IL-2 production by lymphocytes, including cultured cells, patient-derived CD3+ T-cells, and peripheral blood mononuclear cells isolated from human patients, is decreased, although the mechanism underlying this decrease remains unclear.75,76 There have been conflicting reports regarding whether this decreased IL-2 production correlates with disease activity.76,77 Additionally, one study demonstrated that the Immunoglobulin type G (IgG) fraction of SLE patient serum from a subset of study participants was capable of inhibiting IL-2 production by cultured human peripheral blood mononuclear cells. This finding indicates that there may be a factor that inhibits IL-2 production by lymphocytes in the serum of SLE patients, which could impact IL-2s action at wound sites and the systemic effects of IL-2 on wound healing.78 Furthermore, mice bearing the lpr mutation in their Fas Receptor gene, which develop an SLE-like autoimmune disease, are also IL-2 deficient.71,79 Thus, IL-2 signaling may be important in the development of wound healing pathologies found in SLE mouse models, which have similar phenotypes to human SLE patients, and may be a useful model in studying these pathologies.
The IL-2R may also play a role in the wound healing pathologies seen in SLE. Patients experiencing SLE exacerbations express lower levels of IL-2R on their T-cells, indicating that the response to IL-2 may also be playing a role in SLE severity.77 SIL-2R expression is also increased in the peripheral blood of SLE patients, especially in those experiencing discoid skin lesions.80 It is unclear from these studies what role either the soluble or surface IL-2Rs may be playing in the wound healing problems found in SLE patients. Thus, more study of the actions of downstream IL-2R signaling and the behavior of the IL-2R are required in this context.
Together, the results of studies of IL-2 and IL-2R signaling indicate that a lack of IL-2 production, poor IL-2 signaling, or IL-2 inhibition may play a role in SLE and specifically in the cutaneous manifestations of SLE. If IL-2 signaling deficits are demonstrated to be a significant part of the pathogenesis of wound healing impairment and other skin pathologies in SLE, it would represent an attractive therapeutic target.
Sarcoidosis
Another multifactorial inflammatory disease that links IL-2 with wound healing is sarcoidosis. Sarcoidosis is a complex and relatively rare disease that involves immune-mediated damage to multiple organs, including the lungs, kidneys, eyes, and skin.81 Roughly 25–35% of patients experience skin symptoms, including plaque and ulcer formation, which can occur on normal skin or around scars and tattoos. The relationship to prior sites of injury is indicative of aberrant wound healing in patients suffering from this disease. Furthermore, the lung pathology seen in sarcoidosis includes excessive fibrosis, possibly implicating an overactive scarring response in the disease state, a process which may also impact cutaneous healing. Thus, impaired balance of wound healing versus scarring may warrant additional study in sarcoidosis.
Sarcoidosis involves significant immunopathologies, notably the development of granulomas, or collections of macrophages and lymphocytes.81 The pathogenesis of these granulomas in sarcoidosis involves expansion and activation of T-cell subsets, many of which secrete IL-2. IL-2-secreting T-cells are more numerous in the granulomas of patients with active sarcoidosis compared to those with chronic sarcoidosis.82 Additionally, many of the therapies used for the cutaneous lesions of sarcoidosis decrease IL-2 levels, which may be a part of their mechanism of action.83 IL-2 secretion by T-cells has long been recognized to play a role in the pulmonary fibrosis found in sarcoidosis and patients with active sarcoidosis have lung lymphocytes that spontaneously secrete IL-2 in the absence of activation.84,85 This aberrant IL-2 secretion in the context of lung fibrosis could be a reaction to the fibrosis or could be involved in its pathogenesis. It is possible that spontaneous IL-2 production by immune cells also occurs in the skin and leads to some of the cutaneous pathologies associated with sarcoidosis. The IL-2 production by T-cells in sarcoidosis can be overcome by immunosuppression using systemic corticosteroids, as demonstrated in a prospective clinical trial, which could be an attractive therapeutic for this disease.86
SIL-2R is also elevated in sarcoidosis and has been extensively studied in the disease.87,88 Su et al. found that sIL-2R levels were elevated in sarcoidosis compared with healthy controls, although the sIL-2R levels did not correlate with lung disease severity.87 The amount of sIL-2R does correlate, however, with eye inflammation in sarcoidosis patients.88 Methotrexate therapy in sarcoidosis patients is associated with decreased serum sIL-2R, and this decrease correlates with improved lung function.89 These studies do not clarify whether sIL-2R is pathologic or is produced in response to excess IL-2. Furthermore, it is unclear what role sIL-2R may play in the cutaneous lesions accompanying this disease. However, IL-2 and IL-2R signaling likely play a role in the skin damage involved in sarcoidosis and warrant further study in the context of this disease, which may shed light on the role of IL-2 in wound healing in other contexts.
