Abstract
Changes in the regulation, formation, and gating of connexin (Cx)-based gap junction channels occur in various disorders. It has been shown that H+ and Ca2+ are involved in regulation of gap junctional communication. Ischemia-induced intracellular acidification and Ca2+ overload lead to closure of gap junctions and inhibit an exchange by ions and small molecules throughout the network of cells in the heart, brain and other tissues. In this study we examined the role of the polyamines, in the regulation of Cx43-based gap junction channels under elevated intracellular concentrations of hydrogen ([H+]i) and calcium ([Ca2+]i) ions. Experiments, performed in Novikoff and A172 human glioblastoma cells, endogenously express Cx43, revealed that polyamines prevent downregulation of Cx43-mediated gap junctional communication caused by elevated [Ca2+]i and [H+]i, accompanying ischemic and other pathological conditions. siRNA knockdown of Cx43 significantly reduce gap junctional communication, indicating that Cx43 gap junctions are the subjects of spermine regulation.
Keywords: connexins, Cx43, gap junction, polyamines, spermine
Introduction
Connexin-based gap junction channels provide direct intercellular communication for ionic and molecular exchanges between cells allowing to form a functional network in brain, heart and other tissues [1, 2]. Connexin 43 (Cx43) is expressed most abundantly in cardiomyocytes and astrocytes. Under physiological and a number of pathological conditions, gap junctional communication undergoes significant modifications. For example, ischemia-induced intracellular acidification leads to closure of gap junctions and reduces electrical and metabolic intercellular communication [3, 4]. Also, upon malignant transformation, astrocytes significantly reduce the expression of various connexins in the plasma membrane, especially Cx43 [5], and this correlates with increased proliferation rate. Despite this, the gap junctional communication between glioma cells remains high [6], thereby facilitating intercellular communication between cancer cells. Numerous structural and functional studies of connexin-based gap junction channels revealed that changes in the intracellular concentrations of H+ ([H+]i) and Ca2+ ([Ca2+]i) are among the key factors determining gap junctional communication and the functional state of the tissue under various pathological conditions [7, 8, 9, 10, 11]. Previously, we have demonstrated that spermine, an endogenous polyamine, plays an important role in gap junctional communication in astrocytes [12]. Spermine increases intercellular communication and prevents acid-induced uncoupling of Cx43-based gap junctions [13], which makes it vital for the maintenance of cell-to-cell communication under various pathological conditions leading to acidification during ischemia. Polyamines, such as spermine, spermidine and putrescine are organic positively charged compounds that have two or more primary amino groups and are essential for the function of both eukaryotic and prokaryotic cells. Polyamines are involved in gene expression, protein and nucleic acid synthesis, as well as in the regulation of different types of channels and receptors. Polyamines are synthesized in cells via highly regulated pathways [14] and have been associated with cell growth and proliferation. It has been shown that in human cancers, including gliomas, polyamine's concentration is significantly elevated [15]. Intriguingly, in healthy adult brain, polyamines were not found in neurons but abundantly expressed in glial cells: astrocytes of the cortex and hippocampus, Bergmann glia of cerebellum [16] and Müller cells in the retina [17]. These cells exhibit well-developed Cx43-mediated gap junctional communication [8, 10, 11] and contain high concentration of free polyamines [18].
In the present study, we examined Cx43-mediated gap junctional communication regulated by calcium, pHi and intracellular polyamines, such as spermine and spermidine. With the use of A172 human glioblastoma and Novikoff cell lines, which endogenously express Cx43, we have demonstrated that polyamines protect cells from uncoupling under experimental conditions at high [H+]i and [Ca2+]i. Thus, polyamines control and fine-tune Cx43-based gap junctional communication under a number of pathological conditions that are accompanied by tissue acidification and hypercalcemia.
Methods
Cell lines and cell cultures
Novikoff cells (a rat hepatoma cell line), endogenously expressing Cx43 [19], were grown in Swim's S-77 medium with 4 mM glutamine, 20% horse serum, and 5% fetal bovine serum as described previously [20].
The A172 glioma cell line was purchased from ATCC (#CRL1620, American Type Culture Collection (ATCC), Manassas, VA, USA) and maintained in Dulbecco's modified Eagle's medium, supplemented with 10 mM glucose, 2 mM L-glutamine, 10% fetal calf serum, and a mixture of 200 iU/ml penicillin with 200 μg/ml streptomycin at 37°C (5% CO2 in air).
