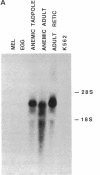
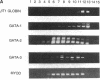
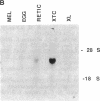
Abstract
Proteins that recognize the core sequence GATA are important regulators of hematopoietic-specific gene transcription. We have characterized cDNAs encoding the Xenopus laevis homologues of three related transcription factors, designated GATA-1, -2, and -3. Comparative sequence analysis reveals strong conservation of the zinc-finger DNA-binding domain among all vertebrate GATA-binding proteins. GATA-2 and GATA-3 polypeptides are homologous throughout their entire sequences, whereas GATA-1 sequence is conserved only in the region responsible for DNA binding. In Xenopus, RNAs encoding GATA-binding proteins are expressed in both larval and adult erythroid cells. GATA-1, -2, and -3 RNAs are first detectable in early gastrula (Nieuwkoop developmental stage 11). This is earlier than the appearance of the early larval alpha T1 globin RNA (stage 15), beta T1 globin RNA (stage 26), or blood island formation (stage 30). The expression of GATA-1, -2, and -3 in early development may signal an early commitment of mesoderm to form hematopoietic tissue.
Full text
PDF

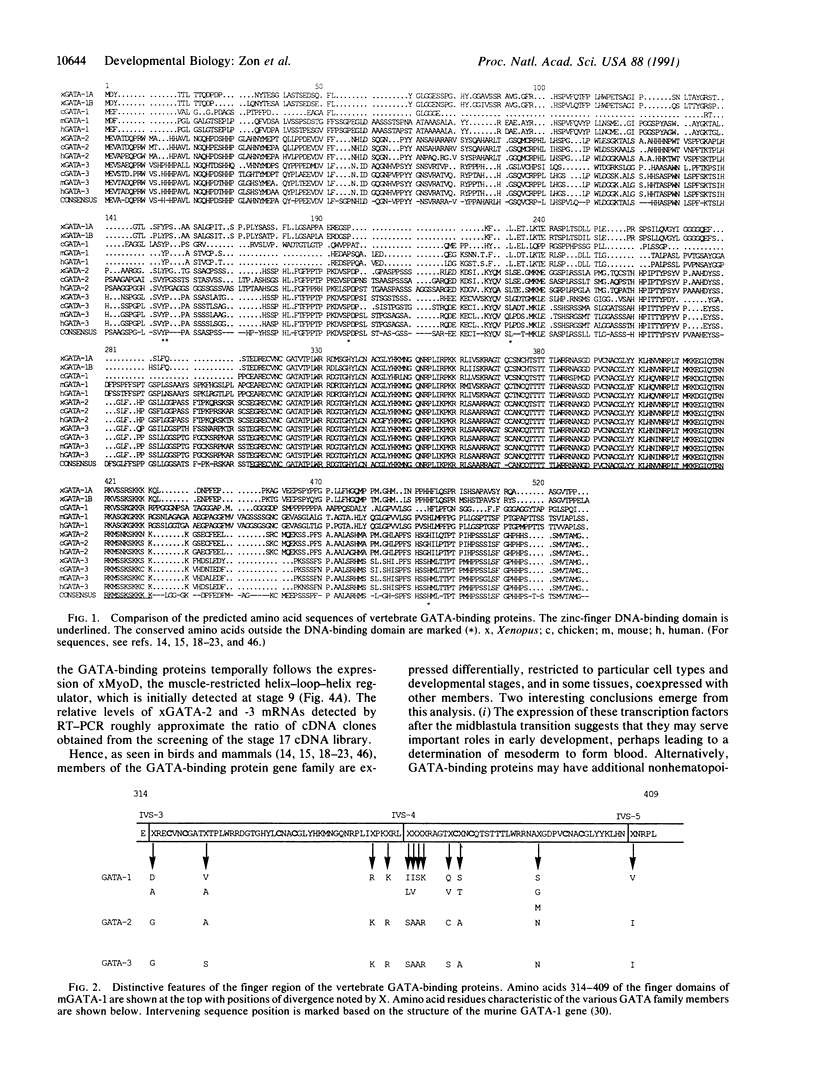

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banville D., Kay R. M., Harris R., Williams J. G. The nucleotide sequence of the mRNA encoding a tadpole beta-globin polypeptide of Xenopus laevis. J Biol Chem. 1983 Jul 10;258(13):7924–7927. [PubMed] [Google Scholar]
- Banville D., Williams J. G. Developmental changes in the pattern of larval beta-globin gene expression in Xenopus laevis. Identification of two early larval beta-globin mRNA sequences. J Mol Biol. 1985 Aug 20;184(4):611–620. doi: 10.1016/0022-2836(85)90307-9. [DOI] [PubMed] [Google Scholar]
- Banville D., Williams J. G. The pattern of expression of the Xenopus laevis tadpole alpha-globin genes and the amino acid sequence of the three major tadpole alpha-globin polypeptides. Nucleic Acids Res. 1985 Aug 12;13(15):5407–5421. doi: 10.1093/nar/13.15.5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans T., Felsenfeld G. The erythroid-specific transcription factor Eryf1: a new finger protein. Cell. 1989 Sep 8;58(5):877–885. doi: 10.1016/0092-8674(89)90940-9. [DOI] [PubMed] [Google Scholar]
- Evans T., Reitman M., Felsenfeld G. An erythrocyte-specific DNA-binding factor recognizes a regulatory sequence common to all chicken globin genes. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5976–5980. doi: 10.1073/pnas.85.16.5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J. B., Howes G., Symes K., Cooke J., Smith J. C. The biological effects of XTC-MIF: quantitative comparison with Xenopus bFGF. Development. 1990 Jan;108(1):173–183. doi: 10.1242/dev.108.1.173. [DOI] [PubMed] [Google Scholar]
