Abstract
Objectives
Low-grade elevation of C-reactive protein (CRP) is a non-specific inflammatory marker, used as a predictor for cardiovascular disease development and chronic inflammatory risks. Research investigating dietary influences on inflammation has focused primarily on the relationship between dietary characteristics, CRP elevation and BMI in the populations at greatest risk for cardiovascular disease, namely those in the overweight and obese ranges, often in clinical settings and/or among those middle aged or older, leaving little information about normal to underweight populations of reproductive age in ecological settings. This study evaluates impacts of dietary nutrients on serum CRP levels in a population of predominantly underweight to normal weight adult women experiencing the additional nutritional demands of lactation.
Methods
Data from non-overweight breastfeeding Ariaal women of Kenya collected in 2006 were used (n = 194). Logistic regression models were applied using low-grade CRP elevation (hsCRP > 3 mg/L) as the outcome variable and dietary nutrients, age, BMI, and serum retinol as predictors.
Results
Models showed that energy intake (Kcal) and age were positive predictors of CRP elevation while folate intake, total vitamin A intake, and serum retinol concentration were protective against CRP elevation. Unlike previous studies among higher BMI populations, this study found no significant effect of dietary lipids/fatty acids or BMI on CRP elevation.
Conclusions
The effects of specific dietary nutrients on inflammatory status may vary with BMI or, in women, reproductive status. Further research should investigate the role of dietary fats, fatty acids, and antioxidant vitamins across populations with a wide range of BMI, including postpartum women.
INTRODUCTION
C-reactive protein (CRP) is a biomarker for nonspecific inflammation (Pepys and Hirschfield, 2003). CRP has long been used in micronutrient biomarker research in developing country settings to adjust for the effect of acute phase reaction (acute infection/inflammation) on micronutrient measurements such as serum vitamin A concentrations (e.g. using CRP >10 mg/l as a cutoff for adults; Thurnham et al., 2003). More recently, the advent of commercial and automated CRP assays with low detection limits (high sensitivity CRP or hsCRP) has facilitated a new body of literature on lower-grade elevation of hsCRP (well below the acute phase cutoff, e.g. >3 mg/L; Pearson et al., 2003; Ridker et al., 2004) as a predictor for future cardiovascular disease, including coronary events, stroke, and peripheral arterial disease (Pepys and Hirschfield, 2003), although its predictive capability is still controversial (Nanri et al., 2007).
Numerous studies have shown that hsCRP has a positive relationship with body mass index (BMI), overweight/obesity and body adiposity across diverse populations (Ford et al., 2001 in US children; Gildiken et al., 2007 among obese patients; Hodge et al., 2010 in Australian adults; Park et al., 2005 in Korean adults; Rexrode et al., 2003 in US women of age ≥45 years; Silva et al. (2010) in Brazilian adolescents). Similarly, hsCRP has been associated with altered blood lipid profiles (Wang et al., 2011 in US overweight university students; Wu et al., 2003 in Taiwanese children) and elevated risk of coronary pathology (Pearson et al., 2003; Ridker et al., 2004).
Despite these associations, it remains unclear how low-grade elevation of hsCRP predicts coronary pathology in apparently healthy populations (Pepys and Hirschfield, 2003). An emerging interest in chronic inflammatory disease research is dietary influence on low-grade hsCRP elevation (Nanri et al., 2007). However, the overwhelming majority of this research is among well-nourished and over-nourished populations or in clinical settings, leaving little information about normal to underweight populations. Clarifying the possible effects of dietary characteristics on hsCRP in relatively lean populations in a different ecological setting would improve the understanding of the link between diets/nutrients and chronic inflammatory diseases, and facilitate more informed interpretations of the low-grade elevation of hsCRP.
According to the literature on chronic low grade inflammation, the adipose tissue plays a central role in the current model of the genesis of the inflammatory state in obesity. Adipose tissue hypoxia, abnormally large adipocyte size and the increased rates of adipocyte apoptosis have been suggested as the major engine of the generation of this condition (Margioris et al., 2013). Given this paradigm, it is logical that much of the research focus on populations with high body adiposity and high BMI. If the adipose tissue model is valid, the reduction of the body adiposity should lead to the resolution of the inflammatory status. This prediction could be tested by examining populations across the wide BMI range. However, the literature currently has insufficient information from populations in low-to-normal BMI range.
Similarly, studies of changes in hsCRP with changes in body weight fail to cover populations across the full BMI range. Previous studies of the relationship between hsCRP and weight loss have focused on cachexia in the very ill (e.g. Wallengren et al., 2013 among cancer patients) or the effects of weight loss on hsCRP among those who are overweight or obese (e.g. Belza et al., 2009). It remains unclear whether decreasing BMI, representing negative energy balance, in healthy individuals leads to decreasing hsCRP in a dose response manner across the full range of BMI values.
CRP and dietary nutrients
There is a growing body of literature on dietary influences on serum levels of hsCRP. Nutrients with antioxidant and anti-inflammatory properties such as carotenoids, vitamin C and vitamin E have been reported as potentially prophylactic against the elevation of hsCRP, although these effects appear to be variable. A review of intervention trials among healthy subjects and patients with type-2 diabetes or cardiovascular disease concluded that administration of carotenoids and vitamin C, but not of vitamin E, can decrease the level of circulating hsCRP (Nanri et al., 2007). Another study of US adults found dietary flavonoid intake to be negatively associated with hsCRP (Chun et al., 2008). Among children and adolescents in the US, lower intakes of grains and vegetables were associated with higher hsCRP (Qureshi et al., 2009). Conversely, a study of older US adults (45–84 years) found no significant effects of vitamin C or β-carotene, a major pro-vitamin A carotenoid, on hsCRP (De Oliveira Otto et al., 2011). Rather, they found significant positive effects of other micronutrients such as dietary zinc and heme iron on hsCRP.
