Abstract
Calculating math problems from memory may seem unrelated to everyday processing of emotions, but they have more in common than one might think. Prior research highlights the importance of the dorsolateral prefrontal cortex (dlPFC) in executive control, intentional emotion regulation, and experience of dysfunctional mood and anxiety. While it has been hypothesized that emotion regulation may be related to ‘cold’ (ie. not emotion-related) executive control, this assertion has not been tested. We address this gap by providing evidence that greater dlPFC activity during ‘cold’ executive control is associated with increased use of cognitive reappraisal to regulate emotions in everyday life. We then demonstrate that in the presence of increased life stress, increased dlPFC activity is associated with lower mood and anxiety symptoms and clinical diagnoses. Collectively, our results encourage ongoing efforts to understand prefrontal executive control as a possible intervention target for improving emotion regulation in mood and anxiety disorders.
Keywords: working memory, prefrontal cortex, depression, anxiety, cognitive reappraisal
Introduction
The ability to adaptively regulate emotional experiences in everyday life is related to a range of outcomes including mental health, relationship quality, academic achievement, and job performance (Garnefski & Kraaij, 2006; Graziano, Reavis, Keane, & Calkins, 2007; Gross & John, 2003; Newman, Joseph, & MacCann, 2010). One of the primary strategies for regulating emotional experiences is cognitive reappraisal, which involves changing one’s interpretation of negative emotions or experiences in an attempt to be more neutral or objective (Goldin, McRae, Ramel, & Gross, 2008; Ochsner, Bunge, Gross, & Gabrieli, 2002). As such, cognitive reappraisal is hypothesized to represent an important specific skill that can be developed through Cognitive Behavioral Therapy (CBT), an umbrella term describing psychotherapies for improving dysfunctional mood and affect by teaching individuals to identify, evaluate, and respond to maladaptive thoughts and beliefs through guided questioning and behavioral experiments (Beck, 2011). Although cognitive reappraisal of emotion has been hypothesized to relate to neural processes supporting ‘cold’ (i.e. not emotion-related) executive control (Ochsner & Gross, 2005), there has only been indirect evidence in support of this hypothesis. Identifying ‘cold’ executive control processes that support the use of cognitive reappraisal will not only deepen our understanding of fundamental mechanisms supporting adaptive emotional functioning but also inform the search for novel approaches to therapy that could target these executive functions and potentially increase the effectiveness of teaching cognitive reappraisal to individuals with mood and anxiety disorders.
Executive control is typically divided into three inter-related subprocesses: (1) updating and monitoring of information in working memory, (2) shifting between information sets, and (3) selecting goal-relevant responses (Miyake et al., 2000). The neural processes supporting executive control have been established as relating to functioning of the dorsolateral prefrontal cortex (dlPFC; Barbey, Colom, & Grafman, 2013; Wager & Smith, 2003). Functional neuroimaging studies of healthy individuals have generally associated higher activity of the dlPFC with better executive control (Braver et al., 1997; D’Esposito et al., 1995).
Functioning of the dlPFC has also been found to be important in controlling emotions, which can be thought of as a state-like skill or as a trait-like disposition (Lee, Heller, van Reekum, Nelson, & Davidson, 2012). Both state- and trait-like emotion regulation have been associated with increased dlPFC activation (Drabant, McRae, Manuck, Hariri, & Gross, 2009; Goldin et al., 2008; Heller et al., 2013; Ochsner et al., 2002), and the specific use of cognitive reappraisal has been found to decrease feelings of distress and reduce symptoms of anxiety and depression (Gross & John, 2003; Hofmann, Heering, Sawyer, & Asnaani, 2009).
