Abstract
Fibroblast growth factor 21 (FGF21) has recently emerged as a novel endocrine hormone involved in the regulation of glucose and lipid metabolism. However, the exact mechanisms whereby FGF21 mediates insulin sensitivity remain not fully understood. In the present study, FGF21was administrated in high-fat diet-induced obese mice and tunicamycin-induced 3T3-L1 adipocytes, and metabolic parameters, endoplasmic reticulum (ER) stress indicators, and insulin signaling molecular were assessed by Western blotting. The administration of FGF21 in obese mice reduced body weight, blood glucose and serum insulin, and increased insulin sensitivity, resulting in alleviation of insulin resistance. Meanwhile, FGF21 treatment reversed suppression of adiponectin expression and restored insulin signaling via inhibiting ER stress in adipose tissue of obese mice. Additionally, suppression of ER stress via the ER stress inhibitor tauroursodeoxycholic acid increased adiponectin expression and improved insulin resistance in obese mice and in tunicamycin-induced adipocytes. In conclusion, our results showed that the administration of FGF21 reversed suppression of adiponectin expression and restored insulin signaling via inhibiting ER stress under the condition of insulin resistance, demonstrating the causative role of ER stress in downregulating adiponectin levels.
Keywords: Adiponectin, endoplasmic reticulum stress, fibroblast growth factor 21, insulin resistance, obese mice, tauroursodeoxycholic acid
Introduction
Fibroblast growth factor 21 (FGF21), a member of the FGF superfamily, has recently emerged as a novel endocrine hormone involved in the regulation of glucose and lipid metabolism.1 Administration of recombinant FGF21 improves glucose metabolism, lowers blood glucose, and protects from insulin resistance in ob/ob mice, db/db mice and Zucker fatty rats, which are rodent models of diabetes.2,3 In vitro, further studies showed that FGF21 stimulated glucose uptake in 3T3-L1 adipocytes via increasing the expression of glucose transporter 1 (GLUT1).4 Although FGF21 has drawn increasing attention to its function, the exact mechanisms whereby FGF21 mediates glucose and lipid metabolism remain not fully understood.
It has been proven that adiponectin plays a critical role in the regulation of glucose and lipid metabolism by elevating insulin sensitivity.5 In several clinical investigations, serum adiponectin levels are markedly decreased in patients with obesity, insulin resistance, and type 2 diabetes.6 Additionally, FGF21 treatment reversed suppression of adiponectin expression in adipocytes, while adiponectin knockout in mice nullified the protective effect of FGF21 on alleviation of insulin resistance,7 suggesting that adiponectin as a prominent adipokine could be a downstream effecter of FGF21.
Although the role of FGF21 in glucose and lipid metabolism has been identified, the intracellular events responsible for FGF21-mediated effects in adiponectin expression remain elusive. The relationship between adiponectin and endoplasmic reticulum (ER) stress was characterized previously. Recent results showed that the administration of tauroursodeoxycholic acid (TUDCA), as an ER stress inhibitor, increased the serum adiponectin levels in db/db mice and diet-induced obese mice.8 In light of these observations, we postulated that FGF21 might reverse suppression of adiponectin expression via inhibiting ER stress in adipose tissue of obese mice.
In the present study, we provide evidence that FGF21 reversed ER stress, increased adiponectin expression, and restored insulin sensitivity in adipose tissue of obese mice. We also assessed the potential insulin signaling required for FGF21-mediated activity. These results supported the hypothesis that FGF21 increased adiponectin expression and protected from insulin resistance via suppressing ER stress.
Materials and methods
Materials
Chemicals of analytical grade were purchased from Sigma (St Louis, MO) except where stated otherwise. The following antibodies were used: anti-CHOP (C/EBP homologous protein), anti-ATF4 (activating transcription factor 4), anti-adiponectin, anti-p-eIF2α (eukaryotic initiation factor 2α; Ser51) and anti-eIF2α (all from Cell Signaling Technology, Danvers, MA); and anti-IRS-1 (insulin receptor substrate 1), anti-pY20, anti-p-Akt (Ser-473), anti-Akt, anti-GAPDH, peroxidase-conjugated goat anti-rabbit IgG, and peroxidase-conjugated goat anti-mouse IgG (all from Santa Cruz Biotechnology, Santa Cruz, CA).