- Groudine M., Weintraub H. Activation of globin genes during chicken development. Cell. 1981 May;24(2):393–401. doi: 10.1016/0092-8674(81)90329-9. [DOI] [PubMed] [Google Scholar]
- Higgs D. R., Wood W. G., Jarman A. P., Sharpe J., Lida J., Pretorius I. M., Ayyub H. A major positive regulatory region located far upstream of the human alpha-globin gene locus. Genes Dev. 1990 Sep;4(9):1588–1601. doi: 10.1101/gad.4.9.1588. [DOI] [PubMed] [Google Scholar]
- Ho I. C., Vorhees P., Marin N., Oakley B. K., Tsai S. F., Orkin S. H., Leiden J. M. Human GATA-3: a lineage-restricted transcription factor that regulates the expression of the T cell receptor alpha gene. EMBO J. 1991 May;10(5):1187–1192. doi: 10.1002/j.1460-2075.1991.tb08059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood N. D., Gurdon J. B. Activation of muscle genes without myogenesis by ectopic expression of MyoD in frog embryo cells. Nature. 1990 Sep 13;347(6289):197–200. doi: 10.1038/347197a0. [DOI] [PubMed] [Google Scholar]
- Hopwood N. D., Pluck A., Gurdon J. B. MyoD expression in the forming somites is an early response to mesoderm induction in Xenopus embryos. EMBO J. 1989 Nov;8(11):3409–3417. doi: 10.1002/j.1460-2075.1989.tb08505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosbach H. A., Wyler T., Weber R. The Xenopus laevis globin gene family: chromosomal arrangement and gene structure. Cell. 1983 Jan;32(1):45–53. doi: 10.1016/0092-8674(83)90495-6. [DOI] [PubMed] [Google Scholar]
- Joulin V., Bories D., Eléouet J. F., Labastie M. C., Chrétien S., Mattéi M. G., Roméo P. H. A T-cell specific TCR delta DNA binding protein is a member of the human GATA family. EMBO J. 1991 Jul;10(7):1809–1816. doi: 10.1002/j.1460-2075.1991.tb07706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko L. J., Yamamoto M., Leonard M. W., George K. M., Ting P., Engel J. D. Murine and human T-lymphocyte GATA-3 factors mediate transcription through a cis-regulatory element within the human T-cell receptor delta gene enhancer. Mol Cell Biol. 1991 May;11(5):2778–2784. doi: 10.1128/mcb.11.5.2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D. I., Orkin S. H. Transcriptional activation and DNA binding by the erythroid factor GF-1/NF-E1/Eryf 1. Genes Dev. 1990 Nov;4(11):1886–1898. doi: 10.1101/gad.4.11.1886. [DOI] [PubMed] [Google Scholar]
- Martin D. I., Zon L. I., Mutter G., Orkin S. H. Expression of an erythroid transcription factor in megakaryocytic and mast cell lineages. Nature. 1990 Mar 29;344(6265):444–447. doi: 10.1038/344444a0. [DOI] [PubMed] [Google Scholar]
- Melton D. A. Pattern formation during animal development. Science. 1991 Apr 12;252(5003):234–241. doi: 10.1126/science.1672778. [DOI] [PubMed] [Google Scholar]