Dysfunction of the dlPFC has also been linked with depression (e.g. Heller et al., 2009). For example, dlPFC lesions specifically have been associated with increased depressive symptoms (Koenigs et al., 2008), and stimulation of the dlPFC through TMS has led to decreased depressive symptoms in patients with Major Depressive Disorder (MDD) and anxiety in Generalized Anxiety Disorder (GAD) (Bystritsky et al., 2008; O’Reardon et al., 2007). Furthermore, abnormal prefrontal activation has been demonstrated in individuals with MDD (Erk et al., 2010; Johnstone, van Reekum, Urry, Kalin, & Davidson, 2007) and individuals at risk for MDD (Joormann, Cooney, Henry, & Gotlib, 2012), specifically during the process of emotion regulation. Similarly consistent patterns have been identified for anxiety disorders, with individuals showing an increase in dlPFC activation pre- to post-CBT treatment during the process of emotion regulation (Goldin et al., 2013).
Though implicit in the associations noted above (Ochsner & Gross, 2005), no direct links have been established between dlPFC function during ‘cold’ executive control with dysfunctional mood and anxiety, and the use of cognitive reappraisal. Establishing such direct links, can help illuminate shared biological foundations of these behavioral processes. Here we used functional magnetic resonance imaging to measure dlPFC activity during working memory computation and evaluated the extent to which variability in this activity was associated with (1) trait-like or habitual regulation of negative emotion using cognitive reappraisal, (2) self-reported mood and anxiety symptoms in the context of stress, and (3) clinical diagnosis of a mood or anxiety disorder. Based on the existing literature, we hypothesized that increased dlPFC activity supporting computation would be associated with increased habitual use of cognitive reappraisal as an emotion regulation strategy, and decreased self-reported symptoms of mood and anxiety as well as clinical disorder. Given the critical role of stressful life events in precipitating increases in symptoms of depression and anxiety (Faravelli, 1985; Kendler, Karkowski, & Prescott, 1999), as well as the necessity to regulate negative emotions (Garnefski, Kraaij, & Spinhoven, 2001), we explicitly tested the moderating role of recent stress on symptoms.
Methods
Participants
Data were available from 186 university students who successfully completed the ongoing Duke Neurogenetics Study (DNS) between September 3rd, 2014 and January 27th, 2016. Informed consent was obtained for all participants in accordance with the Duke University School of Medicine Institutional Review Board. Exclusion criteria included: (1) medical diagnoses of cancer, stroke, head injury with loss of consciousness, untreated migraine headaches, diabetes requiring insulin treatment, chronic kidney or liver disease, or lifetime history of psychotic disorder; (2) use of psychotropic, glucocorticoid, or hypolipidemic medication; and (3) conditions affecting cerebral blood flow and metabolism (e.g. hypertension). The DNS seeks to establish broad variability in multiple behavioral phenotypes related to psychopathology, so other than psychotic disorders, participants were not excluded based on diagnosis of past or current DSM-IV Axis I or select Axis II (borderline and anstisocial personality) disorder. However, no participants were taking psychotropic medication at the time of or at least 10 days prior to study participation.
Self-Report Questionnaires
The Emotion Regulation Questionnaire (ERQ) is a 10-item self-report questionnaire designed to assess individual differences in two emotion regulation strategies: cognitive reappraisal and expressive suppression (Gross & John, 2003). Instructions ask subjects to report on how they control both their emotional experience and their emotional expression. Items are rated on a 7-point-Likert scale from “strongly disagree” to “strongly agree.” The cognitive reappraisal subscale, which prior work links to executive control and dlPFC function, was used in our analyses.
The Mood and Anxiety Symptom Questionnaire – Short Form (MASQ-SF;) is a 62-item self-report questionnaire designed to assess symptoms during the past week. across four subscales: general distress depression, anhedonic depression, general distress anxiety, and anxious arousal (Watson et al., 1995). Items are rated on a 5-point-Likert scale from “not at all” to “extremely.” One item from the anhedonic depression subscale that asked about suicidality was removed from the questionnaire in order to comply with IRB protocol. Items were summed to create a total score for mood and anxiety symptoms.
The Life Events Scale for Students (LESS; Clements & Turpin, 1996) is a 46-item self-report questionnaire that assesses the number of life events that occurred in the past 12 months. Participants rate the impact that the life event had on them on a 1 to 4 scale (4 = severe impact). The impact score for each event reported was summed to yield a LESS total impact score; higher values indicate both greater number and severity of life events.
