Abstract
Introduction
Many primary care patients with multimorbidity (two or more chronic conditions) and depression or anxiety have day-to-day challenges that affect health outcomes, such as having financial or housing concerns, or dealing with social or emotional stressors. Yet, primary care providers (PCPs) are often unaware of patients' daily challenges coping with chronic disease. We developed Customized Care, an intervention, to address the barriers to effective communication about patient's day-to-day challenges.
Methods
In this report we describe the rationale and design of a randomized clinical pilot study to examine the effect of Customized Care on patient-PCP communication and patient health outcomes, including depression, anxiety and functional outcomes. Customized Care comprises two components: (1) a computer-based discussion prioritization tool (DPT) designed to empower patients to communicate their health related priorities; and (2) a customized question prompt list (QPL) tailored to these priorities. Primary care clinic patients and PCPs participated in the study, which consisted of in-person patient assessments, audio recording and transcription of the patient-PCP office visit, and follow-up patient assessments by phone.
Results
We describe study participant demographics and development of a coding manual to assess communication within the office visit. Participants were recruited from an urban primary care clinic. Sixty patients and 12 PCPs were enrolled over six months.
Conclusions
With better communication about everyday challenges, patients and PCPs can have more informed discussions about health care options that positively influence patient outcomes. We expect that Customized Care will improve patient-PCP communication about day-to-day challenges, which can lead to better health outcomes.
Keywords: Doctor-patient communication, Primary care, Multimorbidity, Randomized clinical trial
1. Introduction
In the primary care setting, multimorbidity (2 or more chronic medical conditions) is increasingly common due to population aging and decreases in the age at which chronic diseases are diagnosed [1], [2]. Multimorbidity is associated with worse health function and increased mental health needs and conditions [3], [4], [5], [6], [7]. Given that successful management of chronic disease hinges on patient-primary care provider (PCP) communication [8], [9], prioritizing what to discuss in the medical encounter becomes increasingly important. When PCPs are unaware of the challenges patients face managing their health at home, they may offer treatment recommendations that make adherence difficult for the patient [10], [11]. Moreover, if patients do not feel PCPs care about their day-to-day challenges, they may lose trust in the PCP, which can further affect patient outcomes [12], [13]. Unfortunately, patients with multiple chronic diseases often report poor communications with PCPs [14], [15], perhaps because PCPs rarely inquire about their day-to-day challenges (e.g., financial, housing, social or emotional concerns). In fact, the more medical and mental health conditions a patient has, the less likely the PCP is to recognize patients' health related priorities [16]. The brisk pace of the primary care visit leads many patients to experience time pressure. Patients often feel incompetent in the encounter, and fear being labeled “difficult.” [17] These fears may be exacerbated in socioeconomically disadvantaged patients, such as those with low-income or lower educational attainment.
In order to help patients and primary care physicians discuss patient priorities, it is important to address three barriers patients face getting their needs met: 1. The arbitrary boundaries of medical care, which often marginalize if not ignore patient's life circumstances (e.g. emotional, financial and safety concerns) that affect their health; 2. The historical power asymmetry between PCPs and patients; and 3. The difficulty patients have of knowing what to prioritize for discussion with the PCP.
The tacit assumption that biomedical needs should be the primary focus of the patient visit can have profound effects on patient outcomes. For example, patients may assume that PCPs don't have time or interest to discuss patient's non-biomedical concerns [18] which could lead patients to avoid disclosing critical issues, such as family discord or financial strain; issues that can be barriers to treatment adherence and subsequent health outcomes [19]. PCPs for their part, do feel that day-to-day challenges, such as stress, housing and transportation are important to address, but they are uncertain about how and when to address these challenges in the encounter [20].
Communication around patient needs is further affected by power asymmetries between PCPs and patients because PCPs often drive the agenda [21]. Trained primarily to elicit patients' concerns in a manner that leads to diagnosis [22], PCPs frequently pay less attention to the patient's daily personal experiences and challenges, particularly when patients have multiple chronic diseases [11], [23]. Over the last decade their has been a move to change the power dynamic in medicine, starting with the Institute of Medicine's call for more patient-centered care and the incorporation of patient preferences into treatment decisions [24]. However, empowering patients to discuss what is foremost on their minds requires the recognition that many patients worry about the consequences of being assertive and may need reassurance that PCPs will be able to respond to their concerns [17].
Finally, given the power asymmetries in the patient-physician relationship and the marginalization of patients' psychosocial concerns it is hardly surprising that their day-to-day challenges rarely rise to the fore. Even under the best of circumstances, patients with multimorbidity would have a hard time knowing how and when to discuss their day-to-day challenges in the primary care encounter. Moreover, many patients have multiple challenges. Knowing which particular challenge to discuss is a cognitively complex task [25]. It is difficult for patients to recognize or consider trade-offs between multiple competing challenges intuitively or quickly [26]. Methods are needed to help patients carefully consider their priorities and communicate them in a way that is supported by their PCPs.
In this report, we describe an intervention called Customized Care and the design of a pilot study to examine the effect of Customized Care on patient-PCP communication and patient health outcomes. We describe the rationale and design of our intervention and our proposed analytic plan, including developing a coding manual to assess communication about patients' day-to-day challenges.
