Summary
Genetic risk factors contribute to asymptomatic versus symptomatic visceral leishmaniasis (VL) outcomes following infection with Leishmania infantum. We therefore carried out a family-based (N=918 post-quality control fully genotyped and phenotyped individuals) candidate gene study for symptomatic VL or asymptomatic delayed type hypersensitivity (DTH) skin test phenotypes in highly endemic neighborhoods of northeast Brazil. A total of 248 SNPs were genotyped in 42 genes selected as candidates on the basis of prior genetic, immunological and transcriptional profiling studies. The most significant association with the VL phenotype was with SNP rs6785358 (p=5.7e-04; pcorrected= 0.026) 3.8 kb upstream of TGFBR2, the gene encoding the type 2 receptor for transforming growth factor beta (TGF-β). A second inhibitory member of the TGB-β superfamily signaling pathway, SMAD7, was associated with the DTH phenotype (SNP rs7238442: p=0.001; pcorrected=0.051). The most significant association for the DTH phenotype was with SNP rs10800309 (p=−8.4e-06; pcorrected= 3.9e-04) situated 3.1 kb upstream of FCGR2A, the gene encoding the low affinity IIa receptor for the Fc fragment of IgG. Overall, our results imply a role for IgG-mediated inflammation in determining DTH associated with asymptomatic infection, and contribute to growing evidence that the TGFβ pathway is important in the immunopathogenesis of VL. (196 words; 200 words allowed)
Introduction
Visceral leishmaniasis (VL) is a debilitating parasitic disease of humans caused by protozoa belonging to the Leishmania donovani complex. Symptomatic VL is a severe progressive infection which can be fatal even with treatment. Despite its potential severity, 80–90% of individuals infected with the causative parasites harbor either sub-clinical or asymptomatic infection (Blackwell et al., 2009). The hypothesis that human genetic variants also influence susceptibility to both VL and a positive DTH response is supported by segregation analyses in Brazilian populations (Feitosa et al., 1999, Peacock et al., 2001). Efforts to identify the specific genes conferring susceptibility have inspired candidate gene (reviewed (Blackwell et al., 2009, Blackwell, 2010), as well as genome-wide linkage (Jeronimo et al., 2007a, Jamieson et al., 2007, Miller et al., 2007, Bucheton et al., 2003a) and association (Fakiola et al., 2013) studies. Amongst these, candidate gene studies have not always provided dense coverage across the genes of interest, and relatively few have included analysis of candidate genes as genetic risk factors for both symptomatic and asymptomatic infections, i.e. for genetic variants that potentially act as risk factors for disease and those that may be protective. Here we provide results of a study that included dense single nucleotide polymorphism (SNP) coverage across 42 candidate genes analysed for association with both clinical VL and asymptomatic disease as measured by a delayed type hypersensitivity (DTH) skin test response to crude leishmanial antigen. The results imply a role for IgG-mediated inflammation in determining DTH associated with asymptomatic infection, and provide strong support to growing evidence that the TGF-β pathway is important in the immunopathogenesis of VL.
Materials and methods
Study subjects and phenotype
Details of the study site in Natal, Rio Grande do Norte, Brazil, enrollment of subjects, and clinical phenotyping are described in full in our previous genome-wide linkage (Jeronimo et al., 2007a) and candidate gene (Jeronimo et al., 2007b) studies. Briefly, criteria for diagnosis of VL were a clinical presentation with hepatosplenomegaly, fever, cachexia and pancytopenia, positive parasitologic diagnosis (positive bone marrow aspirate, positive serology), and response to treatment. As before (Jeronimo et al., 2007a, Jeronimo et al., 2007b), the cutoff for a positive Montenegro test for Leishmania antigen was ≥5 mm of induration. The study was approved by the institutional review boards of the Universidade Federal do Rio Grande do Norte (numbers 19–01 and 21–01); the Comissão Nacional de Ética em Pesquisa (CONEP numbers 4581 and 4575); the University of Iowa; Johns Hopkins University; the University of Virginia; and the National Human Genome Research Institute, National Institutes of Health. Written consent was obtained from adults and from parents or guardians of minors <18 years of age, and written assent was obtained from minors 12–17 years of age.
Numbers of subjects
DNA for genotyping was available for 1210 individuals (50% male; 50% female), who all contributed to calculation of allele frequencies and linkage disequilibrium (LD) blocks. Of these, 918 individuals belonged to families for whom phenotype data was available ((141 VL; 419 DTH+; 105 DTH−; 253 individuals of unknown phenotype). Details of family structure are provided in Table S1.
SNP selection and genotyping
SNPs (N=302) were selected to tag 42 candidate genes (Table S2) across intron/exons and including 10 kilobases upstream and downstream of the coding region. Based on our knowledge of admixture in the region of northeast Brazil (Ettinger et al., 2009), tagging SNPs (minor allele frequency >0.05) were selected from LD blocks using the CEU and YRI populations in HapMap (Table S3). SNP selection was based on >1 SNP per LD block with r2>0.8. Additional SNPs between LD blocks were included to ensure coverage. The median distance between SNPs tagging each candidate gene was 2.4 kb. Candidate genes were chosen from the literature based on prior genetic association studies (Blackwell et al., 2009), on their relevance to the polarized immune responses observed in leishmaniasis (Wilson et al., 2005, Sacks & Noben-Trauth, 2002), and/or changes in microarrays of macrophages infected with Leishmania spp. (Rodriguez et al., 2004, Ettinger & Wilson, 2008). Candidate genes included cytokines, chemokines and their receptors, signal transduction molecules, collagens and transporters (Table S2).
