Abstract
The purpose of this study was to investigate the effect of astragalus polysaccharides (APSs), active constituents of astragalus, in the treatment of hepatocellular carcinoma (HCC) and their potential as a promising candidate for future anticancer drug development. Astragalus polysaccharide was administered at different doses to HCC H22-bearing mice to investigate their antitumor effects. Results revealed that APS inhibited the growth of H22 cells with a tumor inhibition rate in the APS 400 mg·kg−1 group of 59.01%. Astragalus polysaccharides significantly increased the spleen and thymus indexes, and also the interleukin (IL) 2, IL-6, and tumor necrosis factor α cytokine concentration in serum, indicating that APS influences immune-regulating properties involved in antitumor activity. In addition, APS increased Bax protein expression and decreased Bcl-2 protein expression; these proteins are apoptosis-regulating factors responsible for cell death or survival. Further development and exploration of APS may enable it to become an effective clinical agent for liver cancer therapy.
Keywords: astragalus, polysaccharides, hepatocellular carcinoma, cytokines, Bax protein, Bcl-2 protein
Introduction
Hepatocellular carcinoma (HCC) is one of the most aggressive malignancies and the leading cause of cancer-related death in the world.1 Today, the main measures used to treat HCC include surgery and intervention chemotherapy, which often cause a variety of adverse events as these methods do not show selectivity between tumor and normal cells.2 The HCC treatment is still far from satisfactory today, partly due to a lack of effective medicines. Treatments that inhibit growth and invasion in early-stage HCC tumors are primordial. Identification of such treatments could ultimately lead to the suppression of cancer metastasis and increase the survival of patients with HCC.
Astragalus is a traditional Chinese medicine that has been studied in depth and widely used in clinics for treatment of diseases, such as hepatitis,3 diabetes, cancer,4 and others.5 Astragalus polysaccharides (APSs) are one of the most important natural active materials extracted from astragalus and are remarkably effective in traditional medicine due to their multitargeting biological activities, including antioxidant,6 anti-inflammatory,7 anti-hepatitis B virus (HBV),3 and immune regulation5 effects.
Astragalus polysaccharides are remarkably effective in inhibiting tumor growth and metastasis in vitro and in vivo. However, the pharmacological effects of APS in the treatment of cancer remain unknown. This study investigated the effect of APS in the treatment of HCC and their implication as a promising candidate for future anticancer drug development.
Materials and Methods
Medicine
Astragalus polysaccharides were purchased from Shanxi Biological Technology Co, Ltd, Yuncheng, Shanxi (batch number: HQ090312, purity: 98%). Before the preparation, APSs should be dissolved with saline water.
Mice
Kunming mice of mean weight 22 ± 2 g were purchased from the Animal Experiment Research Center of the Zhejiang Chinese Medical University.
Cell Culture
The HCC H22 cell line was obtained from the College of Life Science of Zhejiang Chinese Medical University and cultured in Roswell Park Memorial Institute 1640 medium with 10% fetal bovine serum. Cells were incubated at 37°C in 5% CO2 humidified air.
Experimental Models
Ten mice received an intraperitoneal injection of H22 cancer cells (2 × 106 cells per mouse). Seven days after injection, ascitic fluid was extracted and the cells were counted.
Sixty Kunming mice were randomly divided into 6 groups—healthy, vehicle, CTX, and 3 APS groups, 10 mice in each group. Mice were then inoculated with ascitic fluid cells (3 × 106 cells per mouse) subcutaneously in the left armpit, except the healthy group. Animal husbandry was in accordance with institutional guidelines. Mice were housed in the SPF room at 20° ± 2°C.
Antitumor Assays
Treatments were initiated 24 hours after injecting the tumor cells. Drugs were administrated once daily for 15 consecutive days. The vehicle group and the healthy group consisted of normal saline intake mice (20 mL·kg−1). The CTX group was administered cyclophosphamide (CTX; Shanghai Crystal Pure Biological Technology Co., Ltd, Aladdin, CTX purity over 98% by HPLC analysis) at 30 mg·kg−1 body weight. Three APS groups were administered APS (Tianjin Cinorch Pharmaceutical Co, Ltd. Tianjin, China over 98% by HPLC analysis) at 100, 200, and 400 mg·kg−1 body weight, respectively, once per day intragastrically.