In the study presented here, we investigate the possible effects of dietary nutrients on hsCRP elevation in a population of women who are expending calories on lactation, therefore in negative energy balance, and have BMIs in the underweight to normal weight range. Specifically, we test the hypothesis that antioxidant and anti-inflammatory nutrients will be prophylactic against the elevation of serum hsCRP. Additionally, we evaluate whether BMI predicts the elevation of hsCRP in a nonoverweight group of breastfeeding women as previously reported for other populations. We focus on apparently healthy breastfeeding mothers because they are understudied despite their importance in the inter-generational dynamics (Kuzawa et al., 2013) and the developmental programming of health and disease (Brandtzaeg, 2013; Moore, 2013). Maternal inflammation can alter breastmilk constituents such as secretory immunoglobulin A (Groer et al., 2004) and adiponectin (Ozarda et al., 2012), through which nursing infants’ health and immune development will be affected. Clarifying dietary nutrients that may affect CRP elevation among breastfeeding mothers would therefore have far reaching implications.
MATERIALS AND METHODS
Data collection
We used cross-sectional data from 224 breastfeeding women 18 to 46 years of age in rural agro-pastoral communities of Ariaal agro-pastoralists in the Marsabit District of Kenya, for whom dietary and serum hsCRP concentration data were complete. The geographic, ethnographic, and social, health, and economic backgrounds of the agro-pastoral Ariaal have been described in depth elsewhere (Fratkin and Roth, 2005). The data were originally collected for vitamin A research between August and September, 2006 from a sample of 241 women recruited via stratified random sampling, using postpartum months as the sample strata (Fujita, 2008). These 241 women were recruited using the following exclusion criteria: (1) >20 months postpartum, (2) not lactating, (3) taking oral contraceptives, and (4) having visible or reported symptoms of liver and/or kidney diseases or acute infections. Study procedures were approved by the institutional review boards of the University of Washington and Kenya Medical Research Institute.
Dietary nutrients
Dietary nutrients values were estimated using the information from a 24-hr dietary recall interview. In each recall interview, the mother was asked to list all foods that she consumed the previous day and estimate the volume in reference dishes and utensils. After listing all specific foods, the mother was also asked to explain the recipes for any mixed dishes. The amount of each ingredient consumed by the mother from such dishes was estimated from her portion as a fraction the number of individuals who shared the total amount prepared. The list of all recalled foods with volume estimates was then converted into a list of food items with estimated weights, using a volume-weight conversion table constructed during the fieldwork. The nutrient content of diets was then analyzed using SIGHT AND LIFE Vitamin A Intake Calculator (Erhardt, 2003) for the total daily vitamin A intake (μg) and NutriSurvey with a Kenyan food database for energy (Kcal) and all other nutrients (Erhardt, 2005). All recalled food items from individual 24-hr dietary recall interviews were entered into the nutritional software.
The daily energy requirement was calculated using the basal metabolic rate equation appropriate for the age and postpartum time for lactating women and adjusted for the moderate activity level of a rural woman in a developing country, following the method published by the WHO (The Joint FAO/WHO/UNU, 1985).
C-reactive protein determination
Serum hsCRP concentrations were assessed in fasting venous blood samples collected by venipuncture using a sterile needle and syringe. The blood samples were centrifuged and sera frozen in liquid nitrogen, with dry ice, or in freezers until analysis using a microtiter plate-based sandwich enzyme immunoassay developed at the Biodemography Laboratory of the University of Washington (Seattle, WA) 251–553 days after blood collection. The assay method has been described in depth elsewhere (Brindle et al., 2010). Assay controls for 11 assay plates diluted independently for three batches had mean values of 3.25, 5.25, and 8.86 mg/l for low, medium, and high serum controls, respectively. The intra-assay CVs based on these three levels of control specimens were 6.66, 7.39, and 5.46%, and interassay CVs were 20.2, 20.1, and 8.0%, respectively. Analytical sensitivity was 0.00007 mg/L (n = 20 plates).
To avoid the confounding effect of high-grade hsCRP elevation due to acute phase reaction (rather than chronic low-grade inflammation), 25 individuals with serum hsCRP concentrations of >10 mg/L (Thurnham et al., 2003) were excluded from further analysis. For the remaining individuals (n = 199), a dummy variable (elevated hsCRP) for low-grade inflammation was generated where elevated CRP = 1 was assigned if serum hsCRP >3 mg/l (Pearson et al., 2003; Ridker et al., 2004). Otherwise, the value zero was assigned.
Covariates (age, serum retinol, body mass index)
It has been shown that elevated CRP is associated with factors such as age, serum retinol (Stephensen, 2001) and BMI (Bertran et al., 2005). Therefore, data on age, serum retinol concentrations and BMI were utilized as covariates in this study. The information on women’s age, time postpartum, and time since last menstruation was collected using questionnaires.
Although serum retinol has not been routinely utilized in chronic inflammation research, this needs to change (Chai et al., 2010), given a growing body of literature indicating anti-inflammatory effects of serum retinol or its active metabolite, all-trans retinoic acid (Gill et al., 2013; Ouziel et al., 2013; Ribeiro et al., 2009). Serum retinol is considered particularly important in developing countries where vitamin A deficiency is endemic and hence the population variance for serum retinol concentration is substantial.
Serum retinol concentrations were measured in 14 batches using reverse-phase HPLC Waters system (Milford, Massachusetts; Bieri et al., 1979) in the Clinical Nutrition Research Unit Laboratory of the Harborview Medical Center (Seattle, Washington) run 177 to 342 days after blood collection. Assay controls, positioned at the beginning and end of the run and approximately every ten samples throughout the run, were all within the laboratory’s acceptable range (low control <300 μg/l, high control >500 μg/l). Beyond this quality control information, the actual concentration values for these control specimens were not disclosed by the Clinical Nutrition Research Unit Laboratory to the authors, and hence it is not possible to report here the inter-batch CV or any possible assay batch effects in serum retinol concentration values.
BMI (kg/m2) was derived using height and weight measurements taken according to the method described by Frisancho (1990). Height was measured with an anthropometer to the nearest millimeter, while body weight was measured using a digital scale to the nearest 100 g. Of 199 women, 5 were overweight (BMI ≥ 25 kg/m2; BMI cutoffs based on Ferro-Luzzi et al., 1992). Given this small sample size in the overweight range and our research objective to examine non-overweight women, we excluded these five overweight individuals from further analysis.