Genotyping was performed by the Center for Inherited Diseases Research at Johns Hopkins University, Baltimore, MD, USA, using the Illumina Infinium genotyping platform. SNPs with median p<0.001 for deviation from Hardy-Weinberg equilibrium (Wigginton et al., 2005) across unrelated individuals were removed. PEDSTATS (Wigginton & Abecasis, 2005) and MERLIN (Abecasis et al., 2002) software were used to remove Mendelian errors and unlikely genotypes (unlikely recombination events). Individuals or SNPs with more than 2% inconsistent calls or errors were removed from the analysis. Nuclear families with more than 5% errors were also excluded. After quality control, 248 SNPs (Table S3) were retained in the analysis. The call rate for SNPs among genotyped individuals was 99.91% after quality control.
Association analyses
Family-based association tests for qualitative traits (VL present or absent; DTH positive or DTH negative) were conducted on all 248 SNPs using the LAMP software package, with preferences set to “ignore linkage” (Li et al., 2005). For the VL analysis, the 141 individuals with VL were compared to (i) the total of 419 DTH positive plus 105 known DTH negative (total N=524) individuals, and (ii) the 419 DTH positive individuals alone. For the DTH analysis, the 141 VL individuals were set to phenotype unknown (since they may have lost their DTH status secondary to immune suppression as disease progressed), hence making the association comparison between DTH positive (N=419) versus DTH negative (N=105) individuals. In all analyses, phenotype unknown individuals contribute to estimation of allele frequencies. Criteria for families to be considered to be exposed, and therefore included in the study population, were at least 50% of family members to present with VL or a positive DTH response, with DTH negative individuals only being included in the study if they had lived in the household for a minimum of 12 months. A modified Bonferroni threshold for significance was calculated to take account of the number of LD blocks identified using a conservative method (Gabriel et al., 2002) implemented in the Haploview program (Barrett et al., 2005). There were 48 independent blocks (Table S3) across all candidate genes. Considering this total of 48 LD blocks, a threshold nominal p=0.001 (i.e. p=0.05/48) was required to achieve significance at α=0.05. Individual corrected p-values were nominal p-values multiplied by 48 LD blocks. Plots of associations were generated with the Locuszoom software package (Pruim et al., 2010).
Transforming growth factor beta quantification
Latent and endogenously activated transforming growth factor beta (TGFβ) concentrations were measured in platelet-free serum of Brazilian VL patients (N=11) during active VL either prior to or during the first 24 hrs of treatment, or during a relatively healthy state. VL patients were compared with healthy exposed endemic controls (19 DTH positive; 11 DTH negative). Serum was frozen immediately after collection, and underwent only one freeze-thaw before TGFβ measurement to minimize artifactual activation of latent TGFβ. TGFβ was measured using an ELISA kit from R&D systems. Plasma samples were acidified and neutralized according to the manufacturer’s instructions to measure total TGFβ concentrations (i.e. latent plus activated). Endogenously activated TGFβ was measured in non-acidified samples. TGFβ levels were compared between subjects according to genotypes for the TGBR2 rs6785358 SNP. Tests for significant differences between genotypes were performed using a two-tailed student’s T-test assuming unequal variance between groups.
Results
Allelic associations
Table 1 lists the most significant associations (uncorrected p<0.01) between each phenotype and candidate gene SNPs.
Table 1.
Top candidate gene SNP associations for VL and DTH phenotypes.
| SNP | Trait1 | Ref. 2 Allele |
Freq. 2 | LOD | Pnominal3 | Pcorrected3 | Causal Allele |
Gene | Location4 |
|---|---|---|---|---|---|---|---|---|---|
| rs6785358 | VLvsALL | A | 0.836 | 3.25 | 5.7E-04 | 0.027 | G | TGFBR2 | upstream |
| rs6770038 | VLvsALL | C | 0.72 | 3.06 | 8.7E-04 | 0.042 | T | TGFBR2 | intron |
| rs10800309 | VLvsALL | G | 0.699 | 2.77 | 0.0017 | 0.082 | G | FCGR2A | upstream |
| rs2306191 | VLvsALL | C | 0.898 | 2.62 | 0.0024 | 0.115 | G | IL27RA | intron |
| rs2279015 | VLvsALL | T | 0.601 | 2.27 | 0.0054 | 0.259 | T | SLC11A1 | intron |
| rs1882434 | VLvsALL | C | 0.649 | 2.07 | 0.0085 | 0.408 | T | COL4A4 | intron |
| rs6785358 | VLvsDTH | A | 0.836 | 1.87 | 0.013 | 0.642 | G | TGFBR2 | upstream |
| rs6770038 | VLvsDTH | C | 0.72 | 3.08 | 8.3E-04 | 0.039 | T | TGFBR2 | intron |
| rs10800309 | VLvsDTH | G | 0.699 | 2.53 | 0.003 | 0.144 | G | FCGR2A | upstream |
| rs2306191 | VLvsDTH | C | 0.898 | 1.58 | 0.026 | 1.248 | G | IL27RA | intron |
| rs2279015 | VLvsDTH | T | 0.601 | 2.16 | 0.007 | 0.336 | T | SLC11A1 | intron |
| rs1882434 | VLvsDTH | C | 0.649 | 2.42 | 0.0038 | 0.182 | T | COL4A4 | intron |
| rs10800309 | DTH+vsDTH− | G | 0.699 | 5.07 | 8.4E-06 | 3.9E-04 | A | FCGR2A | upstream |
| rs7238442 | DTH+vsDTH− | C | 0.559 | 2.94 | 0.001 | 0.051 | T | SMAD7 | intron |
| rs4586 | DTH+vsDTH− | C | 0.537 | 2.49 | 0.003 | 0.147 | T | CCL2 | intron |
| rs2337106 | DTH+vsDTH− | C | 0.589 | 2.18 | 0.007 | 0.304 | G | SMAD7 | intron |
| rs2239347 | DTH+vsDTH− | G | 0.519 | 2.05 | 0.009 | 0.414 | G | IL4R | intron |
Phenotypes were analysed as qualitative traits (VLvsALL = VL present versus all DTH+ and known DTH− individuals; VLvsDTH = VL present versus DTH+, DTH− set to unknown; DTH+vsDTH− = DTH positive ≥5mm induration versus DTH negative <5mm induration, VL set to unknown).
