Abstract
Teeth have long served as a model system to study basic questions about vertebrate organogenesis, morphogenesis, and evolution. In non-mammalian vertebrates, teeth typically regenerate throughout adult life. Fish have evolved a tremendous diversity in dental patterning in both their oral and pharyngeal dentitions, offering numerous opportunities to study how morphology develops, regenerates, and evolves in different lineages. Threespine stickleback fish (Gasterosteus aculeatus) have emerged as a new system to study how morphology evolves, and provide a particularly powerful system to study the development and evolution of dental morphology. Here we describe the oral and pharyngeal dentitions of stickleback fish, providing additional morphological, histological, and molecular evidence for homology of oral and pharyngeal teeth. Focusing on the ventral pharyngeal dentition in a dense developmental time course of lab-reared fish, we describe the temporal and spatial consensus sequence of early tooth formation. Early in development, this sequence is highly stereotypical and consists of seventeen primary teeth forming the early tooth field, followed by the first tooth replacement event. Comparing this detailed morphological and ontogenetic sequence to that described in other fish reveals that major changes to how dental morphology arises and regenerates have evolved across different fish lineages.
Keywords: tooth development, tooth replacement, tooth patterning, polyphyodonty, stickleback
Introduction
Teeth are a classic model system for understanding vertebrate development and evolution. Mice, a monophyodont (no tooth replacement) rodent, have served as the primary model for understanding tooth formation (Jernvall and Thesleff, 2000; Tucker and Sharpe, 2004; Bei, 2009; Tummers and Thesleff, 2009; O’Connell et al., 2012; Lan et al., 2014). Complementary systems are needed to understand tooth replacement in polyphyodonts (vertebrates with life-long tooth replacement, the primitive jawed vertebrate condition; Reif, 1982; Fraser et al., 2010; Brazeau and Friedman, 2014). Dental patterning has been studied in a variety of polyphyodonts in the context of comparative morphology, development, ecology, and evolution for decades (e.g. Owen, 1845; Osborn, 1971; Wake, 1976). Polyphyodonty has been variously modified in different extant gnathostome lineages and recent work in snakes (Buchtová et al., 2008; Handrigan and Richman, 2010; Gaete and Tucker, 2013), geckos (Handrigan et al., 2010), alligators (Wu et al., 2013), and other reptiles (Juuri et al., 2013) has begun to investigate the cellular and molecular mechanisms of tooth replacement. Fish offer a powerful system to study these mechanisms due to high numbers of offspring, external fertilization, rapid development, and rich diversity in tooth patterning (Evans and Deubler, 1955; Berkovitz, 1977; Wakita et al., 1977; Motta, 1984; Nakajima, 1987; Trapani and Schaefer, 2001; Bemis et al., 2005).
Teeth in fish can be located in the oral jaw (oral teeth) and/or internally in the branchial skeleton (pharyngeal teeth). The branchial skeleton, comprised of posterior segmental homologs of the mandibular and hyoid skeletons, is located in the throat of a fish and functions as an interface between fish and their food (Sibbing, 1991). Prey mastication and manipulation is typically performed by the pharyngeal jaw while oral teeth are primarily used to capture prey (Lauder, 1983; Hulsey et al., 2005; Wainwright, 2006). Oral and pharyngeal teeth have long been thought to be developmentally homologous (Owen, 1845), and have recently been shown to form via similar mechanisms involving similar gene expression patterns (Fraser et al., 2009). Furthermore, similar signaling pathways control tooth development in both fish and mammals (Fraser et al., 2013). For example, genes encoding the Ectodysplasin ligand and receptor, Eda and Edar, are required for oral and pharyngeal teeth in fish (Harris et al., 2008; Atukorala et al., 2011), and for oral teeth in mice and humans (Mikkola and Thesleff, 2003). While mammalian teeth are restricted to a single row along the dental arcade (Mikkola, 2009; Zhang et al., 2009), in non-mammalian vertebrates the tooth field is often much larger and contains multiple rows of teeth.
Numerous fish species have been used to study tooth patterning and replacement including zebrafish (Huysseune and Thesleff, 2004; Huysseune, 2006), cichlids (Huysseune, 1983; Fraser et al., 2013), trout (Fraser, Berkovitz, et al., 2006; Smith, Fraser, et al., 2009), medaka (Abduweli et al., 2014; Mantoku et al., 2015), and many others (Trapani and Schaefer, 2001; Smith, Okabe, et al., 2009; Moriyama et al., 2010; Fraser et al., 2012; Vandenplas et al., 2014; Underwood et al., 2015). These studies have uncovered differences in the location of tooth fields, the arrangement and number of teeth in tooth families, and the positioning and modes of tooth replacement. The spatiotemporal pattern of early tooth positioning within developing tooth fields has also been studied in many of these models (Huysseune et al., 1998; Van der heyden and Huysseune, 2000; Debiais-Thibaud et al., 2007; Stock, 2007; Le Pabic et al., 2009; Atukorala and Franz-Odendaal, 2014) with major differences found in the sequence and pattern of early tooth formation. Collectively, these studies highlight the diversity of tooth patterning and replacement mechanisms among teleosts.
The threespine stickleback fish, Gasterosteus aculeatus, offers an excellent model system to study the developmental and genetic basis of tooth development and replacement. Sticklebacks have undergone an adaptive radiation, with oceanic marine populations repeatedly colonizing and rapidly adapting to freshwater lakes and creeks throughout the northern hemisphere (Bell and Foster, 1994). A suite of morphological changes has evolved including a substantial increase in pharyngeal tooth number in some freshwater populations (Cleves et al., 2014; Miller et al., 2014; Ellis et al., 2015), likely adaptive to a shift in diet towards larger prey (Hynes, 1950; Lemmetyinen and Mankki, 1975). Evolved tooth gain arises late during larval development in two freshwater populations, and is associated with an increased tooth replacement rate in both populations (Cleves et al., 2014; Ellis et al., 2015). The natural variation present in dental patterning and replacement rates provides an entry point to study the developmental genetic basis of tooth regeneration. However, a detailed understanding of the early sequence of tooth formation and replacement is required to interpret the evolved changes arising later in development. Here we present a careful description of early stickleback pharyngeal tooth development and replacement to test three hypotheses about tooth formation and replacement. First, we find morphological and molecular support for the hypothesis of homology of oral and pharyngeal teeth. Second, focusing on the ventral pharyngeal tooth plate, we find support for the hypothesis that the early spatiotemporal pattern of tooth formation and replacement is stereotypical. Third, comparing this early sequence of tooth formation to that described for other species of fish supports the hypothesis that several changes in tooth field patterning have evolved in different fish lineages.
Materials and Methods
Stickleback husbandry
Threespine stickleback fish, Gasterosteus aculeatus (Linnaeus, 1758) were all lab-reared in brackish water (3.5 g/l Instant Ocean salt, 0.217 ml/l 10% sodium bicarbonate) at 18°C in 110 l aquaria. All freshwater fish were from the Paxton benthic population (British Columbia) (Schluter and McPhail, 1992) and marine fish were from either the Rabbit Slough (Alaska) or Little Campbell River (British Columbia) populations. Fish were fed live Artemia as fry, Artemia and frozen Daphnia as juveniles, and frozen bloodworms and Mysis shrimp as adults. All experiments were performed with approval of the Institutional Animal Care and Use Committee of the University of California-Berkeley (protocol # R330).
Skeletal staining and visualization
For skeletal staining, fish were fixed in 10% neutral buffered formalin overnight at 4°C, and washed in water. Juveniles and adults were stained in 0.008% Alizarin Red S in 1% KOH for 24 h. Early time course fish (8-23 days post fertilization) were stained with an acid-free Alizarin Red S and Alcian Blue two color protocol as described (Walker and Kimmel, 2007). Fish were rinsed in water and cleared in 50% glycerol, 0.25% KOH. Dissection and mounting was performed as previously described (Miller et al., 2014; Ellis and Miller, 2016). Brightfield images were taken on a Leica DM2500 except Fig. 1B which was taken on a Leica M165 stereomicroscope. Fluorescent images were taken on a Leica M165 using a rhodamine filter.
