Abstract
While compelling evidence supports the central role of mitochondrial dysfunction in the pathogenesis of heart failure, there is comparatively less information available on mitochondrial alterations that occur prior to failure. Building on our recent work with the dystrophin-deficient mdx mouse heart, this review focuses on how early changes in mitochondrial functional phenotype occur prior to overt cardiomyopathy and may be a determinant for the development of adverse cardiac remodelling leading to failure. These include alterations in energy substrate utilization and signalling of cell death through increased permeability of mitochondrial membranes, which may result from abnormal calcium handling, and production of reactive oxygen species. Furthermore, we will discuss evidence supporting the notion that these alterations in the dystrophin-deficient heart may represent an early “subclinical” signature of a defective nitric oxide/cGMP signalling pathway, as well as the potential benefit of mitochondria-targeted therapies. While the mdx mouse is an animal model of Duchenne muscular dystrophy (DMD), changes in the structural integrity of dystrophin, the mutated cytoskeletal protein responsible for DMD, have also recently been implicated as a common mechanism for contractile dysfunction in heart failure. In fact, altogether our findings support a critical role for dystrophin in maintaining optimal coupling between metabolism and contraction in the heart.
Keywords: Energy substrate metabolism, Cyclic GMP signalling, Duchenne muscular dystrophy, Mitochondrial transition pore, Cardioprotection
1. Introduction
Mitochondria within cardiomyocytes are at considerable risk of undergoing adverse phenotypic changes during the acute and/or chronic physiologic stress events (e.g., ischemic or adrenergic challenge) associated with unfavorable cardiac remodelling. A reduced ability of mitochondria to withstand such stresses may constitute the primary event that places the heart into a vicious cycle of increasing dysfunction and cell death, ultimately resulting in heart failure. In fact, several studies in humans and animal models of adverse cardiac remodelling resulting from either genetic or acquired aetiologies demonstrate that in the failing heart, mitochondria display a number of ultrastructural, biochemical, and functional defects that are believed to contribute to energetic failure, oxidative stress, and activation of cell death (for recent reviews, see refs. [1–6]). While these compelling data support the central involvement of mitochondria in the pathogenesis of heart failure, there is comparatively little information available on the mitochondrial alterations that occur during the period that precedes the transition to heart failure. In particular, measurements of the most commonly used markers of mitochondrial dysfunction have provided little evidence so far to suggest that mitochondrial dysfunction may contribute during the early compensated phases of cardiomyopathy to further deterioration of myocardial function. Accordingly, little changes have been observed during that period in either (i) activity of the mitochondrial respiratory chain complexes, (ii) respiratory performance in isolated mitochondria, (iii) activity, protein and/or mRNA levels of mitochondrial marker enzymes, master regulators of mitochondrial biogenesis (peroxisome proliferator-activated receptor γ coactivator, PGC1α) or uncoupling proteins, (iv) acquired mutations or deletions of mtDNA, or (v) ultrastructural alterations. However, rather than dismissing a role for mitochondria, this lack of evidence may simply reflect the necessity to use different kinds of end-point and experimental models to unravel mitochondrial abnormalities in the early phase of cardiomyopathies. Recent evidence emphasized the importance of using experimental approaches, which preserve the organization of mitochondrial supercomplexes [6,7]. Hence, the use of experimental approaches that capture the dynamics of the mitochondrial functional phenotype under various conditions, including the use of ex vivo experimental models such as the perfused heart, appear particularly relevant in this regard. In addition to the obvious advantage of preserving crucial metabolic and signalling cross-talk between cytosolic and mitochondrial compartments, which may themselves constitute compensatory mechanisms, the use of an intact heart preparation may also unmask abnormalities that are not readily observed in vivo due to the existence of compensatory mechanisms present within the intact organism.
The aforementioned concept is well illustrated by our recent studies in the dystrophin-deficient heart from the mdx mouse, the animal model of Duchenne muscular dystrophy (DMD). Specifically, the use of a perfused, working heart model was instrumental in revealing, in this model, that early mitochondrial alterations in energy substrate metabolism do occur prior to the onset of overt cardiomyopathy [8,9]. Compared to other heart failure aetiologies such as myocardial infarction, atherosclerosis, hypertension and diabetes, much less is known about mitochondrial dysfunction in the dystrophic cardiomyopathy. This question is not only important for the identification of novel and much needed treatment avenues for DMD patients but may also be of relevance for our understanding of the pathogenesis of heart failure in general. Indeed, changes in the integrity of dystrophin, a cytoskeletal protein, have recently been documented in patients with end-stage dilated, ischemic and viral cardiomyopathies and, as thus, may represent a common pathway for contractile dysfunction in the failing heart [10–12].
Hence, building on our recent findings in the dystrophin-deficient heart, this review will focus upon how early mitochondrial alterations may represent a primary factor involved in the pathogenesis of the dystrophic cardiomyopathy and, thus, a potential target for therapeutic interventions. Specifically, we will begin with a brief overview of the clinical course of the dystrophic cardiomyopathy typically observed in humans and in the mdx mouse model, followed by a summary of our recently published findings on the metabolic alterations observed in the hearts of young mdx mice prior to overt cardiomyopathy. We will then present recent unpublished results that further substantiate the presence of early mitochondrial functional alterations in these hearts, specifically cell death signalling through enhanced permeability of mitochondrial membranes, which may result from an enhanced production of reactive oxygen species (ROS), as well as abnormal calcium handling or defective nitric oxide (NO)/cGMP signalling. In each section, we will briefly summarize the current literature supporting the existence of these specific mitochondrial functional alterations in other models of cardiomyopathies. Finally, we will discuss potential therapeutic strategies targeting these early mitochondrial alterations in the dystrophic heart.
2. Characteristics of the dystrophic cardiomyopathy in DMD
DMD is an inherited X-linked disease resulting from mutations in the dystrophin gene located at Xp21, which leads to the absence of this cytoskeletal protein in striated (skeletal and cardiac) muscle cells. Dystrophin is part of a multimeric protein complex, the dystrophin–glycoprotein complex (DGC) that links the cytoskeleton to the extracellular matrix and encompasses both mechanical and signaling roles. Defects in the gene encoding other protein members of this complex, namely, the sarcoglycans, sarcospan, dystroglycans, dystrobrevins and synthrophins, also lead to muscular dystrophy (MD), although the age of onset, affected muscles, and severity may vary considerably (for a recent review, see ref. [13]). Cardiac involvement has been reported for some but not all of these proteins, namely dystrophin (DMD), δ-sarcoglycan (Limb girdle MD), β-sarcoglycan and dystrobrevins (for reviews, see refs. [14,15]).
This review article will mainly focus on DMD, which is the most common form of MD. Although the first clinical manifestations of DMD are generally related to skeletal muscle weakness, cardiac involvement is inescapable with age and can progress to heart failure. Typically, the development of dilated cardiomyopathy occurs progressively over time such that by 18 years of age, 98% of DMD patients display echocardiographic evidence of contractile dysfunction and electrocardiographic abnormalities such as arrhythmias [16,17]. Experimental and clinical evidence also suggests that this cardiac involvement can occur in the absence of clinically apparent skeletal muscle involvement or pathological defects in the vasculature [18–20]. Ultimately progression toward heart failure, characterized by pronounced ventricular fibrosis, will occur in 40% of patients between 10 and 30 years of age [16,17,21,22].
This time course of disease progression is in many ways recapitulated in the mdx mouse, the most widely used animal model of DMD. Indeed, hearts from mdx mice do not display apparent signs of cardiomyopathy prior to 25–30 weeks of age [23]. Selective fibrosis of the left ventricle, cardiac dilation, and more marked deterioration of function usually become evident by 40 weeks of age and worsens over time [23–25].
Currently, the mechanism responsible for the development of dilated cardiomyopathy in DMD is unclear. In skeletal muscle, the primary organ responsible for the debilitating effect of the disease, myofibers lacking dystrophin are abnormally susceptible to load-induced sarcolemmal damage [26]. Similar to skeletal muscles, our group and others showed that increasing cardiac workload either by (i) physical exercise, (ii) acute in vivo administration of a β-adrenergic agonist, (iii) ex vivo heart perfusion in the working mode, or (iv) aortic constriction leads to elevated sarcolemmal injury in dystrophin-deficient mice compared to control mice and, in some conditions, high mortality rates [9,27–29]. While mechanical instability and ensuing rupture of the sarcolemmal membrane are likely a cause of cardiomyocyte death based on the current literature in skeletal muscle [26,30,31], the lack of functional dystrophin in cardiomyocytes may also have other consequences that could play important roles in disease progression, including increased oxidative stress, alterations in cellular Ca2+ handling, and pronounced reductions in NO signaling due to an impaired activity of neuronal nitric oxide synthase (nNOS) [13]. In skeletal muscle, the absence of dystrophin results in displacement of nNOS from the plasma membrane, where it normally binds directly to syntrophins (which are cytoplasmic proteins anchored to dystrophin’s carboxyterminal domain), to the cytoplasm. Although this relocalization has not been demonstrated in the heart, Bia et al. [24] demonstrated that the lack of dystrophin in the mdx mouse resulted in abnormal electrocardiograms that are associated with decreased myocardial nNOS activity. Furthermore, recent studies emphasized the importance of nNOS subcellular (re)localization and interactions with other proteins as a factor regulating myocardial contractility in normal and failing heart [32–34]. However, much remains to be learned about the role and regulation of this nNOS-derived NO signalling. Irrespective of the exact molecular mechanisms involved, abnormalities in NOS signaling, Ca2+ handling and oxidative stress, all have the potential to induce or may be the result of changes in mitochondrial function, as will be detailed below.
3. The dystrophic mouse heart displays early abnormalities of mitochondrial metabolic function
3.1. Alterations in mitochondrial substrate metabolism in other models of cardiac hypertrophy and failure
The importance of mitochondrial substrate metabolism in cardiac hypertrophy and failure has been the subject of numerous recent reviews [35–39], and consequently this topic will only be briefly summarized to highlight certain issues. In brief, alterations in cardiac energy substrate metabolism are currently considered as an independent determining factor that contributes to contractile dysfunction, as well as disease progression from left ventricular hypertrophy to heart failure. However, there is an ongoing debate as to why cardiac remodelling, particularly in the early compensatory phase prior to clinical manifestations, results in a shift in substrate selection for energy production from long chain fatty acids (LCFAs, which supply normally ~70% of the heart’s energy demand through mitochondria β-oxidation) to carbohydrates (CHOs). Such changes are believed to represent a recapitulation of the fetal program, and while this has been shown to be a characteristic of several models of cardiomyopathy, it has not been a consistent finding [38,40,41]. The precise causes, as well as the consequences, of this substrate shift are also unclear, i.e., is it harmful or beneficial for cardiac function? What are the consequences of this shift on the development and progression of cardiac hypertrophy and failure? On one hand, there is evidence that this shift in substrate use from LCFAs to CHOs may be beneficial for the post-ischemic and failing heart. The potential benefits could include (i) the greater ATP-to-oxygen ratio associated with glucose utilization (11–12%), (ii) a more efficient matching between cytosolic glycolysis and mitochondrial oxidation, thereby decreasing the production of protons which can promote detrimental calcium overload, and (iii) improved ion pump function linked to glycolytic flux. On the other hand, potential detrimental consequences of chronic inhibition of mitochondrial LCFA oxidation include intracellular lipid accumulation and its associated lipotoxic sequelae as well as energy starvation, principally under conditions of increased energy demand [36,38,42–47].
Recent studies with genetically modified mouse models of cardioprotection (for, e.g., calcineurin-knockout mouse [48], the glycogen synthase kinase-3 overexpressing mouse [49], cardiomyocyte-specific constitutively active guanylate cyclase (GC+/0) over-expressing mouse [50]), appear, however, to provide some insights in this debate. In fact, we found that working GC+/0 mouse hearts display a metabolic profile that is partly reminiscent of that reported in compensated hypertrophied hearts (namely decreased LCFA β-oxidation, no change in CHO oxidation and increased glycolysis [51–53]). A similar profile was also reported in the other mouse models of cardioprotection, suggesting that it is beneficial. However, GC+/0 mouse hearts exhibited also two striking and distinctive features: (i) the glycolytically derived lactate and pyruvate production ratio (reflecting the cytosolic redox state of the cells) was conserved and (ii) triglyceride synthesis was enhanced concomitantly with lipolysis. In fact, in this model of cardioprotection, the increased triglyceride synthesis/hydrolysis turnover seems to explain the observed decrease in LCFA β-oxidation, which was documented by tracking the fate of exogenously administered and labeled LCFA, a commonly used experimental paradigm. The importance of lipolysis for normal cardiac homeostasis was also recently emphasized by studies with transgenic mice lacking enzymes involved in this process (for, e.g., adipose triglyceride lipase, lipoprotein lipase) [54,55]. However, much remains to be learned about the consequences of alterations in myocardial substrate metabolism – beyond their selection for energy production –on the development of cardiac hypertrophy and failure.
