Abstract
Under the United Network for Organ Sharing (UNOS) policy, deceased donor livers may be offered to ABO-nonidentical candidates at each given Model for End-Stage Liver Disease (MELD) score and to blood type B candidates at MELD ≥30. To evaluate ABO-nonidentical liver transplantation (LT) in the United States, we examined all adult LT non–status 1 candidates, recipients and deceased liver donors from 2013 to 2015. There were 34 920 LT candidates (47% type O, 38% type A, 12% type B, 3% type AB) and 10 479 deceased liver donors (47% type O, 38% type A, 12% type B, 3% type AB). ABO-nonidentical LT occurred in 2%, 3%, 20% and 36% of types O, A, B and AB recipients, respectively, which led to a net liver loss of 6% for type O and 2% for type A recipients but a net liver gain of 14% for type B and 55% for type AB recipients. The LT MELD scores of ABO-identical versus -nonidentical recipients were 29 versus 34 for type O, 29 versus 19 for type A, 25 versus 38 for type B, and 22 versus 28 for type AB (p < 0.01). ABO-nonidentical LT increased liver supply for candidates with blood types B and AB but decreased supply for type O and A candidates. We urge refinement of UNOS policy surrounding ABO-nonidentical LT.
Introduction
Since 1986, the United Network for Organ Sharing (UNOS) has administered the federal Organ Procurement and Transplantation Network (OPTN) for the U.S. Department of Health and Human Services (1). Under this contract, UNOS develops and enforces the policies involving the national allocation, procurement and transportation of deceased organs (2). UNOS Policy 9 governs the allocation of livers to candidates awaiting liver transplantation (LT) (3).
The primary unit by which individual patients are prioritized for LT is the Model for End-Stage Liver Disease (MELD) score within a specific blood type (“identical blood type transplant”; e.g. type A liver to type A recipient, type O liver to type O recipient) (3). OPTN policies, however, explicitly allow for blood type–nonidentical LT for candidates with status 1 (i.e. with fulminant hepatic failure), with MELD scores ≥30 or within a given a MELD score classification (Table 1).
Table 1.
Organ Procurement and Transplantation Network policies that allow for nonidentical blood type matching in liver transplantation (3)
| Policy 9.6.C |
Livers from blood type O deceased donors may be offered to any of the following:
|
| Policy 9.6.D |
Within each status 1A allocation classification, candidates are sorted in the following order:
|
Within each status 1B allocation classification, candidates are sorted in the following order:
|
Within each allocation classification, all candidates (with MELD or PELD >6) are sorted in the following order:
|
MELD, Model for End-Stage Liver Disease; PELD, Pediatric End-Stage Liver Disease.
We aimed to evaluate the impact of these policies on relative liver availability by blood type.
Methods
Data were obtained from UNOS/OPTN Standard Transplant Analysis and Research files as of September 4, 2014 (4). All adult LT candidates and deceased donor livers that were used from July 1, 2013, through May 31, 2015, were eligible for inclusion in this study. Candidates listed for fulminant hepatic failure were excluded (n = 547) because they are at imminent risk of death from their acute liver failure; therefore, the motivation to accept a nonidentical liver for transplantation may be higher than that for a patient listed with end-stage liver disease. Candidates with missing data for ABO blood type (n = 2) were also excluded. No deceased liver donors had missing data for ABO blood type.
Donor–recipient ABO matching was categorized as (i) ABO identical (recipient received a liver with the exact same ABO blood type), (ii) ABO compatible (a blood type A, B or AB recipient received a liver from blood type O donor or a blood type AB recipient received a liver from a blood type O, A or B donor), or (iii) ABO incompatible (all other combinations).
Allocation MELD score was defined as the MELD score at LT, which represents the higher of either the most updated laboratory MELD score (calculated using serum total bilirubin, creatinine and international normalized ratio for prothrombin time (5)) or exception points granted for conditions specified by UNOS/OPTN Policy 9.3.D (e.g. hepatocellular carcinoma [HCC] or hepatopulmonary syndrome) (3). To estimate donor liver quality, a donor risk index (DRI) was calculated for each deceased donor liver (6).