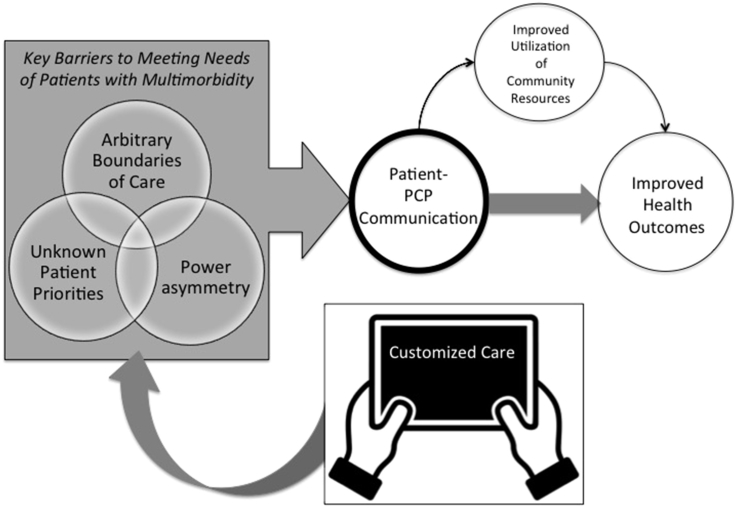
As shown in Fig. 1, the primary goal of the Customized Care intervention is to help reduce the common barriers patients face in getting their needs met. Customized Care does so, first by prompting patients to consider health related concerns that are typically marginalized helping patients prioritize their day-to-day challenges secondly by helping to overcome power asymmetries with specific language for communicating about day-to-day challenges. We anticipate that Customized Care can lead to improvements in patient- PCP communication, specifically with regard to patients' day-to-day challenges. When communication about challenges improves, patients may subsequently also be more likely to utilize community resources, leading to improved health outcomes [27].
Fig. 1.
Conceptual model, pathways to improved communications and health outcomes.
1.1. Development of the Customized Care intervention
Customized Care was developed to address key barriers to effective patient-PCP communication. The intervention builds on previous work assessing patient preferences for depression care [28] and was designed with extensive input from key stakeholders: patients with multimorbidity and PCPs. Through a series of focus groups, we learned that patients with multimorbidity want PCPs to understand more about their daily challenges. For example, one 67 year old woman with hypertension, COPD and depression said: “I like [the PCP] but she just doesn't get how challenging all these problems are on a day-to-day basis. I can barely afford enough food for the week and that makes it hard for me to pay attention to all the other things I'm supposed to do.” Focus groups with PCPs who are part of a large Practice Based Research Network (PBRN) taught us that PCPs want to know about patients' priorities but need help eliciting them in a manner that will not take more time. Furthermore, PCPs wanted more information about how to connect patients with local community services, an interest echoed by a national survey of PCPs [29]. Customized Care is intended to address the needs of both patients and PCPs.
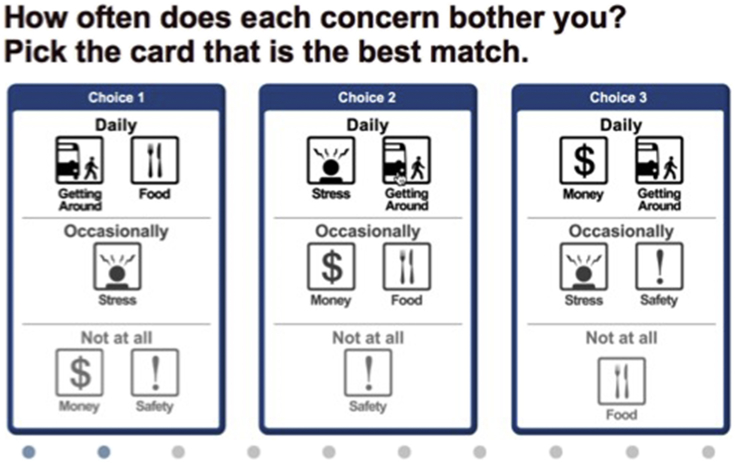
The first component of Customized Care, the computer-based DPT, was designed to help patients prioritize and communicate their day-to-day challenges to the PCP. This is important because patients and PCPs often spend more time discussing biomedical aspects of health over communicating about patient's day-to-day challenges that can affect health. Consider, for example, the patient with hypertension who presents with uncontrolled blood pressure, the PCP may prescribe another medication and order blood tests assuming the patient has progressively worsening hypertension. Yet a conversation about the patient's day-to-day challenges might have revealed that the patient has not been able to afford to buy antihypertensive medication. In order to help patients prioritize their day-to-day challenges, the DPT computer algorithm capitalizes on a type of preference measurement called conjoint analysis (CA). CA technique that requires patients to make a series of trade-offs between competing concerns (Fig. 2 shows an example of a trade-off task patients respond to in the DPT). CA can be used in this way to help reveal patients' priorities. At the same time, CA helps patients become more aware of their options for conversations. The preliminary DPT was developed together with the late Ely Dahan, who developed Adaptive Best-Worst Conjoint (ABC) [30], [31], [32].
Fig. 2.
Example of trade-off task shown in the DPT.