Animal care
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments of Medical School of Xi’an Jiaotong University (Permit number: 2014009). For the in vivo study, C57BL/6 J male mice (4 weeks old) were fed with a chow diet and housed under standard conditions with a 12 h light:12 h darkness cycle (darkness from 19:30 to 07:30). Mice were distributed into four groups (n = 10 per group): (1) vehicle (normal chow diet, 4% of energy as fat, 3.85 kcal/g, plus 10 µL/g of 0.9% NaCl i.p. injection); (2) FGF21 (normal chow plus 1 mg/kg of FGF21 i.p. injection); (3) high-fat + vehicle (diet containing 60% of energy as fat, 5.24 kcal/g, plus 10 µL/g of 0.9% NaCl i.p. injection); (4) high-fat + FGF21 (high-fat diet plus 1 mg/kg of FGF21 i.p. injection). Daily injections of FGF21 or normal saline solution (vehicle) were initiated after mice were fed a high-fat diet for eight weeks. Mice were injected once a day (6 p.m.) intraperitoneally (i.p.) with 1 mg/kg of FGF21 or with vehicle for a further four weeks. At the end of the study period, half of mice in each group were randomly selected and received an intraperitoneal injection of insulin at a dosage of 2 IU/kg. After 15 min, the mice were euthanized, and their omental adipose tissues and blood samples were obtained and stored at −80℃ for subsequent analysis.
Cell culture, differentiation, and treatment
Mouse 3T3-L1 cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA) and cultured in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum (HyClone, Thermo Fisher Scientific, Logan, UT). Induction medium containing 3T3-L1 cells was used for the differentiation of mature fat cells, with differentiation usually being complete by the eighth day. The medium was replaced with fresh medium before each experiment.
Western blotting
The tissues and cells that were subjected to various treatments were lysed in lysis buffer containing 25 mmol/L Tris HCl (pH 6.8), 2% sodium dodecyl sulfate, 6% glycerol, 1% 2-mercaptoethanol, 2 mmol/L phenylmethylsulfonyl fluoride, 0.2% bromophenol blue, and a protease inhibitor cocktail for 20 min. Western blotting was performed in accordance with a standard protocol.9
Immunoprecipitation
Cytoplasmic lysate (200 µg) was incubated for 2 h at 4℃ with the corresponding antibodies coupled to 20 µL of packed protein A+G sepharose beads (Beyotime, Jiangsu, China). Immunocomplexes were resolved by means of sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotted with the indicated antibodies.
Measurement of serum hormones and metabolites
Blood glucose, serum insulin, serum FGF21, and adiponectin were measured as described previously.10
Metabolic tests
Glucose tolerance testing (GTT) was performed after the mice had fasted overnight. Glucose (2 g/kg body weight) was administrated via an i.p. injection, and blood glucose was measured at specific time points. Insulin tolerance testing (ITT) was performed after the animals had fasted for 4 h, at which time 0.75 U/kg insulin was administered via an i.p. injection and blood glucose was measured at specific time points as described previously.11
Glucose uptake
After transfer of 3T3L1 cells to medium without glucose, mouse adipocytes were incubated with 10 nmol/L insulin for 15 min, when glucose transport was determined as uptake of 50 mmol/L (10 mCi/mL) 2-deoxy-D-[1-3H] glucose, and then incubated for 30 min. Uptake was linear for at least 30 min.
Statistics
Statistical analyses were performed using standard statistical software (SPSS version 17.0, Chicago, IL). Statistical analysis between the two groups was performed using unpaired, two-tailed Student’s t-tests. Differences were considered to be statistically significant when the P value was < 0.05.
