Abstract
Objectives
Probiotics are live microorganisms that may provide health benefits to the individual when consumed in sufficient quantities. For studies conducted on health or disease endpoints on probiotics in the United States, the Food and Administration (FDA) has required those studies to be conducted as investigational new drugs. This phase I, double-blinded, randomized, controlled safety study represents the first requirement of this pathway. The purpose of the study was to determine the safety of Bifidobacterium animalis subsp. lactis (B. lactis) strain BB-12® (BB-12®)-supplemented yogurt when consumed by a generally healthy group of children. The secondary aim was to assess the effect of BB-12®-supplemented yogurt on the gut microbiota of the children.
Methods
Sixty children aged 1–5 years were randomly assigned to consume four ounces of either BB-12®-supplemented yogurt or non-supplemented control yogurt daily for 10 days. The primary outcome was to assess safety and tolerability, as determined by the number of reported adverse events.
Results
A total of 186 non-serious adverse events were reported, with no significant differences between the control and BB-12® groups. No significant changes due to probiotic treatment were observed in the gut microbiota of the study cohort.
Conclusions
BB-12®-supplemented yogurt is safe and well-tolerated when consumed by healthy children. This study will form the basis for future randomized clinical trials investigating the potential effects of BB-12®-supplemented yogurt in different disease states.
Keywords: probiotics, clinical trial, gut microbiota, pediatrics
Introduction
Probiotics are defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host.”(1) The probiotics market has grown considerably in recent years, with the value of probiotic ingredients estimated at $33.19 billion in 2015 and projected to grow at an annual rate of 7.0% over the next five years.(2) According to the 2012 National Health Interview Survey (NHIS), probiotics or prebiotics were among the top three natural products used by children (aged 4–17 years).(3) The NHIS showed 0.5% (294,000) of children reported they had used probiotics or prebiotics in the past 30 days.(3) Probiotics or prebiotics were also the third most used dietary supplement (other than vitamins and minerals) in adults, with 1.6% of (or 3.9 million) adults in the U.S. reporting usage of these products in the past 30 days.(4) Despite their widespread use by both consumers and physicians, the efficacy of many probiotic products remains unsupported by rigorous independent research and thus can result in non-evidence-based usage.
The human gastrointestinal tract contains a diverse and dynamic community of microorganisms collectively termed the gut microbiota that contributes to homeostasis of the gut. The gut microbiota contains over 100 trillion microorganisms and hundreds of species of facultative and obligate anaerobes.(5) Interventions, such as antibiotics, can disrupt the normal microbiota, resulting in, among other effects, decreased short chain fatty acid metabolism, accumulation of luminal carbohydrate, subsequent pH changes, water absorption and ultimately, diarrhea.(6) Probiotics may help to normalize the perturbed microbiota of a gastrointestinal tract following antibiotic treatment.(7) Previous systematic reviews of probiotics in the prevention of antibiotic-associated diarrhea (AAD) concluded that probiotics may have a protective effect on AAD.(8, 9) However, most studies were conducted outside the United States and were not subject to Investigational New Drug (IND) guidelines of the Food and Drug Administration, Center for Biologics Evaluation and Research (FDA/CBER).(8) The evidence for the efficacy of yogurt consumption to prevent AAD is further limited.(10)
The most common strains studied and used in probiotic products are from the genera Bifidobacterium and Lactobacillus. Bifidobacterium animalis subsp. lactis (B. lactis) strain BB-12® (BB-12®) is the most widely studied Bifidobacterium strain.(11) In the United States, BB-12® is most commonly marketed as either a dietary supplement or a food, typically in infant formula and yogurt.
The purpose of this study was to assess the safety of strawberry-flavored yogurt supplemented with the probiotic BB-12® in children. The FDA considered the yogurt used in this study to be a drug; as such, we were required to conduct the protocol under IND guidelines. The first stage of this process was to conduct a phase I study in antibiotic-treated adults.(12) Our current study was conducted to establish the safety profile of a BB-12®-supplemented yogurt drink in healthy children aged 1–5 years. The long-term objective is to continue with the FDA/CBER IND staging process toward an efficacy study to determine the effects of BB-12®-supplemented yogurt on gastrointestinal disease states. This study was also the first to evaluate the influence of BB-12® on the gut microbiota of children (aged 1–5 years) and examine changes in the microbial community. The use of DNA sequencing and analysis tools to assess the relationship between the gut microbiota and probiotics provided novel perspective on this complex ecosystem.
Methods
Study design
A phase I, double-blinded, randomized controlled study was conducted with two parallel arms. The study protocol was approved by the Georgetown University Institutional Review Board (IRB# 2012–1112, Washington, DC) and registered at ClinicalTrials.gov (NCT01652287). An independent Data Safety Monitoring Board reviewed the protocol prior to study initiation and adverse event data at approximately 33%, 50% and 66% data completion. Monitoring was also conducted by the FDA/CBER, under IND#13691 and the National Institutes of Health (NIH), National Center for Complementary and Integrative Health (NCCIH), including its Office of Clinical and Regulatory Affairs.
An initial screening was conducted to determine if the caregivers and children were eligible to participate. The children who passed the initial screenings underwent baseline physical examinations conducted by a study physician for vital signs (pulse rate, respiratory rate, blood pressure and oxygen saturation) and to ensure general health. Eligible participants who provided written informed consent were enrolled and randomized (further described later) to either the BB-12® or control yogurt drink by family cluster. The caregivers agreed to refrain from providing any other probiotic foods or supplements 14 days prior to initiating the study yogurts and during the entire intervention period. A list of excluded products was provided to each caregiver. The participating children consumed four ounces (112g) of the assigned yogurt beverage, which delivered at least 1 × 1010 colony forming units of BB-12® per serving, orally per day for ten consecutive days.
To assess the safety of the interventions, research assistants conducted follow-up interviews at days 6, 11, 15 and 180 (all ±2 days), and a second physical examination was performed on day 14. A 14-day daily assessment diary was completed by the caregivers, which captured data on compliance, symptoms and adverse events. Fecal specimens from the children were collected by the caregivers prior to (day 0) and on days 10, 30, 60 and 90 (all ±2 days), following the initiation of the yogurt intervention.