The second component of Customized Care is the QPL (Fig. 3), which is generated after patients use the DPT. The QPL consists of question prompts tailored to the patients' priorities, including those that had previously been implicit or those the patient may have previously dismissed as outside the boundaries of what is traditionally discussed in primary care. Additionally, the QPL provides patients with contact information for relevant community resources (e.g., transportation and housing services) that can help patients address their day-to-day challenges outside of the office visit.
Fig. 3.
Example QPL.
The Customized Care intervention was evaluated and subsequently refined through numerous iterations. We used audio-recorded “think-aloud techniques” [33] and questions adapted from the Health-IT usability scale [34] to gain feedback on ease of use, navigation and usefulness of the DPT. Similarly, follow-up focus groups with patients and PCPs were conducted to make sure the generated QPL output was worded in a way that would be helpful to both parties. The next logical step is to assess whether the DPT can improve patient-PCP communication.
1.2. Study design
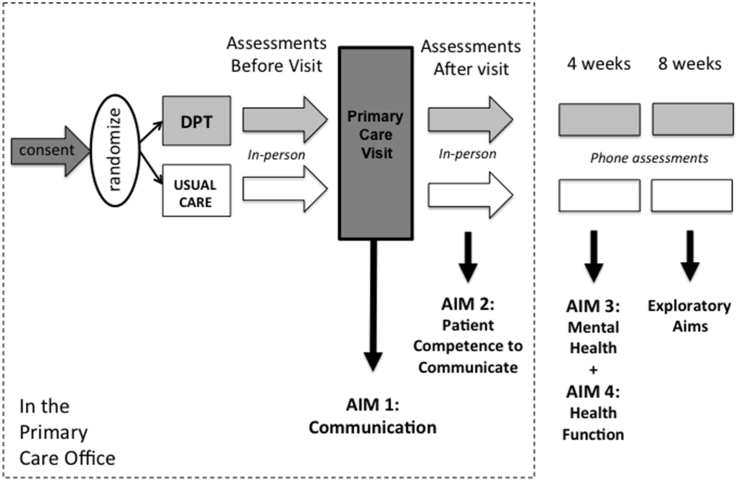
The study was designed as a randomized controlled study to evaluate the effects of Customized Care (the DPT and QPL) on patient-PCP communication and patient competence to communicate day-to-day challenges, Secondary outcomes were patient depression, anxiety and function. Primary care patients and PCPs were the participants in this study. Patient assessments occurred before and immediately after the audio-recorded office visit, and follow-up patient assessments were conducted by phone at 4 and 8 weeks after the visit (Fig. 4).
Fig. 4.
Measurement time-points.
1.3. Inclusion/exclusion criteria
PCP eligibility included all providers (including physicians and Nurse Practitioners) seeing patients at an urban primary care clinic operated by the University of Rochester Medical Center, excluding resident PCPs. Eligible patients included patients attending the clinic, age 40 and older, with diagnoses of two or more common, co-occurring chronic medical conditions (type 2 diabetes, cardiovascular disease, chronic obstructive pulmonary disease, asthma and osteoarthritis), plus a recent diagnosis of depression or anxiety (within 6 months prior to study entry), as determined solely by documentation in the electronic medical record (EMR). Patient exclusion criteria included PCP-identified cognitive deficits or history of psychosis that would limit study participation, as well as inability to read and speak English.
1.4. Recruitment
In order to recruit PCPs to participate in the study, the research staff provided an overview of the study at regularly scheduled medical staff meetings. Additionally, flyers were posted in PCP areas and reminder e-mails regarding the study were sent. Interested PCPs were briefed on the procedures for participation in the study including audio recording of the office visit. Potential PCP participants were told that the study would focus broadly on patient-PCP communication among patients with multimorbidity.
Patients of consenting PCPs were recruited using an established protocol for identifying potential patients in cooperation with the Greater Rochester Practice-Based Research Network (GR-PBRN) and approved by the University of Rochester's Institutional Review Board. An electronic medical record (EMR) database of all patients of the consenting PCPs who had two or more EMR-documented chronic medical conditions plus EMR-documented depression or anxiety was merged with the clinic appointment database generating a list of potentially eligible patients. PCPs were then requested to confirm eligibility and suitability of potential patients for the study (e.g., no cognitive deficits, no history of psychosis, able to read and speak English). Eligible patients were mailed a study information letter. Research staff then called eligible patients who had a scheduled, routine follow-up clinic appointment with their PCPs in the upcoming weeks. Based on these contacts, patients who were interested in the study were asked to arrive 30 min early to their appointment to meet with study staff and complete the consent process.
1.5. Procedures
Upon arrival to the clinic, patient-participants met with the research staff in the clinic waiting room and provided informed consent. Participants were given a brief tutorial on using a tablet computer and then responded to questions about demographics, depression, anxiety and other topics (see Table 1). Next, participants were randomized to intervention or usual care via an embedded computer program. Block randomization by PCP was used to ensure that approximately equal numbers of each participating PCP's patients were assigned to the usual care and intervention groups.
Table 1.
