Abstract
Background/Objectives
Colonic-fermentation of dietary-fibre to short-chain fatty-acids (SCFA) may protect against obesity and diabetes, but excess production of colonic SCFA has been implicated in the promotion of obesity. We aimed to compare the effects of two fermentable-fibres on postprandial SCFA and second-meal glycaemic response in healthy overweight or obese (OWO) vs lean (LN) participants.
Methods
using a randomized cross-over design, 13 OWO and 12 LN overnight fasted participants were studied for 6h on 3 separate days after consuming 300mL water containing 75g glucose(GLU) as control or with 24g inulin(IN) or 28g resistant-starch(RS). A standard lunch was served 4h after the test-drink.
Results
Within the entire group, compared to control, IN significantly increased serum SCFA (p<0.001) but had no effect on FFA or second-meal glucose and insulin responses. In contrast, RS had no significant effect on SCFA but reduced FFA rebound (p<0.001) and second-meal glucose (p=0.002) and insulin responses (p=0.024). OWO had similar postprandial serum SCFA and glucose concentrations but significantly greater insulin and FFA than LN. However, the effects of IN and RS on SCFA, glucose, insulin and FFA responses were similar in LN and OWO.
Conclusions
Resistant-starch has favorable second-meal effects, likely related to changes in FFA rather than SCFA concentrations. However, a longer study may be needed to demonstrate an effect of RS on SCFA. We found no evidence that acute increases in SCFA after IN reduce glycaemic responses in humans, and we were unable to detect a significant difference in SCFA responses between OWO vs LN subjects.
Keywords: sort-chain fatty acid, fermentation, glucose, FFA, obese, microbiota
Introduction
High intake of dietary fibre is associated with reduced risk for type 2 diabetes mellitus (T2DM) and has been shown to improve glycaemic control in T2DM (1). These effects of fibre have been suggested to be due, at least in part, to its fermentation by colonic bacteria into the short-chain fatty acids (SCFA) acetate, propionate and butyrate, which are readily absorbed from the colonic lumen.
SCFA may improve glucose control by reducing serum FFA; High FFA concentrations induce peripheral and hepatic insulin resistance (2,3), and contribute to oxidative stress and thus deteriorate pancreatic β-cell function as well as insulin sensitivity (4). Reductions of FFA have been demonstrated in animals after acetate injections (5,6) and in humans after oral SCFA ingestion (7), rectal SCFA infusion (8) and dietary fibre consumption (9,10). These effects were often, but not always, associated with reduced glycaemic responses.
Different dietary fibres are fermented into different types and amounts of SCFA (11,12), therefore, may affect glycaemic responses differently. Two fermentable dietary-fibres of particular interest in this respect are resistant-starch (RS) and inulin (IN). Resistant-starch is an insoluble type of cereal fibre while IN is a soluble fibre that can be found in many types of plant foods. Resistant-starch has been shown to increase insulin sensitivity in short- and long-term consumption studies (13–16) however, the mechanism is not clear. Inulin has been shown to improve glycaemic control in animals (17–20), however, the results human studies have been equivocal (10,21,22).
The amount of SCFA produced in the colon also depends on the composition of the colonic microbiota. Obese mice harbour an increased abundance of Firmicutes and decreased Bacteroidetes than lean mice (23); it has been suggested that such a microbiota produces SCFA (energy) more efficiently, and may therefore contribute to weight gain (24). Human studies are limited but tend to show that faecal SCFA concentrations were significantly higher in obese than lean participants (25–28). Numerous studies have compared the faecal abundance of Firmicutes and Bacteroidetes in lean vs obese subjects but no consistent findings have emerged (29–31).
However, if obese humans produce SCFA more efficiently than lean humans, then excess SCFA production may contribute to weight gain and an increased risk for T2DM. On the other hand, excess SCFA production may lower FFA concentrations and improve glucose control, and therefore decrease risk for T2DM. Thus, our objective was to compare the effects of adding RS or IN to glucose tolerance test (75g glucose) on acute postprandial responses of SCFA, glucose, insulin and FFA in overweight vs. lean participants. We hypothesized that, in overweight relative to lean subjects, RS and IN would elicit higher postprandial SCFA responses and have less effect in reducing serum FFA and second-mealglucose and insulin responses.
Methods
Participants
Male and non-pregnant, non-lactating females aged 18–65 years with body mass index (BMI) ≥20 and ≤353kg/m2 were recruited from a pool of participants previously involved in similar studies. Participants were excluded for any of the following reasons: presence of diabetes, cardiovascular, bowel, kidney or liver disease; use of medications which affect blood glucose or insulin sensitivity (such as diuretics); any use of antibiotics, laxatives, pre/probiotics or other drugs known to influence gastrointestinal function in the 3 months before the study; smoking; following any unusual dietary practices (such as weight loss diet, Atkins diet, vegan diet); abnormal plasma blood glucose (≥7.0 mmol/L); or anemia. Eligible participants were then divided prospectively into two groups based on their BMI; 12 participants in the LN group (BMI<25) and 13 participants in the OWO group (BMI≥25). All tests were conducted at the Glycemic Index Laboratories, Toronto. Ethical approval for the study was obtained from the Research Ethics Board, University of Toronto. Participants gave written informed consent to participate in the study.
Phase 1
Participants completed questionnaires related to demographics, medical history, drug use, bowel habit and physical activity (32,33). They were given instructions on how to record their dietary intake and asked to keep a 3-day diet record. Participants were also given a faecal collection kit which consisted of the Fisher brand commode specimen collection system (Fisher Scientific, Ottawa, ON) and plastic bags. On the third day of the diet record or the day after, participants collected a faecal sample. The completed 3-day diet record and the plastic bag containing the faecal sample was immediately placed on dry ice, and brought to the lab within 24h of being collected. The frozen faecal samples were stored at −20°C until they were processed.
