Abstract
The main function of small heat shock proteins (sHSPs) as molecular chaperones is to protect proteins from denaturation under adverse conditions. Molecular and physiological data were used to examine the sHSPs underlying cold-hardiness in Harmonia axyridis. Complementary DNA sequences were obtained for six H. axyridis sHSPs based on its transcriptome, and the expression of the genes coding for these sHSPs was evaluated by quantitative real-time PCR (qRT-PCR) in several developmental stages, under short-term cooling or heating conditions, and in black and yellow females of experimental and overwintering populations under low-temperature storage. In addition, we measured water content and the super cooling and freezing points (SCP and FP, respectively) of H. axyridis individuals from experimental and overwintering populations. The average water content was not significantly different between adults of both populations, but the SCP and FP of the overwintering population were significantly lower than that of the experimental population. Overall, the six sHSPs genes showed different expression patterns among developmental stages. In the short-term cooling treatment, Hsp16.25 and Hsp21.00 expressions first increased and then decreased, while Hsp10.87 and Hsp21.56 expressions increased during the entire process. Under short-term heating, the expressions of Hsp21.00, Hsp21.62, Hsp10.87, and Hsp16.25 showed an increasing trend, whereas Hsp36.77 first decreased and then increased. Under low-temperature storage conditions, the expression of Hsp36.77 decreased, while the expressions of Hsp21.00 and Hsp21.62 were higher than that of the control group in the experimental population. The expression of Hsp36.77 first increased and then decreased, whereas Hsp21.56 expression was always higher than that of the control group in the overwintering population. Thus, differences in sHSPs gene expression were correlated with the H. axyridis forms, suggesting that the mechanism of cold resistance might differ among them. Although, Hsp36.77, Hsp16.25, Hsp21.00, and Hsp21.62 regulated cold- hardiness, the only significant differences between overwintering and experimental populations were found for Hsp16.25 and Hsp21.00.
Keywords: Harmonia axyridis, super cooling point, freezing point, cold-hardiness, small heat shock protein, qRT-PCR, gene expression
Introduction
Harmonia axyridis (Coleoptera: Coccinellidae) has strong predation ability on aphids, spider mites, mealy bugs, and other important pests, thus being an important natural enemy (Koch, 2003). Due to its use in agricultural productions worldwide, it is now a cosmopolitan invasive species causing negative ecological impacts (Koch, 2003; Wang et al., 2015). The survival of H. axyridis winter adults is an important factor affecting population's offspring (Zhao et al., 2008). Therefore, several studies on cold hardiness and overwintering strategies, as well as on the relationship between seasonal phenotypic plasticity and cold temperatures, have been developed in this species (Koch et al., 2004; Labrie et al., 2008; Wang et al., 2009; Berkvens et al., 2010; Michie et al., 2011; Ruan et al., 2012).
Under natural conditions, temperature greatly influences insect growth and development, their basic behavior, and evolutionary path (Lee and Denlinger, 1991). Lee (1989) defined cold tolerance as the survival ability of a living creature exposed to low temperature, for a long or short time, according to seasonal environmental changes, genetic factors, nutritional status, and the length of exposure to low temperature. Insects have evolved a variety of cold-resistance measures (Jing and Kang, 2002), mainly including ecological (or behavior) and physiological aspects. Whereas, the former include migrating or hiding to avoid the damages caused by low temperature, the latter include regulating the body's metabolic mechanisms and the accumulation of cold resistant compounds such as glycerol, trehalose, and polyol, among others (Watanabe, 2002; Guo et al., 2006; Gagnon et al., 2013). Many studies on insects' cold tolerance used the super cooling point (SCP) as an important indicator of the strength of cold tolerance (Nedved et al., 1998; Renault et al., 2002; Koch et al., 2004; Colinet et al., 2006). In natural conditions, insects experience a process of cold acclimation before winter that increases cold tolerance, helping to withstand the low temperature environment (Leather et al., 1993). Experiments showed that the cold tolerance of H. axyridis overwintering populations was higher than that of summer populations, due, to some extent, to winter acclimation (Zhao et al., 2010). Studies also indicated that insects moderating cold acclimation before low temperature stress have higher survival rate, lower lethal temperature, prolonged half-lethal time, and decreased SCP (Fields et al., 1998; Renault et al., 2004; Ma et al., 2006; Wang et al., 2006).
Heat shock proteins (HSPs) are highly conserved amino acid sequences, which are widely found in microorganisms, plants, and animals. They function as molecular chaperones to protect proteins from being denatured in high temperature stress (Montfort et al., 2001; Sun and MacRae, 2005), but also protect proteins under cold, drought, oxidation, hypertonic stress, UV, and heavy metals exposure (Dasgupta et al., 1992; Waters et al., 2008; Meijering et al., 2012; Senf, 2013), or under high population density (Wang et al., 2007). The classification of HSPs into five families, namely HSP100, HSP90, HSP70, HSP60, and small HSPs (sHSPs; Carper et al., 1987; Kim et al., 1998), is mainly based on their molecular weight and homologs relationships. With the exception of sHSPs, which are more diverse than the other HSPs, all these families are conserved among organisms (Li et al., 2009). As a family of molecular chaperones, small heat shock proteins (sHSPs) are characterized by a low subunit molecular mass (12–43 kDa), a conserved a-crystallin domain, and for forming large oligomers (Zhang et al., 2015). Small HSPs are conserved across species and play an important role in various developmental, biotic, and abiotic stresses (Bakthisaran et al., 2015; Pandey et al., 2015). The number of genes encoding sHSPs varies greatly among organisms, from as few as one to as many as 19 (Haslbeck et al., 2005). Small HSPs are widely distributed across tissues and play an important role in cell survival under stress conditions (Bakthisaran et al., 2015). The reversible dissociation of the homo-oligomers formed by sHSPs has been reported as important for their enhanced chaperone activity (Haslbeck et al., 1999, 2008).
Since their first description in fruit flies, great advances in the study of HSPs revealed their high conservation throughout evolution, suggesting they might have a vital role in protecting cells from injury (Luo et al., 2007). As molecular chaperones, HSPs participate in protein folding and degradation and in the transport of intracellular material. When stimulated, HSPs can promote protein folding, recovering their original spatial structure and biological activity. In the absence of external stimulation, HSPs can also promote peptide chain folding and ligation. Being a survival gene, Hsp70 can be rapidly expressed to build up protection against several cellular stresses, including elevated temperatures, mechanical damage, hypoxia, lowered pH, and reactive oxygen species generation (Yong et al., 2015). In addition, HSP90 has emerged as a major pharmaceutical target in cancer therapy, and it has been proven responsible for indirectly inducing multiple pathways leading to angiogenesis and metastasis in cancer (Kim et al., 2015; Sharma et al., 2016). Heat shock proteins are ubiquitously present in cells and can modulate several cellular functions; inhibition of Hsp27 and Hsp40 potentiates 5-fluorouracil and carboplatin mediated cell killing in hepatoma cells (Sharma et al., 2009). As different classes of HSPs play several roles in governing proper protein assembly, folding, and translocation (Hightower, 1991; Hartl, 1996), the regulation of their synthesis establishes a unique defense system to maintain cellular protein homeostasis and to ensure cell survival (Hartl, 1996).
The elytra of H. axyridis adults have a rich color, usually black (melanic) or yellow (non-melanic) stained with red, orange, or black dots (Dobzhansky, 1933), which results from a series of expressed alleles (Tan and Li, 1934; Tan, 1946). Most of these alleles are rare in H. axyridis populations, with a combined frequency of <1%, except for the four major alleles after which the four major forms of H. axyridis are named—f. conspicua, f. spectabilis, f. axyridis, and f. succinea (Michie et al., 2010). Because stain ratios differ among areas and seasons within the same area (Heimpel and Lundgren, 2000; Seo and Youn, 2000; Wang et al., 2009; Michie et al., 2011), environmental factors might lead to such elytral diversity (Tang et al., 2012). In the north of China, H. axyridis melanic and non-melanic types vary seasonally: in the summer adults are mainly black and the number of yellow type adults significantly increases in the autumn (Wang et al., 2009); in winter, the proportion of yellow type H. axyridis was significantly higher than that of black adults. To survive through the cold winter, H. axyridis individuals move to concealed and sheltered locations where they aggregate, creating a protective microclimate in which insects experience less extreme temperatures than in the surrounding areas (Berkvens et al., 2010; Durieux et al., 2015). In Northeast China, adults aggregate in some fixed locations where they overwinter (Wang et al., 2011). Although, pre-wintering and overwintering H. axyridis populations increase cold tolerance and compounds' storage during extended periods at low temperature (Ruan et al., 2012), the changes occurring in their metabolism to achieve cold tolerance in winter have rarely been reported. Therefore, the water content, SCP, and freezing point (FP) of experimental and overwintering H. axyridis populations were obtained to analyze their cold tolerance. Six sHSPs were cloned and their differential expression at several developmental stages, short-term cooling temperature stress, and low-temperature storage conditions was determined, to study the potential molecular mechanisms of cold resistance in H. axyridis.
Materials and methods
Insects
Experimental populations of H. axyridis were collected from the Lab of Natural Enemy Research, Beijing Academy of Agriculture and Forestry Science, and reared and maintained in our laboratory over a 3-year period. Non-melanic and melanic populations were separated, maintained at 25°C, 70% relative humidity, and 16:8 h (light:dark) photoperiod, and fed Aphis medicaginis. At each molt, developmental stages were synchronized by collecting new larvae, pupae, or adults. Overwintering populations were collected from Heilongjiang province, Northeast of China, in 2015. After H. axyridis elytrum-staining stabilized, adults were divided into black (melanic) and yellow (non-melanic) types, according to their elytra background.
Water content, SCP, and FP determination
The wet and dry weights of each insect within each group (n = 15) were determined, and the water content of each insect's body was calculated as the difference between wet and dry weight (weight of body water) divided by the wet weight.