Cell transfection with siRNA against Cx43
A172 cells were seeded on round cover glasses at a density of ∼104 cells/cm2 in a 24-well plate. The cells were transfected with a siRNA targeting Cx43 (Qiagen, cat. # SI02780491) by using HiPerfect Transfection reagent, according to the manufacturer's instructions (Qiagen) and our experience [21]. Briefly, 100 μL of serum-free medium containing 0.5 μL of 20 nM Cx43 siRNA and 3 μL of HiPerfect were prepared and incubated for 30 minutes at room temperature to allow formation of transfection complexes. Then, the mixture was added to glioma cells containing 0.4 mL of cell culture medium in a drop-wise fashion and the plate gently swirled to evenly distribute the transfection complex. As a control, we used mock transfections, in which 100 μL of serum-free medium contained 20 μL of HiPerfect, but not siRNA. Cells were used in the experiments 3 days after transfection. The efficiency of Cx43 knockdown was determined by using western blotting.
Electrophysiological studies in cell pairs
Cover glasses with adhered culture cells were transferred to a recording chamber (RC-27L; Warner Instr. Corp., Hamden, Connecticut, USA) adapted on the stage of an upright microscope with infrared and fluorescence attachments (BX51WI; Olympus, Shinjuku-ku, Tokyo, Japan). Cells were visualized using the Nomarski optical infrared attachment equipped with DIC (BX51WI; Olympus) and a DP30BW digital camera with DP Controller software (Olympus). The extracellular perfusion solution (EPS) (in mM): NaCl, 140; CaCl2, 2.5; MgCl2, 2; HEPES, 10; and KCl, 3. Junctional conductance (gj) was measured using a dual whole-cell patch clamp. Briefly, each cell of a pair was voltage clamped with a separate patch clamp amplifier. Transjunctional voltage (Vj) was created by stepping the voltage in one cell and keeping the voltage in the other cell constant. This induces junctional current (Ij) measured as a change in current in the un-stepped cell. Thus, gj was obtained from the ratio, by dividing the change in Ij / Vj. A HEKA (EPC-10) amplifier was used to acquire, store and analyze data. Recordings were digitized at 5 kHz and filtered at 1 kHz.
Electrodes, intracellular solutions, and fluorescent dye
Patch electrodes were made from borosilicate glass tubing (O.D., 1.5 mm; I.D., 0.84 mm; World Precision Instr., Sarasota, FL) pulled in four steps using a P-97 puller (Sutter Instr. Co., Novato, CA), with a resistance of 5–8 MΩ when filled with pipette solution. Lucifer yellow CH potassium salt, LY (L0144; net charge, –2; MW, 521.57; Sigma-Aldrich, St. Louis, MO), at a final concentration of 0.5 mM in the pipette solution (PS), was used throughout the dye-diffusion experiments.
Two micromanipulators (MX7500 with MC-1000 drive; Siskiyou Inc., Grants Pass, Oregon, USA) were used for simultaneous whole-cell voltage-clamp and current-clamp recording from two cells and for positioning micropipettes. We used standard PS containing (in mM): KCl, 140; HEPES, 10; EGTA, 2; CaCl2, 0.2; MgCl2, 1; pH = 7.2. Concentrations of SPM, SPD (0.1–10 mM), and Ca2+ were adjusted according to the experimental protocol. The free concentration of Ca2+ was adjusted based on the Maxchelator program. pH of PSs was adjusted to 6 (with MES buffer), after added SPM or SPD. To evaluate pHi under whole-cell patch-clamp conditions, we used Novikoff cells transfected with EGFP. The fluorescence intensity was measured when the external pH was equilibrated and was then normalized, and compared with the normalized pH dependence of EGFP fluorescence [9].