Results
The administration of FGF21 improves insulin resistance in high-fat diet-induced obese mice
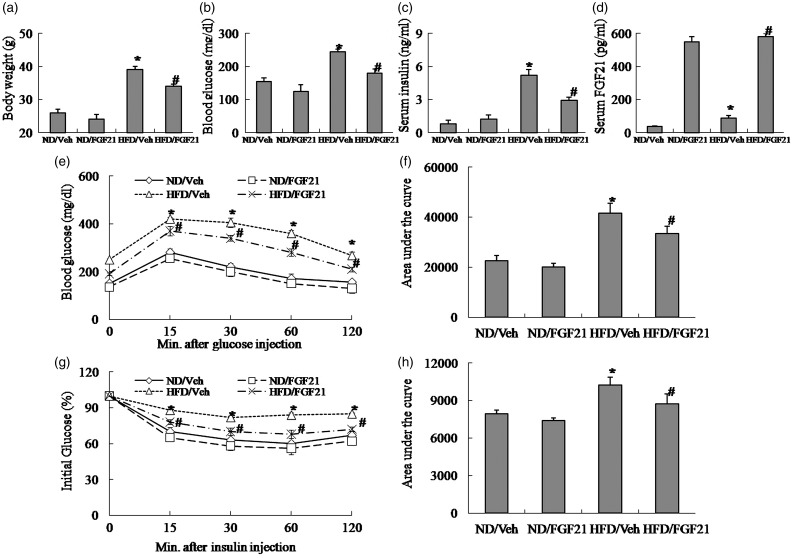
The protective effect of FGF21 on insulin resistance in a model of diet-induced obesity was evaluated by measurement of serum hormones and metabolites, and metabolic tests. In our study, high-fat diet-induced obese mice showed elevated body weight, blood glucose, serum insulin and FGF21, and decreased insulin sensitivity as assessed by GTT and ITT (Figure 1(a)–(h)). As expected, the administration of FGF21 in obese mice reduced body weight, blood glucose and serum insulin, and increased insulin sensitivity as assessed by GTT and ITT (Figure 1(a)–(h)), resulting in alleviation of insulin resistance.
Figure 1.
The administration of FGF21 improves insulin resistance in high-fat diet-induced obese mice. (a) Body weight. (b) Blood glucose. (c) Serum insulin. (d) Serum FGF21. (e) Glucose tolerance testing (GTT). (f) Area under the curve by GTT. (g) Insulin tolerance testing (ITT). (h) Area under the curve by ITT. ND, Normal diet; HFD, high-fat diet; Veh, vehicle. The data were expressed as mean ± SEM in each bar graph. *P < 0.05 (HFD/Veh versus ND/Veh). #P < 0.05 (HFD/FGF21 versus HFD/Veh)
The administration of FGF21 reverses suppression of adiponectin expression and restores insulin signaling via inhibiting ER stress in adipose tissue of high-fat diet-induced obese mice
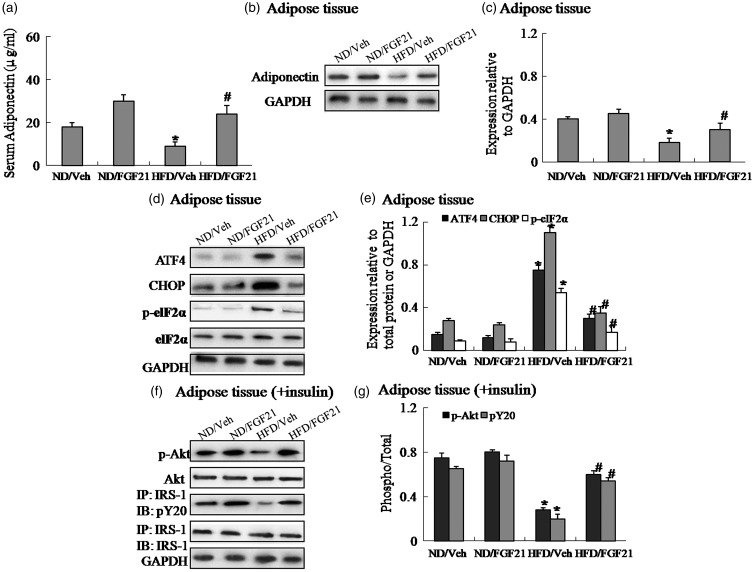
To identify one possible mechanism underlying the effects of FGF21 on alleviation of insulin resistance in obesity, first we measured circulating adiponectin levels and adiponectin expression in adipose tissue. Our results showed that serum adiponectin and adiponectin expression are significantly reduced in obese mice, and the administration of FGF21 in obese mice increased adiponectin expression in adipose tissue and reversed suppression of adiponectin expression compared to obese mice receiving vehicle (Figure 2(a)–(c)). Then, the expression levels of insulin signaling and ER stress indicators in adipose tissue were assessed by Western blotting. As expected high-fat diet-induced obese mice exhibited elevated protein expression of CHOP, ATF4 and the phosphorylation of eIF2α, and decreased insulin-induced IRS-1 tyrosine phosphorylation and Akt Ser-473 phosphorylation (Figure 2(d)–(g)), suggesting activated ER stress and impaired insulin signaling in adipose tissue of obese mice. Of note, FGF21 treatment inhibited ER stress and restored insulin signaling in adipose tissue of obese mice, as evidenced by downregulation of CHOP, ATF4 and the phosphorylation of eIF2α, and upregulation of insulin-stimulated IRS-1 tyrosine phosphorylation and Akt Ser-473 phosphorylation (Figure 2(d)–(g)). Thus, these findings demonstrated the critical role of adiponectin and ER stress in the process of FGF21 action.