Statistical analysis
Comparisons of baseline characteristics by ABO blood type were made using Kruskal-Wallis tests. Expected relative liver availability by ABO blood type was calculated as the ratio of unique waitlisted candidates to deceased liver donors, assuming that all deceased donor livers were matched to ABO-identical candidates. Observed relative liver availability by ABO blood type was calculated as the ratio of unique waitlisted candidates to deceased liver donors that were actually transplanted, regardless of donor blood type.
Cox regression models were used to evaluate the association between donor–recipient ABO combinations and graft loss. Donor–recipient ABO combinations were categorized as ABO identical, ABO compatible or ABO incompatible. Graft loss was defined as death or retransplant. Recipient covariables included age, sex, race/ethnicity, serum creatinine at transplant, etiology of liver disease (categorized as chronic hepatitis C, alcohol, nonalcoholic steatohepatitis, autoimmune/cholestatic disease, chronic hepatitis B and other) and HCC. Donor covariables included the DRI (6) and donor diabetes. Covariables associated with graft loss in univariable analysis using a cutoff of p < 0.20 were evaluated for inclusion in the final multivariable model. The final multivariable model was developed using backward selection of covariables until all covariables in the final model were associated with graft loss at p < 0.05.
Analyses were performed using Stata 14.0 statistical software (Stata-Corp, College Station, TX). The institutional review board at the University of California, San Francisco approved this study.
Results
Characteristics of waitlisted candidates and donors by blood type
A total of 34 920 candidates were on the waitlist during the study period. Baseline characteristics of this cohort are shown in Table 2. Candidates were similar by age and by proportions that were female across blood types. Race/ethnicity differed by blood type; most notably, a greater proportion of blood type O candidates were Hispanic white and a greater proportion of blood type B candidates were Asian or black. The proportions of candidates with HCC were clinically similar across blood types. MELD scores at listing were similar by blood type, as were serum albumin and sodium. Patients with blood type AB had slightly lower rates of ascites and encephalopathy at listing. Wait times were significantly longer for candidates with blood types O and A than with blood type B; candidates with blood type AB waited about half the time as candidates with blood type B.
Table 2.
Characteristics of 34 920 waitlisted candidates in the United States from January 1, 2011, through December 31, 2013, by blood type
| Blood type
|
||||
|---|---|---|---|---|
| O n = 16 418 (47%) |
A n = 13 265 (38%) |
B n = 4134 (12%) |
AB n = 1103 (3%) |
|
| Age, years | 57 (50–62) | 56 (50–62) | 57 (49–62) | 57 (50–62) |
| Female | 40 | 41 | 39 | 39 |
| Race/ethnicity | ||||
| Non-Hispanic white | 67 | 77 | 62 | 68 |
| Hispanic white | 19 | 13 | 13 | 10 |
| Black | 9 | 6 | 14 | 10 |
| Asian | 4 | 3 | 10 | 11 |
| Other | 1 | 1 | 1 | 1 |
| Etiology of liver disease | ||||
| Alcohol | 19 | 19 | 17 | 17 |
| Chronic hepatitis C | 38 | 37 | 38 | 40 |
| Nonalcoholic fatty liver disease | 13 | 13 | 12 | 12 |
| Chronic hepatitis B | 3 | 3 | 5 | 5 |
| Autoimmune/cholestatic | 11 | 11 | 12 | 10 |
| Other | 16 | 17 | 16 | 16 |
| Hepatocellular carcinoma | 13 | 13 | 14 | 15 |
| Allocation MELD | ||||
| At listing | 14 (10–19) | 14 (10–19) | 14 (10–19) | 14 (10–19) |
| At transplant1 | 29 (23–36) | 28 (23–35) | 25 (22–33) | 23 (20–29) |
| Laboratory MELD | ||||
| At listing | 14 (10–19) | 14 (10–19) | 14 (10–19) | 13 (10–19) |
| At transplant1,2 | 27 (18–36) | 26 (18–35) | 24 (18–33) | 22 (16–31) |
| Albumin at listing, g/dL | 3.2 (2.7–3.6) | 3.2 (2.7–3.6) | 3.2 (2.7–3.7) | 3.2 (2.8–3.7) |
| Sodium at listing, mEq | 137 (134–140) | 137 (134–139) | 137 (134–140) | 137 (135–140) |
| Moderate ascites at listing | 26 | 27 | 25 | 25 |
| Encephalopathy at listing | 63 | 63 | 58 | 56 |
| Waitlist time, days | 380 (120–1024) | 365 (125–987) | 281 (96–913) | 196 (54–673) |
Data are shown as median (interquartile range) or percentage.