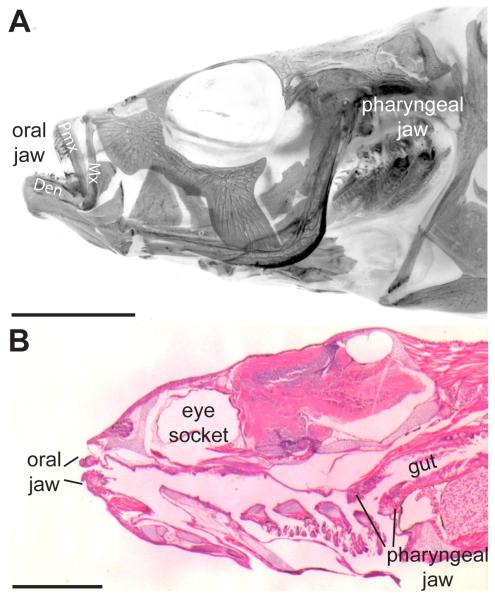
Fig. 1. Stickleback oral and pharyngeal jaws.
(A) Adult 6-month-old freshwater stickleback head (anterior to the left) stained with Alizarin red in whole-mount, imaged under fluorescence (color inverted) after removal of the opercle and subopercle. (B) Hematoxylin and eosin 6 μm sagittal section of 26 days post fertilization marine stickleback. Two sets of toothed jaws are present, the oral jaw in the mouth and the pharyngeal jaw at the back of the branchial skeleton, anterior to the gut. Mx = maxilla, Pmx = premaxilla, Den = dentary bone. Scale bars = 5 mm (A) and 500 μm (B).
To determine tooth positioning and the order of eruption, bilateral tooth plates in 53 Alizarin and Alcian stained (see above) freshwater fish ranging from 8-23 days post fertilization (dpf) were scored. To generate the consensus pattern, each tooth position was scored in individual fish. If at least 50% of the fish had the position filled, it was included for that time point. For two later time points (16 and 18dpf) with more variable standard lengths, fish ± one day were also considered to determine the consensus. See the supplementary materials for raw positioning data.
Histology
Tissue was processed and sectioned as previously described (Ellis et al., 2015). Briefly, samples were fixed overnight in 10% neutral buffered formalin (NBF) at 4°C and decalcified using Humason’s formic acid A (Humason, 1962) at a working concentration of 6% formic acid and 2.5% NBF (~1% formaldehyde). Histoclear (National Diagnostics) was used in the place of xylene as a clearing agent. Tissue was embedded in Paraplast (Fisher), sectioned with a Microm HM340E (Thermo Scientific), baked overnight at 50°C, stained with hematoxylin and eosin, and cover-slipped with Permount (Fisher). The number of samples was: n = 5 26 dpf marine specimens to image and compare oral and pharyngeal teeth; n = 14 freshwater (5 15 millimeters standard length (mm), 5 25 mm, and 4 40 mm) and n = 15 marine (5 15 mm, 6 25 mm, and 40 mm) specimens to characterize tooth replacement.
In situ hybridization
In situ hybridization was performed essentially as described (Xu et al., 1994; Fraser et al., 2008) with the following exceptions: samples were fixed overnight in 4% paraformaldehyde in 1X PBS with 1% DMSO at 4°C, digested with 20 μg/mL proteinase K for 30 minutes at room temperature, and blocked with 20% sheep serum, 2% Boehringer blocking reagent in maleic acid buffer with Tween-20. For sections, whole-mount in situs were embedded in gelatin-albumin cross-linked with 1.75% glutaraldehyde and sectioned at 40 μm on a Pelco 101 Vibratome Series 1000. Pitx2 and Bmp6 riboprobes have been published (Cleves et al., 2014). For each probe, at least four fish were analyzed and representative results presented.
Results
Location and homology of teeth in the threespine stickleback
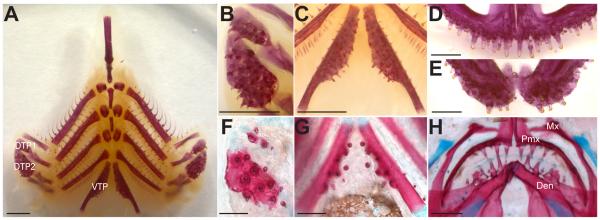
Two sets of jaws are present in threespine stickleback fish, one oral jaw and another at the posterior of the branchial skeleton, the pharyngeal jaw (Fig. 1A, B, Fig. 2A) (Swinnerton, 1902; Anker, 1974). In the oral jaw, teeth are restricted to the premaxilla (upper jaw, Fig. 2D) and the dentary (lower jaw, Fig. 2E). No teeth are present on the maxilla early in development (Fig. 2H) or late (Fig. 1A). Pharyngeal teeth are restricted ventrally to the fifth ceratobranchial (Fig. 2C), hereafter referred to as the ventral tooth plate (VTP), and dorsally to the 2nd and fused 3rd/4th pharyngobranchials (Fig. 2B), hereafter referred to as dorsal tooth plate 1 (DTP1) and dorsal tooth plate 2 (DTP2) respectively. These tooth fields are specified during embryonic development, and then teeth are added continuously from early larval pre-hatching stages throughout adult stages (compare Fig. 2F to 2B, 2G to 2C, and 2H to 2D,E).
Fig. 2. Oral and pharyngeal jaw morphology.
(A-E) Alizarin red stained, flat-mounted branchial skeleton (A-C) and oral jaw (D-E) from a 6-month-old freshwater adult stickleback. (B-C) Magnified view of left dorsal pharyngeal tooth plate 1 and 2 (B) and the ventral pharyngeal tooth plates (C). (D) Premaxilla (upper or dorsal oral jaw) dentition. (E) Dentary (lower or ventral oral jaw) dentition. (F-H) Tooth plates from Alizarin red and Alcian blue stained 20 days post fertilization freshwater larval stickleback. (F) Left dorsal pharyngeal tooth plate 1 and 2. (G) Ventral pharyngeal tooth plates. (H) Oral jaw containing both premaxillary and dentary dentition. DTP1 = dorsal tooth plate 1, DTP2 = dorsal tooth plate 2, VTP = ventral tooth plate, Mx = maxilla, Pmx = premaxilla, Den = dentary bone. Scale bars = 1 mm (A-C), 500 μm (D-E), and 100 μm (F-H).
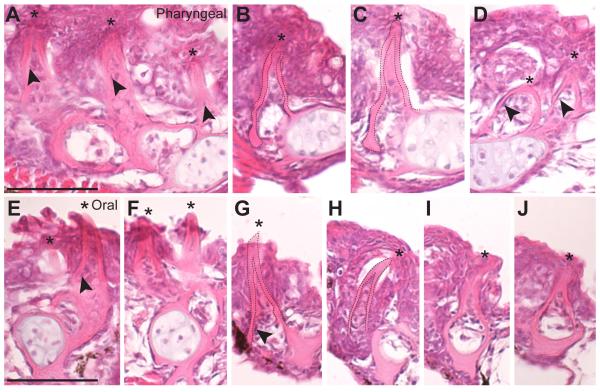
To test whether pharyngeal and oral teeth have similar cellular morphologies, we compared histological sections through both sets of jaws. Detailed comparisons of sections revealed pharyngeal and oral teeth are morphologically similar across the ventral tooth plate (Fig. 3A-C), dorsal tooth plate (Fig. 3D), dentary (Fig. 3E-F), and premaxilla (Fig. 3G-J) with each tooth mostly embedded in a mucosal layer and consisting of an inner dental pulp containing odontoblasts, a mineralized dentine cone, and an enameloid cap covering the tip (apparent in whole mount as yellow tinted tips in Fig. 2B-E). In section, pharyngeal and oral teeth appear morphologically indistinguishable.
Fig. 3. Oral and pharyngeal teeth are morphologically indistinguishable.
Hematoxylin and eosin stained 6 μm sagittal sections of pharyngeal (A-D) and oral (E-J) teeth on the ventral pharyngeal tooth plate (A-C), dorsal pharyngeal tooth plate 1 (D), dentary (E-F) and premaxilla (G-J). Asterisks mark the tips of teeth, all of which are unicuspid mineralized cones (dashed lines in B,C and G,H). Cells in the pulp cavity likely include presumptive odontoblasts (arrowheads). All sections are from a 26 days post fertilization marine fish. Scale bars = 50 μm.