Clinical Interview
All participants were assessed for common mental disorders using the electronic version of the M.I.N.I International Neuropsychiatric Interview (Sheehan et al., 1998), administered by trained staff under the supervision of a licensed clinical psychologist (BDB). The interview follows DSM-IV (American Psychiatric Association, 1994) and ICD-10 (World Health Organization, 2004) diagnostic criteria.
Working Memory fmri Paradigm
Activity of the dlPFC was measured during BOLD fMRI using an event-related working memory paradigm (Tan et al., 2007). This task was chosen because it specifically allows for explicit modeling of component subprocesses of working memory (Figure 1). In particular, it was hypothesized that higher order aspects of executive control, including the manipulation of information in working memory (Miyake et al., 2000), would support the complex process of cognitive reappraisal by allowing for the manipulation of information in constructing new appraisals (Silvers et al., 2014). Previous studies have found that this updating component is related to emotion regulation success (Pe, Raes, & Kuppens, 2013). Here, we focus on this computational function of the dlPFC by isolating activity during the contrast of trials wherein participants subtract 2 or 3 from a remembered number and then make a numerical size judgment against a second number during recall (“E_RCJ,” Figure 1) versus trials wherein participants subtract 2 or 3 from one of 2 numbers during a brief encoding phase before recalling the resulting two numbers and performing a numerical size judgment (“EC_RJ,” Figure 1), in order to isolate the manipulation of information component of working memory. Additional details are provided in Supplemental Methods.
Figure 1. Working Memory Task.
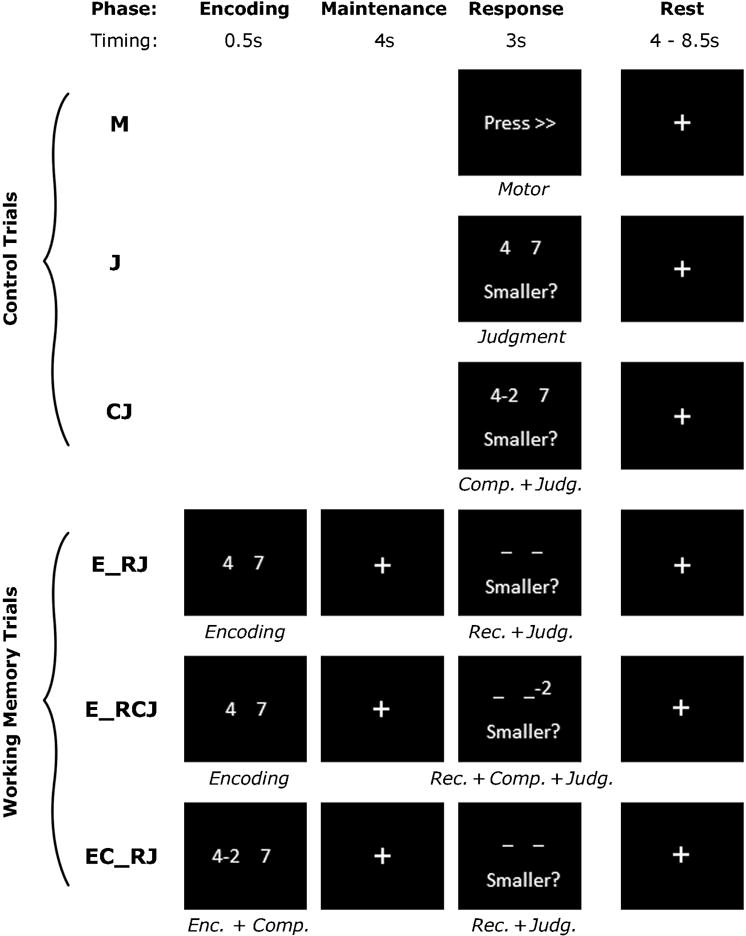
In order to isolate the computational component of working memory, the contrast of E_RCJ>EC_RJ was used in the analyses. This comparison isolates updating/manipulation component of working memory by focusing on computation on information that has been maintained over a delay.