The total of 47 individuals excluded from the study (25 with high-grade CRP elevation, 5 with overweight, and the 17 with no dietary or hsCRP data) were not different from the original sample of 241 individuals or the remaining 194 individuals in age or postpartum time. They had larger BMI (21.2 kg/m2 vs. 19.4 kg/m2 for 194 individuals, one-sample t-test for the equality of means: P = 0.001) by the research design. The subsequent statistical analyses were done using the data from the 194 women.
Statistical methods
Statistical analyses were performed using Stata Corp v11.2 (1985–2009). Logistic regression was used to examine the associations between nutrients and low-grade CRP elevation (hsCRP > 3 mg/l), inclusive and exclusive of covariates (age, BMI, and serum retinol) in statistical models. We hypothesized that in breastfeeding women antioxidant and anti-inflammatory nutrients would be prophylactic against the elevation of hsCRP. Additionally, we tested whether BMI predicts the elevation of hsCRP in this non-overweight group of women as previously reported for general population. The initial list of dietary/nutrient variables available for this study included: energy, protein, carbohydrate, fat, fiber, vitamin A, vitamin C, iron, calcium, vitamin E, vitamin B12, vitamin B6, folate, polyunsaturated fatty acids, fatty acids, saturated fatty acids, cholesterol, zinc, and copper. A forward method was used for selecting the best-fitting model.
RESULTS
Sample characteristics are summarized in Table 1. On average, women were relatively young and thin, and predominantly in negative energy balance. The mean age was 28 years, ranging from 18 to 46, and BMI was 19.4, spanning from 14.4 to 24.7. The mean time postpartum was 237 days, ranging from 32 to 585 days. The majority (173 of 194 or 89%) was amenorrhic. The mean hsCRP concentration was 1.81 mg/l. Of 194 women, 34 or 17.5% had low-grade elevation of serum hsCRP (>3 mg/l).
TABLE 1.
Sample characteristics by body mass index (BMI) categoriesa
| BMI categories
|
|||||
|---|---|---|---|---|---|
| All women (n = 194) | <17 kg/m2 (n = 18) | 17–18.5 kg/m2 (n = 46) | 18.5–25.0 kg/m2 (n = 130) | Pb | |
| Age (years) | 28.1 (6.8) | 25.8 (6.8) | 28.9 (6.7) | 28.1 (6.9) | 0.266 |
| BMI (kg/m2) | 19.4 (2.0) | 15.9 (0.7) | 17.8 (0.5) | 20.4 (1.5) | <0.001 |
| C-reactive protein (mg/l) | 1.81 (1.99) | 0.93 (0.68) | 1.90 (2.30) | 1.89 (1.97) | 0.145 |
| Serum retinol (μmol/l) | 1.54 (0.42) | 1.33 (0.29) | 1.56 (0.44) | 1.55 (0.42) | 0.097 |
| Energy intake (kcal) | 1,683 (554) | 1,739 (564) | 1,595 (538) | 1,707 (559) | 0.945 |
| Folate (μg) | 382.0 (255) | 361.2 (225) | 350.9 (227) | 395.8 (269) | 0.556 |
| Total vitamin A (μg) | 118.9 (76.2) | 112.8 (68.3) | 114.7 (105) | 119.8 (65.1) | 0.902 |
| Elevated hsCRP (>3 mg/l) | 17.5 | 0 | 28.3 | 16.2 | 0.018c |
Values are means and standard deviations. The four BMI categories represent moderate-to-severe chronic energy deficiency, mild chronic energy deficiency, normal weight, and overweight, respectively. Cutoffs based on Ferro-Luzzi et al. (1992).
One-way analysis of variance between BMI categories.
Fisher’s exact test; hsCRP: high-sensitivity C-reactive protein.
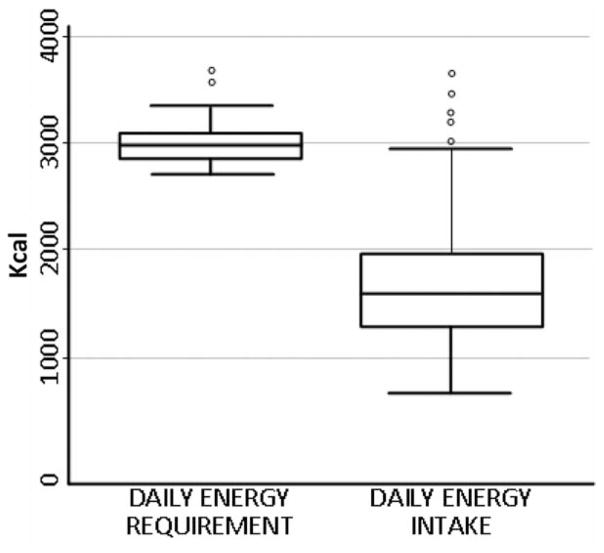
The mean energy intake was 1,683 kcal, ranging from 655 to 3,666 Kcal. The mean daily energy requirement was 2,982 kcal. The great majority of women (187 of 194) had energy intake below the estimated requirements (Fig. 1). Nearly one-third of the sample had BMIs within the chronic energy deficiency range (<18.5 kg/m2). Specifically, of 194 women, 18 women (9%) were moderate-to-severely underweight (BMI < 17 kg/m2), 46 (24%) were mildly underweight (BMI 17–18.5 kg/m2) and 130 (65%) were normal (BMI 18.5 – 25 kg/m2; BMI cutoffs based on Ferro-Luzzi et al., 1992).
Fig. 1.
Boxplots for the estimated daily energy requirement and energy intake of breastfeeding women in northern Kenya. All but 7 of 194 women had energy intake below the estimated requirements. The daily energy requirement was estimated using the basal metabolic rate equation appropriate for the age and postpartum time of lactating women and adjusted for the moderate activity level of a rural woman in a developing country, as per the method published by the WHO (The Joint FAO/WHO/UNU, 1985). The dietary energy intake values were calculated using a list of food items with estimated weights from the 24-hr dietary recall interview and nutrition software NutriSurvey (Erhardt, 2005) with a Kenyan food database.