Diabetes
Another disease that involves both altered IL-2 and impaired wound healing is DM. DM is a growing epidemic within the United States and includes both Type I (T1DM), which is caused by autoimmune destruction of insulin-producing cells, and Type II (T2DM), which is a multifactorial disease involving decreased tissue insulin sensitivity.90 Both T1DM and T2DM diabetes lead to, among other pathologies, aberrant wound healing.90 The nature of the impaired wound healing in DM is not entirely characterized, but it appears to involve a combination of neuropathy leading to decreased sensation of injury, vascular insufficiency causing decreased blood delivery to the wound site, and excessive inflammation.91
In the setting of T1DM, IL-2 and IL-2R have been extensively studied in humans. In T1DM, genome wide association studies (GWAS) have demonstrated allelic variation in multiple IL-2 signaling cascade participants.3,92,93 These include single nucleotide polymorphisms that occur within the IL-2Rα gene and PTPN2, ultimately conferring susceptibility in acquiring T1DM. Moreover, in healthy patients lacking an IL-2R polymorphism known to decrease T1DM risk, IL-2 signaling was attenuated in several T-cell subsets, including memory T-cells and T-regs.94 Interestingly, decreased IL-2 signaling was observed in patients with T1DM as demonstrated by diminished phosphorylation of STAT5 in response to IL-2 treatment. Based on these data, it is unsurprising that there have been clinical trials involving the administration of IL-2 to T1DM patients, with an aim to prevent pathologies. IL-2 administration to these patients appears to increase T-reg frequency, although this IL-2 administration is also associated with an increase in adverse events.95,96 Rapamycin combined with IL-2 therapy further increases T-reg frequency when compared to other T-cell subsets and improves T-reg signaling via the IL-2 pathway, but has a deleterious effect on insulin production by pancreatic β-cells. Additionally, expression of PTPN2 was increased in the T-regs of diabetic patients compared with healthy controls, which may have contributed to a lack of STAT5 signaling in response to IL-2.97 Thus, overactive and underactive IL-2 signaling appears relevant to diabetes pathogenesis. Altered IL-2 signaling in some T1DM patients may impair early immune cell engagement at wound sites, which could lead to an increased risk of wound infection and impairment of early inflammatory events necessary to close the wound. Conversely, other patients may experience excessive IL-2 signaling, either locally at wounds or systemically, which may worsen some diabetes-associated pathologies. Thus, in T1DM, more research is needed to clarify the role of IL-2 in diabetes pathogenesis and in its manifestations.
In T2DM, IL-2 signaling is also likely altered, although this association has not been well-studied. One group, Lagman et al.,98 demonstrated a robust reduction of IL-2 in the plasma of T2DM patients compared with healthy controls.98 Interestingly, other groups have demonstrated an increase in blood sIL-2R in T2DM patients.99,100 One of these studies also demonstrated that increased sIL-2R levels were associated with decreased T-reg frequencies, increased T-helper cell frequencies, and higher percentages of IL-2Rα+ non-T-reg T-cell subsets. These findings implicate IL-2Rα-possessing T-cells in the mechanism by which excessive sIL-2Rα is produced in T2DM patients. Overall, the investigations of IL-2 and T2DM have generally only measured differences in IL-2 and sIL-2R in peripheral blood and have not related these measurements back to severity of T2DM-associated pathologies such as impaired wound healing. However, based upon the evidenced discussed here, it can be hypothesized that the wound healing impairment in T2DM may be due to a decrease in IL-2 signaling early during the skin repair process. These low IL-2 levels, either at the wound site or systemically, may fail to attract relevant inflammatory cells to the wound site or may permit microbial colonization of the injury, ultimately leading to failed wound healing. To further clarify how IL-2 impacts wounds in T2DM patients, however, more study is needed.
Animal studies have been fundamental to further elucidating the contribution of IL-2 to pathologies in DM. Numerous studies in a mouse model of T1DM have demonstrated that IL-2 treatment may prevent or improve T1DM. IL-2 stimulation through a variety of approaches including extrinsic IL-2 treatment, viral vectors containing IL-2, and a combination treatment with an anti-IL-2 antibody and IL-2 have been successful in demonstrating that increasing IL-2 signaling is a potential therapeutic avenue.101–105 IL-2 induction likely acts by expanding T-regs, which prevent the destruction of insulin-producing cells.101 However, the protective nature of IL-2 treatment is dose dependent. Low doses of IL-2 or IL-2-containing virus prevent DM by protecting pancreatic insulin-producing cells, whereas high-dose IL-2 has a deleterious effect on DM prevention.101–103 Furthermore, in order to prevent loss of the insulin-producing cells of the pancreas, the β-cells, the treatment must be administered prior to the loss of these β-cells, indicating that IL-2 is attenuating an immune-mediated destruction of the β-cells rather than preventing downstream diabetes complications.104 In spite of these difficulties in using IL-2 to prevent T1DM initiation, IL-2-related treatments may still prove useful in treating wound complications. DM animal models could serve as a means to test IL-2 for this indication.
Currently there is no direct evidence that IL-2 signaling is disrupted in diabetic wound healing. However, the involvement of the immune system in the wound healing impairment in DM is clear.91,106 Thus, the well-established link between diabetes, especially its immunologic derangements, and impaired wound healing, coupled with the role of IL-2 in diabetes pathogenesis, provides an opportunity to explore the role of IL-2 in wound healing. Such studies might yield attractive therapeutic strategies or a better understanding of the contribution of IL-2 to wound healing, both in DM and in general.
Myocardial infarction
Following a MI, the healing of the necrotic, apoptotic, and autophagic cellular response is strikingly similar to skin wound repair. In an MI, following the irreversible damage of cardiomyocytes by ischemia, the healing process begins immediately. Part of this process includes recruitment of inflammatory cells (neutrophils, macrophages, and lymphocytes) to the injury site, which strikingly resembles the pattern seen in skin healing.7,107 The similarity between MI and cutaneous wounds implicates similar processes in the healing of both types of injuries.
There have been a number of studies investigating IL-2 in cardiac remodeling. A majority of these studies utilized rodent models. Overall, there seems to be a consensus that IL-2 treatment (be that preconditioning or following MI induction) attenuates the destructive cardiac response.108–111 These studies propose multiple mechanisms by which IL-2 regulates post-infarction recovery, including modulating T-regs, decreasing oxygen free radical production, or activating NK cells to promote angiogenesis. Thus, the mechanism(s) of IL-2 efficacy in limiting cardiac injury appear to be immune-mediated and similar mechanisms might lead to a beneficial immune response during wound healing by using IL-2 treatment.