BOLD fMRI
The general linear model of SPM8 (http://www.fil.ion.ucl.ac.uk/spm) was used to conduct analyses of BOLD fMRI data acquired using a standard protocol (see Supplemental Methods for acquisition parameters and details). Following preprocessing, events were modeled for correctly performed trials for the response phase for each of the 6 trial types, and the maintenance and encoding (with and without computation modeled separately) phases for WM trials. Incorrect responses were also modeled as regressors of no interest. A linear contrast employing the canonical hemodynamic response function was used to estimate main effects for each participant for the comparison of E_RCJ > EC_RJ in order to isolate the manipulation of information in working memory above and beyond basic computation and maintenance of information across a delay. Individual contrast images for E_RCJ > EC_RJ were then used in second-level random effects models accounting for scan-to-scan and participant-to-participant variability to determine mean condition-specific regional responses using one-sample t-tests with a voxel-level statistical threshold of p<0.05, family wise error (FWE) corrected for multiple comparisons across the whole brain. Regions of interest for the dlPFC were created using the WFU Pickatlas with the conjunction of bilateral BA9 and BA46. Mean parameter estimates from the primary activation cluster (Figure 2A; −42, 2, 30, k=274) within these anatomical regions of interest surviving FWE correction were extracted to test our hypotheses using IBM SPSS Statistics 23 (Chicago, IL, USA).
Figure 2. ‘Cold’ working memory-related dlPFC activation is related to habitual reappraisal as well as mood and anxiety symptoms in the context of stress.

A) The peak dlPFC cluster extracted for subsequent analyses. B) Increased dlPFC activation during computation of information in working memory is associated with greater levels of self-reported cognitive reappraisal during everyday life as measured by the ERQ (p=0.018). C) At high levels of stress, increased dlPFC activity is associated with fewer mood and anxiety symptoms (p=0.036).
Hypothesis Testing
Extracted values from the dlPFC cluster associated with computation during working memory were entered into a general linear model with ERQ Cognitive Reappraisal scores as the dependent variable. Additionally, a moderation model using PROCESS for SPSS (Hayes, 2012), tested whether the dlPFC activity in interaction with self-reported stressful life events, was associated with MASQ total scores as well as with diagnosis of a mood or anxiety disorder. Lastly, a moderation model tested whether ERQ Cognitive Reappraisal in interaction with stressful life events, was associated with MASQ total scores as well as with clinical diagnosis. Sex was included as a covariate in all analyses.
Results
Demographics
The final sample of 186 participants (118 women; 63.4%) had a mean age of 19.81 (± 1.28) years and self-reported as being European American (110; 59.1%), African American (15; 8.1%), Asian (42; 22.6%), Multi-racial (14; 7.5%), Other (5, 2.7%), and Hispanic/Latino (18; 9.7%). With regards to socioeconomic status, participants were asked to rank on a 0–10 scale where they were in comparison to others in the United States in terms of money, education, respected jobs, etc. The sample mean was 6.97 (1.65). Clinical interview identified 43 participants (23.1%) as having a DSM-IV diagnosis. Additional details regarding demographics of our cohort as a function of DSM-IV diagnoses including comorbidity are provided in Supplemental Table 1.
Working Memory Task Performance and Behavioral Measures
Mean accuracy across all conditions was 95% (±5%), range 71–100%, and mean accuracy on the E_RCJ condition specifically was 89% (±11%), range 50–100%. The mean score for the ERQ Reappraisal subscale was 5.23 (±0.92), range 2–7. The mean score on the MASQ-SF was 110 (±27), range of 65–213. The mean score on the LESS was 10.37 (±8.30), range of 0–48.
Working Memory fmri
The contrast E_RCJ > EC_RJ used to isolate the manipulation of information in working memory, resulted in large clusters of activity across the frontal and parietal cortices as well as the temporal cortices (Supplemental Figure 1). Mean parameter estimates were extracted specifically from a 274-voxel cluster within our dlPFC region of interest.