Participants
The participants in the study were healthy children between the ages of 1–5 years.
Inclusion and exclusion criteria
The inclusion criteria for the parents and caregivers were: ability to read, speak and write English or Spanish; access to a refrigerator for proper storage of the yogurt drink; and access to a telephone for follow-ups. The inclusion criterion for pediatric participants was the age limits of 1–5 years.
Pediatric participants were ineligible if they had any of the following: developmental delays; any chronic condition, such as diabetes or asthma, which required medication; prematurity (birth weight <2500 grams); congenital anomalies; failure to thrive; allergy to strawberry; active diarrhea (defined as three loose stools for two consecutive days); use of any other medicines except anti-pyretic medicines (pro re nata concomitant medications allowed); parental belief of lactose intolerance; history of heart disease, including valvulopathies or cardiac surgery; any implantable device or prosthetic; history of gastrointestinal surgery or disease; milk-protein allergy; allergy to any component of the product or the yogurt vehicle; or during baseline physical exam, had an oxygen saturation rate <96%, or respiratory or pulse rate outside the normal range per age.
Setting
The participants were recruited through the Capital Area Primary Care Research Network, a practice based research network, and from the greater local community through print and web-based advertising.
Interventions
The control and BB-12® interventions were strawberry-flavored yogurt drinks developed and manufactured at the Pennsylvania State University. The live culture used to conduct the fermentation was YF-L702 (supplied as frozen concentrated cultures by Chr. Hansen, Milwaukee, WI), a commercial blend of Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus (methods described previously).(13) Dry ingredients were mixed into the milk and pasteurized at 84.4°C for 41 seconds and homogenized first stage at 2000 psi and second stage at 500 psi. Yogurt mix was then heat treated at 85°C for 30 minutes, cooled to 43.3°C and inoculated with the starter culture YF-L702. Inoculated yogurt mix was allowed to ferment to a pH of 4.6. At this time, a prepared mixture containing strawberry, pectin, corn syrup solids, sugar and water was added and blended into the yogurt until uniform. After addition of the slurry, the yogurt drink was split into two portions; one portion was inoculated with BB-12® (Chr. Hansen, Milwaukee, WI) while the other portion remained BB-12®-free (control). Finally, each of the products was re-homogenized to produce a drinkable yogurt. The two products were identical except for the addition of BB-12®. The microbiological composition of the active yogurt drink at the end of its 30-day shelf life met targets of at least 1 × 1010 colony forming units per 100 mL serving of BB-12®.(13)
To verify the viable count of B. lactis in the yogurt drinks, both control and BB-12® products were analyzed immediately after manufacture and the BB-12® yogurt was analyzed weekly. To determine counts, suitable dilutions were pour-plated on selective MRS agar followed by anaerobic incubation at 37°C for 72 hours. Colonies counted as B. lactis were randomly picked and confirmed to be B. lactis by PCR using subspecies specific primers.(14)
Primary outcome measures
The primary outcome was the frequency and severity of adverse events reported during the study. Data on adverse events were collected via daily assessment diaries, spontaneous calls to the 24-hour study phone line and regularly scheduled phone interviews with the research personnel. An adverse event refers to any untoward event experienced by a participant during a clinical trial, whether or not it is associated with the use of the study products. This includes symptoms that were not present at the start of the study, as well as those symptoms that were present at baseline but worsened in severity during the course of the study. Adverse events were tabulated by type, intensity/severity, solicited or unsolicited and charted over time. The events were graded for severity using the National Institutes of Health, Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events.(15) Serious adverse events were defined as any incidences of death, life-threatening event, hospitalization, prolongation of a hospital stay or an event resulting in permanent disability.
Secondary outcome measures
The secondary aim was to evaluate the influence of BB-12®-supplemented yogurt on the fecal microbiota of patients and determine any changes in the composition of the microbial community.
Fecal DNA extraction
DNA from stool samples provided by participants on days 0, 10, 30, 60 and 90 was isolated using MOBIO PowerSoil DNA isolation kit (Cat. # 12888, Carlsbad, CA) according to the manufacture’s protocol. Isolated genomic DNA was stored at −80°C prior to analysis.
Illumina sequencing
DNA samples were prepared as previously described with the following modifications.(16) Universal primers F515 (5’ –NNNNNNNNGTGTGCCAGCMGCCGCGG – TAA – 3’) and R806 (5’ – GGACTACHVGGGTWTCTAAT – 3’), with the forward primer modified to contain an 8-nt barcode (italicized poly-N section of the primer above) and 2-nt linker sequence (bold portion) at the 5’ end, were used to amplify the V4 region of the 16S rRNA gene. PCR reaction contained 5.0 μl 2 × GoTaq Green Master Mix (Promega, Madison, WI), 0.4 μl 25 mM MgCl2, 2.4 μl water, 0.2 μl reverse primer (10 mM final concentration), 1.0 μl forward primer (2 mM final concentration), and 1.0 μl genomic DNA. Reactions were held at 94°C for 3 min to denature the DNA, with amplification proceeding for 25 cycles at 94°C for 45 s, 50°C for 60 s, and 72°C for 90 s; a final extension of 10 min at 72°C was included to ensure complete amplification. The PCR products were purified using QIAquick PCR Purification Kit (Cat # 28106, Valencia, CA). A composite sample for sequencing was created by combining equimolar ratios of amplicons from individual samples, followed by gel purification and ethanol precipitation to remove any remaining contaminants and PCR artifacts. The sample was sequenced in Dr. David Mills’ laboratory at the University of California, Davis, using the Illumina Genome Analyzer II sequencing platform.
Randomization
Each family cluster was allocated to either the control or BB-12® intervention arm in a 1:1 ratio using permuted block randomization of block size four using SAS® version 9.1 (SAS Institute Inc., Cary, NC). Once eligibility was determined, the participant was randomly assigned a number between 1 and 10, corresponding to a control or BB-12® yogurt drink. True allocation concealment over time was ensured, as research personnel had no methods to alter randomization or enrollment, nor had any knowledge of group assignment while subjects were being followed.
