Abstract
Introduction
Antimicrobial peptides (AMPs) produced by the epithelium are important for innate immune defense. In 2001 a novel AMP Dermcidin (DCD) was described with no homology to other AMPs and an expression pattern restricted to eccrine sweat glands. In contrast to other AMPs, DCD expression has not been shown to be induced under inflammatory conditions in the skin. After identifying DCD by mass spectrometery in a protein sample isolated from human nasal secretions, we sought to determine the role of DCD in innate defense of the sinonasal airway.
Methods
After IRB approval, sinonasal mucosal tissue specimens were acquired from residual clinical material obtained during sinonasal surgery and used to grow cultures in air-liquid-interface (ALI). After stimulation of the cultures with various bitter compounds and PBS, airway surface liquid was collected and a DCD-specific ELISA was used to quantify DCD in each sample. To localize DCD expression ALI cultures were fixed and immunofluorescence performed against DCD, β-tubulin IV, and Muc-5A.
Results
ELISA showed DCD in air-surface liquid and in clinical nasal secretion samples at concentrations comparable to eccrine sweat. There was no evidence of inducible expression with any of the tested stimulants. Confocal microscopy revealed DCD expression in sinonasal mucosal goblet cells.
Conclusion
This is the first report of the presence of DCD in nasal mucosa and demonstration of DCD in clinical samples of human nasal secretions at clinically relevant concentrations which may represent a novel arm of sinonasal airway innate defense.
Keywords: chronic rhinosinusitis, innate immunity, dermcidin, antimicrobial peptide
Introduction
The epithelia of the human body, including both skin and mucosal surfaces, provides a barrier to the environment that is the first line defense against invading pathogens. We have recently described two independent, yet complimentary, arms of upper respiratory innate immunity mediated via taste receptors.1, 2 In the first of these pathways, the bitter taste receptor T2R38, expressed on ciliated cells, detects acyl-homoserine lactones (AHLs) quorum sensing molecules secreted by gram negative bacteria. Upon stimulation of T2R38 by AHLs the ciliated cells, in a calcium dependent manner, produce nitric oxide (NO) which yields two rapid defensive responses against invading bacteria. The gas (NO) diffuses into the mucous layer and then into the bacteria causing direct killing. It also stimulates ciliated cells to increase their beat frequency, increasing mucociliary clearance resulting in accelerated clearance of bacteria trapped in the mucus layer.1 The second pathway begins with discrete non-ciliated unipolar cells called solitary chemosensory cells (SCCs) which also express different bitter taste receptors (T2Rs).2 It is presumed that the bitter receptors on solitary chemosensory cells detect other microbial products (non-AHLs). Activation of SCC T2Rs by bitter receptor agonists such as denatonium benzoate and absinthin, stimulate a rise in intracellular calcium resulting in a calcium induced calcium release yielding a calcium wave which propagates through gap junctions to surrounding epithelial cells. This calcium flux triggers the surrounding epithelial cells to release pre-stored antimicrobial peptides (AMPs).2
Antimicrobial peptides are critical effecter molecules of the innate immune defense and show a broad-spectrum of antimicrobial activity against a wide range of pathogens including bacteria, fungi and enveloped viruses.3 Many AMPs also have a role in cellular processes such as immune modulation, apoptosis and wound healing.4 The mechanism of antimicrobial action of most AMPs is incompletely understood, however, there is evidence that many AMPs effectively increase the permeability of the bacterial membrane as part of their killing mechanism.5, 6
In 2001 Schittek et al. discovered a novel AMP in human sweat, Dermcidin (DCD), which shows no homology to other known AMPs and which has been shown to be active against E. coli, E. faecalis, C. albicans and S. aureus.7 This group found that human DCD gene, codes for a secreted protein found to be specifically and constitutively expressed in eccrine sweat glands, where it is secreted into sweat and transported to the epidermal surface.7 Since its discovery, several groups have reported a restricted expression pattern of dermcidin, observing that with the exception of skin, DCD is not expressed at the RNA level in several tested human adult and fetal tissues.7, 8, 9 In human skin, DCD is expressed in the dark mucous cells of the secretory coil of eccrine sweat glands and is found in the Golgi complex and the secretory granules typical for a secreted protein, however it is not expressed in apocrine sweat glands.7, 10, 11, 12, Additionally, in contrast to inducible AMPs, such as defensins, dermcidin expression has not been shown to be induced under inflammatory conditions.11, 12, 13, 14 Recently, two separate investigators using proteomic approaches have identified dermcidin in basal tears as well as in samples of cervicovaginal fluid.15, 16 However, dermcidin has not yet been identified in any other body fluids, such as nasal secretions, saliva, semen, breast milk, or urine.10, 11, 12, 17, 18 Here, we report the presence of dermcidin in sinonasal secretions both in vitro and in vivo, at concentrations comparable to those found in sweat. This study has important clinical implications, as it identifies a previously-unknown molecular component of sinonasal innate immunity.