Figure 2.
The administration of FGF21 reverses suppression of adiponectin expression and restores insulin signaling via suppressing ER stress in adipose tissue of high-fat diet-induced obese mice. (a) Serum adiponectin. (b) Protein expression of adiponectin in adipose tissue. (c) The relative protein quantity of adiponectin in adipose tissue. (d) Protein expression of ATF4, CHOP and phosphorylation of eIF2α in adipose tissue. (e) The relative protein quantity of ATF4, CHOP and phosphorylation of eIF2α in adipose tissue. (f) Phosphorylation of IRS-1 and Akt in adipose tissue. (g) The relative protein quantity of p-IRS-1 and p-Akt in adipose tissue. ND, Normal diet; HFD, high-fat diet; Veh, vehicle. The relative quantity of proteins was analyzed using Quantity One software. A representative blot is shown and the data were expressed as mean ± SEM in each bar graph. *P < 0.05 (HFD/Veh versus ND/Veh). #P < 0.05 (HFD/FGF21 versus HFD/Veh)
The administration of TUDCA, as an ER stress inhibitor, reverses suppression of adiponectin expression and restores insulin signaling in adipose tissue of obese mice
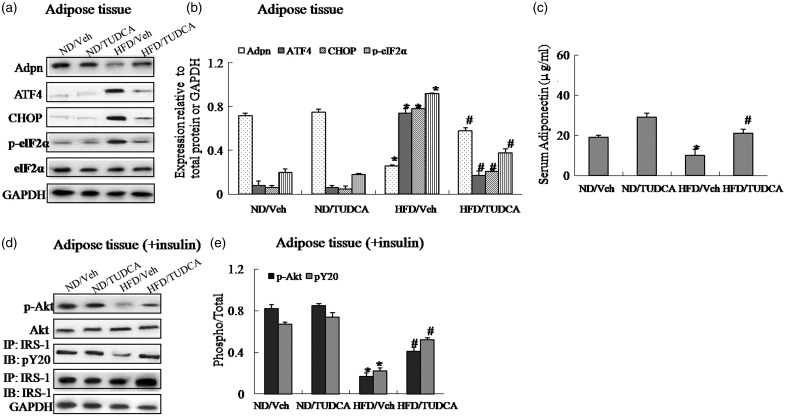
To identify whether ER stress is associated with reduced adiponectin levels in obesity, we measured circulating adiponectin levels and adiponectin expression in adipose tissue of obese mice treated with TUDCA, an ER stress inhibitor or vehicle. TUDCA treatment in adipose tissue of obese mice decreased obesity-induced protein expression of CHOP, ATF4 and the phosphorylation of eIF2α (Figure 3(a) and (b)), concurrent with increased adiponectin expression in adipose tissue and serum adiponectin levels (Figure 3(a)–(c)). Simultaneously, the administration of TUDCA also increased insulin-stimulated IRS-1 tyrosine phosphorylation and Akt Ser-473 phosphorylation in adipose tissue of obese mice, restoring impaired insulin signaling (Figure 3(d)–(e)) and improving insulin resistance in obese mice (Supplemental Figure 1(a)–(g)). Therefore, these results indicated that ER stress mediated downregulation of adiponectin expression in adipose tissue and circulating adiponectin levels in obese mice, and the ER stress inhibitor TUDCA provided the protective effect on insulin resistance.