Reference Allele, and Frequency of Reference Allele.
P-values provided are: pnominal for uncorrected; pcorrectedfor modified Bonferroni correction (i.e. uncorrected p-value multiplied by 48 LD blocks). The overall threshold for significance taking account of the number of LD blocks is p=0.001 (i.e. p=0.05/46 LD blocks) for an α=0.05.
The location of the SNP is given relative to the candidate gene.
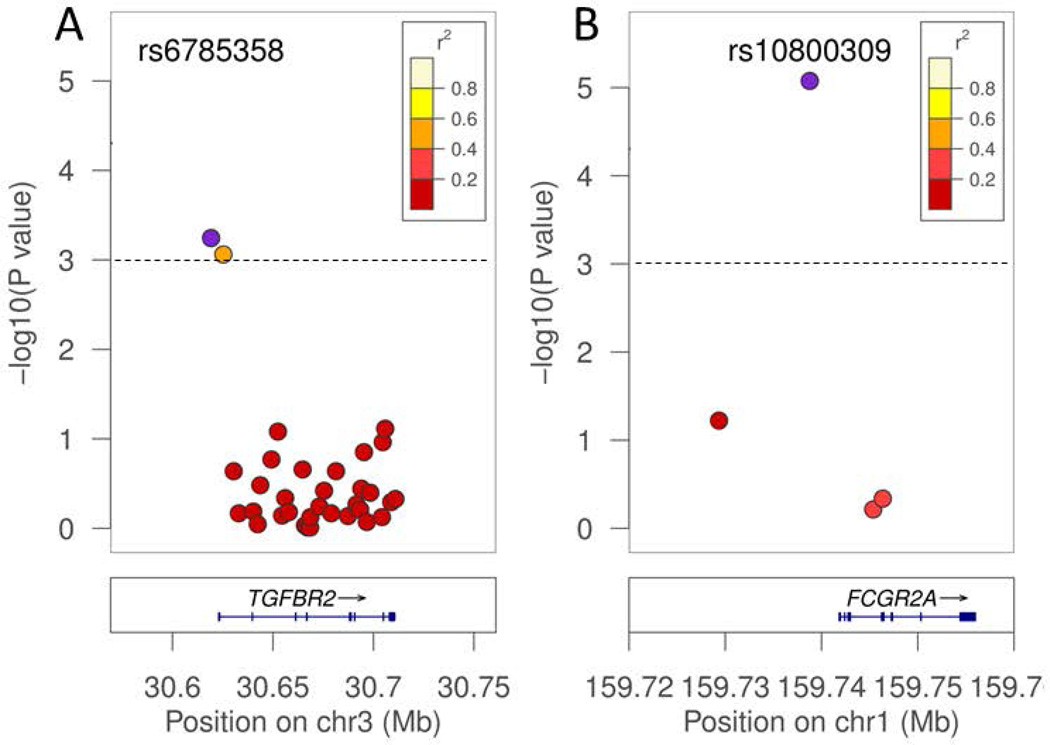
The top association for the VL phenotype was with TGFBR2 (Table 1: SNP rs6785358; nominal p value of p=5.7e-04; Bonferroni corrected p=0.026), the gene encoding the type 2 receptor for transforming growth factor beta (TGFβ). Figure 1A demonstrates that the association was confined to two SNPs at the 5 prime end of the gene, 3.8 kb upstream of TGFBR2 and within intron 1, which were not in LD with the remaining 30 SNPs selected to cover LD blocks across the full length of the gene. This suggests that the two associated SNPs are tagging regulatory variants. SNPs within another inhibitory member of the TGBβ superfamily signaling pathway (Akhurst & Padgett, 2015), SMAD7, were associated with the DTH phenotype (Table 1: SNP rs7238442; nominal p value of p=0.001; Bonferroni corrected p=0.051). Since a positive DTH response has traditionally been accepted as a measure of acquired resistance to infection, we also compared VL individuals to only those with a confirmed DTH positive phenotype. In this analysis the same SNPs showed positive associations at nominal P-values ≤0.026, but only SNP rs6770038 at TGFBR2 remained significant after correction for multiple testing (Table 1: SNP rs6770038; nominal p value of p=8.3e-04; Bonferroni corrected p=0.039). The difference between the two analyses of the VL phenotype likely reflects loss of power due to the smaller sample size of non-VL individuals in the second analysis. It should be noted that DTH negative individuals were only included in our sample if they had lived in an exposed household for a minimum of 12 months, making it highly probably that they are innately resistant to infection.