Materials and Methods
Experimental solution compositions
Physiological experiments were performed with Dulbecco’s PBS (DPBS; containing 1.8 mM Ca2+) on the apical side of the cultures, containing (in mM) 138 NaCl, 2.7 KCl, 1.5 KH2PO4, 8 Na2HPO4, 1.8 CaCl2, and 1.5 MgCl2, with pH adjusted to approximately 7.2 (to prevent any Ca2+ precipitation due to the high phosphate concentrations in PBS).
Sinonasal ALI tissue cultures and Clinical nasal secretion samples
Subjects were recruited from the Division of Rhinology in the Department of Otorhinolaryngology — Head and Neck Surgery at the University of Pennsylvania, and from the Philadelphia Veterans Affairs Medical Center, with full study approval from the Institutional Review Boards at both institutions. Informed consent was obtained during the pre-operative visit. Selection criteria for recruitment were patients undergoing sinonasal surgery. Samples were obtained from patients undergoing sinonasal surgery for skull base pathology. Specimens were obtained from the ethmoid cavity with no overt signs of infection or inflammation. Samples were collected over a 3-month period with analysis performed over the following 3 months. Exclusion criteria included a history of systemic diseases such as Wegner’s granulomatosis, sarcoidosis, cystic fibrosis, immunodeficiencies, and use of antibiotics, oral corticosteroids, or antibiologics (e.g., Xolair) within 1 month of surgery. Tissue or secretions from active smokers or those who quit within three months were not utilized in this study. Sinonasal mucosal tissue specimens were acquired from residual clinical material obtained during surgery and transported to the laboratory in saline placed on ice.
Air-liquid interface cultures were established from this tissue as previously described.2, 19 In brief, ALI cultures were prepared from human sinonasal epithelial cells obtained from enzymatically dissociated human tissue and grown to confluence in tissue culture flasks (75 cm2) using bronchial epithelial basal medium (BEBM; Clonetics, Cambrex, East, NJ) and proliferation medium consisting of DMEM/Ham’s F-12 media containing 100 μg/ml streptomycin and 100 U/ml penicillin for 7 days. Cells were trypsinized and seeded on porous polyester membranes (6–7 × 104 cells per membrane) in cell culture inserts (Transwell-clear, 12-mm diameter, 0.4-μm pores; Corning, Acton, MA) coated with type I bovine collagen [30 μg/ml; BD Biosciences (San Jose, CA)], 100 μl of coating solution (BSA [0.1 mg/ml; Sigma-Aldrich (St. Louis, MO)] and fibronectin [10 μg/ml; BD Biosciences] in LHC basal medium [Invitrogen (Grand Island, NY)]) and placed in a tissue culture laminar flow hood for approximately 12 hours. After five days, the culture media from the upper compartment was removed and the epithelium was allowed to differentiate in differentiation medium consisting of 1:1 BEBM (Clonetics; Cambrex) and DMEM (Invitrogen) with the Clonetics complements for hEGF (0.5 ng/ml), BPE (0.13 mg/ml), hydrocortisone (0.5 g/ml), triiodothyronine (6.5 g/ml), insulin (5 g/ml), epinephrine (5 g/ml), and transferrin (0.5 g/ml), supplemented with 100 g/ml streptomycin, 100 U/ml penicillin, 0.1 nM retinoic acid (Sigma-Aldrich), and 10% FBS (Sigma-Aldrich) in the basal compartment.
Lysates of ALI cultures were made using a lysis buffer containing 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, and 100 mM Tris, pH 7.4, with Roche Complete Protease Inhibitor Cocktail plus PMSF. All lysates were normalized by addition of excess lysis buffer to a protein concentration of 2 mg/ml, measured using a Bio-Rad DC protein assay.