The thermocouple method was used to measure SCP and FP, by determining latent heat release (Ju and Du, 2002). As the insect's body temperature cools below the SCP, body fluids begin to freeze spontaneously. A temperature probe was fixed on the back of each H. axyridis individual and connected to a computer, which automatically recorded and processed body temperature variations. H. axyridis were placed in a refrigerator and their body temperature declined with decreasing temperature down to a point where it stopped decreasing and began to rise. This temperature is the SCP and that at which body temperature begins to decline again is the FP (Liu et al., 2005). A cooling curve was drawn, and the SCP was read from it.
Cloning six sHSPs genes
In our previous study (Tang et al., 2017), two H. axyridis groups were exposed to normal and low temperature conditions (5°C) for 2 h. These groups were named HaRT_Trans and HaCS_Trans, respectively, and their transcriptome sequencing and analysis revealed partial sequences of six sHsps genes. In the present study, total RNA was isolated from H. axyridis using TRIzol® reagent (Invitrogen, Shanghai, China), and first-strand cDNA synthesis was carried out using a PrimeScript RT® with gDNA Eraser kit (TaKaRa, Dalian, China). Based on the six sHSPs found in the previous study, specific primers were designed and used to obtain full length cDNA sequences by Rapid Amplification of cDNA Ends (RACE) technology, together with a SMART™ kit (TaKaRa) and following the manufacturer's protocol. The resulting PCR products were separated by electrophoresis on 1.0% agarose gels, and the cDNA fragments of interest were purified using a DNA gel extraction kit (OMEGA, Hangzhou, China). Purified DNA was ligated into a pMD18-T vector (TaKaRa) and sequenced using the Sanger method.
Sequence and phylogenetic analysis
Nucleic acid sequences of H. axyridis sHSPs were queried for similar sequences on the National Center for Biotechnology Information (NCBI). Multiple sHSPs sequences, belonging to H. axyridis and other insects, were aligned using ClustalW (Julie et al., 2002), and a neighbor-joining (NJ) phylogenetic tree was constructed in Mega 7.0 software and evaluated using 1000 bootstrap replications.
Expression of sHSPs genes in different developmental stages
The experimental population was used in this trial. Total RNA was extracted from larvae, pre-pupae, pupae, and adults (four individuals from each developmental stage; collected 1–3 days after molting or 2 h after eclosion) as described in the previous section. After cDNA synthesis (as described in the previous section), the relative expression of sHSPs genes was detected by quantitative real-time PCR (qRT-PCR). Primers used in qRT-PCR were designed based on the conserved regions of sHSPs genes from differently colored H. axyridis (Table 1). Details of the qRT-PCR are given below, in the section “Quantitative RT-PCR.”
Table 1.
Primer sequences used in quantitative real-time PCR.
| Primer name | Nucleotide sequences (5′–3′) |
|---|---|
| Ha-rp49-QF | GCGATCGCTATGGAAAACTC |
| Ha-rp49-QR | TACGATTTTGCATCAACAGT |
| HaHsp36.77-F | TTCTTCACGGCTGTCTT |
| HaHsp36.77-R | AATCACTGCCTTCCCTC |
| HaHsp16.25-F | GACCCTCAACATACCAGA |
| HaHsp16.25-R | TGATGCTTACCCTTTACTTC |
| HaHsp21.00-F | CACCATAGAAGGCAAGCA |
| HaHsp21.00-R | AGACAATCTCGATTCCACC |
| HaHsp21.62-F | TCTTCTGGACCAACATTTC |
| HaHsp21.62-R | GTGGCTTTAACGGTGATT |
| HaHsp10.87-F | TGCCCTTGTTGGATAGA |
| HaHsp10.87-R | TGTTGCCTTCAGGACTT |
| HaHsp21.56-F | AGGAGCATGGTGGACTG |
| HaHsp21.56-R | ACTTCACTTTCTGGCAATC |
Short-term cooling and heating
The experimental population was used in this trial. Lee and Denlinger (1991) found that rapid cooling contributed to enhance cold-hardiness in arthropods. As the coming of winter or spring is a process of gradual cooling or warming, we designed a series of experiments using different temperatures (25, 15, 10, 5, 0, and −5°C) to measure sHSPs responses to temperature transitions. H. axyridis were placed in rapidly changing temperature environments. In treatment (i), hundreds of individuals were placed in plastic tubes sealed with a sponge (10 individuals per tube) and maintained at 25°C for 2 h; tubes were then rapidly cooled to 15°C, kept at this temperature for 2 h, rapidly cooled to 10°C, kept at this temperature for 2 h, and finally rapidly cooled to −5°C. Treatment (ii) involved a similar procedure, but the starting temperature was −5°C and individuals were heated to 25°C. As a control, 100 adults were maintained at 25°C without any cold stimulation before treatment (ii) (Shi et al., 2016). We randomly sampled three pieces of abdominal tissue from experimental insects at every changing temperature point. The above treatments were repeated three times. Total RNA isolation and cDNA synthesis were performed as described in the “Cloning six sHSPs genes” section, and details of the qRT-PCR are given below, in the “Quantitative RT-PCR” section.
Low temperature storage
Black and yellow females from the experimental and overwintering H. axyridis populations stored at low temperature (5°C) were sampled at 0, 5, 10, 15, and 20 days after storage. At each time point, 5–10 individuals were removed and analyzed. The experiment was repeated three times.
Quantitative RT-PCR
Total RNA was isolated from H. axyridis adults after cold induction, and 1 μg total RNA was used to synthesize first-strand cDNA, as described in the “Cloning six sHSPs genes” section. The expression levels of the six sHSPs genes obtained from cloning, namely Hsp36.77, Hsp16.25, Hsp21.00, Hsp21.62, Hsp10.87, and Hsp21.56, were estimated by qRT-PCR in a CFX96™ system, using the SsoFast™ EvaGreen® Supermix (both Bio-Rad Laboratories, Hercules, CA, USA). The qRT-PCR was performed in a 20 μl total reaction volume containing 1 μl cDNA template, 1 μl (10 μmol/μl) each primer, 7 μl RNase-free and DNase-free water, and 10 μl SsoFast™ EvaGreen® Supermix. Gene expression data was normalized using the housekeeping gene Harp49 (H. axyridis ribosomal protein 49 gene, AB552923) as the internal control (Shi et al., 2016), which was amplified using the primers Harp49-qF (5′-GCGATCGCTATGGAAAACTC-3′) and Harp49-qR (5′-TACGATTTTGCATCAACAGT-3′). Primers for the six sHSPs genes of H. axyridis were designed to target their unique regions; the annealing temperature of each primer pair is shown in Table 1. Target amplification efficiency was identical to that of the reference amplification at each annealing temperature. The cycling parameters were 94°C for 5 min (initial denaturation), followed by 40 cycles at 94°C for 15 s, 59°C for 30 s, and 65°C for 30 s; the fluorescence signal was collected at 68°C. Expression was quantified using the ΔΔCt relative method (Shi et al., 2016).
Statistical analysis
Data normality and variance homogeneity was evaluated based on three replicates at each temperature point. A multiple factorial three-way analyses of variance (ANOVA) was used to evaluated differences in water content, SCP, and FP among groups, considering color, treatments, and sex as independent factors. The expressions of sHSPs genes during cooling or heating processes were analyzed in IBM SPSS 22 (IBM Corporation, Armonk, NY, USA), and multiple comparisons of means were conducted using Tukey's test. Differences between means were considered significant when P < 0.05, and extremely significant when P < 0.01.
Results
Water content, SCP, and FP
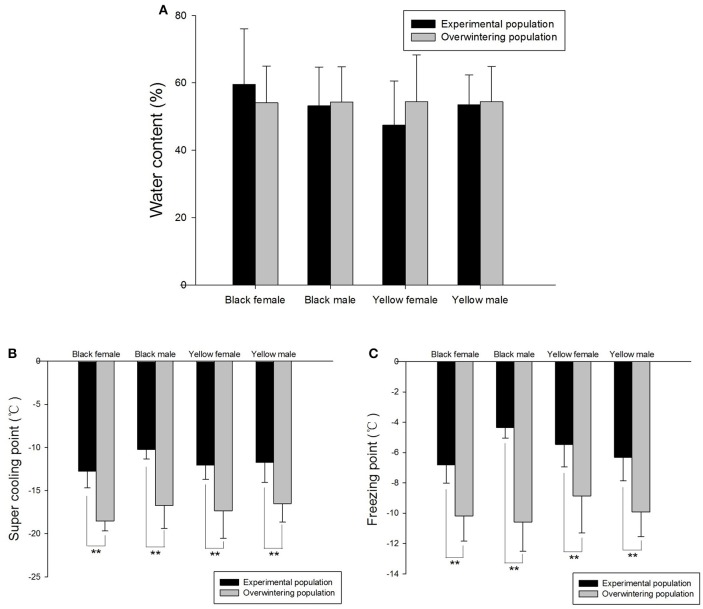
The average water contents of overwintering and experimental populations were 54.27 and 53.42%, respectively. There were no obvious differences between the same forms of H. axyridis adults belonging to overwintering or experimental populations (Figure 1A). The water content of black adults from the experimental population (56.34%) was slighter higher than that of yellow adults (50.50%), but no obvious differences between adult forms were detected in the overwintering population. There was no significant difference in water content between sexes in the overwintering or experimental populations.
Figure 1.
Water content (A), super cooling point (B), and freezing point (C) of Harmonia axyridis in different populations. The overwintering population was obtained from Northeast China and the experimental population from Hangzhou. Bars with double asterisks indicate significant differences (P < 0.01).
The SCP and FP of the overwintering population were −17.28 and −9.89°C, respectively, whereas those of the experimental population were −11.70 and −5.74°C, respectively. For the same forms of H. axyridis adults, SCP and FP were significantly lower in the overwintering than in the experimental population (Figures 1B,C). Three-way ANOVA results showed there was no significant interaction between color, sex, and treatment (Table 2). Females' SCP was significantly lower than that of males within same color H. axyridis adults (Figure 1B). There was a small gap in the FP and SCP of differently colored insects within the overwintering population, with the SCP and FP of black adults being slightly lower than that of yellow adults, although this difference was not significant (Table 2).
Table 2.