Phase 2
A week after completing phase 1, participants began phase 2; a cross-over randomized controlled-trial, in which participants came to the laboratory on 3 separate occasions, separated by a 1-week washout. On each study day, subjects arrived at 8:00am after fasting for 12h; after warming their forearms with a heating pad for 2–3min, a cannula was inserted into a forearm vein and kept clear with periodic saline flushes. After a fasting blood sample was drawn, participants consumed a test drink within 5min, and further blood samples were drawn at 0.5,1,1.5,2,3 and 4h after the start of the test drink. Immediately after the 4-h blood sample, a standard lunch was provided. Participants ate the lunch within 15min, and further blood samples were drawn at 4.5,5,5.5, and 6h. Participants remained seated and awake for the duration of the study. Breath samples were collected at hourly intervals for 6h for breath hydrogen and methane.
Test drinks
Each subject consumed all 3 treatments in random order. The sequence of the test meals were randomly assigned to the subjects by the study coordinator by using Random Integer Subject Generator (http://www.random.org). The test drinks consisted of 75g glucose (GLU) (Glucose; Grain Process Enterprises Ltd, Scarborough, ON, Canada) or 75g glucose +24g Oliggo-Fiber Instant Inulin (Inulin; 90% dietary fibre, 10% free fructose, glucose and saccharose; Inulin; Cargill Inc., Wayzata, MN, USA) or 75g glucose +28g resistant starch (RS) (Nutriose® FM06, Roquette, France; total fibre content of 85%; 24g RS and 4.2g rapidly digestible starch;) dissolved in 300mL water on the morning of the study. The inulin dose of 24g was based on a previous study from our lab (10) and the dose of 28g RS was chosen so that the amount of fibre it contained equaled the dose of inulin.
Standard lunch
The standard lunch consisted of a cheese and tomato sandwich, a drink of apple juice (200mL), a bottle of water (500mL), and two chocolate cookies (for more details see supplementary information).
Biochemistry
The faecal sample was weighed and homogenized in a 400 series masticator (IUL Instruments, S.A., Barcelona, Spain). Aliquots of faeces were then transferred to individual vials for determination of pH and SCFA. Faecal pH was measured using a pH meter, and faecal SCFA were analyzed by gas chromatography as previously described (34). DNA extraction and Ion Torrent V6 16S-rRNA gene sequencing were performed as described elsewhere (35). Compositional analysis of the data using Principle Component Biplots was done as described (36).
Blood for glucose, insulin, C-peptide and FFA was drawn into tubes containing spray-coated silica and a polymer gel for serum separation (BD Canada Inc., Oakville, ON). Blood for SCFA was drawn into serum tube with no additive and an uncoated interior (BD Canada Inc., Oakville, ON, Canada). Serum glucose was measured by a glucose oxidase method (YSI 2300 STAT Plus™, YSI Life Sciences Inc., Yellow Springs, OH, USA) (inter-assay CV <2%), insulin and C-peptide using the ELISA immunoassay (80-INSHU-E01.1 and 80-CPTHU-E01.1, E10, Alpco Diagnostic, Salem, NH) (Insulin: Intra- and inter-assay CVs <10% and <17% respectively. Analytical sensitivity is 0.399μlU/ml. And C-peptide: intra- and inter-assay CVs <5% and <9%, respectively; analytical sensitivity 2.95pmol/L). Serum FFA was measure using enzymatic technique that used acyl-CoA oxidase (Wako NEFA-HR(2), Wako Chemicals USA, Inc., Richmond, VA, USA). All blood samples were allowed to clot at room temperature for 30min, centrifuged at 3000rpm for 15min at 4°C, and the serum aliquoted and stored at −70°C before analysis. Serum SCFA were measured by gas chromatography after microfiltration and vacuum distillation as previously described (37).
Breath samples of methane and hydrogen were measured by gas chromatography (Quintron Microlyzer, Model SC, Milwaukee, WI, USA) as previously described (37).
Power Analysis – see supplementary information.
Statistical analysis
The primary results are reported as means ± standard error of mean (SEM). For glucose, insulin and c-peptide, incremental areas under the curve (iAUC; subtracting area below the baseline) over 0–2h and 2–4h were calculated using the fasting concentration as the baseline. For FFA, iAUC from the nadir to 4h (iAUCmin4) was calculated using the minimum concentration achieved over the first 4h as the baseline. For glucose, insulin, c-peptide and FFA, total areas under the curve (tAUC) were calculated over the 4–6h periods using the trapezoidal method. For SCFA, tAUC were calculated over the 0–4 and 4–6h periods, and iAUC from the nadir to 6h (iAUCmin6) were calculated using the minimum concentration achieved over the first 4h as the baseline. All AUCs were calculated using a computer spreadsheet (Microsoft Office Excel 2007).
To examine for the main effects of groups and treatments, and their interactions, the mixed-effects (random-effects) model was used to account for the within-participant correlation, which was introduced by the cross-over design.
To investigate a possible relationship between serum FFA and SCFA and second-meal glucose responses, the difference in response between IN and Control and between RS and Control were calculated, and the differences in FFA and SCFA were correlated with the differences in glucose tAUC from 4–6h. Two-group t-tests (two sided) were performed to analyze differences in baseline data between the groups. Differences were considered statistically significant if p < =0.05. Statistical analyses were conducted using IBM SPSS Statistics version 22 (SPSS Inc., Chicago, IL, USA) and Stata 13.0 (College Station, TX, USA).
Results
Lean participants were significantly younger than OWO but LN and OWO did not differ significantly with respect to sex and ethnicity (Table 1). Concentrations of faecal SCFA (Supplementary Table 1) and fasting serum SCFA (Table 1) were not significantly different between the groups. However, after age adjustment, faecal propionate was significantly higher in the LN group compared to the OWO group (Supplementary Table 1). Faecal bacterial profiles at the level of phyla, revealed no differences in the relative abundance of the Firmicutes and Bacteroidetes or in their ratio between the LN and the OWO group (Supplementary Figure 1,2). OWO had significantly higher fasting insulin, total- and low-density lipoprotein-cholesterol and triglycerides than LN, but similar glucose, aspartate transaminase, C-reactive protein and high-density lipoprotein-cholesterol concentrations.
Table 1.