Figure 3.
The administration of TUDCA, as an ER stress inhibitor, reverses suppression of adiponectin expression and restores insulin signaling in adipose tissue of obese mice. Mice were distributed into four groups (n = 10 per group): (1) vehicle (normal chow diet, 4% of energy as fat, 3.85 kcal/g, plus 10 µL/g of 0.9% NaCl i.p. injection); (2) TUDCA (normal chow plus 500 mg/kg of TUDCA i.p. injection); (3) high-fat + vehicle (diet containing 60% of energy as fat, 5.24 kcal/g, plus 10 µL/g of 0.9% NaCl i.p. injection); (4) high-fat + TUDCA (high-fat diet plus 500 mg/kg of TUDCA i.p. injection). Daily injections of TUDCA or normal saline solution (vehicle) were initiated after mice were fed a high-fat diet for 8 weeks. Mice were injected once a day (6 p.m.) intraperitoneally (i.p.) with 500 mg/kg of TUDCA or with vehicle for a further 4 weeks. At the end of the study period, half of mice in each group were randomly selected and received an intraperitoneal injection of insulin at a dosage of 2 IU/kg; 15 min after the injection. (a) Protein expression of adiponectin (Adpn), ATF4, CHOP and phosphorylation of eIF2α in adipose tissue. (b) The relative protein quantity of adiponectin, ATF4, CHOP and phosphorylation of eIF2α in adipose tissue. (c) Serum adiponectin. (d) Phosphorylation of IRS-1 and Akt in adipose tissue. (e) The relative protein quantity of p-IRS-1 and p-Akt in adipose tissue. ND, normal diet; HFD, high-fat diet; Veh, vehicle. The relative quantity of proteins was analyzed using Quantity One software. A representative blot is shown and the data were expressed as mean ± SEM in each bar graph. *P < 0.05 (HFD/Veh versus ND/Veh). #P < 0.05 (HFD/TUDCA versus HFD/Veh)
Suppression of ER stress elevates adiponectin expression in tunicamycin-induced 3T3-L1 adipocytes
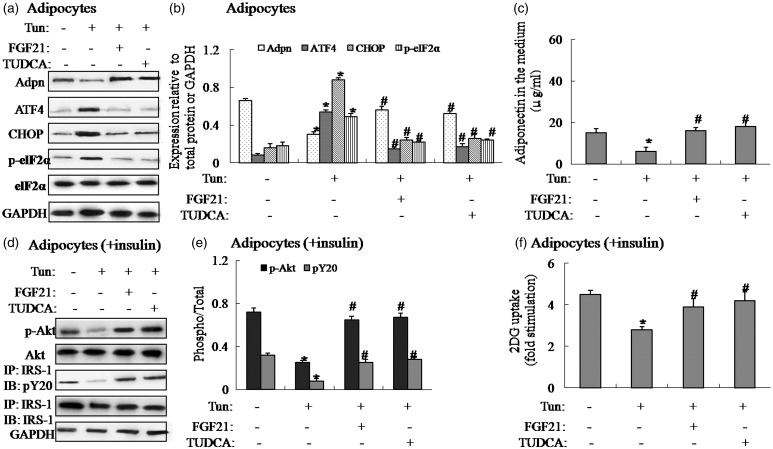
To determine the effect of ER stress on adiponectin expression in vitro, we treated 3T3-L1 adipocytes with tunicamycin, a ER stress inducer, to induce ER stress. Tunicamycin treatment in 3T3-L1 adipocytes resulted in activated ER stress, as demonstrated by increased protein expression of CHOP, ATF4 and the phosphorylation of eIF2α (Figure 4(a) and (b)), and decreased adiponectin expression in adipocytes and adiponectin levels in the medium (Figure 4(a)–(c)). Meanwhile, tunicamycin-induced adipocytes displayed impaired insulin signaling and glucose uptake (Figure 4(d)–(f)). Consistent with the results in vivo, inhibiting ER stress by addition of FGF21 or TUDCA in tunicamycin-induced adipocytes reversed the negative effect of tunicamycin (Figure 4(a)–(f)), suggesting FGF21 has a protective effect on downregulation of ER stress-induced adiponectin in vitro.
Figure 4.