Nasal secretions were obtained from healthy subjects as previously described.20 Briefly, in the absence of any topical anesthetic or decongestant a pre-expanded Pope ear wick (Medtronic ENT) was placed in the nasal cavity under direct visualization between the nasal septum and inferior turbinate bilaterally. Wicks were removed after 5 minutes and placed into an Eppendorf tube. A hole was made in the bottom of the Eppendorf tube with a 20-gauge needle, and this Eppendorf tube was placed inside another Eppendorf tube and centrifuged (1,000 g; 5 minutes); secretions were collected in the outside Eppendorf tube. Samples were collected over a 3-month period with analysis performed over the following 3 months. All mucous samples were normalized to total protein.
Enzyme-Linked Immunosorbent Assay
ELISA kit for Dermcidin was purchased from Biomatik (Cambridge, Ontario, Canada) and performed according to the manufacturer’s instructions. Both ALI samples and clinical nasal secretion samples were diluted 1:10 in PBS. Using three different human-derived sinonasal ALI cultures in duplicate, apical surface liquid was collected, diluted 1:100 in PBS, and used in the DCD-specific ELISA. Additionally, to test for the presence of DCD within the sinonasal cells themselves, the permeable membrane on which the cells were grown was cut out using a razor blade, washed three times with PBS, placed in 500mL of PBS, and then sonicated for 30 minutes to release the cells from the membrane, Subsequently, 100ul of this lysate was then used in the DCD-specific ELISA at 1:100 dilution and performed in quadruplicate. For nasal secretion samples described above, 10 μl of each patient’s collected secretions was diluted in 990 μl PBS and 100 μl aliquots were tested in quadruplicate with DCD-specific ELISA for each of the three patients.
Immunofluorescent staining and confocal microscopy
ALI cultures were fixed in 4% formaldehyde for 20 minutes at 4°C and subsequently washed 3 times in PBS, followed by blocking and permeabilization with 0.3% Triton X-100, 5% normal donkey serum, and 1% bovine serum albumin for 60 minutes at room. Primary antibodies raised from different hosts were chosen for the double immunofluorescent staining: mouse monoclonal anti–β-tubulin IV (1:1,000; Abcam) and rabbit polyclonal anti-Dermcidin (1:500; LS Bioscience) and mouse monoclonal anti-Muc5A (anti-Muc5AC clone 45M1, 1:200 [Abcam]), used previously in lung.21 Visualization was carried out using Alex Fluor 488–conjugated (green) donkey anti-mouse IgG for tubulin IV and Muc5AC and Alex Fluora 555 (red)-conjugated donkey anti-rabbit IgG for DCD. Both secondary antibodies (Invitrogen) were diluted at 1:1000. The incubation time was 2 hours at 4°C for primary antibodies and 1 hour at 4°C for secondary antibodies, respectively. Counterstaining was done using Hoechst (blue), a nuclear dye. Confocal images were acquired using an Olympus Fluoview System at the z-axis step of 0.3 μm. A sequential scanning module was used to prevent bleed-through of fluorophores into other channels.
Statistics
Data was analyzed in Excel (Student’s t test) and all statistical analysis was done through GraphPad Prism (GraphPad Software, Inc., La Jolla, CA) including Student’s t test, χ2 test, and ANOVA as indicated with P< 0.05 was considered statistically significant. For multiple comparisons, ANOVA with the Bonferroni post hoc test was used when pre-selected pairwise comparisons were performed, ANOVA with the Turkey-Kramer post hoc test was used when all values in the data set were compared, and ANOVA with the Dunnett’s post hoc test was used when all values were compared with a control value. All data are reported as means ± standard deviation.
Results
Prior work in our lab was focused on determining the proteins secreted following stimulation of nasal solitary chemosensory cells with denatonium benzoate.2 In these efforts we performed mass spectrometry on a low-molecular weight protein band isolated from the airway surface liquid of human sinonasal epithelial cells grown at an air-liquid interface (ALI) cultures.2, 22, 23, 24 This analysis identified dermcidin as a component of the band of interest. To confirm this, ASL and cell lysates from ALI cultures established from three different individuals were interrogated for dermcidin utilizing an enzyme-linked Immunosorbent assay (ELISA) specific for DCD (Biomatik, Cambridge, Ontario, Canada). We found that DCD was in fact present in the ASL as well as in the cell lysates of these sinonasal cultures (Figure 1).