Characteristics of study participants at screening
| Whole group (n=25) | Lean (n=12) | Overweight/obese (n=13) | P value* | |
|---|---|---|---|---|
| Body Mass Index (kg/m2) | 27.5 ± 1.0 | 23.2 ± 0.4 | 31.5 ± 1.0 | <0.001 |
| Age (y) | 39.8 ± 2.8 | 33.4 ± 3.7 | 45.8 ± 3.5 | 0.025 |
| Ethnicity A:C:O (n)** | 5:16:4 | 2:9:1 | 3:7:3 | 0.494 |
| Sex (M:F) (n) | 12:13 | 7:5 | 5:8 | 0.320 |
| Blood Samples | ||||
| Acetate (μmol/L) | 36 ± 4 | 42 ± 6 | 32 ± 5 | 0.230 |
| Propionate (μmol/L) | 1.1 ± 0.5 | 1.2 ± 0.2 | 1.0 ± 0.1 | 0.347 |
| Butyrate (μmol/L) | 0.40 ± 0.04 | 0.39 ± 0.04 | 0.41 ± 0.06 | 0.756 |
| Fasting Glucose (mmol/L) | 4.87 ± 0.84 | 4.76 ± 0.12 | 4.98 ± 0.11 | 0.202 |
| Fasting Insulin (pmol/L) | 43.1 ± 5.0 | 25.7 ± 3.0 | 57.77 ± 6.5 | <0.001 |
| Hematocrit | 0.40 ± 0.01 | 0.39 ± 0.01 | 0.40 ± 0.01 | 0.710 |
| AST (U/L) | 22.4 ± 1.4 | 21.7 ± 1.44 | 23.0 ± 2.31 | 0.657 |
| CRP (mg/L) | 3.3 ± 1.5 | 0.7 ± 0.2 | 5.7 ± 2.8 | 0.098 |
| Total cholesterol (mmol/L) | 4.70 ± 0.22 | 4.23 ± 0.19 | 5.11 ± 0.35 | 0.046 |
| Triglycerides (mmol/L) | 0.94 ± 0.10 | 0.68 ± 0.08 | 1.18 ± 0.15 | 0.009 |
| HDL cholesterol (mmol/L) | 1.47 ± 0.05 | 1.53 ± 0.09 | 1.41 ± 0.06 | 0.300 |
| LDL cholesterol (mmol/L) | 2.85 ± 0.18 | 2.46 ± 0.16 | 3.19 ± 0.28 | 0.038 |
Values are means ±SEM except for Ethnicity and sex which are number of subjects.
Significance of difference between Lean and Overweight/obese groups;
A=Asian; C=Caucasian; O=Other
Lean had lower mean intakes of fat(g), protein(g), CHO(g) sugars(g) and total calories than the OWO group, but the differences were not significant (Supplementary Table 2).
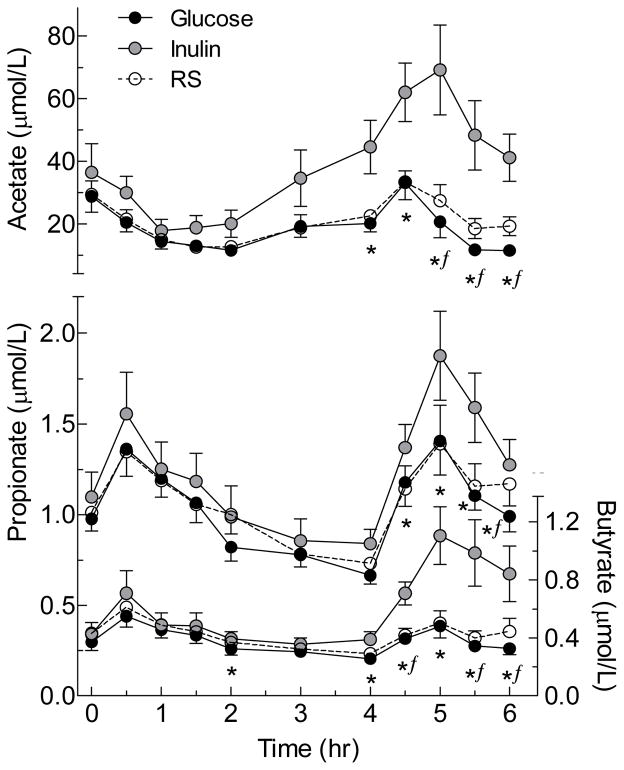
Serum acetate, propionate and butyrate concentrations began to increase 2 to 4h after IN and were significantly higher than GLU from 4 to 6h (Figure 1); thus, both the incremental AUC and the total AUC of all 3 SCFA after IN were significantly greater than those after GLU (Table 2). By contrast, it took 5–6h for mean serum SCFA concentrations after RS to begin to exceed those after GLU; by 6h after RS the concentrations of all 3 SCFA were significantly greater those after GLU by paired t-test when uncorrected for multiple comparisons (Figure 1), but the differences were small and there were no significant differences between RS and GLU in incremental or total AUC (Table 2). There were no significant differences in serum SCFA responses between LN and OWO subjects (Table 4).
FIGURE 1. Serum short-chain fatty acid responses elicited by the test meals.
Values are means±SEM for n=25 subjects.
* Significant difference between Inulin and Glucose by related samples Wilcoxon signed rank test (p<0.05).
ƒSignificant difference between resistant starch and glucose by related samples Wilcoxon signed rank test (p<0.05).
Table 2.