Analysis of variance table realized by GLMM.
| Source | Sum Sq. | df | Mean Sq. | F | Sig. |
|---|---|---|---|---|---|
| Color | 0.787 | 1 | 0.787 | 0.174 | 0.677 |
| Treats | 932.084 | 1 | 932.084 | 206.109 | 0 |
| Sex | 55.733 | 1 | 55.733 | 12.324 | 0.001 |
| Color*treats | 9.163 | 1 | 9.163 | 2.026 | 0.157 |
| Color*sex | 18.961 | 1 | 18.961 | 4.193 | 0.043 |
| Treats*sex | 0.08 | 1 | 0.08 | 0.018 | 0.894 |
| Color*treats*sex | 2.682 | 1 | 2.682 | 0.593 | 0.443 |
| Error | 506.496 | 112 | 4.522 | ||
| Total | 26718.881 | 120 |
R2 = 0.668 (Corrected R2 = 0.647).
Phylogenetic analysis of sHSPs
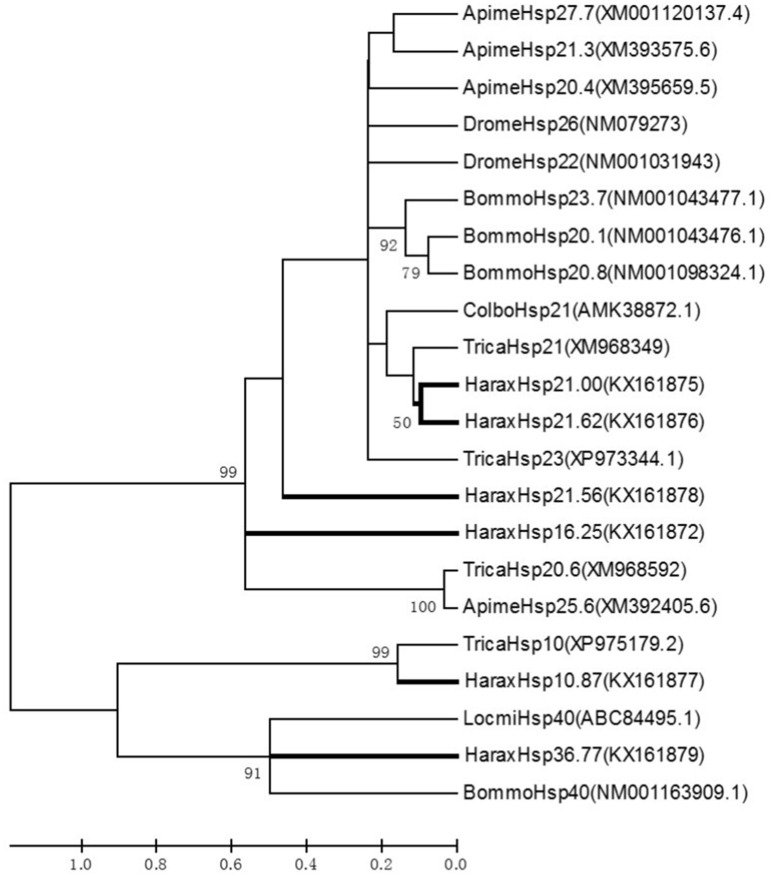
Among the numerous insect sHSPs genes logged into NCBI, those belonging the following representative species were selected for this study: Tribolium castaneum, Drosophila melanogaster, Bombyx mori, Apis mellifera, Locusta migratoria, and Colaphellus bowringi. Multiple protein alignments showed that H. axyridis sHSPs were highly homologous to that known and predicted for other insects, with HaraxHsp10.87 being 99.0% identical to TricaHsp10 (XP975179.2). Similar molecular weight sHSPs belonging to different insect species appeared in the same branch of the phylogenetic tree (Figure 2).
Figure 2.
Phylogenetic analysis of sHSPs in insects. Phylogenetic tree constructed by the neighbor-joining method. Bootstrap values for 1000 replications are indicated at each node. Scale bar = 0.2 PAM. Harax, Harmonia axyridis; Apime, Apis mellifera; Bommo, Bombyx mori; Colbo, Colaphellus bowringi; Drome, Drosophila melanogaster; Locmi, Locusta migratoria; Trica, Tribolium castaneum.
Developmental expression patterns of sHSPs
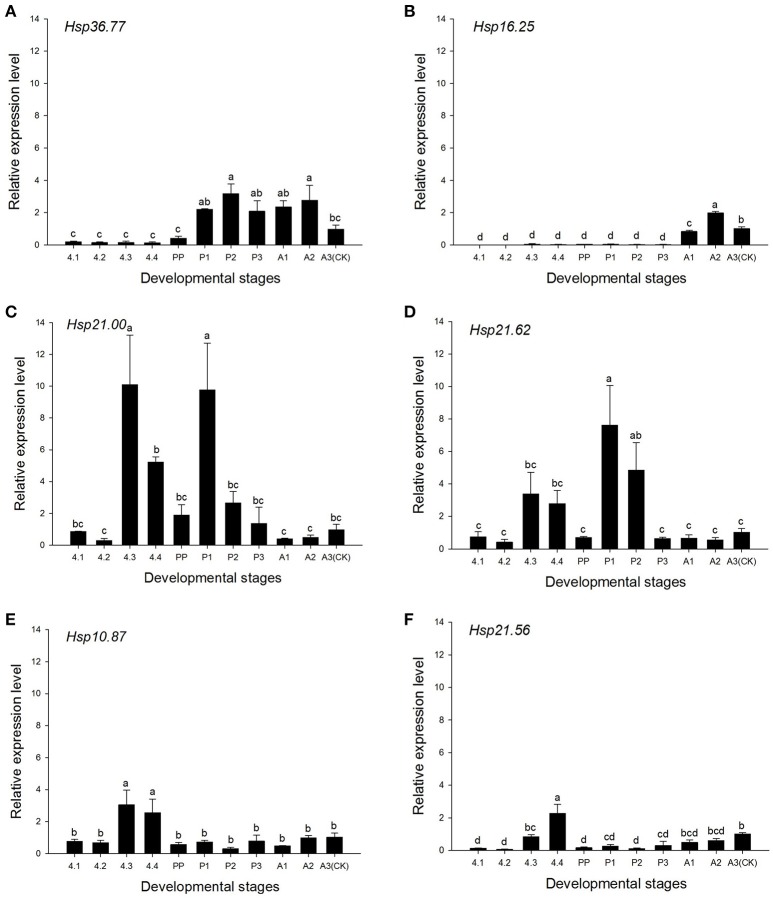
According to the patterns of the six sHSPs genes determined by qRT-PCR, Hsp36.7 was continuously expressed from pupal stages to 3-day adults in the H. axyridis experimental population [F(10, 25) = 23.544, P < 0.001; Figure 3A]. The expression of Hsp16.25 was high in adults but low in larvae and pupae [F(10, 29) = 391.653, P < 0.001; Figure 3B], and Hsp21.00 [F(10, 24) = 18.652, P < 0.001] and Hsp21.62 [F(10, 26) = 13.078, P < 0.001] were expressed at higher levels in the later period of fourth-instar larvae and in early pupae than in 3-day adults (Figures 3C,D). A similar trend was found for the expression of Hsp10.87 [F(10, 28) = 14.906, P < 0.001] and Hsp21.56 [F(10, 26) = 29.573, P < 0.001], which were highly expressed in the later period of fourth-instar larvae (Figures 3E,F).
Figure 3.
Relative expression levels of several sHSPs in Harmonia axyridis in different development stages. Experimental population is used for this trial. (A) Hsp36.77. (B) Hsp16.25. (C) Hsp21.00. (D) Hsp21.62. (E) Hsp10.87. (F) Hsp21.56.Changes in H. axyridis sHSPs mRNA levels from day 1 of fourth-instar larvae to adult stage in relation to those of Harp49 (H. axyridis ribosomal protein 49 gene) were measured by quantitative real-time PCR. 4, fourth-instar larva; PP, pre-pupae; P, pupae; and A, adult. Each combination of letters and numbers represents the age of the individual at a certain developmental stage (e.g., 4.1: the 1st day of fourth-instar larva). Data are presented as means ± s.d. (n = 3). Bars with different letters indicate significant differences (P < 0.05) and used Tukey's test, α = 0.05, a > b > c.
