Abstract
The lack of a reliable immunosuppressive regimen that effectively suppresses both renal and islet allograft rejection without islet toxicity hampers a wider clinical application of simultaneous islet and kidney transplantation (SIK). Seven MHC mismatched SIKs were performed in diabetic cynomolgus monkeys. Two recipients received rabbit ATG induction followed by daily tacrolimus and rapamycin (ATG/Tac/Rapa) and five recipients were treated with anti-CD40 monoclonal antibody (mAb) and rapamycin (aCD40/Rapa). Anti-inflammatory therapy, including anti-IL-6R mAb and anti-TNF alpha mAb, was given in both groups. The ATG/Tac/Rapa recipients failed to achieve long-term islet allograft survival (19, 26 days) due to poor islet engraftment and CMV pneumonia. In contrast, the aCD40/Rapa regimen provided long-term islet and kidney allograft survival (90, 94, >120, >120, >120 days), with only one recipient developing evidence of allograft rejection. The aCD40/Rapa regimen was also tested in four kidney alone transplant recipients. All four recipients achieved long-term renal allograft survival (100% at day 120), which was superior to renal allograft survival (62.9% at day 120) with triple immunosuppressive regimen (tacrolimus, MMF and steroids). The combination of anti-CD40 mAb and rapamycin is an effective and non-toxic immunosuppressive regimen that utilizes only clinically available agents for kidney and islet recipients.
Introduction
Pancreatic islet transplantation (PITx) can restore beta cell function in type I diabetes (T1D) (1, 2) to achieve both euglycemia and potential prevention and even reversal of diabetic complications (3–5). Since the advent of the Edmonton protocol that achieved long-term islet allograft survival without steroids (6, 7), the results of islet transplantation have steadily improved, with recent report indicating that 5 year insulin independence can be achieved in nearly 50% of recipients treated with T cell depletion and anti-inflammatory therapy after isolated islet allograft transplantation (8, 9).
Approximately 10% of T1D patients develop end stage renal disease (ESRD) by 30 years after diagnosis (10) and over 1000 simultaneous pancreas and kidney transplants (SPK) have been performed yearly in the United States with pancreas graft function being achieved in over 80% of these recipients. The major disadvantage of SPK continues to be the morbidity associated with the procedure. Perez-Saez et al. (11) reported that more than 75% of SPK recipients developed infectious complications in the early postoperative period and nearly one third required reoperation primarily because of bleeding or infection. In contrast, simultaneous islet and kidney transplantation (SIK) has proved to be a safer procedure compared to SPK (12, 13). Nevertheless, SIK is rarely performed clinically, partially because a reliable immunosuppressive regimen that effectively suppresses rejection of both kidney and islet allografts without toxic effects on the islet allograft remains to be defined (14). An ideal immunosuppressive regimen for SIK should not include long-term steroid or high dose calcineurin inhibitor administration (15), both of which have significant adverse effects on pancreatic beta cell function and glucose metabolism. In the current study, we evaluated chronic anti-CD40 monoclonal antibody (mAb) and rapamycin therapy for SIK in cynomolgus monkeys.
MATERIALS AND METHODS
Animals and pair selections
A total of 22 cynomolgus monkeys (including donor animals) (Charles River Primates, Wilmington, MA) that weighed 3–8 kg were used for this study. Donors and recipients were paired on the basis of ABO blood type compatibility and major histocompatibility complex (MHC) mismatching (Supplemental Figure 1). MHC characterization was performed as previously described (16, 17). In each SIK case, one donor (weight range 4.9–7.9 kg) was used for one recipient (weight range 3.1–7.5 kg) (Table 1). All surgical procedures and postoperative care of animals were performed in accordance with National Institute of Health guidelines for the care and use of primates and were approved by the Massachusetts General Hospital Institutional Animal Care and Use Committee. The historical data of the rejection free allograft survival on kidney alone transplant (KTx) were from our previous studies on the delayed tolerance where the KTx recipients were treated with a triple immunosuppressive regimen (tacrolimus, mycophenolate mofetil and prednisone) until delayed donor bone marrow transplantation at day 120 after kidney transplantation (18, 19).
Table 1.
Summary of donors, recipients, and transplanted islets.
| Animal No. | M3513 | M3213 | M5113 | M5813 | M7113 | M6813 | M2414 |
|---|---|---|---|---|---|---|---|
| MHC class I | 4/4 | 4/4 | 3/4 | 2/4 | 2/4 | 2/4 | 2/4 |
| mismatch class II | 6/6 | 6/6 | 3/6 | 3/6 | 3/6 | 3/6 | 3/6 |
| Recipient (kg) | 6.1 | 6.4 | 5.6 | 5.9 | 7.5 | 5.9 | 3.1 |
| Donor (kg) | 7.2 | 6.5 | 5.3 | 5 | 7.9 | 7.9 | 4.9 |
| Pancreas weight (g) | 8.9 | 8.5 | 7.5 | 7.1 | 11.2 | 10.8 | 8.4 |
| Islet particle number | 188300 | 217500 | 135000 | 114000 | 126000 | 193500 | 106500 |
| Islet Equivalent (IEQ) | 110500 | 115800 | 118900 | 93300 | 121000 | 123900 | 100400 |
| Islet purity (%) | 80 | 90 | 75 | 85 | 80 | 75 | 80 |
| Islet viability (%) | 99 | 99 | 98 | 90 | 95 | 95 | 95 |
| IEQ/BW (kg) | 18115 | 18094 | 21232 | 15814 | 16133 | 21000 | 32387 |
Treatment regimens
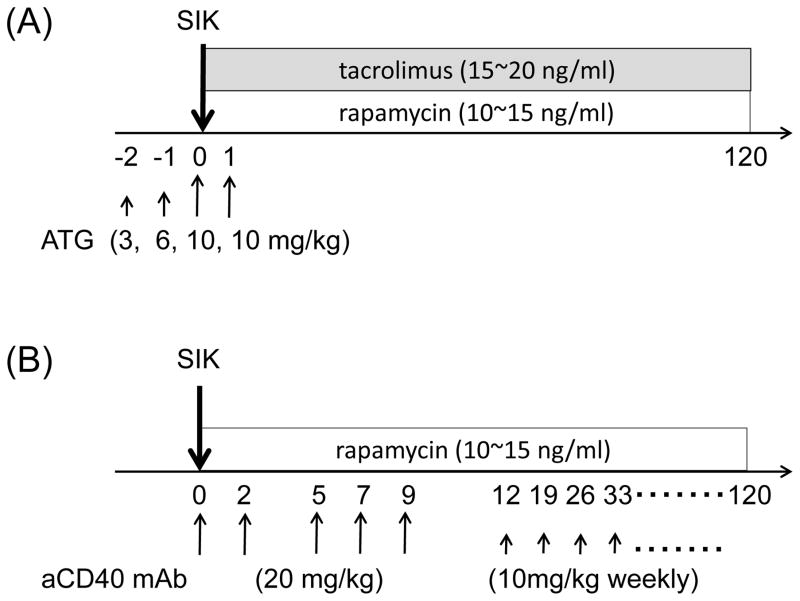
ATG/Tac/Rapa regimen (Figure 1A): anti-thymocyte globulin-rabbit (ATG) (Genzyme, Cambridge, MA) was given intravenously (i.v.) at day −2 (3 mg/kg), −1 (6 mg/kg), 0 (10 mg/kg), and +1 (10 mg/kg). Daily tacrolimus (Astellas Pharma US, Inc., Deerfield, IL) (trough; 15~20 ng/ml) and rapamycin (LC laboratories, Woburn, MA) (trough; 10~15 ng/ml) were administered intramuscularly (i.m.) from day 0.