Serum short chain fatty acid responses elicited by the test meals
| Variable | Time after treatment (h) | Glucose | Treatment | |
|---|---|---|---|---|
| Inulin | Resistant Starch | |||
| Acetate (μmol×h/L) | tAUC 0–4 h | 79.7 ± 7.6 | 108.4 ± 12.4a | 80.4 ± 8.1 |
| tAUC 4–6 h | 41.9 ± 3.8 | 102 ± 11.9b | 52.9 ± 4.2 | |
| iAUC min-6 h | 24.4 ± 2.8 | 94.5 ± 13.1b | 36.1 ± 3.8 | |
|
| ||||
| Propionate (μmol×h/L) | tAUC 0–4 h | 3.98 ± 0.21 | 4.25 ± 0.29 | 4.05 ± 0.27 |
| tAUC 4–6 h | 2.26 ± 0.11 | 3.04 ± 0.24b | 2.48 ± 0.17 | |
| iAUC min-6 h | 1.01 ± 0.09 | 1.71 ± 0.20b | 1.09 ± 0.13 | |
|
| ||||
| Butyrate (μmol×h/L) | tAUC 0–4 h | 1.4 ± 0.1 | 1.67 ± 0.14a | 1.49 ± 0.14 |
| tAUC 4–6 h | 0.71 ± 0.05 | 1.51 ± 0.16b | 0.83 ± 0.06 | |
| iAUC min-6 h | 0.27 ± 0.03 | 1.11 ± 0.15b | 0.40 ± 0.05 | |
Values are mean±SEM for n=25; iAUC, incremental area under the curve; tAUC, total area under the curve; min6, measured from nadir to 6h.
Significantly different from Glucose (p<0.02).
Significantly different from Glucose (p<0.001).
Table 4.
The mean serum SCFA, glucose, insulin, C-peptide and free-fatty acid (FFA) responses elicited by the test meals of the 3 visits in lean vs. overweight and obese participants
| Variable | Time | Lean (n=12) | Overweight and obese (n=13) | P value* |
|---|---|---|---|---|
| Acetate (μmol×h/L) | iAUC min6 | 46.2 + 6.1 | 56.7 + 9 | 0.319 |
| tAUC 0–6h | 158.2 + 14 | 152.2 + 19.8 | 0.799 | |
|
| ||||
| Propionate (μmol×h/L) | iAUC min6 | 1.3 + 0.2 | 1.2 + 0.2 | 0.386 |
| tAUC 0–6h | 6.9 + 0.5 | 6.5 + 0.5 | 0.254 | |
|
| ||||
| Butyrate (μmol×h/L) | iAUC min6 | 0.5 + 0.1 | 0.6 + 0.1 | 0.78 |
| tAUC 0–6h | 2.3 + 0.1 | 2.7 + 0.3 | 0.617 | |
|
| ||||
| Glucose (mmol×h/L) | iAUC 0–2h | 3.3 ± 0.5 | 4.8 ± 0.8 | 0.076 |
| iAUC 2–4h | −1.1 ± 0.2 | −0.7 ± 0.4 | 0.663 | |
| tAUC 4–6h | 11.7 ± 0.4 | 11.0 ± 0.3 | 0.208 | |
|
| ||||
| FFA (mEq×h/L) | tAUC 0–4h | 0.97 ± 0.11 | 1.07 ± 0.08 | 0.425 |
| tAUC 4–6h | 0.45 ± 0.03 | 0.57 ± 0.04 | 0.038 | |
| iAUC min4 | 0.19 ± 0.05 | 0.17 ± 0.03 | 0.699 | |
|
| ||||
| Insulin (pmol×h/L) | iAUC 0–2h | 65.5 ± 738 | 152.8 ± 30.6 | 0.005 |
| iAUC 2–4h | 16.1 ± 3.1 | 43.5 ± 16.5 | 0.166 | |
| tAUC 4–6h | 49.5 ± 5.3 | 96.2 ± 14.4 | 0.002 | |
|
| ||||
| C-peptide (pmol×h/L) | iAUC 0–2h | 2139 ± 138 | 3250 ± 324 | 0.001 |
| iAUC 2–4h | 698 ± 108.7 | 1410 ± 304.6 | 0.136 | |
| tAUC 4–6h | 2030 ± 133.5 | 3056 ± 291 | 0.001 | |
Values are mean ± SEM;
, P value for the difference between the lean group to the overweight and obese group; FFA, free fatty acids; iAUC, incremental area under the curve; min4, measured from nadir to 4h; tAUC, total area under the curve
Breath hydrogen 2h after IN was significantly greater than after GLU and increased rapidly to reach a peak concentration at 5h which was about 60ppm greater than that after GLU. Breath hydrogen after RS increased gradually to became significantly greater than GLU at 4h, reaching a peak concentration at 5h of approximately 10ppm (Supplementary Figure 3). There was no difference in breath hydrogen responses between LN and OWO subjects (not shown). There were 8 methane producers, and their mean breath methane concentration tended to fall throughout the day with no difference among treatments.
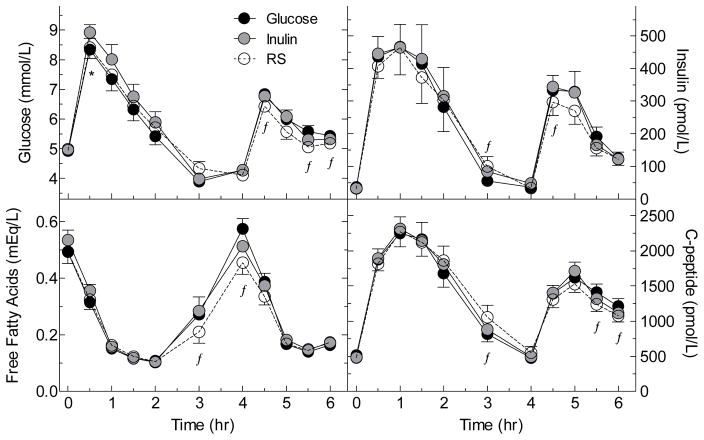
Glucose iAUC from 0–2h was significantly greater after IN than GLU but the difference between RS and GLU not significant. However, after the standard lunch, tAUC 4–6h was significantly less after RS than GLU but there was no difference between IN and GLU (Figure 2, Table 3). There were no significant differences in glycaemic response between the LN and OWO groups (Table 4).
FIGURE 2. Serum glucose, insulin, c-peptide and FFA responses elicited by the test meals.
Values are means±SEM in n=25 subjects.
* Significant difference between Inulin and Glucose by related samples Wilcoxon signed rank test (p<0.05).
ƒSignificant difference between resistant starch and glucose by related samples Wilcoxon signed rank test (p<0.05).
Table 3.