MELD, Model for End-Stage Liver Disease.
Among those who underwent liver transplant: n = 4665 for blood type O, n = 3835 for blood type A, n = 1456 for blood type B and n = 523 for blood type AB.
Among those without MELD exception points.
A total of 10 479 deceased liver donors were transplanted during the study period (Table 3). Deceased liver donors were similar by age and by proportions that were female across blood types. Substantially greater proportions of blood type B or AB donors were black. Blood type O donors were shorter by height but similar to the other blood types by BMI. A substantially greater proportion of blood type O recipients received split transplants. Rates of donation after cardiac death were slightly higher among blood type O and A donors. A substantially higher proportion of blood type AB livers were shared nationally.
Table 3.
Characteristics of 10 479 deceased liver donors and grafts in the United States from January 1, 2011, through December 31, 2013, by blood type
| Blood type
|
||||
|---|---|---|---|---|
| O n = 4966 (47%) |
A n = 3898 (37%) |
B n = 1278 (12%) |
AB n = 337 (3%) |
|
| Donor characteristics | ||||
| Age, years | 42 (28–54) | 41 (27–54) | 40 (26–53) | 38 (26–52) |
| Female | 41 | 40 | 38 | 42 |
| Race/ethnicity | ||||
| Non-Hispanic white | 64 | 73 | 54 | 62 |
| Hispanic white | 15 | 10 | 10 | 11 |
| Black | 17 | 13 | 29 | 21 |
| Asian | 2 | 2 | 6 | 4 |
| Other | 2 | 2 | 2 | 1 |
| HCV antibody positive | 6 | 5 | 3 | 5 |
| Diabetes | 13 | 13 | 13 | 5 |
| Hypertension | 37 | 33 | 35 | 36 |
| Height, cm | 172 (165–178) | 173 (165–180) | 173 (165–180) | 170 (163–178) |
| Weight, kg | 80 (68–93) | 80 (68–93) | 79 (68–93) | 76 (66–90) |
| BMI, kg/m2 | 27 (24–31) | 27 (23–31) | 27 (23–30) | 26 (23–30) |
| Cause of death | ||||
| Trauma | 33 | 31 | 33 | 33 |
| Anoxia | 30 | 32 | 32 | 31 |
| Cerebrovascular accident | 36 | 34 | 32 | 34 |
| Other | 2 | 3 | 3 | 2 |
| Transplant-related characteristics | ||||
| Partial/split | 7 | 3 | 2 | 1 |
| Donation after cardiac death | 6 | 7 | 5 | 4 |
| Share type | ||||
| Regional | 30 | 28 | 22 | 30 |
| National | 3 | 3 | 3 | 20 |
| Cold ischemia time, h | 6.0 (4.5–7.4) | 6.0 (4.7–7.5) | 6.0 (4.6–7.4) | 6.3 (5.0–8.0) |
| Donor risk index | 1.5 (1.2–1.8) | 1.5 (1.2–1.7) | 1.5 (1.2–1.8) | 1.5 (1.2–1.8) |
Data are shown as median (interquartile range) or percentage.
HCV, hepatitis C virus.