Figure 1. Treatment of ATG/Tac/Rapa or aCD40/Rapa based on regimen.
Two to three weeks before simultaneous islet and kidney transplantation (SIK), all seven cynomolgus monkey recipients received streptozotocin (75 mg/kg) for induction of diabetes. (A) Two cynomolgus monkey recipients received 4 doses of anti-thymocyte globulin (ATG: 3, 6, 10, 10 mg/kg) on days −2, −1, 0, and 1 relative to SIK. Daily tacrolimus (trough; 15~20 ng/ml) and rapamycin (trough; 10~15 ng/ml) were administered starting from day 0. (B) Five cynomolgus monkey recipients received 5 doses of anti-CD40 monoclonal antibody (mAb) (2C10R4: 20 mg/kg) on days 0, 2, 5, 7, and 9 relative to SIK, and 10 mg/kg weekly thereafter. Daily rapamycin (trough; 10~15 ng/ml) was given from day 0. Anti-inflammatory therapies including anti-IL-6R mAb (10 mg/kg) on days 0 and 5, and anti-TNF alpha mAb (25 mg) on days 0, 3, 7, and 10 were given in both groups.
aCD40/Rapa regimen (Figure 1B): anti-CD40 mAb (Nonhuman Primate Reagent Resource, Boston, MA) (2C10R4; 20 mg/kg) was given i.v. on days 0, 2, 5, 7, and 9. Thereafter, weekly administration at 10 mg/kg/dose was continued. Daily rapamycin (trough; 10~15 ng/ml) was administered i.m. from day 0.
Anti-inflammatory therapy
Both ATG/Tac/Rapa and aCD40/Rapa groups received identical anti-inflammatory therapy. Tocilizumab (anti-IL-6R mAb; 10 mg/kg) (Chugai Pharm, Tokyo, Japan) was administered i.v. on days 0 and 5. Etanercept (anti-TNF alpha mAb; 25 mg) (Immunex, Seattle, WA) was given i.v. one hour before islet transplantation and was administered subcutaneously on days 3, 7, and 10.
Diabetes induction and management
Two to three weeks before SIK, recipients received streptozotocin (STZ) 75 mg/kg i.v. (Zanosar, Teva Parenteral Medicines, Irvine, CA). Thereafter, blood glucose (BG) levels were monitored twice daily via tail pricking Accu-Check active II (Roche, Indianapolis, IN). Diabetes was defined as three consecutive fasting BG (FBG) readings >250 mg/dL and C-peptide levels <50 pg/mL (C-peptide, Luminex, Merck Millipore, Billerica, MA). Insulin (Neutral Protamine Hagedorn and Humulin R, Eli Lilly Co., Indianapolis, IN) and Lantus (Eli Lilly Co.) was administered by sliding scale to achieve BG <200 mg/dL pretransplant.
Islet isolation and SIK
As previously reported, the protocol of islet isolation was based on modified human islet isolation techniques (20).
Under general anesthesia, heterotopic kidney transplantation was performed as previously described (21) following which the islet allograft was infused into the portal vein. Islet infusion was performed via cannulation of a branch of the superior mesenteric vein with an 18-gauge catheter to access the portal system. The islet preparation was slowly infused by gravity over a period of approximately 10–15 min.
Definition of islet rejection and BG control after rejection
The loss of Islet function was diagnosed when the FBG level exceeded 250 mg/dL for three consecutive days. The date of graft loss was defined as the first of the three consecutive days. Subsequent to the loss of islet function, recipient animals were treated with exogenous insulin to maintain BG levels in the range of <200 mg/dL. Definitive diagnosis of the islet loss was made by liver biopsy or autopsy.
Measurement of plasma C-peptide
Serum C-peptide levels were measured by a Luminex (Merck Millipore, Billerica, MA).
Intravenous glucose tolerance test (IVGTT)
After fasting for 15 hours, the test animals were given 0.5 g/kg glucose in 25% glucose solution intravenously over 1 min. Blood samples were collected before glucose injection and at 1, 3, 5, 10, 15, 30, 45 and 60 min after glucose injection for assessment of BG, serum C-peptide level. IVGTTs were performed before and after STZ-administration, and sequentially after SIK.
Flow cytometric analyses
PBMCs were labeled with a combination of the following mAbs: CD3 (SP 34-2), CD4 (L-200), CD8 (SK1), CD20 (2H7), CD21 (B-ly4), CD27 (M-T 271), CD28 (CD28.2), CD95 (DX2), and IgM (G20-127) (BD Pharmingen, San Jose, CA). CD38 (OKT10) was purchased from Nonhuman Primate Reagent Resource (Boston, MA). Phenotypic markers for memory and naive T cells in cynomolgus monkeys were chosen based on the studies by Pitcher et al (22). And also mature and immature B cells were chosen based on the studies by Liu et al (23). The fluorescence of the stained samples was analyzed using FACSverse (BD Biosciences) and Acculi flow cytometers (BD PharMingen, San Jose, CA), and FlowJo software (Tree Star, Inc., Ashland, OR).
Measurement of T cell mediated alloresponses by ELIspot
The frequencies of donor-reactive interferon (IFN)-γ–secreting T cells in the periphery were determined by the Monkey IFN-γ kit (Mabtech, Cleveland, OH, USA) (24).
Alloantibody measurement
Anti-donor-specific alloantibody (DSA) was detected by flow cytometric analysis (25).