Serum glucose, insulin, C-peptide and free-fatty acid responses elicited by the test meals
| Time after treatment (h) | Treatment
|
|||
|---|---|---|---|---|
| Glucose | Inulin | Resistant Starch | ||
| Glucose (mmol×h/L) | iAUC 0–2 h | 3.73 ± 0.49 | 4.60 ± 0.55a | 3.93 ± 0.56 |
| iAUC 2–4 h | −1.13 ± 0.23 | −0.89 ± 0.25 | −0.66 ± 0.30 | |
| tAUC 4–6 h | 11.6 ± 0.3 | 11.5 ± 0.3 | 10.9 ± 0.3b | |
|
| ||||
| Insulin (pmol×h/L) | iAUC 0–2 h | 111.0 ± 18.8 | 114.9 ± 21.0 | 106.8 ± 16.8 |
| iAUC 2–4 h | 23.7 ± 7.2 | 33.3 ± 11.6 | 34.0 ± 10.1 | |
| tAUC 4–6 h | 77.8 ± 10.5 | 76.7 ± 9.2 | 66.7 ± 8.9a | |
|
| ||||
| C-peptide (pmol×h/L) | iAUC 0–2 h | 2660 ± 238 | 2770 ± 212 | 2720 ± 203 |
| iAUC 2–4 h | 857 ± 151 | 1055 ± 179 | 1290 ± 241b | |
| tAUC 4–6 h | 2632 ± 211 | 2617 ± 188 | 2444 ± 197a | |
|
| ||||
| FFA (mEq×h/L) | tAUC 0–4 h | 1.05 ± 0.07 | 1.06 ± 0.08 | 0.94 ± 0.06a |
| tAUC 4–6 h | 0.53 ± 0.03 | 0.52 ± 0.03 | 0.48 ± 0.03a | |
| iAUC min4 | 0.21 ± 0.03 | 0.19 ± 0.03 | 0.15 ± 0.03b | |
Values are mean ± SEM for n=25; iAUC, incremental area under the curve; min4, measured from nadir to 4h; tAUC, total area under the curve.
Significantly different from Glucose (p<0.03).
Significantly different from Glucose (p<0.01).
There was no difference among treatments in serum insulin or c-peptide responses between 0–2h; however, the mean iAUC from 2–4h was greater after RS compared to GLU, and the difference was significant for c-peptide. During the 4–6-h period, serum insulin and c-peptide responses after IN were similar to those after GLU, but, after RS, tAUC 4–6h for serum insulin and c-peptide were significantly less than those after GLU (Figure 2, Table 3). Serum insulin and c-peptide responses between 0–2h and 4–6h were significantly greater in OWO compared to LN (Table 4) but the effects of IN and RS were similar in the 2 groups.
Serum FFA responses were similar after IN compared to GLU. However, over the 0–4h and 4–6h periods the total AUC for FFA was significantly less after RS than GLU, due to a significantly smaller rebound between the nadir and 4h (Figure 2, Table 3). FFA responses in LN did not differ significantly from those in OWO (Table 4).
The interaction term was not significant for any of the outcomes (probably due to small sample size), therefore, the interaction term was dropped from the statistical model to save power for the main effects.
The changes in serum butyrate iAUC from minimum (nadir) to 6h elicited by IN were negatively related to the changes in glucose tAUC at 4–6h (p=0.055) (Supplementary Table 3A). The changes in serum FFA rise (minimum nadir to peak nadir) elicited by RS tend to negatively relate to glucose tAUC 4–6h (p=0.059)(Supplementary Table 3A). The changes in acetate tAUC at 0–4h elicited by IN were negatively correlated to the changes in FFA rise (p=0.034)(Supplementary Table 3B).
DISCUSSION
It has been suggested, on one hand that excess production of colonic SCFA is a cause of obesity but on the other that colonic fermentation of dietary fibre improves insulin sensitivity, and therefore reduces the risk for T2DM (14,15,38). Our results show no significant differences in postprandial SCFA responses between LN and OWO participants after acute fibre consumption. Moreover, unlike previous reports (26,34,39), no significant differences in faecal SCFA concentrations between LN and OWO participants were seen. This does not support the hypothesis that excess SCFA production is a cause of obesity. Our results also suggest that any effect of the colonic fermentation of dietary fibre on glycaemic responses is not due to SCFA per-se, since IN increased serum SCFA without reducing the second-meal glycaemic response, whereas RS reduced the second-meal glycaemic response without increasing serum SCFA.
Although both IN and RS are fermentable, only IN elicited a significant increase in SCFA. The earlier elevation of acetate after IN compared to RS could reflect faster gastric emptying (which may also explain the increased glucose concentrations we saw after the IN breakfast), a more rapid transit of IN than RS through the small intestine, and more rapid and more proximal fermentation of IN than RS in the large intestine (40). To our knowledge, no acute human studies have compared the fermentation of IN and RS. However, 12h fermentation of faecal inocula from humans (41) showed that RS and IN produced similar amounts of total SCFA, with increased butyrate production from IN. Therefore, a study period longer than 6h may be required to detect rises in serum SCFA after RS.
In this study, the lower glucose response after RS at 4–6h compared to control, together with the reduced insulin and c-peptide responses, are consistent with other studies showing that RS treatment improves insulin sensitivity (13,15,42,43). This reduced glycaemic response is likely not related to SCFA (as there was no significant increase in SCFA after RS), but rather to the reduced FFA rebound. The acute reduction in FFA could have been explained by the presence of a larger than expected amount of available, but slowly digested starch in the RS ingredient used, since both prolonged carbohydrate absorption (44) and an increase in the amount of carbohydrate absorbed (3) have been shown to reduce postprandial FFA rebound. The presence of enough additional available carbohydrate in the RS test meal compared to the control to reduce FFA at 3 and 4 hours might be expected to increase serum glucose and insulin concentrations at these times. By contrast, we found that, compared to control, RS only elicited a small but significant increase in insulin at 3hr and a small non-significant increase in glucose at 3hr. However, these differences are quite consistent with those of a study showing that increasing the dose of glucose consumed from 75 to 100g was associated with only a small increase in serum glucose (~0.4mmol/L) at 3h, a small reduction in glycose at 4h, small increases in insulin (~50–70 pmol/L) at 3 and 4h, and large reductions in FFA (>0.1 mEq/L) at both 3 and 4h (45).