- Meunier H., Rivier C., Evans R. M., Vale W. Gonadal and extragonadal expression of inhibin alpha, beta A, and beta B subunits in various tissues predicts diverse functions. Proc Natl Acad Sci U S A. 1988 Jan;85(1):247–251. doi: 10.1073/pnas.85.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhof W., Stalder J., Köster M., Wirthmüller U., Knöchel W. Sequence analysis of the upstream regions of Xenopus laevis beta-globin genes and arrangement of repetitive elements within the globin gene clusters. Mol Biol Rep. 1990 Feb;14(1):17–26. doi: 10.1007/BF00422711. [DOI] [PubMed] [Google Scholar]
- Orkin S. H. Globin gene regulation and switching: circa 1990. Cell. 1990 Nov 16;63(4):665–672. doi: 10.1016/0092-8674(90)90133-y. [DOI] [PubMed] [Google Scholar]
- Patient R. K., Banville D., Brewer A. C., Elkington J. A., Greaves D. R., Lloyd M. M., Williams J. G. The organization of the tadpole and adult alpha globin genes of Xenopus laevis. Nucleic Acids Res. 1982 Dec 20;10(24):7935–7945. doi: 10.1093/nar/10.24.7935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevny L., Simon M. C., Robertson E., Klein W. H., Tsai S. F., D'Agati V., Orkin S. H., Costantini F. Erythroid differentiation in chimaeric mice blocked by a targeted mutation in the gene for transcription factor GATA-1. Nature. 1991 Jan 17;349(6306):257–260. doi: 10.1038/349257a0. [DOI] [PubMed] [Google Scholar]
- Romeo P. H., Prandini M. H., Joulin V., Mignotte V., Prenant M., Vainchenker W., Marguerie G., Uzan G. Megakaryocytic and erythrocytic lineages share specific transcription factors. Nature. 1990 Mar 29;344(6265):447–449. doi: 10.1038/344447a0. [DOI] [PubMed] [Google Scholar]
- Rupp R. A., Weintraub H. Ubiquitous MyoD transcription at the midblastula transition precedes induction-dependent MyoD expression in presumptive mesoderm of X. laevis. Cell. 1991 Jun 14;65(6):927–937. doi: 10.1016/0092-8674(91)90545-a. [DOI] [PubMed] [Google Scholar]
- Smith J. C., Cooke J., Green J. B., Howes G., Symes K. Inducing factors and the control of mesodermal pattern in Xenopus laevis. Development. 1989;107 (Suppl):149–159. doi: 10.1242/dev.107.Supplement.149. [DOI] [PubMed] [Google Scholar]
- Smith J. C., Price B. M., Van Nimmen K., Huylebroeck D. Identification of a potent Xenopus mesoderm-inducing factor as a homologue of activin A. Nature. 1990 Jun 21;345(6277):729–731. doi: 10.1038/345729a0. [DOI] [PubMed] [Google Scholar]
- Smith P. B., Flajnik M. F., Turpen J. B. Experimental analysis of ventral blood island hematopoiesis in Xenopus embryonic chimeras. Dev Biol. 1989 Feb;131(2):302–312. doi: 10.1016/s0012-1606(89)80003-x. [DOI] [PubMed] [Google Scholar]
- TILL J. E., MCCULLOCH E. A., SIMINOVITCH L. A STOCHASTIC MODEL OF STEM CELL PROLIFERATION, BASED ON THE GROWTH OF SPLEEN COLONY-FORMING CELLS. Proc Natl Acad Sci U S A. 1964 Jan;51:29–36. doi: 10.1073/pnas.51.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen G., Woolf T., Whitman M., Sokol S., Vaughan J., Vale W., Melton D. A. Activins are expressed early in Xenopus embryogenesis and can induce axial mesoderm and anterior structures. Cell. 1990 Nov 2;63(3):485–493. doi: 10.1016/0092-8674(90)90445-k. [DOI] [PubMed] [Google Scholar]
