Abstract
The skeletal musculature is usually thought of as the primary organ of locomotion, and, like the tyres of a high-performance racing car, their composition, design, preparation and plasticity can make the difference between winner and ‘wannabe’. The similarities do not end there, however. Their primary components (cells of the mesodermal layer in the embryo and latex from the rubber tree) begin their existence in locations that can be quite distant from their final point of use and in forms that bear no resemblance to the final product. Their differentiation from primary material to final product entails extensive processing, and the integration of other materials and structures are essential to ensure their function. A fundamental difference, however, is that, in the case of muscle, once the embryo is formed, the progression from relatively undifferentiated mesodermal cells to the final structures is on autopilot, provided there are no contextual aberrations either from genetic or environmental causes. Our current understanding of how muscles develop is a synthesis of observations made on a wide array of organisms, including nematode worms, fruitflies, fish, frogs, birds and various mammals, as well as from the in vitro study of cells isolated from these species. The study of myogenesis in mammals, although less amenable to experimental manipulation, has been facilitated by the recent advances in mouse genetic engineering which has enabled the function of individual genes and cell types to be investigated, as well as the lineage of cells to be traced back to their origin. In this rapid trek through the life of a muscle, how the production of a mature functional muscle from its early inception is orchestrated will be outlined in exceedingly broad strokes so as to convey the wide range of processes that must be engaged in order to generate a functional muscle. Hopefully, enough information will be provided to encourage those interested to explore further.
Keywords: hypertrophy muscle, myogenesis, myogenic regulatory factors, satellite cell
The basic cellular unit of mature skeletal muscle is the myofibre (Figure 1). This is a complex multinucleated cell containing the contractile proteins organized into myofibrils and sarcomeres, a complex membrane system that includes the sarcoplasmic reticulum, T-tubules and outer membranes, and the sarcoplasmic proteins and organelles necessary for energy production and maintenance of other housekeeping cell functions. Each fibre is supplied by a network of blood vessels and, when mature, is innervated by a single motor neuron via the neuromuscular junction. The basement membrane, composed of extracellular matrix materials (primarily collagen and laminin), surrounds each fibre and is linked to the sarcolemma via integrin proteins. The basement membrane is responsible for most of the muscle's tensile strength and elasticity and also acts as a scaffold anchoring the fibres to the endomysium and to the muscle tendons. Sandwiched between the basal membrane and the sarcolemma are satellite cells that represent a heterogeneous population of stem cells. As the nuclei in the differentiated muscle fibre are post-mitotic, satellite cell division is responsible for the provision of myonuclei necessary for postnatal fibre hypertrophy and muscle repair in the adult (it is unfortunate that the ability to self-repair is a feature of muscles that is not shared by tyres). Although this basic structure applies to all muscles, there is considerable heterogeneity in their metabolic and contractile properties that serve to accommodate the wide range of functional demands of the body's musculature1.
Figure 1.