All mice were killed and tumors were dissected and weighed on the 16th day. Body weights and tumor weights were recorded. The tumor inhibition rate was calculated as follows:
Organ Index Assays
The day after treatment administration, the mice were killed. Thymuses and spleens were dissected and weighed to calculate the thymus and spleen indexes. The thymus index was calculated as the thymus weight of each mouse divided by its body weight. The spleen index was calculated as the spleen weight of each mouse divided by its body weight.
Determination of Cytokines
Blood samples were acquired from the sinus by extracting the eyes of the mice. Blood serum was separated by centrifugation at 3000 rpm for 10 minutes. Cytokines including interleukin (IL) 2, IL-6, tumor necrosis factor α (TNF-α) were measured using the quantitative sandwich enzyme immunoassay technique (Hangzhou MultiSciences Biotech Co, Ltd, Hangzhou, Zhejiang).
Bax and Bcl-2 Protein Expression
Bax and Bcl-2 protein expression was measured using the streptavidin–biotin complex (SABC). Tumors were fixed in 10% formalin and the paraffin sections were prepared with a thickness of 5 μm. The sections were high-pressure repaired in 0.01 mol/L citrate buffer. Bax and Bcl-2 primary antibodies (NOVUS Biologicals) were added and the sections were incubated overnight at 4°C. Subsequently, they were washed in phosphate-buffered saline (PBS) and secondary antibodies were added and incubated at 37°C for 20 minutes. The sections were then washed in PBS. The SABC reagent was added and the sections were incubated at 37°C for 20 minutes. Phosphate-buffered saline was used to wash the sections and DAB to stain them. They were then observed using light microscopy.
Statistical Analysis
Bax and Bcl-2 protein expression was analyzed using the IPP pathological image analysis system. The field of view from each tissue section was selected randomly and collected at 400× to calculate its mean optical density (OD).
Data were analyzed using SPSS 19.0 and expressed as the mean (SD). Statistical significance of the values was evaluated by analysis of variance. A P value <.05 was considered as significantly different.
Results
Histopathological Observation
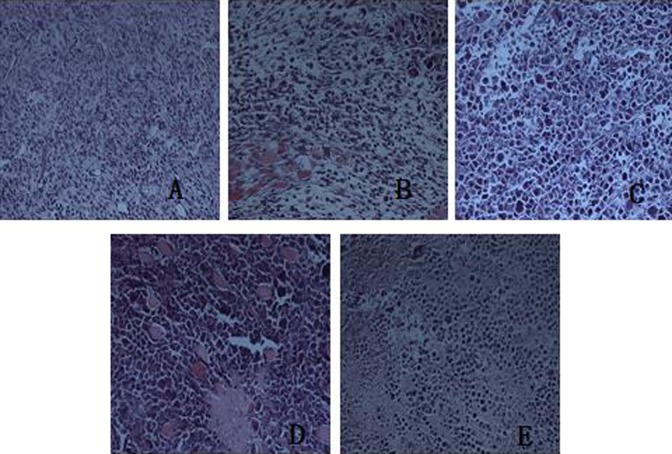
Histopathological observation of tumor tissues of the H22-bearing mice vehicle group showed that tumor cells were densely arranged with different sizes and diffuse distribution; some of them showed invasive growth. Conversely, tumor cells of the CTX group were restrained and showed obvious necrosis. In the APS groups, tumor cells were arranged loosely and showed swelling and degeneration (Figure 1).
Figure 1.
Histopathological observation of tumor tissues from H22-bearing mice (hematoxylin eosin [HE] staining, ×400). A, Vehicle group. B, CTX group. C, APS 100 mg kg−1 group. D, APS 200 mg kg−1 group. E, APS 400 mg kg−1 group. APS indicates astragalus polysaccharide.