Fig. 1.
Most highly associated SNPs in candidate genes. Associations between VL phenotype and rs6785358 upstream of TGFBR2 (A), and the DTH positive phenotype and the rs10800309 upstream of FCGR2A (B), are plotted along with tagging SNPs covering the candidate gene. The blue points represent the strongest association for the trait within the candidate gene. The LD structure between the most closely associated SNP and other flanking SNPs is indicated by a color gradient on flanking SNPs, with white in strong LD, and red being in weak LD. The dashed line represents the global adjusted Bonferroni threshold of significance at p=0.001 cutoff.
The top association for the DTH phenotype was with FCGR2A (SNP rs10800309; nominal p value of p=8.4e-06; Bonferroni corrected p=3.9e-04), the gene encoding the low affinity IIa receptor for the Fc fragment of IgG. Figure 1B demonstrates that the association is confined to SNP rs10800309 located 3.1kb upstream of the FCGR2A, which was not in LD with the remaining 3 SNPs selected to cover LD blocks across the full length of the gene. This suggests that the associated SNP is tagging regulatory variants. This SNP also showed suggestive association with the VL phenotype (Table 1: SNP rs10800309; nominal p value of p=0.0017; Bonferroni corrected p=0.078). The association with DTH was with the minor A allele, whereas the association with VL was with the major G allele.
Association of the VL phenotype with SNP rs2279015 (nominal p value of p=0.005; Bonferroni corrected p=0.248) at SLC11A1, the gene encoding solute carrier family 11, member A1, is also worthy of note given prior genetic evidence for association between variants at SLC11A1 and VL in other geographic regions (Bucheton et al., 2003b, Mohamed et al., 2004, Ejghal et al., 2014).
Since LD tagging is never complete, with a number of singleton SNPs identified (Table S2), we cannot discount the possibility that further associations between the VL and DTH positive phenotypes and candidate genes could be identified with denser coverage of SNPs across each gene.
TGF-β levels by TGFBR2 genotype
Previous research suggests that TGF-β induces expression of specific micro-RNAs that inhibit TGFBR2 expression as a self-limiting homeostatic response (Fierro-Fernandez et al., 2015) Associations observed at TGFBR2 therefore prompted us to determine whether TGF-β levels differed between individuals with different phenotypes (VL, DTH positive, DTH negative), and whether this was influenced by genotype for the top SNP rs6785358 (Table 2). Given previously observed (MEW and SMJ, unpublished observations) wide fluctuations in TGF-β levels during acute VL, we assessed TGF-β levels in VL patients who were not acutely ill. Amongst 30 non-VL subjects (DTH positive and DTH negative) assessed for endogenously active and total TGF-β levels, 25 were homozygous for the major allele (A/A) of the SNP rs6785358, 4 were heterozygous (A/G), and one (DTH positive) was homozygous for the minor allele (G/G). Amongst 12 VL individuals studied, 6 were homozygous A/A, and 6 were heterozygous A/G, consistent with over-representation of the disease-associated minor G allele in this group. Overall levels of total TGF-β did not differ significantly between the 3 phenotypic groups (Table 2). However, when broken down by genotype, VL individuals homozygous for the A allele had significantly lower levels of total TGF-β than individuals carrying the VL-associated G allele (Table 2, p=0.002). There was no significant difference between genotypes for DTH positive individuals (Table 2, p=0.792), who all had similarly higher levels of total TGF-β. It was not possible to make comparisons within the DTH negative group as there was only one G allele carrier. For active TGF-β, both non-VL groups (DTH positive and DTH negative) had significantly lower levels of activated TGF-b compared to the VL group (Table 2, DTH positive p=0.051; DTH negative p=0.007). Broken down by genotype, DTH positive individuals homozygous for the A allele had significantly higher levels of activated TGF-β than individuals carrying the VL-associated G allele (Table 2, p=0.033), whereas all VL individuals had similarly high levels of activated TGF-β. Comparing within genotypes, A/A homozygous VL individuals had, on average, ~2-fold the level activated TGF-β compared to A/A DTH positive indiduals, whereas for carriers of the G allele, VL patients had ~15-fold activated (p=0.056) TGF-β compared to DTH positive individuals. Hence, the effect of the G allele, which is the risk allele for VL, was to enhance the levels of activated TGF-β compared to DTH positive individuals.
Table 2.