Analysis of variance (ANOVA) to compare group means showed that these BMI groups had marginally different mean serum retinol concentrations (F = 2.36, P = 0.097, underweight women tended to have lower retinol). Otherwise no significant differences were found in age, hsCRP or nutrient intake values between these BMI groups. However, the BMI groups had significantly different proportions of women with elevated CRP (P = 0.018, Fisher’s exact test). The mildly underweight group had the highest proportion (28%), followed by the normal weight group (16%). None of moderate-to-severely underweight individuals had elevated CRP.
The logistic regression model for the low-grade elevation of hsCRP is presented in Table 2, Model 1. Age and energy intake were positive predictors (OR: 1.097, 95% CI: 1.029–1.169, P = 0.005 for age; OR: 1.002, 95% CI: 1.001–1.003, P = 0.002 for energy; n = 194), while folate intake (OR: 0.997, 95% CI: 0.995–0.9995, P = 0.020), vitamin A intake (OR: 0.988, 95% CI: 0.979–0.998, P = 0.015), and serum retinol concentration (OR: 0.165, 95% CI: 0.045–0.603, P = 0.006) were negative predictors. BMI was not a significant predictor of hsCRP (OR: 1.037, 95% CI: 0.853–1.261, P = 0.716). Odds ratios for dietary nutrients changed only slightly in a model exclusive of covariates (Table 2, Model 2). The individual nutrients did not relate to hsCRP significantly when they were examined alone. However, their relationships were significant on hsCRP only when they were conditional with each other. As a result, the combined models (Model 1 and 2 reported above), were considered to represent more accurate estimated effects of the nutrients on hsCRP after we control for the nutrients simultaneously.
TABLE 2.
Logistic regression models for low-grade elevation of serum hsCRP (>3 mg/l)
| Predictor | Model 1a
|
Model 2b
|
||
|---|---|---|---|---|
| OR | CI | OR | CI | |
| Energy (kcal) | 1.002* | 1.001–1.003 | 1.002* | 1.001–1.003 |
| Folate | 0.997** | 0.995–0.9995 | 0.997** | 0.995–0.9996 |
| Total vitamin A | 0.988** | 0.979–0.998 | 0.990** | 0.982–0.999 |
| Age | 1.097* | 1.029–1.169 | – | – |
| Serum retinol | 0.165* | 0.045–0.603 | – | – |
| Body mass index | 1.037 | 0.853–1.261 | – | – |
Pseudo R2 = 0.139, P = 0.0004, n = 194; the model with covariates.
Pseudo R2 = 0.062, P = 0.0109, n = 194; the model without covariates.
hsCRP: high-sensitivity C-reactive protein, OR: odds ratio.
P < 0.05,
P < 0.01.
DISCUSSION
There are significant gaps in our understanding of the influence of diet on hsCRP. This study sought to address one of the missing pieces in the literature by investigating this relationship in a population unlike those included in previous research. Two important themes in recent global health research have been maternal and child health and the rise in non-communicable disease in developing countries. CRP is a biomarker used commonly in studies addressing both of those topics: it is used in maternal and child health studies, particularly those addressing vitamin A or iron deficiency, as a marker of acute infection and in non-communicable disease research as a marker of risk for cardiovascular and other metabolic diseases. However, our understanding of CRP dynamics is lacking for populations unlike those found in western and/or clinical settings. Our study of lactating rural Kenyan women provides information about a population who, unlike most western populations, have relatively low BMI and whose diet might not provide adequate calories and micronutrients.
This study examined the relationship between serum hsCRP and dietary characteristics in a group of relatively thin breastfeeding women in rural Kenya who were not exhibiting acute phase reaction and were seemingly healthy. The majority of them had inadequate calorie intakes to satisfy high daily energy requirements for lactating women, and nearly one-third of them had BMIs falling under the chronic energy deficiency range.
Among these women, we found that some nutrients have small but significant effects on CRP elevation; energy intake increased the probability of having elevated hsCRP while folate and total vitamin A intake decreased the probability. These findings are consistent with several studies demonstrating prophylactic effects of pro-vitamin A carotenoids, vitamin A, folate, and other nutrients/food items with anti-inflammatory or anti-oxidative properties (e.g. Bertran et al., 2005 among Spanish adults with dietary folate; Kobayashi et al., 2012 with tea rich in antioxidants among healthy Japanese women; Lima et al., 2010 vegetables/fruits rich in beta-carotene among Brazilian women), but at the same time contradict another study among older US adults reporting no benefits of pro-vitamin A carotenoids (De Oliveira Otto et al., 2011). Our study further found that saturated fatty acids was not a significant predictor of the odds of elevated CRP. This also contradicts a previous study among reproductive-aged US women with substantially greater BMI which found saturated fatty acids significantly associated with elevated hsCRP (Gaskins et al., 2010). It is unclear what factors contribute to these conflicting findings among studies. Possible reasons may relate to the variations in BMI or energy balance, or perhaps differences in other factors (e.g. reproductive status such as lactation) among the study populations.
Aside from nutrients, our study also found that even in this relatively slender population, the odds of low-grade elevation of hsCRP appear to increase with advancing age. This is concordant with a study among Spanish women with a greater BMI range (Bertran et al., 2005) and US adults (Alley et al., 2005), and suggests the importance of age as a determinant for low-grade inflammatory status regardless of body weight.
Another significant finding in the current study was the serum retinol concentration as a negative predictor of CRP elevation. The presence of a negative relationship between serum retinol and CRP has long been recognized in the field of micronutrient research (Stephensen, 2001). However, this relationship has been invariably considered a transient phenomenon in response to the acute phase reaction (due to acute infection or acute inflammation). This response occurs as the liver synthesis and the blood circulation of CRP increase while those of retinol-binding protein (the transporter protein for serum retinol) declines in acute phase reaction (Rosales et al., 1996). The current study, however, showed that the inverse relationship between serum retinol and CRP also exists in individuals without a sign of acute phase reaction and that this relationship is different from the transient relationship associated with acute phase reaction.