Results
Allogeneic Islet/Kidney graft survival and outcome
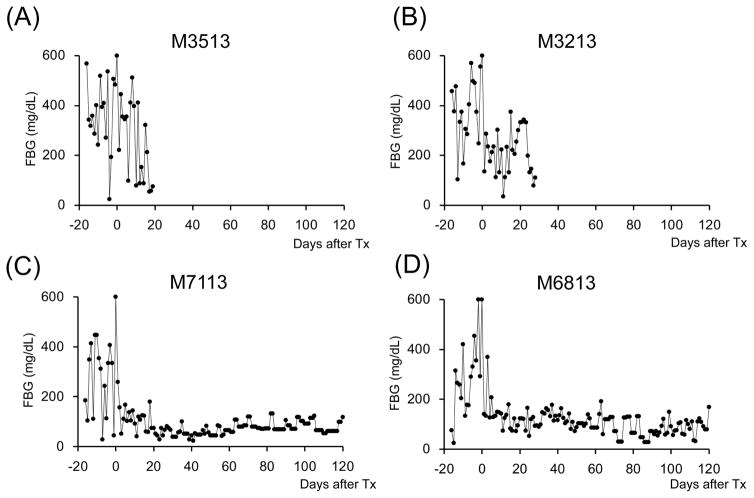
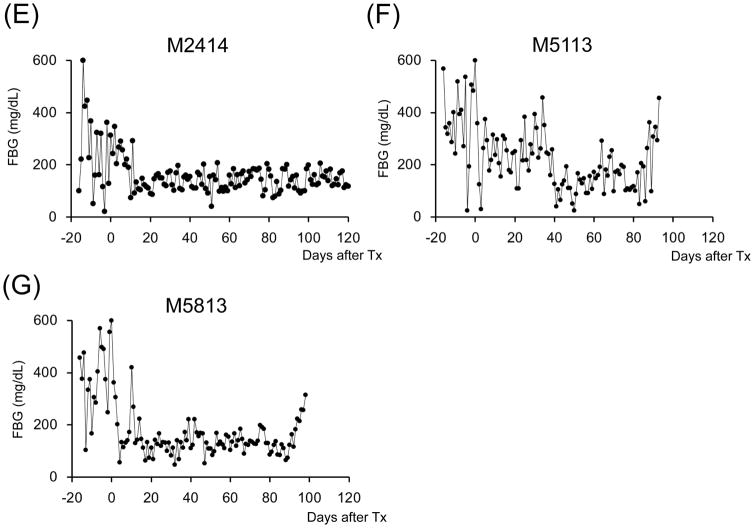
A total of seven cynomolgus monkey recipients were rendered diabetic by STZ and received single donor SIK and immunosuppression with either the ATG/Tac/Rapa (Figure 1A, n=2) or aCD40/Rapa regimen (Figure 1B, n=5). Post-STZ administration, all animals manifested robust diabetes pretransplant even with 20–40 units of daily insulin administration (Supplemental Figure 2). The MHC incompatibility with the donor and the quality of the transplanted islets are summarized in Table 1. Both recipients treated with the ATG/Tac/Rapa regimen achieved only partial engraftment with limited islet function (Figures 2A and 2B) and both died on days 19 and 26 due to cytomegalovirus (CMV) pneumonia (Table 2). There was no histopathologic evidence of kidney (Figure 3A) or islet (Figure 3B) rejection at the time of euthanasia. In the aCD40/Rapa group, all recipients achieved prolonged islet (Figures 2C–G) and kidney allograft survival (90, 94, >120, >120, >120 days) without any major complications (Table 2). Islet and kidney allograft function of the three aCD40/Rapa recipients that survived >120 days were excellent during the entire observation time (Table 2) (Figures 2C–E and Supplemental Figure 3) with no evidence of rejection in either allograft (Figures 3C–D). In the one recipient (M5113) that achieved only partial engraftment of islets and eventually lost islet function after day 90 (Figure 2F and Supplemental Figure 3), there was no evidence of rejection in either the islet or the kidney allograft at biopsy (Figure 3E). In this group, one recipient (M5813) did develop acute antibody mediated rejection (ABMR) and lost islet and kidney allografts on days 94 and 98 (Figures 2G, 3F, and 3G), respectively (Table 2). The rejection observed in this particular animal may have been related to multiple blood transfusions required after the recipient developed sepsis from a tail infection.
Figure 2. Glycemic control before and after SIK.
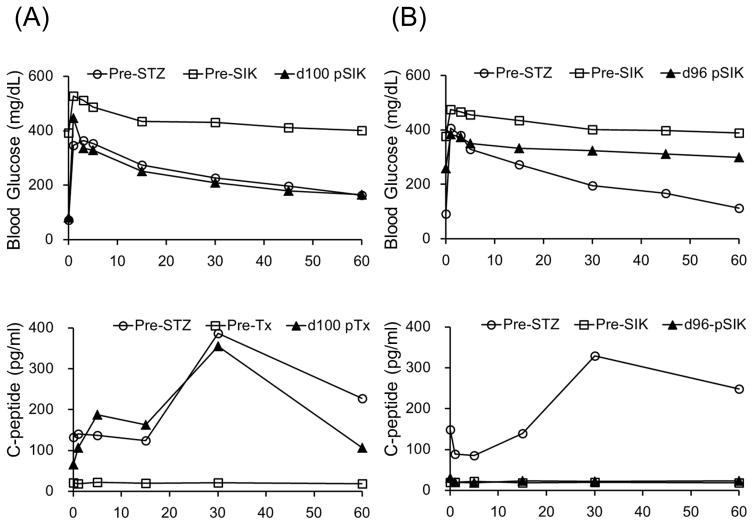
All cynomolgus monkey recipients after diabetic induction by streptozotocin (STZ) infusion showed very poor glycemic control even taking 20–40 units per day of insulin administration. Post-transplantation daily low-dose (2–4 units) insulin was administered to all animals to help initial islet engraftment and prevent exhaustions of islet allografts. The two ATG/Tac/Rapa-treated recipients showed insufficient islet allograft function (A and B). In contrast, all aCD40/Rapa-treated animals (C, D, E, and G) except for M5113 (F) showed promptly normalized fasting blood glucose (FBS) levels. Although two animals (F and G) lost islet allograft functions at days 90 and 94 after SIK, the remaining three recipients manifested normoglycemic conditions more than 120 days after SIK.
Table 2.
Treatment regimens and allograft survivals
| Animal No. | Treatment
|
Islet | Kidney | Comments | |||
|---|---|---|---|---|---|---|---|
| ATG | CD40 | Tac/Rapa | Rapa | ||||
| M3513 | + | + | 1 PELF | >19 | 2 CMV (19 3 POD) | ||
| M3213 | + | + | 1 PELF | >26 | 2 CMV (26 3 POD) | ||
| M5113 | + | + | 90 | >120 | Kidney: No rejection | ||
| M5813 | + | + | 94 | 98 | 4 ABMR | ||
| M7113 | + | + | >120 | >120 | Both: No rejection | ||
| M6813 | + | + | >120 | >120 | Both: No rejection | ||
| M2414 | + | + | >120 | >120 | Both: No rejection | ||
| M4514 | + | + | - | >120 | No rejection | ||
| M3115 | + | + | - | >120 | No rejection | ||
| M2815 | + | + | - | >120 | No rejection | ||
| M4315 | + | + | - | >120 | No rejection | ||
Partial Engraftment with Limited Function,
Cytomegalovirus pneumonia,
Post Operative Day,
Antibody Mediated Rejection
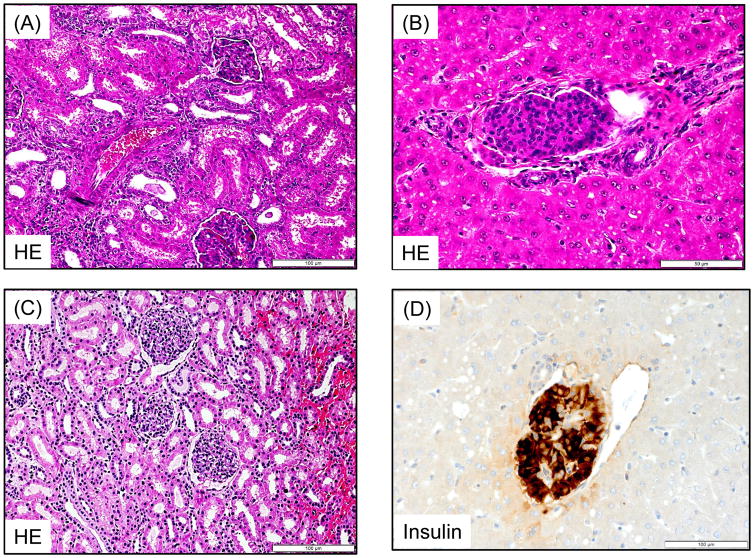
Figure 3. Islet and Kidney allograft histopathological findings.