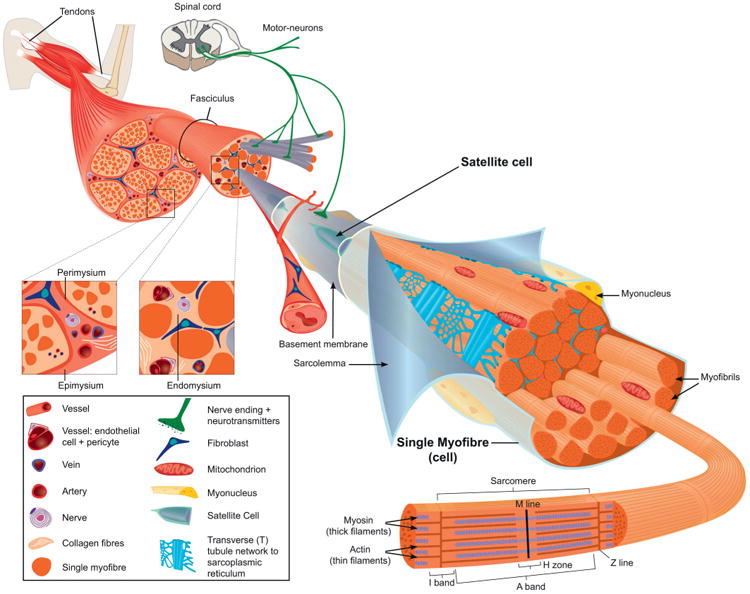
The structural organization of a fully mature skeletal muscle and its accessories. The sarcomeres are the basic contractile units of the muscle, and are arranged in series to form myofibrils. The myofibrils are aligned along their longitudinal axis and constitute the largest component of the muscle fibre. The muscle fibres are packaged in three connective tissue layers which serve to form the muscle: the endomysium surrounds individual fibres and electrically insulates them from each other; the perimysium bundles groups of myofibres together into fascicules and, in turn, groups of fascicules are wrapped together by the epimysium which is also contiguous with muscle tendons and the bone endosteum. The endomysium and epimysium also provide a matrix to house the nerves and blood vessels that supply the muscle fibres. The satellite cells are sandwiched between the basement membrane and the sarcolemma and often are found near blood vessels. Myonuclei are located at the periphery of the myofibres just underneath the sarcolemma; in muscles that have been injured and repaired, they may be located centrally. There are two populations of mitochondria that have either subsarcolemmal or intermyofibrillar locations and differ in their functional properties. The sarcoplasmic reticulum is a network of flattened tubules around each myofibril and is responsible for the release and uptake of Ca2+ associated with muscle contraction. The T-tubules are perpendicular to the main axis of the fibre, and formed from invaginations of the sarcolemma; their function is to transmit the action potential from the muscle fibre surface to its interior. Each mature myofibre is innervated by a single motor neuron that makes contact with the myofibre at the neuromuscular junction. (Reprinted from Tajbakhsh 3; © 2009 Blackwell Publishing Ltd.)
In the adult, the skeletal muscles constitute the largest protein pool in the body and comprise approximately 45% of fat-free mass in healthy adult women and 53% in healthy adult men. These levels are attained after puberty having started out at only approximately 25% of the fat-free mass in the newborn2. This increase in the proportion of the lean mass in skeletal muscle indicates that its growth out-paces that of the other lean components of the body during childhood. The formation of muscles therefore occurs exclusively in prenatal life and involves cell differentiation, migration and fusion, in contrast with postnatal growth which largely entails expansion in mass. These fundamentally different growth processes are regulated by distinct molecular mechanisms that, in turn, are engaged by the extracellular cues they receive and the intracellular signalling pathways and regulatory networks they activate. During early myogenesis, the extracellular cues are derived from the surrounding cellular environment and via receptors convey spatial and temporal information to the cells for the specification of muscle founder cells and to guide their subsequent differentiation and organization into the muscles of the body. The mechanisms employed ultimately lead to chromatin remodelling and histone modifications that permit the expression of transcription factors. It is through these transcription factors and the associated expression of microRNAs (miRNAs) that the genes and their products that confer cells with their appropriate identity are expressed. Once the muscle is differentiated, the primary signals that regulate its growth are those that provide information on substrate availability, environmental conditions and the functional demands required of the muscle. At the intracellular level, the regulatory processes engaged are similar, but with some differences in the families of factors engaged to regulate gene expression.