Compliance
Compliance was assessed in two ways: first, by self-report on the follow-up interview forms, and also through PCR analysis of the fecal samples collected at day 10 of the intervention. A child was defined as compliant if he or she reported consuming one-half (two fluid ounces/56g) or more of the daily serving of yogurt beverage at least 80% of the intervention days. Detection of B. lactis in the feces of the BB-12® group by PCR in the treatment group and absence of B. lactis in the feces of the control group were taken as evidence of compliance. Results showing the presence of B. lactis in the control group or the absence of B. lactis in the BB-12® group, or missing fecal samples, were considered to be noncompliant.
Data analysis
Sample size calculations are not applicable for a phase I safety study. A sample size of 30 children per group was selected in accordance with the FDA. Statistical analyses were conducted using Stata® 14 (StataCorp LP, College Station, TX) statistical software. Continuous variables were summarized using means, medians and standard deviations, and frequency percentages were calculated for categorical variables. The frequency and severity of the adverse events were described using frequencies and percentages. Baseline demographics and health characteristics were compared between the treated and control groups using Chi-square or Fisher’s exact test for categorical variables and t-tests and Wilcoxon rank test for continuous variables. The frequencies of adverse and serious adverse outcomes were compared using Fisher’s exact test. No adjustments for multiple comparisons were used.
The data analysis pipeline employed was modified based on a previous study.(17) Briefly, the QIIME software package was used to analyze the results of the Illumina sequencing run. Raw Illumina fastq files were first demultiplexed and quality filtered.(18) Reads were truncated after a maximum number of 3 consecutive low quality scores (< 1e-5), and any read containing one or more ambiguous base calls was discarded. Reads with a minimum pairwise identity of 97% were clustered into operational taxonomic units (OTUs) using QIIME’s open-reference OTU-picking workflow, which was based on UCLUST (19) software. The Greengenes bacterial 16S rRNA database (13_8 release) was used for the open-reference OTU-picking.(20) The most abundant sequence was chosen to represent each OTU. Taxonomy was assigned to each OTU using QIIME-based wrapper of the Ribosomal Database Project (RDP) classifier (21) against a representative subset of the Greengenes 16S rRNA database 13_8 release, using a 0.50 confidence threshold for taxonomic assignment. Bacterial 16S rRNA gene sequences were aligned using PyNAST (22) against a template alignment of the Greengenes core set filtered at 97% similarity. During the process, chimeras were identified and removed using the ChimeraSlayer (23) algorithm and a phylogenetic tree was built from the filtered alignment using FastTree.(24) Any OTU representing less than 0.001% of the total filtered sequences was removed to avoid erroneous reads that could lead to inflated estimates of diversity.(25) After these quality-filtering steps, each sample was represented by fewer than 150 sequences and the filtered OTU tables were ready for downstream analyses, such as diversity comparisons and biomarker discoveries. Alpha-diversity (within-sample species richness) and beta-diversity (between-sample community dissimilarity) were calculated within QIIME based on weighted UniFrac (26) distance between samples. Principle coordinates were calculated from the Unifrac distance matrices to decrease the dimensionality of the taxonomic dataset into 3D principal coordinate analysis (PCoA) plots, enabling visualization of sample relationships (Figure S1). To determine whether sample classification (treatment, time points) caused differences in phylogenetic or species diversity, ANOSIM (27) and permutational MANOVA (28) were used to test significant differences between sample groups based on weighted Unifrac. Significant taxonomic differences between sample groups were also tested using the Linear Discriminant Analysis (LDA) effect size (LEfSe).(29)
Results
Recruitment, enrollment and participant flow
One hundred potential participants called the recruitment hotline and expressed interest in the study. Eighty-seven parents were screened for eligibility and sixty-two children were enrolled and randomized in the study (Figure S2). Two children withdrew from the study prior to receiving the allocated intervention. Thirty-one children received the control intervention and twenty-nine children received the BB-12® intervention, totaling sixty participants (Figure S2). One child from each intervention group discontinued the intervention during the follow-up period. All sixty children who initiated the intervention were included in the analyses as per the intention-to-treat principle, and all participants were included for assessment of stool samples.
Baseline health and demographics
There were no significant differences in any of the demographic or baseline health characteristics between the BB-12® and control groups (Table S1).
Interventions
Viable counts of BB-12® and pH of the BB-12® probiotic-containing and control yogurt-based drinkable products were evaluated over the 30 days the products were stored at 4°C. All product information has been previously described elsewhere.(13) We found a stable, viable BB-12® population in the test product and the absence (<100 colony forming units/gram) of BB-12® in the control product. The pH of the drinkable products remained constant over time. The maintenance of BB-12® viability and constant pH of the drinkable products indicated that the product was stable throughout the experiment.(13)
Compliance
The rates of compliance, as measured by the self-reported consumption days, were 81% in the BB-12® group and 89% in the control group; the difference was not significant (P=0.469). The PCR results for day 10 fecal samples show that overall, 92% of the participants were compliant; of the participants in the BB-12® group, 100% tested positive for B. lactis (97% compliance due to one missing sample in the BB-12® group) and of the participants in the control group, 87% tested negative for B. lactis. The difference between the groups was not significant (P=0.355). Additionally, blinding worked appropriately as, when surveyed at the end of the intervention period as to which yogurt beverage the parent believed his or her child consumed, 51% of participants (29 out of 57 respondents) correctly guessed their assignment.
Primary outcome
A total of 186 adverse events were reported in this study, all of which were self-limited (Table 1). A similar number of adverse events were reported during the intervention period (days 0–10) and the post-intervention period (days 11–180), with 96 and 90 adverse events, respectively. There were 73 adverse events reported in the control group and 113 adverse events reported in the BB-12® intervention group. There were also no reported allergic reactions or hypersensitivity to the yogurts. There were three reported serious adverse events: one occurred at the start of the yogurt intervention and two occurred months after the yogurt intervention concluded. The serious adverse events were all hospitalizations that resolved within days and were determined to be unrelated to the study interventions. There were no withdrawals from the study due to adverse events related to product consumption. Fisher’s exact test showed none of the symptoms having significantly different frequencies.