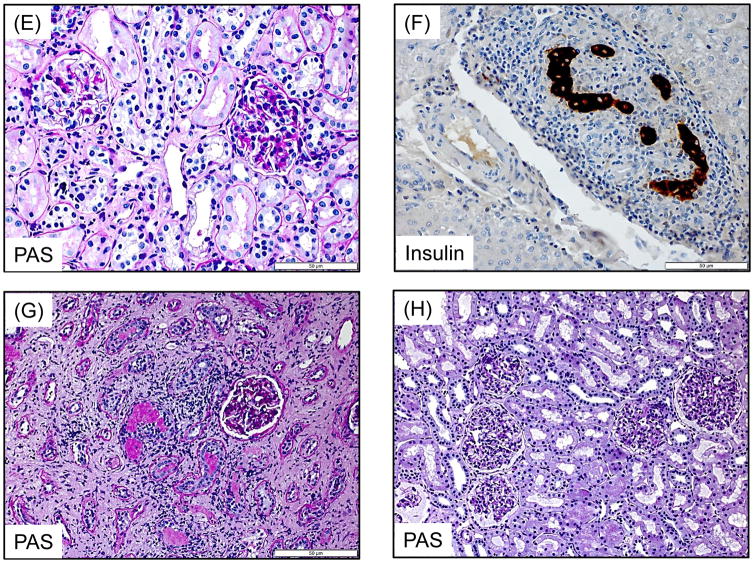
A recipient (M3213) treated with the ATG/Tac/Rapa regimen showed no evidence of rejection in the kidney (H&E, 200x) (A) and islet allograft (H&E, 400x) (B) at autopsy. In the aCD40/Rapa group, a representative recipient that survived for >120 days (M2414) showed no evidence of rejection in the kidney (H&E, 400x) (C) and islet (Insulin, 400x) (D) allografts on day 96. M5113 lost islet allograft function on day 90 but showed no evidence of rejection in the kidney allograft (PAS, 400x) (E) on day 104. M5813 which lost islet allograft function on day 94 showed peri-islet dense mononuclear infiltrates (Insulin, 400x) (F). In the kidney allograft, extensive interstitial fibrosis with focal mononuclear infiltration (PAS, 200x) (G) was observed on day 98. Renal allograft biopsy on day 104 after KTx (M2815) showed no diagnostic abnormality (PAS, 200x) (H).
Anti-CD40/Rapa regimen for KTx
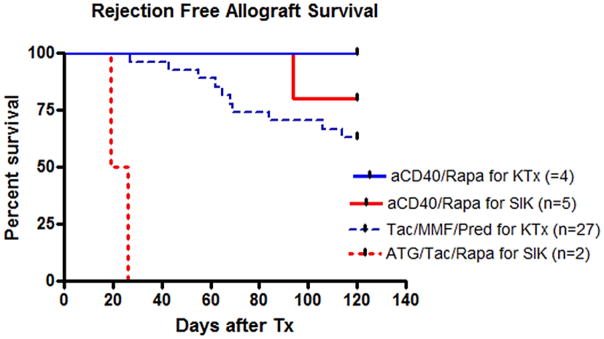
To further evaluate the clinical value of the aCD40/Rapa regimen, four additional KTx recipients were treated with aCD40/Rapa. All four recipients achieved long-term renal allograft survival without rejection (100% graft survival at day 120), which was superior to our historical results of the renal allograft survival (62.9% at day 120) in 27 recipients treated with triple immunosuppressive regimen (tacrolimus, mycophenolate mofetil, and prednisone) (Figure 4).
Figure 4. aCD40/Rapa for kidney alone transplantation.
Four kidney alone transplant recipients were treated with aCD40/Rapa. All four recipients achieved long-term renal allograft survival without rejection or infectious complications. This result was clearly superior to the historical results of the renal allograft survival in recipients treated with triple immunosuppressive regimen, where renal allograft survival at day 120 was only 62.9%. Blue solid line: KTx graft survival by aCD40/Rapa (n=4), Blue dotted line: KTx survival by triple immunosuppression (n=27), red solid line: SIK graft survival by aCD40/Rapa (n=5), and Red dotted line: SIK survival by ATG/Tac/Rapa (n=2).
Histological features of transplanted islet and kidney allografts
Autopsy of the two recipients in the ATG/Tac/Rapa group showed no rejection in either kidney or islet allografts (Figures 3A and 3B). Although their islet function was limited, there was no inflammation around islets (Figure 3B). In the aCD40/Rapa group, the biopsy from three recipients with stable kidney and islet function showed no rejection or inflammation (Figures 3C and 3D). Although M5113 lost islet allograft function after day 90, there was no histological evidence of rejection in his kidney allograft (Figure 3E). In contrast, M5813 that lost islet and kidney allograft function after day 94 due to ABMR showed islets with positive insulin staining surrounded by dense mononuclear cell infiltration (Figure 3F). The kidney allograft of this recipient also showed focal mononuclear cell infiltration with extensive interstitial fibrosis (Figure 3G). No diagnostic abnormality was found in four KTx recipients of renal allografts (Fig. 3H).
FBG and function of islet allografts in aCD40/Rapa-treated cynomolgus monkeys
To evaluate the function of the transplanted islet allografts, IVGTT was performed sequentially at pre-STZ administration, just before SIK, and post-SIK (Figure 5). After STZ administration, complete induction of insulin dependent diabetes was confirmed in all animals based on blood glucose levels and the absence C-peptide on IVGTT. Three recipients in the aCD40/Rapa group (M7113, M6813, and M2414) showed normal IVGTT after SIK, which was comparable to pre-STZ administration (Figure 5A). Two recipients (M5113 and M5813) that lost islet function showed abnormal IVGTT on days 92 and 96, respectively (Figure 5B).
Figure 5. Islet allograft function in aCD40/Rapa-treated cynomolgus monkeys.
IVGTT was performed. Representative data for rejection free (M6813) and rejected (M5813) animals are shown. IVGTT was performed before (Pre-STZ; white circle), just before SIK (Pre-SIK; white square), and at d100 post-SIK (black triangle) or rejection (black triangle). After receiving STZ treatment, no C-peptide responses were observed against IVGTT in both monkeys. In the animal showing rejection free, BG and C-peptide levels remained normal even at d100 post-SIK (A). In contract, In the animal showing rejection (M5813), BG in response to IVGTT performed at 2 days after rejection showed a diabetic pattern and C-peptide level did not response to glucose injection (B).