From mesoderm to myoblast
Myogenesis begins in early embryonic life and can be divided into two main phases that culminate in the formation of myofibres within anatomically discrete muscles (Figure 2)3. The onset of a third phase of muscle growth begins with the fusion of myoblasts and the expression of muscle-specific proteins which result in the formation of the muscle structures responsible for the functional development of the tissue. The first myogenic phase occurs in the early embryo and results in the formation of the dermomyotome from the paraxial mesoderm derived from the primitive germ layers in the embryo4. The paraxial mesoderm is organized into aggregates of epithelial cells, the somites, on either side of the neural tube and notochord and proceeds from head to tail. The formation of the somites is orchestrated by synchronous oscillations in the expression of genes (termed the segmentation clock) associated with Notch signalling and by the action of rostrocaudal morphogen gradients that controls which cells will differentiate. Specifically, there is posterior to anterior gradient in the activity of the fibroblast growth factor (FGF) and Wnt/β-catenin signalling pathways which is responsible for maintenance of the presomitic mesodermal cells in the undifferentiated state. This is opposed by an anterior to posterior gradient in the activity of the retinoic acid (vitamin A) signalling pathway which promotes differentiation and segmentation into the somites when FGF and Wnt levels are low. Once formed, positive and negative regulators, in the form of diffusible molecules produced by the adjacent tissues, induce the somites to undergo further differentiation. The signals to cells on the ventral aspect of the somite specify the formation of the sclerotome which gives rise to the ribs and skeleton. The dorsal surface of the somite forms the dermomyotome and contains the progenitor cells for the skeletal musculature (including the muscle fibres, tendons, endothelial cells and connective tissue) in the limbs and trunk, and some head muscles (the remainder of the head muscles, such as the anterior neck, jaw, facial and extraocular muscles, derive from cells of the paraxial head mesoderm and the prechordal mesoderm) as well as for the dermis, smooth muscles, angiogenic cells, and brown adipose tissue. Specification of the muscle lineage in the cells of the dermomyotome is through the expression of the paired domain homeobox transcription factors Pax3 and Pax75. Pax3 is expressed early throughout the cells of the dermomyotome, whereas later Pax7 expression is restricted primarily to its central portion. Cells from the dorsomedial (epaxial) and ventrolateral (hypaxial) edges as well as the central part of the dermomyotome migrate under the dermomyotome to form the myotome, where the cells become determined muscle precursor cells (Figure 2). The epaxial myotome gives rise to the deep back muscles, whereas the lateral trunk muscles form from the hypaxial myotome. Additionally, Pax3-expressing progenitor cells from the ventromedial lip of the dermomyotomes located at the level of the limbs undergo an epithelial-to-mesenchymal transformation, delaminate and, under the guidance of the positional cues from their surroundings, migrate to regions of the limbs, ventral body wall, diaphragm and tongue where they undergo further rounds of replication before differentiating into muscles. This process requires the activation of the c-met receptor by hepatocyte growth factor (HGF) produced by non-somitic mesenchymal cells. Transcription of the c-met gene is activated by Pax3, and, in its absence, no limb muscles form. Pax3+ somitic cells also contribute to the endothelial lineage in the limbs and are critical for muscle formation.
Figure 2.
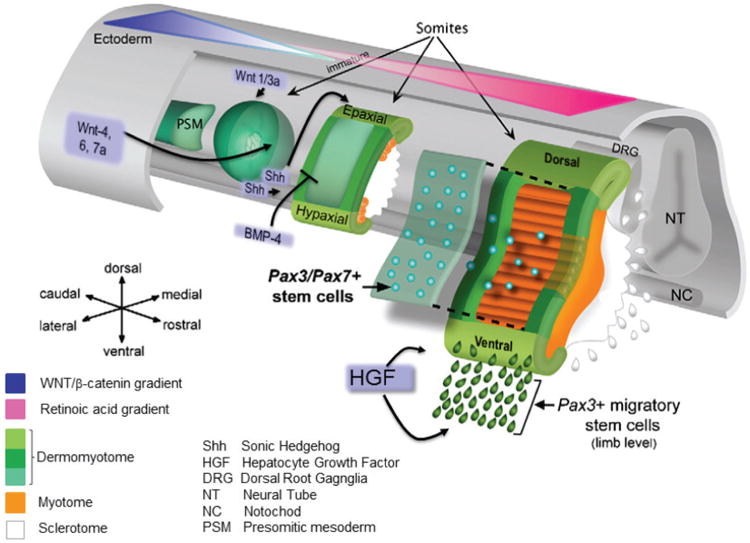
A schematic representation of the formation of the myotome from the presomitic mesoderm in a mouse embryo. Under the control of morphogen gradients and the segmentation clock, the presomitic mesoderm forms immature epithelial somites. Diffusible molecules (e.g., Wnts and BMPs) from surrounding cells induce differentiation of somites into the dorsal dermomyotome (DM) and ventral sclerotome. Pax3+ muscle progenitor cells along the edges of the DM migrate under the DM to form the myotome where they elongate along the axis of the embryo to span the entire somite. These are later supplemented by Pax3/Pax7+ cells from the central portion of the DM. Under HGF stimulation, Pax3+ myoblast cells from the ventrolateral edge of the DM of some somites delaminate and migrate into the limbs, diaphragm and tongue and differentiate into these muscles. (Modified from Figure 1A from Sambasivan, R. and Tajbakhsh, S. (2007) Skeletal muscle stem cell birth and properties. Semin. Cell Dev. Biol. 18, 870–882 ©2007, with permission from Elsevier14.)