Interestingly, in contrast to many of these more recent animal studies, clinical trials revealed that high-dose treatment of IL-2 (usually for cancer treatment) increased the risk of death from MI and myocarditis.112,113 The authors of these studies speculate that the observed increase in the ischemic events was due to hypotension caused by the vascular leak associated with IL-2 administration because infarctions occurring in patients sometimes occurred in the absence of atherosclerotic lesions. Furthermore, it is also possible that in this study too much IL-2 led to an overaggressive immune response. Either hypotension or excessive inflammation would be detrimental to both cardiac and cutaneous wound healing, which argues for careful dosing, timing, and patient selection or local administration when using IL-2 to treat a healing wound or cardiac insult.
Furthermore, there have been a number of investigations looking at IL-2 signaling in patients with MI. Blum et al.114 found a significantly higher blood sIL-2R in patients who experienced a second MI in the week following an initial MI.114 Higher sIL-2R also inversely correlated with ejection fraction and was associated with an increased mortality risk, although it is unclear how the increased sIL-2R is involved in these effects. Using a 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase inhibitor, atorvastatin, Zhang et al.115 were able to improve infarct volume in a mechanism involving increased T-regs at the site of infarct. While this study does not directly address IL-2, IL-2 signaling may be involved in the T-reg recruitment in response to atorvastatin. T-reg engagement by atorvastatin and other drugs in its class might also be exploited to improve cutaneous wound healing. Taken together, evidence suggests that supraphsyiological doses of IL-2 may not be advantageous for MI as they might stimulate effector cell expansion and increase the overall inflammatory response. However, low-dose IL-2 treatment aimed at stimulating T-reg or NK cells may be cardioprotective. A similar mechanism may improve wound healing by careful administration of IL-2 following cutaneous injury.
Conclusions
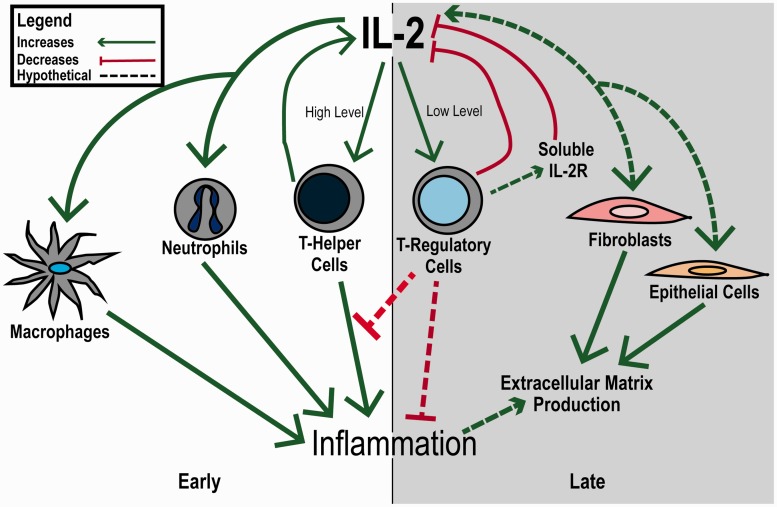
Wound healing appears to involve IL-2 signaling, suggesting that the IL-2 pathway may be an important therapeutic target (Figure 3). However, the contribution of IL-2 signaling, both systemically and locally, to wound healing needs more study before it can be exploited clinically. It appears that early in the wound healing process, IL-2 signaling may play a role in attracting immune mediators for initiating the healing process and preventing colonization of the wound site by microbes. It may also contribute to skin and blood vessel cell proliferation. Later, decreasing IL-2 may help promote resolution of inflammation by attracting and expanding regulatory immune cells. Immunologic derangements, especially those involving the IL-2 signaling pathways, may impair wound healing. Evidence for this role for IL-2 in wound healing comes from studies that explore the cytokine mediators of wound repair and from evidence in diseases involving both aberrant wound healing and derangements of IL-2. Taken together, the evidence indicates that the role IL-2 plays in wound healing needs further exploration but that IL-2 signaling is a promising therapeutic target in improving wound healing rate and quality.
Figure 3.
Overview of IL-2 signaling in wound healing. IL-2 levels appear to influence the immune system to promote wound healing. Early in the healing process, high levels of IL-2, both systemically and locally, favor the actions of immune effector cells, including as T-Helper cells, macrophages, and neutrophils. Later, IL-2 levels drop, possibly in response to T-regulatory cell signaling or high levels of soluble IL-2 receptor, which leads to a decrease in inflammation and an environment that favors skin cell growth. (A color version of this figure is available in the online journal.)
Acknowledgements
The authors would like to thank Jonathon Ruland for assistance with figure development and the American Physician Scientists Association and the Society for Experimental Biology and Medicine the opportunity to write this review as part of their joint abstract competition. Support was provided by both Baylor/Scott and White and Texas A&M Health Science Center College of Medicine to MKN.
Authors’ contributions
All authors contributed to the literature review, writing, and revision of this manuscript. KMD is the recipient of the Sigma Xi Grant-in-Aid (identification number: G201603152030013) and DJD is the recipient of the American Heart Association Predoctoral Fellowship Grant (identification number: 15PRE24470072).
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, Gottrup F, Gurtner GC, Longaker MT. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen 2009; 17: 763–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khalil H, Cullen M, Chambers H, Carroll M, Walker J. Elements affecting wound healing time: An evidence based analysis. Wound Repair Regen 2015; 23: 550–6. [DOI] [PubMed] [Google Scholar]
- 3.Wound dressings market (traditional wound dressings: wound closure products, basic wound care, anti-infective dressings; & advanced wound dressings: films, foams, hydrofiber, hydrocolloids, hydrogels, collagen, alginates) – global industry analysis, size, share, growth, trends, and forecast, 2014-2020. [press release]. Available from: http://www.transparencymarketresearch.com/wound-dressing-market.html.