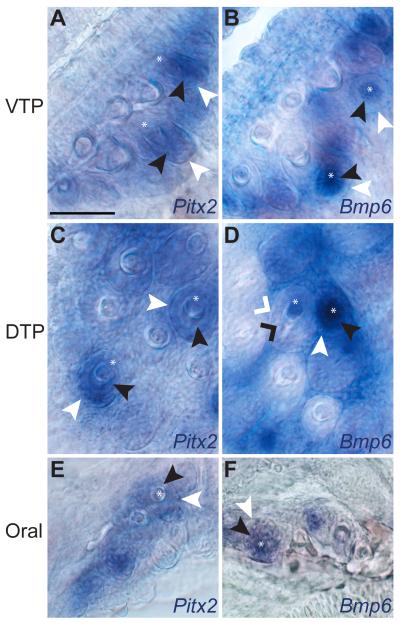
To test the hypothesis that similar gene expression patterns occur during stickleback pharyngeal and oral tooth development, we performed in situ hybridization with two known markers of tooth development. Pitx2, a marker of the inner and outer dental epithelium (Fraser et al., 2004, 2008; Fraser, Graham, et al., 2006), is expressed in both domains in all developing pharyngeal (Fig. 4A, C, Fig. S1A, B, B'), and oral teeth (Fig. 4E, Fig. S1A', A", B"). Bmp6, a marker dynamically expressed in both the inner dental epithelium and odontogenic mesenchyme of early developing tooth germs, but restricted to the dental mesenchyme in later stage tooth germs (Cleves et al., 2014), is also expressed in a similar pattern across all teeth (Fig. 4B,D,F, Fig. S1C, C', D, D'). Thus, as was found in trout (Fraser et al., 2004) and cichlids (Fraser et al., 2009), similar gene expression patterns are seen in developing stickleback oral and pharyngeal teeth.
Fig. 4. Oral and pharyngeal teeth have similar gene expression patterns during development.
Whole-mount in situ hybridization of Pitx2 (A, C, E) and Bmp6 (B, D, F) expression in the ventral pharyngeal tooth plate (VTP) (A, B), dorsal pharyngeal tooth plate (DTP) (C, D), and in the oral jaw (E, F) in 15 day post fertilization freshwater larvae. Pitx2 expression is detected in the inner and outer dental epithelium and not in dental mesenchyme. Bmp6 is detected in the inner (but not outer) dental epithelium and condensing dental mesenchyme in early tooth germs. After calcification begins, Bmp6 expression is only detected in dental mesenchyme (see panel D). Expression of both genes is detected in similar patterns in developing oral and pharyngeal tooth germs. Inner dental epithelium = black arrowheads (black caret on late stage), outer dental epithelium = white arrowheads (white caret on late stage), dental mesenchyme = black caret. Also see Fig. S1. Scale bar = 50 μm.
Spatial and temporal development of pharyngeal teeth
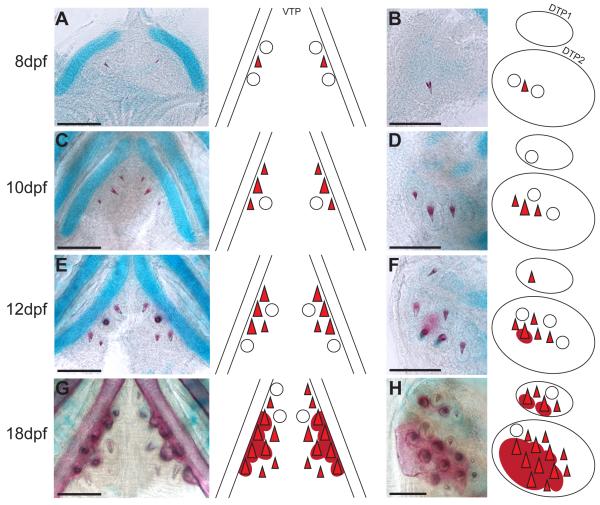
Tooth development begins prior to hatching with a pioneer tooth germ specified on VTP and DTP2 by 6 days post fertilization (dpf). Upon hatching at 8 dpf, both of these teeth are calcified and are flanked by two developing tooth germs (Fig. 5A, B). At 10 dpf, the first developing DTP1 tooth germ is visible and medial positions are added to both VTP and DTP2 (Fig. 5C, D). By 12 dpf, the first DTP1 tooth is calcified and individual teeth on VTP and DTP2 further ossify around the base of the tooth, forming the tooth plate (Fig. 5E, F). Both DTPs form juxtaposed and immediately ventral to the pharyngobranchial cartilages (Fig. S2A-B, white arrowhead). In contrast, VTP teeth form posterior and medial to chondrocytes of the fifth ceratobranchial (Fig. S2B). By 18 dpf, many teeth are calcified and the underlying tooth plate has formed by fusing the extended ossification around the base of teeth together (Fig. 5G, H). Notably, the DTP2 tooth plate extends medially past the field of developing teeth (Fig. 5H).
Fig. 5. Developmental time course of pharyngeal tooth formation.
In each panel, bilateral ventral pharyngeal tooth plates (A, C, E, G) or unilateral dorsal pharyngeal tooth plates 1 and 2 (B, D, F, H) of freshwater larvae are shown on the left, and a diagram depicting the tooth positions at each stage on the right. In all panels, anterior is towards the top. (A, B) 8 days post fertilization (dpf), (C, D) 10 dpf, (E, F) 12 dpf, and (G, H) 18 dpf tooth plates stained with Alizarin red and Alcian blue to label bone and cartilage, respectively. Open circles depict developing, but uncalcified tooth germs. Red triangles depict calcified, developing teeth and dark red circles beneath depict ossification of the tooth plate. Scale bars = 100 μm.
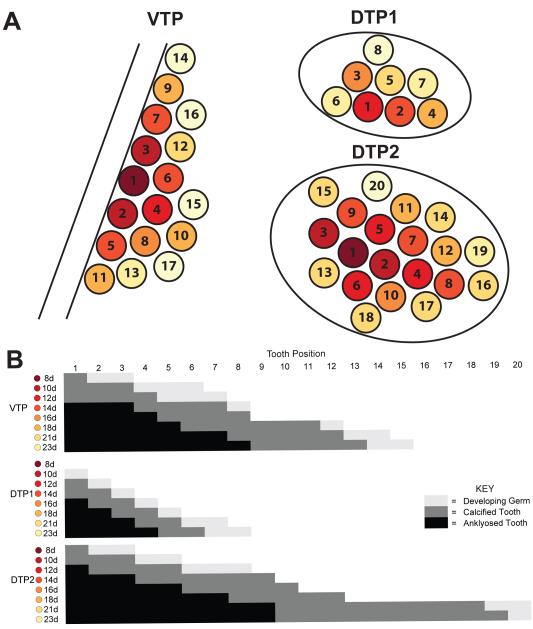
To test the hypothesis that the early sequence of tooth formation is hard-wired and stereotypical during early development, we scored each tooth position across a dense developmental time course of freshwater fish. Each position was ordered numerically by when a tooth typically arises in that position and scored as absent, developing germ (present, but uncalcified), calcified, or ankylosed (attached to the tooth plate). The pioneer tooth on each tooth plate was numbered as one. We generated a diagram summarizing the consensus sequence of tooth formation, with teeth shown as circles with colors ranging from dark red to light yellow corresponding to the day the individual tooth typically calcifies. For example, the pioneer tooth on VTP and DTP2 calcified at 8 dpf and is colored dark red while the DTP1 pioneer tooth did not calcify until 12 dpf and is colored lighter red (Fig. 6A).
Fig. 6. Sequence of pharyngeal tooth development.
(A) Unilateral (left side) tooth plate diagrams following the flat-mounted orientation from Fig. 2A. Each circle indicates the position where a tooth will form and each number corresponds to the order in which that position typically arises on each tooth plate. The color scheme follows the consensus sequence (B) and is color-coded dark red to light yellow for each day of development. Circles are colored according to the day they typically first ossify. (B) Consensus sequence of the number of teeth present across early development. Each position is scored as no tooth (white), developing germ (light gray), calcified tooth (dark gray), and ankylosed tooth (black) for each day. See Supplementary Materials for raw data. Note that the VTP diagram is a dorsal view and the DTP diagrams are a ventral view, as the tooth plates appear in flat-mounts (as in Figures 2 and 5).
The order of teeth developing on the tooth plate was highly stereotypical early and became more variable with later positions. Focusing on the VTP, the order of tooth formation and ankylosing to the tooth plate was invariant through position 7. However, here position 6 arose and calcified before position 7, but position 7 ankylosed to the tooth plate first, suggesting the sequences of tooth calcification and ankylosing to the tooth plate are different. Also at this stage, around 12 dpf, symmetry broke down between the left and right tooth plates where each side occasionally had a different number of calcified teeth. After 12 dpf and after position 7, the order of teeth arising had more variation. However, by scoring an additional 35 fish at time points between 13 and 23 dpf, a consensus emerged (Fig. 6B). For example, position 10 generally formed before position 11, but this was not always the case (see Supplementary Materials) and the sequence of later positions was even more variable. Qualitatively, an early marine time course appears similar in patterning through 20dpf (pers. obs.)