Plasma TGF-β concentrations by phenotype and genotype.
| TGF-β | Genotype | DTH− | DTH+ | VL | DTH+vsDTH− | VLvsDTH+ | VLvsDTH− | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | pg/ml | N | pg/ml | N | pg/ml | T-test: DTH−vsDTH+ |
Fold: VL/DTH+ |
T-test: VLvsDTH+ |
Fold: VL/DTH− |
T-test: VLvsDTH− |
||
| Total | All* | 11 | 707±192 | 19 | 940±178 | 11 | 1006±215 | P=0.382 | 1.1 | P=0.817 | 1.4 | P=0.313 |
| Total | AA | 10 | 723±211 | 15 | 906±186 | 6 | 488±166 | P=0.522 | 0.5 | P=0.113 | 0.7 | P=0.397 |
| Total | AG/GG | 1 | 552 | 4 | 1067±538 | 5 | 1627±190 | - | 1.5 | P=0.386 | - | - |
| T-test AAvsAG/GG |
- | P=0.792 | P=0.002 | |||||||||
| Activated | All* | 11 | 22±12 | 19 | 52±19 | 11 | 125±30 | P=0.198 | 2.4 | P=0.051 | 5.7 | P=0.007 |
| Activated | AA | 10 | 24±14 | 15 | 64±7 | 6 | 143±47 | P=0.154 | 2.2 | P=0.171 | 5.9 | P=0.052 |
| Activated | AG/GG | 1 | 0 | 4 | 6.9±6.9 | 5 | 104±35 | - | 15.1 | P=0.056 | - | |
| T-test AAvsAG/GG |
- | P=0.033 | P=0.285 | |||||||||
Subjects genotyped for the SNP rs6785358 near the TGFBR2 promoter were assayed for plasma levels of total and of endogenously activated forms of TGF-β.
Discussion
The current study was designed as a comprehensive analysis of candidate genes for both clinical VL and asymptomatic DTH positive phenotypes associated with L. infantum infection in northeastern Brazil. Amongst 42 candidate genes studied, only SNPs at TGFBR2 provided associations with VL that were robust to correction for multiple testing, whilst SNPs at FCGR2A and SMAD7 were robust to correction for the DTH phenotype. The opposing allele for the same SNP at FCGR2A was also marginally associated with VL. FCGR2A encodes the low affinity IIa receptor for the Fc fragment of IgG. A functional immunological role for antibodies in VL disease pathogenesis remained equivocal for many years (Kumar & Nylen, 2012). More recently, however, Fc gamma receptors have been shown to mediate uptake by inflammatory macrophages of L. major amastigotes coated with host-derived IgG, preferentially inducing production of high amounts of IL-10 which suppress parasite clearance (Kane & Mosser, 2001). Passive administration of anti-Leishmania IgG to L. major infected mice which lack IgG enhanced lesion sizes, accompanied by high IL-10 production (Miles et al., 2005). The effect of IgG administration could be reversed by blocking the murine IL-10 receptor. The same authors (Miles et al., 2005) showed that L. infantum amastigotes grown axenically induced little or no detectable IL-10 from human monocytes, but when incubated in VL patient (but not normal donor) serum to facilitate specific IgG opsonisation, high levels of IL-10 were produced. Furthermore, patients from our study area with ongoing VL disease have high levels of IgG and no DTH responses, a pattern that is reversed upon treatment (Miles et al., 2005). In the light of these findings, a strong association between variants at FCGR2A and DTH responses at first seems counter-intuitive, since there would be no antigen-specific IgG in the circulation at the time of administration of skin test antigen that could mediate an antigen-specific Fc gamma receptor-mediated local inflammatory response, even if this was now diverted to pro-inflammatory IL-12 rather than anti-inflammatory IL-10. Further work will be required to replicate these findings in other populations and to understand the mechanism behind this association, and how this relates to the marginal association observed for the alternative allele with VL disease.
The two strongly VL associated SNPs at TGFBR2 were in LD with each other, and located in the 5 prime region of the gene – consistent with a regulatory role in determining TGFBR2 expression. Although the TGFBR2 gene is not expected to directly regulate TGF-β levels, there are reports of co-regulated expression of both receptor and TGF-β by third party factors including microRNAs (Wei et al., 2013, Li et al., 2012, Fu et al., 2014) and the ets-1 transcription factor flightless encoded by FLI1 (Im et al., 2000). One report (Fierro-Fernandez et al., 2015) suggests that TGF-β specifically induces expression of micro-RNA miR-9-5p to inhibit TGFBR2 expression as a self-limiting homeostatic response. Thus our observed relationships between TGF-β levels and TGFBR2 genotypes may be indicative of such a homeostatic mechanism. Furthermore, it was of interest that the VL-associated minor G allele of SNP rs6785358 3.8 kb upstream of TGFBR2 was strongly associated with higher levels of active circulating TGF-β in symptomatic VL subjects compared to non-VL controls. The availability of extracellular TGF-β stores, and the efficiency with which the active form of the cytokine is released, could greatly influence disease outcome. As part of a study fine mapping regions of the genome linked to VL susceptibility, we recently (Weirather et al., 2016) demonstrated a robust association between VL disease and a group of intronic SNPs at LTBP4 encoding Latent Transforming Growth Factor Beta Binding Protein 4. The LTBP4 complex remains in extracellular tissues until activated via a number of mechanisms that alters its physiochemical characteristics, causing it to release its activated TGF-β cargo. It was also of interest that the DTH+ phenotype was associated with the inhibitory member of the TGF-β super-signaling pathway SMAD7, which will inhibit TGFBR2-mediated signaling events. DTH responses in our population have also previously been associated with genetic variants at TGFBI on chromosome 5q31.1 (Jeronimo et al., 2007b), while VL has been associated with TGFB promoter region SNPs elsewhere in Brazil (Frade et al., 2011). TGFBI encodes the protein keratoepithelin, which is upregulated by TGF-β, and is expressed in skin epithelial cells where it could modulate the DTH phenotype. This effect on the skin response to leishmanial antigen is consistent with our prior reports of association between cutaneous leishmaniasis and wound healing genes in, or affecting, the TGF-β pathway (Castellucci et al., 2012, Castellucci et al., 2011). Genetic regulation of the TGF-β pathway may be providing a balance between positive wound healing responses reflected in the DTH phenotype, as opposed to the disease-promoting role of TGF-β in relation to the VL phenotype.