- 4.Gauglitz GG, Korting HC, Pavicic T, Ruzicka T, Jeschke MG. Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Mol Med 2011; 17: 113–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stebbins WG, Gusev J, Higgins HW, 2nd, Nelson A, Govindarajulu U, Neel V. Evaluation of patient satisfaction with second intention healing versus primary surgical closure. J Am Acad Dermatol 2015; 73: 865–7 e1. [DOI] [PubMed] [Google Scholar]
- 6.Reinholz M, Poetschke J, Schwaiger H, Epple A, Ruzicka T, Gauglitz GG. The dermatology life quality index as a means to assess life quality in patients with different scar types. J Eur Acad Dermatol Venereol 2015; 29: 2112–9. [DOI] [PubMed] [Google Scholar]
- 7.Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci 2004; 9: 283–9. [DOI] [PubMed] [Google Scholar]
- 8.Broughton G, 2nd, Janis JE, Attinger CE. The basic science of wound healing. Plast Reconstr Surg 2006; 117(7 Suppl): 12S–34S. [DOI] [PubMed] [Google Scholar]
- 9.Lopez N, Cervero S, Jimenez MJ, Sanchez JF. Cellular characterization of wound exudate as a predictor of wound healing phases. Wounds 2014; 26: 101–7. [PubMed] [Google Scholar]
- 10.Leoni G, Neumann PA, Sumagin R, Denning TL, Nusrat A. Wound repair: role of immune-epithelial interactions. Mucosal Immunol 2015; 8: 959–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med 2015; 6: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu RH, Weinstein AL, Chang MM, Argenziano M, Ascherman JA, Rohde CH. Risk factors of infected sternal wounds versus sterile wound dehiscence. J Surg Res 2016; 200: 400–7. [DOI] [PubMed] [Google Scholar]
- 13.Owens PL, Barrett ML, Raetzman S, Maggard-Gibbons M, Steiner CA. Surgical site infections following ambulatory surgery procedures. JAMA 2014; 311: 709–16. [DOI] [PubMed] [Google Scholar]
- 14.Leaper D, Assadian O, Edmiston CE. Approach to chronic wound infections. Br J Dermatol 2015; 173: 351–8. [DOI] [PubMed] [Google Scholar]
- 15.Wolfram D, Tzankov A, Pulzl P, Piza-Katzer H. Hypertrophic scars and keloids – a review of their pathophysiology, risk factors, and therapeutic management. Dermatol Surg 2009; 35: 171–81. [DOI] [PubMed] [Google Scholar]
- 16.Toulon A, Breton L, Taylor KR, Tenenhaus M, Bhavsar D, Lanigan C, Rudolph R, Jameson J, Havran WL. A role for human skin-resident T cells in wound healing. J Exp Med 2009; 206: 743–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishio N, Ito S, Suzuki H, Isobe K. Antibodies to wounded tissue enhance cutaneous wound healing. Immunology 2009; 128: 369–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Komori HK, Witherden DA, Kelly R, Sendaydiego K, Jameson JM, Teyton L, Havran WL. Cutting edge: dendritic epidermal gammadelta T cell ligands are rapidly and locally expressed by keratinocytes following cutaneous wounding. J Immunol 2012; 188: 2972–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liao W, Lin JX, Wang L, Li P, Leonard WJ. Modulation of cytokine receptors by IL-2 broadly regulates differentiation into helper T cell lineages. Nat Immunol 2011; 12: 551–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin JX, Leonard WJ. The role of Stat5a and Stat5b in signaling by IL-2 family cytokines. Oncogene 2000; 19: 2566–76. [DOI] [PubMed] [Google Scholar]
- 21.Lin JX, Leonard WJ. Signaling from the IL-2 receptor to the nucleus. Cytokine Growth Factor Rev 1997; 8: 313–32. [DOI] [PubMed] [Google Scholar]
- 22.Lan RY, Selmi C, Gershwin ME. The regulatory, inflammatory, and T cell programming roles of interleukin-2 (IL-2). J Autoimmun 2008; 31: 7–12. [DOI] [PubMed] [Google Scholar]
- 23.Granucci F, Feau S, Angeli V, Trottein F, Ricciardi-Castagnoli P. Early IL-2 production by mouse dendritic cells is the result of microbial-induced priming. J Immunol 2003; 170: 5075–81. [DOI] [PubMed] [Google Scholar]
- 24.Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood 2008; 112: 1557–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao W, Lin JX, Leonard WJ. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity 2013; 38: 13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelson BH, Willerford DM. Biology of the interleukin-2 receptor. Adv Immunol 1998; 70: 1–82. [DOI] [PubMed] [Google Scholar]
- 27.Khan SH, Martin MD, Starbeck-Miller GR, Xue HH, Harty JT, Badovinac VP. The timing of stimulation and IL-2 signaling regulate secondary CD8 T cell responses. PLoS Pathog 2015; 11: e1005199–e1005199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang LH, Kirken RA, Erwin RA, Yu C, Farrar WL. JAK3, STAT, and MAPK signaling pathways as novel molecular targets for the tyrphostin AG-490 regulation of IL-2-mediated T cell response. J Immunol 1999; 162: 3897–904. [PubMed] [Google Scholar]
- 29.Orton RJ, Sturm OE, Vyshemirsky V, Calder M, Gilbert DR, Kolch W. Computational modelling of the receptor-tyrosine-kinase-activated MAPK pathway. Biochem J 2005; 392(Pt 2): 249–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Downward J. Targeting RAS and PI3K in lung cancer. Nat Med 2008; 14: 1315–6. [DOI] [PubMed] [Google Scholar]