Pharyngeal tooth replacement in sticklebacks
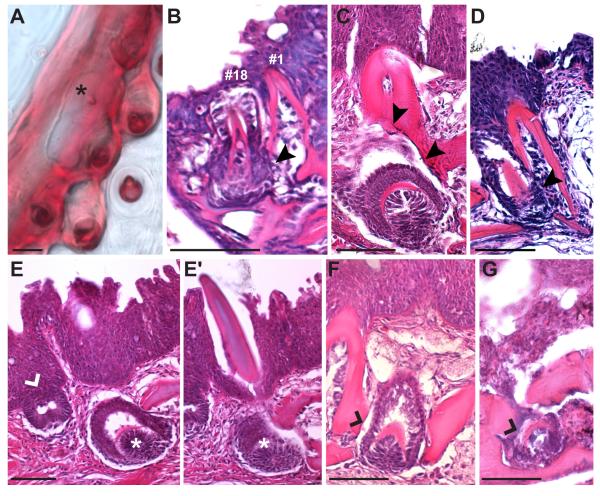
To examine the histological basis of tooth replacement, we cut serial sections through developing tooth plates and focused on the VTP. We used four histological criteria to distinguish primary from replacement teeth. First, replacement teeth appear at the base of an existing tooth, and asymmetrically erode bone away from the base of the primary tooth they are replacing (Wakita et al., 1977). Second, primary teeth form superficially, near the buccal cavity, while replacement teeth form deeper in the tooth plate tissue. Third, primary teeth form extraosseously (outside of bone; Trapani and Schaefer, 2001), while replacement teeth form intraosseously in sticklebacks (Huysseune and Witten, 2006). Fourth, the epithelium of replacement teeth appears locally connected to the epithelium of the tooth they are replacing, and extends deep into the tooth plate tissue (Huysseune and Thesleff, 2004; Huysseune, 2006).
Using these criteria, seventeen primary teeth typically form on the tooth plate before the first replacement tooth forms laterally to position one (Fig. 7A). In section, this replacement tooth formed intraosseously within the fifth ceratobranchial and immediately adjacent to position one. The base of the tooth located at position one was eroded on the side of the new replacement tooth (Fig. 7B), a phenomenon also seen in adult stage tooth replacement events (Fig. 7C, D). Other late stage replacement teeth also form intraosseously (Fig. 7F, G). While primary teeth form at the interface of the epithelium and mesenchyme (Fig. 7E), replacement teeth formed deep in the mesenchyme (Fig. 7E, E'). However, the epithelium of all replacement teeth was continuous with the lumenal pharyngeal epithelium in adjacent serial sections (see Fig. S3). The first replacement tooth on VTP erupted between ~25-30 dpf while the first DTP2 replacement tooth erupted between ~20-24 dpf.
Fig. 7. Histological sections of tooth replacement.
(A) Whole-mount 29 days post fertilization Alizarin red stained freshwater ventral pharyngeal tooth plate with position #1 and position #2 shed and the first replacement tooth arising (black asterisk). (B) Hematoxylin and eosin section through the first ventral pharyngeal tooth replacement event in a 30 day marine larvae. The eighteenth tooth to form is the replacement of the initial pioneer tooth forming lateral to and at the base of position one, within the fifth ceratobranchial bone. Bone resorption (black arrowheads) has occurred at the base of position one adjacent to the replacement tooth. (C,D) Examples of adult tooth replacement occurring similarly. Bone resorption is present on the side with the replacement tooth (black arrowheads). (E) Primary tooth germs form at the interface of the epithelium and the mesenchyme (left tooth germ, white caret), replacement teeth form deep in the mesenchyme (right germ, white asterisks), but with deeply invaginating epithelium that is continuous with the lumenal pharyngeal epithelium (see Fig. S3). (E') Adjacent serial section showing the adult tooth above the replacement germ (white asterisks). (F, G) Stickleback replacement teeth form intraosseously, though not completely encased in bone (tooth germs marked with black caret). (C-F) Adult freshwater, (G) adult marine. Scale bars = 50 μm.
Discussion
Location and use of stickleback teeth
Fish retain the primitive jawed vertebrate condition of constant tooth regeneration (Reif, 1982; Fraser et al., 2010; Brazeau and Friedman, 2014) and provide model systems to study tooth replacement. Toothed locations are highly modular and variable across teleosts. While some fish have teeth covering most bones of their branchial skeletons (Liem and Greenwood, 1981), others, such as zebrafish, have lost all oral and dorsal pharyngeal teeth and rely solely on the ventral pharyngeal tooth plate attached to the fifth ceratobranchial (Stock, 2007). As previously described (Swinnerton, 1902; Anker, 1974) sticklebacks have teeth on the premaxilla and dentary in the oral jaw, and on the fifth ceratobranchial and the 2nd and fused 3rd/4th pharyngobranchials in the branchial skeleton.
Variation in tooth number has been identified in the oral premaxillary (upper jaw) (Caldecutt et al., 2001), ventral pharyngeal (Cleves et al., 2014; Miller et al., 2014; Ellis et al., 2015), and dorsal pharyngeal (Ellis et al., 2015) dentitions in sticklebacks. Variation in pharyngeal tooth number is heritable, and appears to correlate with trophic niche, as benthic lake and creek freshwater populations are higher toothed than oceanic populations (Cleves et al., 2014; Ellis et al., 2015). Stickleback feeding kinematics suggest the oral jaw is primarily used for prey capture and suction feeding (McGee et al., 2013). Instead, food mastication likely involves the pharyngeal jaw and teeth. In cichlid fish, evolved changes in pharyngeal jaw morphology (Meyer, 1990; Huysseune, 1995) also correlate with trophic niche (Muschick et al., 2012), suggesting pharyngeal jaw patterning is likely an important ecological trait. Tooth number in cichlids has a phenotypically plastic component in response to diet (Huysseune, 1995). It remains to be determined whether shifts in diet can result in phenotypically plastic changes in tooth patterning in sticklebacks.
Oral and pharyngeal tooth homology
Stickleback pharyngeal teeth resemble oral teeth morphologically in whole-mount and in histological sections, and molecularly by gene expression patterns. These morphological and molecular parallels further support the classically proposed homology between oral and pharyngeal teeth (Owen, 1845), also supported by recent developmental genetic studies. For example, both oral and pharyngeal tooth formation require Ectodysplasin signaling (reviewed in Sadier et al., 2014). Furthermore, ectopic expression of Ectodysplasin is remarkably sufficient to restore dorsal pharyngeal tooth formation in zebrafish (Aigler et al., 2014). A previous study found evidence for the conservation of a dental gene network between oral and pharyngeal teeth in two species of cichlids, M. zebra and L. fuelleborni (Fraser et al., 2009). In sticklebacks, Pitx2, Bmp6, and Shh are expressed in developing pharyngeal teeth (Cleves et al., 2014). Here we show Pitx2 and Bmp6 are also expressed in similar patterns in developing stickleback oral teeth. Pitx2 and Shh are also expressed in epithelia of developing pharyngeal teeth in zebrafish (Jackman et al., 2004, 2010) and in developing oral teeth in Mexican tetra (Stock et al., 2006). Interestingly, two transcriptional enhancers have been identified which drive expression in all developing oral and pharyngeal teeth. A 4 kb enhancer of the zebrafish dlx2b gene drives expression in teeth in both sets of jaws in tetras, despite zebrafish not having oral teeth (Jackman and Stock, 2006). Similarly, in sticklebacks, a 190 bp Bmp6 enhancer drives expression in all teeth in both sets of jaws (Erickson et al., 2015). In axolotls, oral tooth epithelia have been shown to be derived from the ectoderm, endoderm, or even mixed ecto/endoderm origin within a single tooth, suggesting the embryonic source of the dental epithelium can be either ectoderm or endoderm, and further supporting homology of oral and pharyngeal teeth (Soukup et al., 2008). Collectively the current and these former studies strongly support developmental homology between oral and pharyngeal teeth.
Initial patterning of pharyngeal dentition
Compared to other fish such as medaka where adult teeth are in a regular array (Debiais-Thibaud et al., 2007; Abduweli et al., 2014) the adult pharyngeal dentition seems relatively unorganized in sticklebacks. However, the early sequence of tooth formation follows a highly stereotypical pattern. Initially, a pioneer tooth arises on each pharyngeal tooth plate. Subsequent teeth then form in a stereotypical sequence around this pioneer tooth. The pioneer tooth is calcified by 8 dpf on VTP and DTP2 while DTP1 calcifies by 12 dpf. The second tooth forms posterior and lateral to position one with the third tooth forming anterior and medial to position one in quick succession. This initial pattern of three teeth is conserved across all three pharyngeal tooth plates with a slight shift of position 3 on DTP1. After the fourth tooth forms, each tooth plate follows a unique pattern with no two consecutive teeth forming next to one another. Several similarities in extended positions are shared between DTP1 and DTP2. Notably, position 1-5, and 7 all are generally conserved while position 6 and 8 form in a different spatial location (see Fig. 6). As tooth number and complexity of the pattern increases, more variation is present in the order of eruption of teeth, however each discrete position is present (see Supplementary Materials). Variation in eruption times in humans also increases at later developmental stages (Parner et al., 2001; Woodroffe et al., 2010).