3.2. Alterations in mitochondrial substrate metabolism in the early preclinical stages of the dystrophic cardiomyopathy
Using dystrophic (mdx) mice at the young age of 10–12 weeks (where histological or echocardiographic evidence of cardiac disease cannot be detected yet), we measured the metabolic and functional responses of their hearts, using ex vivo whole organ perfusion in the working mode with carbon 13 (13C)-labeled substrates [56]. Results that were obtained in these experiments are summarized in Table 1. Briefly, at a physiological afterload (50 mm Hg) and with buffer substrate concentrations designed to mimic the in vivo milieu, mdx hearts demonstrated (i) compromised cardiac contractile function and efficiency and (ii) reduced cellular integrity as reflected by a greater lactate dehydrogenase release [8]. It is noteworthy, however, that the performance of mdx hearts in the above study, which were perfused with multiple substrates consisting of CHOs (glucose, lactate, pyruvate) and a LCFA (oleate), was by far superior to that previously reported with glucose as the sole substrate [27], pointing to a need for an increased and balanced substrate supply in order to achieve adequate ATP production in the mdx heart.
Table 1.
Comparison of metabolic flux and physiological parameters assessed in 12-week-old C57BL/10 and mdx mouse hearts perfused with 13C-labeled substrates.
| Measured parameters | mdx vs. C57BL/10 (ratio) |
|---|---|
| (1) Metabolic flux parameters assessed at 30 min | |
| (A) Cytosolic | ↑(1.62)* |
| Glycolysis (production of lactate and pyruvate) | |
| (B) Mitochondria | |
| (i) Relative contribution of substrates to citrate synthesis | |
| –Carbohydrate oxidation: pyruvate decarboxylation (PDC/CS) | ↑ (2.20)** |
| –Fatty acid oxidation: oleate β-oxydation (OLE/CS) | ↓ (0.73)* |
| –Oxidation of other substrates (OS/CS) | NS |
| –Anaplerosis: pyruvate carboxylation (PC/CS) | ↓ (0.81)* |
| (ii) Citric acid cycle-related parameters | |
| –Citric acid cycle intermediate levels | ↓ (0.66)** |
| –Aconitase activity | ↓ (0.72)* |
| –Citrate synthase activity | NS |
| –Calculated ATP production rates | ↑ (1.17)* |
| (2) Physiological parameters assessed at 25–30 min | |
| –Aortic flow | ↑ (0.83)* |
| –Coronary flow | NS |
| –Left ventricular systolic pressure | ↓ (0.90)* |
| –Left ventricular end diastolic pressure | NS |
| –Heart rate | NS |
| –dP/dtmax | ↓ (0.84)** |
| –dP/dtmin | ↓ (0.87)* |
| –Oxygen consumption | ↑ (1.18)** |
| –Lactate dehydrogenase release | ↑ (2.60)** |
Metabolic and functional data are from [8]. The following metabolic parameters were assessed in working hearts perfused for 30 min with physiological concentrations of carbohydrates (11 mM glucose, 1.5 mM lactate and 0.2 mM pyruvate) and a fatty acid (0.4 mM oleate bound to albumin) using 13C-labeled substrates and mass isotopomer analysis by gas chromatography-mass spectrometry: (A) Glycolysis reflecting the production of lactate and pyruvate from 13C-glucose (μmol×min−1). (B.i) Substrate flux ratios reflecting the contribution of exogenous fatty acids (oleate) and carbohydrates (CHOs: lactate, pyruvate and glucose) to citrate formation via (i) acetyl-CoA (energy) from oleate β-oxidation (OLE) and pyruvate decarboxylation (PDC), or (ii) oxaloacetate (anaplerosis) from pyruvate carboxylation (PC), all expressed relative to citrate synthesis (CS); (B.ii) Tissue concentration of citric acid cycle (CAC) intermediates (in μmol×g wet weight−1), tissue aconitase and citrate synthase activity (μmol×min−1×mg protein−1) and calculated ATP production rates.
p<0.05,
p< 0.001 for mdx versus control mouse hearts; NS: not significant.
In addition, at the metabolic level, we found that mdx mouse hearts displayed (i) a marked shift from LCFA to CHO oxidation for energy production associated with enhanced oxygen consumption, (ii) a reduction in citric acid cycle (CAC) intermediates tissue levels, and (iii) a 20% lower activity of the CAC enzyme aconitase. The metabolic shift from LCFA to CHO that we observed in young mdx mice concurs with findings from cardiac positron emission tomography studies performed in patients with DMD using 18F-deoxyglucose or a radioiodinated branched fatty acid ([123I]15-(p-iodophenyl)-3-(R, S)-methylpentadecanoic acid (BMIPP)) [57–60]. Although metabolic flux parameters relative to LCFA partitioning between oxidation and triglyceride synthesis were not specifically assessed in our study, our results indicated that the contribution of endogenous substrates (postulated to be triglycerides) to acetyl-CoA production was similar to controls at this early stage of the cardiomyopathic process, presumably because lower LCFA oxidation was compensated by increased CHO oxidation. Taken together, these results strongly suggest the existence of early mitochondrial metabolic alterations in the young dystrophic mdx mouse heart at a time that clearly precedes the onset of overt cardiomyopathy [8]. To the best of our knowledge, cardiac energy substrate metabolism has not been assessed in any of the other MD mouse models.
3.3. Putative mechanisms responsible for the mitochondrial metabolic alterations in the dystrophic heart
Numerous candidate mechanisms were considered to explain the documented substrate shift in dystrophic hearts. We initially examined two interacting nutrient signaling pathways, Akt and AMPK, given their roles in regulating glucose uptake and utilization [61,62]. Compared to controls, mdx mouse hearts showed a significantly lower Akt phosphorylation state and no differences in AMPK phosphorylation or activity (unpublished data), the latter being consistent with the lack of significant differences in the AMP-to-ATP ratio between the two groups of isolated hearts. However, neither the Akt nor the AMPK results could directly account for the observed substrate shift towards CHO. However, another signaling pathway that could help explain the metabolic shift observed in the perfused mdx heart is the p38 MAPK pathway, which has been shown to be involved in regulating substrate energy metabolism [63] through the nuclear receptor peroxisome proliferator-activated receptor (PPARα), a transcriptional regulator of FA oxidation enzyme expression. Phosphorylation of PPARα by p38 may lead to an increase in ligand-dependent transactivating functions and enhanced functional cooperation with PGC-1α. We have found a significant 2-fold decrease in p38 phosphorylation (unpublished data), which may account, at least in part, for the observed decrease in LCFA oxidation in the dystrophic heart.
To further explore the possible relationship between our observations and evolution of the dystrophic cardiomyopathy in mdx mice, we undertook a quantitative comparison of young (10–12 weeks) versus older (25 weeks) dystrophic hearts with respect to PPARα-regulated genes involved in FA β-oxidation. In young mdx mice, where there are little obvious signs of cardiomyopathy, there were no changes in gene expression that could account for the concurrent shift in substrate utilization. However, in 25-week-old mdx mice, the developing cardiomyopathy was accompanied by slight changes in the gene expression levels of PPARα, pyruvate dehydrogenase kinase-4 and myosin heavy chain β, which might be compatible with a trend to recapitulate the fetal gene expression phenotype. Furthermore, this is associated with a significant rise in anf gene expression and a significant increase in transcript levels of sgcα1. The latter is a well-known target gene of the NO/cGMP pathway, which has been shown to negatively correlate with cGMP levels [64]. In addition, although anf expression is often used as a marker of cardiac remodelling, recent studies have also emphasized new cardioprotective roles for natriuretic peptides, particularly under conditions of defective NO signaling (for review, see ref. [65]).
In this regard, existing literature did support the presence of defective NO signaling within mdx hearts [24,66,67], as well as a crucial role for this pathway in optimizing metabolism-contraction coupling [68,69]. In our denervated mdx heart perfusion model, in which the buffer is not recirculated preventing compounds released by the heart from accumulating, the natriuretic peptide signaling pathway is unlikely to be optimally active despite a potential autocrine action of natriuretic peptides. This provided a potential explanation for the fact that metabolic changes observed in mdx hearts were most evident when these hearts were perfused ex vivo [8]. In addition, the pattern of metabolic alterations in the perfused mdx hearts, namely the observed metabolic shift in substrate utilization from LCFA to CHO oxidation and the enhanced MVO2, is consistent with decreased NO signaling [69]. Finally, a defect in NO/cGMP signaling is also compatible with decreased levels of phosphorylated Akt, an activator of endothelial NO synthase, in ex vivo perfused mdx hearts [61].
3.4. Establishing a role for the NO/cGMP pathway in mitochondrial metabolic alterations of the dystrophic heart
To more firmly establish a link between defective NO/cGMP signaling and mitochondrial function abnormalities, we recently increased cGMP signaling downstream of NO formation in mdx mice by using either (1) a targeted genetic approach, where mdx mice were crossed to transgenic mice overexpressing guanylyl cyclase in a cardiomyocyte-specific manner, or (2) a pharmacological approach, where cGMP breakdown was inhibited by chronically treating young (6 weeks old) mdx mice with the PDE5 inhibitor sildenafil. We found that increasing cGMP signaling significantly improved mitochondrial metabolic and function status of mdx mouse hearts, along with cardiac contractility and sarcolemmal integrity [9].
Specifically, at the metabolic level, 12-week-old mdx/GC+/o hearts displayed a pattern of substrate selection for energy production that was similar to that of their mdx counterparts. This finding raises the following question: what are the potential cause(s) or consequence (s) of the previously documented shift from LCFA to CHO utilization in 12-week-old mdx hearts compared to controls? At least two explanations for this observation can be put forward. First, this shift may be a direct or indirect consequence of dystrophin deficiency. Indeed, cytoskeletal disturbances subsequent to dystrophin deficiency may impact on the compartmentalization of cytosolic carbohydrate metabolism [70,71]. Furthermore, dystrophin deficiency also results in abnormalities in calcium handling, a factor that may also independently impact substrate metabolism [72].
Another possibility is that the metabolic shift previously reported in mdx hearts may represent an adaptive response rather than a deleterious consequence of cardiac dysfunction. In fact, our finding of a substrate shift from LCFA to CHO utilization associated with enhanced glycolysis is in agreement with our findings in the cardioprotected GC+/0 mouse hearts, as discussed earlier [50]. However, it appears warranted to further investigate (i) how the mdx heart handles lipids, including the partitioning of exogenous fatty acids between oxidation (for energy production) and triglycerides (for storage) as well as triglyceride hydrolysis particularly as the cardiomyopathy progresses, and (ii) how this would be affected by enhancing cGMP signaling.
Beyond considerations about substrate selection, our finding of a marked increase in the mitochondrial CAC pool size in the perfused mdx/GC+/o heart compared to their mdx counterparts suggested an improved mitochondrial metabolic status in the former group. Such a change would be expected to enable the mdx heart to enhance CAC flux and hence mitochondrial NADH and energy production, especially under conditions of high energy demand. It is noteworthy that a downregulation of mitochondrial gene expression has been reported in dystrophic skeletal muscle [73]. Conversely, the induction of PGC-1α, a factor governing mitochondrial biogenesis [5,74], was found to improve indices of skeletal muscle damage in mdx mice [75]. However, we did not observe a difference in mitochondrial citrate synthase activity (a marker of mitochondrial volume-density) in young 12-week-old mdx mouse hearts [8], although it was found to be decreased by 18% at 8 months [76]. These results suggest that, at least at a young age, factors other than PGC-1α levels modulate the mitochondrial metabolic status of the mdx mouse heart. This improved mitochondrial metabolic status in mdx/GC+/o mouse hearts reconciles many findings in the literature that support the role of cGMP signalling in preventing mitochondria-mediated cell death which will be further discussed in Section 4.3.