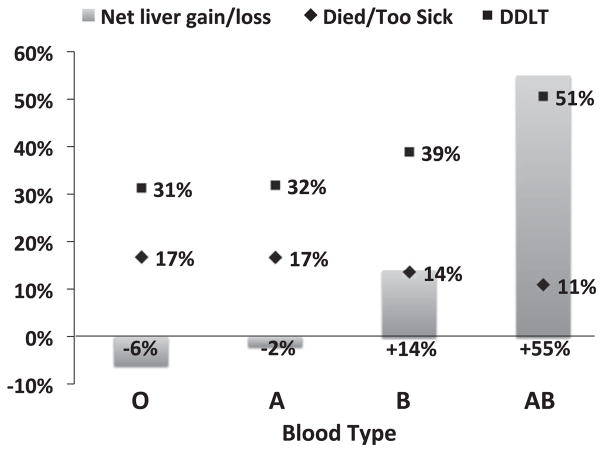
At a median wait time of 347 days (interquartile range 109–973 days) for the entire cohort, the proportion of candidates who died or were delisted for being too sick for transplant was highest for blood type O and A candidates, lower for blood type B candidates and lowest for blood type AB candidates. The opposite was true with respect to deceased donor LT (DDLT), with type AB candidates having the highest proportion of DDLT, followed by type B candidates and then type A and O candidates (Figure 1).
Figure 1. The proportion of patients who died or were delisted for being too sick for transplant (diamond) or underwent deceased donor liver transplant (square).
The columns represent the net gain or loss of livers for candidates of each blood type, accounting for ABO-nonidentical transplants, relative to the number of deceased donor livers of each blood type available during the study period. DDLT, deceased donor liver transplant.
Identical versus nonidentical transplants
Of the 10 479 DDLTs performed during the study period, 9966 (92%) were ABO identical, 704 (7%) were ABO compatible and 129 (1%) were ABO incompatible. For blood type O, A, B, and AB recipients, 98%, 97%, 80%, and 64%, respectively, received an ABO-identical liver (Table 4).
Table 4.
Number of recipients who received a liver from a donor by recipient blood type
| Donor | Recipient
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| O | Percentage of O recipients | A | Percentage of A recipients | B | Percentage of B recipients | AB | Percentage of AB recipients | Donor total | |
| O | 4554 | 98% | 117 | 3% | 292 | 20% | 3 | 1% | 4966 |
| Percentage of O donors | 92% | 2% | 6% | 0% | |||||
| A | 108 | 2% | 3715 | 97% | 1 | 0% | 74 | 14% | 3898 |
| Percentage of A donors | 3% | 95% | 0% | 2% | |||||
| B | 2 | 0% | 3 | 0% | 1162 | 80% | 111 | 21% | 1278 |
| Percentage of B donors | 0% | 0% | 91% | 9% | |||||
| AB | 1 | 0% | 0 | 0% | 1 | 0% | 335 | 64% | 337 |
| Percentage of AB donors | 1% | 0% | 0% | 99% | |||||
| Recipient total | 4665 | 3835 | 1456 | 523 | 10 479 | ||||
The proportion of recipients is reported in the column to the right of the absolute number, and the proportion of donors is reported in the row below (e.g. 65% of blood type AB recipients received a blood type AB liver, 99% of blood type AB donors were transplanted into blood type AB recipients). The boxes shaded in gray represent ABO-identical liver transplants.
Among the 111 (2%) blood type O recipients who received a liver from a donor with a nonidentical blood type, the vast majority of livers were from blood type A donors. Among the 120 (3%) blood type A recipients of an ABO-nonidentical liver, the majority were from blood type O donors. For the 294 (20%) blood type B recipients, the majority of livers were from blood type O donors. For the 188 (36%) blood type AB recipients, 111 of 188 (59%) livers were from blood type B donors, 74 of 188 (39%) were from blood type A donors and 3 of 188 (2%) were from blood type O donors (Table 4).
There was significant variation in ABO-nonidentical transplantation by UNOS region (Table 5). The proportion of ABO-nonidentical LTs ranged from 3% in region 6 to 19% in region 5. In each UNOS region, the most common nonidentical blood type pairing was O donor livers transplanted in non–type O recipients (Table 5).
Table 5.