Table 1.
Frequency of adverse and serious adverse events reported by group
| Days 0–10 (During Intervention) | Days 11–180 (Post-Intervention) | |||||
|---|---|---|---|---|---|---|
| Adverse Event | Control | BB-12® | Total | Control | BB-12® | Total |
| Allergies (e.g. seasonal) | 1 | 2 | 3 | 1 | 3 | 4 |
| Broken finger | 1 | 1 | ||||
| Bronchiolitis | 1 | 1 | ||||
| Cold | 2 | 2 | 2 | 1 | 3 | |
| Constipation | 3 | 3 | 2 | 1 | 3 | |
| Cough | 5 | 10 | 15 | 1 | 5 | 6 |
| Croup | 1 | 1 | ||||
| Cut finger | 1 | 1 | ||||
| Diarrhea | 2 | 2 | 4 | 2 | 5 | 7 |
| Ear aches | 1 | 1 | ||||
| Ear infection | 2 | 1 | 3 | |||
| Fever | 2 | 2 | 2 | 3 | 5 | |
| Flatulence | 2 | 2 | 4 | 1 | 1 | 2 |
| Headache | 1 | 1 | ||||
| Hives | 1 | 1 | ||||
| Irritability | 4 | 1 | 5 | 3 | 5 | 8 |
| Laceration | 1 | 1 | ||||
| Lack of/decreased appetite | 2 | 2 | 4 | 3 | 1 | 4 |
| Lethargy | 1 | 5 | 6 | |||
| Loose stool | 2 | 6 | 8 | 3 | 3 | 6 |
| Lump on back of head | 1 | 1 | ||||
| Nasal congestion | 4 | 6 | 10 | 2 | 2 | |
| Pain | 2 | 1 | 3 | 2 | 2 | |
| Physical injury | 1 | 1 | ||||
| Pink eye | 1 | 1 | ||||
| Pneumonia | 1 | 1 | ||||
| Rash | 2 | 2 | 1 | 2 | 3 | |
| Runny nose | 6 | 12 | 18 | 6 | 9 | 15 |
| Skin infection | 1 | 1 | ||||
| Sore foot | 1 | 1 | ||||
| Sore throat | 1 | 2 | 3 | |||
| Strep throat | 1 | 1 | ||||
| Umbilical hernia | 1 | 1 | ||||
| Vomiting | 2 | 2 | 1 | 2 | 3 | |
| Total | 35 | 61 | 96 | 38 | 52 | 90 |
| Serious adverse eventsa | 1 | 1 | 2 | 2 | ||
Serious adverse events included: grade 4/potentially life-threatening fever reported on day 2; grade 4/potentially life-threatening bronchiolitis reported at day 180; and grade 4/potentially life-threatening pneumonia reported at day 180. All serious adverse events were unrelated to the interventions and resolved.
Secondary outcomes
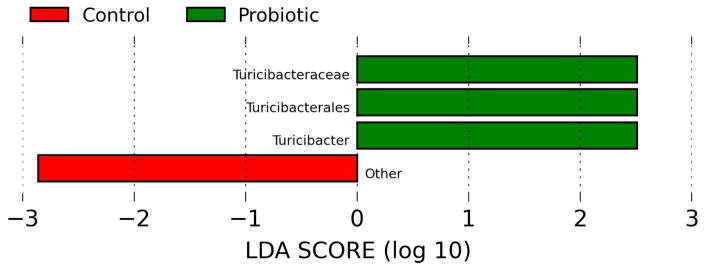
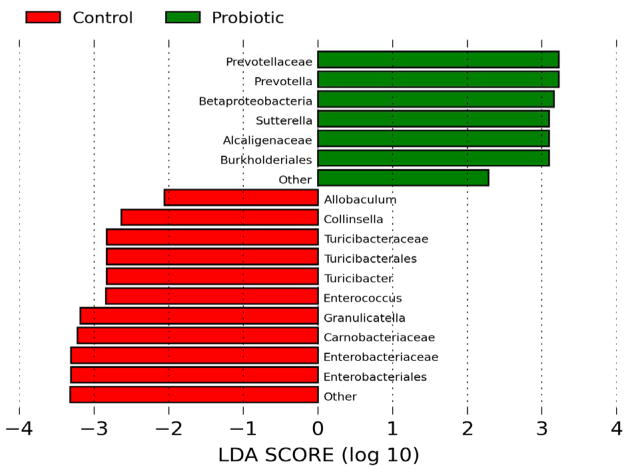
Illumina sequencing of the 288 fecal samples generated over 5 million total reads. After removing samples with poor compliances, about 4.4 million sequences from 251 fecal samples were used for data analyses. Overall, 8 phyla and 103 genera were identified in the participants. Firmicutes, Bacteroidetes, and Actinobacteria accounted for > 90% of the sequences at the phylum level. No significant differences in relative abundance between treatments were observed at this level. The large variations observed among individuals might account for failure to detect any small changes. Lower level comparisons showed that 8 differentially abundant taxonomic clades with an LDA score higher than 2.0 were detected at baseline between the two arms (Figure 1a). Differentially abundant bacterial taxa at different levels (order, family, genus) in the BB-12® arm belong to the phylum of Firmicutes, including Turicibacterales, Turicibacteraceae, and Turicibacter while another member of Firmicutes, Dialister, was overrepresented in the control arm. This differential abundance pattern was slightly shifted after 10 days of intervention (Figure 1b). Specifically, a few members in the phyla Bacteroidetes and Proteobacteria including Prevotella and Burkholderiales were more abundant in the BB-12® group whereas Turicibacterales, Turicibacteraceae, Eubacteriaceae, Enterococcaceae, Turicibacter were overrepresented in the control group after 10 days of intervention. Within each treatment, no significant changes over time were observed except the relative abundance of Streptococcus increased in both groups after 10 days of yogurt consumption. The relative abundance of Bifidobacterium did not differ between treatments or among time points (data not shown), possibly because of the significant variations observed among individuals (P < 0.001).
Figure 1.