Figure 1.
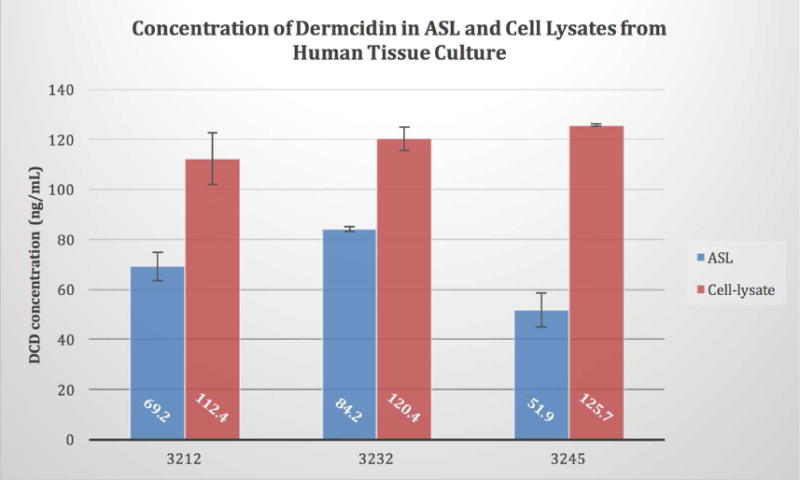
Identification of Dermcidin in Sinonasal Cells. Primary human sinonasal air-liquid interface cultures established from three individuals (n=2 per subject). Apical surface was washed and 30 minutes later the ASL was collected. Additionally, the trans-well membranes were harvested and the cells lysed via sonication. ASL and cell lysate samples were normalized for total protein and dermcidin concentrations determined by DCD ELISA. The average DCD concentration overall in ASL (68.4 ng/ml 14.97) was roughly half of what was evident in cell lysates (119.5 ng/ml 7.87).
We next evaluated clinical samples of nasal secretions to determine if DCD is present in vivo and if it is secreted in clinically-relevant concentrations. Human nasal secretion samples were obtained as previously described.20 Using the DCD ELISA, the concentration of DCD found in these three in vivo samples was between 14077ng/mL and 2405ng/mL which is similar to the concentration of DCD-1L found in sweat (1–10ug/mL=1000–10000ng/mL) (Figure 2).
Figure 2.
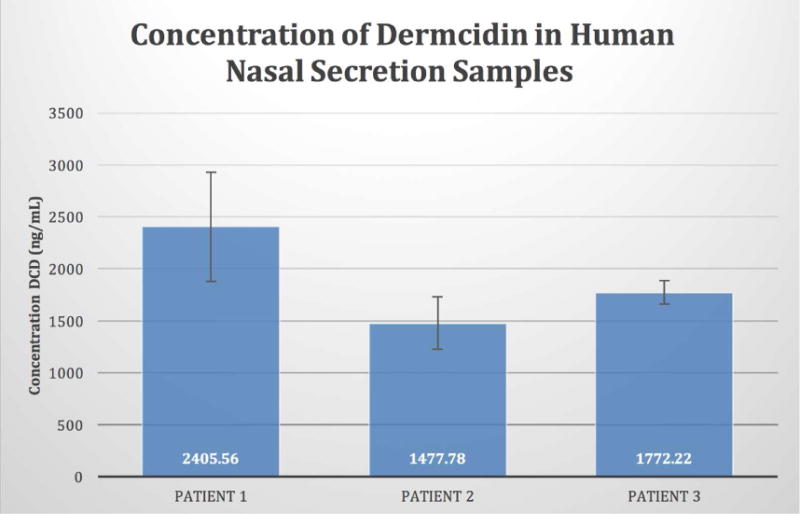
Identification of Dermcidin in Nasal Mucus. Human nasal secretions were obtained from three subjects and tested in quadruplicate using DCD-specific ELISA. Concentration of DCD in nasal mucus were as follows: Patient 1: 2,406ng/ml (562); Patient 2: 1478 (251); Patient 3: 1772 (112). These concentrations are similar to the concentration of DCD-1L found in sweat (1,000–10,000ng/mL).