Western Blot Analysis
Cells were sonicated briefly in ice-cold homogenization buffer (pH 7.5) containing (in mM): Tris-HCl, 20; NaCl, 150; MgCl2, 10; EDTA, 1.0; EGTA, 1.0, PMSF, 1.0; 1% Triton X-100. The buffer also contained an additional mixture of peptide inhibitors (leupeptin, antipain, bestatin, chymostatin, pepstatin, each at a final concentration of 1.6 μg/ml). The protein concentration of cell homogenates was determined with a DC protein assay (Bio-Rad Laboratories, Hercules, CA), followed by addition of an appropriate volume of urea sample buffer (62 mM Tris/HCl pH 6.8, 4% SDS, 8 M urea, 20 mM EDTA, 5% β-mercaptoethanol, 0.015% Bromophenol Blue) for a final concentration of 0.5–1.5 μg protein/μl and incubation in a water bath at 100°C for 10 min. Clarified cell lysates were separated on 10% SDS-PAGE gels as follows: samples were loaded (10 μg/lane) onto 10% SDS-polyacrylamide gels (Protean mini-gel system; Bio-Rad Laboratories, Hercules, CA), and run for 45 min (200 V, constant). After electrophoresis, separated proteins were transferred overnight (12.5 hr, 4°C) to a PVDF membrane with a Mini Trans-Blot apparatus (12 V, constant) (Bio-Rad Laboratories, Hercules, CA) and immediately stained with India ink. Subsequently, the membranes were incubated overnight with blocking solution containing 5% BSA in 10 mM Tris, 100 mM NaCl, and 0.1% Tween 20 (TBST, pH 7.5). The proteins transferred to PVDF membranes were incubated with the corresponding primary antibody overnight at 4°C. We used rabbit polyclonal anti-Cx43 primary antibody (LifeSpan, ## LS-C175973) diluted 1:1000, followed by anti-rabbit conjugated immunoglobulins (Sigma-Aldrich). Final detection was performed with enhanced chemiluminescence methodology (SuperSignal® West Dura Extended Duration Substrate; Pierce, Rockford, IL). Signal intensity was measured using a gel documentation system (Versa Doc Model 1000, Bio Rad). In all cases, the intensity of the chemiluminescence signal was corrected for minor changes in protein content after densitometry analysis of the India ink-stained membrane.
Data Analysis
Data were analyzed using Origin 9.1 software (OriginLab, Northampton, MA, USA) and are reported as mean ± standard error of the mean. Significant differences between groups were evaluated using the t-test.
Results
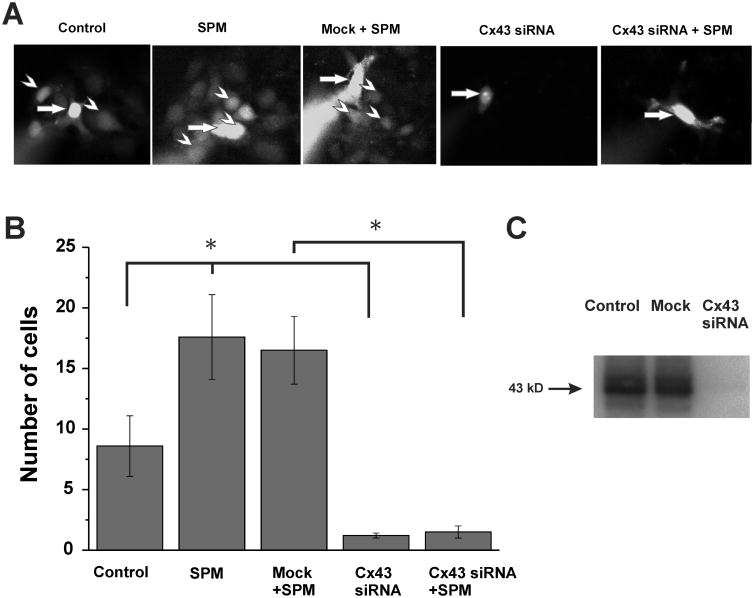
We examined the effect of spermine on Lucifer yellow (LY) diffusion between A172 cells (Fig. 1). We found that 20 min after loading of a single A172 cell with LY throughout a patch pipette (0.5 mM), the fluorescence was detected in eight neighboring cells (see Fig. 1A (Control), and averaged data are shown in Fig. 1B (Control column)). Intracellular application of spermine (0.5 mM), given together with LY, almost doubled the number of cells exhibiting fluorescence (Fig. 1A (SPM), and Fig. 1B (SPM column); p<0.02, n=5 in each group). Therefore, spermine increased noticeably and confidently the diffusion of LY between A172 cells.
Figure 1. Spermine increases gap junctional communication between A172 human glioma cells.