Associations between dlPFC Activity, Symptoms, and Cognitive Reappraisal
A significant positive association was found between dlPFC activity and ERQ Reappraisal (Fig 2B; b=0.18, p=0.018, R2 change=3.0%). The effects were specific to Reappraisal, as dlPFC activity was not associated with ERQ Suppression (p=0.50). The magnitude of dlPFC activity interacted with stress to predict symptoms of mood and anxiety (p=0.009, R2-change=3.4%). In particular, for individuals with high levels of stressful life events, higher dlPFC activity was associated with fewer symptoms of anxiety and depression (Figure 2C). The effects did not change significantly after controlling for presence or absence of DSM-IV diagnosis or after controlling for task accuracy. A similar pattern was observed when predicting mood or anxiety diagnoses (p=0.050, R2-change =2.0%).
Lastly, ERQ Cognitive Reappraisal interacted with stress to predict symptoms of mood and anxiety (p=0.043, R2-change =1.9%). In particular, for individuals with intermediate or high levels of stressful life events, greater reappraisal was associated with fewer symptoms of anxiety and depression (Supplemental Figure 2). After controlling for presence or absence of DSM-IV diagnosis, this interaction was reduced to a trend effect (p=0.06). A similar pattern was observed when predicting mood or anxiety diagnoses (p=0.003, R2-change =4.4%).
Discussion
Here we provide initial evidence that dlPFC function supporting general executive control is associated with the everyday use of cognitive reappraisal as a strategy for regulating emotions. Furthermore, we find that the use of cognitive reappraisal is associated with decreased symptoms of mood and anxiety in the context of greater life stress as well as clinical diagnosis of a mood or anxiety disorder. Although the cross-sectional nature of our data makes formal tests of mediation unfeasible, the findings are consistent with prior behavioral work finding that coping strategies, including cognitive reappraisal, mediate the relationship between executive control and mood (Campbell et al., 2009; Evans, Kouros, Samanez-Larkin, & Garber, 2015). Thus, our current findings fill a gap in understanding the cognitive control of emotion. Specifically, our results suggest that cognitive and emotional processing may not just utilize the same brain regions, but instead rely on shared information processing itself. Our analyses lend support for the theory that coping with emotional distress relies on the ability to construct expectations, select among alternative explanations, and make judgments about emotional stimuli through ‘cold’ executive control processes (Ochsner & Gross, 2005).
Recently, cognitive training programs that impact working memory ability and other neuropsychological function have begun to show reliable and generalizable effects (e.g. Anguera et al., 2013). In light of this work, our current results suggest the intriguing hypothesis that such domain general strategies could be implemented as an adjunct to cognitive behavioral therapy techniques as a means of enhancing efficacy. Given that working memory and other executive control functions have been shown to be impaired in depression (Snyder, 2013), the enhancement of general working memory might facilitate cognitive reappraisal, leading to improved outcomes. Additionally, methods of directly strengthening dlPFC function may buttress executive control and associated emotion regulation leading to decreased anxiety and depression. In support of this strategy, functional neuroimaging studies have demonstrated that individuals are able to upregulate dlPFC activity supporting working memory using real-time neurofeedback leading to improved performance (Zhang, Yao, Zhang, Long, & Zhao, 2013). Similarly, transcranial magnetic stimulation (TMS) of the dlPFC is associated not only with improved working memory performance (Fregni et al., 2005), but also decreased symptoms of anxiety and depression (Bystritsky et al., 2008; O’Reardon et al., 2007), although the effects of dlPFC-targeted TMS on executive control and mood symptoms have not been considered jointly in a single experiment. Given that changes in working memory performance would be an immediately observable outcome when using TMS, one possibility is that working memory performance could act as an intermediate index of the effectiveness of stimulation parameters in treating mood and anxiety disorders.