Expression of sHSPs under short-term cooling and heating
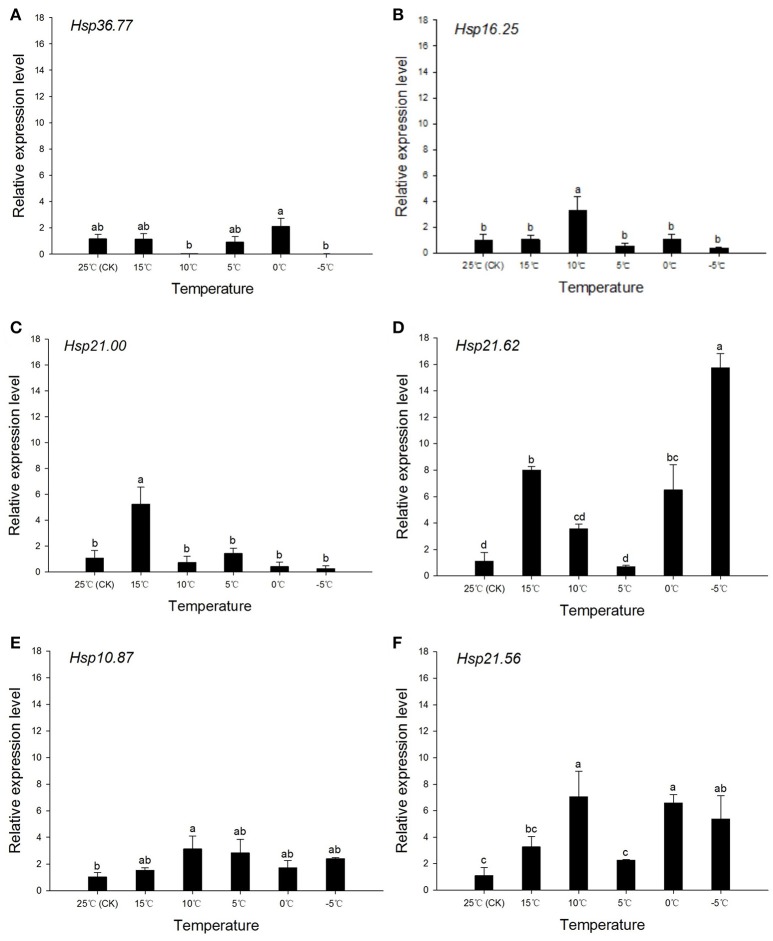
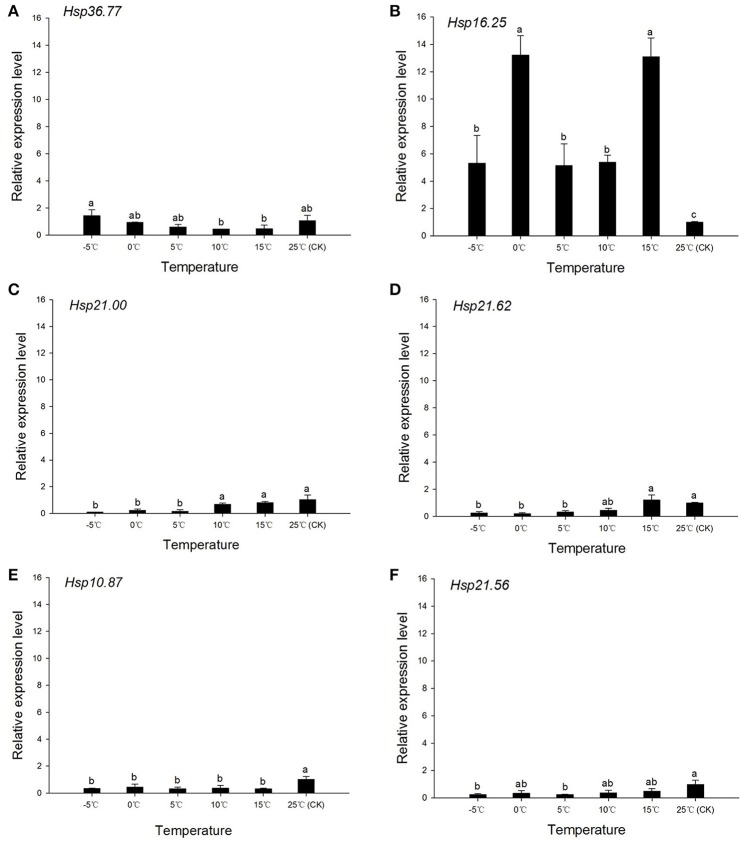
The mRNA levels of Hsp36.77, Hsp16.25, Hsp21.00, Hsp21.62, Hsp10.87, and Hsp21.56 determined during cooling and heating conditions in the H. axyridis experimental populations, revealed complex patterns under cooling conditions. In the short-term cooling treatment, the expression at 25°C was used as reference. The expression of Hsp36.77 was highest at 0°C and significantly higher than that registered at −5 and 10°C [F(5, 12) = 5.421, P = 0.023; Figure 4A]; however, it was not significantly different from that observed in the control group (CK). The expressions of Hsp16.25 [F(5, 11) = 7.658, P = 0.014] and Hsp21.00 [F(5, 13) = 20.905, P < 0.001] were significantly higher at 10 and 15°C, respectively, than at other temperatures (Figures 4B,C). The expression of Hsp21.62 first increased when temperature dropped from 25 to 15°C, decreased in the following cooling to 5°C, and reached its highest level at −5°C [F(5, 11) = 69.016, P < 0.001; Figure 4D]; at this temperature, expression was significantly higher than at any other temperature. The expression of Hsp10.87 was highest at 10°C [F(5, 13) = 4.032, P = 0.040; Figure 4E], although it was only significantly different from that obtained at 25°C. Expression of Hsp21.56 was significantly higher than that of the CK group at 10, 0, and −5°C [F(5, 12) = 7.441, P = 0.010; Figure 4F].
Figure 4.
Relative expression of Harmonia axyridis sHSPs under the short-term cooling treatment. Experimental population is used for this trial. (A) Hsp36.77. (B) Hsp16.25. (C) Hsp21.00. (D) Hsp21.62. (E) Hsp10.87. (F) Hsp21.56. Expression of sHSPs was examined in a gradually cooled environment, from 25 to −5°C.During the cooling process, the control group (CK) comprised adults reared at an optimum temperature (25°C) without any cold stimulation. Data are presented as means ± s.d. (n = 3). Bars with different letters indicate significant differences (P < 0.05) and used Tukey's test, α = 0.05, a > b > c > d.
In the short-term heating treatment, the expression of Hsp36.77 decreased when temperature changed from −5 to 10°C, increasing afterwards [F(5, 13) = 5.946, P = 0.014; Figure 5A]; however, expression levels were not significantly different from those registered in the CK. The expression of Hsp16.25 was significantly higher than that of the CK, especially at 0 and 15°C [F(5, 12) = 42.868, P < 0.001; Figure 5B]. The expression levels of Hsp21.00 [F(5, 12) = 61.788, P < 0.001] and Hsp21.62 [F(5, 12) = 10.262, P = 0.004] generally increased with increasing temperature and significantly differed between temperatures below or above 10°C (Figures 5C,D). There was no significant difference in the expressions of Hsp10.87 and Hsp21.56 [F(5, 14) = 2.438, P = 0.116] from −5 to 15°C during the heating process, but the expression level of these genes at 25°C the expression level Hsp10.87 [F(5, 13) = 14.561, P = 0.001] was significantly higher than that obtained for the CK (Figures 5E,F).
Figure 5.
Relative expression of Harmonia axyridis sHSPs under the short-heating treatment. Experimental population is used for this trial. (A) Hsp36.77. (B) Hsp16.25. (C) Hsp21.00. (D) Hsp21.62. (E) Hsp10.87. (F) Hsp21.56. Expression of sHSPs was examined in a gradually heated environment, from −5 to 25°C. During the heating process, the control group (CK) comprised adults reared at an optimum temperature (25°C) without any heat stimulation. Data are presented as means ± s.d. (n = 3). Bars with different letters indicate significant differences (P < 0.05) and used Tukey's test, α = 0.05, a > b > c.
Expression of sHSPs in yellow and black forms during low temperature storage
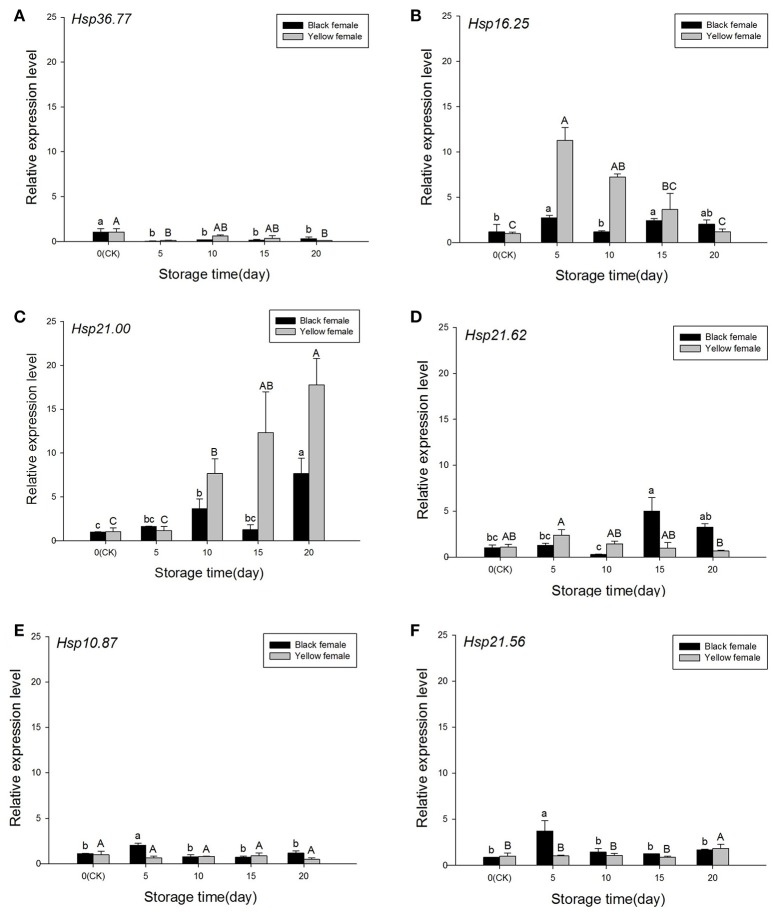
During storage at low temperature, the expression levels of sHSPs recorded at day 0 were considered the CK. The expression of Hsp36.77 was always lower in black [F(4, 12) = 10.562, P = 0.030] and yellow females [F(4, 9) = 6.933, P = 0.028] than in the CK (Figure 6A). Compared to the CK, the expression of Hsp16.25 in yellow females was significantly higher at days 5 and 10 [F(4, 9) = 35.738, P = 0.001], whereas in black females it was significantly higher at days 5 and 15 [F(4, 12) = 5.170, P = 0.024; Figure 6B]. The expression of Hsp21.00 in yellow females was significantly higher than that of the CK at days 10–20 [F(4, 9) = 4.147, P = 0.075], and it increased with increasing exposure to low temperature; expression levels of Hsp21.00 in black females at days 10 and 20 were significantly higher than that of the CK [F(4, 9) = 16.895, P = 0.004; Figure 6C). The expression level of Hsp21.62 in yellow females was highest at day 5, but not significantly different from that of the CK [F(4, 9) = 4.147, P = 0.075], while the expression of this gene in black females was significantly higher than that of the CK group at day 15 [F(4, 9) = 16.895, P = 0.004; Figure 6D]. The expressions of Hsp10.87 [black females F(4, 11) = 15.007, P = 0.002; yellow females F(4, 12) = 1.909, P = 0.202] and Hsp21.56 [black females F(4, 11) = 11.074, P = 0.004; yellow females F(4, 11) = 4.934, P = 0.033] presented a similar trend, and were generally not significantly different from those in the CK group, except in black females at day 5 (both genes) and in yellow females at day 20 (Hsp21.56; Figures 6E,F).