Overall, our findings in mdx/GC+/o and GC+/o mice suggest that the metabolic shift from LCFA to CHO in mdx heart may be an adaptive response. While additional studies are needed to clarify how the enhancement of NO/cGMP signaling in the mdx heart might impact on CHO and LFCA utilization based on the marked improvement in mitochondrial CAC intermediate levels in perfused mdx/GC+/o hearts, the mitigation of the dystrophic cardiomyopathy found in these mice may be mediated, at least in part, by an improved mitochondrial function resulting from enhanced cGMP signalling.
4. Is the dystrophic heart more vulnerable to induction of mitochondrial cell death pathways?
4.1. Mitochondrial cell death pathways in other models of cardiac hypertrophy and failure
In addition to energy production, mitochondria play several important roles in other cellular functions: (i) regulation of intracellular Ca2+dynamics by taking up and releasing large amounts of Ca2+[77–80], (ii) ROS production that can trigger signalling pathways or induce cell injury [81–84], and (iii) regulation of cell death through their ability to trigger necrosis and apoptosis [85–88] by changing mitochondrial membrane permeability [88–90].
Studies employing models of acquired cardiomyopathy have shown that, in addition to overt structural [91,92] and respiratory defects [93–101], mitochondria within the failing myocardium display enhanced ROS production [94,95,102,103]. This is associated with signs of oxidative stress-related damage such as accumulation of lipid peroxidation by-products and mutations or deletions of mtDNA [94,104,105]. In addition, experimental evidence obtained both in humans and animal models indicates that failing hearts display enhanced permeability of mitochondrial outer [106–108] and inner [97,109] membranes, leading to release of mitochondrial proapoptotic factors such as cytochrome c [110,111] and activation of other downstream events, including cleavage of procaspases 3 [106,110] and 9 [111]. Hence, these results indicate that the mitochondrial death pathway is active in the failing heart and likely plays a role in the associated cardiomyocyte death.
Although the molecular events involved in mitochondrial membrane permeation are not well understood, the current consensus is that several mechanisms coexist and may be recruited in response to specific death stimuli [88,112]. One major mechanism involves the oligomerization of proapoptotic proteins of the Bcl-2 family to form pores across the outer mitochondrial membrane [88,112]. Another important and well-characterized mechanism involves the opening of the PTP, a high conductance nonspecific channel in the mitochondrial inner membrane [88,90].
Recently published work from our group supports the notion that increased susceptibility of mitochondria to opening of the permeability transition pore (PTP) during periods of stress could be an important early factor in the progression towards heart failure by promoting cell death [113,114]. Specifically, we have assessed myocardial PTP opening in the intact rat heart as well as in isolated mitochondria during the early stages of ventricular remodelling, prior to the onset of systolic dysfunction or overt mitochondrial structural and respiratory defects. Mitochondria isolated from hypertrophied rat hearts displayed an increased susceptibility to opening of the PTP in response to a physiological stressor in vitro (i.e., Ca2+overload, anoxia reoxygenation). This was also observed in the intact perfused heart following ischemia–reperfusion (I-R) [113] and was associated with an impaired functional recovery, greater tissue release of lactate dehydrogenase (LDH), and mitochondrial release of proapoptotic factors upon reperfusion. Other studies have shown that hypertrophied hearts are more susceptible to contractile dysfunction and cell death when exposed to stimuli such as H2O2 [115] and I-R [116–118], which are known to promote PTP opening. Importantly, in at least one of these studies [115], susceptibility to stress-induced cell death could be detected in cardiomyocytes isolated from hypertrophied hearts prior to detection of apoptotic cell death in the whole organ in situ. The latter became substantial only once the heart had reached the failing stage, further suggesting that mitochondrial vulnerability to stress is a precursor event in the progression toward organ failure.
4.2. Activation of mitochondrial death pathways in the dystrophic heart
Considering our findings of increased CAC intermediate levels in perfused mdx/GC+/o hearts compared to their mdx counterparts [9] and of an increased susceptibility to PTP opening in the early stages of cardiomyopathy in our rat studies [113,114], we recently determined whether vulnerability to PTP opening could be involved in the enhanced cardiomyocyte injury previously observed in the hearts of young mdx mice challenged by physiological stressors [8,9,27]. To accomplish this, we adapted the mitochondrial [3H]-deoxyglucose ([3H]-DOG) entrapment method used to quantify PTP opening in rat hearts [97,113,119,120] to the mouse. Using this approach, we found that when hearts of young mdx mice are subjected to I-R, the number of mitochondria undergoing PTP opening is significantly enhanced compared to normal hearts (Fig. 1). Enhanced PTP opening is accompanied by greater release of cytochrome c (Cyt-c) into the cytosolic fraction (Fig. 1E), which further supports enhanced mitochondrial membrane permeability. As opening of the PTP allows for ions and solutes of <1,500 Da to equilibrate across mitochondrial membranes, we postulate that this phenomenon could account, at least in part, for the reduction in citrate and total CAC pool size that we previously observed in ex vivo perfused working hearts from mdx mice [8,9]. Furthermore, because this susceptibility to PTP opening is present prior to any histological or hemodynamic evidence of cardiomyopathy, we believe that it may play an important pathogenic role in the ensuing development of the dystrophic cardiomyopathy. In support of this interpretation, Millay et al. [121] recently reported that genetic ablation of the PTP-sensitizing protein cyclophilin-D (Cyp-D) in δ-sarcoglycan (scgd−/−) null mice, another model of MD in which there is associated sarcolemmal instability, resulted in significantly reduced cardiac fibrosis and improved ejection fraction. Recent data obtained in skeletal muscle from other forms of MD, including Bethlem myopathy, Ullrich congenital dystrophy, and Limb girdle MD [121–124] also support a role for PTP opening, but whether this also applies to the heart has not yet been established.
Fig. 1.
Mitochondrial vulnerability to permeability transition pore (PTP) opening in 12 week-old mdx mice. PTP opening was quantified in situ in isolated Langendorff perfused hearts from mdx and C57BL/10 mice using an adaptation of the mitochondrial [3H]-2-deoxyglucose (DOG) entrapment technique previously described for rat hearts [113,119]. As depicted in (A), this method relies on the fact that [3H]DOG accumulates in the cytosol of cardiomyocytes as [3H]DOG-6-phosphate (P) and does not enter mitochondria unless PTP opening occurs. Therefore, quantification of [3H]DOG-6P levels in mitochondria isolated at the end of perfusion provides a quantitative index of the number of mitochondria in which PTP opening occurred during the experiment. Specifically, hearts from control and mdx mice were initially perfused for 15 min in the nonrecirculating mode with a buffer containing 11 mM glucose, 1.5 mM lactate, 0.2 mM pyruvate, 0.8 nM insulin and 0.5 mM [3H]DOG (10 μCi/mL) to load the cardiomyocytes with this tracer. Then, following a 5-min washout of extracellular [3H]DOG, hearts were submitted to 20 min of low-flow ischemia (10% initial coronary flow) followed by 40 min reperfusion in presence of 1 μM norepinephrine. Prior to ischemia (I), mdx hearts showed no major contractile dysfunction as reflected by the rate pressure product (RPP= Left ventricular developed pressure in mm Hg×heart rate in beats per min) (C) but released greater amounts of lactate dehydrogenase (LDH; values normalized for contractile function) in the coronary effluent compared to controls (D). However, at reperfusion (R), mdx heart displayed (i) poorer functional recovery (C), (ii) enhanced LDH release (D), (iii) greater opening of the PTP (B), and (iv) greater release of cytochrome c (Cyt-c) into the cytosolic fraction (E). Data represent means±SEM, n= 10–11 per group. *p< 0.05 vs. control C57BL/10 mice.
4.3. Potential factors underlying greater vulnerability to PTP opening in the dystrophic heart
There are several factors that could potentially account for the enhanced propensity of the dystrophin-deficient heart to PTP opening. These include mitochondrial calcium accumulation, enhanced oxidative stress, and altered cGMP signalling, which could act alone or in synergy. Acute ischemic episodes, similar to what has been described for skeletal muscles, could also contribute to this phenomenon, although this remains to be substantiated in the dystrophin-deficient heart [125,126]. Firstly, accumulation of Ca2+ in the mitochondrial matrix is recognized as one of the most important triggers for permeability transition [127] and the lack of dystrophin (or δ-sarcoglycan) predisposes to disruption of the sarcolemmal membrane during mechanical stress, resulting in increased Ca2+ entry. In addition, it is now well recognized that there are other important sources of increased Ca2+ levels within dystrophic muscle cells, even in the absence of sarcolemmal disruption. These include an increased Ca2+ influx through voltage-independent stretch-sensitive Ca2+ leak channels (SACs) [128,129] and store-operated Ca2+ channels (SOCs) [130–132], as well as potentially via the ryanodine receptor [133]. Several studies in cardiac [134,135] and skeletal muscle cells [132,136] indicate that because of their localization in the vicinity of the sarcoplasmic reticulum and T-tubules, mitochondria rapidly take up Ca2+ entering the cytosol during depolarization. Thus mitochondria from dystrophic myocytes are likely to be more susceptible to Ca2+ overload due to increased Ca2+ influx from several sources [131,132].
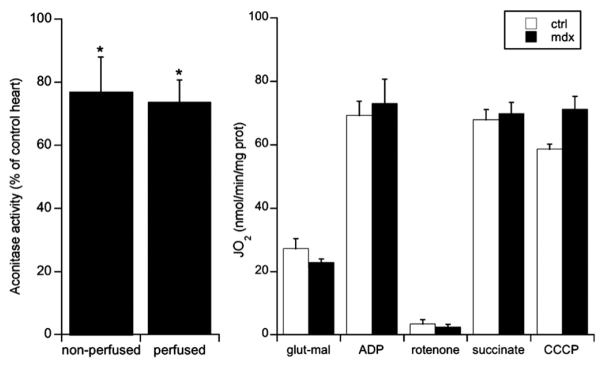
Enhanced production of ROS is another major factor, which may act synergistically with Ca2+ overload to induce PTP opening [127]. The activities of aconitase and NADP-isocitrate dehydrogenase, two mitochondrial enzymes that are notoriously sensitive to inactivation by ROS, are reduced in adult skeletal muscle from mdx mice compared to control [137]. Importantly, the muscle glutathione pool was significantly more oxidized in 14-day-old mdx mice (i.e., prior to the onset of skeletal muscle fiber necrosis, which occurs at ~4 weeks of age) compared to normal mice, consistent with the notion that, in dystrophic muscle, chronic oxidative stress constitutes a primary defect rather than being solely a consequence of necrosis [137]. Although little data are available on mitochondrial oxidative stress in the dystrophic heart, we found that aconitase activity was reduced (25%) in mdx hearts compared to controls at 12 weeks of age (i.e., prior to any overt sign of cardiomyopathy) [8] (Fig. 2). However, respiratory function measured in isolated mitochondria from young mdx hearts was similar to controls (Fig. 2, unpublished observations), as also previously described by Kuznetsov et al. [138], suggesting that there are no major defects in respiratory chain complexes at this early stage. While these results clearly indicate that factors which promote oxidative stress are present in mitochondria from dystrophic hearts, it is still unclear whether this is caused by enhanced H2O2 production or by a reduction in the capacity of one or more of the mitochondrial H2O2 scavenging systems such as the glutathione and thioredoxin systems, catalase, or other nonenzymatic reactions.
Fig. 2.
Mitochondrial oxidative stress in mdx mouse hearts. (A) shows the activity of the mitochondrial oxidative stress marker, aconitase, measured in tissue homogenates prepared from either freshly isolated 12-week-old mouse hearts or perfused ex vivo in the working mode [8]. Data are expressed in percentage of values measured in control animals (C57BL/10) and represent means ± SEM, n= 6–11 per group. (B) shows the results of experiments in which changes in respiration (JO2) were recorded in response to the sequential addition of glutamate–malate (5:2.5 mM), ADP (1 mM), rotenone (1 μM), succinate (5 mM), and carbonyl cyanide m-chlorophenylhydrazone (CCCP: 0.1 μM). Data shown in (B) are expressed per mg of total protein and represent means ± SEM, n= 4–6 per group. *p< 0.05 vs. control C57BL/10 mice.