Study outcomes table.
| Construct | Measures | Time points |
|||||
|---|---|---|---|---|---|---|---|
| Entry | Post-random | Visit | Post-visit | 4 week | 8 week | ||
| Demographics | Individual items about gender, age, education, marital status, employment status, income, race, ethnicity | X | |||||
| Medical conditions | Modified Charlson comorbidity index | X | |||||
| Health literacy | 3- item health literacy screening item | X | |||||
| Patient-PCP Asymmetry | Perceived Autonomy Support | X | |||||
| COLLABORATE | X | ||||||
| Patient-PCP Communication | Patient competence to communicate day-to-day challenges | X | X | ||||
| Patients' Everyday Dilemmas (PED) Coding of Visit Transcripts | X | ||||||
| Active Patient Participation Coding of Visit Transcripts | X | ||||||
| Community Resources/Health Services Use | Chart review | X | |||||
| Cornell Health Services Index | X | X | |||||
| Mental Health | Generalized Anxiety Disorder-7 | X | X | X | |||
| Patient Health Questionnaire-8 | X | X | X | ||||
| Health Function | Modified 12-Item Short Form Health Function | X | X | ||||
In the intervention arm, participants completed baseline measures and the DPT on the tablet computer. Upon completion of the DPT, a customized question prompt list (QPL) was automatically generated, printed and provided to the patient to share with their PCP during their visit. Patients randomized to usual care completed baseline measures on the tablet computer and then proceeded as usual with the primary care visit.
In both intervention and usual care conditions, the ensuing routine patient-PCP office visit was audio recorded. The research staff started the audio recorder before the PCP entered the room. Immediately after the visit, post-visit questionnaires were administered to assess patients' confidence that they effectively communicated with their PCP about their day-to-day challenges.
Follow-up patient assessments were performed by telephone at 4 and 8 weeks after the PCP visit to assess mental health and functional outcomes. These follow-up times were selected in order to coincide with the common clinical practice for routine follow up in the primary care practice used for the study.
Table 1 shows the timing and type of assessments. The following were completed at study entry: demographic questionnaire, medical conditions questionnaire, items related to health literacy, mental health symptoms questionnaires and several questions about health function. The brief demographic questionnaire includes items on gender, age, education, race and ethnicity, employment, and household income. The medical conditions questionnaire contains items to assess presence of various chronic conditions, whether the patient currently takes medications for the condition, and whether the condition is felt to limit the patients' activities. Questions were included for 13 chronic medical conditions based on the Charlson comorbidity index [35]; heart disease, high blood pressure, lung disease - chronic obstructive pulmonary disease (COPD), asthma, diabetes, stomach disease (or ulcer), kidney disease, liver disease, anemia (or blood disease), cancer, osteoarthritis (or degenerative arthritis), back pain, and rheumatoid arthritis, as well as items for depression and ‘other medical problems’ allowing participants to report on additional conditions. Health literacy was assessed with a 3-item assessment which has been shown to accurately detect inadequate health literacy [36], [37]. The Patient Health Questionnaire (PHQ) [38] was used to assess depression symptoms, the GAD-7 [39] was used to assess anxiety symptoms and items modified from the Short form Health function questionnaire [40] were used to assess health functioning.
Immediately after randomization, participants completed an assessment of their confidence communicating about day-to-day challenges with their PCP using items adapted from a perceived competence scale [41], [42]. The primary care office visit was audio-recorded and transcribed. Transcripts will be coded using two coding schemes to assess patient-PCP communication: the Patients' Everyday Dilemmas (PED) Coding [43] (described in more detail below) and Active Patient Participation Coding [44]. Immediately after the visit, patients again completed the communication confidence items [45] and two assessments of their perceptions of asymmetry in the patient-PCP relationship: perceived autonomy support items, adapted from the Health Climate Questionnaire, and the Collaborate [46], [47].
At the 4 and 8 week telephone follow-up assessments patients completed the PHQ and GAD-7 in addition to a health services questionnaire which was adapted from the Cornell Health Services Index [48] and comprised of 10 items (dichotomized as yes or no) regarding the patient's involvement in the past four weeks with services that provide mental health care or social, financial, legal, housing and other practical assistance. Finally, chart review will be used to assess the number of appointments made and attended within the 8 week period of the study.
1.6. Statistical analysis plan
For this pilot study we aimed to enroll 60 patients and 10 providers. Considering this is an exploratory-developmental study, it is not powered to detect statistically significant effects of a particular size. Instead, sample size is set by the availability of resources (i.e., funding, recruitment sites and rates), and our goal is to estimate the treatment effect as precisely as possible at the highest achievable sample size (in this case, 60 patients with up to three assessments each). Our plan for the primary analysis is as follows: 1) Demographic and baseline variables will be compared between intervention and usual care groups, 2) categorical variables will be compared with chi-square tests, and 3) continuous variables will be compared using t-tests if they are normally distributed or can be transformed to a normal distribution. Otherwise, the Wilcoxon rank sum test will be used. The analytic plan provides for an intent-to-treat approach based on published guidelines [49].