Changes in Lymphocyte Subsets in aCD40/Rapa
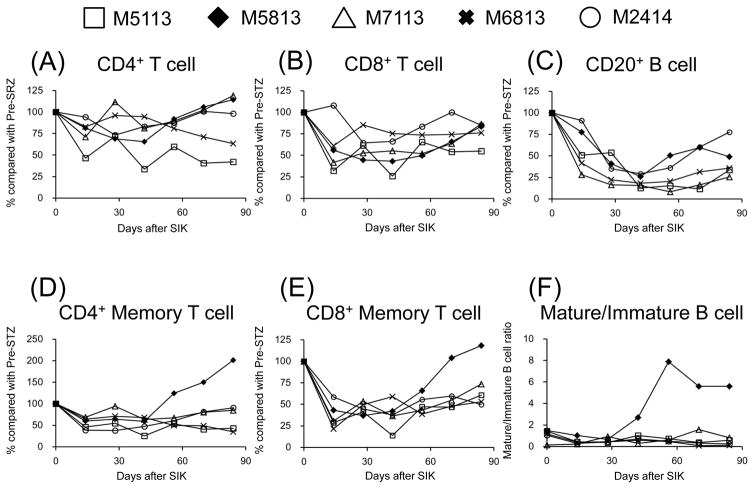
Figure 6 shows changes in lymphocyte subsets during treatment with aCD40/Rapa. Although no significant changes were noted in total CD4+ T cell and CD8+ cell counts (Figures 6A and 6B), suppression of both CD4+ and CD8+ memory T cells was observed during the treatment (Figures 6D and 6E). These memory T cells remained suppressed in the recipients without rejection, while both CD4+ and CD8+ memory T cells increased after day 50 in the recipient (M5813) that developed rejection (Figures 6D and 6E). CD3−CD20+ B cell counts decreased in all recipients (Figure 6C), but a higher mature B cell/immature B cell ratio was observed in the recipient with ABMR (Figure 6F).
Figure 6. Changes in peripheral lymphocyte counts following aCD40/Rapa treatments.
Counts of peripheral CD4+ (A) and CD8+ (B) T cell, and CD3-CD20+ B cell (C) compared with those of Pre-STZ following aCD40/Rapa treatment are shown. Memory phenotype (CD28+−CD95+) of CD4+ (D) and CD8+ (E) T cells, and B cell ratio of mature (CD20brightCD21−CD38+ + CD27+IgM−) and immature (CD20dimCD21+CD38+ + CD27−IgM+) (F) are also examined. White square, black diamond, white triangle, X-mark, and white circle represents M5113, M5813, M7113, M6813, and M2414, respectively. Although no marked differences were noted in the behaviors of peripheral CD4+, CD8+, and CD3−CD20+ lymphocytes between immunologically rejected and non-rejected (A, B, and C), rejected animals showed preponderances of memory T cells (D and E) and maturation of B cell (F).
Cellular immune responses
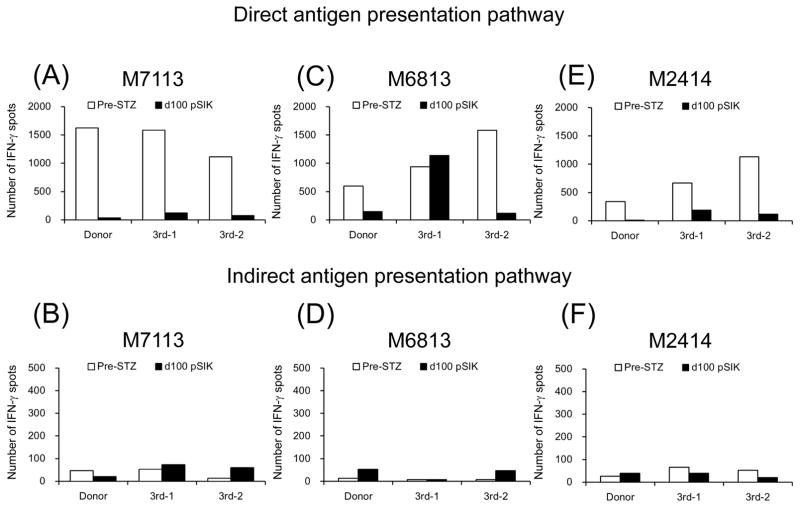
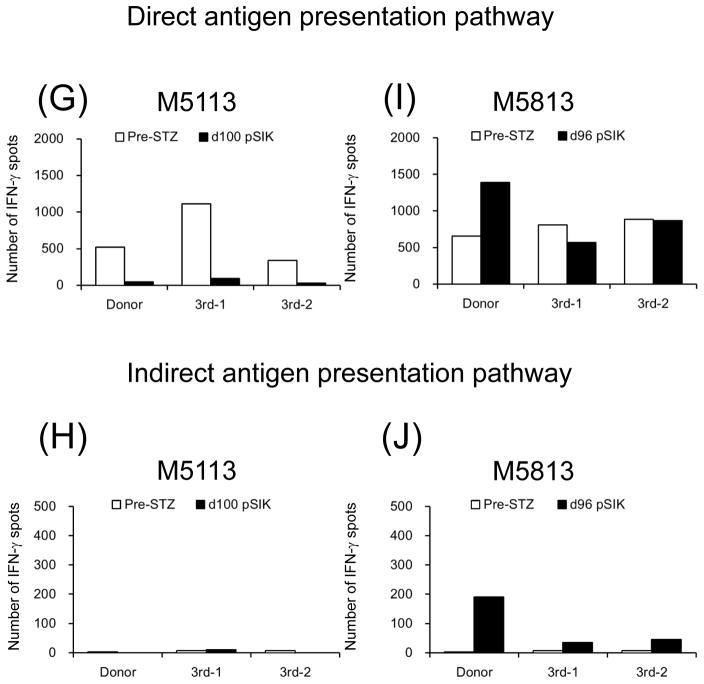
Figure 7 shows direct and indirect anti-donor T cell responses examined by the IFN-γ ELIspot assay. In the four aCD40/Rapa recipients without rejection, both direct and indirect pathways, anti-donor and anti-third party responses were effectively suppressed (Figures 7A–H), except for anti-third party responses observed in M6813 on day 100 (Figure 7C). In contrast, strong anti-donor responses were observed in both direct and indirect pathways in the recipient (M5813) that rejected the kidney and islet allografts (Figures 7I and 7J).
Figure 7. Cellular alloimmune responses to allografts.
The frequencies of directly and indirectly stimulated, donor- or 3rd party-reactive IFN-γ–secreting T cells were evaluated with ELIspot assay. White and black bars represent numbers of IFN-γ spots per 1 million T cells at pre-STZ and d100 post-SIK or rejected, respectively. Except for immunologically rejected animal (M5813) (I and J), all aCD40/Rapa-treated recipients (A, B, E, F, G, H, I, and J) showed hypo-responsiveness against donor antigen stimulations at d100 post-SKT compared with those of Pre-STZ.
Humoral immune responses
In the five aCD40/Rapa treated animals, all recipients except for M5813 did not develop DSA during treatment course. M5813 developed DSA on days 98 after SIK.