After confirming the presence of DCD in the ASL of primary human sinonasal epithelial cultures grown under ALI conditions, we set out to determine whether DCD secretion could be stimulated by various compounds. ALI cultures were stimulated from the apical side with various potential stimulants or PBS as control for 30 minutes while incubated at 37°C.2 The stimulants used were; the bitter taste receptor agonist denatonium benzoate (10 mM), the general purinergic agonist ATP (100 μM), histamine (100 μM) responsible for local immune response, Neuro-peptide-Y (5 μM) an autonomic nervous system transmitter, and Vasoactive Intestinal Peptide (10 μM) a neuropeptide that is a ligand for class II G-protein coupled receptors. After exposure, the ASL was removed and evaluated by DCD-specific ELISA. Our results indicated that dermcidin secretion was not elevated above basal levels during any of the tested stimulated conditions, similar to its reported constitutive expression in the eccrine glands of the skin (Table 1).
Table 1.
The average concentration of DCD in ASL from human sinonasal ALI cultures after 30-minute incubation with various potential stimulants versus PBS (negative control). There was no statistically significant increase (p<.05) in DCD concentration after stimulation when compared with a 1-tailed t-test.
| DCD Concentration | Standard Deviation | P value | |
|---|---|---|---|
| Denatonium | 77.6 ng/ml | ± 11.2 | .0656 |
| ATP | 100.2 ng/ml | ± 18.25 | .4708 |
| Histamine | 84.5 ng/ml | ± 5.68 | .1751 |
| VIP | 152.5 ng/ml | ± 12.92 | .1606 |
| Neuropeptide-Y | 78.8 ng/ml | ± 3.88 | .0827 |
| PBS | 95.68 ng/ml | ± 5.3 |
Lastly, to determine which cells may be responsible for the production of DCD in the sinuses, we used immunocytochemical staining, to localize DCD within our human sinonasal cultures. DCD expression was observed near the apical surface of non-ciliated cells and found to co-localize with the mucin marker MUC5AC, indicative that DCD expression is limited to goblet cells, as DCD expression was absent in ciliated cells visualized using a marker for motile cilia, type IV beta-tubulin (Figure 3).
Figure 3.
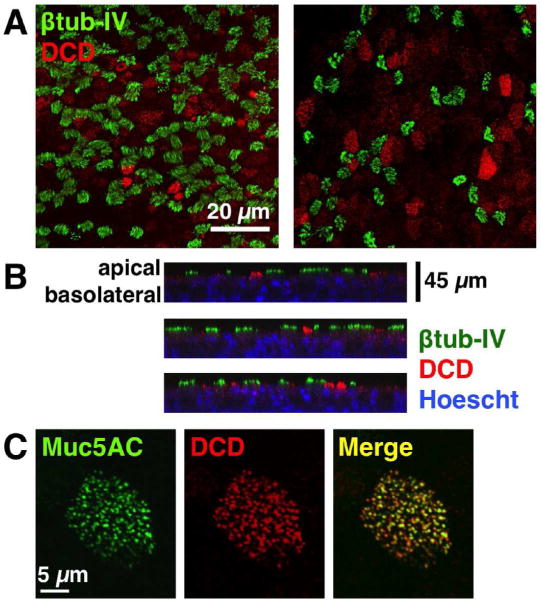
Dermcidin Expression Localizes to Goblet Cells. Human sinonasal ALI cultures were subjected to double immunofluorescent staining for Type IV β-tubulin (cilia marker), and Dermcidin, as well as Muc5A (goblet cell marker). Counterstaining was done using Hoechst (blue), a nuclear dye. (A) DCD did not co-localize to ciliated cells stained with Type IV β-tubulin and appeared in distinct non-ciliated cells; (B) Z-stacks of (A) reveals that DCD localizes toward the apical surface of the cells and is absent in ciliated cells; (C) Merge image (yellow) shows co-localization of DCD and Muc5A which is a marker for goblet cells.