Comparing the stickleback early tooth formation sequence in the ventral pharyngeal dentition to the sequence described in other fish reveals some significant evolved differences in how the tooth field in different fish arises. In zebrafish (D. rerio), the first four teeth follow the same spatial pattern as sticklebacks (Van der heyden and Huysseune, 2000). However in this system, the fourth tooth to form (termed 4V3) is described as a replacement of the pioneer tooth (termed 4V1). Additionally, the first row to form is the medially most row (termed ventral) and subsequent primary positions form 2 distinct rows lateral to the pioneer tooth in zebrafish (termed mediodorsal and dorsal rows) (Van der heyden and Huysseune, 2000; Huysseune and Witten, 2006) (see Nakajima, 1984, 1987; Stock, 2007 for discussion of other cypriniform patterns). In stark contrast, all stickleback subsequent primary positions form medially to the pioneer tooth. The only teeth that form lateral to the pioneer tooth in sticklebacks are replacements for the primary teeth adjacent to the fifth ceratobranchial. Compared to the Mexican tetra (A. mexicanus), the first 2 positions match. However, the third tooth in Mexican tetra instead forms medially and all subsequent teeth form medially or posteriorly to the pioneer tooth (Atukorala and Franz-Odendaal, 2014) whereas sticklebacks continue to add teeth anteriorly as well. Medaka (O. latipes) early pharyngeal tooth patterning is quite similar to the early stickleback positions. Positions 1-5 are spatially identical but temporally different, with positions 2 and 3 having opposite sequence of formation relative to sticklebacks. Despite this early similarity, the medaka pharyngeal dentition resolves into discrete rows late in development (Debiais-Thibaud et al., 2007), which is not seen in sticklebacks. Thus, within the ventral pharyngeal jaw, different fish species have evolved changes in (1) the number of primary teeth that form before replacement occurs, (2) the spatial sequence of tooth addition in both the mediolateral and anteroposterior directions, (3) the temporal sequence of early tooth position formation, and (4) the regularity of tooth row formation.
Early tooth patterning data for the dorsal pharyngeal tooth plates is more rare, likely in part due to their absence in many cypriniforms (Stock, 2007). In medaka, the first four dorsal teeth form in the same spatial pattern as the first four teeth in stickleback DTP2. The fifth medaka tooth, referred to as the ‘second tooth ridge’ forms more medially, but in close proximity to the fifth stickleback position. The next few medaka positions continue these two mediolateral ‘ridges’ or rows of teeth, while the next few stickleback positions form across the tooth field in an anteroposterior direction as well (Debiais-Thibaud et al., 2007). Compared to the Mexican tetra, the first two dorsal positions again match the stickleback positions with the next few tetra positions all forming posteriorly (Atukorala and Franz-Odendaal, 2014) while sticklebacks add tooth positions anteriorly as well. In one species of cichlid (Astatotilapia elegans) with the dorsal tooth pattern recorded, positions after the pioneer tooth form anterolateral through 20 dpf (Huysseune, 1983), the opposite direction of the tetra. These early similarities yet late differences highlight the diversity of developmental programs governing teleost pharyngeal tooth patterning. Future studies will address the underlying molecular genetic and cellular bases of these programs, and how evolved changes in establishing and replacing these tooth fields arise.
Tooth replacement
Similar to cichlids (Huysseune, 1995; Fraser et al., 2013) and rainbow trout (Fraser, Berkovitz, et al., 2006), stickleback tooth replacement appears to occur on a one-for-one basis, where a replacement tooth directly replaces a primary tooth and is not connected to an extended tooth family with other replacement teeth already forming. A new epithelial down growth, termed the successional dental lamina, forms for each replacement tooth, budding off the reduced enamel organ of the predecessor tooth (Huysseune, 2006). The dental lamina in sticklebacks appears discontinuous (separate invagination for each tooth family) and nonpermanent (reforms for each individual replacement tooth; after the definitions in Reif, 1982): some but not all positions have a detectable dental lamina at a given time point. This pattern of replacement is dissimilar to other teleosts such as zebrafish and medaka where multiple replacements have been proposed to be present for a single position (Van der heyden and Huysseune, 2000; Abduweli et al., 2014).
First generation teeth form extraosseously (outside the bone) in teleosts, while replacement teeth can from either extraosseously or intraosseously (reviewed in Trapani and Schaefer, 2001). First generation teeth arise individually from the pharyngeal epithelium in zebrafish, while replacement teeth form in crypts associated with the erupted primary tooth (Huysseune et al., 1998; Huysseune and Thesleff, 2004; Huysseune, 2006; Stock, 2007). These replacement teeth form extraosseously in zebrafish, but intraosseously in cichlids (Huysseune et al., 1994; Huysseune and Witten, 2006; Fraser et al., 2013). In sticklebacks, primary teeth form extraosseously at the interface of the epithelium and the mesenchyme. Replacement teeth in sticklebacks form intraosseously, surrounded by a bony crypt, though not fully encased on all sides (see Fig. 7F, G and Fig. S3). Replacement teeth form deep in the tooth plate, connected to but several cell diameters away from the pharyngeal epithelium, below or adjacent to the predecessor tooth. While stickleback replacement teeth share an epithelium with the tooth they will replace (see Fig. 7), this epithelium extends directly to the lumenal pharyngeal epithelium (see Fig. S3). The replacement may displace or arise next to the original tooth. The pattern of tooth replacement appears to initially follow the primary tooth eruption pattern with position one generally replacing first and positions 2 and 3 following. The early sequence and pattern of stickleback tooth development and replacement described here will facilitate future developmental genetic studies of tooth formation and replacement, as well as provide another comparison for understanding how dental morphology forms and is regenerated throughout development, and is modified during evolution.
Supplementary Material
Fig. S1: Pitx2 and Bmp6 tooth expression in section and whole mount.
In situ hybridization for Pitx2 (A-A", B-B") and Bmp6 (C, C', D, D') in section (A-D) and whole mount (A'-D', A', B'). Tooth developmental stages are vertically matched for early germs (A, A', C, C') and late germs (B, B', D, D') for pharyngeal (A-D, A’-D’) and oral (A”, B”) teeth. Pitx2 expression is detected in the dental epithelium (A-B, A'-B', A"-B") while Bmp6 expression is detected in the inner dental epithelium and mesenchyme early (C, C'), and appears restricted to the mesenchyme only late (D, D'). The black dotted line outlines the developing tooth germ while the white dotted line separates the inner and outer dental epithelium (when discernable). Black arrowheads denote mineralized teeth and white asterisks denote mesenchyme. Scale bar = 15 μm.
Fig. S2: Dorsal pharyngeal tooth plates develop on pharyngobranchial cartilage templates.
(A) Alizarin red (bone) and Alcian blue (cartilage) stained 11 days post fertilization (dpf) marine dorsal pharyngeal tooth plates. The pharyngobranchial cartilages chondrify before dorsal pharyngeal teeth form adjacently. (B) Transverse H&E sections of 20 dpf marine dorsal and ventral pharyngeal tooth plates. The dorsal pharyngeal tooth plate contains chondrocytes (white arrowhead) and teeth ossify directly on the thin layer of perichondral bone surrounding them (white arrow). The fifth ceratobranchial contains chondrocytes (black arrowhead), but the ventral pharyngeal tooth plate does not (black arrow), instead forming from subsequent ossification ventral to individual teeth. Note the medial tooth germ not surrounded by bone (black caret). Scale bars = 50 μm.
Fig. S3: Replacement teeth form deep but with invaginated epithelia that is continuous with lumenal pharyngeal epithelium. H&E stained 6 μm serial sections from adult sticklebacks showing pharyngeal teeth forming deep in the mesenchyme with an epithelial connection to the lumenal pharyngeal epithelium. (A-A", B-B", C-C", D-D") Each series indicates an individual tooth germ forming deep in the mesenchyme (A, B, C, D), yet with a clear epithelial connection to the lumenal pharyngeal epithelium in subsequent serial sections (A', A", B', B", C', C", D', D". (A) Arrowhead indicates the stratum compactum, the boundary between the pharyngeal epithelium and the underlying connective tissue. Scale bar is 50 μm and applies to all panels. (A-C) Adult freshwater, (D) adult marine. Note B and C' appear in Fig. 7C and Fig. 7F respectively.