The transition from muscle progenitor to muscle precursor cell or myoblast, a higher level of commitment to the muscle lineage, occurs upon the activation of myogenic regulatory factors (MRFs), specifically those that encode the basic helix–loop–helix (bHLH) transcription factors Myf5, MyoD and MRF4 in Pax3/7-expressing cells3,6,7 (Figure 3). Myogenin is the fourth MRF that is expressed once cell division has stopped and is responsible for differentiation of committed precursors that culminates in the expression of muscle-specific proteins. MRF expression appears to be spatially and temporally regulated by a balance between positive regulators, such as the Wnts and Sonic hedgehog (Shh), and negative regulators, such as the bone morphogenetic proteins (BMPs) and the Notch signalling pathway that inhibit myogenic differentiation. The net signalling intensity sensed by the cells, and hence the molecular mechanisms that are activated, depends on their exact location with respect to the signalling source, and the cells' sensitivity and responsiveness to individual factors. As only a few migratory progenitor cells reach their target location, inhibition of differentiation by negative regulators is critical to ensure that proliferation can continue to expand the precursor pool for generating a full complement of muscle fibres8. Indeed, all known instances of muscle hypertrophy that are associated with increased or reduced myofibre number have their origin in embryonic and fetal life.
Figure 3.
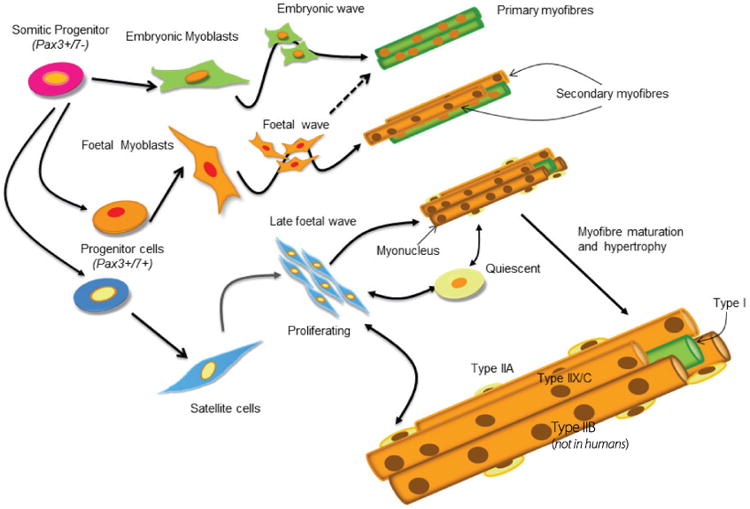
Illustration of lineage progression and the multiple waves of myogenesis. Cells from the dermomyotome gives rise to the progenitor cells that will enter the muscle lineage. These progenitor cells give rise to embryonic and fetal myoblasts, and satellite cells. These precursors undergo variable levels of proliferation, before undergoing further differentiation into myocytes and fusing to form myotubes and myofibres. There are three waves of myogenesis; the embryonic wave gives rise to primary fibres that go on to form type I fibres; the fetal wave gives rise to secondary fibres that mostly develop into type II fibres; the third wave consists of the satellite cells that either proliferate and contribute myonuclei to the hypertrophying newly formed fibres, or become quiescent until they are needed for muscle repair or to support workload-induced hypertrophy.