Linear discriminant analysis (LDA) scores computed for features differentially abundant between control yogurt and BB-12® yogurt drink. (a) Operational taxonomic unit (OTU) comparison at day 0 (prior to interventions) (b) OTU comparison at day 10 (after interventions)
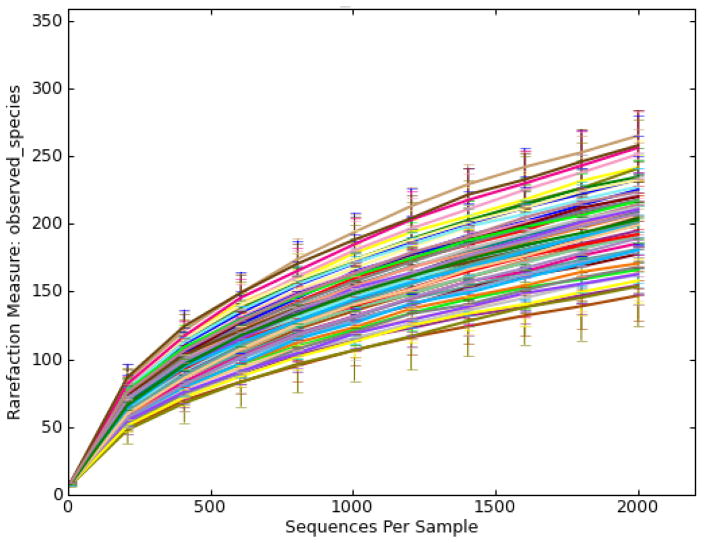
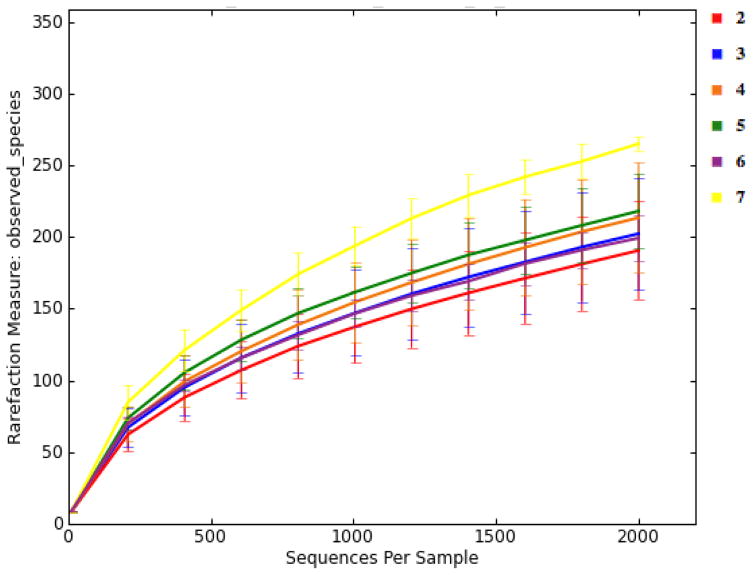
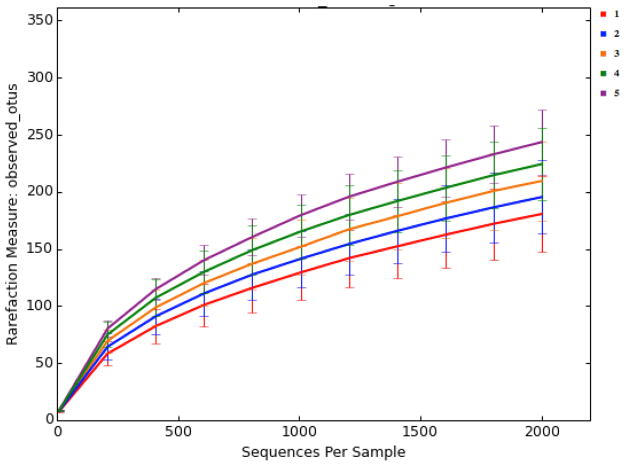
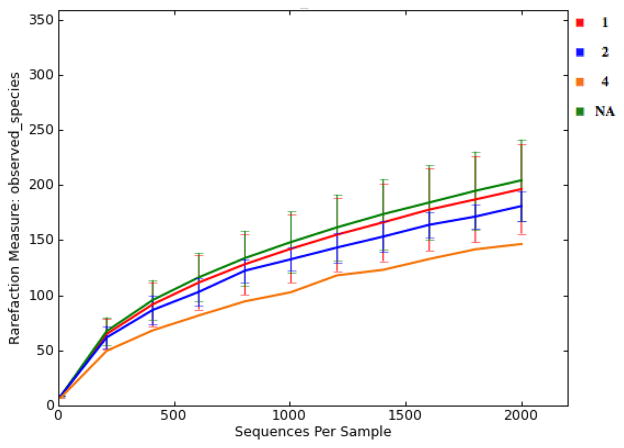
There were large differences in alpha-diversity among individuals (Figure 2). This made it challenging to compare treatments, because significant variations in the pooled data would make it difficult to see small treatment effects. Interestingly, subjects with high number of housemates tended to present a more diverse gut microbiota (Figure 3). The gut microbiota of older children appeared to be more diverse when they are older (Figure 4). Moreover, subjects that reported an adverse event tended to have less diverse microbiota (Figure 5). Nonparametric permutational statistical tests confirmed that there was weak or no significant treatment effect on subjects’ gut microbiota (weighted UniFrac RANOSIM = 0.023, P = 0.01; R2ADONIS = 0.007, P = 0.009) while subjects differed significantly (RANOSIM = 0.271, P = 0.01; R2ADONIS = 0.342, P = 0.001) from each other in terms of gut microbiota regardless of treatment. This suggests that subject biology plays a bigger role than the intervention in shaping gut microbiota. Alpha-diversity rarefaction curves indicated total number of housemates, age, and adverse event might lead to different gut microbial structure in the host. Beta-diversity comparison substantiated that gut microbiota dissimilarities were marginally significant if grouped by age (RANOSIM = -0.031, P = 0.92; R2ADONIS = 0.018, P = 0.001) or by total number of housemates (RANOSIM = -0.008, P = 0.68; R2ADONIS = 0.008, P = 0.004), but significant if grouped by adverse event (RANOSIM = 0.104, P = 0.03). In order to do more detailed comparisons, the OTU table was divided by treatment and time. Within the control drink group, the structure of gut microbiota did not change with time while subjects differed significantly from each other (RANOSIM = 0.269, P = 0.01; R2ADONIS = 0.334, P = 0.001) and among age groups (RANOSIM = 0.068, P = 0.04; R2ADONIS = 0.028, P = 0.001). The same was observed in the BB-12® probiotic group (data not shown). Interestingly, there was weak difference between control group and BB-12® probiotic group at baseline (RANOSIM = 0.047, P = 0.05; R2ADONIS = 0.02, P = 0.272). However, this difference disappeared after 10 days of treatment and never presented again during the 90-day study window.