The suggestion that the reduced FFA concentration accounted for the reduced second-meal glucose response is supported by the positive correlation between the FFA rise elicited by RS before lunch and glucose concentrations after lunch. Robertson et al. demonstrated that supplementation of 30 g/day of RS for 4wks in healthy subjects significantly improved insulin sensitivity, reduced subcutaneous abdominal adipose tissue FFA (but not systemic FFA) and increased serum SCFA concentrations (14). However, in two other studies in healthy subjects, though a beneficial effect of RS on insulin sensitivity was seen, SCFA and FFA were either not measured (46) or showed no significant change (15). In a 12wk study in T2DM humans, RS supplementation resulted in lower postprandial glucose concentrations, and a reduction in fasting and postprandial FFA. However, no effects on insulin sensitivity or on serum SCFA concentrations were seen (42). Thus, more human studies are needed to understand how RS reduce glycaemic responses
The inability of IN to elicit a significant reduction in postprandial FFA rebound, despite a large increase in serum SCFA concentrations, was unexpected and not consistent with the results of several previous studies from our laboratory (10,37). The increased glucose response before lunch was also unexpected and inconsistent with our previous studies which showed that the glycaemic response after adding 24 g IN to either high-fructose corn syrup (10) or to 75g glucose (47) was virtually identical to that after the control. The inverse relation between butyrate (min-6h) and glycaemic responses at 4–6h may show that butyrate may has a role in reducing glucose (48), though its elevation might not be consistent and high enough to significantly reduce blood glucose. Though animal studies have shown a positive effect of the inulin-type fructans on glucose control (17–19), a systematic review in humans did not find a conclusive result (21), possibly due to the differences among studies in fructan types, doses, durations of studies, health status of the participants, and analytical methods. Further investigations are needed in order to determine whether inulin has any effect on glycaemic control.
Surprisingly, we found no significant differences in serum SCFA responses between LN and OWO groups. However, this do not rule out increased colonic SCFA production in OWO compared to LN participants, since if this was also accompanied by increased hepatic and peripheral SCFA clearance, postprandial serum SCFA responses could be similar in the 2 groups. Moreover, a modest difference in SCFA responses between OWO and LN could have been missed, as about 85 subjects per group would be required to detect, with statistical significance, the difference we actually observed. With our current sample size, and with 80% power, a significant difference would have been detected only if the OWO group had produced almost twice the amount of acetate after IN than the amount the LN group produced. However, the lack of differences between our groups in faecal SCFA concentrations (as opposed to previous human studies (25–27)), in faecal bacterial phyla, and in habitual dietary intakes, may suggest that the group of OWO subjects we studied did not produce more SCFA than the LN subjects. Whether the similar faecal and serum SCFA responses in our groups were due to their similar relative abundance of Firmicutes and Bacteroides, due to other factors that were not explored in our study (i.e., genetic variations (49)), or due to a small sample size, should be further explored.
It is concluded that IN and RS differ in their acute postprandial effects: IN is rapidly fermented in the colon eliciting prompt and large increases in breath hydrogen and serum SCFA concentrations, whereas RS is more slowly fermented with much smaller increases in breath hydrogen and serum SCFA for 6h after consumption. The results provide no evidence that acute elevations in SCFA per se influence glucose metabolism. In addition, the results do not support the hypothesis that overweight/obesity is associated with increased colonic SCFA production. However, a larger sample size would be needed to rule out a modest increase in SCFA production in obesity.
Supplementary Material
Acknowledgments
We are thankful to Kervan Rivera-Rufner for analyzing serum and faecal SCFA. Supported by grant no. 486906 from the Canadian Institutes of Health Research (CIHR), Institute of Nutrition, Metabolism and Diabetes.
Footnotes
Supplementary information is available at European Journal of Clinical Nutrition’s website.
Clinical Trials registration number (at www.ClinicalTrials.gov): NCT02562014
Conflict of interest: The authors declared no conflict of interest
References
- 1.Weickert MO, Pfeiffer AFH. Metabolic effects of dietary fiber consumption and prevention of diabetes. J Nutr. 2008;138(3):439–442. doi: 10.1093/jn/138.3.439. [DOI] [PubMed] [Google Scholar]
- 2.Nilsson A, Granfeldt Y, Östman E, Preston T, Björck I. Effects of GI and content of indigestible carbohydrates of cereal-based evening meals on glucose tolerance at a subsequent standardised breakfast. Eur J Clin Nutr. 2006;60(9):1092–1099. doi: 10.1038/sj.ejcn.1602423. [DOI] [PubMed] [Google Scholar]
- 3.Wolever TMS, Bentum-Williams A, Jenkins DJA. Physiological modulation of plasma free fatty acid concentrations by diet: Metabolic implications in nondiabetic subjects. Diabetes Care. 1995;18(7):962–970. doi: 10.2337/diacare.18.7.962. [DOI] [PubMed] [Google Scholar]