A limitation of the present study is the cross-sectional nature of the data. Therefore, while we hypothesize that habitual use of cognitive reappraisal may mediate the association between dlPFC function and stress-related mood, it is also possible that dlPFC function may act as the mediator. Future longitudinal studies will be better positioned to explore these alternate models. Another limitation of the present sample is that it was comprised of relatively high-functioning undergraduate students. While a range of clinical psychopathology was observed in our sample, the prevalence rates were slightly below population norms (Moffitt et al., 2010). Thus, it will be important to extend our findings to samples with higher base rates of psychopathology or at higher risk for disorder. A further limitation is the conceptualization of recent life stress, which was developed specifically for students (e.g., failing a course), and thus may not capture stressors more common in the general population (e.g., unemployment). While the measure of stress we use is easily administered via self-report and accounts for both the number and subjective severity of these stressful life events, it may be subject to retrospective bias or confounded with current symptoms (i.e., more depressed individuals may be more likely to rate past life events more severely). These limitations can be addressed in future work using different measures of common life stressors not unique to students as well as through longitudinal studies capable of establishing temporal order of events.
These limitations notwithstanding, our results show that dlPFC function supporting general executive control is related to the habitual use of cognitive reappraisal to regulate negative emotions and the experience of stress-related mood and anxiety dysfunction. As such, our results encourage further consideration of the importance of domain general executive control processes in emotion regulation and in the experience of mood and anxiety dysfunction particularly in the context of stressful life events, motivating ongoing efforts to better understand top-down prefrontal executive control as a target for clinical intervention.
Supplementary Material
Acknowledgments
We thank ALL LAB MEMBERS NOT AUTHORS for their assistance in conducting the Duke Neurogenetics Study. M.A.S. is supported by an NSF Graduate Research Fellowship. J.R.S. is supported by P30DA023026 and R01AG049789. The Duke Neurogenetics Study is supported by Duke University and NIH grant R01DA033369. A.R.H. is further supported by NIH grant R01AG049789.
Footnotes
Author contributions
A.R.H. conceived and designed the parent Duke Neurogenetics Study. M.A.S. developed the study concept. B.D.B. designed the clinical interview and neuropsychological testing protocols. Data analyses were performed by A.R.K, J.R.S. and M.A.S. under the supervision of A.R.H. M.A.S., A.R.K., and A.R.H. drafted the paper. All authors reviewed and approved the final version of the manuscript.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington, DC: 1994. [Google Scholar]
- Anguera Ja, Boccanfuso J, Rintoul JL, Al-Hashimi O, Faraji F, Janowich J, Gazzaley a. Video game training enhances cognitive control in older adults. Nature. 2013;501(7465):97–101. doi: 10.1038/nature12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbey AK, Colom R, Grafman J. Dorsolateral prefrontal contributions to human intelligence. Neuropsychologia. 2013;51(7):1361–9. doi: 10.1016/j.neuropsychologia.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck JS. Cognitive behavior therapy: Basics and beyond. Guilford Press; 2011. [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. NeuroImage. 1997;5(1):49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- Bystritsky A, Kaplan JT, Feusner JD, Kerwin LE, Wadekar M, Burock M, Iacoboni M. A preliminary study of fMRI-guided rTMS in the treatment of generalized anxiety disorder. Journal of Clinical Psychiatry. 2008;69(7):1092–1098. doi: 10.4088/JCP.v69n0708. [DOI] [PubMed] [Google Scholar]
- Campbell LK, Scaduto M, Van Slyke D, Niarhos F, Whitlock JA, Compas BE. Executive Function, Coping, and Behavior in Survivors of Childhood Acute Lymphocytic Leukemia*. 2009;34(3):317–327. doi: 10.1093/jpepsy/jsn080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements K, Turpin G. The life events scale for students: Validation for use with British samples. Personality and Individual Differences. 1996;20(6):747–751. doi: 10.1016/0191-8869(96)00005-0. [DOI] [Google Scholar]