Acknowledgements
We thank Priscilla Erickson and Phillip Cleves for generating and dissecting a subset of fish, Marvalee Wake for sectioning and decalcification suggestions, the He lab for assistance staining slides, and Gareth Fraser for advice on in situ hybridization.
Funding
This work was supported by the National Institutes of Health (NIH) [R01-DE021475 to C.T.M.]; National Science Foundation (NSF) Graduate Research Fellowship (N.A.E.) and an Achievement Rewards for College Scientists (ARCS) Fellowship (N.A.E.). Deposited in PMC for release after 12 months.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
N.A.E. and C.T.M. conceived and designed the experiments; N.A.E. and N.N.D. performed the experiments; N.A.E., N.N.D., and C.T.M. analyzed the data; N.A.E. and C.T.M. wrote the manuscript, with input from all authors.
References
- Abduweli D, Baba O, Tabata MJ, Higuchi K, Mitani H, Takano Y. Tooth replacement and putative odontogenic stem cell niches in pharyngeal dentition of medaka (Oryzias latipes) Microscopy. 2014:1–13. doi: 10.1093/jmicro/dft085. [DOI] [PubMed] [Google Scholar]
- Aigler SR, Jandzik D, Hatta K, Uesugi K, Stock DW. Selection and constraint underlie irreversibility of tooth loss in cypriniform fishes. Proc Natl Acad Sci U S A. 2014;111:7707–7712. doi: 10.1073/pnas.1321171111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker GC. Morphology and kinetics of the head of the stickleback, Gasterosteus aculeatus. Trans Zool Soc London. 1974;32:311–416. [Google Scholar]
- Atukorala ADS, Franz-Odendaal TA. Spatial and temporal events in tooth development of Astyanax mexicanus. Mech Dev. 2014;134:42–54. doi: 10.1016/j.mod.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Atukorala ADS, Inohaya K, Baba O, Tabata MJ, Ratnayake RARK, Abduweli D, Kasugai S, Mitani H, Takano Y. Scale and tooth phenotypes in medaka with a mutated ectodysplasin-A receptor: implications for the evolutionary origin of oral and pharyngeal teeth. Arch Histol Cytol. 2011;73:139–148. doi: 10.1679/aohc.73.139. [DOI] [PubMed] [Google Scholar]
- Bei M. Molecular genetics of tooth development. Curr Opin Genet Dev. 2009;19:504–510. doi: 10.1016/j.gde.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell M, Foster S. The Evolutionary Biology of the Threespine Stickleback. Oxford University Press; New York: 1994. [Google Scholar]
- Bemis WE, Giuliano A, McGuire B. Structure, attachment, replacement and growth of teeth in bluefish, Pomatomus saltatrix (Linnaeus, 1776), a teleost with deeply socketed teeth. Zoology. 2005;108:317–327. doi: 10.1016/j.zool.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Berkovitz B. The order of tooth development and eruption in the rainbow trout (Salmo gairdneri) J Exp Zool. 1977:221–225. doi: 10.1002/jez.1401930211. [DOI] [PubMed] [Google Scholar]
- Brazeau MD, Friedman M. The characters of Palaeozoic jawed vertebrates. Zool J Linn Soc. 2014;170:779–821. doi: 10.1111/zoj.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchtová M, Handrigan GR, Tucker AS, Lozanoff S, Town L, Fu K, Diewert VM, Wicking C, Richman JM. Initiation and patterning of the snake dentition are dependent on Sonic hedgehog signaling. Dev Biol. 2008;319:132–145. doi: 10.1016/j.ydbio.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Caldecutt WJ, Bell MA, Buckland-Nicks JA. Sexual dimorphism and geographic variation in dentition of threespine stickleback, Gasterosteus aculeatus. Copeia. 2001;4:936–944. [Google Scholar]
- Cleves PA, Ellis NA, Jimenez MT, Nunez SM, Schluter D, Kingsley DM, Miller CT. Evolved tooth gain in sticklebacks is associated with a cis-regulatory allele of Bmp6. Proc Natl Acad Sci. 2014;111:13912–13917. doi: 10.1073/pnas.1407567111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiais-Thibaud M, Borday-Birraux V, Germon I, Bourrat F, Metcalfe CJ, Casane D, Laurenti P. Development of oral and pharyngeal teeth in the medaka (Oryzias latipes): comparison of morphology and expression of eve1 gene. J Exp Zool. 2007;708:693–708. doi: 10.1002/jez.b.21183. [DOI] [PubMed] [Google Scholar]
- Ellis NA, Glazer AM, Donde NN, Cleves PA, Agoglia RM, Miller CT. Distinct developmental and genetic mechanisms underlie convergently evolved tooth gain in sticklebacks. Development. 2015;142:2442–2451. doi: 10.1242/dev.124248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis NA, Miller CT. Dissection and flat-mounting of the threespine stickleback branchial skeleton. J Vis Exp. 2016:e54056. doi: 10.3791/54056. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson PA, Cleves PA, Ellis NA, Schwalbach KT, Hart JC, Miller CT. A 190 base pair, TGF-β responsive tooth and fin enhancer is required for stickleback Bmp6 expression. Dev Biol. 2015;401:310–323. doi: 10.1016/j.ydbio.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans HE, Deubler EE. Pharyngeal tooth replacement in Semotilus atromaculatus and Clinostomus elongatus, two species of cyprinid fishes. Copeia. 1955;1:31–41. [Google Scholar]
- Fraser G, Graham A, Smith M. Developmental and evolutionary origins of the vertebrate dentition: molecular controls for spatio-temporal organisation of tooth sites in osteichthyans. J Exp Zool. 2006;306:183–203. doi: 10.1002/jez.b.21097. [DOI] [PubMed] [Google Scholar]
- Fraser GJ, Berkovitz BK, Graham A, Smith MM. Gene deployment for tooth replacement in the rainbow trout (Oncorhynchus mykiss): a developmental model for evolution of osteichthyan dentition. Evol Dev. 2006;8:446–457. doi: 10.1111/j.1525-142X.2006.00118.x. [DOI] [PubMed] [Google Scholar]
- Fraser GJ, Bloomquist RF, Streelman JT. A periodic pattern generator for dental diversity. BMC Biol. 2008;6:32. doi: 10.1186/1741-7007-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser GJ, Bloomquist RF, Streelman JT. Common developmental pathways link tooth shape to regeneration. Dev Biol. 2013;377:399–414. doi: 10.1016/j.ydbio.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser GJ, Britz R, Hall A, Johanson Z, Smith MM. Replacing the first-generation dentition in pufferfish with a unique beak. Proc Natl Acad Sci U S A. 2012:2–7. doi: 10.1073/pnas.1119635109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser GJ, Cerny R, Soukup V, Bronner-Fraser M, Streelman JT. The odontode explosion: the origin of tooth-like structures in vertebrates. BioEssays. 2010;32:808–817. doi: 10.1002/bies.200900151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser GJ, Graham A, Smith MM. Conserved deployment of genes during odontogenesis across osteichthyans. Proc Biol Sci. 2004;271:2311–2317. doi: 10.1098/rspb.2004.2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser GJ, Hulsey CD, Bloomquist RF, Uyesugi K, Manley NR, Streelman JT. An ancient gene network is co-opted for teeth on old and new jaws. PLoS Biol. 2009;7:e1000031. doi: 10.1371/journal.pbio.1000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaete M, Tucker AS. Organized emergence of multiple-generations of teeth in snakes is dysregulated by activation of Wnt/beta-catenin signalling. PLoS One. 2013;8:e74484. doi: 10.1371/journal.pone.0074484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handrigan GR, Leung KJ, Richman JM. Identification of putative dental epithelial stem cells in a lizard with life-long tooth replacement. Development. 2010;137:3545–3549. doi: 10.1242/dev.052415. [DOI] [PubMed] [Google Scholar]