Suppression of ER stress elevates adiponectin expression in tunicamycin-induced 3T3-L1 adipocytes. Pretreatment of 5 µg/mL tunicamycin (Tun) for 4 h was used to induce ER stress in 3T3-L1 adipocytes. The cells were cultured in the presence or absence of tunicamycin with or without 1 µg/mL FGF21 or 1 mmol/L TUDCA for 4 h. For insulin signaling, cells were stimulated with 10 nmol/L of insulin for 10 min. (a) Protein expression of adiponectin, ATF4, CHOP and phosphorylation of eIF2α in adipocytes. (b) The relative protein quantity of adiponectin (Adpn), ATF4, CHOP and phosphorylation of eIF2α in adipocytes. (c) Adiponectin in the medium. (d) Phosphorylation of IRS-1 and Akt in adipocytes. (e) The relative protein quantity of p-IRS-1 and p-Akt in adipocytes. (f) Glucose uptake. The relative quantity of proteins was analyzed using Quantity One software. A representative blot is shown and the data were expressed as mean ± SEM in each bar graph. *P < 0.05 (Tun versus Veh). #P < 0.05 (Tun/FGF21 or TUDCA versus Veh)
Discussion
Recent studies have proved the positive effects of FGF21 on insulin resistance in high-fat diet-induced obesity,12 but the underlying mechanisms remain elusive. In our study, the results showed that the administration of FGF21 reverses suppression of adiponectin expression and restores insulin signaling via inhibiting ER stress in adipose tissue of high-fat diet-induced obese mice and in tunicamycin-induced 3T3-L1 adipocytes, resulting in alleviation of insulin resistance. However, the intracellular events responsible for FGF21-mediated effects in insulin resistance are still complex. FGFR1 and the single-pass membrane protein b-klotho have been identified to act as the receptors of FGF21, which are highly expressed in adipocytes.13 Recent findings revealed that tunicamycin treatment reduced FGFR1 mRNA levels, but did not influence β-klotho mRNA levels,14 suggesting ER stress might result in the decline of FGF21 function via decreasing its receptor FGFR1 expression. Therefore, we extrapolated that increased circulating FGF21 levels in obesity could be a protective and decompensatory response to increased ER stress and impaired insulin signaling.
It has been proven that serum adiponectin levels are negatively correlated with insulin resistance.15 However, the underlying mechanisms remain unclear. Obesity exhibited activated ER stress and increased inflammatory cytokines expression, leading to reduced adiponectin expression.16,17 In accordance with these findings, our results also showed that suppression of ER stress elevates adiponectin expression in diet-induced obese mice and tunicamycin-induced 3T3-L1 adipocytes, demonstrating the causative role of ER stress in downregulating adiponectin levels.