Figure 2.
Alpha rarefaction plots grouped by subject (Each rarefaction plot represents species richness or alpha-diversity of one subject or one group within the sample metadata. It is a curve of the number of species as a function of the number of samples or simulated sequencing effort.)
Figure 3.
Alpha rarefaction plots grouped by total number of housemates
Figure 4.
Alpha rarefaction plots grouped by age of participant (in years)
Figure 5.
Alpha rarefaction plots grouped by adverse event severity grade NA: not applicable/no AE reported; 1: mild; 2: moderate; 4: potential life threatening
Discussion
The aim of this randomized, controlled study was to assess the safety of a probiotic-supplemented yogurt containing the probiotic strain BB-12® when consumed by healthy children. The baseline and demographic characteristics of the study participants, the duration of the study and the compliance of the product consumption were similar for the BB-12®-supplemented and control groups. There was no significant difference in the number of adverse events between the BB-12®-supplemented and control groups. There were no serious adverse events related to the interventions or withdrawals from the study for adverse events related to product consumption.
The majority of studies on probiotics are conducted outside of the United States and are not conducted under IND regulations. As one of the few studies conducted under IND regulations, and the first to study BB-12® in a pediatric population under an IND, this study is novel. This study provides the safety profile necessary to continue with a phase II study on the efficacy and safety of BB-12® yogurt in preventing AAD in children.
Comparison between control group and BB-12® probiotic group at baseline and day 10 revealed that a few members in the phyla Firmicutes, Bacteroidetes, and Proteobacteria were differently represented in each group before and after interventions. Specifically, the relative abundance of Turicibacter of Firmicutes decreased while Prevotella of Bacteroidetes increased in the BB-12® arm compared to the control arm after 10 days of treatment. Such a shift of relative abundance of a few bacterial family/genera was rather subtle as there was no difference between treatments at phylum level. The significance of changes in relative abundance of specific taxa is unclear. In one study, African children who ate fiber-rich diets had a higher abundance of specific Bacteroidetes (Prevotella and Xylanibacter), a reduced abundance of Firmicutes and decreased level of certain Proteobacteria (Shigella and Escherichia), compared with European children.(30) Increased Firmicutes:Bacteroidetes ratio in gut microbiota has been associated with metabolic disorders, such as obesity.(31–33) However, other studies found that obese individuals had reduced Firmicutes and increased Bacteroidetes (34) or no change in proportion of either Firmicutes or Bacteroidetes.(35) The discrepancies found across studies could be linked to heterogeneity in age of subjects as the ratio of Firmicutes:Bacteroidetes has been found to change with age, or to many other factors such as overall diet, lifestyle, experimental setup, methodology of data analysis.(36) However, focusing on the ratio of Firmicutes:Bacteroidetes does not completely capture the compositional changes in microbiota associated with treatments in our study.
Diversity analyses indicated that individuals varied significantly in alpha-diversity (within-sample diversity) and beta diversity (between-sample diversity), although a few factors (family size, age, and self-reported adverse event) seemed to be associated with these variations. These observations are in agreement with previous studies.(37, 38) The present study shows that subjects with a high number of housemates tend to have more diverse gut microbiota (Figure 3). Song et al. showed that family members that live together share microbiota with one another and with their dogs.(37) In the present work, older children within a household seemed to have a more diverse gut microbiota (Figure 4). This is in agreement with a previous study reporting that the gut microbiota of newborns increase rapidly in diversity through early childhood and become more adult-like during the first 1–3 years of life.(38) Moreover, self-reported adverse events seemed to be associated with the level of diversity observed in the gut microbiota of this study cohort. While numerous studies have shown that a low-diversity gut microbiota are often linked to disease states,(39–43) it is acknowledged that the interaction between gut microbiota and host is much more complicated than just a high or low microbial diversity. Meanwhile, it is noteworthy that the gut microbial communities of healthy individuals are relatively stable.(44) Probiotics have shown promising effects on gut microbiota in health-compromised subjects.(45, 46) However, healthy people with stable gut microbiota are likely to be more resilient to probiotic-induced changes in gut microbiota.
A few limitations to this study should be noted. The sample size was fixed at 30 children per treatment group in order to obtain sufficient phase I trial results that can be used for future planning. As the research proceeds with additional studies, larger sample sizes, more diverse populations and longer intervention periods are necessary to further assess adverse events. While others have shown that probiotic administration does not impact compositional changes in gut microbiota, important differences in gene expression and transcript diversity may result from probiotic administration.(47) We hope to examine metabolomics changes elicited by BB-12® administration along with composition changes in future studies.
In conclusion BB-12® is safe and well tolerated in healthy children aged 1–5 years, and this randomized, controlled trial provided the evidence for safety needed for the next stage of the IND process to include a pediatric population concurrently receiving antibiotic treatment. Further research is necessary to understand the effects of BB-12® on improving health outcomes and on the relationship between factors such as number of housemates, age, and self-reported adverse events and the host gut microbiota under the influence of BB-12® intervention in patients receiving antibiotic treatment.
Supplementary Material
Jackknife-supported principal coordinate analysis (PCoA) plot demonstrates the beta-diversity of the gut microbiota of the participants. Red: BB-12® group; blue: control group.
Flow diagram of study participation
What is known?
Despite their widespread use by both consumers and physicians, the efficacy of many probiotic products remains unsupported by rigorous independent research. What is new?