- D’Esposito M, Detre JA, Alsop DC, Shin RK, Atlas S, Grossman M. The neural basis of the central executive system of working memory. Nature. 1995;378(6554):279–81. doi: 10.1038/378279a0. [DOI] [PubMed] [Google Scholar]
- Drabant EM, McRae K, Manuck SB, Hariri AR, Gross JJ. Individual Differences in Typical Reappraisal Use Predict Amygdala and Prefrontal Responses. Biological Psychiatry. 2009;65(5):367–373. doi: 10.1016/j.biopsych.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erk S, Mikschl A, Stier S, Ciaramidaro A, Gapp V, Weber B, Walter H. Acute and sustained effects of cognitive emotion regulation in major depression. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2010;30(47):15726–34. doi: 10.1523/JNEUROSCI.1856-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans LD, Kouros CD, Samanez-Larkin S, Garber J. Concurrent and Short-Term Prospective Relations Among Neurocognitive Functioning, Coping, and Depressive Symptoms in Youth. Journal of Clinical Child and Adolescent Psychology: The Official Journal for the Society of Clinical Child and Adolescent Psychology, American Psychological Association, Division 53. 2015 Feb;:1–15. doi: 10.1080/15374416.2014.982282. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faravelli C. Life events preceding the onset of panic disorder. Journal of Affective Disorders. 1985;9(1):103–105. doi: 10.1016/0165-0327(85)90016-3. [DOI] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Nitsche M, Bermpohl F, Antal A, Feredoes E, Pascual-Leone A. Anodal transcranial direct current stimulation of prefrontal cortex enhances working memory. Experimental Brain Research. 2005;166(1):23–30. doi: 10.1007/s00221-005-2334-6. [DOI] [PubMed] [Google Scholar]
- Garnefski N, Kraaij V. Relationships between cognitive emotion regulation strategies and depressive symptoms: A comparative study of five specific samples. Personality and Individual Differences. 2006;40(8):1659–1669. doi: 10.1016/j.paid.2005.12.009. [DOI] [Google Scholar]
- Garnefski N, Kraaij V, Spinhoven P. Negative life events, cognitive emotion regulation and emotional problems. Personality and Individual Differences. 2001;30(8):1311–1327. doi: 10.1016/S0191-8869(00)00113-6. [DOI] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biological Psychiatry. 2008;63(6):577–86. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, Ziv M, Jazaieri H, Hahn K, Heimberg R, Gross JJ, Author C. Impact of Cognitive Behavioral Therapy for Social Anxiety Disorder on the Neural Dynamics of Cognitive Reappraisal of Negative Self-beliefs Randomized Clinical Trial. JAMA Psychiatry. 2013;70(10):1048–1056. doi: 10.1001/jamapsychiatry.2013.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano PA, Reavis RD, Keane SP, Calkins SD. The role of emotion regulation in children’s early academic success. Journal of School Psychology. 2007;45(1):3–19. doi: 10.1016/j.jsp.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ, John OP. Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. Journal of Personality and Social Psychology. 2003;85(2):348–362. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Hayes AF. PROCESS: A versatile computational tool for observed variable mediation, moderation, and conditional process modeling [White Paper] 2012 [Google Scholar]
- Heller AS, Johnstone T, Peterson MJ, Kolden GG, Kalin NH, Davidson RJ. Increased prefrontal cortex activity during negative emotion regulation as a predictor of depression symptom severity trajectory over 6 months. JAMA Psychiatry. 2013;70(11):1181–9. doi: 10.1001/jamapsychiatry.2013.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller AS, Johnstone T, Shackman AJ, Light SN, Peterson MJ, Kolden GG, Davidson RJ. Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(52):22445–50. doi: 10.1073/pnas.0910651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Heering S, Sawyer AT, Asnaani A. How to handle anxiety: The effects of reappraisal, acceptance, and suppression strategies on anxious arousal. Behaviour Research and Therapy. 2009;47(5):389–394. doi: 10.1016/j.brat.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2007;27(33):8877–84. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, Cooney RE, Henry ML, Gotlib IH. Neural correlates of automatic mood regulation in girls at high risk for depression. Journal of Abnormal Psychology. 2012;121(1):61–72. doi: 10.1037/a0025294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA. Causal Relationship Between Stressful Life Events and the Onset of Major Depression. Psychiatry Interpersonal and Biological Processes. 1999 Jun;156:837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Huey ED, Calamia M, Raymont V, Tranel D, Grafman J. Distinct regions of prefrontal cortex mediate resistance and vulnerability to depression. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2008;28(47):12341–12348. doi: 10.1523/JNEUROSCI.2324-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Heller AS, van Reekum CM, Nelson B, Davidson RJ. Amygdala-prefrontal coupling underlies individual differences in emotion regulation. NeuroImage. 2012;62(3):1575–81. doi: 10.1016/j.neuroimage.2012.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki aH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cognitive Psychology. 2000;41(1):49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Caspi a, Taylor a, Kokaua J, Milne BJ, Polanczyk G, Poulton R. How common are common mental disorders? Evidence that lifetime prevalence rates are doubled by prospective versus retrospective ascertainment. Psychological Medicine. 2010;40(6):899–909. doi: 10.1017/S0033291709991036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman DA, Joseph DL, MacCann C. Emotional Intelligence and Job Performance: The Importance of Emotion Regulation and Emotional Labor Context. Industrial and Organizational Psychology. 2010;3(2):159–164. doi: 10.1111/j.1754-9434.2010.01218.x. [DOI] [Google Scholar]
- O’Reardon JP, Solvason HB, Janicak PG, Sampson S, Isenberg KE, Nahas Z, Sackeim HA. Efficacy and Safety of Transcranial Magnetic Stimulation in the Acute Treatment of Major Depression: A Multisite Randomized Controlled Trial. Biological Psychiatry. 2007;62(11):1208–1216. doi: 10.1016/j.biopsych.2007.01.018. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JDE. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14(8):1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Pe ML, Raes F, Kuppens P. The Cognitive Building Blocks of Emotion Regulation: Ability to Update Working Memory Moderates the Efficacy of Rumination and Reappraisal on Emotion. PLoS ONE. 2013;8(7) doi: 10.1371/journal.pone.0069071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Dunbar GC. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- Silvers JA, Buhle JT, Ochsner KN. The neuroscience of emotion regulation: Basic mechanisms and their role in development, aging and psychopathology. In: Ochsner KN, SM K, editors. The Oxford Handbook of Cognitive Neuroscience, Vol 2: The Cutting Edges. New York, NY: Oxford University Press; 2014. pp. 53–78. [Google Scholar]
- Snyder HR. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: A meta-analysis and review. Psychological Bulletin. 2013;139(1):81–132. doi: 10.1037/a0028727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan HY, Chen Q, Goldberg TE, Mattay VS, Meyer-Lindenberg A, Weinberger DR, Callicott JH. Catechol-O-methyltransferase Val158Met modulation of prefrontal-parietal-striatal brain systems during arithmetic and temporal transformations in working memory. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2007;27(49):13393–401. doi: 10.1523/JNEUROSCI.4041-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: A meta-analysis. Cognitive, Affective, & Behavioral Neuroscience. 2003;3(4):255–274. doi: 10.3758/CABN.3.4.255. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Weber K, Assenheimer JS, Strauss ME, McCormick Ra. Testing a tripartite model: II. Exploring the symptom structure of anxiety and depression in student, adult, and patient samples. Journal of Abnormal Psychology. 1995;104(1):15–25. doi: 10.1037/0021-843X.104.1.15. [DOI] [PubMed] [Google Scholar]
- World Health Organization. International statistical classification of diseases and health related problems (The) ICD-10. World Health Organization; 2004. [Google Scholar]
- Zhang G, Yao L, Zhang H, Long Z, Zhao X. Improved Working Memory Performance through Self-Regulation of Dorsal Lateral Prefrontal Cortex Activation Using Real-Time fMRI. PLoS ONE. 2013;8(8):1–9. doi: 10.1371/journal.pone.0073735. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