Several clinical studies showed that the patients with obesity or type 2 diabetes exhibited increased serum FGF21 levels and reduced serum adiponectin levels,18,19 but likely multiple signaling could account for the link between FGF21 and adiponectin. Some study showed that over-expressing FGF21 in mice decreased circulating ceramide levels, while FGF21 knockout in mice increased circulating ceramide levels, which was strongly associated with adiponectin, resulting in regulation of glucose and lipid metabolism.20 On the basis of these results, large numbers of factors could regulate serum adiponectin levels and influence glucose and lipid metabolism during the development of insulin resistance,21 so FGF21, just one of the regulators, reverses suppression of adiponectin expression partially via inhibiting ER stress in adipose tissue and protects from insulin resistance in obese mice. Additionally, FGF21 resistance has been found in adipose tissue of obese mice,22 which may be the causative role of the progression of insulin resistance. Therefore, the insulin-like effects of FGF21 still need to further explore in vivo and in vitro.
In conclusion, our results showed that the administration of FGF21 reverses suppression of adiponectin expression and restored insulin signaling via inhibiting ER stress in adipose tissue of high-fat diet-induced obese mice. Although further studies need to address the insulin-like effects of FGF21 on glucose and lipid metabolism, our findings further verify the key role of FGF21 in treatment of obesity and type 2 diabetes.
Supplementary Material
Acknowledgments
This work was supported by the programs from the National Natural Science Foundation of China (no. 81370899, no. 81472038 and no.81500016), and the Young Innovators Awards of the First Affiliated Hospital of Xi’an Jiaotong University (2014YK3 and 2015YK6).
Authors’ contribution
BZ and QG designed and executed the experiments and drafted the manuscript. LX, JL, and HL conducted most of the experiments and contributed to manuscript preparation. BZ, HS, and SW contributed to the experimental design. All authors revised, edited, and approved the final version of the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, Gromada J, Brozinick JT, Hawkins ED, Wroblewski VJ, Li DS, Mehrbod F, Jaskunas SR, Shanafelt AB. FGF-21 as a novel metabolic regulator. J Clin Invest 2005; 115: 1627–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berglund ED, Li CY, Bina HA, Lynes SE, Michael MD, Shanafelt AB, Kharitonenkov A, Wasserman DH. Fibroblast growth factor 21 controls glycemia via regulation of hepatic glucose flux and insulin sensitivity. Endocrinology 2009; 150: 4084–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y, Moller DE, Kharitonenkov A. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 2008; 149: 6018–27. [DOI] [PubMed] [Google Scholar]
- 4.Kharitonenkov A, Larsen P. FGF21 reloaded: challenges of a rapidly growing field. Trends Endocrinol Metab 2011; 22: 81–6. [DOI] [PubMed] [Google Scholar]
- 5.Turer AT, Scherer PE. Adiponectin: mechanistic insights and clinical implications. Diabetologia 2012; 55: 2319–26. [DOI] [PubMed] [Google Scholar]
- 6.Shetty S, Kusminski CM, Scherer PE. Adiponectin in health and disease: evaluation of adiponectin-targeted drug development strategies. Trends Pharmacol Sci 2009; 30: 234–9. [DOI] [PubMed] [Google Scholar]
- 7.Lin Z, Tian H, Lam KS, Lin S, Hoo RC, Konishi M, Itoh N, Wang Y, Bornstein SR, Xu A, Li X. Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metab 2013; 17: 779–89. [DOI] [PubMed] [Google Scholar]