Overall, these genetic studies provide strong support for the importance of the TGF-β pathway in determining the outcome of infection with L. infantum in our study population, supporting a growing body of functional data on the role of the TGF-β pathway in VL disease including its importance both as a suppressor of T cell responses (Gantt et al., 2003, Gomes et al., 2000) and potentially as an activator of T helper 17 development (Gantt et al., 2003). Replication in other populations and further understanding the role of key pathways in the host response to L. infantum may have future implications for the development of efficient and cost-effective therapeutic strategies against Leishmania infection.
Supplementary Material
Acknowledgments
This work was supported by NIH grant R01 AI076233 (JMB and MEW), AI045540 (MEW) and AI067874 (MEW, JMB). Data were collected under the Tropical Medicine Research Center grant P50 AI-30639 (SMBJ, MEW, JMB). Partial support was received from Merit Review grants from the Department of Veterans’ Affairs (1i01BX001983 and 5I01BX000536; MEW). The work was performed in part during the tenure of J.L.W. on NIH training grants T32 GM008629 and T32 GM082729. The authors are grateful to Anne Kwitek, Ph.D. and to Jeffrey Murray, M.D. for their helpful advice and discussions regarding genetic data analysis.
Footnotes
Competing Interests
The authors declare they have no competing interests.
Author contributions
JLW performed statistical genetics analyses and drafted the manuscript. PD performed and advised on statistical genetics analyses. ELN participated in subject entry and performed TGF-β assays. GRM participated in subject entry and sample processing in Brazil. DRM participated in subject entry and sample processing in Brazil. HGL entered subjects, participated in data and sample collection and data gathering. MF advised on statistical genetics analyses, and assisted in manuscript revisions. JMB advised on statistical genetics analyses, and produced the final version of the manuscript. SMBJ and MEW oversaw the study design. SMBJ initiated subject entry, data collection and sample processing, supervised field site activities, was responsible for accuracy of data entry, and revised the manuscript. MEW supervised statistical genetics analyses and drafting of the original manuscript with JLW.
Supporting Information
Table S1 contains a list of the candidate genes targeted in this study, and the numbers of SNPs genotyped in each gene. Table S2 contains the full list of SNPs that were genotyped, and the corresponding LD blocks.
References
- Abecasis GR, Cherny SS, Cookson WO, Cardon LR. Merlin--rapid analysis of dense genetic maps using sparse gene flow trees. Nat. Genet. 2002;30:97–101. doi: 10.1038/ng786. [DOI] [PubMed] [Google Scholar]
- Akhurst RJ, Padgett RW. Matters of context guide future research in TGFbeta superfamily signaling. Science signaling. 2015;8:re10. doi: 10.1126/scisignal.aad0416. [DOI] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Blackwell JM. Chapter 35: Immunogenetics of host response to parasites in humans. In: Kaufmann SHE, Rouse B, Sacks D, editors. Immunology of Infectious Diseases. Washington: ASM Publications; 2010. Immunology of Infectious Diseases. [Google Scholar]
- Blackwell JM, Fakiola M, Ibrahim ME, Jamieson SE, Jeronimo SB, Miller EN, Mishra A, Mohamed HS, Peacock CS, Raju M, Sundar S, Wilson ME. Genetics and visceral leishmaniasis: of mice and man. Parasite Immunol. 2009;31:254–266. doi: 10.1111/j.1365-3024.2009.01102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucheton B, Abel L, El-Safi S, Kheir MM, Pavek S, Lemainque A, Dessein AJ. A major susceptibility locus on chromosome 22q12 plays a critical role in the control of kala-azar. Am. J. Hum. Genet. 2003a;73:1052–1060. doi: 10.1086/379084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucheton B, Abel L, Kheir MM, Mirgani A, El-Safi SH, Chevillard C, Dessein A. Genetic control of visceral leishmaniasis in a Sudanese population: candidate gene testing indicates a linkage to the NRAMP1 region. Genes Immun. 2003b;4:104–109. doi: 10.1038/sj.gene.6363927. [DOI] [PubMed] [Google Scholar]