Astragalus Polysaccharides Inhibit the Growth of H22 Cells
Different doses of APS were administrated orally to mice to determine the suppression of the H22 tumor. Body weight was not significantly different between the groups, except in the CTX group where it decreased significantly. Compared to the vehicle group, the tumor weight of the APS 400 mg·kg−1 and APS 200 mg·kg−1 groups and the CTX group was significantly lighter (P < .05). The tumor weight of the APS 400 mg·kg−1 group was similar to the CTX group (P > .05), while the APS 100 mg·kg−1 and APS 200 mg·kg−1 groups were significantly different from it (P < .05). Tumor inhibition rates of each group were as follows—CTX group, 80.74%; APS 100 mg·kg−1 group, 29.19%; APS 200 mg·kg−1 group, 35.71%; and APS 400 mg·kg−1 group, 59.01%. These investigations indicated that APS had an inhibitory effect on H22 tumor in mice (Table 1).
Table 1.
Inhibitory Effect of APSs on the Growth of H22 Cells.a
| Group | Body Weight, g | Tumor Weight, g | Tumor-Inhibition Rate |
|---|---|---|---|
| Healthy group | 38.96 ± 2.09 | – | – |
| Vehicle group | 41.28 ± 3.88 | 3.22 ± 1.09 | – |
| CTX group | 34.269 ± 2.75 | 0.62 ± 0.08b | 80.74% |
| APS 100 mg·kg−1 | 38.93 ± 3.43 | 2.28 ± 0.75c | 29.19% |
| APS 200 mg·kg−1 | 39.42 ± 5.35 | 2.07 ± 0.53b,c | 35.71% |
| APS 400 mg·kg−1 | 40.58 ± 3.47 | 1.32 ± 0.51b | 59.01% |
Abbreviations: APSs, astragalus polysaccharides; SD, standard deviation.
aValues are expressed as mean (SD) (n = 10).
b P < .05 compared to the vehicle group.
c P < .05 compared to the CTX group.
Effect of APS on Organ Indexes
In addition to tumor death, the CTX group caused immunosuppression compared to vehicle group, and the thymus and spleen index values decreased significantly (P < .05), indicating significant side effects. Astragalus polysaccharides groups had an effect on the thymus and spleen indexes, although not significantly, compared to the vehicle group (P < .05), indicating APS with low toxicity (Table 2).
Table 2.
Effect of APSs on the Thymus and Spleen Indexes.a
| Group | Thymus Index, mg/g | Spleen Index, mg/g |
|---|---|---|
| Healthy group | 3.01 ± 0.51 | 0.87 ± 0.20 |
| Vehicle group | 7.25 ± 1.21b | 1.95 ± 0.48b |
| CTX group | 3.29 ± 0.64c | 0.72 ± 0.25c |
| APS 100 mg·kg−1 | 6.96 ± 1.38b,d | 1.76 ± 0.31b,d |
| APS 200 mg·kg−1 | 7.35 ± 0.93b,d | 1.87 ± 0.32b,d |
| APS 400 mg·kg−1 | 7.96 ± 1.30b,d | 2.25 ± 0.38b,d |
Abbreviations: APSs, astragalus polysaccharides; SD, standard deviation.
aValues are expressed as mean (SD) (n = 10).
b P < .05 compared to the healthy group.
c P < .05 compared to the vehicle group.
d P < .05 compared to the CTX group.
Effect of APS on Cytokines
The effect of APS on IL-2, IL-6, and TNF-α cytokines is shown in Table 3. All three cytokines decreased in the vehicle group compared to the healthy group (P < .05). Interleukin 2 in each APS treatment group and IL-6 and TNF-α in the CTX group, APS 100 mg·kg−1 and 200 mg·kg−1 groups decreased compared to the healthy group; IL-2 and IL-6 in the CTX group and IL-2 and TNF-α in the APS 100 mg kg−1 group decreased significantly (P < .05). Compared to the vehicle group, IL-2, IL-6, and TNF-α increased in the CTX group and all APS groups (P < .05), though TNF-α did not increase significantly in the APS 100 mg kg−1 group (P > .05). Interleukin 2 and IL-6 in all APS groups and TNF-α in the APS 400 mg kg−1 group increased significantly compared to the CTX group (P < .05). These results indicated that APS promotes the expression of IL-2, IL-6, and TNF-α as an H22 tumor treatment mechanism (Table 3).