Primary and secondary fibre formation
The second chapter of muscle growth starts after the muscle anlagen are formed in the embryo3,6 (Figure 3). During this phase, the muscle progenitor cells have become committed to the muscle lineage and undergo fusion to form multinucleated fibres and express muscle-specific proteins9. This first wave of myogenesis results in the formation of primary fibres that act as a scaffold to shape and position the orientation of individual muscles. The process of cell fusion is complex and also highly regulated10; following migration, cells make contact and adhere to each other before fusing to form nascent myotubes. These are enlarged by the progressive fusion of additional myoblasts. Subsequently, there is a secondary wave of myogenesis, sometimes called fetal myogenesis, when a population of Pax7+ fetal myoblasts derived from Pax3+ myoblasts and located under the basal lamina of the primary fibres proliferate using the plasma membrane of the primary fibres as scaffolding. These then fuse among themselves to form secondary myotubes, which then gradually separate from the primaries by forming their own basal lamina.
Satellite cells
The muscle fibres also acquire a third population of muscle precursor cells, called satellite cells11. Recent lineage-tracing studies have identified that satellite cells are of embryonic/fetal origin, specifically from the central region of the dermomyotome, and, with a few exceptions, express Pax7 after arriving at their destination. In the muscle, they are lodged in a niche between the sarcolemma and the basal lamina. The satellite cell niche defines its physical location, the cell's axis of symmetry and the nature of extracellular molecules to which it is exposed, and these factors can all influence their activity and fate. These cells have stem cell characteristics in that they can proliferate to expand the myonuclear population, as well as self-renew to maintain their own population. Recent studies on the role of Pax7 in the regulation of satellite cell function suggest that they are a heterogeneous population of cells that vary in the MRF profile they express, and also according to their necessity for Pax7 for proliferative function at various stages of muscle maturity11. The proliferative Pax7-dependent activities of the satellite cells are most prevalent in the growth phase soon after secondary myogenesis has been completed and are essential for myofibre hypertrophy. In the newly formed muscle fibres, satellite cells comprise a significant proportion of the total muscle nuclei, which decreases as the number of myonuclei per fibre increase. In newborn rats, satellite cells comprise approximately 35% of muscle nuclei, and decrease to 10% at 4 weeks of age and less than 5% at sexual maturity. This pattern is replicated in other mammals, but with the maturational age of the muscle serving as the time scale. Once myofibres have attained their adult size, satellite cell activity becomes quiescent to be activated only when muscles are in need of repair or in response to specific patterns of muscle use. The mechanisms responsible for the decrease in the cells' proliferative activity and induction of quiescence with muscle maturation are unclear. They probably involve a temporal change in the balance between expression and/or responsiveness to factors (paracrine, autocrine and endocrine) that promote satellite cell proliferation while restraining differentiation, and those that advance differentiation and drive the nuclei into a post-mitotic state. For example, HGF, which is essential for satellite cell activation, is high in neonatal rat muscle and decreases rapidly after 10 days of age; the local expression of the insulin-like growth factors (IGFs) and the type 1 IGF receptor are down-regulated with maturation and this might be expected to mitigate cell cycle activity. Recently, the cross-talk between the Notch and Wnt signalling pathways has been implicated in the regulation of satellite cell function and fate. How and if alterations in the balance of these factors influences the number of satellite cells present in a mature muscle is uncertain. There is evidence from studies of young healthy adult humans that there is variability in satellite cell abundance among humans12. This variability was shown to have significant bearing on the magnitude of an individual's hypertrophic response to resistance exercise. It would not be unfeasible for this variability to also affect the severity of sarcopenia that occurs with aging or the capacity to repair injured muscles
Maturation and hypertrophic growth
This third phase of muscle growth is sometimes called ‘the postnatal growth phase’, which is something of a misnomer because the stage of muscle development when birth occurs varies among species: in humans, the third phase probably begins during the second trimester of pregnancy, whereas in rodents, in which a significant part of the research on muscle development has been performed, ‘postnatal’ is appropriate. Moreover, within an individual, maturation proceeds in a rostral to caudal direction, and from proximal to distal in the appendages. Hence altricial species, such as rabbits, rodents and most carnivores, are born with more mature head and thoracic muscles, whereas their lower abdominal and limb muscles, especially those of the hindlimbs, are still immature. In contrast, precocial species, such as ungulates, have relatively longer gestations and the newborn muscle is at a fairly advanced stage of maturity. Indeed, locomotor function is attained very soon after birth.