Finally, our finding [9] that expression of the GC+/o transgene in mdx cardiomyocytes largely prevented the loss of CAC intermediates in hearts perfused ex vivo in the working mode suggests that defective NO/cGMP signalling may contribute to the enhanced propensity of the dystrophic heart to opening of the PTP. Although unequivocal proof requires a comparative assessment of PTP opening in mdx and mdx/GC+/o hearts, there is published evidence in support of a link between cGMP signalling and mitochondrial PTP opening, including results from studies that examined the impact of enhancing cGMP signalling by either inhibiting its breakdown with the phosphodiesterase-5 inhibitor sildenafil, or increasing its formation by stimulating the natriuretic receptors in the setting of I-R or doxorubicin-induced cardiotoxicity [139–141]. The underlying mechanisms appear to involve the capacity of cGMP-dependent protein kinase (PKG) to inhibit the PTP through opening of mitochondrial KATP channels (mKATP) [140–143]. Recent studies by Kukreja and colleagues provide compelling evidence that ERK1/2 may be a key mediator that bridges PKG to mKATP [144]. In addition, cGMP-dependent signalling has the potential to reduce PTP opening indirectly through its impact on intracellular Ca2+ dynamics. In fact, data obtained in various cell types including cardiomyocytes show that PKG can potentially reduce intracellular calcium levels by inhibiting (i) the synthesis of inositol triphosphate (IP3), (ii) the release of Ca2+from intracellular stores through the IP3 receptor, (iii) SERCA2a-mediated Ca2+reuptake, and (iv) the influx of extracellular Ca2+ through L-type Ca2+ channels [145,146].
Taken together, these results suggest that, similar to what has been observed in volume overload-induced cardiac hypertrophy [113,114], mitochondria within dystrophin-deficient cardiomyocytes are more prone to PTP opening than are those from normal cardiomyocytes when challenged with a stressor. More importantly, this phenomenon is present at the early stages of disease progression and contributes directly to the development of histological and hemodynamic evidence of cardiomyopathy [121]. Although all of the mechanisms underlying these phenomena remain to be clarified, increased Ca2+ entry, enhanced oxidative stress within mitochondria, and impaired NO/cGMP signalling are likely to play key roles.
5. Mitochondrial protection: a potential strategy to protect the dystrophic heart
While the dystrophic cardiomyopathy can be symptomatically treated directly with conventional heart failure therapy such as β-adrenergic blockers or angiotensin-converting enzyme inhibitors, novel therapies are being sought to specifically correct functional defects resulting from dystrophin deficiency [147–151]. In this regard, current strategies that are being developed include stem cell approaches as well as gene transfer of dystrophin, utrophin, or functional dystrophin fragments (microdystrophins). However, the effectiveness of these interventions remains to be established, particularly with respect to cardiac muscle function given that for the most part, these interventions have been tested predominantly on dystrophic skeletal muscle. In the meantime, alternative adjunct therapies are being sought to slow disease progression, and these could ameliorate both the cardiac and skeletal muscle dysfunction [152]. In view of the evidence presented in this review, pharmacological strategies aimed at preventing mitochondrial dysfunction, particularly with respect to reducing oxidative stress and opening of the PTP, appear to be a promising avenue. In this regard, three strategies will be discussed briefly: the use of cyclophilin-D ligands, mitochondria-targeted antioxidants, and PDE-5 inhibitors.
5.1. Cyclophilin-D ligands
Cyclophilin-D (Cyp-D) is a chaperone-like protein of the immunophilin family located in the mitochondrial matrix, which acts as a potent PTP sensitizer when translocated to the inner membrane and bound to putative pore components [153–155]. In fact, cyclosporin-A (CsA), an inhibitor of PTP opening, has long been known to act in vitro by binding Cyp-D, thus preventing its recruitment to the mitochondrial inner membrane [155,156]. Until recently, however, the potential benefits of chronic treatment with CsA were limited by its capacity to also inhibit calcineurin complexed to cyclophilin-A, causing a number of deleterious effects in myocytes, including enhancement of apoptosis [157–159]. Fortunately, several CsA analogs that do not inhibit calcineurin have now been developed, including NIM811, Sanglifehrin-A, and Debio025. To date, the therapeutic potential of Cyp-D ligands in the treatment of DMD has only been evaluated in skeletal muscle [121,124]. Specifically, administration of Debio025 for six weeks in young mdx mice significantly reduced fibrosis and the percentage of fibers containing central nuclei in the diaphragm, EDL, TA, and quadriceps muscles [121]. Based on our recent results (Fig. 1), as well as the recently reported improvement of cardiac outcomes following genetic ablation of Cyp-D in the δ-sarcoglycan deficient mouse [121], one would anticipate that Cyp-D ligands could also be effective in slowing progression of the cardiomyopathy in DMD patients.
5.2. Mitochondria-targeted antioxidants
Given the evidence that oxidative stress within mitochondria is increased at very early stages of disease progression in mdx mice [8,137] (Fig. 2), antioxidant therapies appear to be another logical approach for the treatment of DMD. In fact, buffering excessive ROS production within mitochondria could be beneficial not only by limiting oxidative damage to mitochondrial and cellular structures, but also by reducing the likelihood of PTP opening [127]. Various antioxidants, such as vitamin E and selenium [160], were considered but not found to be beneficial against the progression of DMD. However, given the complexity of free radical biology, the design of antioxidant therapies remains difficult [161]. Nevertheless, Buyse et al. [162] recently reported the benefits of the antioxidant idebenone (SNT-MC17) in a long-term placebo-controlled study in the dystrophin-deficient mdx mouse. Specifically, this treatment was found to (i) improve diastolic dysfunction, (ii) limit mortality from cardiac pump failure induced by dobutamine stress testing in vivo, (iii) reduce cardiac inflammation and fibrosis, and (iv) enhance exercise performance.
In recent years, a novel class of antioxidants that specifically accumulate within mitochondria in large amounts has emerged. The development of these agents was motivated by the recognition that the lack of effectiveness of classical antioxidant therapies in some pathologies in which mitochondrial oxidative stress is known to develop, could be caused by the inability to mobilize sufficient amounts of antioxidants within these organelles. Among the agents available, Mito-Q, developed by Murphy et al., has reached clinical trials [163,164]. The superiority of these mitochondria-targeted antioxidants over untargeted coenzyme Q derivatives, such as idebenone, has been demonstrated in Friedreich Ataxia fibroblasts [164], but has not yet been tested in the dystrophin-deficient heart.
5.3. NO/cGMP signalling
Enhancing cGMP signalling, specifically downstream and independent of NO formation, improved contractile performance, mitochondrial metabolic alterations, and sarcolemmal integrity in the young mdx mouse heart [9]. More recently, Pasrchen et al. reported that 3-month sildenafil treatment in mdx mice started at 12 months of age was able to reverse the existing cardiac dysfunction [165]. These findings point to defective cGMP signalling as being an important component of disease pathogenesis in the dystrophin-deficient heart and also suggest the basis for a novel therapeutic approach to prevent or delay the onset of dystrophin-related cardiomyopathies. Since there are currently safe and well-tolerated pharmacological means to enhance cGMP signalling, such drugs could provide the basis for a new therapeutic strategy based upon PDE5 inhibition in the dystrophic heart. This type of intervention may be considered as an adjunct therapy to other available treatments [151]. Asai et al. [166] also showed that treatment with tadalafil, another PDE5 inhibitor, ameliorates contraction-induced skeletal muscle damage. While the mechanisms underlying the beneficial effects of enhanced cGMP signalling in the dystrophic heart remain to be established, our studies suggest that preservation of mitochondrial function could play an important role [9]. This “mito-protective” effect could be partly related to a better maintenance of anaplerotic fluxes responsible for the maintenance of CAC pool size and/or to an improved mitochondrial integrity due to inhibition of PTP opening. This latter possibility would be consistent with the existing literature showing that inhibition of mitochondrial PTP opening through activation of KATP channels is an end effector of preconditioning induced by cGMP-dependent signalling [140–143]. Interestingly, dystrophin has also been recently proposed to be an end-target of ischemic preconditioning [167].
6. Conclusion
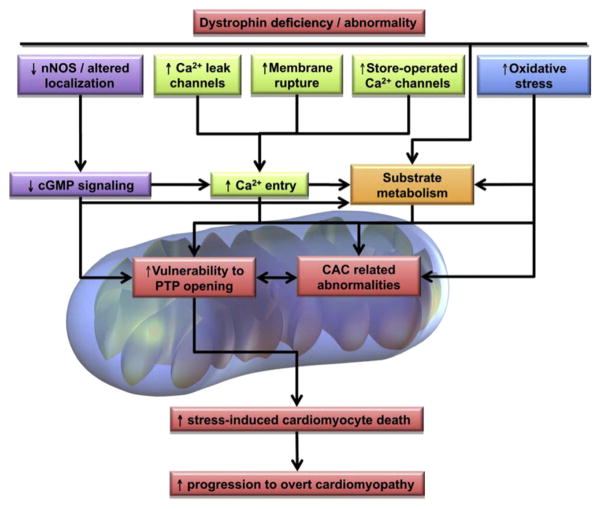
Based on the evidence discussed in this review, we propose a simplified integrated mechanistic scheme of potential players and consequences of early mitochondrial dysfunction in the dystrophin-deficient heart (Fig. 3). Taken together, recent results highlight early mitochondrial functional alterations in the dystrophic heart that precede overt cardiomyopathy. These mitochondrial alterations include (i) CAC-related metabolic parameters such as decreased aconitase activity and lower levels of CAC intermediate levels, which are factors that may limit the capacity of the dystrophin-deficient to withstand an increase in energy demand as occurs during an adrenergic challenge, and (ii) an enhanced propensity of the dystrophin-deficient heart to PTP opening. We also demonstrated that ex vivo perfused working hearts from young mdx mice display early alterations in substrate selection for energy production, namely a shift from LCFA to CHO oxidation. While our results suggest that this substrate shift may be an adaptive response, further investigations are needed to better understand the causes and potential consequences of this metabolic alteration. However, the mitochondrial alterations are likely to be maladaptive, contributing to the pathophysiology of the disease. Overall, defective NO/cGMP signalling appears to be a likely candidate mechanism contributing to these mitochondrial alterations, particularly the enhanced opening of the PTP, which in itself may be a factor governing mitochondrial efflux of CAC intermediates. Additional factors that may contribute to mitochondrial dysfunction in the dystrophin-deficient heart include augmented ROS production and abnormal calcium handling. Therefore, it appears warranted to consider therapeutic interventions which specifically target these mitochondrial abnormalities, including PDE inhibitors (e.g., sildenafil), along with newly emerging agents. Given dystrophin’s implication in other end-stage cardiomyopathies and the central role of mitochondria in heart failure, the aforementioned mitochondria-targeted therapeutic approaches appear to offer promising avenues for patient management that extend beyond the dystrophic cardiomyopathy.
Fig. 3.
Simplified integrated mechanistic scheme of potential players and consequences of early mitochondrial dysfunction in the dystrophin-deficient heart. Refer to Section 6 for details. Abbreviations: nNOS, neuronal nitric oxide synthase; Ca2+, calcium; cGMP, cyclic guanosine monophosphate; PTP, permeability transition pore; CAC, citric acid cycle.
Acknowledgments
This work was supported by the Canadian Institutes of Health Research (CIHR grants 9575 to C.D.R. 77791 to B.G.A.) and a Pfizer Cardiovascular Award (to Y.B.). Y.B. is a Junior II Research Scholar and B.J.P. is a National Research Scholar from the Fonds de la Recherche en Santé du Québec (FRSQ). The authors gratefully acknowledge the secretarial assistance of Antoinette Paolitto.