For aim 1, patient-PCP communication, we will compare the intervention and usual care groups using a coding manual developed for the study [43]. All audio-recorded visits will be transcribed by a certified medical transcriptionist. All personal identifiers will be removed. An independent coding team will be assembled who will be blinded to randomization and given instructions to code each de-identified transcript using the coding manual. For aim 2, patient competence will be compared by treatment group at the time of the assessment immediately after the office visit. Depression, anxiety and health function will be compared by treatment group at 4 weeks (aim 3, 4) and at 8 weeks (exploratory aim). Generalized linear mixed models (GLMM) [51] will be applied with the appropriate link function and distribution for each outcome, random effects for PCP and time point, and fixed effects for treatment group (an indicator variable for treatment condition) and indicator variables for 4 and 8 weeks against a reference category of baseline. GLMMs provide unbiased estimates when data is “missing at random”. Drop out rate itself is considered a factor of interest in this study, however, given the need to assess the tolerability of new interventions. Interaction models will be used by the treatment condition and each time indicator variable, whereas outcomes such as depression and anxiety scores are liable to be roughly normally distributed, aim 1 outcomes are count-based and will be modeled as Poisson or Negative Binomial, as appropriate. For outcomes that do not conform to any suitable exponential family distribution, we will switch to a semi-parametric approach such as generalized estimating equations. Our goal in each model is to obtain an estimate of the difference between groups at 4 and 8 weeks, around which we will construct 95% confidence intervals using the appropriate variance estimate.
1.7. Patient participant characteristics at study entry
60 patients and 12 PCPs were recruited over 6 months for the study. Of the total patient sample, 44 (73.3%) are women and the mean age of participants is 55.4 (standard deviation [SD] 8.1) years. More than one half of the sample are African American (n = 32 [54.2%]) and four participants (6.7%) are Hispanic.
1.8. Development of the Patients' Everyday Dilemmas (PED) coding manual
A coding manual was developed in order to systematically determine and track the following key aspects of communication within the office visit: 1. How often patients disclose their day-to-day challenges; 2. At what point in the conversation are day-to-day challenges brought up; 3. Which day-to-day challenges are discussed; and 4. How often and when patients and PCPs discuss resources or services available outside of the office or health system (e.g., legal, childcare, housing services).
Since we want to ensure that our coding manual reflects the range of day-to-day challenges that are likely to be discussed in the patient–PCP visit, we read transcripts from audio recorded patient-PCP conversations (see Fig. 2). We created a coding manual development team comprised of four of the authors (MW, SY, PW and PD). Each of the team members independently read 14 transcripts, roughly half of the transcripts were from patients who had been exposed to the intervention. Through a series of in-person meetings over 3 months, we developed a set of instructions for coding and created broad content codes based on the types of day-to-day challenges that were discussed in the transcripts [43]. We categorized the day-to-day challenges into three overarching content domains: Socio-emotional, safety and services-related challenges. Socio-emotional challenges were defined as challenges related to social interactions, relationships or emotions. Sub-domains include challenges with particular emotions (e.g. grief, anger); challenges with specific and non-specific relationships (e.g. a relationship with a spouse vs. relationships with others in general); individual and institutional-level trust issues (e.g. when the patient describes a lack of trust in a particular individual, such as a nurse or a lack of trust in an entire institution, such as the pharmaceutical industry). Safety related challenges were defined as content in which patients describe feeling unsafe or worrying about safety. Safety subdomains included feeling unsafe due to home or neighborhood violence; feeling unsafe due to worries about injuries or falls; and feeling unsafe due to medications or medication interactions. For each of the general content domains and sub-domains we developed a description and provided examples of coded text, loosely based on actual quotes from the de-identified transcripts.
2. Discussion
This paper describes our plan to test an innovative solution to address key barriers to communication in the primary care encounter with the goal of improving health outcomes for patients with multimorbidity. When patients become more engaged in their health care, their outcomes are better [52]. Helping patients communicate their day-to-day challenges prior to seeing the PCP could increase their sense of competence, which has been shown to drive positive health outcomes [45].
As with any study, there are several limitations to consider. Although PCPs and patients are both blinded to the study outcomes, PCPs are not blinded to randomization arm. Moreover, PCPs will see patients in each study arm. To offset the concern about PCPs potentially treating control patients as though they were in the intervention arm, we considered randomizing at the PCP or practice levels. However, this preliminary study's inherent budgetary and time constraints preclude cluster randomization. Further, while we recruited patients with a recent EMR diagnosis of depression or anxiety, we acknowledge that some participants may have few symptoms of depression and anxiety at study entry, reducing the likelihood that we will detect an effect on mental health outcomes. This is not a major concern, as the primary outcome is patient-centered communication, not mental health. Finally, we recognize that not all primary care practices will have access to tablet-computers. The technology we developed can be easily adapted to other types of technology however, such as mobile or smart-phone applications. Moreover the application can be made accessible through patient portals to the electronic medical record that could be accessed from home.
When patients have multiple chronic medical conditions in the context of multiple day-to-day challenges, patient–provider communication becomes increasingly important. Our conceptualization of Customized Care recognizes patients as the experts in their own circumstances [53]. This patient-centered approach to communication builds on our observational and experimental research in primary care [54], [55], [56], [57], [58], [59] and the recognition that brief technology-based interventions can be used to do more than administer questionnaires. Increasingly, technology interventions are being used at the point-of-care, such as in patient waiting rooms, to educate patients and elicit patients' preferences. [60], [61] We propose that such tools can also empower patients to bring up aspects of their health, such as day-to-day challenges, that providers care about but may not explicitly elicit [62]. With the aid of technology, patients and PCPs might more efficiently communicate about what matters most to patients, by for example, focusing earlier in the visit on the pragmatic aspects of coping with chronic disease. Ideally such conversations could help patients and PCPs discuss treatments and resources that are customized to patient's particular circumstances, leading to improved outcomes.