- 8.Zhou L, Liu M, Zhang J, Chen H, Dong LQ, Liu F. DsbA-L alleviates endoplasmic reticulum stress-induced adiponectin downregulation. Diabetes 2010; 59: 2809–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo Q, Shi Q, Li H, Liu J, Wu S, Sun H, Zhou B. Glycolipid metabolism disorder in the liver of obese mice is improved by TUDCA via the restoration of defective hepatic autophagy. Int J Endocrinol 2015; 2015: 687938–687938. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Zhou B, Li H, Liu J, Xu L, Guo Q, Zang W, Sun H, Wu S. Autophagic dysfunction is improved by intermittent administration of osteocalcin in obese mice. Int J Obes (Lond) 2016; 40: 833–43. [DOI] [PubMed] [Google Scholar]
- 11.Zhou B, Li H, Xu L, Zang W, Wu S, Sun H. Osteocalcin reverses endoplasmic reticulum stress and improves impaired insulin sensitivity secondary to diet-induced obesity through nuclear factor-kB signaling pathway. Endocrinology 2013; 154: 1055–68. [DOI] [PubMed] [Google Scholar]
- 12.Han S, Kim JD, Lee S, Jeong AL, Park JS, Yong HJ, Boldbaatar A, Ka HI, Rhee EJ, Lee WY, Yang Y. Circulating CTRP1 levels in type 2 diabetes and their association with FGF21. Int J Endocrinol 2016; 2016: 5479627–5479627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kharitonenkov A, Dunbar JD, Bina HA, Bright S, Moyers JS, Zhang C, Ding L, Micanovic R, Mehrbod SF, Knierman MD, Hale JE, Coskun T, Shanafelt AB. FGF-21/FGF-21 receptor interaction and activation is determined by betaKlotho. J Cell Physiol 2008; 215: 1–7. [DOI] [PubMed] [Google Scholar]
- 14.Jiang S, Yan C, Fang QC, Shao ML, Zhang YL, Liu Y, Deng YP, Shan B, Liu JQ, Li HT, Yang L, Zhou J, Dai Z, Liu Y, Jia WP. Fibroblast growth factor 21 is regulated by the IRE1α-XBP1 branch of the unfolded protein response and counteracts ER stress-induced hepatic steatosis. J Biol Chem 2014; 289: 29751–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindsay RS, Funahashi T, Hanson RL, Matsuzawa Y, Tanaka S, Tataranni PA, Knowler WC, Krakoff J. Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet 2002; 360: 57–8. [DOI] [PubMed] [Google Scholar]
- 16.Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, Matsuda M, Shimomura I. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 2007; 56: 901–11. [DOI] [PubMed] [Google Scholar]
- 17.Chen B, Lam KS, Wang Y, Wu D, Lam MC, Shen J, Wong L, Hoo RL, Zhang J, Xu A. Hypoxia dysregulates the production of adiponectin and plasminogen activator inhibitor-1 independent of reactive oxygen species in adipocytes. Biochem Biophys Res Commun 2006; 341: 549–56. [DOI] [PubMed] [Google Scholar]
- 18.Zhang X, Yeung DC, Karpisek M, Wu D, Lam MC, Shen J, Wong L, Hoo RL, Zhang J, Xu A. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes 2008; 57: 1246–53. [DOI] [PubMed] [Google Scholar]
- 19.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab 2001; 86: 1930–5. [DOI] [PubMed] [Google Scholar]
- 20.Holland WL, Adams AC, Brozinick JT, Bui HH, Miyauchi Y, Kusminski CM, Bauer SM, Wade M, Singhal E, Cheng CC, Volk K, Kuo MS, Gordillo R, Kharitonenkov A, Scherer PE. An FGF21-adiponectin-ceramide axis controls energy expenditure and insulin action in mice. Cell Metab 2013; 17: 790–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu M, Liu F. Transcriptional and post-translational regulation of adiponectin. Biochem J 2010; 425: 41–52. [DOI] [PubMed] [Google Scholar]
- 22.Fisher FM, Chui PC, Antonellis PJ, Bina HA, Kharitonenkov A, Flier JS, Maratos-Flier E. Obesity is a fibroblast growth factor 21 (FGF21)-resistant state. Diabetes 2010; 59: 2781–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.