- 4.Nilsson A, Östman E, Preston T, Björck I. Effects of GI vs content of cereal fibre of the evening meal on glucose tolerance at a subsequent standardized breakfast. Eur J Clin Nutr. 2008;62(6):712–720. doi: 10.1038/sj.ejcn.1602784. [DOI] [PubMed] [Google Scholar]
- 5.Ge H, Li X, Weiszmann J, Wang P, Baribault H, Chen J, et al. Activation of G protein-coupled receptor 43 in adipocytes leads to inhibition of lipolysis and suppression of plasma free fatty acids. Endocrinology. 2008;149(9):4519–4526. doi: 10.1210/en.2008-0059. [DOI] [PubMed] [Google Scholar]
- 6.Yamashita H, Fujisawa K, Ito E, Idei S, Kawaguchi N, Kimoto M, et al. Improvement of obesity and glucose tolerance by acetate in type 2 diabetic Otsuka Long-Evans Tokushima Fatty (OLETF) rats. Biosci Biotechnol Biochem. 2007;71(5):1236–1243. doi: 10.1271/bbb.60668. [DOI] [PubMed] [Google Scholar]
- 7.Crouse JR, Gerson CD, DeCarli LM, Lieber CS. Role of acetate in the reduction of plasma free fatty acids produced by ethanol in man. J Lipid Res. 1968;9(4):509–512. [PubMed] [Google Scholar]
- 8.Wolever TMS, Brighenti F, Royall D, Jenkins AL, Jenkins DJA. Effect of rectal infusion of short chain fatty acids in human subjects. Am J Gastroenterol. 1989;84(9):1027–1033. [PubMed] [Google Scholar]
- 9.Brighenti F, Benini L, Del Rio D, Casiraghi C, Pellegrini N, Scazzina F, et al. Colonic fermentation of indigestible carbohydrates contributes to the second-meal effect. Am J Clin Nutr. 2006;83(4):817–822. doi: 10.1093/ajcn/83.4.817. [DOI] [PubMed] [Google Scholar]
- 10.Tarini J, Wolever TMS. The fermentable fibre inulin increases postprandial serum short-chain fatty acids and reduces free-fatty acids and ghrelin in healthy subjects. Applied Physiology, Nutrition and Metabolism. 2010;35(1):9–16. doi: 10.1139/H09-119. [DOI] [PubMed] [Google Scholar]
- 11.Slavin J, Stewart M, Timm D, Grabitske H, Hospattankar A. Fermentation patterns and short chain fatty acid profiles of wheat dextrin and other functional fibres. Dietary Fibre: New Frontiers for Food and Health. 2010:177–191. [Google Scholar]
- 12.Jenkins DJA, Vuksan V, Kendall CWC, Würsch P, Jeffcoat R, Waring S, et al. Physiological effects of resistant starches on fecal bulk, short chain fatty acids, blood lipids and glycemic index. J Am Coll Nutr. 1998;17(6):609–616. doi: 10.1080/07315724.1998.10718810. [DOI] [PubMed] [Google Scholar]
- 13.Johnston KL, Thomas EL, Bell JD, Frost GS, Robertson MD. Resistant starch improves insulin sensitivity in metabolic syndrome. Diabetic Med. 2010;27(4):391–397. doi: 10.1111/j.1464-5491.2010.02923.x. [DOI] [PubMed] [Google Scholar]
- 14.Robertson MD, Bickerton AS, Dennis AL, Vidal H, Frayn KN. Insulin-sensitizing effects of dietary resistant starch and effects on skeletal muscle and adipose tissue metabolism. Am J Clin Nutr. 2005;82(3):559–567. doi: 10.1093/ajcn.82.3.559. [DOI] [PubMed] [Google Scholar]
- 15.Robertson MD, Currie JM, Morgan LM, Jewell DP, Frayn KN. Prior short-term consumption of resistant starch enhances postprandial insulin sensitivity in healthy subjects. Diabetologia. 2003;46(5):659–665. doi: 10.1007/s00125-003-1081-0. [DOI] [PubMed] [Google Scholar]
- 16.Robertson MD, Wright JW, Loizon E, Debard C, Vidal H, Shojaee-Moradie F, et al. Insulin-sensitizing effects on muscle and adipose tissue after dietary fiber intake in men and women with metabolic syndrome. J Clin Endocrinol Metab. 2012;97(9):3326–3332. doi: 10.1210/jc.2012-1513. [DOI] [PubMed] [Google Scholar]
- 17.Diez M, Hornick J, Baldwin P, Istasse L. Influence of a blend of fructo-oligosaccharides and sugar beet fiber on nutrient digestibility and plasma metabolite concentrations in healthy Beagles. Am J Vet Res. 1997;58(11):1238–1242. [PubMed] [Google Scholar]
- 18.Rozan P, Nejdi A, Hidalgo S, Bisson J, Desor D, Messaoudi M. Effects of lifelong intervention with an oligofructose-enriched inulin in rats on general health and lifespan. Br J Nutr. 2008;100(6):1192–1199. doi: 10.1017/S0007114508975607. [DOI] [PubMed] [Google Scholar]
- 19.Diez M, Hornick JL, Baldwin P, Van Eenaeme C, Istasse L. The influence of sugar-beet fibre, guar gum and inulin on nutrient digestibility, water consumption and plasma metabolites in healthy Beagle dogs. Res Vet Sci. 1998;64(2):91–96. doi: 10.1016/s0034-5288(98)90001-7. [DOI] [PubMed] [Google Scholar]
- 20.Beylot M. Effects of inulin-type fructans on lipid metabolism in man and in animal models. Br J Nutr. 2005;93(SUPP):S163–S168. doi: 10.1079/bjn20041339. [DOI] [PubMed] [Google Scholar]
- 21.Bonsu NK, Johnson CS, Mcleod KM. Can dietary fructans lower serum glucose? Journal of Diabetes. 2011;3(1):58–66. doi: 10.1111/j.1753-0407.2010.00099.x. [DOI] [PubMed] [Google Scholar]
- 22.Raninen K, Lappi J, Mykkänen H, Poutanen K. Dietary fiber type reflects physiological functionality: Comparison of grain fiber, inulin, and polydextrose. Nutr Rev. 2011;69(1):9–21. doi: 10.1111/j.1753-4887.2010.00358.x. [DOI] [PubMed] [Google Scholar]
- 23.Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102(31):11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 25.Schwiertz A, Taras D, Schäfer K, Beijer S, Bos NA, Donus C, et al. Microbiota and SCFA in lean and overweight healthy subjects. Obesity. 2010;18(1):190–195. doi: 10.1038/oby.2009.167. [DOI] [PubMed] [Google Scholar]
- 26.Fernandes J, Su W, Rahat-Rozenbloom S, Wolever TMS, Comelli EM. Adiposity, gut microbiota and faecal short chain fatty acids are linked in adult humans. Nutr Diabetes. 2014 Jun;4 doi: 10.1038/nutd.2014.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teixeira TFS, Grześkowiak L, Franceschini SCC, Bressan J, Ferreira CLLF, Peluzio MCG. Higher level of faecal SCFA in women correlates with metabolic syndrome risk factors. Br J Nutr. 2013;109(5):914–919. doi: 10.1017/S0007114512002723. [DOI] [PubMed] [Google Scholar]