Conflict of interest
There are no conflicts of interest to disclose. All study procedures were approved by the Research Subjects Review Board of the University of Rochester School of Medicine.
Acknowledgements
This work was supported by the National Institute of Mental Health (R34101236) and Leonard Salzman and Hendershot Research Development Funds, Department of Psychiatry, University of Rochester School of Medicine.
We also wish to acknowledge the important contribution of the late Ely Dahan, PhD for his work on the early versions of the Discussion Prioritization Tool and we wish to thank the primary care patients and providers who provided invaluable input and feedback in developing and testing the intervention.
References
- 1.Fortin M., Hudon C., Haggerty J., van den Akker M., Almirall J. Prevalence estimates of multimorbidity: a comparative study of two sources. BMC Health Serv. Res. 2010;10 doi: 10.1186/1472-6963-10-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van den Akker M., Buntinx F., Metsemakers J.F.M., Roos S., Knottnerus J.A. Multimorbidity in general practice: prevalence, incidence, and determinants of co-occurring chronic and recurrent diseases. J. Clin. Epidemiol. 1998;51(5):367–375. doi: 10.1016/s0895-4356(97)00306-5. [DOI] [PubMed] [Google Scholar]
- 3.Blazer D.G., Kessler R.C., McGonagle K.A., Swartz M.S. The prevalence and distribution of major depression in a national community sample: the National Comorbidity Survey. Am. J. psychiatry. 1994;151(7):979–986. doi: 10.1176/ajp.151.7.979. [DOI] [PubMed] [Google Scholar]
- 4.McGaw J.L. Whole patient care: reaching beyond traditional healthcare. Front. health Serv. Manag. 2008;25(2):39–42. discussion 43, 45-36. [PubMed] [Google Scholar]
- 5.Tinetti M.E., Fried T.R., Boyd C.M. Designing health care for the most common chronic condition–multimorbidity. JAMA J. Am. Med. Assoc. 2012;307(23):2493–2494. doi: 10.1001/jama.2012.5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gijsen R., Hoeymans N., Schellevis F.G., Ruwaard D., Satariano W.A., van den Bos G.A. Causes and consequences of comorbidity: a review. J. Clin. Epidemiol. 2001;54(7):661–674. doi: 10.1016/s0895-4356(00)00363-2. [DOI] [PubMed] [Google Scholar]
- 7.Neeleman J., Ormel J., Bijl R.V. The distribution of psychiatric and somatic III health: associations with personality and socioeconomic status. Psychosom. Med. 2001;63(2):239–247. doi: 10.1097/00006842-200103000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Bayliss E.A., Steiner J.F., Fernald D.H., Crane L.A., Main D.S. Descriptions of barriers to self-care by persons with comorbid chronic diseases. Ann. Fam. Med. 2003;1(1):15–21. doi: 10.1370/afm.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bayliss E.A., Edwards A.E., Steiner J.F., Main D.S. Processes of care desired by elderly patients with multimorbidities. Fam. Pract. 2008;25(4):287–293. doi: 10.1093/fampra/cmn040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin L.R., Williams S.L., Haskard K.B., Dimatteo M.R. The challenge of patient adherence. Ther. Clin. risk Manag. 2005;1(3):189–199. [PMC free article] [PubMed] [Google Scholar]
- 11.Weiner S.J., Schwartz A. Contextual errors in medical decision making: overlooked and understudied. Acad. Med. 2016;91(5):657–662. doi: 10.1097/ACM.0000000000001017. [DOI] [PubMed] [Google Scholar]
- 12.Street R.L., Jr. How clinician-patient communication contributes to health improvement: modeling pathways from talk to outcome. Patient Educ. Couns. 2013;92(3):286–291. doi: 10.1016/j.pec.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Street R.L., Jr., Makoul G., Arora N.K., Epstein R.M. How does communication heal? Pathways linking clinician-patient communication to health outcomes. Patient Educ. Couns. 2009;74(3):295–301. doi: 10.1016/j.pec.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 14.Bayliss E.A., Bosworth H.B., Noel P.H., Wolff J.L., Damush T.M., McIver L. Supporting self-management for patients with complex medical needs: recommendations of a working group. Chronic Illn. 2007;3(2):167–175. doi: 10.1177/1742395307081501. [DOI] [PubMed] [Google Scholar]
- 15.Jerant A.F., von Friederichs-Fitzwater M.M., Moore M. Patients' perceived barriers to active self-management of chronic conditions. Patient Educ. Couns. 2005;57(3):300–307. doi: 10.1016/j.pec.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Zulman D.M., Kerr E.A., Hofer T.P., Heisler M., Zikmund-Fisher B.J. Patient-provider concordance in the prioritization of health conditions among hypertensive diabetes patients. J. general Intern. Med. 2010;25(5):408–414. doi: 10.1007/s11606-009-1232-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frosch D.L., May S.G., Rendle K.A., Tietbohl C., Elwyn G. Authoritarian physicians and patients' fear of being labeled 'difficult' among key obstacles to shared decision making. Health Aff. (Millwood). 2012;31(5):1030–1038. doi: 10.1377/hlthaff.2011.0576. [DOI] [PubMed] [Google Scholar]