Previous research among premenopausal women in Hawaii focusing on chronic systemic inflammation (Chai et al., 2010) reported an obesity-related reduction in serum concentrations of pro-vitamin A carotenoids and suggested a possibility that serum lipid-soluble micronutrients are inversely associated to the chronic inflammation status. The authors of this Hawaiian study have hypothesized that this association is due to an increase in the tissue requirement for vitamin A in obese individuals, leading to faster metabolism of pro-vitamin A carotenoids which in turn depletes serum retinol. While this is a plausible explanation for overweight and obese individuals, it is inadequate to explain the same relationship found here in the current study among the underweight to normal weight population.
It is possible that in our study, serum retinol is a negative predictor of the low-grade CRP elevation because of the anti-inflammatory effects of serum retinol or retinoic acid (Gill et al., 2013, Ouziel et al., 2013; Ribeiro et al., 2009). The idea that serum retinol or its active metabolites may play an important role in the genesis, or the prevention of chronic inflammatory diseases is relatively new but increasingly plausible (Chai et al., 2010). Better understanding how vitamin A or other anti-inflammatory nutrients may be involved in the relationships between hsCRP, BMI, and cardiovascular risks is particularly important in the context of the dual burden of underweight and overweight as well as micronutrient insufficiencies/deficiencies found in developing and middle income countries (Doak et al., 2005; Tanumihardjo et al., 2007).
In this study, BMI was not a significant predictor of CRP elevation, contradicting the widely reported positive association between BMI and CRP elevation. However, we are not the first to report this observation. A study among diabetic Kenyans, for example, found no significant differences in the median CRP values across BMI categories (Joshi et al., 2008). It is plausible that the positive association between CRP elevation and BMI is conditional rather than universal.
The frequency distribution of CRP elevation by BMI categories in our study (Table 1) indeed suggested the possibility that the probability of CRP elevation may vary in a curvilinear manner by BMI categories. Mildly underweight group had a higher proportion of individuals with CRP elevation than did either the normal BMI group or the moderate-to-severely underweight. An additional analysis using BMI groups as predictors in a logistic regression model (not shown in the results) found that being mildly underweight (BMI 17–18.5 kg/m2) marginally increased the probability of having elevated CRP relative to being normal weight (OR: 2.292, 95% CI: 0.927–5.664, Pseudo R2 = 0.169, P = 0.001, n = 176; this model dropped individuals with moderate-to-severe underweight because none of them had elevated CRP and hence predicted the failure perfectly).
It is unclear to us what this association represents. If the body adiposity is the primary generator of the inflammatory status, the BMI spectrum in a population should show a unilinear, step-wise increase in the frequency of CRP elevation. This was not the case in our study. It is possible that the surprisingly high rate of elevated CRP among mildly underweight individuals reflects special conditions (e.g. response to minor infection) that are qualitatively different from the low-grade inflammatory status associated with cardiovascular disease development and chronic inflammatory disease risks, the condition of our interest. Another possibility is that hsCRP and BMI have a curvilinear relationship, in which both under- and overweight segments of a human population exhibit higher probabilities of hsCRP elevation than do normal weight or severely underweight segments. These possibilities should be evaluated in future research with a larger sample size.
Taken together, the above findings appear to suggest that the effects of specific dietary nutrients on inflammatory status may vary with BMI or energy balance. Further research should investigate the role of dietary fats, fatty acids, and antioxidant vitamins across populations with a wide spectrum of BMI and energy profiles, including postpartum women.
This study suffers from some limitations. First, the nutrient intake values were estimated from a single session of the 24-hr dietary recall interview for each woman instead of multiple recalls due to logistical challenges. Ideally, nutrient intake values should be estimated from multiple recalls per individual so that the day-to-day variation in their diets is reflected (Gibson, 1990). The compromise to depend on a single interview may have contributed to underestimation of the day-today variation in diets. However, previous ethnographic observations (Fratkin and Smith, 1995) and in-depth interviews on food availability and accessibility conducted in a related study (Fujita, 2008) suggests that Ariaal women’s diets are relatively monotonous with minimal day-to-day variation during the dry season when all the data were collected. Second, the variation in the serum retinol concentration values we utilized in this study may reflect assay batch effects which we are not able to assess or adjust for. The possibility that serum retinol is a significant inverse predictor for low-grade CRP elevation should be carefully reevaluated in future studies.
Third, our study results may reflect the selection effect of breastfeeding women. If breastfeeding women have very different CRP profiles from non-lactating women, our findings may be applicable only to sub-groups of breastfeeding women and not generalizable to all reproductively aged women. Since endogenous or exogenous reproductive hormones have been shown to have relationships with CRP levels (Khera, 2005; Wander et al., 2008), it is possible that the influences from lactation-related hormones may contribute to a distinct CRP profile for breastfeeding women. It has been reported that postpartum women in 4 to 6 weeks postpartum, particularly those who are exclusively breastfeeding, have “heightened and activated innate and specific immune defenses” than those who are not in postpartum period (Groer et al., 2005; p 222), possibly because the inflammatory activation is protective for the mothers toward recovery from the birth, or alternatively related to the stages of uterine involution (Groer et al., 2005). A recent study among Filipino women, however, found no evidence that lactation is inflammatory (Kuzawa et al., 2013).
Another limitation is that this study was not able to investigate the possible effects of the overall qualitative characteristics of women’s diets. For example, dietary diversity characteristics may have exerted an additional effect on hsCRP levels, over and above the aforementioned effects of individual nutrients. Previous studies by others showed that dietary diversity was associated with reduced cardiovascular disease risks such as stunting and overweight among Chinese children (Li et al., 2011), Bolivian Amazonian women (Benefice et al., 2007), and Mexican adults (Flores et al., 2010). Similarly, an association between diverse diets and lower risks of mortality from cardiovascular disease and cancer has been reported in a study of cause-specific mortality risks (Kant et al., 1995).