- 31.DuPage M, Bluestone JA. Harnessing the plasticity of CD4(+) T cells to treat immune-mediated disease. Nat Rev Immunol 2016; 16: 149–63. [DOI] [PubMed] [Google Scholar]
- 32.Goldstein JD, Perol L, Zaragoza B, Baeyens A, Marodon G, Piaggio E. Role of cytokines in thymus- versus peripherally derived-regulatory T cell differentiation and function. Front Immunol 2013; 4: 155–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Long SA, Cerosaletti K, Wan JY, Ho JC, Tatum M, Wei S, Schilling HG, Buckner JH. An autoimmune-associated variant in PTPN2 reveals an impairment of IL-2R signaling in CD4(+) T cells. Genes Immun 2011; 12: 116–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rubin LA, Kurman CC, Fritz ME, Biddison WE, Boutin B, Yarchoan R, Nelson DL. Souble interleukin-2 receptors are released from activated human lymphoid cells in vitro. J Immunol 1985; 135: 3172–7. [PubMed] [Google Scholar]
- 35.Kondo N, Kondo S, Shimizu A, Honjo T, Hamuro J. A soluble ‘Anchorminus' interleukin 2 receptor suppresses in vitro interleukin 2-mediated immune responses. Immunol Lett 1988; 19: 299–308. [DOI] [PubMed] [Google Scholar]
- 36.Jacques Y, Le Mauff B, Boeffard F, Godard A, Soulillou JP. A soluble interleukin 2 receptor produced by a normal alloreactive human T cell clone binds interleukin 2 with low affinity. J Immunol 1987; 139: 2308–16. [PubMed] [Google Scholar]
- 37.Symons JA, Wood NC, Di Giovine FS, Duff GW. Soluble IL-2 receptor in rheumatoid arthritis. Correlation with disease activity, IL-1 and IL-2 inhibition. J Immunol 1988; 141: 2612–8. [PubMed] [Google Scholar]
- 38.Furtado GC, de Lafaille MAC, Kutchukhidze N, Lafaille JJ. Interleukin 2 signaling is required for CD4+regulatory T cell function. J Exp Med 2002; 196: 851–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ray JP, Staron MM, Shyer JA, Ho PC, Marshall HD, Gray SM, Laidlaw BJ, Araki K, Ahmed R, Kaech SM, Craft J. The interleukin-2-mTORc1 kinase axis defines the signaling, differentiation, and metabolism of T helper 1 and follicular B helper T cells. Immunity 2015; 43: 690–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dooms H, Wolslegel K, Lin P, Abbas AK. Interleukin-2 enhances CD4+ T cell memory by promoting the generation of IL-7R alpha-expressing cells. J Exp Med 2007; 204: 547–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Busse D, de la Rosa M, Hobiger K, Thurley K, Flossdorf M, Scheffold A, Hofer T. Competing feedback loops shape IL-2 signaling between helper and regulatory T lymphocytes in cellular microenvironments. Proc Natl Acad Sci U S A 2010; 107: 3058–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leclercq G, De Smedt M, Tison B, Plum J. Preferential proliferation of T cell receptor V gamma 3-positive cells in IL-2 stimulated fetal thymocytes. J Immunol 1990; 145: 3992–7. [PubMed] [Google Scholar]
- 43.Kjeldsen-Kragh J, Quayle AJ, Skalhegg BS, Sioud M, Forre O. Selective activation of resting human γδ T lymphocytes by interleukin-2. Eur J Immunol 1993; 23: 2092–9. [DOI] [PubMed] [Google Scholar]
- 44.Orsini DLM, Kooy YMC, Van Der Tol MA, Struyk L, Van Den Elsen P. T-cell receptor usage of interleukin-2-responsive peripheral gd T cells. Immunology 1995; 86: 385–91. [PMC free article] [PubMed] [Google Scholar]
- 45. Litwin SD. Product: proleukin (aldesleukin), proleukin, interleukin-2 (IL-2). Silver Sping, MD: Food and Drug Administration, 1998.
- 46.McDermott DF, Regan MM, Atkins MB. Interleukin-2 therapy of metastatic renal cell carcinoma: update of phase III trials. Clin Genitourin Cancer 2006; 5: 114–9. [DOI] [PubMed] [Google Scholar]
- 47.Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, Abrams J, Sznol M, Parkinson D, Hawkins M, Paradise C, Kunkel L, Rosenberg SA. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol 1999; 17: 2105–16. [DOI] [PubMed] [Google Scholar]
- 48.Siegel RL, Puri RK. Interleukin-2 toxicity. J Clin Oncol 1991; 9: 694–704. [DOI] [PubMed] [Google Scholar]
- 49.Shi VY, Tran K, Patel F, Leventhal J, Konia T, Fung MA, Wilken R, Garcia MS, Fitzmaurice SD, Joo J, Monjabez AM, Burrall BA, King B, Martinez S, Christensen SD, Maverakis E. 100% Complete response rate in patients with cutaneous metastatic melanoma treated with intralesional interleukin (IL)-2, imiquimod, and topical retinoid combination therapy: results of a case series. J Am Acad Dermatol 2015; 73: 645–54. [DOI] [PubMed] [Google Scholar]
- 50.Woo KY. Exploring the effects of pain and stress on wound healing. Adv Wound Care 2012; 25: 38–44. [DOI] [PubMed] [Google Scholar]
- 51.Cozzolino F, Torcia M, Lucibello M, Morbidelli L, Ziche M, Platt J, Fabiani S, Brett J, Stern D. Interferon-alpha and interleukin 2 synergistically enhance basic fibroblast growth factor synthesis and induce release, promoting endothelial cell growth. J Clin Invest 1993; 91: 2504–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kowal-Vern A, Walenga JM, Hoppensteadt D, Sharp-Pucci M, Gamelli RL. Interleukin-2 and interleukin-6 in relation to burn wound size in the acute phase of thermal injury. J Am Coll Surg 1994; 178: 357–62. [PubMed] [Google Scholar]