Figure 6.
Relative expression of sHSPs in the experimental population of Harmonia axyridis under the low-temperature storage treatment. (A) Hsp36.77. (B) Hsp16.25. (C) Hsp21.00. (D) Hsp21.62. (E) Hsp10.87. (F) Hsp21.56. Selected black and yellow females of the experimental population stored in the refrigerator (5°C) were remove at days 0, 5, 10, 15, and 20. Data are presented as means ± s.d. (n = 3). Bars with different letters indicate significant differences (P < 0.05) and used Tukey's test, α = 0.05, a > b > c or A > B > C.
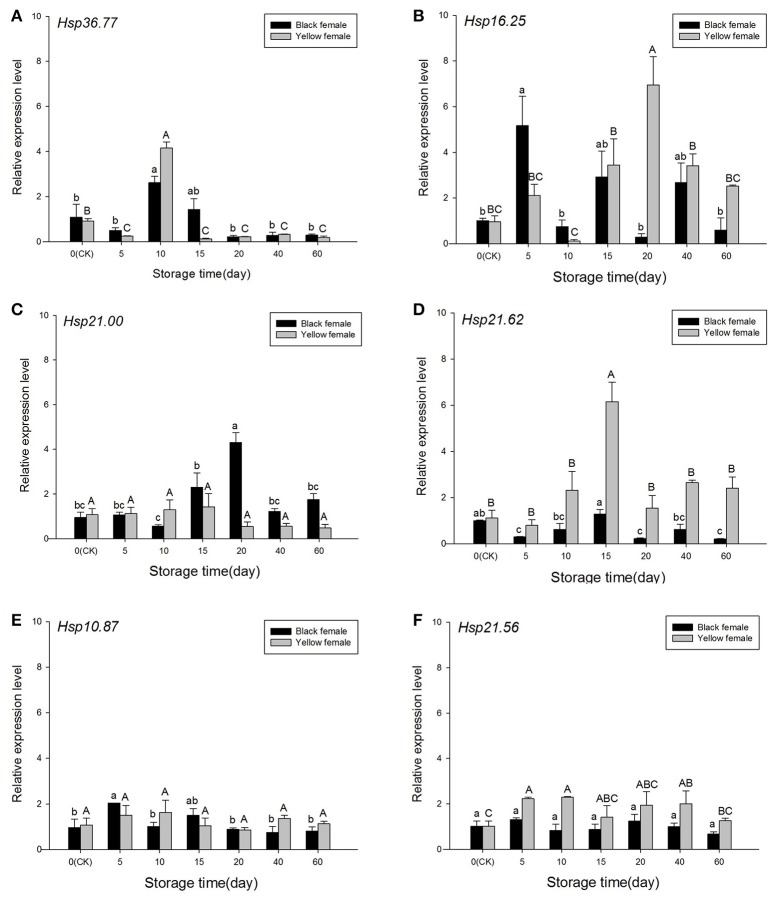
Analyses of expression levels in the H. axyridis overwintering population revealed Hsp36.77 was highly expressed at day 10 in both black [F(6, 13) = 16.136, P = 0.01] and yellow females [F(6, 15) = 420.816, P < 0.001; Figure 7A], generally differing significantly from expression levels in the control groups. In black females, the expression of Hsp16.25 was significantly higher than that of the CK group at day 5 [F(6, 13) = 10.691, P = 0.03], and in yellow females it generally increased from day 0 to day 20 (when it significantly differed from that in CK), decreasing afterwards [F(6, 13) = 19.867, P < 0.001; Figure 7B]. The highest expression level of Hsp21.00 in black females found at day 20 significantly differed from that of CK [F(6, 15) = 14.069, P < 0.001], but there were no significant differences in the expression of this gene in yellow females [F(6, 15) = 1.879, P = 0.190; Figure 7C]. The expression of Hsp21.62 was significantly highest at day 15 in yellow females [F(6, 14) = 21.062, P < 0.001] and black females [F(6, 13) = 15.579, P = 0.001; Figure 7D], whereas Hsp10.87 expression was significantly higher at day 5 in black females [F(6, 16) = 9.523, P = 0.001], but no significant differences were detected in yellow females [F(6, 19) = 1.521, P = 0.247; Figure 7E]. The expression of Hsp21.56 in yellow females was significantly higher at days 5, 10, and 40 [F(6, 16) = 3.234, P = 0.049], whereas no significant differences were detected in black females [F(6, 16) = 2.808, P = 0.072; Figure 7F].
Figure 7.
Relative expression of sHSPs in the overwintering population of Harmonia axyridis under the low-temperature storage treatment. (A) Hsp36.77. (B) Hsp16.25. (C) Hsp21.00. (D) Hsp21.62. (E) Hsp10.87. (F) Hsp21.56. Selected black and yellow females of the overwintering population stored in the refrigerator (5°C) were removed at days 0, 5, 10, 15, and 20. Data are presented as means ± s.d. (n = 3). Bars with different letters indicate significant differences (P < 0.05) and used Tukey's test, α = 0.05, a > b > c or A > B > C.
Expression of sHSPs in experimental and overwintering populations during low temperature storage
Results showed that the six sHsps have different functions in the regulation of cold hardness in H. axyridis. The expression level of Hsp36.77 decreased in the experimental population (Figure 6A), while it first increased and then decreased in the overwintering population (Figure 7A). Although, Hsp16.25 expression pattern was similar in experimental and overwintering population, in yellow females the expression of this gene first increased and then decreased while black females showed a different pattern (Figures 6B, 7B). In addition, Hsp16.25 expression increased with increasing storage time in the experimental population (Figure 6C) and presented an identical trend to Hsp36.77 and Hsp21.62 in the overwintering population (Figures 7C,D). The expression of Hsp21.62 increased in black females from the experimental population and in yellow females from the overwintering population and kept relatively high levels as storage time increased (Figures 6D, 7D). The expression levels of Hsp10.87 and Hsp21.56 were stable during storage times and similar between experimental and overwintering population (Figures 6E,F, 7E,F).
Discussion
Insects are heterothermic organisms, and their survival through winter determines the perpetuation of their populations (Zhang and Ma, 2013). In temperate and cold zones, winter temperature is usually lower than the insects' FP and their overwintering stages generally have a lower SCP than insects living in other zones. This is the case of L. migratoria eggs (−26°C), Liriomyza spp. pupae (−19°C) (Jing and Kang, 2002), Chrysoperla sinica (−13°C) (Guo et al., 2006), Ectomyelois ceratoniae diapausing larvae (−17.3°C) (Heydari and Izadi, 2014), and Pityogenes chalcographus (−26.3°C) (Koštál et al., 2014). In the present study, H. axyridis were divided into black (melanic) and yellow (non-melanic) forms according to the background of their elytra, and into experimental and overwintering populations according to their source. The SCP and FP of the overwintering population was significantly lower than that of the experimental population for the same forms of H. axyridis (Figure 1), and females had a lower SCP than males, in either black or yellow H. axyridis (Figure 1B). Thus, overwintering populations seem to decrease their SCP to avoid the damages caused by fluid freezing, which agrees with the previously described seasonal changes in H. axyridis SCP and its significant decrease in winter (Zhao et al., 2008). Some studies reported that insects with low SCP had high levels of trehalose in the winter (Heydari and Izadi, 2014; Koštál et al., 2014; Vallières et al., 2015). This is an important winter adaptation strategy for survival at low temperature. Although, we found no significant differences in average water content between experimental and overwintering populations (Figure 1A), previous research found seasonal variations in the water contents of male and female H. axyridis adults, which decreased with decreasing temperature (Zhao et al., 2008). Water loss before winter has also been reported in other insects (Nedved et al., 1998; Holmstrup et al., 1999).
The sHSPs are HSPs that function as molecular chaperones (Li et al., 2009). Their molecular weight ranges from 12 to 43 kDa and is usually bellow 30 kDa (Kim et al., 1998; Waters et al., 2008). In H. axyridis, one Hsp90 and three Hsp70 family genes were cloned and reported as highly conserved (Tang et al., 2010; Shen et al., 2015). The homology of HaHsp90 with other insect Hsp90s varied from 81 to 90%, which was the homology with T. castaneum (Tang et al., 2010); HaHsp70 also had a high sequence homology with other eukaryotic Hsp70s, the highest also with T. castaneum (93%) (Yang et al., 2009; Shen et al., 2015). Insect HSP70s have three signatures -“IDLGTTYSCVGV,” “IFDLGGGTFDVSIL,” and “IVLVGGSTRIPKIQ,” one ATP/GTP binding site motif, “AEAYLG(K/T)T,” and a “MEEVD” motif at the C terminus (Shen et al., 2015). Members of the HSP90 family also have an “MEEVD” motif at the C terminus, but this was not found in sHSPs (Figure S1). These were reported to have a conserved secondary structure and functional domain (Sun and MacRae, 2005), and sHSPs with similar molecular weight were found in the same branch of the phylogenetic tree (Figure 2).
Small HSPs are molecular chaperones not only under stress conditions, but also during normal development (Sun and MacRae, 2005). Under thermal and other damaging stresses, sHSPs bind to other cellular proteins protecting them from denaturation; in addition, they participate in protein folding and transportation, embryo development, and immunization mechanisms (Li et al., 2009). In addition, the overexpression of sHSPs can enhance the tolerance of cells to heat shock and to temperature changes. Under normal or stress conditions, HSP90 is present in the cytoplasm of all cell types, where it acts as a molecular chaperone to recover the folding state of the denatured protein (Yonehara et al., 1996). Studies have shown that sHSPs are widely distributed in insects, and are closely related to their growth and development (Sun and MacRae, 2005). In the present study, Hsp16.25 was highly expressed in adults and Hsp36.77 was highly expressed from 1-day pupae to 3-day adults (Figures 3A,B). A previous study reported that HaHsp68 was highly expressed from 2-day fourth-instar larvae to 3-day adults, while HaHsp70A was highly expressed in larvae (Shen et al., 2015). In the present study, Hsp21.00, Hsp21.62, and Hsp10.87 expression levels varied according to developmental stages, whereas Hsp21.56 expression increased gradually from 2-day pupae to 3-day adults, suggesting Hsp21.56 and Hsp16.25 might have important functions in adults (Figure 3). Thus, sHSPs genes might play different roles during H. axyridis growth and development.