- Handrigan GR, Richman JM. A network of Wnt, hedgehog and BMP signaling pathways regulates tooth replacement in snakes. Dev Biol. 2010;348:130–141. doi: 10.1016/j.ydbio.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Harris MP, Rohner N, Schwarz H, Perathoner S, Konstantinidis P, Nüsslein-Volhard C. Zebrafish eda and edar mutants reveal conserved and ancestral roles of Ectodysplasin signaling in vertebrates. PLoS Genet. 2008;4:e1000206. doi: 10.1371/journal.pgen.1000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsey CD, Fraser GJ, Streelman JT. Evolution and development of complex biomechanical systems: 300 million years of fish jaws. Zebrafish. 2005;2:243–257. doi: 10.1089/zeb.2005.2.243. [DOI] [PubMed] [Google Scholar]
- Humason G. Animal Tissue Techniques. W. H. Freeman and Company; San Francisco: 1962. [Google Scholar]
- Huysseune A. Observations on tooth development and implantation in the upper pharyngeal jaws in Astatotilapia elegans (Teleostei, Cichlidae ) J Morphol. 1983;175:217–234. doi: 10.1002/jmor.1051750302. [DOI] [PubMed] [Google Scholar]
- Huysseune A. Phenotypic plasticity in the lower pharyngeal jaw dentition of Astatoreochromis alluaudi (Teleostei: Cichlidae) Arch Oral Biol. 1995;40:1005–1014. doi: 10.1016/0003-9969(95)00074-y. [DOI] [PubMed] [Google Scholar]
- Huysseune A. Formation of a successional dental lamina in the zebrafish (Danio rerio): support for a local control of replacement tooth initiation. Int J Dev Biol. 2006;50:637–643. doi: 10.1387/ijdb.052098ah. [DOI] [PubMed] [Google Scholar]
- Huysseune A, Sire JY, Meunier FJ. Comparative-study of lower pharyngeal jaw structure in two phenotypes of Astatoreochromis alluaudi (Teleostei, Cichlidae) J Morphol. 1994;221:25–43. doi: 10.1002/jmor.1052210103. [DOI] [PubMed] [Google Scholar]
- Huysseune A, Thesleff I. Continuous tooth replacement: the possible involvement of epithelial stem cells. Bioessays. 2004;26:665–671. doi: 10.1002/bies.20039. [DOI] [PubMed] [Google Scholar]
- Huysseune A, Van der heyden C, Sire J-Y. Early development of the zebrafish (Danio rerio) pharyngeal dentition (Teleostei, Cyprinidae) Anat Embryol (Berl) 1998;198:289–305. doi: 10.1007/s004290050185. [DOI] [PubMed] [Google Scholar]
- Huysseune A, Witten PE. Developmental mechanisms underlying tooth patterning in continuously replacing osteichthyan dentitions. J Exp Zool Part B Mol Dev Evol. 2006;306:204–215. doi: 10.1002/jez.b.21091. [DOI] [PubMed] [Google Scholar]
- Hynes HBN. The food of fresh-water sticklebacks (Gasterosteus aculeatus and Pygosteus pungitius), with a review of methods used in studies of the food of fishes. J Anim Ecol. 1950;19:36–58. [Google Scholar]
- Jackman WR, Draper BW, Stock DW. Fgf signaling is required for zebrafish tooth development. Dev Biol. 2004;274:139–157. doi: 10.1016/j.ydbio.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Jackman WR, Stock DW. Transgenic analysis of Dlx regulation in fish tooth development reveals evolutionary retention of enhancer function despite organ loss. Proc Natl Acad Sci U S A. 2006;103:19390–19395. doi: 10.1073/pnas.0609575103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman WR, Yoo JJ, Stock DW. Hedgehog signaling is required at multiple stages of zebrafish tooth development. BMC Dev Biol. 2010;10:119. doi: 10.1186/1471-213X-10-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernvall J, Thesleff I. Reiterative signaling and patterning during mammalian tooth morphogenesis. Mech Dev. 2000;92:19–29. doi: 10.1016/s0925-4773(99)00322-6. [DOI] [PubMed] [Google Scholar]
- Juuri E, Jussila M, Seidel K, Holmes S, Wu P, Richman J, Heikinheimo K, Chuong C-M, Arnold K, Hochedlinger K, Klein O, Michon F, Thesleff I. Sox2 marks epithelial competence to generate teeth in mammals and reptiles. Development. 2013;140:1424–1432. doi: 10.1242/dev.089599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan Y, Jia S, Jiang R. Molecular patterning of the mammalian dentition. Semin Cell Dev Biol. 2014;25-26:61–70. doi: 10.1016/j.semcdb.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauder G. Functional design and evolution of the pharyngeal jaw apparatus in euteleostean fishes. Zool J Linn Soc. 1983;77:1–38. [Google Scholar]
- Le Pabic P, Stellwag EJ, Scemama JL. Embryonic development and skeletogenesis of the pharyngeal jaw apparatus in the cichlid Nile tilapia (Oreochromis niloticus) Anat Rec. 2009;292:1780–1800. doi: 10.1002/ar.20960. [DOI] [PubMed] [Google Scholar]
- Lemmetyinen R, Mankki J. The three-spined stickleback (Gasterosteus aculeatus) in the food chains of the northern Baltic. Merentutkimuslait Julk/Havsforskningsinst. 1975;239:155–161. [Google Scholar]
- Liem K, Greenwood P. A functional approach to the phylogeny of the pharyngognath teleosts. Am Zool. 1981;21:83–101. [Google Scholar]
- Mantoku A, Chatani M, Aono K, Inohaya K, Kudo A. Osteoblast and osteoclast behaviors in the turnover of attachment bones during medaka tooth replacement. Dev Biol. 2015:1–12. doi: 10.1016/j.ydbio.2015.12.002. [DOI] [PubMed] [Google Scholar]
- McGee MD, Schluter D, Wainwright PC. Functional basis of ecological divergence in sympatric stickleback. BMC Evol Biol. 2013;13:277. doi: 10.1186/1471-2148-13-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A. Morphometrics and allometry in the trophically polymorphic cichlid fish, Cichlusomu citrinelfum: alternative adaptations and ontogenetic changes in shape. J Zool, Lond. 1990;221:237–260. [Google Scholar]
- Mikkola ML. Controlling the number of tooth rows. Sci Signal. 2009;2:pe53. doi: 10.1126/scisignal.285pe53. [DOI] [PubMed] [Google Scholar]
- Mikkola ML, Thesleff I. Ectodysplasin signaling in development. Cytokine Growth Factor Rev. 2003;14:211–224. doi: 10.1016/s1359-6101(03)00020-0. [DOI] [PubMed] [Google Scholar]
- Miller CT, Glazer AM, Summers BR, Blackman BK, Norman AR, Shapiro MD, Cole BL, Peichel CL, Schluter D, Kingsley DM. Modular skeletal evolution in sticklebacks is controlled by additive and clustered quantitative trait loci. Genetics. 2014;197:405–420. doi: 10.1534/genetics.114.162420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama K, Watanabe S, Iida M, Sahara N. Plate-like permanent dental laminae of upper jaw dentition in adult gobiid fish, Sicyopterus japonicus. Cell Tissue Res. 2010;340:189–200. doi: 10.1007/s00441-010-0935-2. [DOI] [PubMed] [Google Scholar]
- Motta PJ. Tooth attachment, replacement, and growth in the butterfly fish, Chaetodon miliaris (Chaetodontidae, Perciformes) Can J Zool. 1984;62:183–189. [Google Scholar]
- Muschick M, Indermaur A, Salzburger W. Convergent evolution within an adaptive radiation of cichlid fishes. Curr Biol. 2012;22:2362–2368. doi: 10.1016/j.cub.2012.10.048. [DOI] [PubMed] [Google Scholar]
- Nakajima T. Larval vs. adult pharyngeal dentition in some Japanese cyprinid fishes. J Dent Res. 1984;63:1140–1146. doi: 10.1177/00220345840630090901. [DOI] [PubMed] [Google Scholar]
- Nakajima T. Development of pharyngeal dentition in the cobitid fishes, Misgurnus anguillicaudatus and Cobitis biwae, with a consideration of evolution of cypriniform. Copeia. 1987;1987:208–213. [Google Scholar]
- O’Connell DJ, Ho JWK, Mammoto T, Turbe-Doan A, O’Connell JT, Haseley PS, Koo S, Kamiya N, Ingber DE, Park PJ, Maas RL. A Wnt-Bmp feedback circuit controls intertissue signaling dynamics in tooth organogenesis. Sci Signal. 2012;5:1–10. doi: 10.1126/scisignal.2002414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn J. The ontogeny of tooth succession in Lacerta vivipara Jacquin (1787) Proc R Soc London Ser B, Biol Sci. 1971;179:261–289. doi: 10.1098/rspb.1971.0097. [DOI] [PubMed] [Google Scholar]
- Owen R. Odontography; a treatise on the comparative anatomy of the teeth. Vol. 1. Hippolyte Bailliere; London: 1845. pp. 1–178. [Google Scholar]
- Parner ET, Heidmann JM, Vath M, Poulsen S. A longitudinal study of time trends in the eruption of permanent teeth in Danish children. Arch Oral Biol. 2001;46:425–431. doi: 10.1016/s0003-9969(01)00002-4. [DOI] [PubMed] [Google Scholar]