- Castellucci L, Jamieson SE, Almeida L, Oliveira J, Guimaraes LH, Lessa M, Fakiola M, Jesus AR, Nancy Miller E, Carvalho EM, Blackwell JM. Wound healing genes and susceptibility to cutaneous leishmaniasis in Brazil. Infect Genet Evol. 2012;12:1102–1110. doi: 10.1016/j.meegid.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellucci L, Jamieson SE, Miller EN, De Almeida LF, Oliveira J, Magalhaes A, Guimaraes LH, Lessa M, Lago E, De Jesus AR, Carvalho EM, Blackwell JM. FLI1 polymorphism affects susceptibility to cutaneous leishmaniasis in Brazil. Genes Immun. 2011;12:589–594. doi: 10.1038/gene.2011.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejghal R, Hida M, Idrissi ML, Hessni AE, Lemrani M. SLC11A1 polymorphisms and susceptibility to visceral leishmaniasis in Moroccan patients. Acta Trop. 2014;140:130–136. doi: 10.1016/j.actatropica.2014.08.013. [DOI] [PubMed] [Google Scholar]
- Ettinger NA, Duggal P, Braz RF, Nascimento ET, Beaty TH, Jeronimo SM, Pearson RD, Blackwell JM, Moreno L, Wilson ME. Genetic admixture in Brazilians exposed to infection with Leishmania chagasi. Ann. Hum. Genet. 2009;73:304–313. doi: 10.1111/j.1469-1809.2009.00510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger NA, Wilson ME. Macrophage and T-cell gene expression in a model of early infection with the protozoan, Leishmania chagasi. PLoS Neglect. Dis. 2008 doi: 10.1371/journal.pntd.0000252. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakiola M, Strange A, Cordell HJ, Miller EN, Pirinen M, Su Z, Mishra A, Mehrotra S, Monteiro GR, Band G, Bellenguez C, Dronov S, Edkins S, Freeman C, Giannoulatou E, Gray E, Hunt SE, Lacerda HG, Langford C, Pearson R, Pontes NN, Rai M, Singh SP, Smith L, Sousa O, Vukcevic D, Bramon E, Brown MA, Casas JP, Corvin A, Duncanson A, Jankowski J, Markus HS, Mathew CG, Palmer CN, Plomin R, Rautanen A, Sawcer SJ, Trembath RC, Viswanathan AC, Wood NW, Wilson ME, Deloukas P, Peltonen L, Christiansen F, Witt C, Jeronimo SM, Sundar S, Spencer CC, Blackwell JM, Donnelly P. Common variants in the HLA-DRB1-HLA-DQA1 HLA class II region are associated with susceptibility to visceral leishmaniasis. Nat Genet. 2013;45:208–213. doi: 10.1038/ng.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feitosa MF, Axevedo E, Lima AM, Krieger H. Genetic causes involved in Leishmania chagasi infection in northeastern Brazil. Genet. Mol. Biol. 1999;22:1–5. [Google Scholar]
- Fierro-Fernandez M, Busnadiego O, Sandoval P, Espinosa-Diez C, Blanco-Ruiz E, Rodriguez M, Pian H, Ramos R, Lopez-Cabrera M, Garcia-Bermejo ML, Lamas S. miR-9-5p suppresses pro-fibrogenic transformation of fibroblasts and prevents organ fibrosis by targeting NOX4 and TGFBR2. EMBO Rep. 2015;16:1358–1377. doi: 10.15252/embr.201540750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frade AF, Oliveira LC, Costa DL, Costa CH, Aquino D, Van Weyenbergh J, Barral-Netto M, Barral A, Kalil J, Goldberg AC. TGFB1 and IL8 gene polymorphisms and susceptibility to visceral leishmaniasis. Infect Genet Evol. 2011;11:912–916. doi: 10.1016/j.meegid.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Fu Y, Liu X, Zhou N, Du L, Sun Y, Zhang X, Ge Y. MicroRNA-200b stimulates tumour growth in TGFBR2-null colorectal cancers by negatively regulating p27/kip1. J Cell Physiol. 2014;229:772–782. doi: 10.1002/jcp.24497. [DOI] [PubMed] [Google Scholar]
- Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, Higgins J, Defelice M, Lochner A, Faggart M, Liu-Cordero SN, Rotimi C, Adeyemo A, Cooper R, Ward R, Lander ES, Daly MJ, Altshuler D. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- Gantt KR, Schultz-Cherry S, Rodriguez N, Jeronimo SM, Nascimento ET, Goldman TL, Recker TJ, Miller MA, Wilson ME. Activation of TGF-beta by Leishmania chagasi: importance for parasite survival in macrophages. J. Immunol. 2003;170:2613–2620. doi: 10.4049/jimmunol.170.5.2613. [DOI] [PubMed] [Google Scholar]
- Gomes NA, Gattass CR, Barreto-De-Souza V, Wilson ME, Dosreis GA. TGF-beta mediates CTLA-4 suppression of cellular immunity in murine kalaazar. J. Immunol. 2000;164:2001–2008. doi: 10.4049/jimmunol.164.4.2001. [DOI] [PubMed] [Google Scholar]
- Im YH, Kim HT, Lee C, Poulin D, Welford S, Sorensen PH, Denny CT, Kim SJ. EWS-FLI1, EWS-ERG, and EWS-ETV1 oncoproteins of Ewing tumor family all suppress transcription of transforming growth factor beta type II receptor gene. Cancer Res. 2000;60:1536–1540. [PubMed] [Google Scholar]