A typical daily diet among the study participants consisted of a cup of tea with milk for breakfast, a maize-based dish such as uji, ugali, or githeri for lunch, and another maize-based meal for dinner. Beyond this basic plan, variations were seen in the diversity of ingredients women included in their meals (Fujita et al., 2012). For example, those who were less fortunate took their morning tea without milk. They also consumed lunch/dinner made of fewer ingredients (e.g. ugali prepared only with maize flour, cooking fat, and water) than their better-off neighbors, who added sauce prepared with vegetables (e.g. onion, cabbage, kale) and/or meats. Future studies should explore not just nutrient intakes but also other qualitative characteristics of diets such as dietary diversity/variety associated with low-grade elevation of hsCRP.
Acknowledgments
Contract grant sponsor: NSF Dissertation Improvement Grant; Contract grant number: BCS-0622358 (to M.F.); Contract grant sponsor: Wenner-Gren Foundation Grant; Contract grant number: 7460 (to M.F.); Contract grant sponsors: Micronutrient Initiative (to M.F.) and NICHD Research Infrastructure Grant; Contract grant number: R24 HD042828 (to E.B.).
The authors are grateful to the anonymous reviewers and the Editor of this journal for constructive comments and suggestions. The authors express their appreciation to the study participants and the research staff of the Marsabit District. The authors also thank Dr. David Mwaniki and Mr. Philip Ndemwa and Dr. Yeri Kombe of the Centre for Public Health Research, Kenya Medical Research Institute, and Drs. Jonathan Gorstein, Kathleen O’Connor, Thomas McDade, Eric Roth, and Bettina Shell-Duncan for their guidance and support for the original research. Janine Baranski, and Allison Apland of the MSU Biomarker Laboratory for Anthropological Research have greatly contributed to this study through data coding, literature search and manuscript preparation under the Professorial Assistantship of the Michigan State University Honors College.
LITERATURE CITED
- Alley DE, Seeman TE, Kim JK, Karlamangla A, Hu P, Crimmins EM. Socioeconomic status and C-reactive protein levels in the US population: NHANES IV. Brain Behav Immun. 2005;20:498–504. doi: 10.1016/j.bbi.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Belza A, Toubro S, Stender S, Astrup A. Effect of diet-induced energy deficit and body fat reduction on high-sensitive CRP and other inflammatory markers in obese subjects. Int J Obes (Lond) 2009;33:456–464. doi: 10.1038/ijo.2009.27. [DOI] [PubMed] [Google Scholar]
- Benefice E, Lopez R, Monroy SL, Rodríguez S. Fatness and overweight in women and children from riverine Amerindian communities of the Beni River (Bolivian Amazon) Am J Hum Biol. 2007;19:61–73. doi: 10.1002/ajhb.20580. [DOI] [PubMed] [Google Scholar]
- Bertran N, Camps J, Fernandez-Ballart J, Arija V, Ferre N, Tous M, Simo D, Murphy MM, Vilella E, Joven J. Diet and lifestyle are associated with serum C-reactive protein concentrations in a population-based study. J Lab Clin Med. 2005;145:41–46. doi: 10.1016/j.lab.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Bieri JG, Tolliver TJ, Catignani GL. Simultaneous determination of alpha-tocopherol and retinol in plasma or red cells by high pressure liquid chromatography. Am J Clin Nutr. 1979;32:2143–2149. doi: 10.1093/ajcn/32.10.2143. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P. Mucosal immunity in a healthy gut. In: Calder PC, Yaqoob P, editors. Diet, immunity and inflammation. Cambridge: Wood-head Publishing; 2013. pp. 34–80. [Google Scholar]
- Brindle E, Fujita M, Shofer J, O’Connor KA. Serum, plasma, and dried blood spot high sensitivity C-reactive protein enzyme immunoassay for population research. J Immunol Methods. 2010;362:112–120. doi: 10.1016/j.jim.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai W, Conroy SM, Maskarinec G, Franke AA, Pagano IS, Cooney RV. Associations between obesity and serum lipid-soluble micronutrients among premeopausal women. Nutr Res. 2010;30:227–232. doi: 10.1016/j.nutres.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun OK, Chun S-J, Claycombe KJ, Son WO. Serum C-reactive protein concentrations are inversely associated with dietary flavonoid intake in U.S. adults. J Nutr. 2008;138:753–760. doi: 10.1093/jn/138.4.753. [DOI] [PubMed] [Google Scholar]
- De Oliveira Otto MCC, Alonso A, Lee DH, Delclos GL, Jenny NS, Jiang R, Lima JA, Symanski E, Jacobs DR, Jr, Nettleton JA. Dietary micronutrient intakes are associated with markers of inflammation but not with markers of subclinical atherosclerosis. J Nutr. 2011;141:1508–1515. doi: 10.3945/jn.111.138115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doak CM, Adair LS, Bentley M, Monteiro C, Popkin BM. The dual burden household and the nutrition transition paradox. Int J Obesity (Lond) 2005;29:129–136. doi: 10.1038/sj.ijo.0802824. [DOI] [PubMed] [Google Scholar]
- Erhardt J. SIGHT AND LIFE vitamin A intake calculation program. A free program made available by SIGHT and LIFE. 2003 http://www.sight-andlife.org/vac/vac.html. Kindly provide the last accessed date for the references Erhardt, 2003, 2005.
- Erhardt J. Nutrition surveys and calculations: guidelines, software and additional information. 2005 Available from www.nutrisurvey.de.