Table 3.
Effect of APSs on Cytokines.a
| Group | IL-6, pg/mL | IL-2, pg/mL | TNF-α, pg/mL |
|---|---|---|---|
| Healthy group | 74.53 ± 6.65 | 10.24 ± 0.75 | 40.11 ± 3.54 |
| Vehicle group | 51.45 ± 2.73b | 6.74 ± 0.48b | 23.25 ± 4.36b |
| CTX group | 65.56 ± 4.13b,c | 7.78 ± 1.31b | 36.56 ± 4.75c |
| APS 100 mg·kg−1 | 70.71 ± 3.54c,d | 8.14 ± 0.59b,c,d | 28.64 ± 5.57b |
| APS 200 mg·kg−1 | 76.54 ± 5.74c,d | 9.23 ± 0.60c,d | 34.75 ± 5.75c |
| APS 400 mg·kg−1 | 85.21 ± 10.46b,c,d | 9.58 ± 0.53c,d | 43.16 ± 6.53c,d |
Abbreviations: APSs, astragalus polysaccharides; IL, interleukin; SD, standard deviation; TNF, tumor necrosis factor.
aValues are expressed as mean (SD) (n = 10).
b P < .05 compared to the healthy group.
c P < .05 compared to the vehicle group.
d P < .05 compared to the CTX group.
Effect of APS on Bax and Bcl-2 Protein Expression
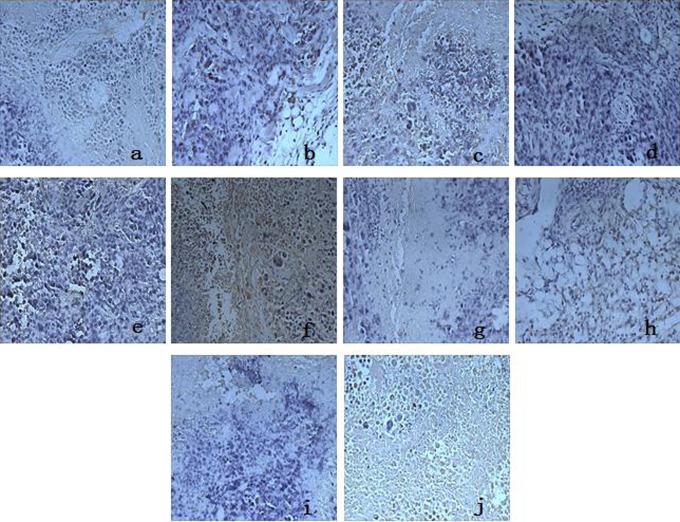
Immunohistochemical detection revealed the diffuse distribution of Bax- and Bcl-2-positive cells (Figure 2). Combined with the OD values of Bax and Bcl-2 protein expression in Table 4, Bax protein expression in the vehicle group was weakly positive while Bcl-2 protein expression was strongly positive. Conversely, in the CTX group and APS groups, Bax protein expression was strongly positive and Bcl-2 was weakly positive (Table 4).
Figure 2.
Effect of APSs on Bax and Bcl-2 protein expression (×400). A-E, Effect of APS on Bax protein expression. F-J, Effect of APS on Bcl-2 protein expression. Vehicle group (A and F); CTX group (B and G); APS 100 mg kg−1 group (C and H); APS 200 mg kg−1 group (D and I); APS 400 mg kg−1 group (E and J). APSs indicate astragalus polysaccharides
Table 4.