‘If you don't eat, you can't grow’
Because the total myofibre complement of muscles is largely defined after the second wave of myogenesis, the last stage of muscle development involves the hypertrophy of these existing fibres. This is brought about by the expansion of the protein content of each fibre necessitating the addition of myonuclei, and by the maturation of the muscles' biochemical and structural composition. Net protein accretion occurs when the rate of protein synthesis is higher than that of protein degradation. The synthesis rate of skeletal muscle proteins in newly formed muscles is substantially higher than their degradation rates and results in the high accretion rates typical of the neonatal period. As maturation proceeds, synthesis rates decrease more than degradation rates until the two processes are in balance in adult non-growing muscles. The maximal rate of protein synthesis is dictated by the abundance of ribosomes in a tissue and the efficiency with which they translate mRNA into protein. The elevated capacity for protein synthesis in immature muscle is in part driven by an elevated concentration of ribosomes, in conjunction with a heightened responsiveness and sensitivity of the translational machinery to the presence of nutrients2. In the immature animal, feeding therefore becomes a prime regulator of muscle growth. The aphorism ‘if you don't eat, you can't grow’ is highly pertinent to the neonatal muscle.
Although feeding stimulates protein synthesis in all tissues of the newborn, the magnitude of the increase is highest for skeletal muscle, and this response diminishes as muscles mature2. This capacity of the muscle to accelerate protein synthesis when nutrients are available channels amino acids towards anabolic processes so that they are not available for oxidation, thereby promoting the efficient use of dietary nutrients. The protein synthetic response to feeding is mediated primarily by the postprandial rise in insulin and amino acids. The effects of insulin are mediated by activation of the insulin receptor and the downstream signalling pathway that lead to translation initiation via Akt and mammalian target of rapamycin (mTOR) (Figure 4). This stimulation of protein synthesis by physiological increases in plasma insulin concentration is blunted in the mature muscle. Amino acids promote translation initiation independently of Akt by activating mTOR by an as yet undetermined mechanism. The actions of amino acids on protein synthesis can be replicated by providing the branched-chain amino acid leucine, but the acute leucine-induced stimulation is not sustained (despite continued activation of the mTOR signalling pathway) unless essential amino acids are also provided to prevent their depletion as they are utilized for protein synthesis. The stimulation of protein synthesis by amino acids is more widespread among tissues and organs than that of insulin. Thus it is the capacity of the immature muscle to respond to both insulin and amino acids that underlies its enhanced response to feeding, and that distinguishes it from other major organs. The age-related decline in the postprandial stimulation of protein synthesis in skeletal muscle can be attributed to an overall reduction in ribosomal abundance and a diminution in the capacity of the intracellular insulin signalling pathway to propagate to the translational apparatus the stimulus provided by the feeding-induced rise in insulin and amino acid concentrations.
Figure 4.