Discussion
Although the clinical benefits of SPK have been clearly demonstrated (26), SPK has been associated with high postoperative morbidity and mortality (11). Therefore, SIK could provide a better option with fewer surgical complications. Nevertheless, SIK has been reported only from a few small trials. Lehmann et al. (27) reported successful SIK with daclizumab induction followed by a combination of rapamycin and tacrolimus. This steroid-free regimen resulted in significant improvement in HbA1c. However, 5-year insulin independence was observed in only 9.3% of the recipients which was comparable to the results of islet alone transplantation by the Edmonton group (7) and significantly lower than that in achieved after SPK (73.6%) (27). Tan et al. (28) reported seven cases of SIK treated with a steroid-free immunosuppressive regimen that consisted of alemtuzumab, rapamycin and tacrolimus. Although four patients achieved insulin independence, three of them required sequential islet transplants from multiple donors in order to achieve insulin independence. Although the cause of transplant islet loss may be multifactorial, the toxicity of immunosuppressive drugs, especially calcineurin inhibitor (CNI), may play a role. In addition, tacrolimus/sirolimus therapy has been associated with numerous toxicities including renal dysfunction, anemia and recurrent aphthous ulcers. Addition of mycophenolate mofetil to the regimen may be helpful (29), but development of a better immunosuppressive regimen that does not include steroids and CNI seems imperative to allow more widespread clinical application of SIK.
In this study, we have shown that SIK could be successfully performed by the therapeutic regimen including CD40 costimulatory blockade and rapamycin without the need for steroids, CNI, or T cell depletion. Our initial attempts to develop the nonhuman primate (NHP) model of SIK used an immunosuppressive regimen that has been used successfully in clinical islet transplantation (8, 9). This resulted in poor islet engraftment and death by cytomegalovirus (CMV) in our NHP model. The cause of poor engraftment of islets in two monkeys is not clear, since anti-T cell antibodies such as ATG or alemtuzumab have been successfully included as an induction therapy for isolated islet transplantation (8, 9). We speculate that the poor engraftment of islets with this regimen may be attributed to inflammatory cytokines released after ATG treatment (30) as very high C-peptide levels have been detected as early as one hour after SIK (M3213: 792 pg/ml, M5113: 281 pg/ml, M7113: 267 pg/ml) which may indicate immediate destruction of islets. CMV pneumonia might have been caused by higher tacrolimus and rapamycin levels used in these monkeys (Supplemental Figure 4). However, in our experience in NHPs, the renal allograft survival with triple immunosuppressive regimen (tacrolimus, MMF and prednisone) has been significantly inferior to clinical patients treated with the same triple immunosuppressive regimen despite higher trough levels of tacrolimus used in NHPs. Monkeys have never developed neurotoxicity or nephrotoxicity by tacrolimus and drug metabolism and optimal drug levels may be markedly different in monkeys. Inadequate prophylaxis against CMV in monkeys resulted in death due to CMV pneumonia. Interestingly, CMV disease was not observed in recipients treated with the anti-CD40/Rapa regimen despite effective suppression of rejection.
Based on previous reports in cynomolgus monkey kidney (31, 32) and islet (33) transplantation studies that used anti-CD40 mAb, we adopted anti-CD40 mAb with rapamycin for SIK (34). The aCD40/Rapa regimen appeared to be advantageous in engraftment of islets compared with the conventional immunosuppression with T cell depletion and CNI. Excellent engraftment of islets was achieved in all of the recipients treated with this regimen except for M5113 that did not quickly achieve blood glucose normalization. In terms of prevention of rejection, since two recipients treated with the ATG/Tac/Rapa regimen died early due to CMV, we could not evaluate whether the ATG/Tac/Rapa regimen can effectively suppress rejection of both islet and kidney allografts long-term. However, aCD40/Rapa regimen appeared markedly effective in preventing rejection of both islet and kidney allografts as four out of five recipients achieved long-term (>90 days) survival in SIK.
ABMR observed in one recipient (M5813) is likely the result of sensitization after repeated blood transfusions that were required during the episode of infection. Such ABMR has rarely been reported during aCD40 treatment presumably because CD40 blockade is likely to inhibit B cell sensitization or maturation. Indeed, ABMR has never been observed in kidney (31, 32), liver (24), and islet (33) transplantation during CD40 blockade in other NHP studies.
To further evaluate the clinical value of the aCD40/Rapa regimen, we tested the regimen in four KTx recipients. All four recipients achieved long-term allograft survival (100% at day 120) with no evidence of rejection or other infectious complications. Although it was not statistically significant (p=0.17), this results were clearly superior to the renal allograft survival (with the triple immunosuppressive regimen). Although Phase 2 clinical trial on the efficacy of anti-CD40 mAb in KTx is currently in progress, it has been combined with tacrolimus. The results of this clinical trial have not been reported but, based on the results in our current study, we would encourage to combine with rapamycin in future clinical trials..
In aCD40/Rapa treated NHP recipients, deletion of CD20+ B cells has been observed without expansion of the mature B cell population (23) except for a DSA developing recipient (M5813). In addition, both CD4+ and CD8+ memory T cells were effectively suppressed with negative direct and indirect anti-donor T cell responses being observed in all recipients except for one rejected recipient (M5813). The aCD40/Rapa treatment effectively suppressed effector T cell generation with inhibition of B cell maturation, which resulted in long-term SIK survival. Ligation of CD40 to CD154 on T cells plays a crucial role in cellular immunity. Signals via CD40 on dendritic cells and monocytes engender maturation of these cells into fully competent antigen-presenting cells, which can reciprocally trigger the cognate T cells to clonally expand and differentiate (35). Also, CD40 activation leads to cell proliferation, antibody production and immunoglobulin isotype switching in B cells that intensifies humoral immunity responses (36). On the basis of the role of CD40 signaling in immunity, interruption of CD40/CD154 interaction can result in inadequate antigen presentation and a deficiency in both cellular and humoral immune responses. Indeed, in this study, we have demonstrated that inhibition of CD40 signal completely suppressed anti-donor cellular responses and inhibited generation of DSA during the treatment course in the SIK recipients.
A limitation of the current study is the relatively short follow-up. The three SIK recipients that survived for >120 days without rejection were subjected to attempted tolerance induction with delayed donor bone marrow transplantation (18, 19). Thus, the longer term immunosuppressive effect of the aCD40/Rapa regimen was not studied. Nevertheless, to date, this is the only therapeutic protocol that we have found reliably provides islet engraftment and survival for months in SIK NHP recipients. In addition, the effect of aCD40 monotherapy on SIK was not evaluated in this study. Anti-CD40 monotherapy was previously reported by the Hokkaido group in NHP kidney (32) or islet (33) transplantation. Since both anti-donor cellular responses and development of DSA were abolished during the aCD40 treatment period in those studies, it is possible that a short-term immunosuppressive effect similar to what we observed could be achieved by aCD40 alone. However, it may be difficult to space out aCD40 treatment if it is administered as monotherapy. Our hypothesis has been that, for clinical application, a therapeutic protocol must be defined that requires even less frequent aCD40 treatment than weekly (current protocol in this study) and that by combining the agent with rapamycin this may be more feasible.
In conclusion, these preliminary studies provide encouraging evidence that a combination of aCD40 mAb and rapamycin can be a basis of a clinically applicable immunosuppressive regimen for SIK. Since clear immunosuppressive effects were successfully achieved without toxicity on the transplanted islets, our observation presented here warrant further efforts to extend this approach to clinical trials.