Regional analysis of the percentage of deceased donor LTs that were ABO nonidentical, the absolute number of ABO-nonidentical LTs, and the percentages of nonidentical LTs that were O→B, A→AB, and B→AB (representing the three most common nonidentical LTs)
| UNOS region | Allocation MELD score | ABO-nonidentical LTs among all LTs, % | ABO-nonidentical LTs, n | Among ABO-nonidentical LTs, %
|
|||
|---|---|---|---|---|---|---|---|
| O→non-O | A→non-A | B→non-B | AB → non-AB | ||||
| 1 | 31 | 4 | 32 | 69 | 25 | 6 | 0 |
| 2 | 29 | 13 | 94 | 47 | 32 | 21 | 0 |
| 3 | 25 | 13 | 95 | 62 | 14 | 24 | 0 |
| 4 | 31 | 11 | 77 | 68 | 21 | 11 | 0 |
| 5 | 35 | 19 | 136 | 62 | 30 | 8 | 0 |
| 6 | 28 | 3 | 23 | 65 | 26 | 9 | 0 |
| 7 | 30 | 10 | 69 | 72 | 14 | 14 | 0 |
| 8 | 25 | 7 | 48 | 50 | 25 | 21 | 4 |
| 9 | 31 | 4 | 29 | 55 | 34 | 11 | 0 |
| 10 | 24 | 6 | 45 | 44 | 36 | 20 | 0 |
| 11 | 25 | 9 | 65 | 42 | 32 | 26 | 0 |
LT, liver transplantation.
We next evaluated the allocation and laboratory MELD scores of recipients of ABO-identical versus -nonidentical livers (Table 6). Among all recipients, the allocation MELD score for all recipients of an ABO-nonidentical liver was higher than that for ABO-identical recipients (31 vs. 27; p < 0.001). Among those listed without MELD exception points, laboratory MELD score was also higher for ABO-nonidentical versus -identical LT recipients (32 vs. 25; <0.001). Both the median allocation and laboratory MELD scores were significantly higher for type O, A and AB recipients of an ABO-nonidentical liver compared with recipients of an ABO-identical liver (Table 6). Nevertheless, blood type A recipients of an ABO-nonidentical liver had significantly lower median allocation and laboratory MELD scores than blood type A recipients of blood type A livers (Table 6). The DRI for ABO-identical versus -nonidentical transplants are also shown in Table 6. As a point of reference, the DRI for all livers transplanted in the United States is 1.47. The DRI of type A liver grafts was significantly higher at 1.62 than those for all other livers; the DRIs of ABO-nonidentical livers transplanted into both B and AB recipients were significantly lower (Table 6)
Table 6.
AMELD score and DRI of recipients of ABO-identical versus -nonidentical liver transplants by recipient blood type, reported as median (interquartile range)
| Recipient–donor ABO match
|
p-value | ||||
|---|---|---|---|---|---|
| Identical | Nonidentical | ||||
| Recipient ABO | O | AMELD | 29 (23–36) | 34 (29–40) | <0.001 |
| LMELD1 | 27 (18–36) | 33 (24–39) | <0.001 | ||
| DRI | 1.49 (1.22–1.79) | 1.48 (1.17–1.81) | 0.47 | ||
| A | AMELD | 29 (24–35) | 19 (14–25) | <0.001 | |
| LMELD1 | 26 (19–35) | 16 (12–21) | <0.001 | ||
| DRI | 1.46 (1.20–1.75) | 1.62 (1.48–1.84) | <0.001 | ||
| B | AMELD | 25 (22–29) | 38 (33–40) | <0.001 | |
| LMELD1 | 21 (16–27) | 35 (30–41) | <0.001 | ||
| DRI | 1.47 (1.20–1.77) | 1.43 (1.18–1.68) | 0.19 | ||
| AB | AMELD | 22 (19–25) | 28 (22–37) | <0.001 | |
| LMELD1 | 19 (15–24) | 29 (19–36) | <0.001 | ||
| DRI | 1.47 (1.22–1.81) | 1.36 (1.19–1.62) | 0.002 | ||
AMELD, allocation Model of End-Stage Liver Disease score; DRI, donor risk index; LMELD, laboratory Model of End-Stage Liver Disease score.