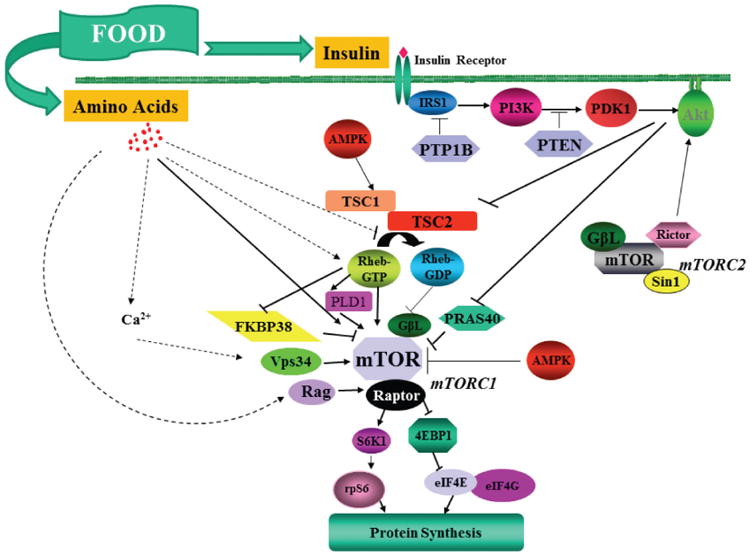
The intracellular signalling pathways that are activated by insulin and amino acids after feeding and that lead to the activation of translation initiation in the immature skeletal muscle. The broken lines represent potential pathways that are engaged by amino acids to signal to mTOR. Insulin activates the insulin receptor (IR), IRS-1, phosphoinositide 3-kinase (PI3K) and Akt1. Akt1 inactivates tuberous sclerosis complex (TSC) 1/2 and proline-rich Akt substrate of 40 kDa (PRAS40) which thereby relieves inhibition on mTOR complex (mTORC) 1; mTORC1 promotes phosphorylation of ribosomal S6 kinase1 (S6K1) and eukaryotic initiation factor 4E-binding protein 1 (4E-BP1) that enable mRNA translation to proceed. At a specific insulin concentration, the activation of this pathway is greater in immature than mature muscle. Amino acids specifically activate mTORC1 and not mTORC2 and therefore do not produce Akt activation. The exact mechanism responsible for the enhanced stimulation of protein synthesis by leucine in the immature muscle is still uncertain.
In addition to this acute level of regulation, there are significant changes in the expression of hormones and growth factors, e.g., growth hormone and thyroid hormone, the sex steroids, transforming growth factor (TGF)-β family members, and IGF-I and -II at various developmental stages in the period from birth to puberty. Their specific mechanisms of action have been studied extensively using experimental paradigms or in medical conditions where their production or the capacity to respond to them deviates from the norm. It is clear that they can enhance or inhibit muscle growth at the level of the satellite cell and/or protein metabolism by direct action through specific receptors and intracellular signalling pathways. However, as important are their indirect effects that serve to modulate insulin and amino acid signalling, or the consequences that are secondary to the effects they exert on other tissues and cells. Thus, although it is certain that these molecules are involved in the orchestration of skeletal muscle growth, their exact roles are age-dependent and vary according to the nutritional state of the organism. The actions of insulin and amino acids on muscle protein anabolism are blunted by the presence of glucocorticoids and/or cytokines, through mechanisms that dampen the propagation of the insulin and amino acid signals, and also by stimulating protein degradation. Thus, in addition to an inadequate diet, stress and illness will have detrimental consequences on skeletal muscle growth and maturation.
In addition to undergoing a rapid expansion in fibre size, the muscles are also undergoing profound changes in their composition and structure during the third phase of muscle growth1. The maturation of myotubes into fully functional myofibres involves the co-ordinated development of the metabolic machinery of the cells, the ion transport membrane system, and the contractile elements. This process of maturation is critical for postnatal survival of developing offspring as they are a prerequisite for independent breathing and suckling. By approximately 2 weeks of age in the rat, the muscles' complex membrane system constitutes approximately 40% of the non-myofibrillar compartment, and the contractile proteins, not present in the myotube at fusion, comprise 55–65% of total muscle protein. The diversity in fibre type that originated in the embryonic and fetal stages becomes fully manifested over this time with input from innervation, hormones and growth factors. These external inputs serve to adjust the genetic programme inherited by the muscle fibres so that the contractile, ion transport and metabolic activities developed support the specific contractile functions of the muscle.
One of many unanswered questions
An ongoing debate concerns the mechanism that coordinates the addition of myonuclei to the growing myofibre, and therefore satellite cell proliferative function, with the anabolic process in the fibre that promote protein deposition. In the growing muscle, these processes appear to be closely linked, but, as revealed in several recent experimental animal models, this may not necessarily be the case when adult muscles hypertrophy (see O'Connor and Pavlath13 and related discussion). This is highly relevant in understanding what regulates ‘maximal’ muscle mass and therefore of great interest to the athletic and medical communities. However, this is a ‘chicken and egg’ situation. Do satellite cells receive environmental cues to proliferate, and increase myonuclear number which then in turn facilitates mechanisms that promote protein accretion; or do muscle fibres expand, until the nuclear domain size can no longer be supported by existing myonuclei, which then signals to the satellite cells to divide? Both may be possible, but it is likely that the answer will lie in understanding what limits the amount of sarcoplasm that a nucleus can support. This is unlikely to be as simple as a specific volume, but is probably a function of volume and the metabolic activity of the sarcoplasm; this is best exemplified in the changes in nuclear domain size that occur during maturation when growth is perturbed2, as well as by the difference among fibre types. Such a possibility has the added advantage that it provides a mechanism for the generation of diffusible signals which, in contrast with the embryo, enables the adult fibre to instruct the surrounding cells of its needs.