- 53.Mikhal'chik EV, Piterskaya JA, Budkevich LY, Pen'kov LY, Facchiano A, De Luca C. Comparative study of cytokine content in the plasma and wound exudate from children with severe burns. Bull Exp Biol Med 2009; 148: 771–5. [DOI] [PubMed] [Google Scholar]
- 54.Currie HN, Loos MS, Vrana JA, Dragan K, Boyd JW. Spatial cytokine distribution following traumatic injury. Cytokine 2014; 66: 112–8. [DOI] [PubMed] [Google Scholar]
- 55.Han AA, Currie HN, Loos MS, Vrana JA, Fabyanic EB, Prediger MS, Boyd JW. Spatiotemporal phosphoprotein distribution and associated cytokine response of a traumatic injury. Cytokine 2016; 79: 12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barbul A, Knud-Hansen J, Wasserkrug HL, Efron G. Interleukin 2 enhances wound healing in rats. J Surg Res 1986; 40: 315–9. [DOI] [PubMed] [Google Scholar]
- 57.Sakakibara S, Inouye K, Shudo K, Kishida Y, Kobayashi Y, Prockop DJ. Synthesis of (Pro-Hyp-Gly)n of defined molecular weights. Evidence for the stabilization of collagen triple helix by hydroxyproline. Biochem Biophys Acta 1973; 303: 198–202. [DOI] [PubMed] [Google Scholar]
- 58.Byrd VM, Ballard DW, Miller GG, Thomas JW. Fibroblast growth factor-1 (FGF-1) enhances IL-2 production and nuclear translocation of NF-kB in FGF receptor-bearing Jurkat T cells. J Immunol 1999; 162: 5853–9. [PubMed] [Google Scholar]
- 59.Ozawa A, Tada H, Tamai R, Uehara A, Watanabe K, Yamaguchi T, Shimauchi H, Takada H, Sugawara S. Expression of IL-2 receptor B and Γ chains by human gingival fibroblasts and up-regulation of adhesion to neutrophils in response to IL-2. J Leukocyte Biol 2003; 74: 352–9. [DOI] [PubMed] [Google Scholar]
- 60.Corrigall VM, Arastu M, Khan S, Shah C, Fife M, Smeets T, Tak P, Panayi GS. Functional IL-2 receptor B (CD122) and Γ (CD132) chains are expressed by fibroblast-like synoviocytes: activation by IL-2 stimulates monocyte chemoattractant protein-1 production. J Immunol 2001; 166: 4141–7. [DOI] [PubMed] [Google Scholar]
- 61.Kang R, Tang D, Lotze MT, Zeh Iii HJ. Autophagy is required for IL-2-mediated fibroblast growth. Exp Cell Res 2013; 319: 556–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Serban AI, Stanca L, Geicu OI, Munteanu MC, Dinischiotu A. RAGE and TGF-beta1 cross-talk regulate extracellular matrix turnover and cytokine synthesis in AGEs exposed fibroblast cells. PLoS One 2016; 11: e0152376–e0152376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Breslin RJ, Barbul A, Kupper TS, Knud-Hansen J, Wasserkrug HL, Efron G. Generation of an anti-interleukin-2 factor in healing wounds. Arch Surg 1988; 123: 305–8. [DOI] [PubMed] [Google Scholar]
- 64.Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol 2012; 12: 180–90. [DOI] [PubMed] [Google Scholar]
- 65.Boyman O, Kolios AGA, Raeber ME. Modulation of T cell responses by IL-2 and IL-2 complexes. Clin Exp Rheumatol 2015; 33(Suppl. 92): S54–S7. [PubMed] [Google Scholar]
- 66.Lehmann P, Homey B. Clinic and pathophysiology of photosensitivity in lupus erythematosus. Autoimmun Rev 2009; 8: 456–61. [DOI] [PubMed] [Google Scholar]
- 67.Kuhn A, Sontheimer RD. Cutaneous lupus erythematosus: molecular and cellular basis of clinical findings. Curr Dir Autoimmun 2008; 10: 119–40. [DOI] [PubMed] [Google Scholar]
- 68.Robinson ES, Werth VP. The role of cytokines in the pathogenesis of cutaneous lupus erythematosus. Cytokine 2015; 73: 326–34. [DOI] [PubMed] [Google Scholar]
- 69.Hanssen AD, Cabanela ME, Michet CJ. Hip arthroplasty in patients with systemic lupus erythematosus. J Bone Joint Surg 1987; 69: 807–14. [PubMed] [Google Scholar]
- 70.Heydemann A. The super super-healing MRL mouse strain. Front Biol 2012; 7: 522–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Petitto JM, Huang Z, Lo J, Beck RD, Rinker C, Hartemink DA. Relationship between the development of autoimmunity and sensorimotor gating in MRL-lpr mice with reduced IL-2 production. Neurosci Lett 2002; 328: 304–8. [DOI] [PubMed] [Google Scholar]
- 72.Kench JA, Russell DM, Fadok VA, Young SK, Worthen GS, Jones-Carson J, Henson JE, Henson PM, Nemazee D. Aberrant wound healing and TGF-β production in the autoimmune-prone MRL/+ mouse. Clin Immunol 1999; 92: 300–10. [DOI] [PubMed] [Google Scholar]
- 73.Katsiari CG, Tsokos GC. Transcriptional repression of interleukin-2 in human systemic lupus erythematosus. Autoimmun Rev 2006; 5: 118–21. [DOI] [PubMed] [Google Scholar]
- 74.Carneiro JRM, Fuzii HT, Kayser C, Alberto FL, Soares FA, Sato EI, Andrade LEC. IL-2, IL-5, TNF-α and IFN-γ mRNA expression in epidermal keratinocytes of systemic lupus erythematosus skin lesions. Clinics 2011; 66: 77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Solomou EE, Juang Y, Gourley MF, Kammer GM, Tsokos GC. Molecular basis of deficient IL-2 production in T cells from patients with systemic lupus erythematosus. J Immunol 2011; 166: 4216–22. [DOI] [PubMed] [Google Scholar]