The expression of Hsp70, Hsp74, and Hsp83 among the several developmental stages of Spodoptera exigua differed between fat body and whole body tissues (Xu et al., 2011). Drosophila spp. long-term exposure to 0°C induced HSPs expression in the whole body (Burton et al., 1998) and cold-shock treatment induced the expression of Hsp90, Hsp70, Hsp40, and some sHSPs in Liriomyza sativae (Huang and Kang, 2007). The expression level of SeHsp70 and SeHsp74 increased quickly at 40°C, while SeHsp83 expression increased with heat-shock exposure time. These genes were also differently expressed during cold-shock treatment at 0°C: whereas SeHsp70 was highly expressed after 15 min of cold-shock, followed by a decrease from min 15 to min 60, SeHsp74 and SeHsp83 expressions increased from min 15 to min 45 (Xu et al., 2011). When H. axyridis adults were subject to different low temperatures, Hsp70B expression at 0°C was higher than that of the control group and higher than at other temperature conditions (Shen et al., 2015). Under the short-term cooling treatment performed in the present study, the expression of Hsp16.25 increased significantly at 10°C, followed by a decrease (Figure 4B), Hsp21.00 expression increased significantly at 15°C and then decreased (Figure 4C), and Hsp10.87 expression was highest at 10°C (Figure 4E); Hsp21.62 and Hsp21.56 expressions were highest at 15, 0, and −5°C, and were always higher than that of the control group (25°C; Figures 4D,F). These results indicated that sHSPs might increase their expression to provide insects' with cold-hardiness. Given that, during the short-term heating treatment, Hsp21.00, Hsp21.62, and Hsp21.56 expressions increased gradually from 10 to 25°C (Figures 5C,D,F), and Hsp10.87 expression was higher than that of the control group (at −5°C; Figure 5E), sHSPs might also play a key role in heat-stress conditions.
Populations of the ladybird H. axyridis contain both melanic and non-melanic forms, and changes in allele frequency in some populations suggested melanism might be advantageous in winter but costly in summer (Michie et al., 2010; Wang et al., 2011). The ratio of H. axyridis elytral coloring varied seasonally (Wang et al., 2009), which might be related to the protection conferred by color (Geng and Tan, 1980), and is related to temperature changes (Michie et al., 2010; Purse et al., 2015; Roy et al., 2016). Previous research indicated that black and yellow populations of H. axyridis might have had different roles in temperature or climatic adaptation (Roy et al., 2016), so we analyzed sHSPs expression under different storage times in black and yellow female populations, separately. In the yellow population, Hsp16.25 and Hsp21.62 expressions increased significantly at day 5, decreasing gradually afterwards (Figures 6B,D), while Hsp21.00 and Hsp10.87 expressions increased gradually (Figures 6C,E), and Hsp21.56 expression was relatively stable, except at day 20 (Figure 6F). In the black population, Hsp16.25, Hsp10.87, and Hsp21.56 were highly expressed at day 5, decreasing and stabilizing afterwards (Figures 6B,E,F), while Hsp21.62 expression was higher after day 15 (Figure 6D). In the experimental population, five of the studied genes played a role during cold-temperature storage and their function varied between melanic and non-melanic forms of H. axyridis adults. Thus, there might be differences between cold-resistance in H. axyridis melanic and non-melanic forms. There are many reasons for the elytral variety observed in H. axyridis (Tang et al., 2012), including mating selection, so the differences found in gene expression need to be further studied.
Winter in temperate zones imposes a substantial environmental stress on arthropods (Kang et al., 2009). Many studies revealed that cold acclimation, especially between 0 and 5°C, significantly improves cold tolerance in insects (Broufas and Koveos, 2001). Cold acclimation may induce the accumulation of cryoprotectants (such as trehalose, glycerol, and polyol; Koštál et al., 2001; Slachta et al., 2002), and the synthesis of antifreeze proteins (HSP20.5, HSP70, and HSP90; Wang et al., 2006; Feng et al., 2007). Many insects mitigate seasonal stresses by entering diapause (Dong et al., 2014). Under the low-temperature storage experiment, the overwintering black population revealed a high expression of Hsp36.77 at day 10 and of Hsp16.26 and Hsp10.87 at day 5 followed by a decrease (Figures 7A,B,E), while Hsp21.00 presented a relatively high and stable expression from day 15 to day 60 (Figure 7C). In the yellow population, Hsp21.56 was highly expressed during the 60 days of cold storage (Figure 7F), Hsp36.77 was highly expressed at day 10, Hsp16.25 at day 20, and Hsp21.62 at day 15, all followed by a decrease (Figures 7A,B,D). The high expression of these genes might promote the expression of other cold-resistant genes and protect H. axyridis, as sHSPs seem to participate in stress response under low-temperature storage. As the cold winter starts, overwintering populations are ready to survive it, because they have adapted their physiology and behavior by entering diapause, decreasing body water content and SCP, accumulating fat, finding hiding places, etc. (Zhao et al., 2008). Moreover, overwintering populations live longer than experimental populations at appropriate storage temperatures (Ruan et al., 2012).
In summary, the present study found that average water content was not significantly different between adults of both populations, but the SCP and FP of the overwintering population were significantly lower than that of the experimental population. Differences in sHSPs expression between experimental and overwintering populations were correlated with H. axyridis elytral coloring, cooling, and heating and low temperature storage, suggesting that the mechanism of cold resistance might differ among black and yellow females of H. axyridis. In addition, different sHsps might play different roles in the cold hardness process of H. axyridis populations.
Ethics statement
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Author contributions
Conceived and designed the experiments: SW, ZM, S-GW, BW, and BT. Performed the experiments and analyzed the data: HW, ZS, QS, and CX. Contributed reagents/materials/analysis tools: SW, ZM, S-GW, and BT. Wrote the paper: HW, SW, and BT.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the National Basic Research Program of China (Grant Nos. 2013CB127600), National Natural Science Foundation of China (Grant Nos. 31071731), the Beijing NOVA Program (Grant No. Z121105002512039), and the Program for Excellent Young Teachers at Hangzhou Normal University (Grant No. JTAS 2011-01-031).
Supplementary material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fphys.2017.00060/full#supplementary-material
The cDNA sequence of Harmonia axyridis sHSPs. The initiation and termination codons are indicated in bold and underlined. (A) Hsp36.77, (B) Hsp16.25, (C) Hsp21.00, (D) Hsp21.62, (E) Hsp10.87, (F) Hsp21.56.
References
- Bakthisaran R., Tangirala R., Rao C. H. M. (2015). Small heat shock proteins: role in cellular functions and pathology. Biochim. Biophys. Acta 1854, 291–319. 10.1016/j.bbapap.2014.12.019 [DOI] [PubMed] [Google Scholar]
- Berkvens N. B. J. S., Berkvens D., Tirry L., Clercq P. D. (2010). Cold tolerance of the harlequin ladybird Harmonia axyridis in Europe. J. Insect Physiol. 56, 438–444. 10.1016/j.jinsphys.2009.11.019 [DOI] [PubMed] [Google Scholar]
- Broufas G. D., Koveos D. S. (2001). Rapid cold hardening in the predatory mite Euseius (Amblyseius) finlaandicus (Acari: Phytoseiidae). J. Insect Physiol. 47, 699–708. 10.1016/S0022-1910(00)00162-1 [DOI] [PubMed] [Google Scholar]
- Burton V., Mitchell H. K., Young P., Petersen N. S. (1998). Heat shock protection against cold stress of Drosophila melanogaster. Mol. Cell. Biol. 8, 3550–3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carper S. W., Duffy J. J., Gerner E. W. (1987). Heat shock proteins in thermo tolerance and other cellular physiological processes. Cancer Res. 47, 5249–5255. [PubMed] [Google Scholar]
- Colinet H., Renault D., Hance T., Vernon P. (2006). The impact of fluctuating thermal regimes on the survival of a cold-exposed parasitic wasp, Aphidius colemani. Physiol. Entomol. 31, 234–240. 10.1111/j.1365-3032.2006.00511.x [DOI] [Google Scholar]
- Dasgupta S., Hohman T. C., Carper D. (1992). Hypertonic stress induces α B-crystallin expression. Exp. Eye Res. 54, 461–470 10.1016/0014-4835(92)90058-Z [DOI] [PubMed] [Google Scholar]
- Dobzhansky T. (1933). Geographic variation in ladybeetles. Am. Nat. 67, 97–126. 10.1086/280472 [DOI] [Google Scholar]