Abbreviations
- [3H]-DOG
[3H]-deoxyglucose
- 13C
carbon 13
- AMPK
AMP kinase
- anf
atrial natriuretic factor
- CAC
citric acid cycle
- cGMP
cyclic guanosine monophosphate
- CHO
carbohydrates
- CsA
cyclosporine-A
- Cyp-D
cyclophilin-D
- DMD
Duchenne muscular dystrophy
- FA
fatty acid
- GC
guanylate cyclise
- I-R
ischemia–reperfusion
- LCFA
long chain fatty acid
- LDH
lactate dehydrogenase
- mKATP
mitochondrial KATP channels
- mtDNA
mitochondrial DNA
- NO
nitric oxide
- nNOS
neuronal nitric oxide synthase
- PDE5
phosphodiesterase-5
- PGC
peroxisome proliferator-activated receptor γ coactivator
- PPAR
peroxisome proliferator-activated receptor
- PTP
permeability transition pore
- ROS
reactive oxygen species
- SACs
stretch-sensitive Ca2+-leak channels
- Scgd−/−
δ-sarcoglycan
- Sgcα1
soluble guanylate cyclase α1
- SOCs
store-operated Ca2+channels
References
- 1.Huss JM, Kelly DP. Mitochondrial energy metabolism in heart failure: a question of balance. J Clin Invest. 2005;115:547–55. doi: 10.1172/JCI200524405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marin-Garcia J, Goldenthal MJ. Mitochondrial centrality in heart failure. Heart Fail Rev. 2008;13(2):137–50. doi: 10.1007/s10741-007-9079-1. [DOI] [PubMed] [Google Scholar]
- 3.Murray AJ, Edwards LM, Clarke K. Mitochondria and heart failure. Curr Opin Clin Nutr Metab Care. 2007;10:704–11. doi: 10.1097/MCO.0b013e3282f0ecbe. [DOI] [PubMed] [Google Scholar]
- 4.Tsutsui H, Kinugawa S, Matsushima S. Mitochondrial oxidative stress and dysfunction in myocardial remodelling. Cardiovasc Res. 2009;81:449–56. doi: 10.1093/cvr/cvn280. [DOI] [PubMed] [Google Scholar]
- 5.Ventura-Clapier R, Garnier A, Veksler V. Transcriptional control of mitochondrial biogenesis: the central role of PGC-1alpha. Cardiovasc Res. 2008;79:208–17. doi: 10.1093/cvr/cvn098. [DOI] [PubMed] [Google Scholar]
- 6.Lemieux H, Hoppel CL. Mitochondria in the human heart. J Bioenerg Biomembr. 2009;41:99–106. doi: 10.1007/s10863-009-9211-0. [DOI] [PubMed] [Google Scholar]
- 7.Rosca MG, Vazquez EJ, Kerner J, Parland W, Chandler MP, Stanley W, et al. Cardiac mitochondria in heart failure: decrease in respirasomes and oxidative phosphorylation. Cardiovasc Res. 2008;80:30–9. doi: 10.1093/cvr/cvn184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khairallah M, Khairallah R, Young ME, Dyck JR, Petrof BJ, Des Rosiers C. Metabolic and signaling alterations in dystrophin-deficient hearts precede overt cardiomyopathy. J Mol Cell Cardiol. 2007;43:119–29. doi: 10.1016/j.yjmcc.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 9.Khairallah M, Khairallah RJ, Young ME, Allen BG, Gillis MA, Danialou G, et al. Sildenafil and cardiomyocyte-specific cGMP signaling prevent cardiomyopathic changes associated with dystrophin deficiency. Proc Natl Acad Sci USA. 2008;105:7028–33. doi: 10.1073/pnas.0710595105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Badorff C, Lee GH, Lamphear BJ, Martone ME, Campbell KP, Rhoads RE, et al. Enteroviral protease 2A cleaves dystrophin: evidence of cytoskeletal disruption in an acquired cardiomyopathy. Nat Med. 1999;5:320–6. doi: 10.1038/6543. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez M, Cai WJ, Kostin S, Lucchesi BR, Schaper J. Ischemia depletes dystrophin and inhibits protein synthesis in the canine heart: mechanisms of myocardial ischemic injury. J Mol Cell Cardiol. 2005;38:723–33. doi: 10.1016/j.yjmcc.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 12.Vatta M, Stetson SJ, Perez-Verdia A, Entman ML, Noon GP, Torre-Amione G, et al. Molecular remodelling of dystrophin in patients with end-stage cardiomyopathies and reversal in patients on assistance-device therapy. Lancet. 2002;359:936–41. doi: 10.1016/S0140-6736(02)08026-1. [DOI] [PubMed] [Google Scholar]
- 13.Wallace GQ, McNally EM. Mechanisms of muscle degeneration, regeneration, and repair in the muscular dystrophies. Annu Rev Physiol. 2009;71:37–57. doi: 10.1146/annurev.physiol.010908.163216. [DOI] [PubMed] [Google Scholar]
- 14.Towbin JA, Bowles NE. The failing heart. Nature. 2002;415:227–33. doi: 10.1038/415227a. [DOI] [PubMed] [Google Scholar]
- 15.Goodwin FC, Muntoni F. Cardiac involvement in muscular dystrophies: molecular mechanisms. Muscle Nerve. 2005;32:577–88. doi: 10.1002/mus.20352. [DOI] [PubMed] [Google Scholar]
- 16.Nigro G, Comi LI, Limongelli FM, Giugliano MA, Politano L, Petretta V, et al. Prospective study of X-linked progressive muscular dystrophy in Campania. Muscle Nerve. 1983;6:253–62. doi: 10.1002/mus.880060403. [DOI] [PubMed] [Google Scholar]
- 17.Nigro G, Comi LI, Politano L, Bain RJ. The incidence and evolution of cardiomyopathy in Duchenne muscular dystrophy. Int J Cardiol. 1990;26:271–7. doi: 10.1016/0167-5273(90)90082-g. [DOI] [PubMed] [Google Scholar]
- 18.Cox GF, Kunkel LM. Dystrophies and heart disease. Curr Opin Cardiol. 1997;12:329–43. [PubMed] [Google Scholar]
- 19.Hainsey TA, Senapati S, Kuhn DE, Rafael JA. Cardiomyopathic features associated with muscular dystrophy are independent of dystrophin absence in cardiovasculature. Neuromuscular Disord. 2003;13:294–302. doi: 10.1016/s0960-8966(02)00286-9. [DOI] [PubMed] [Google Scholar]
- 20.Zhu X, Wheeler MT, Hadhazy M, Lam MY, McNally EM. Cardiomyopathy is independent of skeletal muscle disease in muscular dystrophy. FASEB J. 2002;16:1096–8. doi: 10.1096/fj.01-0954fje. [DOI] [PubMed] [Google Scholar]
- 21.de Kermadec JM, Becane HM, Chenard A, Tertrain F, Weiss Y. Prevalence of left ventricular systolic dysfunction in Duchenne muscular dystrophy: an echocardiographic study. Am Heart J. 1994;127:618–23. doi: 10.1016/0002-8703(94)90672-6. [DOI] [PubMed] [Google Scholar]
- 22.Mukoyama M, Kondo K, Hizawa K, Nishitani H. Life spans of Duchenne muscular dystrophy patients in the hospital care program in Japan. J Neurol Sci. 1987;81:155–8. doi: 10.1016/0022-510x(87)90092-x. [DOI] [PubMed] [Google Scholar]
- 23.Quinlan JG, Hahn HS, Wong BL, Lorenz JN, Wenisch AS, Levin LS. Evolution of the mdx mouse cardiomyopathy: physiological and morphological findings. Neuromuscul Disord. 2004;14:491–6. doi: 10.1016/j.nmd.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Bia BL, Cassidy PJ, Young ME, Rafael JA, Leighton B, Davies KE, et al. Decreased myocardial nNOS, increased iNOS and abnormal ECGs in mouse models of Duchenne muscular dystrophy. J Mol Cell Cardiol. 1999;31:1857–62. doi: 10.1006/jmcc.1999.1018. [DOI] [PubMed] [Google Scholar]
- 25.Wilding JR, Schneider JE, Sang AE, Davies KE, Neubauer S, Clarke K. Dystrophin-and MLP-deficient mouse hearts: marked differences in morphology and function, but similar accumulation of cytoskeletal proteins. FASEB J. 2005;19:79–81. doi: 10.1096/fj.04-1731fje. [DOI] [PubMed] [Google Scholar]
- 26.Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci USA. 1993;90:3710–4. doi: 10.1073/pnas.90.8.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Danialou G, Comtois AS, Dudley R, Karpati G, Vincent G, Des Rosiers C, et al. Dystrophin-deficient cardiomyocytes are abnormally vulnerable to mechanical stress-induced contractile failure and injury. FASEB J. 2001;15:1655–7. doi: 10.1096/fj.01-0030fje. [DOI] [PubMed] [Google Scholar]
- 28.Kamogawa Y, Biro S, Maeda M, Setoguchi M, Hirakawa T, Yoshida H, et al. Dystrophin-deficient myocardium is vulnerable to pressure overload in vivo. Cardiovasc Res. 2001;50:509–15. doi: 10.1016/s0008-6363(01)00205-x. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura A, Yoshida K, Takeda S, Dohi N, Ikeda S. Progression of dystrophic features and activation of mitogen-activated protein kinases and calcineurin by physical exercise in hearts of mdx mice. FEBS Lett. 2002;520:18–24. doi: 10.1016/s0014-5793(02)02739-4. [DOI] [PubMed] [Google Scholar]
- 30.Petrof BJ. The molecular basis of activity-induced muscle injury in Duchenne muscular dystrophy. Mol Cell Biochem. 1998;179:111–23. doi: 10.1023/a:1006812004945. [DOI] [PubMed] [Google Scholar]
- 31.Petrof BJ. Molecular pathophysiology of myofiber injury in deficiencies of the dystrophin-glycoprotein complex. Am J Phys Med Rehabil. 2002;81:S162–74. doi: 10.1097/00002060-200211001-00017. [DOI] [PubMed] [Google Scholar]
- 32.Oceandy D, Cartwright EJ, Emerson M, Prehar S, Baudoin FM, Zi M, et al. Neuronal nitric oxide synthase signaling in the heart is regulated by the sarcolemmal calcium pump 4b. Circulation. 2007;115:483–92. doi: 10.1161/CIRCULATIONAHA.106.643791. [DOI] [PubMed] [Google Scholar]
- 33.Kanai AJ, Pearce LL, Clemens PR, Birder LA, VanBibber MM, Choi SY, et al. Identification of a neuronal nitric oxide synthase in isolated cardiac mitochondria using electrochemical detection. Proc Natl Acad Sci USA. 2001;98:14126–31. doi: 10.1073/pnas.241380298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bendall JK, Damy T, Ratajczak P, Loyer X, Monceau V, Marty I, et al. Role of myocardial neuronal nitric oxide synthase-derived nitric oxide in beta-adrenergic hyporesponsiveness after myocardial infarction-induced heart failure in rat. Circulation. 2004;110:2368–75. doi: 10.1161/01.CIR.0000145160.04084.AC. [DOI] [PubMed] [Google Scholar]
- 35.Ashrafian H, Frenneaux MP, Opie LH. Metabolic mechanisms in heart failure. Circulation. 2007;116:434–48. doi: 10.1161/CIRCULATIONAHA.107.702795. [DOI] [PubMed] [Google Scholar]
- 36.Neubauer S. The failing heart-an engine out of fuel. N Engl J Med. 2007;356:1140–51. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- 37.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85:1093–129. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- 38.van Bilsen M, van Nieuwenhoven FA, van der Vusse GJ. Metabolic remodelling of the failing heart: beneficial or detrimental? Cardiovasc Res. 2009;81:420–8. doi: 10.1093/cvr/cvn282. [DOI] [PubMed] [Google Scholar]
- 39.Ingwall JS. Energy metabolism in heart failure and remodelling. Cardiovasc Res. 2009;81:412–9. doi: 10.1093/cvr/cvn301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chandler MP, Kerner J, Huang H, Vazquez E, Reszko A, Martini WZ, et al. Moderate severity heart failure does not involve a downregulation of myocardial fatty acid oxidation. Am J Physiol Heart Circ Physiol. 2004;287:H1538–43. doi: 10.1152/ajpheart.00281.2004. [DOI] [PubMed] [Google Scholar]
- 41.de Brouwer KF, Degens H, Aartsen WM, Lindhout M, Bitsch NJ, Gilde AJ, et al. Specific and sustained down-regulation of genes involved in fatty acid metabolism is not a hallmark of progression to cardiac failure in mice. J Mol Cell Cardiol. 2006;40:838–45. doi: 10.1016/j.yjmcc.2006.03.429. [DOI] [PubMed] [Google Scholar]
- 42.Borradaile NM, Schaffer JE. Lipotoxicity in the heart. Curr Hypertens Rep. 2005;7:412–7. doi: 10.1007/s11906-005-0035-y. [DOI] [PubMed] [Google Scholar]