- Trainor C. D., Evans T., Felsenfeld G., Boguski M. S. Structure and evolution of a human erythroid transcription factor. Nature. 1990 Jan 4;343(6253):92–96. doi: 10.1038/343092a0. [DOI] [PubMed] [Google Scholar]
- Tsai S. F., Martin D. I., Zon L. I., D'Andrea A. D., Wong G. G., Orkin S. H. Cloning of cDNA for the major DNA-binding protein of the erythroid lineage through expression in mammalian cells. Nature. 1989 Jun 8;339(6224):446–451. doi: 10.1038/339446a0. [DOI] [PubMed] [Google Scholar]
- Tsai S. F., Strauss E., Orkin S. H. Functional analysis and in vivo footprinting implicate the erythroid transcription factor GATA-1 as a positive regulator of its own promoter. Genes Dev. 1991 Jun;5(6):919–931. doi: 10.1101/gad.5.6.919. [DOI] [PubMed] [Google Scholar]
- Turpen J. B., Smith P. B. Location of hemopoietic stem cells influences frequency of lymphoid engraftment in Xenopus embryos. J Immunol. 1989 Dec 1;143(11):3455–3460. [PubMed] [Google Scholar]
- Wall L., deBoer E., Grosveld F. The human beta-globin gene 3' enhancer contains multiple binding sites for an erythroid-specific protein. Genes Dev. 1988 Sep;2(9):1089–1100. doi: 10.1101/gad.2.9.1089. [DOI] [PubMed] [Google Scholar]
- Whitelaw E., Tsai S. F., Hogben P., Orkin S. H. Regulated expression of globin chains and the erythroid transcription factor GATA-1 during erythropoiesis in the developing mouse. Mol Cell Biol. 1990 Dec;10(12):6596–6606. doi: 10.1128/mcb.10.12.6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmer H. J., Andres A. C., Niessing J., Hosbach H. A., Weber R. Comparative analysis of cloned larval and adult globin cDNA sequences of Xenopus laevis. Dev Biol. 1981 Dec;88(2):325–332. doi: 10.1016/0012-1606(81)90176-7. [DOI] [PubMed] [Google Scholar]
- Widmer H. J., Hosbach H. A., Weber R. Globin gene expression in Xenopus laevis: anemia induces precocious globin transition and appearance of adult erythroblasts during metamorphosis. Dev Biol. 1983 Sep;99(1):50–60. doi: 10.1016/0012-1606(83)90253-1. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Ko L. J., Leonard M. W., Beug H., Orkin S. H., Engel J. D. Activity and tissue-specific expression of the transcription factor NF-E1 multigene family. Genes Dev. 1990 Oct;4(10):1650–1662. doi: 10.1101/gad.4.10.1650. [DOI] [PubMed] [Google Scholar]
- Zon L. I., Tsai S. F., Burgess S., Matsudaira P., Bruns G. A., Orkin S. H. The major human erythroid DNA-binding protein (GF-1): primary sequence and localization of the gene to the X chromosome. Proc Natl Acad Sci U S A. 1990 Jan;87(2):668–672. doi: 10.1073/pnas.87.2.668. [DOI] [PMC free article] [PubMed] [Google Scholar]