BB-12® probiotic-supplemented yogurt is safe when consumed by generally healthy children aged 1–5 years for 10 consecutive days.
This was the first trial to study BB-12® in a pediatric population as an investigational new drug.
This study was also the first to evaluate the influence of BB-12® on the gut microbiota of children (aged 1–5 years) and examine changes in the microbial community.
Acknowledgments
We would like to express our gratitude to Linda C. Duffy, National Institutes of Health program officer for the Division of Extramural Research at NCCIH, and the Office of Clinical and Regulatory Affairs for their guidance and support. We also thank all the individuals who participated in this study, Jessy Sparenborg, Emily Furumoto, the Berkey Creamery at the Pennsylvania State University, research assistants, the Capital Area Primary Care Research Network, and Data Safety Monitoring Board Chair Elizabeth Carter and members Tamar Ringel-Kulka, Felice Roggen, and Alan Simon. We would like to thank Chr. Hansen for their assistance in the IND and DMF process with the FDA, and for supplying the BB-12® to the Pennsylvania State University. Chr. Hansen had no role in design, data interpretation, or analysis of the study data.
Financial Support
The research described was supported by the National Center for Complementary and Integrative Health of the National Institutes of Health under Award Number U01AT003600. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- BB-12®
Bifidobacterium animalis subsp. lactis strain BB-12®
- IND
investigational new drug
- FDA
Food and Drug Administration
- CBER
Center for Biologics Evaluation and Research
- NIH
National Institutes of Health
- NCCIH
National Center for Complementary and Integrative Health
Footnotes
Trial registration: NCT01652287; ClinicalTrials.gov
Conflicts of Interest
D.J.M. previously served as a paid expert witness for General Mills, Inc., Nestlé Nutrition, Bayer and the Proctor & Gamble Company. M.E.S. consults for numerous probiotic manufacturers. The remaining authors have no conflicts of interest to declare.
Authorship
D.J.M., R.F.R. T.P.T., and M.E.S. designed research; D.J.M., K.H.S., T.P.T., and Z.B. conducted research and acquired data; F.J.D., D.J.M., T.P.T., Z.B. and R.F.R. analyzed and interpreted data; F.J.D., T.P.T. and Z.B. performed the statistical analyses; T.P.T., Z.B., and D.J.M. drafted the initial manuscript; M.E.S., F.J.D., and R.F.R. provided critical revision for important intellectual content; D.J.M. had primary responsibility for the final content. All authors have seen and approved the final manuscript.
References
- 1.Hill C, Guarner F, Reid G, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506–14. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 2.marketsandmarkets.com. Probiotic Ingredients Market by Function (Regular, Preventative, Therapy), Application (Food & Beverage, Dietary Supplements, & Animal Feed), End Use (Human & Animal Probiotics), Ingredient (Bacteria & Yeast), and by Region - Global Trends & Forecast to 2020. [Accessed November 13, 2015]; http://www.marketsandmarkets.com/Market-Reports/probiotic-market-advanced-technologies-and-global-market-69.html.
- 3.Black LI, Clarke TC, Barnes PM, et al. National health statistics reports. Hyattsville, MD: National Center for Health Statistics; 2015. Use of complementary health approaches among children aged 4–17 years in the United States: National Health Interview Survey, 2007–2012. [PMC free article] [PubMed] [Google Scholar]
- 4.Clarke TC, Black LI, Stussman BJ, et al. National health statistics reports. Hyattsville, MD: National Center for Health Statistics; 2015. Trends in the use of complementary health approaches among adults: United States, 2002–2012. [PMC free article] [PubMed] [Google Scholar]
- 5.Tuohy KM, Rouzaud GC, Bruck WM, et al. Modulation of the human gut microflora towards improved health using prebiotics - assessment of efficacy. Curr Pharm Des. 2005;11(1):75–90. doi: 10.2174/1381612053382331. [DOI] [PubMed] [Google Scholar]
- 6.Clausen MR, Bonnen H, Tvede M, et al. Colonic Fermentation to Short-Chain Fatty-Acids Is Decreased in Antibiotic-Associated Diarrhea. Gastroenterology. 1991;101(6):1497–504. doi: 10.1016/0016-5085(91)90384-w. [DOI] [PubMed] [Google Scholar]
- 7.Engelbrektson AL, Korzenik JR, Sanders ME, et al. Analysis of treatment effects on the microbial ecology of the human intestine. FEMS Microbiol Ecol. 2006;57(2):239–50. doi: 10.1111/j.1574-6941.2006.00112.x. [DOI] [PubMed] [Google Scholar]
- 8.Johnston BC, Goldenberg JZ, Vandvik PO, et al. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev. 2011;(11):CD004827. doi: 10.1002/14651858.CD004827.pub3. [DOI] [PubMed] [Google Scholar]
- 9.Hempel S, Newberry SJ, Maher AR, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA. 2012;307(18):1959–69. doi: 10.1001/jama.2012.3507. [DOI] [PubMed] [Google Scholar]
- 10.Patro-Golab B, Shamir R, Szajewska H. Yogurt for treating antibiotic-associated diarrhea: Systematic review and meta-analysis. Nutrition. 2015;31(6):796–800. doi: 10.1016/j.nut.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 11.Jungersen M, Wind A, Johansen E, et al. The Science behind the Probiotic Strain Bifidobacterium animalis subsp. lactis BB-12®. Microorganisms. 2014;2(2):92. doi: 10.3390/microorganisms2020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Merenstein DJ, Tan TP, Molokin A, et al. Safety of Bifidobacterium animalis subsp. lactis (B lactis) strain BB-12-supplemented yogurt in healthy adults on antibiotics: a phase I safety study. Gut Microbes. 2015;6(1):66–77. doi: 10.1080/19490976.2015.1005484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merenstein D, Gonzalez J, Young AG, et al. Study to investigate the potential of probiotics in children attending school. Eur J Clin Nutr. 2011;65(4):447–53. doi: 10.1038/ejcn.2010.290. [DOI] [PubMed] [Google Scholar]
- 14.Ventura M, Reniero R, Zink R. Specific identification and targeted characterization of Bifidobacterium lactis from different environmental isolates by a combined multiplex-PCR approach. Appl Environ Microbiol. 2001;67(6):2760–5. doi: 10.1128/AEM.67.6.2760-2765.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events, Version 1.0. U.S. Department of Health and Human Services, National Institutes of Health, National Institute of Allergy and Infectious Diseases, Division of AIDS; [Updated August 2009]. Available from: http://rsc.tech-res.com/Document/safetyandpharmacovigilance/Table_for_Grading_Severity_of_Adult_Pediatric_Adverse_Events.pdf. [Google Scholar]