Abbreviations
- BMP
bone morphogenetic protein
- FGF
fibroblast growth factor
- HGF
hepatocyte growth factor
- IGF
insulin-like growth factor
- MRF
myogenic regulatory factor
- mTOR
mammalian target of rapamycin
Biography

Marta Fiorotto is Associate Professor of Pediatrics at the Children's Nutrition Research Center in the Department of Pediatrics at Baylor College of Medicine in Houston, TX, USA. She received her BSc in Biochemistry from the University of Manchester Institute of Science and Technology and her PhD from the University of Cambridge, where she contributed to the studies on the pathophysiology of protein-energy malnutrition at the Dunn Nutritional Laboratory. Her research is concerned with the regulation of growth and body composition by nutrition in perinatal life, with particular focus on protein metabolism and skeletal muscle development. martaf@bcm.edu
References
- 1.Schiaffino S, Reggiani C. Physiol Rev. 2011;91:1447–1531. doi: 10.1152/physrev.00031.2010. [DOI] [PubMed] [Google Scholar]
- 2.Davis TA, Fiorotto ML. Curr Opin Clin Nutr Metab Care. 2009;12:78–85. doi: 10.1097/MCO.0b013e32831cef9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tajbakhsh S. J Intern Med. 2009;266:372–389. doi: 10.1111/j.1365-2796.2009.02158.x. [DOI] [PubMed] [Google Scholar]
- 4.Aulehla A, Pourquie O. Cold Spring Harbor Perspect Biol. 2010;2:a000869. doi: 10.1101/cshperspect.a000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buckingham M, Relaix F. Annu Rev Cell Dev Biol. 2007;23:645–673. doi: 10.1146/annurev.cellbio.23.090506.123438. [DOI] [PubMed] [Google Scholar]
- 6.Hutcheson DA, Zhao J, Merrell A, Haldar M, Kardon G. Genes Dev. 2009;23:997–1013. doi: 10.1101/gad.1769009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bentzinger CF, Wang YX, Rudnicki MA. Cold Spring Harbor Perspect Biol. 2012;4:a008342. doi: 10.1101/cshperspect.a008342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuchtbauer EM. Results Probl Cell Differ. 2002;38:143–161. doi: 10.1007/978-3-540-45686-5_7. [DOI] [PubMed] [Google Scholar]
- 9.Ontell M. In: Biochemical Development of the Fetus and Neonate. Jones CT, editor. Elsevier Biomedical Press; Amsterdam: 1982. pp. 213–247. [Google Scholar]
- 10.Abmayr, S.M. and Pavlath, G.K. (2012) 139, 641–656
- 11.Aziz A, Sebastian S, Dilworth FJ. Stem Cell Rev. 2012 doi: 10.1007/s12015-012-9352-0. [DOI] [PubMed] [Google Scholar]
- 12.Petrella JK, Kim JS, Mayhew DL, Cross JM, Bamman MM. J Appl Physiol. 2008;104:1736–1742. doi: 10.1152/japplphysiol.01215.2007. [DOI] [PubMed] [Google Scholar]
- 13.O'Connor RS, Pavlath GK. J Appl Physiol. 2007;103:1099–1100. doi: 10.1152/japplphysiol.00101.2007. [DOI] [PubMed] [Google Scholar]
- 14.Sambasivan R, Tajbakhsh S. Semin Cell Dev Biol. 2007;18:870–882. doi: 10.1016/j.semcdb.2007.09.013. [DOI] [PubMed] [Google Scholar]