- 76.Linker-Israeli M, Bakke AC, Kitridou RC, Gendler S, Gillis S, Horwitz DA. Defective production of interleukin 1 and interleukin 2 in patients with systemic lupus erythematosus (SLE). J Immunol 1983; 130: 2651–5. [PubMed] [Google Scholar]
- 77.Alcocer-Varela J, Alarcon-Segovia D. Longitudinal study of the production of and cellular response to interleukin-2 in patients with systemic lupus erythematosus. Rheumatol Int 1995; 15: 57–63. [DOI] [PubMed] [Google Scholar]
- 78.Miyagi J, Minato N, Sumiya M, Kasahara T, Kano S. Two typesof antibodies inhibiting interleukin-2 production by normal lymphocytes in patients with systemic lupus erythematosus. Arth Rheum 1989; 32: 1356–64. [DOI] [PubMed] [Google Scholar]
- 79.Wofsy D, Murphy ED, Roths RB, Dauphinee MJ, Kipper SB, Talal N. Deficient Interleukin 2 activity in MRL/Mp and C57BL6/J mice bearing the LPR gene. J Exp Med 1981; 154: 1671–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sawada S, Hashimoto H, Iijma S, Tokano Y, Matsukawa Y, Takei M, Ishikawa H, Kang H, Tomura K, Mitamura K, Hashimoto S, Obara T. Increased soluble Il-2 receptor in serum of patients with systemic lupus erythematosus. Clin Rheumatol 1993; 12: 204–9. [DOI] [PubMed] [Google Scholar]
- 81.Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med 2007; 357: 2153–65. [DOI] [PubMed] [Google Scholar]
- 82.Buechner SA, Winkelmann RK, Banks PM. T-cell subsets in cutaneous sarcoidosis. Arch Dermatol 1983; 119: 728–32. [PubMed] [Google Scholar]
- 83.Badgwell C, Rosen T. Cutaneous sarcoidosis therapy updated. J Am Acad Dermatol 2007; 56: 69–83. [DOI] [PubMed] [Google Scholar]
- 84.Saltini C, Spurzem JR, Lee JJ, Pinkston P, Crystal RG. Spontaneous release of interleukin 2 by lung T lymphocytes in active pulmonary sarcoidosis is primarily from the Leu3+DR+ T cell subset. J Clin Invest 1986; 77: 1962–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pinkston P, Bitterman PB, Crystal RG. Spontaneous release of interleukin-2 by lung T lymphocytes in active pulmonary sarcoidosis. N Engl J Med 1983; 308: 793–800. [DOI] [PubMed] [Google Scholar]
- 86.Pinkston P, Saltini C, Muller-Quernheim J, Crystal RG. Corticosteroid therapy suppresses spontaneous interleukin 2 release and spontaneous proliferation of lung T lymphocytes of patients with active pulmonary sarcoidosis. J Immunol 1987; 139: 755–60. [PubMed] [Google Scholar]
- 87.Su R, Nguyen MT, Agarwal MR, Kirby C, Nguyen CP, Ramstein J, Darnell EP, Gomez AD, Ho M, Woodruff PG, Koth LL. Interferon-inducible chemokines reflect severity and progression in sarcoidosis. Respir Res 2013; 14: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gundlach E, Hoffmann MM, Prasse A, Heinzelmann S, Ness T. Interleukin-2 receptor and angiotensin-converting enzyme as markers for ocular sarcoidosis. PLoS One 2016; 11: e0147258–e0147258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vorselaars AD, van Moorsel CH, Zanen P, Ruven HJ, Claessen AM, van Velzen-Blad H, Grutters JC. ACE and sIL-2R correlate with lung function improvement in sarcoidosis during methotrexate therapy. Respir Med 2015; 109: 279–85. [DOI] [PubMed] [Google Scholar]
- 90.Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev 2013; 93: 137–88. [DOI] [PubMed] [Google Scholar]
- 91.Ackermann PW, Hart DA. Influence of comorbidities: neuropathy, vasculopathy, and diabetes on healing response quality. Adv Wound Care 2013; 2: 410–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lowe CE, Cooper JD, Brusko T, Walker NM, Smyth DJ, Bailey R, Bourget K, Plagnol V, Field S, Atkinson M, Clayton DG, Wicker LS, Todd JA. Large-scale genetic fine mapping and genotype-phenotype associations implicate polymorphism in the IL2RA region in type 1 diabetes. Nat Genet 2007; 39: 1074–82. [DOI] [PubMed] [Google Scholar]
- 93.van Belle TL, Coppieters KT, von Herrath MG. Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol Rev 2011; 91: 79–118. [DOI] [PubMed] [Google Scholar]
- 94.Garg G, Tyler JR, Yang JH, Cutler AJ, Downes K, Pekalski M, Bell GL, Nutland S, Peakman M, Todd JA, Wicker LS, Tree TI. Type 1 diabetes-associated IL2RA variation lowers IL-2 signaling and contributes to diminished CD4+CD25+ regulatory T cell function. J Immunol 2012; 188: 4644–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Long SA, Reick M, Sanda S, Bollyky JB, Samuels PL, Goland R, Ahmann A, Rabinovitch A, Aggarwal S, Phippard D, Turka LA, Ehlers MR, Bianchine PJ, Boyle KD, Adah SA, Bluestone JA, Buckner JH, Greenbaum CJ. Rapamycin/IL-2 combination therapy in patients with type 1 diabetes augments Tregs yet transiently impairs b-cell function. Diabetes 2012; 61: 2340–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hartemann A, Bensimon G, Payan CA, Jacqueminet S, Bourron O, Nicolas N, Fonfrede M, Rosenzwajg M, Bernard C, Klatzmann D. Low-dose interleukin 2 in patients with type 1 diabetes: a phase 1/2 randomised, double-blind, placebo-controlled trial. Lancet Diab Endocrinol 2013; 1: 295–305. [DOI] [PubMed] [Google Scholar]