- 18.Wittink M.N., Barg F.K., Gallo J.J. Unwritten rules of talking to doctors about depression: integrating qualitative and quantitative methods. Ann. Fam. Med. 2006;4(4):302–309. doi: 10.1370/afm.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weiner S.J., Schwartz A., Weaver F. Contextual errors and failures in individualizing patient care: a multicenter study. Ann. Intern Med. 2010;153(2):69–75. doi: 10.7326/0003-4819-153-2-201007200-00002. [DOI] [PubMed] [Google Scholar]
- 20.RWJF . Robert Wood Johnson Foundation; 2011. Health Care's Blind Side: the Overlooked Connection between Social Needs and Good Health. [Google Scholar]
- 21.Roter D., Hall J. Praeger Publishers; Westport, CT: 2005. Doctors Talking with Patients/Patients Talking with Doctors. [Google Scholar]
- 22.Voigt I., Wrede J., Diederichs-Egidi H., Dierks M.L., Junius-Walker U. Priority setting in general practice: health priorities of older patients differ from treatment priorities of their physicians. Croat. Med. J. 2010;51(6):483–492. doi: 10.3325/cmj.2010.51.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marvel M.K., Epstein R.M., Flowers K., Beckman H.B. Soliciting the patient's agenda: have we improved? JAMA J. Am. Med. Assoc. 1999;281(3):283–287. doi: 10.1001/jama.281.3.283. [DOI] [PubMed] [Google Scholar]
- 24.(IOM) IoM . National Academy Press; Washington DC: 2001. Crossing the Quality Chasm: a new health system for the 21st century. [PubMed] [Google Scholar]
- 25.Thaler R.H., Sunstein C.R. Penguin Books; New York: 2009. Nudge : Improving Decisions about Health, Wealth, and Happiness. Rev. and expanded ed. [Google Scholar]
- 26.Kahneman D. first ed. Farrar, Straus and Giroux; New York: 2011. Thinking, Fast and Slow. [Google Scholar]
- 27.Bertakis K.D., Azari R. Patient-centered care is associated with decreased health care utilization. J. Am. Board Fam. Med. JABFM. 2011;24(3):229–239. doi: 10.3122/jabfm.2011.03.100170. [DOI] [PubMed] [Google Scholar]
- 28.Wittink M.N., Cary M., Tenhave T., Baron J., Gallo J.J. Towards patient-centered care for depression: conjoint methods to tailor treatment based on preferences. Patient. 2010;3(3):145–157. doi: 10.2165/11530660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Health Care's Blind Side: the Overlooked Connection between Social Needs and Good Health. Robert Wood Johnson Foundation; 2011. [Google Scholar]
- 30.Dahan E. Medicine 2.0 World Congress on Social Media, Mobile Apps, Internet/Web 2.0. 2012. Enhancing in-clinic doctor-patient communication in real time through adaptive best-worst conjoint (ABC) analysis. (Boston, USA) [Google Scholar]
- 31.E. Dahan, S. Lambrechts, R. Kaplan, C. Saigal. Treatment Preferecnes Derived Using Adaptive Best-Worst Conjoint (ABC) Analysis. Paper presented at: Society for Medical Decision Making 2012; Phoenix, AZ.
- 32.Dahan E. 2012. Conjoint Analysis in Healthcare Conference March 21, 2012. (Orlando, Florida) [Google Scholar]
- 33.Jaspers M.W.M., Steen T., van den Bos C., Geenen M. The think aloud method: a guide to user interface design. Int. J. Med. Inf. 2004;73(11–12):781–795. doi: 10.1016/j.ijmedinf.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 34.Yen P, Wantland D, Bakken S. Development of a customizable health it usability evaluation scale. Paper Presented at: AMIA Annual Symosium 2010. [PMC free article] [PubMed]
- 35.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 36.Chew L.D., Bradley K.A., Boyko E.J. Brief questions to identify patients with inadequate health literacy. Fam. Med. 2004;36(8):588–594. [PubMed] [Google Scholar]
- 37.Chew L.D., Griffin J.M., Partin M.R. Validation of screening questions for limited health literacy in a large VA outpatient population. J. Gen. Intern Med. 2008;23(5):561–566. doi: 10.1007/s11606-008-0520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kroenke K., Strine T.W., Spitzer R.L., Williams J.B., Berry J.T., Mokdad A.H. The PHQ-8 as a measure of current depression in the general population. J. Affect Disord. 2009;114(1–3):163–173. doi: 10.1016/j.jad.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 39.Spitzer R.L., Kroenke K., Williams J.B., Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch. Intern Med. 2006;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 40.Ware J., Kosinski M., Keller S.D. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med. Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 41.Williams G.C., Deci E.L. Internalization of biopsychosocial values by medical students: a test of self-determination theory. J. Pers. Soc. Psychol. 1996;70(4):767–779. doi: 10.1037//0022-3514.70.4.767. [DOI] [PubMed] [Google Scholar]