- 43.Gélinas R, Labarthe F, Bouchard B, Mc DJ, Charron G, Young ME, et al. Alterations in carbohydrate metabolism and its regulation in PPARalpha null mouse hearts. Am J Physiol Heart Circ Physiol. 2008;294:H1571–80. doi: 10.1152/ajpheart.01340.2007. [DOI] [PubMed] [Google Scholar]
- 44.Labarthe F, Khairallah M, Bouchard B, Stanley WC, Des Rosiers C. Fatty acid oxidation and its impact on response of spontaneously hypertensive rat hearts to an adrenergic stress: benefits of a medium-chain fatty acid. Am J Physiol Heart Circ Physiol. 2005;288:H1425–36. doi: 10.1152/ajpheart.00722.2004. [DOI] [PubMed] [Google Scholar]
- 45.Lewin TM, Coleman RA. Regulation of myocardial triacylglycerol synthesis and metabolism. Biochim Biophys Acta. 2003;1634:63–75. doi: 10.1016/j.bbalip.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 46.Russell LK, Finck BN, Kelly DP. Mouse models of mitochondrial dysfunction and heart failure. J Mol Cell Cardiol. 2005;38:81–91. doi: 10.1016/j.yjmcc.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 47.Sharma S, Adrogue JV, Golfman L, Uray I, Lemm J, Youker K, et al. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J. 2004;18:1692–700. doi: 10.1096/fj.04-2263com. [DOI] [PubMed] [Google Scholar]
- 48.Bueno OF, Wilkins BJ, Tymitz KM, Glascock BJ, Kimball TF, Lorenz JN, et al. Impaired cardiac hypertrophic response in Calcineurin Abeta-deficient mice. Proc Natl Acad Sci USA. 2002;99:4586–91. doi: 10.1073/pnas.072647999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Antos CL, McKinsey TA, Frey N, Kutschke W, McAnally J, Shelton JM, et al. Activated glycogen synthase-3 beta suppresses cardiac hypertrophy in vivo. Proc Natl Acad Sci USA. 2002;99:907–12. doi: 10.1073/pnas.231619298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khairallah RJ, Khairallah M, Gelinas R, Bouchard B, Young ME, Allen BG, et al. Cyclic GMP signaling in cardiomyocytes modulates fatty acid trafficking and prevents triglyceride accumulation. J Mol Cell Cardiol. 2008;45:230–9. doi: 10.1016/j.yjmcc.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Allard MF, Wambolt RB, Longnus SL, Grist M, Lydell CP, Parsons HL, et al. Hypertrophied rat hearts are less responsive to the metabolic and functional effects of insulin. Am J Physiol Endocrinol Metab. 2000;279:E487–93. doi: 10.1152/ajpendo.2000.279.3.E487. [DOI] [PubMed] [Google Scholar]
- 52.Nascimben L, Ingwall JS, Lorell BH, Pinz I, Schultz V, Tornheim K, et al. Mechanisms for increased glycolysis in the hypertrophied rat heart. Hypertension. 2004;44:662–7. doi: 10.1161/01.HYP.0000144292.69599.0c. [DOI] [PubMed] [Google Scholar]
- 53.Sambandam N, Lopaschuk GD, Brownsey RW, Allard MF. Energy metabolism in the hypertrophied heart. Heart Fail Rev. 2002;7:161–73. doi: 10.1023/a:1015380609464. [DOI] [PubMed] [Google Scholar]
- 54.Haemmerle G, Lass A, Zimmermann R, Gorkiewicz G, Meyer C, Rozman J, et al. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312:734–7. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- 55.Yamashita H, Bharadwaj KG, Ikeda S, Park TS, Goldberg IJ. Cardiac metabolic compensation to hypertension requires lipoprotein lipase. Am J Physiol Endocrinol Metab. 2008;295:E705–13. doi: 10.1152/ajpendo.90338.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khairallah M, Labarthe F, Bouchard B, Danialou G, Petrof BJ, Des Rosiers C. Profiling substrate fluxes in the isolated working mouse heart using 13C-labeled substrates: focusing on the origin and fate of pyruvate and citrate carbons. Am J Physiol Heart Circ Physiol. 2004;286:H1461–70. doi: 10.1152/ajpheart.00942.2003. [DOI] [PubMed] [Google Scholar]
- 57.Momose M, Iguchi N, Imamura K, Usui H, Ueda T, Miyamoto K, et al. Depressed myocardial fatty acid metabolism in patients with muscular dystrophy. Neuromuscul Disord. 2001;11:464–9. doi: 10.1016/s0960-8966(01)00191-2. [DOI] [PubMed] [Google Scholar]
- 58.Naruse H, Miyagi J, Arii T, Ohyanagi M, Iwasaki T, Jinnai K. The relationship between clinical stage, prognosis and myocardial damage in patients with Duchenne-type muscular dystrophy: five-year follow-up study. Ann Nucl Med. 2004;18:203–8. doi: 10.1007/BF02985001. [DOI] [PubMed] [Google Scholar]
- 59.Perloff JK, Henze E, Schelbert HR. Alterations in regional myocardial metabolism, perfusion, and wall motion in Duchenne muscular dystrophy studied by radionuclide imaging. Circulation. 1984;69:33–42. doi: 10.1161/01.cir.69.1.33. [DOI] [PubMed] [Google Scholar]
- 60.Quinlivan RM, Lewis P, Marsden P, Dundas R, Robb SA, Baker E, et al. Cardiac function, metabolism and perfusion in Duchenne and Becker muscular dystrophy. Neuromuscul Disord. 1996;6:237–46. doi: 10.1016/0960-8966(96)00007-7. [DOI] [PubMed] [Google Scholar]
- 61.Matsui T, Rosenzweig A. Convergent signal transduction pathways controlling cardiomyocyte survival and function: the role of PI 3-kinase and Akt. J Mol Cell Cardiol. 2005;38:63–71. doi: 10.1016/j.yjmcc.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 62.Young LH, Li J, Baron SJ, Russell RR. AMP-activated protein kinase: a key stress signaling pathway in the heart. Trends Cardiovasc Med. 2005;15:110–8. doi: 10.1016/j.tcm.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 63.Barger PM, Browning AC, Garner AN, Kelly DP. p38 mitogen-activated protein kinase activates peroxisome proliferator-activated receptor alpha: a potential role in the cardiac metabolic stress response. J Biol Chem. 2001;276:44495–501. doi: 10.1074/jbc.M105945200. [DOI] [PubMed] [Google Scholar]
- 64.Krumenacker JS, Hanafy KA, Murad F. Regulation of nitric oxide and soluble guanylyl cyclase. Brain Res Bull. 2004;62:505–15. doi: 10.1016/S0361-9230(03)00102-3. [DOI] [PubMed] [Google Scholar]
- 65.Nishikimi T, Maeda N, Matsuoka H. The role of natriuretic peptides in cardioprotection. Cardiovasc Res. 2006;69:318–28. doi: 10.1016/j.cardiores.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 66.Heydemann A, Huber JM, Kakkar R, Wheeler MT, McNally EM. Functional nitric oxide synthase mislocalization in cardiomyopathy. J Mol Cell Cardiol. 2004;36:213–23. doi: 10.1016/j.yjmcc.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 67.Wehling-Henricks M, Jordan MC, Roos KP, Deng B, Tidball JG. Cardiomyopathy in dystrophin-deficient hearts is prevented by expression of a neuronal nitric oxide synthase transgene in the myocardium. Hum Mol Genet. 2005;14:1921–33. doi: 10.1093/hmg/ddi197. [DOI] [PubMed] [Google Scholar]
- 68.Paulus WJ, Bronzwaer JG. Nitric oxide’s role in the heart: control of beating or breathing? Am J Physiol Heart Circ Physiol. 2004;287:H8–13. doi: 10.1152/ajpheart.01147.2003. [DOI] [PubMed] [Google Scholar]
- 69.Recchia FA. Role of nitric oxide in the regulation of substrate metabolism in heart failure. Heart Fail Rev. 2002;7:141–8. doi: 10.1023/a:1015324508556. [DOI] [PubMed] [Google Scholar]
- 70.Henning SL, Wambolt RB, Schonekess BO, Lopaschuk GD, Allard MF. Contribution of glycogen to aerobic myocardial glucose utilization. Circulation. 1996;93:1549–55. doi: 10.1161/01.cir.93.8.1549. [DOI] [PubMed] [Google Scholar]
- 71.Lewandowski ED. Metabolic heterogeneity of carbon substrate utilization in mammalian heart: NMR determinations of mitochondrial versus cytosolic compartmentation. Biochemistry. 1992;31:8916–23. doi: 10.1021/bi00152a031. [DOI] [PubMed] [Google Scholar]
- 72.Schonekess BO, Brindley PG, Lopaschuk GD. Calcium regulation of glycolysis, glucose oxidation, and fatty acid oxidation in the aerobic and ischemic heart. Can J Physiol Pharmacol. 1995;73:1632–40. doi: 10.1139/y95-725. [DOI] [PubMed] [Google Scholar]
- 73.Chen YW, Zhao P, Borup R, Hoffman EP. Expression profiling in the muscular dystrophies: identification of novel aspects of molecular pathophysiology. J Cell Biol. 2000;151:1321–36. doi: 10.1083/jcb.151.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Arany Z. PGC-1 coactivators and skeletal muscle adaptations in health and disease. Curr Opin Genet Dev. 2008;18:426–34. doi: 10.1016/j.gde.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Handschin C, Kobayashi YM, Chin S, Seale P, Campbell KP, Spiegelman BM. PGC-1alpha regulates the neuromuscular junction program and ameliorates Duchenne muscular dystrophy. Genes Dev. 2007;21:770–83. doi: 10.1101/gad.1525107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang W, ten Hove M, Schneider JE, Stuckey DJ, Sebag-Montefiore L, Bia BL, et al. Abnormal cardiac morphology, function and energy metabolism in the dystrophic mdx mouse: an MRI and MRS study. J Mol Cell Cardiol. 2008;45:754–60. doi: 10.1016/j.yjmcc.2008.09.125. [DOI] [PubMed] [Google Scholar]
- 77.Duchen MR. Contributions of mitochondria to animal physiology: from homeostatic sensor to calcium signalling and cell death. J Physiol. 1999 Apr 1;516(Pt 1):1–17. doi: 10.1111/j.1469-7793.1999.001aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ichas F, Jouaville LS, Mazat JP. Mitochondria are excitable organelles capable of generating and conveying electrical and calcium signals. Cell. 1997;89:1145–53. doi: 10.1016/s0092-8674(00)80301-3. [DOI] [PubMed] [Google Scholar]
- 79.Ichas F, Jouaville LS, Sidash SS, Mazat JP, Holmuhamedov EL. Mitochondrial calcium spiking: a transduction mechanism based on calcium-induced permeability transition involved in cell calcium signalling. FEBS Lett. 1994;348:211–5. doi: 10.1016/0014-5793(94)00615-6. [DOI] [PubMed] [Google Scholar]
- 80.Ichas F, Mazat JP. From calcium signaling to cell death: two conformations for the mitochondrial permeability transition pore. Switching from low-to high-conductance state. Biochim Biophys Acta. 1998;1366:33–50. doi: 10.1016/s0005-2728(98)00119-4. [DOI] [PubMed] [Google Scholar]
- 81.Cadenas E, Poderoso JJ, Antunes F, Boveris A. Analysis of the pathways of nitric oxide utilization in mitochondria. Free Radic Res. 2000;33:747–56. doi: 10.1080/10715760000301271. [DOI] [PubMed] [Google Scholar]
- 82.Lenaz G. The mitochondrial production of reactive oxygen species: mechanisms and implications in human pathology. IUBMB Life. 2001;52:159–64. doi: 10.1080/15216540152845957. [DOI] [PubMed] [Google Scholar]
- 83.Poderoso JJ, Boveris A, Cadenas E. Mitochondrial oxidative stress: a self-propagating process with implications for signaling cascades. Biofactors. 2000;11:43–5. doi: 10.1002/biof.5520110112. [DOI] [PubMed] [Google Scholar]
- 84.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–44. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Green DR. Apoptotic pathways: the roads to ruin. Cell. 1998;94:695–8. doi: 10.1016/s0092-8674(00)81728-6. [DOI] [PubMed] [Google Scholar]