- Flores M, Macias N, Rivera M, Lozada A, Barquera S, Rivera-Dommarco J, Tucker KL. Dietary patterns in Mexican adults are associated with risk of being overweight or obese. J Nutr. 2010;140:1869–1873. doi: 10.3945/jn.110.121533. [DOI] [PubMed] [Google Scholar]
- Ferro-Luzzi A, Sette S, Frandlin M, James WPT. A simplified approach of assessing adult energy deficiency. Eur J Clin Nutr. 1992;46:173–186. [PubMed] [Google Scholar]
- Ford ES, Galuska DA, Gillespie C, Will JC, Giles WH, Dietz WH. C-reactive protein and body mass index in children: findings from the Third National Health and Nutrition Examination Survey, 1988–1994. J Pediatr. 2001;138:486–492. doi: 10.1067/mpd.2001.112898. [DOI] [PubMed] [Google Scholar]
- Fratkin E, Roth E. The social, health, and economic consequences of pastoral sedentarization in Marsabit District, Northern Kenya. In: Fratkin E, Roth E, editors. As pastoralists settle: Social, health, and economic consequences of pastoral sedentarization in Marsabit District, Kenya. New York: Kluwer Academic; 2005. pp. 1–28. [Google Scholar]
- Fratkin E, Smith K. Women’s changing economic roles with pastoral sedentarization: varying strategies in alternate Rendille communities. Hum Ecol. 1995;23:433–454. [Google Scholar]
- Frisancho AR. Anthropometric standards for the assessment of growth and nutritional status. Ann Arbor: The University of Michigan Press; 1990. pp. 1–30. [Google Scholar]
- Fujita M. Doctoral Dissertation. University of Washington; Seattle: 2008. An epidemiological and evolutionary investigation of mother-offspring Vitamin A transfer. [Google Scholar]
- Fujita M, Lo Y, Baranski JR. Dietary diversity score is a useful indicator of vitamin A status of adult women in northern Kenya. Am J Hum Biol. 2012;24:829–834. doi: 10.1002/ajhb.22327. [DOI] [PubMed] [Google Scholar]
- Fujita M, Roth EA, Lo Y, Hurst C, Vollner J, Kendell A. Low serum vitamin A mothers breastfeed daughters more often than sons in drought-ridden northern Kenya: a test of the Trivers-Willard hypothesis. Evol Hum Behav. 2012;33:357–364. [Google Scholar]
- Gaskins AJ, Mumford SL, Rovner AJ, Zhang C, Chen L, Wactawski-Wende J, Perkins NJ, Schisterman EF BioCycle Study Group. Whole grains are associated with serum concentrations of high sensitivity C-reactive protein among premenopausal women. J Nutr. 2010;140:1669–1676. doi: 10.3945/jn.110.124164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson RS. Principles of nutritional assessment. New York: Oxford University Press; 1990. pp. 37–54. [Google Scholar]
- Gill N, Bijjem KR, Sharma PL. Anti-inflammatory and anti-hyperalgesic effect of all-trans retinoic acid in carrageenan-induced paw edema in Wistar rats: involvement of peroxisome proliferator-activated receptor-β/δ receptors. Indian J Pharmacol. 2013;45:278–282. doi: 10.4103/0253-7613.111944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groer M, Davis M, Steele K. Associations between human milk SIgA and maternal immune, infectious, endocrine, and stress variables. J Hum Lact. 2004;20:153–158. doi: 10.1177/0890334404264104. [DOI] [PubMed] [Google Scholar]
- Groer MW, Davis MW, Smith K, Casey K, Kramer V, Bukovsky E. Immunity, inflammation and infection in postpartum breast and formula feeders. Am J Reprod Immunol. 2005;54:222–231. doi: 10.1111/j.1600-0897.2005.00301.x. [DOI] [PubMed] [Google Scholar]
- Guldiken S, Demir M, Arikan E, Turgut B, Azcan S, Gerenli M, Tugrul A. The levels of circulating markers of atherosclerosis and inflammation in subjects with different degrees of body mass index: soluble CD40 ligand and high-sensitivity C-reactive protein. Thromb Res. 2007;119:79–84. doi: 10.1016/j.thromres.2005.12.019. [DOI] [PubMed] [Google Scholar]
- Hodge AM, Maple-Brown L, Cunningham J, Boyle J, Dunbar T, Weeramanthri T, Shaw J, O’Dea K. Abdominal obesity and other risk factors largely explain the high CRP in indigenous Australians relative to the general population, but not gender differences: a cross sectional study. BMC Public Health. 2010;10:700. doi: 10.1186/1471-2458-10-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi MD, Wala J, Acharya KS, Amayo A. High-sensitivity C-reactive protein in type 2 diabetic patients with and without the metabolic syndrome. East Afr Med J. 2008;85:178–186. doi: 10.4314/eamj.v85i4.9642. [DOI] [PubMed] [Google Scholar]
- Kant AK, Schatzkin A, Ziegler RG. Dietary diversity and subsequent cause-specific mortality in the NHANES I epidemiologic follow-up study. J Am Coll Nutr. 1995;14:233–238. doi: 10.1080/07315724.1995.10718501. [DOI] [PubMed] [Google Scholar]
- Khera A, McGuire DK, Murphy SA, Stanek HG, Das SR, Vongpatanasin W, Wians FH, Jr, Grundy SM, de Lemos JA. Race and gender differences in C-reactive protein levels. J Am Coll Cardiol. 2005;46:464–469. doi: 10.1016/j.jacc.2005.04.051. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Murakami K, Sasaki S, Uenishi K, Yamasaki M, Hayabuchi H, Goda T, Oka J, Baba K, Ohki K, Watanabe R, Sugiyamama Y. Dietary total antioxidant capacity from different assays in relation to serum C-reactive protein among young Japanese women. Nutr J. 2012;11:91. doi: 10.1186/1475-2891-11-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzawa CW, Adair LS, Borja J, McDade TW. C-reactive protein by pregnancy and lactational status among Filipino young adult women. Am J Hum Biol. 2013;25:131–134. doi: 10.1002/ajhb.22351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wedick NM, Lai J, He Y, Hu X, Liu A, Du S, Zhang J, Yang X, Chen C, Hu FB, Ma G. Lack of dietary diversity and dyslipidaemia among stunted overweight children: the 2002 China National Nutrition and Health Survey. Public Health Nutr. 2011;14:896–903. doi: 10.1017/S1368980010002971. [DOI] [PubMed] [Google Scholar]