(A) Examples of intercellular diffusion of Lucifer yellow (LY) in confluent cultures of A172 human glioma cells expressing Cx43. Each image was acquired 20 minutes after loading a single cell (arrow) with LY (0.5 mM) through the patch pipette. In the absence of spermine (SPM) (Control), LY was detected in 8.6±2.5 (B; p<0.02, n=5) neighboring cells (arrowhead). Under presence of 0.5 mM SPM in the patch pipette LY was detected in 17.6±3.5 cells (B; p<0.02, n=5). After knockdown of Cx43 by siRNA treatment, was no detectable Lucifer yellow diffusion to neighboring cells (B; p<0.02, n=5). (B) Summary of the number of fluorescent cells following injection of LY without or with 0.5 mM SPM into control glioma cells or those treated with Cx43 siRNA. Error bars represent standard errors of the mean, and asterisks indicate significant differences (p<0.02, n=5). (C) Western blot analysis of Cx43 in A172 human glioma cells with and without siRNA knock-down of Cx43. India Ink staining used as a loading control for densitometry analysis.
Western blot analysis using antibodies against Cx43 revealed high expression of Cx43 in A172 cells (Fig. 1C). Furthermore, siRNA knockdown Cx43 expression (Fig. 1C) and reduced LY diffusion significantly below control level (see Fig. 1A (Cx43 siRNA) and Fig. 1B (Cx43 siRNA column); p<0.02, n=5 in each group), indicating that the Cx43 GJs provide a major pathway for LY diffusion between A172 cells and that SPM highly promote the diffusion of the fluorescence dye through Cx43 GJs.
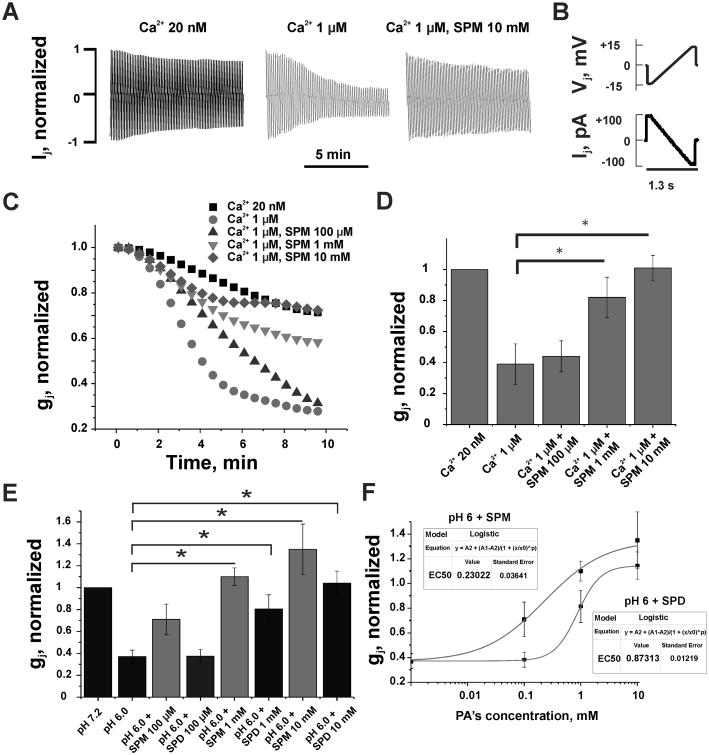
Furthermore, we examined electrical cell-cell coupling under hypercalcemic and acidic conditions in Novikoff cells (Fig. 2), which endogenously express only Cx43 [22]. Expression of other connexins in Novikoff cells was not detected [22], which makes them the best available model to study the regulation of Cx43 gap junctions. To investigate the influence of polyamines on high Ca2+-mediated uncoupling of Cx43 gap junctions, we performed dual whole-cell patch clamp recordings in Novikoff cell pairs. We found that an increase in [Ca2+]i from physiological (∼20 nM) to 1 μM caused more than 65% reduction of junctional conductance (Fig. 2; p<0.05, n=7 in each group). Intracellular application of spermine (0.1, 1, 10 mM) prevented uncoupling in a concentration-dependent manner, with full elimination of gj decay at 10 mM spermine.
Figure 2. Polyamines prevented uncoupling of Novikoff cells induced by enhanced intracellular calcium or hydrogen concentrations.