- Reif W. Evolution of dermal skeleton and dentition in vertebrates. In: Hecht MK, editor. Evol. Biol. Plenum Press; New York: 1982. pp. 287–368. [Google Scholar]
- Sadier A, Viriot L, Pantalacci S, Laudet V. The ectodysplasin pathway: from diseases to adaptations. Trends Genet. 2014;30:24–31. doi: 10.1016/j.tig.2013.08.006. [DOI] [PubMed] [Google Scholar]
- Schluter D, McPhail JD. Ecological character displacement and speciation in sticklebacks. Am Nat. 1992;140:85–108. doi: 10.1086/285404. [DOI] [PubMed] [Google Scholar]
- Sibbing F. In: Food capture and oral processing. Winfield IJ, Nelson JS, editors. Cyprinid Fishes Chapman and Hall; 1991. pp. 377–412. [Google Scholar]
- Smith MM, Fraser GJ, Mitsiadis TA. Dental lamina as source of odontogenic stem cells: evolutionary origins and developmental control of tooth generation in gnathostomes. J Exp Zool B Mol Dev Evol. 2009;312B:260–280. doi: 10.1002/jez.b.21272. [DOI] [PubMed] [Google Scholar]
- Smith MM, Okabe M, Joss J. Spatial and temporal pattern for the dentition in the Australian lungfish revealed with sonic hedgehog expression profile. Proc Biol Sci. 2009;276:623–631. doi: 10.1098/rspb.2008.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soukup V, Epperlein H-H, Horácek I, Cerny R. Dual epithelial origin of vertebrate oral teeth. Nature. 2008;455:795–798. doi: 10.1038/nature07304. [DOI] [PubMed] [Google Scholar]
- Stock D. Zebrafish dentition in comparative context. J Exp Zool B Mol Dev Evol. 2007;308B:523–549. doi: 10.1002/jez.b.21187. [DOI] [PubMed] [Google Scholar]
- Stock DW, Jackman WR, Trapani J. Developmental genetic mechanisms of evolutionary tooth loss in cypriniform fishes. Development. 2006;133:3127–3137. doi: 10.1242/dev.02459. [DOI] [PubMed] [Google Scholar]
- Swinnerton HH. A contribution to the morphology of the Teleostean head skeleton, based upon a study of the developing skull of the three-spined stickleback (Gasterosteus aculeatus) Q J Microsc Sci. 1902;45:503–601. [Google Scholar]
- Trapani J, Schaefer S. Position of developing replacement teeth in teleosts. Copeia. 2001;2001:35–51. [Google Scholar]
- Tucker A, Sharpe P. The cutting-edge of mammalian development; how the embryo makes teeth. Nat Rev Genet. 2004;5:499–508. doi: 10.1038/nrg1380. [DOI] [PubMed] [Google Scholar]
- Tummers M, Thesleff I. The importance of signal pathway modulation in all aspects of tooth development. J Exp Zool B Mol Dev Evol. 2009;312B:309–319. doi: 10.1002/jez.b.21280. [DOI] [PubMed] [Google Scholar]
- Underwood CJ, Johanson Z, Welten M, Metscher B, Rasch LJ, Fraser GJ, Smith MM. Development and evolution of dentition pattern and tooth order in the skates and rays (Batoidea; Chondrichthyes) PLoS One. 2015;10:e0122553. doi: 10.1371/journal.pone.0122553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der heyden C, Huysseune A. Dynamics of tooth formation and replacement in the zebrafish (Danio rerio) (Teleostei, Cyprinidae) Dev Dyn. 2000;219:486–496. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1069>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Vandenplas S, De Clercq A, Huysseune A. Tooth replacement without a dental lamina: the search for epithelial stem cells in Polypterus senegalus. J Exp Zool B Mol Dev Evol. 2014;322:281–293. doi: 10.1002/jez.b.22577. [DOI] [PubMed] [Google Scholar]
- Wainwright P. In: Functional morphology of the pharyngeal jaw apparatus. Shadwick RE, Lauder GV, editors. Fish Biomechanics Academic Press; Fish Physiology: 2006. pp. 77–102. [Google Scholar]
- Wake M. The development and replacement of teeth in viviparous caecilians. J Morphol. 1976;148:33–64. doi: 10.1002/jmor.1051480104. [DOI] [PubMed] [Google Scholar]
- Wakita M, Itoh K, Kobayashi S. Tooth replacement in the teleost fish Prionurus microlepidotus Lacépède. J Morphol. 1977;153:129–141. doi: 10.1002/jmor.1051530109. [DOI] [PubMed] [Google Scholar]
- Walker MB, Kimmel CB. A two-color acid-free cartilage and bone stain for zebrafish larvae. Biotech Histochem. 2007;82:23–28. doi: 10.1080/10520290701333558. [DOI] [PubMed] [Google Scholar]
- Woodroffe S, Mihailidis S, Hughes T, Bockmann M, Seow WK, Gotjamanos T, Townsend GC. Primary tooth emergence in Australian children: timing, sequence and patterns of asymmetry. Aust Dent J. 2010;55:245–251. doi: 10.1111/j.1834-7819.2010.01230.x. [DOI] [PubMed] [Google Scholar]
- Wu P, Wu X, Jiang T-X, Elsey RM, Temple BL, Divers SJ, Glenn TC, Yuan K, Chen M-H, Widelitz RB, Chuong C-M. Specialized stem cell niche enables repetitive renewal of alligator teeth. Proc Natl Acad Sci. 2013:1–10. doi: 10.1073/pnas.1213202110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Holder N, Patient R, Wilson SW. Spatially regulated expression of three receptor tyrosine kinase genes during gastrulation in the zebrafish. Development. 1994;120:287–299. doi: 10.1242/dev.120.2.287. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Lan Y, Chai Y, Jiang R. Antagonistic actions of Msxl and Osr2 pattern mammalian teeth into a single row. Science. 2009;323:1232–1234. doi: 10.1126/science.1167418. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1: Pitx2 and Bmp6 tooth expression in section and whole mount.
In situ hybridization for Pitx2 (A-A", B-B") and Bmp6 (C, C', D, D') in section (A-D) and whole mount (A'-D', A', B'). Tooth developmental stages are vertically matched for early germs (A, A', C, C') and late germs (B, B', D, D') for pharyngeal (A-D, A’-D’) and oral (A”, B”) teeth. Pitx2 expression is detected in the dental epithelium (A-B, A'-B', A"-B") while Bmp6 expression is detected in the inner dental epithelium and mesenchyme early (C, C'), and appears restricted to the mesenchyme only late (D, D'). The black dotted line outlines the developing tooth germ while the white dotted line separates the inner and outer dental epithelium (when discernable). Black arrowheads denote mineralized teeth and white asterisks denote mesenchyme. Scale bar = 15 μm.
Fig. S2: Dorsal pharyngeal tooth plates develop on pharyngobranchial cartilage templates.
(A) Alizarin red (bone) and Alcian blue (cartilage) stained 11 days post fertilization (dpf) marine dorsal pharyngeal tooth plates. The pharyngobranchial cartilages chondrify before dorsal pharyngeal teeth form adjacently. (B) Transverse H&E sections of 20 dpf marine dorsal and ventral pharyngeal tooth plates. The dorsal pharyngeal tooth plate contains chondrocytes (white arrowhead) and teeth ossify directly on the thin layer of perichondral bone surrounding them (white arrow). The fifth ceratobranchial contains chondrocytes (black arrowhead), but the ventral pharyngeal tooth plate does not (black arrow), instead forming from subsequent ossification ventral to individual teeth. Note the medial tooth germ not surrounded by bone (black caret). Scale bars = 50 μm.
Fig. S3: Replacement teeth form deep but with invaginated epithelia that is continuous with lumenal pharyngeal epithelium. H&E stained 6 μm serial sections from adult sticklebacks showing pharyngeal teeth forming deep in the mesenchyme with an epithelial connection to the lumenal pharyngeal epithelium. (A-A", B-B", C-C", D-D") Each series indicates an individual tooth germ forming deep in the mesenchyme (A, B, C, D), yet with a clear epithelial connection to the lumenal pharyngeal epithelium in subsequent serial sections (A', A", B', B", C', C", D', D". (A) Arrowhead indicates the stratum compactum, the boundary between the pharyngeal epithelium and the underlying connective tissue. Scale bar is 50 μm and applies to all panels. (A-C) Adult freshwater, (D) adult marine. Note B and C' appear in Fig. 7C and Fig. 7F respectively.