- 16.Caporaso JG, Lauber CL, Walters WA, et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. PNAS. 2011;108(Suppl 1):4516–22. doi: 10.1073/pnas.1000080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bokulicha NA, Thorngated JH, Richardsone PM, et al. Microbial biogeography of wine grapes is conditioned by cultivar, vintage, and climate. PNAS. 2014;111(1):E139–48. doi: 10.1073/pnas.1317377110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7(5):335–6. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26(19):2460–1. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 20.DeSantis TZ, Hugenholtz P, Larsen N, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 2006;72(7):5069–72. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Q, Garrity GM, Tiedje JM, et al. Naïve bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73(16):5261–7. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caporaso JG, Bittinger K, Bushman FD, et al. PyNAST: A flexible tool for aligning sequences to a template alignment. Bioinformatics. 2010;26(2):266–7. doi: 10.1093/bioinformatics/btp636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haas BJ, Gevers D, Earl AM, et al. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 2011;21(3):494–504. doi: 10.1101/gr.112730.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Price MN, Dehal PS, Arkin AP. FastTree 2—approximately maximum-likelihood trees for large alignments. PLoS One. 2010;5(3):e9490. doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bokulich NA, Subramanian S, Faith JJ, et al. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat Methods. 2013;10(1):57–9. doi: 10.1038/nmeth.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lozupone C, Hamady M, Knight R. UniFrac--an online tool for comparing microbial community diversity in a phylogenetic context. BMC Bioinformatics. 2006;7(371) doi: 10.1186/1471-2105-7-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clarke KR. Non-parametric multivariate analyses of changes in community structure. Aust J Ecol. 1993;18(1):117–43. [Google Scholar]
- 28.Anderson MJ. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2008;26(1):32–46. [Google Scholar]
- 29.Segata N, Izard J, Waldron L, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12(6):R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Filippoa C, Cavalieri D, Paolab MD, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. PNAS. 2010;107(33):14691–6. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen TLA, Vieira-Silva S, Liston A, et al. How informative is the mouse for human gut microbiota research? Dis Model Mech. 2015;8(1):1–16. doi: 10.1242/dmm.017400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ley RE, Turnbaugh PJ, Klein S, et al. Human gut microbes associated with obesity. Nature. 2006;444:1022–3. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 33.Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–4. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwiertz A, Taras D, Schäfer K, et al. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring) 2010;18(1):190–5. doi: 10.1038/oby.2009.167. [DOI] [PubMed] [Google Scholar]
- 35.Duncan SH, Lobley GE, Holtrop G, et al. Human colonic microbiota associated with diet, obesity and weight loss. Int J Obes (Lond) 2008;32:1720–4. doi: 10.1038/ijo.2008.155. [DOI] [PubMed] [Google Scholar]
- 36.Mariat D, Firmesse O, Levenez F, et al. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009;9(123) doi: 10.1186/1471-2180-9-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song SJ, Lauber C, Costello EK, et al. Cohabiting family members share microbiota with one another and with their dogs. eLife. 2013;2:e00458. doi: 10.7554/eLife.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dominguez-Bello MG, Blaser MJ, Ley RE, et al. Development of the human gastrointestinal microbiota and insights from high-throughput sequencing. Gastroenterology. 2011;140(6):1713–9. doi: 10.1053/j.gastro.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dicksved J, Halfvarson J, Rosenquist M, et al. Molecular analysis of the gut microbiota of identical twins with Crohn's disease. ISME J. 2008;2(7):716–27. doi: 10.1038/ismej.2008.37. [DOI] [PubMed] [Google Scholar]
- 40.Bisgaard H, Li N, Bonnelykke K, et al. Reduced diversity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. J Allergy Clin Immunol. 2011;128(3):646–52. doi: 10.1016/j.jaci.2011.04.060. [DOI] [PubMed] [Google Scholar]
- 41.Abrahamsson TR, Jakobsson HE, Andersson AF, et al. Low diversity of the gut microbiota in infants with atopic eczema. J Allergy Clin Immunol. 2012;129(2):434–40. doi: 10.1016/j.jaci.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 42.Marsland BJ, Salami O. Microbiome influences on allergy in mice and humans. Curr Opin Immunol. 2015;36:94–100. doi: 10.1016/j.coi.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 43.Giongo A, Gano KA, Crabb DB, et al. Toward defining the autoimmune microbiome for type 1 diabetes. ISME J. 2011;(5):1. doi: 10.1038/ismej.2010.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ursell LK, Metcalf JL, Parfrey LW, et al. Defining the human microbiome. Nutr Rev. 2012;70(Suppl 1):S38–44. doi: 10.1111/j.1753-4887.2012.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kirjavainen PV, Arvola T, Salminen SJ, et al. Aberrant composition of gut microbiota of allergic infants: a target of bifidobacterial therapy at weaning? Gut. 2002;51(1):51–5. doi: 10.1136/gut.51.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mohan R, Koebnick C, Schildt J, et al. Effects of Bifidobacterium lactis BB-12 supplementation on intestinal microbiota of preterm infants: a double-blind placebo controlled randomized study. J Clin Microbiol. 2006;44(11):4025–31. doi: 10.1128/JCM.00767-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eloe-Fadrosh EA, Brady A, Crabtree J, et al. Functional dynamics of the gut microbiome in elderly people during probiotic consumption. MBio. 2015;6(2) doi: 10.1128/mBio.00231-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Jackknife-supported principal coordinate analysis (PCoA) plot demonstrates the beta-diversity of the gut microbiota of the participants. Red: BB-12® group; blue: control group.
Flow diagram of study participation