- Jamieson SE, Miller EN, Peacock CS, Fakiola M, Wilson ME, Bales-Holst A, Shaw MA, Silveira F, Shaw JJ, Jeronimo SM, Blackwell JM. Genome-wide scan for visceral leishmaniasis susceptibility genes in Brazil. Genes Immun. 2007;8:84–90. doi: 10.1038/sj.gene.6364357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeronimo SM, Duggal P, Ettinger NA, Nascimento ET, Monteiro GR, Cabral AP, Pontes NN, Lacerda HG, Queiroz PV, Gomes CE, Pearson RD, Blackwell JM, Beaty TH, Wilson ME. Genetic predisposition to self-curing infection with the protozoan Leishmania chagasi: a genomewide scan. J. Infect. Dis. 2007a;196:1261–1269. doi: 10.1086/521682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeronimo SM, Holst AK, Jamieson SE, Francis R, Martins DR, Bezerra FL, Ettinger NA, Nascimento ET, Monteiro GR, Lacerda HG, Miller EN, Cordell HJ, Duggal P, Beaty TH, Blackwell JM, Wilson ME. Genes at human chromosome 5q31.1 regulate delayed-type hypersensitivity responses associated with Leishmania chagasi infection. Genes Immun. 2007b;8:539–551. doi: 10.1038/sj.gene.6364422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane MM, Mosser DM. The role of IL-10 in promoting disease progression in leishmaniasis. J. Immunol. 2001;166:1141–1147. doi: 10.4049/jimmunol.166.2.1141. [DOI] [PubMed] [Google Scholar]
- Kumar R, Nylen S. Immunobiology of visceral leishmaniasis. Front Immunol. 2012;3:251. doi: 10.3389/fimmu.2012.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Shi JY, Zhu GQ, Shi B. MiR-17-92 cluster regulates cell proliferation and collagen synthesis by targeting TGFB pathway in mouse palatal mesenchymal cells. Journal of cellular biochemistry. 2012;113:1235–1244. doi: 10.1002/jcb.23457. [DOI] [PubMed] [Google Scholar]
- Li M, Boehnke M, Abecasis GR. Joint modeling of linkage and association: identifying SNPs responsible for a linkage signal. Am J Hum Genet. 2005;76:934–949. doi: 10.1086/430277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles SA, Conrad SM, Alves RG, Jeronimo SM, Mosser DM. A role for IgG immune complexes during infection with the intracellular pathogen Leishmania. J. Exp. Med. 2005;201:747–754. doi: 10.1084/jem.20041470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EN, Fadl M, Mohamed HS, El Zein A, Jamieson SE, Cordell HJ, Peacock CS, Fakiola M, Raju M, Khalil EA, El Hassan AM, Ibrahim ME, Blackwell JM. Y chromosome lineage- and village-specific genes on chromosomes 1p22 and 6q27 that control visceral leishmaniasis in The Sudan. PLoS Genet. 2007;3:679–688. doi: 10.1371/journal.pgen.0030071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed HS, Ibrahim ME, Miller EN, White JK, Cordell HJ, Howson JMM, Peacock CS, Khalil EaG, Elhassan AM, Blackwell JM. SLC11A1 (formerly NRAMP1) and susceptibility to visceral leishmaniasis in The Sudan. Eur J Hum Genet. 2004;12:66–74. doi: 10.1038/sj.ejhg.5201089. [DOI] [PubMed] [Google Scholar]
- Peacock CS, Collins A, Shaw MA, Silveira F, Costa J, Coste CH, Nascimento MD, Siddiqui R, Shaw JJ, Blackwell JM. Genetic epidemiology of visceral leishmaniasis in northeastern Brazil. Genet Epidemiol. 2001;20:383–396. doi: 10.1002/gepi.8. [DOI] [PubMed] [Google Scholar]
- Pruim RJ, Welch RP, Sanna S, Teslovich TM, Chines PS, Gliedt TP, Boehnke M, Abecasis GR, Willer CJ. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics. 2010;26:2336–2337. doi: 10.1093/bioinformatics/btq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez NE, Chang HK, Wilson ME. Novel program of macrophage gene expression induced by phagocytosis of Leishmania chagasi. Infect Immun. 2004;72:2111–2122. doi: 10.1128/IAI.72.4.2111-2122.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks D, Noben-Trauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nat Rev Immunol. 2002;2:845–858. doi: 10.1038/nri933. [DOI] [PubMed] [Google Scholar]
- Wei W, Hou J, Alder O, Ye X, Lee S, Cullum R, Chu A, Zhao Y, Warner SM, Knight DA, Yang D, Jones SJ, Marra MA, Hoodless PA. Genome-wide microRNA and messenger RNA profiling in rodent liver development implicates mir302b and mir20a in repressing transforming growth factor-beta signaling. Hepatology. 2013;57:2491–2501. doi: 10.1002/hep.26252. [DOI] [PubMed] [Google Scholar]
- Weirather JL, Duggal P, Nascimento EL, Monteiro GR, Martins DR, Lacerda HG, Fakiola M, Blackwell JM, Jeronimo SMB, Wilson ME. Fine mapping under linkage peaks for symptomatic or asymptomatic outcomes of Leishmania infantum infection in Brazil. Infection, Genetics and Evoluation. 2016 doi: 10.1016/j.meegid.2016.05.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigginton JE, Abecasis GR. PEDSTATS: descriptive statistics, graphics and quality assessment for gene mapping data. Bioinformatics. 2005;21:3445–3447. doi: 10.1093/bioinformatics/bti529. [DOI] [PubMed] [Google Scholar]
- Wigginton JE, Cutler DJ, Abecasis GR. A note on exact tests of Hardy-Weinberg equilibrium. Am J Hum Genet. 2005;76:887–893. doi: 10.1086/429864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson ME, Jeronimo SM, Pearson RD. Immunopathogenesis of infection with the visceralizing Leishmania species. Microb Pathog. 2005;38:147–160. doi: 10.1016/j.micpath.2004.11.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.