Among those without Model of End-Stage Liver Disease exception points.
In univariable analysis, receipt of an ABO-nonidentical versus -identical liver was not associated with an increased risk of graft failure (ABO compatible: hazard ratio [HR] 1.24, 95% confidence interval [CI] 0.98–1.57; p = 0.08; ABO incompatible: HR 1.11, 95% CI 0.64–1.92; p = 0.70). Other variables associated with graft failure in univariable analysis with p < 0.20 were female sex, age at transplant, recipient ethnicity, etiology of liver disease, HCC and DRI. In a final multivariable model, adjustment for all of these variables did not change the hazard of graft failure for a recipient of an ABO-nonidentical versus -identical liver (ABO compatible: HR 1.19, 95% CI 0.94–1.52, p = 0.15; ABO incompatible: HR 1.05, 95% CI 0.61–1.82, p = 0.86).
Observed versus expected relative availability of donors by blood type
If all LTs had been ABO matched identically during this study period, the expected ratio of candidates to donors would be 3.3 for blood type O (16 418 candidates, 4966 donors), 3.4 for blood type A (13 265 candidates, 3898 donors), 3.2 for blood type B (4134 candidates, 1278 donors) and 3.3 for blood type AB (1103 candidates, 337 donors). In practice, nonidentically matched transplants (accounting for both losses and gains of livers) resulted in a net loss of 301 of 4966 livers (6%) for type O candidates and 63 of 3898 livers (2%) for type A candidates but a net gain of 178 of 1278 livers (14%) for type B candidates and 186 of 337 livers (55%) for type AB candidates (Figure 1). The observed ratio of candidates to donors, accounting for these nonidentical LTs, was 3.5 for blood type O, 3.5 for blood type A, 2.8 for blood type B, and 2.1 for blood type AB.
Discussion
It would stand to reason that the timing of LT would be the same regardless of the blood type of the candidate. Barring major differences in progression of liver disease and death or donation rates by blood type, the allocation MELD score would be expected to be the same for each blood type because the numbers of candidates relative to liver donors should be proportional. This is precisely what we found. Looking purely at the total numbers of available donor livers and waitlisted candidates during the study period, we found that the ratios of candidates to donors were remarkably similar by blood type, between 2.5 and 2.6.
In practice, the waitlist experience for an LT candidate varied significantly by blood type, as we described in this paper. Wait times and transplant MELD scores (both allocation and laboratory) varied dramatically: blood type O and A candidates waited the longest and required the highest MELD scores to undergo LT, blood type AB candidates waited the shortest time and had the lowest allocation MELD score. Paralleling this pattern, blood type O and A candidates experienced the lowest rates of transplant and the highest rates of death, whereas blood type AB candidates experienced the highest rates of transplant and the lowest rates of death.
In this study, we investigated the extent to which nonidentical donor–recipient liver matching, as explicitly allowed by UNOS/OPTN, contributes to this variation in outcomes. Although only 6% of all LTs in the nation were ABO nonidentical, more than one-third of all blood type AB recipients received a liver from a non-AB donor, whereas only 2% of all blood type O recipients received a liver from a non-O donor. Transplantation with ABO-nonidentical livers resulted in a net loss of 6% for type O candidates and a net gain of 55% of livers for type AB candidates, offering a strong advantage to patients with this uncommon blood type. Consequently, the observed ratio of candidates to donors tipped strongly in favor of type AB candidates, dropping from 2.6 to 1.8.
We observed significant regional variation in utilization of ABO-nonidentical LT. As one might expect, UNOS region 5, the region with the highest allocation MELD scores, performed the highest proportion of ABO-nonidentical LTs; however, the regions that performed the lowest proportion (3–4%) of ABO-nonidentical transplants (UNOS regions 1, 6 and 9) were not the regions with the lowest allocation MELD scores. Consequently, allocation MELD score is not the only driver of ABO-nonidentical LT. Further research to identify factors associated with this practice is much needed.