(A) Examples of junctional currents (Ij) records in response to repeated (0.7 Hz) Vj ramps shown in (B). (C) Changes in the time course of averaged and normalized gj at different [Ca2+]i and spermine (SPM). An increase of [Ca2+]i from 20 nM to 1 μM accelerated gj decay (filled circles over filled squares). Addition of SPM at 0.1 mM (up triangles), 1 mM (down triangles) and 10 mM (diamonds) decelerated gj decay up to full recovery at 10 mM of SPM. (D) A summary of data demonstrating a lessening of Ca2+-induced uncoupling by SPM (p<0.05, n=7 in each group). (E) Averaged and normalized gj show that spermidine (SPD) diminish acidosis-mediated gj decay in a concentration dependent manner (p<0.05, n=6 in each group). Data shown in grey columns (SPM) demonstrate that SPD effect is lower than that of SPM at all three concentrations. (F) Dose-response relationships of normalized gj recovery under an influence of SPM and SPD. Curves are fits of a Logistic equation to the experimental data.
Previously, we reported that spermine prevented uncoupling of Novikoff cells by intracellular acidification to pHi=6 [13]. Now, in Fig. 2E, we showed that spermidine (black columns) added to pipette solution also reduce acidification-induced closure of Cx43 GJs (when the intracellular pHi changed from normal/control (pHi=7.2) to acidic (pHi = 6), gj decreased by 70% (Fig. 2E; p<0.05, n=6 in each group)), but less efficiently then spermine (gray columns). Spermidine at a concentration of 10 mM completely eliminated uncoupling caused by acidification. Dose-response relationships shown in Fig. 2F demonstrate that spermine is ∼4-fold more effective than spermidine in preventing uncoupling caused by acidification.
Discussion
Previously we reported that spermidine increases diffusion of fluorescent dye between astrocytes through gap junction channels [12]. These data correlate with our current results obtained in A172 cells (Fig. 1) and demonstrating enhanced LY diffusion under increased intracellular concentration of spermine. In both cell types Cx43 forms majority of gap junction channels and are responsible for spermine-mediated enhancement of the gap junctional communication. A172 glioblastoma cells are originated from glia cells, and can express several connexines [23, 24]. However, our studies revealed that knock-down of Cx43 in human glioma cells eliminated not only spermine-mediated increase in LY permeability but even reduced it below control level (Fig. 1A, B). This suggests that Cx43 is a major player in polyamines induced enhancement of gap junctional communication. It has been shown that in human cancers, including gliomas, the level of polyamines is significantly elevated compared with their tissues of origin [15]. Also, it was shown that malignant cells exhibit significantly reduced, when compared with healthy astrocytes, an expression of connexins in the plasma membrane, mainly through the mechanisms of cytoplasmic mis-localization of connexins [5]. A decrease of Cx43 expression in the plasma membrane, and consequently the number of gap junction channels, accelerates proliferation due to weakening of mechanical cell-cell interaction that assists cells separation, which is necessary for cells division. However, despite a reduction of connexins expression in glioma cells, the gap junctional communication between them remained high [6]. We speculate that high level of polyamines in cancer cells maintain Cx43 gap junctions in an open state resulting to relatively high functional gap junctional communication [13]. It is possible that positively charged polyamines, especially spermine4+, shield connexins from H+ and Ca2+ ions.
In Novikoff cells expressing only Cx43, polyamines enhance gap junctional communication, and rescue gap junction channels against hypercalcemia and acidosis - mediated uncoupling (Fig. 2). These results extend our previous studies [12, 13] and demonstrate the ability of polyamines to increase diffusion of fluorescent dyes and preserve gap junction channels formed of Cx43 from their closure during an overload of cells with hydrogen and calcium ions. During physiological and a number of pathological conditions, such as ischemia and cancer, the intracellular concentration of H+ and Ca2+ in brain tissue may vary significantly. The predominant accumulation of polyamines, mostly in glial cells [16] may be related to the importance of maintaining active gap junctional communication between astrocytes in the panglial syncytium or network under physiological and pathological conditions. For example, during local ischemia, when intracellular acidification and Ca2+ levels are increased and gap junctional communication is reduced, polyamines hold Cx43 gap junctions open for diffusion of ions and molecules to enhance cell survival.