- 86.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–9. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 87.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–12. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 88.Mattson MP, Kroemer G. Mitochondria in cell death: novel targets for neuroprotection and cardioprotection. Trends Mol Med. 2003;9:196–205. doi: 10.1016/s1471-4914(03)00046-7. [DOI] [PubMed] [Google Scholar]
- 89.Waterhouse NJ, Ricci JE, Green DR. And all of a sudden it’s over: mitochondrial outer-membrane permeabilization in apoptosis. Biochimie. 2002;84:113–21. doi: 10.1016/s0300-9084(02)01379-2. [DOI] [PubMed] [Google Scholar]
- 90.Zoratti M, Szabo I, De Marchi U. Mitochondrial permeability transitions: how many doors to the house? Biochim Biophys Acta. 2005;1706:40–52. doi: 10.1016/j.bbabio.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 91.Ozcan C, Bienengraeber M, Hodgson DM, Mann DL, Terzic A. Mitochondrial tolerance to stress impaired in failing heart. J Mol Cell Cardiol. 2003;35:1161–6. doi: 10.1016/s0022-2828(03)00204-9. [DOI] [PubMed] [Google Scholar]
- 92.Sabbah HN, Sharov V, Riddle JM, Kono T, Lesch M, Goldstein S. Mitochondrial abnormalities in myocardium of dogs with chronic heart failure. J Mol Cell Cardiol. 1992;24:1333–47. doi: 10.1016/0022-2828(92)93098-5. [DOI] [PubMed] [Google Scholar]
- 93.Buchwald A, Till H, Unterberg C, Oberschmidt R, Figulla HR, Wiegand V. Alterations of the mitochondrial respiratory chain in human dilated cardiomyopathy. Eur Heart J. 1990;11:509–16. doi: 10.1093/oxfordjournals.eurheartj.a059743. [DOI] [PubMed] [Google Scholar]
- 94.Ide T, Tsutsui H, Hayashidani S, Kang D, Suematsu N, Nakamura K, et al. Mitochondrial DNA damage and dysfunction associated with oxidative stress in failing hearts after myocardial infarction. Circ Res. 2001;88:529–35. doi: 10.1161/01.res.88.5.529. [DOI] [PubMed] [Google Scholar]
- 95.Ide T, Tsutsui H, Kinugawa S, Utsumi H, Kang D, Hattori N, et al. Mitochondrial electron transport complex I is a potential source of oxygen free radicals in the failing myocardium. Circ Res. 1999;85:357–63. doi: 10.1161/01.res.85.4.357. [DOI] [PubMed] [Google Scholar]
- 96.Jarreta D, Orus J, Barrientos A, Miro O, Roig E, Heras M, et al. Mitochondrial function in heart muscle from patients with idiopathic dilated cardiomyopathy. Cardiovasc Res. 2000;45:860–5. doi: 10.1016/s0008-6363(99)00388-0. [DOI] [PubMed] [Google Scholar]
- 97.Javadov S, Huang C, Kirshenbaum L, Karmazyn M. NHE-1 inhibition improves impaired mitochondrial permeability transition and respiratory function during postinfarction remodelling in the rat. J Mol Cell Cardiol. 2005;38:135–43. doi: 10.1016/j.yjmcc.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 98.Moe GW, Marin-Garcia J, Konig A, Goldenthal M, Lu X, Feng Q. In vivo TNF-alpha inhibition ameliorates cardiac mitochondrial dysfunction, oxidative stress, and apoptosis in experimental heart failure. Am J Physiol Heart Circ Physiol. 2004;287:H1813–20. doi: 10.1152/ajpheart.00036.2004. [DOI] [PubMed] [Google Scholar]
- 99.Scheubel RJ, Tostlebe M, Simm A, Rohrbach S, Prondzinsky R, Gellerich FN, et al. Dysfunction of mitochondrial respiratory chain complex I in human failing myocardium is not due to disturbed mitochondrial gene expression. J Am Coll Cardiol. 2002;40:2174–81. doi: 10.1016/s0735-1097(02)02600-1. [DOI] [PubMed] [Google Scholar]
- 100.Sharov VG, Todor AV, Silverman N, Goldstein S, Sabbah HN. Abnormal mitochondrial respiration in failed human myocardium. J Mol Cell Cardiol. 2000;32:2361–7. doi: 10.1006/jmcc.2000.1266. [DOI] [PubMed] [Google Scholar]
- 101.Sordahl LA, McCollum WB, Wood WG, Schwartz A. Mitochondria and sarcoplasmic reticulum function in cardiac hypertrophy and failure. Am J Physiol. 1973;224:497–502. doi: 10.1152/ajplegacy.1973.224.3.497. [DOI] [PubMed] [Google Scholar]
- 102.Guarnieri C, Muscari C, Caldarera CM. Oxygen radicals and tissue damage in heart hypertrophy. Adv Myocardiol. 1985;5:191–9. doi: 10.1007/978-1-4757-1287-2_15. [DOI] [PubMed] [Google Scholar]
- 103.Kobayashi A, Yamashita T, Kaneko M, Nishiyama T, Hayashi H, Yamazaki N. Effects of verapamil on experimental cardiomyopathy in the Bio 14. 6 Syrian hamster. J Am Coll Cardiol. 1987;10:1128–38. doi: 10.1016/s0735-1097(87)80356-x. [DOI] [PubMed] [Google Scholar]
- 104.Marin-Garcia J, Goldenthal MJ, Pierpont EM, Ananthakrishnan R, Perez-Atayde A. Is age a contributory factor of mitochondrial bioenergetic decline and DNA defects in idiopathic dilated cardiomyopathy? Cardiovasc Pathol. 1999;8:217–22. doi: 10.1016/s1054-8807(99)00010-1. [DOI] [PubMed] [Google Scholar]
- 105.Matsushima S, Ide T, Yamato M, Matsusaka H, Hattori F, Ikeuchi M, et al. Overexpression of mitochondrial peroxiredoxin-3 prevents left ventricular remodeling and failure after myocardial infarction in mice. Circulation. 2006;113:1779–86. doi: 10.1161/CIRCULATIONAHA.105.582239. [DOI] [PubMed] [Google Scholar]
- 106.Moe GW, Naik G, Konig A, Lu X, Feng Q. Early and persistent activation of myocardial apoptosis, bax and caspases: insights into mechanisms of progression of heart failure. Pathophysiology. 2002;8:183–92. doi: 10.1016/s0928-4680(02)00008-1. [DOI] [PubMed] [Google Scholar]
- 107.Olivetti G, Abbi R, Quaini F, Kajstura J, Cheng W, Nitahara JA, et al. Apoptosis in the failing human heart. N Engl J Med. 1997;336:1131–41. doi: 10.1056/NEJM199704173361603. [DOI] [PubMed] [Google Scholar]
- 108.Saraste A, Pulkki K, Kallajoki M, Heikkila P, Laine P, Mattila S, et al. Cardiomyocyte apoptosis and progression of heart failure to transplantation. Eur J Clin Invest. 1999;29:380–6. doi: 10.1046/j.1365-2362.1999.00481.x. [DOI] [PubMed] [Google Scholar]
- 109.Sharov VG, Todor A, Khanal S, Imai M, Sabbah HN. Cyclosporine A attenuates mitochondrial permeability transition and improves mitochondrial respiratory function in cardiomyocytes isolated from dogs with heart failure. J Mol Cell Cardiol. 2007;42:150–8. doi: 10.1016/j.yjmcc.2006.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Narula J, Pandey P, Arbustini E, Haider N, Narula N, Kolodgie FD, et al. Apoptosis in heart failure: release of cytochrome c from mitochondria and activation of caspase-3 in human cardiomyopathy. Proc Natl Acad Sci USA. 1999;96:8144–9. doi: 10.1073/pnas.96.14.8144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Scheubel RJ, Bartling B, Simm A, Silber RE, Drogaris K, Darmer D, et al. Apoptotic pathway activation from mitochondria and death receptors without caspase-3 cleavage in failing human myocardium: fragile balance of myocyte survival? J Am Coll Cardiol. 2002;39:481–8. doi: 10.1016/s0735-1097(01)01769-7. [DOI] [PubMed] [Google Scholar]
- 112.Crow MT, Mani K, Nam YJ, Kitsis RN. The mitochondrial death pathway and cardiac myocyte apoptosis. Circ Res. 2004;95:957–70. doi: 10.1161/01.RES.0000148632.35500.d9. [DOI] [PubMed] [Google Scholar]
- 113.Marcil M, Ascah A, Matas J, Belanger S, Deschepper CF, Burelle Y. Compensated volume overload increases the vulnerability of heart mitochondria without affecting their functions in the absence of stress. J Mol Cell Cardiol. 2006;41:998–1009. doi: 10.1016/j.yjmcc.2006.08.117. [DOI] [PubMed] [Google Scholar]
- 114.Matas J, Young NT, Bourcier-Lucas C, Ascah A, Marcil M, Deschepper CF, et al. Increased expression and intramitochondrial translocation of cyclophilin-D associates with increased vulnerability of the permeability transition pore to stress-induced opening during compensated ventricular hypertrophy. J Mol Cell Cardiol. 2009;46:420–30. doi: 10.1016/j.yjmcc.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 115.Kang PM, Yue P, Liu Z, Tarnavski O, Bodyak N, Izumo S. Alterations in apoptosis regulatory factors during hypertrophy and heart failure. Am J Physiol Heart Circ Physiol. 2004;287:H72–80. doi: 10.1152/ajpheart.00556.2003. [DOI] [PubMed] [Google Scholar]
- 116.Allard MF, Flint JD, English JC, Henning SL, Salamanca MC, Kamimura CT, et al. Calcium overload during reperfusion is accelerated in isolated hypertrophied rat hearts. J Mol Cell Cardiol. 1994;26:1551–63. doi: 10.1006/jmcc.1994.1175. [DOI] [PubMed] [Google Scholar]
- 117.Anderson PG, Allard MF, Thomas GD, Bishop SP, Digerness SB. Increased ischemic injury but decreased hypoxic injury in hypertrophied rat hearts. Circ Res. 1990;67:948–59. doi: 10.1161/01.res.67.4.948. [DOI] [PubMed] [Google Scholar]
- 118.Gaasch WH, Zile MR, Hoshino PK, Weinberg EO, Rhodes DR, Apstein CS. Tolerance of the hypertrophic heart to ischemia. Studies in compensated and failing dog hearts with pressure overload hypertrophy. Circulation. 1990;81:1644–53. doi: 10.1161/01.cir.81.5.1644. [DOI] [PubMed] [Google Scholar]
- 119.Ciminelli M, Ascah A, Bourduas K, Burelle Y. Short term training attenuates opening of the mitochondrial permeability transition pore without affecting myocardial function following ischemia–reperfusion. Mol Cell Biochem. 2006;291:39–47. doi: 10.1007/s11010-006-9192-9. [DOI] [PubMed] [Google Scholar]
- 120.Griffiths EJ, Halestrap AP. Mitochondrial non-specific pores remain closed during cardiac ischaemia, but open upon reperfusion. Biochem J. 1995;307:93–8. doi: 10.1042/bj3070093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Millay DP, Sargent MA, Osinska H, Baines CP, Barton ER, Vuagniaux G, et al. Genetic and pharmacologic inhibition of mitochondrial-dependent necrosis attenuates muscular dystrophy. Nat Med. 2008;14:442–7. doi: 10.1038/nm1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hicks D, Lampe AK, Laval SH, Allamand V, Jimenez-Mallebrera C, Walter MC, et al. Cyclosporine A treatment for Ullrich congenital muscular dystrophy: a cellular study of mitochondrial dysfunction and its rescue. Brain. 2009;132:147–55. doi: 10.1093/brain/awn289. [DOI] [PubMed] [Google Scholar]
- 123.Angelin A, Tiepolo T, Sabatelli P, Grumati P, Bergamin N, Golfieri C, et al. Mitochondrial dysfunction in the pathogenesis of Ullrich congenital muscular dystrophy and prospective therapy with cyclosporins. Proc Natl Acad Sci USA. 2007;104:991–6. doi: 10.1073/pnas.0610270104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Reutenauer J, Dorchies OM, Patthey-Vuadens O, Vuagniaux G, Ruegg UT. Investigation of Debio 025, a cyclophilin inhibitor, in the dystrophic mdx mouse, a model for Duchenne muscular dystrophy. Br J Pharmacol. 2008;155:574–84. doi: 10.1038/bjp.2008.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gnecchi-Ruscone T, Taylor J, Mercuri E, Paternostro G, Pogue R, Bushby K, et al. Cardiomyopathy in duchenne, becker, and sarcoglycanopathies: a role for coronary dysfunction? Muscle Nerve. 1999;22:1549–56. doi: 10.1002/(sici)1097-4598(199911)22:11<1549::aid-mus10>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 126.Mendell JR, Engel WK, Derrer EC. Duchenne muscular dystrophy: functional ischemia reproduces its characteristic lesions. Science. 1971;172:1143–5. doi: 10.1126/science.172.3988.1143. [DOI] [PubMed] [Google Scholar]