- 42.Williams G.C., Freedman Z.R., Deci E.L. Supporting autonomy to motivate patients with diabetes for glucose control. Diabetes Care. 1998;21(10):1644–1651. doi: 10.2337/diacare.21.10.1644. [DOI] [PubMed] [Google Scholar]
- 43.Wittink M.N., Walsh P., Yilmaz S., Chapman B., Duberstein P. University of Rochester School of Medicine; 2016. Assessing Patients' Disclosure of Day-to-day Health-related Challenges: Development of the Patients' Everyday Dilemmas Coding Scheme. [Google Scholar]
- 44.Street R.L., Jr., Millay B. Analyzing patient participation in medical encounters. Health Commun. 2001;13(1):61–73. doi: 10.1207/S15327027HC1301_06. [DOI] [PubMed] [Google Scholar]
- 45.Williams G.C., Lynch M., Glasgow R.E. Computer-assisted intervention improves patient-centered diabetes care by increasing autonomy support. Health Psychol. official J. Div. Health Psychol. Am. Psychol. Assoc. 2007;26(6):728–734. doi: 10.1037/0278-6133.26.6.728. [DOI] [PubMed] [Google Scholar]
- 46.Barr P.J., Thompson R., Walsh T., Grande S.W., Ozanne E.M., Elwyn G. The psychometric properties of CollaboRATE: a fast and frugal patient-reported measure of the shared decision-making process. J. Med. Internet Res. 2014;16(1):e2. doi: 10.2196/jmir.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elwyn G., Barr P.J., Grande S.W., Thompson R., Walsh T., Ozanne E.M. Developing CollaboRATE: a fast and frugal patient-reported measure of shared decision making in clinical encounters. Patient Educ. Couns. 2013;93(1):102–107. doi: 10.1016/j.pec.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 48.Sirey J.A., Meyers B.S., Teresi J.A. The Cornell Service Index as a measure of health service use. Psychiatr. Serv. 2005;56(12):1564–1569. doi: 10.1176/appi.ps.56.12.1564. [DOI] [PubMed] [Google Scholar]
- 49.Lachin J.M. Statistical considerations in the intent-to-treat principle. Control Clin. Trials. 2000;21(3):167–189. doi: 10.1016/s0197-2456(00)00046-5. [DOI] [PubMed] [Google Scholar]
- 51.Davidian M., Giltinan D.M. Nonlinear models for repeated measurement data: an overview and update. J. Agric. Biol. Environ. Statistics. 2003;8(4):387–419. [Google Scholar]
- 52.Clever S.L., Ford D.E., Rubenstein L.V. Primary care patients' involvement in decision-making is associated with improvement in depression. Med. care. 2006;44(5):398–405. doi: 10.1097/01.mlr.0000208117.15531.da. [DOI] [PubMed] [Google Scholar]
- 53.Coulter A., Collins A. The King's Fund; London: 2011. Making Shared Decision-making a Reality: No Decision about Me, without Me. [Google Scholar]
- 54.Wittink M.N., Givens J.L., Knott K.A., Coyne J.C., Barg F.K. Negotiating depression treatment with older adults: primary care providers' perspectives. J. Ment. Health. 2011;20(5):429–437. doi: 10.3109/09638237.2011.556164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wittink M.N., Morales K.H., Cary M., Gallo J.J., Bartels S.J. Towards personalizing treatment for depression: developing treatment values markers. Patient. 2013;6(1):35–43. doi: 10.1007/s40271-013-0003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fernandez Y-Garcia E., Duberstein P., Paterniti D.A., Cipri C.S., Kravitz R.L., Epstein R.M. Feeling labeled, judged, lectured, and rejected by family and friends over depression: cautionary results for primary care clinicians from a multi-centered, qualitative study. BMC Fam. Pract. 2012;13:64. doi: 10.1186/1471-2296-13-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wittink M.N., Duberstein P., Lyness J.M. Oxford University Press; 2013. Late-life Deprssion in the Primary Care Setting: toward a Patient Centered Future. [Google Scholar]
- 58.Poleshuck E., Wittink M., Crean H. Using patient engagement in the design and rationale of a trial for women with depression in obstetrics and gynecology practices. Contemp. Clin. Trials. 2015;43:83–92. doi: 10.1016/j.cct.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 59.Kravitz R.L., Franks P., Feldman M.D. Patient engagement programs for recognition and initial treatment of depression in primary care: a randomized trial. JAMA J. Am. Med. Assoc. 2013;310(17):1818–1828. doi: 10.1001/jama.2013.280038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grant R.W., Uratsu C.S., Estacio K.R. Pre-Visit Prioritization for complex patients with diabetes: randomized trial design and implementation within an integrated health care system. Contemp. Clin. Trials. 2016;47:196–201. doi: 10.1016/j.cct.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kinnersley P., Edwards A., Hood K. Interventions before consultations to help patients address their information needs by encouraging question asking: systematic review. BMJ. 2008;337:a485. doi: 10.1136/bmj.a485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weiner S.J. From research evidence to context: the challenge of individualising care. Equine Vet. J. 2006;38(3):195–196. doi: 10.2746/042516406776866426. [DOI] [PubMed] [Google Scholar]