Supplementary Material
Figure S1: MHC disparity information. The MHC class I (A and B loci) and II (DP, DO, and DR loci) genes expressed by each of the recipient monkey both ATG/Tac/Rapa-treated (M3513 and M3213) (A) and aCD40/Rapa-treated (M5113, M5813, M7113, M6813, and M2414) (B), and its respective donor are shown and color-coded to highlight allelic differences.
Figure S2: Immunohistochemical studies using anti-insulin antibody. Normal native pancreas control tissue showed intense and diffuse staining in islet beta cells in low (200x, A) and high magnification (400x, B). After STZ administration, M3513 native pancreas showed reduced and focal staining in islet beta cells (arrow) (200x, C) and with staining in a few isolated beta cells (arrows) (400x, D) on 19 days after SIK.
Figure S3: Representative fasting C-peptide levels after SIK. Representative fasting C-peptide levels after SIK were presented. After receiving STZ treatment, serum C-peptide levels were undetectable in both M5113 (black square) and M2414 (white square). For M2414, C-peptide levels were restored and maintained over time. In contrast, the recovery of C-peptide levels was delayed. And C-peptide levels were gradually reduced and eventually undetectable for M5113.
Figure S4: Rapamycin and tacrolimus trough levels after SIK. Both rapamycin and tacrolimus trough levels in ATG treated group were shown (n=2, A) and rapamycin trough level in aCD40 treated group shown (n=5, B) after SIK.
Acknowledgments
This work was supported by grant U19 AI102405-01.
Abbreviations
- ABMR
antibody mediated rejection
- ATG
anti-thymocyte globulin-rabbit
- BG
blood glucose
- DSA
donor-specific allo-antibody
- ESRD
end-stage renal disease
- FBG
fasting blood glucose
- IEQ
islet equivalents
- IVGTT
intravenous glucose tolerance test
- KTx
kidney transplantation
- mAb
monoclonal antibody
- MHC
major histocompatibility complex
- NHP
nonhuman primate
- PBMC
peripheral blood mononuclear cell
- PITx
pancreas islet transplantation
- SIK
simultaneous islet and kidney transplantation
- SPK
simultaneous islet and pancreas transplantation
- STZ
streptozotocin
- T1D
type 1 diabetes
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by American Journal of Transplantation.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Ballinger WF, Lacy PE. Transplantation of intact pancreatic islets in rats. Surgery. 1972;72:175–186. [PubMed] [Google Scholar]
- 2.Reckard CR, Ziegler MM, Barker CF. Physiological and immunological consequences of transplanting isolated pancreatic islets. Surgery. 1973;74:91–99. [PubMed] [Google Scholar]
- 3.Fiorina P, Folli F, Bertuzzi F, Maffi P, Finzi G, Venturini M, et al. Long-term beneficial effect of islet transplantation on diabetic macro-/microangiopathy in type 1 diabetic kidney-transplanted patients. Diabetes Care. 2003;26:1129–1136. doi: 10.2337/diacare.26.4.1129. [DOI] [PubMed] [Google Scholar]
- 4.Warnock GL, Thompson DM, Meloche RM, Shapiro RJ, Ao Z, Keown P, et al. A multi-year analysis of islet transplantation compared with intensive medical therapy on progression of complications in type 1 diabetes. Transplantation. 2008;86:1762–1766. doi: 10.1097/TP.0b013e318190b052. [DOI] [PubMed] [Google Scholar]
- 5.Thompson DM, Meloche M, Ao Z, Paty B, Keown P, Shapiro RJ, et al. Reduced progression of diabetic microvascular complications with islet cell transplantation compared with intensive medical therapy. Transplantation. 2011;91:373–378. doi: 10.1097/TP.0b013e31820437f3. [DOI] [PubMed] [Google Scholar]
- 6.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 7.Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, et al. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060–2069. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 8.Bellin MD, Kandaswamy R, Parkey J, Zhang HJ, Liu B, Ihm SH, et al. Prolonged insulin independence after islet allotransplants in recipients with type 1 diabetes. Am J Transplant. 2008;8:2463–2470. doi: 10.1111/j.1600-6143.2008.02404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bellin MD, Barton FB, Heitman A, Harmon JV, Kandaswamy R, Balamurugan AN, et al. Potent induction immunotherapy promotes long-term insulin independence after islet transplantation in type 1 diabetes. Am J Transplant. 2012;12:1576–1583. doi: 10.1111/j.1600-6143.2011.03977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finne P, Reunanen A, Stenman S, Groop PH, Gronhagen-Riska C. Incidence of end-stage renal disease in patients with type 1 diabetes. Jama. 2005;294:1782–1787. doi: 10.1001/jama.294.14.1782. [DOI] [PubMed] [Google Scholar]
- 11.Perez-Saez MJ, Toledo K, Navarro MD, Redondo MD, Leon C, Arjona A, et al. Long-term survival of simultaneous pancreas-kidney transplantation: Influence of early posttransplantation complications. Transplant Proc. 2011;43:2160–2164. doi: 10.1016/j.transproceed.2011.05.025. [DOI] [PubMed] [Google Scholar]
- 12.Gerber PA, Pavlicek V, Demartines N, Zuellig R, Pfammatter T, Wuthrich R, et al. Simultaneous islet-kidney vs pancreas-kidney transplantation in type 1 diabetes mellitus: A 5 year single centre follow-up. Diabetologia. 2008;51:110–119. doi: 10.1007/s00125-007-0860-4. [DOI] [PubMed] [Google Scholar]
- 13.Hirshberg B. Benefits of simultaneous islet-kidney transplantation versus simultaneous pancreas-kidney transplantation. Curr Diab Rep. 2008;8:307–309. doi: 10.1007/s11892-008-0054-6. [DOI] [PubMed] [Google Scholar]
- 14.Khan MH, Harlan DM. Counterpoint: Clinical islet transplantation: Not ready for prime time. Diabetes Care. 2009;32(8):1570–1574. doi: 10.2337/dc09-0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oura T, Ko DS, Boskovic S, O’Neil JJ, Chipashvili V, Koulmanda M, et al. Kidney versus islet allograft survival after induction of mixed chimerism with combined donor bone marrow transplantation. Cell Transplant. 2015;2:2. doi: 10.3727/096368915X688966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Connor SL, Blasky AJ, Pendley CJ, Becker EA, Wiseman RW, Karl JA, et al. Comprehensive characterization of mhc class ii haplotypes in mauritian cynomolgus macaques. Immunogenetics. 2007;59:449–462. doi: 10.1007/s00251-007-0209-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pendley CJ, Becker EA, Karl JA, Blasky AJ, Wiseman RW, Hughes AL, et al. Mhc class i characterization of indonesian cynomolgus macaques. Immunogenetics. 2008;60:339–351. doi: 10.1007/s00251-008-0292-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koyama I, Nadazdin O, Boskovic S, Ochiai T, Smith RN, Sykes M, et al. Depletion of cd8 memory t cells for induction of tolerance of a previously transplanted kidney allograft. Am J Transplant. 2007;7:1055–1061. doi: 10.1111/j.1600-6143.2006.01703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamada Y, Boskovic S, Aoyama A, Murakami T, Putheti P, Smith RN, et al. Overcoming memory t-cell responses for induction of delayed tolerance in nonhuman primates. Am J Transplant. 2012;12:330–340. doi: 10.1111/j.1600-6143.2011.03795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lei J, Kim JI, Shi S, Zhang X, Machaidze Z, Lee S, et al. Pilot study evaluating regulatory t cell-promoting immunosuppression and nonimmunogenic donor antigen delivery in a nonhuman primate islet allotransplantation model. Am J Transplant. 2015;26:13329. doi: 10.1111/ajt.13329. [DOI] [PubMed] [Google Scholar]