The mechanism of polyamines protecting effect from hydrogen and calcium ion-mediated closure of Cx43 gap junctions remains to be determined. We observed that the efficacy of this mechanism is proportional to the number of positively charged amino groups in polyamine molecules. Polyamines with higher net charge affect stronger an open probability of the gap junction channel. Since spermine has four positively charged amino groups and spermidine has only three, spermine causes a stronger Cx43-mediated, coupling-promoting effect than spermidine and higher efficacy of protecting gap junctions from hydrogen and Ca2+-mediated closure (Fig. 2) (putrescine has two positively charged amino groups and is less effective than spermidine (data not shown)). Combinations of polyamines, pHi, and Ca2+ concentrations in cells expressing Cx43 determine the potentiality of cell-to-cell communication and therefore the functionality of the entire network.
Conclusion
In this study, we highlighted the role of polyamines in the regulation of Cx43 gap junctions. In particular, we found that polyamines augment cell-to-cell communication and prevent uncoupling of Cx43 gap junctions induced by acidification and high [Ca2+]i.
Acknowledgments
Source of funding: This work was supported by National Institutes of Health (NIH) grants: SC2 GM095410 from NIGMS (to Y.V.K.), SC2 GM102040 (to LYK), R01 NS065201 from NINDS (to SNS), G12-MD007583 from NIMHD (to the core facilities at UCC), Title V grant number P031S130068 from the U.S. Department of Education, R01 NS072238 from NINDS (to F.F.B), and by MIP-76/2015 grant from the Research Council of Lithuania (to F.F.B). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Statement of conflicts of interest: none declared
References
- 1.Giaume C, Koulakoff A, Roux L, Holcman D, Rouach N. Astroglial networks: a step further in neuroglial and gliovascular interactions. Nat Rev. 2010;11:87–99. doi: 10.1038/nrn2757. [DOI] [PubMed] [Google Scholar]
- 2.Griemsmann S, Höft SP, Bedner P, Zhang J, von Staden E, Beinhauer A, et al. Characterization of Panglial Gap Junction Networks in the Thalamus, Neocortex, and Hippocampus Reveals a Unique Population of Glial Cells. Cereb Cortex. 2014 doi: 10.1093/cercor/bhu157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spray DC, Harris AL, Bennett MV. Gap junctional conductance is a simple and sensitive function of intracellular pH. Science. 1981;211:712–715. doi: 10.1126/science.6779379. [DOI] [PubMed] [Google Scholar]
- 4.Palacios-Prado N, Briggs SW, Skeberdis VA, Pranevicius M, Bennett MV, Bukauskas FF. pH-dependent modulation of voltage gating in connexin45 homotypic and connexin45/connexin43 heterotypic gap junctions. Proc Natl Acad Sci U S A. 2010;107(21):9897–902. doi: 10.1073/pnas.1004552107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shinoura N, Chen L, Wani MA, Kim YG, Larson JJ, Warnick RE, et al. Protein and messenger RNA expression of connexin43 in astrocytomas: implications in brain tumor gene therapy. J Neurosurg. 1996;84:839–845. doi: 10.3171/jns.1996.84.5.0839. [DOI] [PubMed] [Google Scholar]
- 6.Cottin S, Ghani K, Caruso M. Bystander effect in glioblastoma cells with a predominant cytoplasmic localization of connexin43. Cancer Gene Therapy. 2008;15:823–831. doi: 10.1038/cgt.2008.49. [DOI] [PubMed] [Google Scholar]
- 7.Bennett MV, Contreras JE, Bukauskas FF, Saez JC. New roles for astrocytes: gap junction hemichannels have something to communicate. Trends Neurosci. 2003;26:610–617. doi: 10.1016/j.tins.2003.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Contreras JE, Sáez JC, Bukauskas FF, Bennett MV. Gating and regulation of connexin 43 (Cx43) hemichannels. Proc Natl Acad Sci U S A. 2003;100(20):11388–11393. doi: 10.1073/pnas.1434298100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bukauskas FF, Kreuzberg MM, Rackauskas M, Bukauskiene A, Bennett MV, Verselis VK, et al. Properties of mouse connexin 30.2 and human connexin 31.9 hemichannels: implications for atrioventricular conduction in the heart. Proc Natl Acad Sci U S A. 2006;103(25):9726–9731. doi: 10.1073/pnas.0603372103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirst-Jensen BJ, Sahoo P, Kieken F, Delmar M, Sorgen PL. Characterization of the pH-dependent interaction between the gap junction protein connexin43 carboxyl terminus and cytoplasmic loop domains. J Biol Chem. 2006;282(8):5801–5813. doi: 10.1074/jbc.M605233200. [DOI] [PubMed] [Google Scholar]