- 28.Murugesan S, Ulloa-Martínez M, Martínez-Rojano H, Galván-Rodríguez FM, Miranda-Brito C, Romano MC, et al. Study of the diversity and short-chain fatty acids production by the bacterial community in overweight and obese Mexican children. Eur J Clin Microbiol Infect Dis. 2015;34(7):1337–1346. doi: 10.1007/s10096-015-2355-4. [DOI] [PubMed] [Google Scholar]
- 29.Armougom F, Henry M, Vialettes B, Raccah D, Raoult D. Monitoring bacterial community of human gut microbiota reveals an increase in Lactobacillus in obese patients and Methanogens in anorexic patients. PLoS ONE. 2009;4(9) doi: 10.1371/journal.pone.0007125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santacruz A, Collado MC, García-Valdés L, Segura MT, Marítn-Lagos JA, Anjos T, et al. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br J Nutr. 2010;104(1):83–92. doi: 10.1017/S0007114510000176. [DOI] [PubMed] [Google Scholar]
- 31.Furet J, Kong L, Tap J, Poitou C, Basdevant A, Bouillot J, et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: Links with metabolic and low-grade inflammation markers. Diabetes. 2010;59(12):3049–3057. doi: 10.2337/db10-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ainsworth BE, Haskell WL, Leon AS, Jacobs DR, Jr, Montoye HJ, Sallis JF, et al. Compendium of physical activities: Classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25(1):71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 33.Modifiable Activity Questionnaire. Med Sci Sports Exerc. 1997;29(6 SUPPL):S73–S78. [PubMed] [Google Scholar]
- 34.Rahat-Rozenbloom S, Fernandes J, Gloor GB, Wolever TMS. Evidence for greater production of colonic short-chain fatty acids in overweight than lean humans. Int J Obes. 2014;38(12):1525–1531. doi: 10.1038/ijo.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petrof EO, Gloor GB, Vanner SJ, Weese SJ, Carter D, Daigneault MC, et al. Stool substitute transplant therapy for the eradication of Clostridium difficile infection: ‘RePOOPulating’ the gut. Microbiome. 2013 Jan 09;1:3. doi: 10.1186/2049-2618-1-3. 2013/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gloor GB, Reid G. Compositional analysis: a valid approach to analyze microbiome high-throughput sequencing data. Can J Microbiol. 2016 Apr 12;:1–12. doi: 10.1139/cjm-2015-0821. 2016/06. [DOI] [PubMed] [Google Scholar]
- 37.Fernandes J, Vogt J, Wolever TMS. Inulin increases short-term markers for colonic fermentation similarly in healthy and hyperinsulinaemic humans. Eur J Clin Nutr. 2011;65(12):1279–1286. doi: 10.1038/ejcn.2011.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Freeland KR, Wilson C, Wolever TMS. Adaptation of colonic fermentation and glucagon-like peptide-1 secretion with increased wheat fibre intake for 1 year in hyperinsulinaemic human subjects. Br J Nutr. 2010;103(1):82–90. doi: 10.1017/S0007114509991462. [DOI] [PubMed] [Google Scholar]
- 39.Schwiertz A, Taras D, Schäfer K, Beijer S, Bos NA, Donus C, et al. Microbiota and SCFA in lean and overweight healthy subjects. Obesity. 2010;18(1):190–195. doi: 10.1038/oby.2009.167. [DOI] [PubMed] [Google Scholar]
- 40.James SL, Muir JG, Curtis SL, Gibson PR. Dietary fibre: a roughage guide. Intern Med J. 2003;33(7):291–296. doi: 10.1046/j.1445-5994.2003.00404.x. [DOI] [PubMed] [Google Scholar]
- 41.Yang J, Martínez I, Walter J, Keshavarzian A, Rose DJ. Invitro characterization of the impact of selected dietary fibers on fecal microbiota composition and short chain fatty acid production. Anaerobe. 2013;23:74–81. doi: 10.1016/j.anaerobe.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 42.Bodinham CL, Smith L, Thomas EL, Bell JD, Swann JR, Costabile A, et al. Efficacy of increased resistant starch consumption in human type 2 diabetes. Endocrine connections. 2014;3(2):75–84. doi: 10.1530/EC-14-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klosterbuer AS, Thomas W, Slavin JL. Resistant starch and pullulan reduce postprandial glucose, insulin, and GLP-1, but have no effect on satiety in healthy humans. J Agric Food Chem. 2012;60(48):11928–11934. doi: 10.1021/jf303083r. [DOI] [PubMed] [Google Scholar]
- 44.Jenkins DJA, Wolever TMS, Ocana AM, Vuksan V, Cunnane SC, Jenkins M, et al. Metabolic effects of reducing rate of glucose ingestion by single bolus versus continuous sipping. Diabetes. 1990;39(7):775–781. doi: 10.2337/diab.39.7.775. [DOI] [PubMed] [Google Scholar]
- 45.Christensen NJ, Ørskov H, Hansen AP. Significance of glucose load in oral glucose tolerance tests. Acta Medica Scand. 1972;192:337–342. [PubMed] [Google Scholar]
- 46.Bodinham CL, Al-Mana NM, Smith L, Robertson MD. Endogenous plasma glucagon-like peptide-1 following acute dietary fibre consumption. Br J Nutr. 2013;110(8):1429–1433. doi: 10.1017/S0007114513000731. [DOI] [PubMed] [Google Scholar]
- 47.Fernandes J, Vogt J, Wolever TMS. Intravenous acetate elicits a greater free fatty acid rebound in normal than hyperinsulinaemic humans. Eur J Clin Nutr. 2012;66(9):1029–1034. doi: 10.1038/ejcn.2012.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, et al. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156(1–2):84–96. doi: 10.1016/j.cell.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 49.Goodrich JK, Waters JL, Poole AC, Sutter JL, Koren O, Blekhman R, et al. Human Genetics Shape the Gut Microbiome. Cell. 2014;159(4):789–799. doi: 10.1016/j.cell.2014.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.