- Lima RLFC, Costa MJC, et al. Consumption of fruits and vegetables and C-reative protein in women undergoing cosmetic surgery. Nutr Hosp. 2010;25:763–767. [PubMed] [Google Scholar]
- Margioris AN, Dermitzaki E, Venihaki M, Tsatsanis C. Chronic low-grade inflammation. In: Calder PC, Yaqoob P, editors. Diet, immunity and inflammation. Cambridge: Woodhead Publishing; 2013. pp. 105–120. [Google Scholar]
- Moore SE. Early nutritional programming. In: Calder PC, Yaqoob P, editors. Diet, immunity and inflammation. Cambridge: Woodhead Publishing; 2013. pp. 612–630. [Google Scholar]
- Nanri A, Moore MA, Kono S. Impact of C-reactive protein on disease risk and its relation to dietary factors: literature review. Asian Pacific J Cancer Prev. 2007;8:167–177. [PubMed] [Google Scholar]
- Ouziel R, Trépo E, Cremer A, Moreno C, Degré D, Chaouni M, Vercruysse V, Quertinmont E, Devière J, Lemmers A, Gustot T. Correction of all-trans retinoic acid deficiency in alcoholic cirrhosis lessens the excessive inflammatory monocyte response: a translational study. Liver Int. 2013;34:343–352. doi: 10.1111/liv.12249. [DOI] [PubMed] [Google Scholar]
- Ozarda Y, Gunes Y, Tuncer GO. The concentration of adiponectin in breast milk is related to maternal hormonal and inflammatory status during 6 months of lactation. Clin Chem Lab Med. 2012;50:911–917. doi: 10.1515/cclm-2011-0724. [DOI] [PubMed] [Google Scholar]
- Park HS, Park JY, Yu R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-α and IL-6. Diabet Res Clin Practice. 2005;69:29–35. doi: 10.1016/j.diabres.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, III, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Jr, Taubert K, Tracy RP, Vinicor F. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi MM, Singer MR, Moore LL. A cross-sectional study of food group intake and C-reactive protein among children. Nutr Metab (Lond) 2009;6:40. doi: 10.1186/1743-7075-6-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rexrode KM, Pradhan A, Manson JE, Buring JE, Ridker PM. Relationship of total and abdominal adiposity with CRP and IL-6 in women. Ann Epidemiol. 2003;13:674–682. doi: 10.1016/s1047-2797(03)00053-x. [DOI] [PubMed] [Google Scholar]
- Ribeiro Nogueira C, Ramalho A, Lameu E, Da Silva Franca CA, David C, Accioly E. Serum concentrations of vitamin A and oxidative stress in critically ill patients with sepsis. Nutr Hosp. 2009;24:312–317. [PubMed] [Google Scholar]
- Ridker PM, Wilson PW, Grundy SM. Should C-reactive protein be added to metabolic syndrome and to assessment of global cardiovascular risk? Circulation. 2004;109:2818–2825. doi: 10.1161/01.CIR.0000132467.45278.59. [DOI] [PubMed] [Google Scholar]
- Rosales FJ, Ritter SJ, Zolfaghari R, Smith JE, Ross AC. Effects of acute inflammation on plasma retinol, retinol-binding protein, and its mRNA in the liver and kidneys of vitamin A-sufficient rats. J Lipid Res. 1996;37:962–971. [PubMed] [Google Scholar]
- Silva IT, Sanches LB, Mello AP, Damasceno NR. Impact of C-reactive protein on cardiovascular risk in adolescents. Arquivos Brasileiros de Cardiologia. 2010;94:585–591. doi: 10.1590/s0066-782x2010005000027. [DOI] [PubMed] [Google Scholar]
- StataCorp LP. Stata statistical software, version 11.2. College Station, TX: StataCorp LP; 1985–2009. [Google Scholar]
- Stephensen CB. Vitamin A, infection, and immune function. Annu Rev Nutr. 2001;21:167–192. doi: 10.1146/annurev.nutr.21.1.167. [DOI] [PubMed] [Google Scholar]
- Tanumihardjo SA, Anderson C, Kaufer-Horwitz M, Bode L, Emenaker NJ, Haqq AM, Satia JA, Silver HJ, Stadler DD. Poverty, obesity, and malnutrition: an international perspective recognizing the paradox. J Am Diet Assoc. 2007;107:1966–1972. doi: 10.1016/j.jada.2007.08.007. [DOI] [PubMed] [Google Scholar]
- The Joint FAO/WHO/UNU Expert Consultation on Energy and Protein. Energy and Protein Requirements. WHO. FAO Corporate Document Repository; 1985. World Health Organization Technical Report Series 724. http://www.fao.org/DOCREP/003/AA040E/AA040E00.HTM. [Google Scholar]
- Thurnham DI, McCabe GP, Northrop-Clewes CA, Nestel P. Effects of subclinical infection on plasma retinol concentrations and assessment of prevalence of VA deficiency: meta-analysis. Lancet. 2003;362:2052–2058. doi: 10.1016/s0140-6736(03)15099-4. [DOI] [PubMed] [Google Scholar]
- Wallengren O, Bosaeus I, Lundholm K. Dietary energy density, inflammation and energy balance in palliative care cancer patients. Clin Nutr. 2012;32:88–92. doi: 10.1016/j.clnu.2012.05.023. [DOI] [PubMed] [Google Scholar]
- Wander K, Brindle E, O’Connor KA. C-reactive protein across the menstrual cycle. Am J Phys Anthropol. 2008;136:138–146. doi: 10.1002/ajpa.20785. [DOI] [PubMed] [Google Scholar]
- Wang LY, Wu SL, Yang XL, Wang TJ, Su LR, Gao JS, Zheng XM, Liu XR. Association between baseline high sensitivity C reactive protein level and the first cardio-cerebral vascularevent in diabetic population: a prospective cohort study. Zhonghua Xin Xue Guan Bing Za Zhi. 2011;39:749–754. [PubMed] [Google Scholar]
- Wu D, Chu Nain-Feng, Shen Muh-Han, Chang Jin-Biou. Plasma C-reactive protein levels and their relationship to anthropometric and lipid characteristics among children. J Clin Epidemiol. 2003;56:94–100. doi: 10.1016/s0895-4356(02)00519-x. [DOI] [PubMed] [Google Scholar]