- 127.Zoratti M, Szabo I. The mitochondrial permeability transition. Biochim Biophys Acta. 1995;1241:139–76. doi: 10.1016/0304-4157(95)00003-a. [DOI] [PubMed] [Google Scholar]
- 128.Gailly P. New aspects of calcium signaling in skeletal muscle cells: implications in Duchenne muscular dystrophy. Biochim Biophys Acta. 2002;1600:38–44. doi: 10.1016/s1570-9639(02)00442-9. [DOI] [PubMed] [Google Scholar]
- 129.Williams IA, Allen DG. Intracellular calcium handling in ventricular myocytes from mdx mice. Am J Physiol Heart Circ Physiol. 2007;292:H846–55. doi: 10.1152/ajpheart.00688.2006. [DOI] [PubMed] [Google Scholar]
- 130.Huang J, van Breemen C, Kuo KH, Hove-Madsen L, Tibbits GF. Store-operated Ca2+ entry modulates sarcoplasmic reticulum Ca2+ loading in neonatal rabbit cardiac ventricular myocytes. Am J Physiol Cell Physiol. 2006;290:C1572–82. doi: 10.1152/ajpcell.00226.2005. [DOI] [PubMed] [Google Scholar]
- 131.Robert V, Massimino ML, Tosello V, Marsault R, Cantini M, Sorrentino V, et al. Alteration in calcium handling at the subcellular level in mdx myotubes. J Biol Chem. 2001;276:4647–51. doi: 10.1074/jbc.M006337200. [DOI] [PubMed] [Google Scholar]
- 132.Vandebrouck A, Ducret T, Basset O, Sebille S, Raymond G, Ruegg U, et al. Regulation of store-operated calcium entries and mitochondrial uptake by minidystrophin expression in cultured myotubes. FASEB J. 2006;20:136–8. doi: 10.1096/fj.04-3633fje. [DOI] [PubMed] [Google Scholar]
- 133.Bellinger AM, Reiken S, Carlson C, Mongillo M, Liu X, Rothman L, et al. Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle. Nat Med. 2009;15:325–30. doi: 10.1038/nm.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rizzuto R, Brini M, Murgia M, Pozzan T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science. 1993;262:744–7. doi: 10.1126/science.8235595. [DOI] [PubMed] [Google Scholar]
- 135.Robert V, Gurlini P, Tosello V, Nagai T, Miyawaki A, Di Lisa F, et al. Beat-to-beat oscillations of mitochondrial [Ca2+] in cardiac cells. EMBO J. 2001;20:4998–5007. doi: 10.1093/emboj/20.17.4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gilabert JA, Parekh AB. Respiring mitochondria determine the pattern of activation and inactivation of the store-operated Ca2+ current I(CRAC) EMBO J. 2000;19:6401–7. doi: 10.1093/emboj/19.23.6401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dudley RW, Khairallah M, Mohammed S, Lands L, Des Rosiers C, Petrof BJ. Dynamic responses of the glutathione system to acute oxidative stress in dystrophic mouse (mdx) muscles. Am J Physiol Regul Integr Comp Physiol. 2006;291:R704–10. doi: 10.1152/ajpregu.00031.2006. [DOI] [PubMed] [Google Scholar]
- 138.Kuznetsov AV, Winkler K, Wiedemann FR, von Bossanyi P, Dietzmann K, Kunz WS. Impaired mitochondrial oxidative phosphorylation in skeletal muscle of the dystrophin-deficient mdx mouse. Mol Cell Biochem. 1998;183:87–96. doi: 10.1023/a:1006868130002. [DOI] [PubMed] [Google Scholar]
- 139.Fisher PW, Salloum F, Das A, Hyder H, Kukreja RC. Phosphodiesterase-5 inhibition with sildenafil attenuates cardiomyocyte apoptosis and left ventricular dysfunction in a chronic model of doxorubicin cardiotoxicity. Circulation. 2005;111:1601–10. doi: 10.1161/01.CIR.0000160359.49478.C2. [DOI] [PubMed] [Google Scholar]
- 140.Ockaili R, Salloum F, Hawkins J, Kukreja RC. Sildenafil (Viagra) induces powerful cardioprotective effect via opening of mitochondrial K(ATP) channels in rabbits. Am J Physiol Heart Circ Physiol. 2002;283:H1263–9. doi: 10.1152/ajpheart.00324.2002. [DOI] [PubMed] [Google Scholar]
- 141.Salloum F, Yin C, Xi L, Kukreja RC. Sildenafil induces delayed preconditioning through inducible nitric oxide synthase-dependent pathway in mouse heart. Circ Res. 2003;92:595–7. doi: 10.1161/01.RES.0000066853.09821.98. [DOI] [PubMed] [Google Scholar]
- 142.Costa AD, Garlid KD, West IC, Lincoln TM, Downey JM, Cohen MV, et al. Protein kinase G transmits the cardioprotective signal from cytosol to mitochondria. Circ Res. 2005;97:329–36. doi: 10.1161/01.RES.0000178451.08719.5b. [DOI] [PubMed] [Google Scholar]
- 143.Costa AD, Quinlan CL, Andrukhiv A, West IC, Jaburek M, Garlid KD. The direct physiological effects of mitoK(ATP) opening on heart mitochondria. Am J Physiol Heart Circ Physiol. 2006;290:H406–15. doi: 10.1152/ajpheart.00794.2005. [DOI] [PubMed] [Google Scholar]
- 144.Das A, Salloum FN, Xi L, Rao YJ, Kukreja RC. ERK phosphorylation mediates sildenafil-induced myocardial protection against ischemia–reperfusion injury in mice. Am J Physiol Heart Circ Physiol. 2009;296:H1236–43. doi: 10.1152/ajpheart.00100.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mendelsohn ME. Viagra: now mending hearts. Nat Med. 2005;11:115–6. doi: 10.1038/nm0205-115. [DOI] [PubMed] [Google Scholar]
- 146.Schlossmann J, Feil R, Hofmann F. Signaling through NO and cGMP-dependent protein kinases. Ann Med. 2003;35:21–7. doi: 10.1080/07853890310004093. [DOI] [PubMed] [Google Scholar]
- 147.Yue Y, Li Z, Harper SQ, Davisson RL, Chamberlain JS, Duan D. Microdystrophin gene therapy of cardiomyopathy restores dystrophin-glycoprotein complex and improves sarcolemma integrity in the mdx mouse heart. Circulation. 2003;108:1626–32. doi: 10.1161/01.CIR.0000089371.11664.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Perkins KJ, Davies KE. The role of utrophin in the potential therapy of Duchenne muscular dystrophy. Neuromuscul Disord. 2002;12(Suppl 1):S78–89. doi: 10.1016/s0960-8966(02)00087-1. [DOI] [PubMed] [Google Scholar]
- 149.Deconinck AE, Rafael JA, Skinner JA, Brown SC, Potter AC, Metzinger L, et al. Utrophin-dystrophin-deficient mice as a model for Duchenne muscular dystrophy. Cell. 1997;90:717–27. doi: 10.1016/s0092-8674(00)80532-2. [DOI] [PubMed] [Google Scholar]
- 150.Janssen PM, Hiranandani N, Mays TA, Rafael-Fortney JA. Utrophin deficiency worsens cardiac contractile dysfunction present in dystrophin-deficient mdx mice. Am J Physiol Heart Circ Physiol. 2005;289:H2373–8. doi: 10.1152/ajpheart.00448.2005. [DOI] [PubMed] [Google Scholar]
- 151.McNally EM. New approaches in the therapy of cardiomyopathy in muscular dystrophy. Annu Rev Med. 2007;58:75–88. doi: 10.1146/annurev.med.58.011706.144703. [DOI] [PubMed] [Google Scholar]
- 152.Jung C, Martins AS, Niggli E, Shirokova N. Dystrophic cardiomyopathy: amplification of cellular damage by Ca2+signalling and reactive oxygen species-generating pathways. Cardiovasc Res. 2008 Mar 1;77:766–73. doi: 10.1093/cvr/cvm089. [DOI] [PubMed] [Google Scholar]
- 153.Connern CP, Halestrap AP. Recruitment of mitochondrial cyclophilin to the mitochondrial inner membrane under conditions of oxidative stress that enhance the opening of a calcium-sensitive non-specific channel. Biochem J. 1994;302:321–4. doi: 10.1042/bj3020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Connern CP, Halestrap AP. Chaotropic agents and increased matrix volume enhance binding of mitochondrial cyclophilin to the inner mitochondrial membrane and sensitize the mitochondrial permeability transition to [Ca2+] Biochemistry. 1996;35:8172–80. doi: 10.1021/bi9525177. [DOI] [PubMed] [Google Scholar]
- 155.Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999;341:233–49. [PMC free article] [PubMed] [Google Scholar]
- 156.Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion-a target for cardioprotection. Cardiovasc Res. 2004;61:372–85. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- 157.Morioka M, Hamada J, Ushio Y, Miyamoto E. Potential role of calcineurin for brain ischemia and traumatic injury. Prog Neurobiol. 1999;58:1–30. doi: 10.1016/s0301-0082(98)00073-2. [DOI] [PubMed] [Google Scholar]
- 158.Nishinaka Y, Sugiyama S, Yokota M, Saito H, Ozawa T. Protective effect of FK506 on ischemia/reperfusion-induced myocardial damage in canine heart. J Cardiovasc Pharmacol. 1993;21:448–54. doi: 10.1097/00005344-199303000-00015. [DOI] [PubMed] [Google Scholar]
- 159.Ruiz F, Alvarez G, Ramos M, Hernandez M, Bogonez E, Satrustegui J. Cyclosporin A targets involved in protection against glutamate excitotoxicity. Eur J Pharmacol. 2000;404:29–39. doi: 10.1016/s0014-2999(00)00584-7. [DOI] [PubMed] [Google Scholar]
- 160.Backman E, Nylander E, Johansson I, Henriksson KG, Tagesson C. Selenium and vitamin E treatment of Duchenne muscular dystrophy: no effect on muscle function. Acta Neurol Scand. 1988;78:429–35. doi: 10.1111/j.1600-0404.1988.tb03681.x. [DOI] [PubMed] [Google Scholar]
- 161.Tidball JG, Wehling-Henricks M. The role of free radicals in the pathophysiology of muscular dystrophy. J Appl Physiol. 2007;102:1677–86. doi: 10.1152/japplphysiol.01145.2006. [DOI] [PubMed] [Google Scholar]
- 162.Buyse GM, Van der Mieren G, Erb M, D’Hooge J, Herijgers P, Verbeken E, et al. Long-term blinded placebo-controlled study of SNT-MC17/idebenone in the dystrophin deficient mdx mouse: cardiac protection and improved exercise performance. Eur Heart J. 2009;30:116–24. doi: 10.1093/eurheartj/ehn406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Adlam VJ, Harrison JC, Porteous CM, James AM, Smith RA, Murphy MP, et al. Targeting an antioxidant to mitochondria decreases cardiac ischemia–reperfusion injury. FASEB J. 2005;19(9):1088–95. doi: 10.1096/fj.05-3718com. [DOI] [PubMed] [Google Scholar]
- 164.Jauslin ML, Meier T, Smith RA, Murphy MP. Mitochondria-targeted antioxidants protect Friedreich Ataxia fibroblasts from endogenous oxidative stress more effectively than untargeted antioxidants. FASEB J. 2003;17:1972–4. doi: 10.1096/fj.03-0240fje. [DOI] [PubMed] [Google Scholar]
- 165.Parchen MC, Percival JM, Dao-Fu D, Gray HN, Frohner SC, Beavo JA. Sildenafil Ameliorates Cardiomyopathy in mdx Mice. FASEB J. 2009;23 (abstract #823.1) [Google Scholar]
- 166.Asai A, Sahani N, Kaneki M, Ouchi Y, Martyn JA, Yasuhara SE. Primary role of functional ischemia, quantitative evidence for the two-hit mechanism, and phosphodiesterase-5 inhibitor therapy in mouse muscular dystrophy. PLoS ONE. 2007;e806:2. doi: 10.1371/journal.pone.0000806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kyoi S, Otani H, Hamano A, Matsuhisa S, Akita Y, Fujiwara H, et al. Dystrophin is a possible end-target of ischemic preconditioning against cardiomyocyte oncosis during the early phase of reperfusion. Cardiovasc Res. 2006;70:354–63. doi: 10.1016/j.cardiores.2006.01.004. [DOI] [PubMed] [Google Scholar]