Mean OD Values of Bax and Bcl-2 Protein Expression.a
| Group | Bax | Bcl-2 | |
|---|---|---|---|
| Healthy group | – | – | – |
| Vehicle group | 0.31 ± 0.04 | 0.57 ± 0.05 | 0.54 ± 0.05 |
| CTX group | 0.42 ± 0.06b | 0.40 ± 0.08b | 1.05 ± 0.08b |
| APS 100 mg·kg−1 | 0.41 ± 0.05b | 0.50 ± 0.05c | 0.82 ± 0.05b |
| APS 200 mg·kg−1 | 0.44 ± 0.05b | 0.42 ± 0.07b | 1.04 ± 0.07b |
| APS 400 mg·kg−1 | 0.47 ± 0.06b | 0.38 ± 0.04b | 1.23 ± 0.06b |
Abbreviations: APS, astragalus polysaccharide; OD, optical density; SD, standard deviation.
aValues are expressed as mean (SD) (n = 10).
b P < .05 compared to the vehicle group.
c P < .05 compared to the CTX group.
Discussion
This study demonstrated that APS exerts anticancer activities on H22 cells by inhibiting cell growth and increasing the spleen and thymus indexes. In the CTX group, spleen and thymus weights decreased significantly despite having the highest inhibitory rate of 80.74% than all groups. As suggested by Zhao,8 spleen and thymus indexes reflect the immune function of the organism. In our study, APS groups had not decreased the thymus and spleen indexes significantly compared to the vehicle group; therefore, APS could inhibit H22 tumor with low toxicity.
Cytokines are essential immune response components and play a pivotal antitumor role.4,9 In this study, IL-2, IL-6, TNF-α cytokines in the APS groups increased significantly compared to the vehicle group, indicating APS influenced immune-regulating properties involved in antitumor activity. Interleukin 2 is one of the important cytokines of immune response regulation involved in cell that reflect the cellular immune ability to some extent; therefore, decreased IL-2 levels indicate impaired cellular immunity in patients with cancer.8–10 Interleukin 6 is one of the important members of interleukins family involved in tumor-infiltrating lymphocyte immunosuppression. Interleukin 6 promotes T-cell proliferation, helper T-cell differentiation, and development of T-cell-mediated cytotoxicity.11–13 Astragalus polysaccharides can strengthen the body’s immune response by promoting the production of IL-6 and play a regulatory role in the tumor inhibition. Tumor necrosis factor α, mainly produced by mononuclear cells and macrophages, is able to cause tumor cell apoptosis and plays an important role in immunoregulatory and inflammatory mediators.8,14 In this study, the serum cytokines—IL-2 of APS in the treatment group, IL-6, and TNF-α—were significantly higher in the vehicle group, indicating the APS can promote the production of IL-2, IL-6, TNF-α in H22 tumor burdened mice and enhancing the body’s immune response, which play an important role in the inhibition of tumor.
Tumor tissue sections (HE staining) showed Bcl-2 protein expression in the inner mitochondrial membrane, which plays a key role in apoptosis. Bax protein expression in the APS groups increased, while Bcl-2 protein expression decreased with the dose administered. The Bcl-2 protein family includes proapoptosis proteins such as Bax, Bad, Bak, Bid, and apoptosis protein inhibitors, such as Bcl-2 and Bcl-xl. Members of the Bcl-2 protein family regulate the release of apoptosis-activating factors that determine cell survival or death14,15 and therefore have important antitumor activity.
In conclusion, APS influenced immune-regulating properties involved in antitumor activity. Astragalus polysaccharides inhibited the growth of H22 cells and significantly increased the spleen and thymus indexes and the IL-2, IL-6, and TNF-α concentrations in serum. In addition, APS increased Bax protein expression and decreased Bc1-2 protein expression, which are active components in determining cell death and survival. Further development and exploration of APS may enable it to become an effective clinical agent for liver cancer therapy.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the Project of Zhejiang Provincial Department of Education (Y201019151), Zhejiang Provincial Natural Science Foundation, China (LY14H270014), the Public Welfare Technology Application Research Project of Zhejiang Province, China (2016C33086), and the Scientific Research Fund Project of Zhejiang Chinese Medicine University (2015ZG02).