- 97.Long SA, Cerosaletti K, Bollyky PL, Tatum M, Shilling H, Zhang S, Zhang Z, Pihoker C, Sanda S, Greenbaum C, Buckner JH. Defects in IL-2R signaling contribute to diminished maintenance of FOXP3 expression in CD4+CD25+ regulatory T-cells of type 1 diabetic subjects. Diabetes 2010; 59: 407–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lagman M, Ly J, Saing T, Kaur Singh M, Vera Tudela E, Morris D, Chi PT, Ochoa C, Sathananthan A, Venketaraman V. Investigating the causes for decreased levels of glutathione in individuals with type II diabetes. PLoS One 2015; 10: e0118436–e0118436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cai B, Zhang J, Zhang M, Li L, Feng W, An Z, Wang L. Micro-inflammation characterized by disturbed Treg/Teff balance with increasing sIL-2R in patients with type 2 diabetes. Exp Clin Endocrinol Diab 2013; 121: 214–9. [DOI] [PubMed] [Google Scholar]
- 100.Pereira FO, Frode TS, Medeiros YS. Evaluation of tumour necrosis factor alpha, interleukin-2 soluble receptor, nitric oxide metabolites, and lipids as inflammatory markers in type 2 diabetes mellitus. Mediat Inflamm 2006; 2006: 39062–39062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Grinberg-Bleyer Y, Baeyens A, You S, Elhage R, Fourcade G, Gregoire S, Cagnard N, Carpentier W, Tang Q, Bluestone J, Chatenoud L, Klatzmann D, Salomon BL, Piaggio E. IL-2 reverses established type 1 diabetes in NOD mice by a local effect on pancreatic regulatory T cells. J Exp Med 2010; 207: 1871–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Flores RR, Zhou L, Robbins PD. Expression of IL-2 in beta cells by AAV8 gene transfer in pre-diabetic NOD mice prevents diabetes through activation of FoxP3-positive regulatory T cells. Gene Ther 2014; 21: 715–22. [DOI] [PubMed] [Google Scholar]
- 103.Johnson MC, Garland AL, Nicolson SC, Li C, Samulski RJ, Wang B, Tisch R. B-cell-specific IL-2 therapy increases islet Foxp3+Treg and suppresses type 1 diabetes in NOD mice. Diabetes 2013; 62: 3775–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Diaz-de-Durana Y, Lau J, Knee D, Filippi C, Londei M, McNamara P, Nasoff M, DiDonato M, Glynne R, Herman AE. IL-2 immunotherapy reveals potential for innate beta cell regeneration in the non-obese diabetic mouse model of autoimmune diabetes. PLoS One 2013; 8: e78483–e78483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Baeyens A, Perol L, Fourcade G, Cagnard N, Carpentier W, Woytschak J, Boyman O, Hartemann A, Piaggio E. Limitations of IL-2 and rapamycin in immunotherapy of type 1 diabetes. Diabetes 2013; 62: 3120–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nunan R, Harding KG, Martin P. Clinical challenges of chronic wounds: searching for an optimal animal model to recapitulate their complexity. Dis Model Mech 2014; 7: 1205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Frangogiannis NG. Regulation of the inflammatory response in cardiac repair. Circ Res 2012; 110: 159–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cao CM, Xia Q, Tu J, Chen M, Wu S, Wong TM. Cardioprotection of interleukin-2 is mediated via kappa-opioid receptors. J Pharmacol Exp Ther 2004; 309: 560–7. [DOI] [PubMed] [Google Scholar]
- 109.Bouchentouf M, Williams P, Forner KA, Cuerquis J, Michaud V, Paradis P, Schiffrin EL, Galipeau Interleukin-2 enhances angiogenesis and preserves cardiac function following myocardial infarction. Cytokine 2011; 56: 732–8. [DOI] [PubMed] [Google Scholar]
- 110.Koch M, Savvatis K, Scheeler M, Dhayat S, Bonaventura K, Pohl T, Riad A, Bulfone-Paus S, Schultheiss HP, Tschope C. Immunosuppression with an interleukin-2 fusion protein leads to improved LV function in experimental ischemic cardiomyopathy. Int Immunopharmacol 2010; 10: 207–12. [DOI] [PubMed] [Google Scholar]
- 111.Zeng Z, Yu K, Chen L, Li W, Xiao H, Huang Z. Interleukin-2/anti-interleukin-2 immune complex attenuates cardiac remodeling after myocardial infarction through expansion of regulatory T cells. J Immunol Res 2016; 2016: 8493767–8493767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vial T, Descotes J. Clinical toxicity of interleukin-2. Drug Saf 1992; 7: 417–33. [DOI] [PubMed] [Google Scholar]
- 113.Kragel AH, Travis WD, Steis RG, Rosenberg SA, Roberts WC. Myocardial or acute myocardial infarction associated with interleukin-2 therapy for cancer. Cancer 1990; 66: 1513–6. [DOI] [PubMed] [Google Scholar]
- 114.Blum A, Sclarovsky S, Rehavia E, Shohat B. Levels of T-lymphocytes subpopulations, interleukin-1b, and soluble interleukin-2 receptor in acute myocardial infarction. Am Heart J 1994; 127: 1226–30. [DOI] [PubMed] [Google Scholar]
- 115.Zhang D, Wang S, Guan Y, Wang L, Xie W, Li N, Zhao P, Su G. Effect of oral atorvastatin on CD4+CD25+ regulatory T cells, FoxP3 expression, and prognosis in patients with ST-segment elevated myocardial infarction before primary percutaneous coronary intervention. J Cardiovasc Pharmacol 2011; 57: 536–41. [DOI] [PubMed] [Google Scholar]