- 21.Cosimi AB, Delmonico FL, Wright JK, Wee SL, Preffer FI, Jolliffe LK, et al. Prolonged survival of nonhuman primate renal allograft recipients treated only with anti-cd4 monoclonal antibody. Surgery. 1990;108:406–413. [PubMed] [Google Scholar]
- 22.Pitcher CJ, Hagen SI, Walker JM, Lum R, Mitchell BL, Maino VC, et al. Development and homeostasis of t cell memory in rhesus macaque. J Immunol. 2002;168:29–43. doi: 10.4049/jimmunol.168.1.29. [DOI] [PubMed] [Google Scholar]
- 23.Liu C, Noorchashm H, Sutter JA, Naji M, Prak EL, Boyer J, et al. B lymphocyte-directed immunotherapy promotes long-term islet allograft survival in nonhuman primates. Nat Med. 2007;13:1295–1298. doi: 10.1038/nm1673. [DOI] [PubMed] [Google Scholar]
- 24.Oura T, Yamashita K, Suzuki T, Fukumori D, Watanabe M, Hirokata G, et al. Long-term hepatic allograft acceptance based on cd40 blockade by askp1240 in nonhuman primates. Am J Transplant. 2012;12:1740–1754. doi: 10.1111/j.1600-6143.2012.04014.x. [DOI] [PubMed] [Google Scholar]
- 25.Boskovic S, Kawai T, Smith RN, Wee SL, Nadazdin O, Koyama I, et al. Monitoring antidonor alloantibodies as a predictive assay for renal allograft tolerance/long-term observations in nonhuman primates. Transplantation. 2006;82:819–825. doi: 10.1097/01.tp.0000234786.26511.a4. [DOI] [PubMed] [Google Scholar]
- 26.Israni AK, Feldman HI, Propert KJ, Leonard M, Mange KC. Impact of simultaneous kidney-pancreas transplant and timing of transplant on kidney allograft survival. Am J Transplant. 2005;5:374–382. doi: 10.1111/j.1600-6143.2004.00688.x. [DOI] [PubMed] [Google Scholar]
- 27.Lehmann R, Weber M, Berthold P, Zullig R, Pfammatter T, Moritz W, et al. Successful simultaneous islet-kidney transplantation using a steroid-free immunosuppression: Two-year follow-up. Am J Transplant. 2004;4:1117–1123. doi: 10.1111/j.1600-6143.2004.00468.x. [DOI] [PubMed] [Google Scholar]
- 28.Tan J, Yang S, Cai J, Guo J, Huang L, Wu Z, et al. Simultaneous islet and kidney transplantation in seven patients with type 1 diabetes and end-stage renal disease using a glucocorticoid-free immunosuppressive regimen with alemtuzumab induction. Diabetes. 2008;57:2666–2671. doi: 10.2337/db08-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaplan B, West P, Neeley H, Martellotto J, Iqbal R, Gangemi A, et al. Use of low dose tacrolimus, mycophenolate mofetil and maintenance il-2 receptor blockade in an islet transplant recipient. Clin Transplant. 2008;22:250–253. doi: 10.1111/j.1399-0012.2007.00757.x. [DOI] [PubMed] [Google Scholar]
- 30.Denny JT, Burr AT, Balzer F, Tse JT, Denny JE, Chyu D. Methylene blue treatment for cytokine release syndrome-associated vasoplegia following a renal transplant with ratg infusion: A case report and literature review. Exp Ther Med. 2015;9:1915–1920. doi: 10.3892/etm.2015.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Imai A, Suzuki T, Sugitani A, Itoh T, Ueki S, Aoyagi T, et al. A novel fully human anti-cd40 monoclonal antibody, 4d11, for kidney transplantation in cynomolgus monkeys. Transplantation. 2007;84:1020–1028. doi: 10.1097/01.tp.0000286058.79448.c7. [DOI] [PubMed] [Google Scholar]
- 32.Aoyagi T, Yamashita K, Suzuki T, Uno M, Goto R, Taniguchi M, et al. A human anti-cd40 monoclonal antibody, 4d11, for kidney transplantation in cynomolgus monkeys: Induction and maintenance therapy. Am J Transplant. 2009;9:1732–1741. doi: 10.1111/j.1600-6143.2009.02693.x. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe M, Yamashita K, Suzuki T, Kamachi H, Kuraya D, Koshizuka Y, et al. Askp1240, a fully human anti-cd40 monoclonal antibody, prolongs pancreatic islet allograft survival in nonhuman primates. Am J Transplant. 2013;13:1976–1988. doi: 10.1111/ajt.12330. [DOI] [PubMed] [Google Scholar]
- 34.Lowe M, Badell IR, Thompson P, Martin B, Leopardi F, Strobert E, et al. A novel monoclonal antibody to cd40 prolongs islet allograft survival. Am J Transplant. 2012;12:2079–2087. doi: 10.1111/j.1600-6143.2012.04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quezada SA, Jarvinen LZ, Lind EF, Noelle RJ. Cd40/cd154 interactions at the interface of tolerance and immunity. Annu Rev Immunol. 2004;22:307–328. doi: 10.1146/annurev.immunol.22.012703.104533. [DOI] [PubMed] [Google Scholar]
- 36.van Kooten C, Banchereau J. Cd40-cd40 ligand. J Leukoc Biol. 2000;67:2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: MHC disparity information. The MHC class I (A and B loci) and II (DP, DO, and DR loci) genes expressed by each of the recipient monkey both ATG/Tac/Rapa-treated (M3513 and M3213) (A) and aCD40/Rapa-treated (M5113, M5813, M7113, M6813, and M2414) (B), and its respective donor are shown and color-coded to highlight allelic differences.
Figure S2: Immunohistochemical studies using anti-insulin antibody. Normal native pancreas control tissue showed intense and diffuse staining in islet beta cells in low (200x, A) and high magnification (400x, B). After STZ administration, M3513 native pancreas showed reduced and focal staining in islet beta cells (arrow) (200x, C) and with staining in a few isolated beta cells (arrows) (400x, D) on 19 days after SIK.
Figure S3: Representative fasting C-peptide levels after SIK. Representative fasting C-peptide levels after SIK were presented. After receiving STZ treatment, serum C-peptide levels were undetectable in both M5113 (black square) and M2414 (white square). For M2414, C-peptide levels were restored and maintained over time. In contrast, the recovery of C-peptide levels was delayed. And C-peptide levels were gradually reduced and eventually undetectable for M5113.
Figure S4: Rapamycin and tacrolimus trough levels after SIK. Both rapamycin and tacrolimus trough levels in ATG treated group were shown (n=2, A) and rapamycin trough level in aCD40 treated group shown (n=5, B) after SIK.