- Dong Y. C., Nicolas D., Lei C. L., Niu C. Y. (2014). Transcriptome characterization analysis of Bactrocera minax and new insights into its pupal diapause development with gene expression analysis. Int. J. Biol. Sci. 10, 1051–1063. 10.7150/ijbs.9438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durieux D., Fassotte B., Deneubourg J. L., Brostaux Y., Vandereycken A., Joie E., et al. (2015). Aggregation behavior of Harmonia axyridis under non-wintering conditions. Insect Sci. 22, 670–678. 10.1111/1744-7917.12144 [DOI] [PubMed] [Google Scholar]
- Feng C. J., Lu W. J., Dong Q. A., Chen J., Fu W. J. (2007). Effect of low Temperature treatment on larvae of the Asian cornborer, Ostrinia furnacalis (Guenée) (Lepidoptera:Pyralidae). Acta Entomol. Sin. 50, 1–6. 10.1360/aas-007-0001 [DOI] [Google Scholar]
- Fields P. G., Fleurat-Lessard F., Lavenseau L., Febvay G., Peypelut L., Bonnot G. (1998). The effect of cold acclimation and deacclimation on cold tolerance, trehalose and free amino acid levels in Sitophilus granaries and Cryptolestes ferrugineus Coleoptera. J. Insect Physiol. 44, 955–965. 10.1016/S0022-1910(98)00055-9 [DOI] [PubMed] [Google Scholar]
- Gagnon D. D., Rintamäki H., Gagnon S. S., Cheung S. S., Herzig K. H., Porvari K., et al. (2013). Cold exposure enhances fat utilization but not non-esterified fatty acids, glycerol or catecholamines availability during submaximal walking and running. Front. Physiol. 4:99 10.3389/fphys.2013.00099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Z. C., Tan J. Z. (1980). Several genetic problems of Harmonia axyridis. J. Nat. 3, 512–518. [Google Scholar]
- Guo H. B., Xu Y. Y., Ju Z., Li M. G. (2006). Seasonal changes of cold hardiness of the green lacewing, Chrysoperla sinica (Tjeder) (Neuroptera: Chrysopidae). Acta Ecol. Sin. 26, 3238–3244. [Google Scholar]
- Hartl F. (1996). Molecular chaperones in cellular protein folding. Nature 381, 571–579. 10.1038/381571a0 [DOI] [PubMed] [Google Scholar]
- Haslbeck M., Franzmann T., Weinfurtner D., Buchner J. (2005). Some like it hot: the structure and function of small heat-shock proteins. Nat. Struct. Mol. Biol. 12, 842–846. 10.1038/nsmb993 [DOI] [PubMed] [Google Scholar]
- Haslbeck M., Kastenmüller A., Buchner J., Weinkauf S., Braun N. (2008). Structural dynamics of archaeal small heat shock proteins. J. Mol. Biol. 378, 362–374. 10.1016/j.jmb.2008.01.095 [DOI] [PubMed] [Google Scholar]
- Haslbeck M., Walke S., Stromer T., Ehrnsperger M., White H. E., Chen S., et al. (1999). Hsp26: a temperature-regulated chaperone. EMBO J. 18, 6744–6751. 10.1093/emboj/18.23.6744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimpel G. E., Lundgren J. G. (2000). Sexual ration of commercially reared biological control agent. Biol. Control 19, 77–93. 10.1006/bcon.2000.0849 [DOI] [Google Scholar]
- Heydari M., Izadi H. (2014). Effects of seasonal acclimation on cold tolerance and biochemical status of the carob moth, Ectomyelois ceratoniae Zeller, last instar larvae. Bull. Entomol. Res. 104, 592–600. 10.1017/S0007485314000364 [DOI] [PubMed] [Google Scholar]
- Hightower L. E. (1991). Heat shock, stress proteins, chaperones, and proteotoxicity. Cell 66, 191–197. 10.1016/0092-8674(91)90611-2 [DOI] [PubMed] [Google Scholar]
- Holmstrup M., Costanzo J., Lee R. E. (1999). Cryoprotective and osmotic responses to cold acclimation and freezing in freeze-tolerant and freeze in tolerant earthworms. J. Compar. Physiol. B 169, 207–214 10.1007/s003600050213 [DOI] [Google Scholar]
- Huang L. H., Kang L. (2007). Cloning and interspecific altered expression of heat shock protein genes in two leafminer species in response to thermal stress. Insect Mol. Biol. 16, 491–500. 10.1111/j.1365-2583.2007.00744.x [DOI] [PubMed] [Google Scholar]
- Jing X. H., Kang L. (2002). Research progress in insect cold hardiness. Acta Ecol. Sin. 22, 2202–2207. 10.1111/j.1744-7917.1997.tb00100.x [DOI] [Google Scholar]
- Ju R. T., Du Y. Z. (2002). Mensuration of super cooling point and cold hardiness of insects. Wuyi. Sci. J. 18, 252–257. 10.15914/j.cnki.wykx.2002.00.054 [DOI] [Google Scholar]
- Julie D. T., Toby J. G., Des G. H. (2002). Multiple sequence alignment using ClustalW and ClustalX. Curr. Protoc. Bioinform. Chapter 2, 2.3. 1–22. 10.1002/0471250953.bi0203s00 [DOI] [PubMed] [Google Scholar]
- Kang L., Chen B., Wei J. N., Liu T. X. (2009). Roles of thermal adaptation and chemical ecology in Liriomyza distribution and control. Annu. Rev. Entomol. 54, 127–145. 10.1146/annurev.ento.54.110807.090507 [DOI] [PubMed] [Google Scholar]
- Kim H. B., Lee S. H., Um J. H., Kim M. J., Hyun S. K., Gong E. J., et al. (2015). Sensitization of chemo-resistant human chronic myeloid leukemia stem-like cells to Hsp90 inhibitor by SIRT1 inhibition. Int. J. Biol. Sci. 11, 923–934. 10.7150/ijbs.10896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. K., Kim R., Kim S. H. (1998). Crystal structure of a small heat-shock protein. Nature 394, 595–599. 10.1038/29106 [DOI] [PubMed] [Google Scholar]
- Koch R. L. (2003). The multicolored Asian lady beetle, Harmonia axyridis: a review of its biology, uses in biological control, and non-target impacts. J. Insect Sci. 3, 1–16. 10.1673/031.003.3201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch R. L., Carrillo M. A., Venette R. C., Cannon C. A., Hutchison W. D. (2004). Cold hardiness of the multicolored Asian lady beetle (Coleoptera: Coccinellidae). Environ. Entomol. 33, 815–822. 10.1603/0046-225X-33.4.815 [DOI] [Google Scholar]
- Koštál V., Miklas B., Doležal P., Rozsypal J., Zahradníčková H. (2014). Physiology of cold tolerance in the bark beetle, Pityogenes chalcographus and its overwintering in spruce stands. J. Insect Physiol. 63, 62–70. 10.1016/j.jinsphys.2014.02.007 [DOI] [PubMed] [Google Scholar]
- Koštál V., Slachta M., Simek P. (2001). Cryoprotective role of polyols Independent of the increase in super cooling capacity in diapausing adults of Pyrrhocoris apterus (Heteroptera: Insecta). Compar. Biochem. Physiol. 130B, 365–374. 10.1016/S1096-4959(01)00441-9 [DOI] [PubMed] [Google Scholar]
- Labrie G., Coderre D., Lucas É. (2008). Overwintering strategy of multicolored Asian lady beetle (Coleoptera: Coccinellidae): cold-free space as a factor of invasive success. Ann. Entomol. Soc. Am. 101, 860–866. 10.1093/aesa/101.5.860 [DOI] [Google Scholar]
- Leather S. R., Walters K. F. A., Bale J. S. (1993). The Ecology of Insect Over Wintering. Cambridge: Cambridge University Press. [Google Scholar]
- Lee R. E. (1989). Insect cold hardiness: to freeze or not to freeze. Bioscience 39, 308–313. 28150589 [Google Scholar]
- Lee R. E., Denlinger D. L. (1991). Insects at Low Temperature. New York, NY: Chapman and Hall. [Google Scholar]
- Li Z. W., Li X., Yu Q. Y., Xiang Z. H., Kishino H., Zhang Z. (2009). The small heat shock protein (sHSP) genes in the silkworm, Bombyx mori, and comparative analysis with other insect sHSP genes. BMC Evol. Biol. 9:215. 10.1186/1471-2148-9-215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K., Peng Z. Q., Li W. D., Fu Y. G., Jin Q. A. (2005). The super-cooling point measure of Brontispa lngissima. Plant Quarantine 19, 24–26. [Google Scholar]
- Luo G. R., Chen S., Le W. D. (2007). Are heat shock proteins therapeutic target for Parkinson's disease? Int. J. Biol. Sci. 3, 20–26. 10.7150/ijbs.3.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma R. Y., Hao S. G., Tian J., Sun J. H., Kang L. (2006). Seasonal variation in cold-hardiness of the Japanese pine sawyer Monochamus alternatus (Coleoptera: Cerambycidae). Environ. Entomol. 35, 881–886. 10.1603/0046-225X-35.4.881 [DOI] [Google Scholar]
- Meijering R. A., Zhang D., Hoogstra-Berends F., Henning R. H., Brundel B. J. (2012). Loss of proteostatic control as a substrate for atrial fibrillation: a novel target for upstream therapy by heat shock proteins. Front. Physiol. 3:36. 10.3389/fphys.2012.00036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michie L. J., Mallard F., Majerus M. E. N., Jiggins F. M. (2010). Melanic through nature or nurture: genetic polymorphism and phenotypic plasticity in Harmonia axyridis. J. Evol. Biol. 23, 1699–1707. 10.1111/j.1420-9101.2010.02043.x [DOI] [PubMed] [Google Scholar]
- Michie L. J., Masson A., Ware R. L., Jiggins F. M. (2011). Seasonal phenotypic plasticity: wild ladybirds are darker at cold temperatures. Evol. Ecol. 25, 1259–1268. 10.1007/s10682-011-9476-8 [DOI] [Google Scholar]
- Montfort R. L. M., Slingsby C., Vierling E. (2001). Structure and function of the small heat shock protein/alpha-crystallin family of molecular chaperones. Adv. Protein Chem. 59, 105–156. 10.1016/S0065-3233(01)59004-X [DOI] [PubMed] [Google Scholar]
- Nedved O., Lavy D., Verhoef H. A. (1998). Modelling the time-temperature relationship in cold injury and effect of high temperature interruptions on survival in a chill-sensitive collembolan. Funct. Ecol. 12, 816–824. 10.1046/j.1365-2435.1998.00250.x [DOI] [Google Scholar]