- 11.Zayas-Santiago A, Agte S, Rivera Y, Benedikt J, Ulbricht E, Karl A, et al. Unidirectional photoreceptor-to-Müller glia coupling and unique K+ channel expression in Caiman retina. PLoS One. 2014;9(5):e97155. doi: 10.1371/journal.pone.0097155. doi:10.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benedikt J, Inyushin M, Kucheryavykh YV, Rivera Y, Kucheryavykh LY, Nichols CG, et al. Intracellular polyamines enhance astrocytic coupling. Neuroreport. 2012;23(17):1021–1025. doi: 10.1097/WNR.0b013e32835aa04b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skatchkov SN, Bukauskas FF, Benedikt J, Inyushin M, Kucheryavykh YV. Intracellular spermine prevents acid-induced uncoupling of Cx43 gap junction channels. Neuroreport. 2015;26(9):528–532. doi: 10.1097/WNR.0000000000000385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pegg AE, McCann PP. Polyamine metabolism and function. Am J Physiol Cell Physiol. 1982;243:C212–C221. doi: 10.1152/ajpcell.1982.243.5.C212. [DOI] [PubMed] [Google Scholar]
- 15.Röhn G, Els T, Hell K, Ernestus RI. Regional distribution of ornithine decarboxylase activity and polyamine levels in experimental cat brain tumors. Neurochem Int. 2001;39(2):135–140. doi: 10.1016/s0197-0186(01)00011-0. [DOI] [PubMed] [Google Scholar]
- 16.Laube G, Veh RW. Astrocytes, not neurons, show most prominent staining for spermidine/spermine-like immunoreactivity in adult rat brain. Glia. 1997;19:171–179. doi: 10.1002/(sici)1098-1136(199702)19:2<171::aid-glia8>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 17.Skatchkov SN, Eaton MJ, Krusek J, Veh RW, Biedermann B, Bringmann A, et al. Spatial distribution of spermine/spermidine content and K(+)-current rectification in frog retinal glial (Muller) cells. Glia. 2000;31:84–90. doi: 10.1002/(sici)1098-1136(200007)31:1<84::aid-glia80>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 18.Kucheryavykh YV, Shuba YM, Antonov SM, Inyushin MY, Cubano L, Pearson WL, et al. Complex rectification of Muller cell Kir currents. Glia. 2008;56:775–790. doi: 10.1002/glia.20652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lampe PD, Qiu Q, Meyer RA, TenBroek EM, Walseth TF, Starich TA, et al. Gap junction assembly: PTX-sensitive G proteins regulate the distribution of connexin43 within cells. Am J Physiol Cell Physiol. 2001;281(4):C1211–22. doi: 10.1152/ajpcell.2001.281.4.C1211. [DOI] [PubMed] [Google Scholar]
- 20.Bukauskas FF, Bukauskiene A, Bennett MV, Verselis VK. Gating properties of gap junction channels assembled from connexin43 and connexin43 fused with green fluorescent protein. Biophys J. 2001;81(1):137–52. doi: 10.1016/S0006-3495(01)75687-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kucheryavykh YV, Kucheryavykh LY, Nichols CG, Maldonado HM, Baksi K, Reichenbach A, et al. Downregulation of Kir4.1 Inward Rectifying Potassium Channel Subunit Expression by RNAi Impairs Potassium Transfer and Glutamate Uptake by Cultured Cortical Astrocytes. Glia. 2007;55:274–281. doi: 10.1002/glia.20455. [DOI] [PubMed] [Google Scholar]
- 22.Meyer RA, Laird DW, Revel JP, Johnson RG. Inhibition of gap junction and adherens junction assembly by connexin and A-CAM antibodies. J Cell Biol. 1992;119(1):179–189. doi: 10.1083/jcb.119.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Artesi M, Kroonen J, Bredel M, Nguyen-Khac M, Deprez M, Schoysman L, et al. Connexin 30 expression inhibits growth of human malignant gliomas but protects them against radiation therapy. Neuro Oncol. 2015;17(3):392–406. doi: 10.1093/neuonc/nou215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hitomi M, Deleyrolle LP, Mulkearns-Hubert EE, Jarrar A, Li M, Sinyuk M, et al. Differential connexin function enhances self-renewal in glioblastoma. Cell Rep. 2015;11(7):1031–42. doi: 10.1016/j.celrep.2015.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]