There is a clear rationale for allowing ABO-nonidentical LTs in the national liver allocation system. Transplantation with an ABO-nonidentical liver may be life-saving for a patient who is at high risk of death while waiting for an identically matched liver. This may be particularly true for candidates with blood type AB, for whom blood type AB livers are available rarely (albeit proportionally to the number of AB candidates). We observed that the allocation MELD score was significantly higher at 31 for recipients of ABO-nonidentical versus -identical livers (who were transplanted at a median MELD score of 27); however, for blood type AB recipients of non-AB livers, that transplant MELD score was only 28. Allowing ABO-nonidentical LTs may also provide a mechanism to use livers that otherwise would have been discarded. The median allocation MELD score for, for example, blood type A recipients of nonidentical livers was only 16, but these livers were significantly lower in quality than the average transplanted liver. Of note, half of AB livers were transplanted regionally or nationally (compared with 25–33% for the other ABO blood types), suggesting an effort to reduce discard of AB livers that could not otherwise be transplanted into non-AB recipients.
Given the tension between introducing disparities in outcomes by blood type versus the potential utility of a system that allows for ABO-nonidentical LT, we advocate not for the abandonment of such a system but rather for its refinement. A system in which livers of any blood type could be transplanted into a nonidentical recipient only when a candidate has reached a specific MELD threshold—for example, 30, as is currently the policy for blood type B recipients receiving type O livers (3)—may encourage acceptance of livers in both ABO-identical and -nonidentical recipients at higher MELD scores. Such a system would theoretically reduce disparities in waitlist outcomes by blood type. As evidence of this, blood type B recipients of blood type O livers have a median allocation MELD score of 38 (and median laboratory MELD score of 35), well above the median MELD score of the average O recipient, suggesting effective prioritization of waitlist candidates by medical urgency. As a safeguard against excessive discard of livers, the policy could also allow for ABO-compatible liver allocation once all ABO-identical candidates have been exhausted at the regional level. A concern regarding a change in the current policy is whether reducing the supply of livers to type B and AB recipients might disproportionately disadvantage black and Asian candidates, who represent a greater proportion of type B and AB recipients. Thorough exploration of the impact of this policy on multiple dimensions of transplant, not just mortality, is needed prior to implementation of new policy surrounding ABO-nonidentical transplantation.
In conclusion, our study is the first to investigate the impact of ABO-nonidentical LT on waitlist disparities by blood type. Future work will focus on center-related variation in ABO-nonidentical transplantation and the impact of this practice on racial/ethnic disparities in transplantation. Our data provide powerful justification for what is needed next: thorough reevaluation of the UNOS/OPTN policies that allow for ABO-nonidentical matching and modification of these policies to promote a more equitable liver allocation system that provides livers in a timely fashion to those with the greatest medical urgency.
Acknowledgments
This study was funded by P30 DK026743 (UCSF Liver Center).
Abbreviations
- AMELD
allocation Model of End-Stage Liver Disease score
- CI
confidence interval
- DDLT
deceased donor liver transplant
- DRI
donor risk index
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HR
hazard ratio
- LMELD
laboratory Model of End-Stage Liver Disease score
- LT
liver transplantation
- MELD
Model for End-Stage Liver Disease
- OPTN
Organ Procurement and Transplantation Network
- PELD
Pediatric End-Stage Liver Disease
- UNOS
United Network for Organ Sharing
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.[cited 2015 Jun 22]. Available from: http://optn.transplant.hrsa.gov.
- 2.[cited 2015 Jun 22]. Available from: https://www.unos.org/policy/policy-development.
- 3.UNOS. OPTN Policies. 2015 May;:1–221. [Google Scholar]
- 4.Based on UNOS/OPTN data as of September 5, 2014.
- 5.Kamath P. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 6.Feng S, Goodrich NP, Bragg Gresham JL, et al. Characteristics associated with liver graft failure: The concept of a donor risk index. Am J Transplant. 2006;6:783–790. doi: 10.1111/j.1600-6143.2006.01242.x. [DOI] [PubMed] [Google Scholar]