References
- 1. Xiang JF, Xiang YJ, Lin SM, et al. Anticancer effects of deproteinized asparagus polysaccharide on hepatocellular carcinoma in vitro. Tumor Biol. 2014;35(4):3517–3524. [DOI] [PubMed] [Google Scholar]
- 2. Paraskevi AF, Jonathan G, James H, Depinho RA. Cooperative interactions of p53 mutation, telomere dysfunction, and chronic liver damage in hepatocellular carcinoma progression. Cancer Res. 2006;66(9):4766–4773. [DOI] [PubMed] [Google Scholar]
- 3. Dang SS, Jia XL, Song P, et al. Inhibitory effect of emodin and astragalus polysaccharide on the replication of HBV. World J Gastroenterol. 2009;15(45):5669–5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yang B, Xiao B, Sun TY. Antitumor and immunomodulatory activity of Astragalus membranaceus polysaccharides in H22 tumor-bearing mice. Int J Biol Macromol. 2013;62(11):287–290. [DOI] [PubMed] [Google Scholar]
- 5. Zhao HM, Wang Y, Huang XY, et al. Astragalus polysaccharide attenuates rat experimental colitis by inducing regulatory T cells in intestinal Peyer’s patches. World J Gastroenterol. 2016;22(11):3175–3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang J, Gu JY, Chen ZS, Xing KC, Sun B. Astragalus polysaccharide suppresses palmitate-induced apoptosis in human cardiac myocytes: the role of Nrf1 and antioxidant response. Int J Clin Exp Pathol. 2015;8(3):2515–2524. [PMC free article] [PubMed] [Google Scholar]
- 7. Huang WM, Liang YQ, Tang LJ, Ding Y, Wang XH. Antioxidant and anti-inflammatory effects of astragalus polysaccharide on EA.hy926 cells. Exp Ther Med. 2013;6(1):199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhao T, Mao GH, Mao RW, et al. Antitumor and immunomodulatory activity of a water-soluble low molecular weight polysaccharide from Schisandra chinensis (Turcz.) Baill. Food Chem Toxicol. 2013;55(3):609–616. [DOI] [PubMed] [Google Scholar]
- 9. Sun X, Gao RL, Xiong YK, Huang QC, Xu M. Antitumor and immunomodulatory effects of a water-soluble polysaccharide from Lilii Bulbus in mice. Carbohydr Polym. 2014;102(4):543–549. [DOI] [PubMed] [Google Scholar]
- 10. Jin AH, Piao L, Yin XZ, Quan JS. Anti-tumor effect of iridoid glucosides from Boschniakia rossica in H22-bearing mice. Chinese Tradit Herb Drug. 2012;43(2):332–335. [Google Scholar]
- 11. Xu L. The intervention effect of Schisandra polysaccharide on hepatoma mice serum IL-6, TNF-α and VEGF expression. Chin J Pract Med. 2013;40(15):18–19. [Google Scholar]
- 12. Meng LZ, Xie J, Lv GP, et al. A comparative study on immunomodulatory activity of polysaccharides from two official species of Ganoderma (Lingzhi). Nutr Cancer. 2014;66(7):1124–1131. [DOI] [PubMed] [Google Scholar]
- 13. Li JF, Wu XY, Li J, et al. Clinical significance of serum and tumor tissue IL-6 levels in patients with gastric cancer. J Diagn Concepts Pract. 2012;11(3):302–305. [Google Scholar]
- 14. Yuan GP, Dai SJ, Yin ZQ, et al. Sub-chronic lead and cadmium co-induce apoptosis protein expression in liver and kidney of rats. Int J Clin Exp Pathol. 2014;7(6):2905–2914. [PMC free article] [PubMed] [Google Scholar]
- 15. Mu XY, Dong XL, Sun J, et al. Simultaneous blockage of epidermal growth factor receptor and cyclooxygenase-2 in a human xenotransplanted lung cancer vehicle. Asian Pac J Cancer P. 2014;15(1):69–73. [DOI] [PubMed] [Google Scholar]