- Pandey B., Kaur A., Gupta O. P., Sharma I., Sharma P. (2015). Identification of HSP20 gene family in wheat and barley and their differential expression profiling under heat stress. Appl. Biochem. Biotechnol. 175, 2427–2446. 10.1007/s12010-014-1420-2 [DOI] [PubMed] [Google Scholar]
- Purse B. V., Comont R., Butler A., Brown P. M. J., Kessel C., Roy H. E. (2015). Landscape and climate determine patterns of spread for all colour morphs of the alien ladybird Harmonia axyridis. J. Biogeogr. 42, 575–588. 10.1111/jbi.12423 [DOI] [Google Scholar]
- Renault D., Nedvěd O., Hervant F., Vernon P. (2004). The importance of Fluctuating thermal regimes for repairing chill injuries in the tropical Beetle Alphitobius diaperinus (Coleoptera:Tenebrionidae) during exposure to low temperature. Physiol. Entomol. 29, 139–145. 10.1111/j.0307-6962.2004.00377.x [DOI] [Google Scholar]
- Renault D., Salin C., Vannier G., Vernon P. (2002). Survival at low temperatures in insects: what is the ecological significance of the super cooling point? Cryoletters 23, 217–228. [PubMed] [Google Scholar]
- Roy H. E., Brown P. M. J., Adriaens T., Berkvens N., Borges I., Clusella-Trullas S., et al. (2016). The harlequin ladybird, Harmonia axyridis: global perspectives on invasion history and ecology. Biol. Invasions 18, 1–48. 10.1007/s10530-016-1077-6 [DOI] [Google Scholar]
- Ruan C. C., Du W. M., Wang X. M., Zhang J. J., Zang L. S. (2012). Effect of long-term cold storage on the fitness of pre-wintering Harmonia axyridis, (Pallas). Biocontrol 57, 95–102. 10.1007/s10526-011-9414-2 [DOI] [Google Scholar]
- Senf S. M. (2013). Skeletal muscle heat shock protein 70: diverse functions and therapeutic potential for wasting disorders. Front. Physiol. 4:330. 10.3389/fphys.2013.00330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo M. J., Youn Y. N. (2000). The asian lady-bird, Harmonia axyridis, as biological control agents: I. predacious behavior and feeding ablity. Korean J. Appl. Entomol. 39, 59–71. [Google Scholar]
- Sharma A., Upadhyay A. K., Bhat M. K. (2009). Inhibition of Hsp27 and Hsp40 potentiates 5-fluorouracil and carboplatin mediated cell killing in hepatoma cells. Cancer Biol. Ther. 8, 2106–2113. 10.4161/cbt.8.22.9687 [DOI] [PubMed] [Google Scholar]
- Sharma N., Akhtar S., Jamal Q. M., Kamal M. A., Khan M. K., Siddiqui M. H., et al. (2016). Elucidation of antiangiogenic potential of Vitexin obtained from Cucumis sativus targeting Hsp90 protein: a novel multipathway targeted approach to restrain angiogenic phenomena. Med. Chem. [Epub ahead of print]. 10.2174/1573406413666161111152720 [DOI] [PubMed] [Google Scholar]
- Shen Q. D., Zhao L. N., Xie G. Q., Wei P., Yang M. M., Wang S. G., et al. (2015). Cloning three Harmonia axyridis (Coleoptera: Coccinellidae) heat shock protein 70 family genes: regulatory function related to heat and starvation stress. J. Entomol. Sci. 50, 168–185. 10.18474/JES14-30.1 [DOI] [Google Scholar]
- Shi Z. K., Liu X. J., Xu Q. Y., Qin Z., Wang S., Zhang F., et al. (2016). Two novel soluble trehalase genes cloned from Harmonia axyridis and regulation of the enzyme in a rapid changing temperature. Compar. Biochem. Physiol. 198B, 10–18. 10.1016/j.cbpb.2016.03.002 [DOI] [PubMed] [Google Scholar]
- Slachta M., Berková P., Vambera J., Kostá V. (2002). Physiology of cold-acclimation in non-diapausing adults of Pyrrhocoris apterus (Heteroptera). Eur. J. Entomol. 99, 181–187. 10.14411/eje.2002.026 [DOI] [Google Scholar]
- Sun Y., MacRae T. H. (2005). Small heat shock proteins: molecular structure and chaperone function. Cell. Mol. Life Sci. 62, 2460–2476. 10.1007/s00018-005-5190-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan C. C. (1946). Mosaic dominance in the inheritance of color patterns in the lady-bird beetle, Harmonia axyridis. Genetics 31, 195–210. [PubMed] [Google Scholar]
- Tan C. C., Li J. C. (1934). Inheritance of the elytral colour patterns in the lady-bird beetle, Harmonia axyridis. Am. Nat. 68, 252–265. 10.1086/280543 [DOI] [Google Scholar]
- Tang B., Liu X. J., Shi Z. K., Shen Q. D., Wang S., Zhang F., et al. (2017). Transcriptome analysis of differential gene expression related to cold hardiness in harmonia axyridis. Comp. Biochem. Physiol. Part D. Genomics Proteomics. [DOI] [PubMed] [Google Scholar]
- Tang B., Wang S. G., Wang F. W., Pang H., Zhang F. (2010). Cloning and characterization analysis of heat shock protein 90 gene from Harmonia axyridis (Pallas). Acta Sci. Nat. Sun. Yat. Sen. Univ. 49, 72–78. [Google Scholar]
- Tang B., Zhu J., Guo H. S., Fang D., Chen Q. D., Zheng X. X., et al. (2012). Studies of the diversity of multiple elytral color morphs of Harmonia axyridis (Pallas). J. Hangzhou. Norm. Univ. 11, 132–136. [Google Scholar]
- Vallières R., Rochefort S., Berthiaume R., Hébert C., Bauce É. (2015). Effect of simulated fall heat waves on cold hardiness and winter survival of hemlock looper, Lambdina fiscellaria (Lepidoptera: Geometridae). J. Insect Physiol. 73, 60–69. 10.1016/j.jinsphys.2014.12.001 [DOI] [PubMed] [Google Scholar]
- Wang H. S., Wang X. H., Zhou C. S., Huang L. H., Zhang S. F., Guo W., et al. (2007). cDNA cloning of heat shock proteins and their expression in the two phases of the migratory locust. Insect Mol. Biol. 16, 207–219. 10.1111/j.1365-2583.2006.00715.x [DOI] [PubMed] [Google Scholar]
- Wang H. S., Zhou C. S., Guo W., Kang L. (2006). Thermoperiodic acclimations enhance cold hardiness of the eggs of the migratory locust. Cryobiology 53, 206–217. 10.1016/j.cryobiol.2006.06.003 [DOI] [PubMed] [Google Scholar]
- Wang S., Michaud J. P., Tan X. L., Zhang F., Guo X. J. (2011). The aggregation behavior of Harmonia axyridis in its native range in Northeast China. Biocontrol 56, 193–206. 10.1007/s10526-010-9325-7 [DOI] [Google Scholar]
- Wang S., Michaud J. P., Zhang R., Zhang F., Liu S. (2009). Seasonal cycles of assortative mating and reproductive behaviour in polymorphic populations of Harmonia axyridis in China. Ecol. Entomol. 34, 483–494. 10.1111/j.1365-2311.2008.01075.x [DOI] [Google Scholar]
- Wang S., Tan X. L., Michaud J. P., Shi Z. K., Zhang F. (2015). Sexual selection drives the evolution of limb regeneration in Harmonia axyridis (Coleoptera: Coccinellidae). Bull. Entomol. Res. 105, 1–8. 10.1017/s0007485315000036 [DOI] [PubMed] [Google Scholar]
- Watanabe M. (2002). Cold tolerance and myo-inositol accumulation in overwintering adults of a lady beetle, Harmonia axyridis (Coleoptera: Coccinellidae). Eur. J. Entomol. 99, 5–9. 10.14411/eje.2002.002 [DOI] [Google Scholar]
- Waters E. R., Aevermann B. D., Sanders-Reed Z. (2008). Comparative analysis of the small heat shock proteins in three angiosperm genomes. Cell Stress Chaperones 13, 127–142. 10.1007/s12192-008-0023-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q., Zou Q., Zheng H. Z., Zhang F., Tang B., Wang S. G. (2011). Three heat shock proteins from Spodoptera exigua: gene cloning, characterization and comparative stress response during heat and cold shocks. Compar. Biochem. Physiol. 159B, 92–102. 10.1016/j.cbpb.2011.02.005 [DOI] [PubMed] [Google Scholar]
- Yang C. H., Pang H., Zhang F. (2009). Cloning and sequence analysis of heat shock protein70 gene in Harmonia axyridis (Pallas). J Environ. Entomol. 2, 124–131. [Google Scholar]
- Yonehara M., Minami Y., Kawata Y., Nagai J., Yahara I. (1996). Heat-induced chaperone activity of HSP90. J. Biol. Chem. 271, 2641–2645. [DOI] [PubMed] [Google Scholar]
- Yong E. L., Chung Y. H., Yi L. L., Ruei M. C. (2015). MicroRNA-1 participates in nitric oxide-induced apoptotic insults to MC3T3-E1 Cells by targeting heat-shock protein-70. Int. J. Biol. Sci. 11, 246–255. 10.7150/ijbs.11138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Ezemaduka A. N., Wang Z., Hu H., Shi X., Yin C. C., et al. (2015). A novel mechanism for small heat shock proteins to function as molecular chaperones. Sci. Rep. 5:8811. 10.1038/srep08811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Ma J. (2013). Insect super cooling point and its influence factors. Tianjin. Agric. Sci. 11, 76–84. 10.16380/j.kcxb.2010.02.010 [DOI] [Google Scholar]
- Zhao J., Chen Z. Z., Qu J. J., Zhang F., Yin X. C., Xu Y. Y. (2010). Responses of Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae) adults to cold acclimation and the related changes of activities of several enzymes in their bodies. Acta Entomol. Sin. 53, 147–153. [Google Scholar]
- Zhao J., Yu L. Y., Li M., Zheng F. Q., Zhang F., Xu Y. Y. (2008). Seasonal variation in cold tolerance of the multicolored ladybeetle, Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae) adults. Acta Entomol. Sin. 51, 1271–1278. 10.16380/j.kcxb.2008.12.001 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The cDNA sequence of Harmonia axyridis sHSPs. The initiation and termination codons are indicated in bold and underlined. (A) Hsp36.77, (B) Hsp16.25, (C) Hsp21.00, (D) Hsp21.62, (E) Hsp10.87, (F) Hsp21.56.