Discussion
The discovery of DCD expression and secretion by sinonasal epithelial cells has important clinical relevance because it introduces a new potential component of sinonasal innate immunity. DCD may play an important role in sinonasal secretions because it has been previously shown to have broad antimicrobial effects. Many antimicrobial peptides, including defensins, are produced as inactive precursor proteins that are then proteolytically processed to give rise to active peptides.25 Although the DCD amino acid sequence shared no homology with other known AMPs, the size of DCD and its processed peptides resembles the structural characteristics of the defensin family of AMPs. One of these processed peptides, DCD-1L, a 47aa peptide secreted into sweat at approximately 1–10ug/mL, was found to be antimicrobially active against E. coli, E. faecalis, C. albicans and S. aureus under a broad pH range and in high salt concentrations resembling human sweat.7, 26, 27 This wide range of antimicrobial activity as well as the fact that DCD- 1L is anionic, in contrast to most other AMPs, suggested that the functional mechanism of DCD-1L might be different from many other AMPs. It was recently shown that DCD-1L interacts preferentially with negatively charged bacterial phospholipids, forming oligomeric complexes that create ion channels in the bacterial membrane.28 Another recent paper demonstrated the detection of a higher concentration of antimicrobially active dermcidin peptides at certain body sites which are most likely to come into contact with pathogenic organisms, such as the face and hands.10 Dermcidin was found to be the most abundant AMP in sweat from these areas.10, 29 Because not all DCD-derived peptides are antimicrobial, future work is required to isolate the peptide itself from the nasal secretions to determine which post-processed form(s) of DCD are present which may shed some light on the functional role of DCD in the sinuses.
DCD may also play other important roles in the sinonasal environment. Peptides processed from the dermcidin precursor protein exhibit a range of biological functions. In addition to the well-described host-defense function of peptides derived from the AMP domain, peptides derived from the pro-domain have been reported to exert a survival-promoting effect on neuronal and tumor cells (Y-P30), and to induce muscle proteolysis causing cancer cachexia (PIF). Dermcidin has also been reported to be a putative oncogene in cancer cells.30 Additionally, Niyonsaba et al. suggest that dermcidin participates in the regulation of skin innate immunity not only by direct bacterial killing, but also by regulation of skin inflammation via DCD-1 and DCD-1L activation pro-inflammatory cytokines TNF-alpha, IL-8, interferon-inducible protein 10 and macrophage inflammatory protein-3.31 Further work is required to determine whether a signaling role for CDC in the sinonasal epithelium exists.
The fact that DCD secretion is not altered by stimulation of the respiratory epithelium, while fitting with prior observations of the sweat gland, is highly intriguing as it differs from other sinonasal AMPs (e.g., the defensins) that we recently demonstrated to be regulated by T2R stimulation. Basal DCD concentrations may have important implications for sinonasal disease. Future clinical studies should be aimed at determining if sinonasal DCD concentrations differ in healthy individuals and CRS patients or patients with upper respiratory disease, as well as whether any such differences are independent (i.e., potentially under genetic control) or acquired after the onset of disease. Additionally, correlating DCD levels in nasal secretions to microbiology or microbiome data may shed additional information on the role DCD is playing in maintaining a healthy mucosal environment. It is still possible that DCD is regulated through a stimulation pathway that we did not test (e.g., via cytokines or TLRs) or that its regulation is gradual and up-regulation would only be seen after chronic stimulation, such as might occur during hours or days of infection. One shortcoming of the experiments described herein is that the relatively short (30 minute) exposure time of the sinonasal ALI cultures to tested stimulants. Future work must be carried out to understand the regulation of DCD production and secretion from sinonasal goblet cells.
In summary, this study is, to our knowledge, the first to demonstrate that dermcidin protein is expressed in the airway, specifically within sinonasal goblet cells, and that it is secreted into the nose at clinically relevant concentrations similar to those found in human sweat. While future work must be done to clarify the role of DCD in the upper respiratory system, the data shown here suggest that dermcidin is a potentially important and previously-overlooked player in respiratory innate immunity.
Acknowledgments
This study was supported by grants from National Institutes of Health (R01DC013588 to N.A.C.), a charitable donation from the RLG Foundation, Inc (to N.A.C.)
Footnotes
Financial Disclosure: None
This work was presented at the American Rhinologic Society Spring Meeting on 5/19/2016, in Chicago, IL, USA.
References
- 1.Lee RJ, Xiong G, Kofonow JM, Chen B, et al. T2R38 taste receptor polymorphisms underlie susceptibility to upper respiratory infection. The Journal of Clinical Investivation. 2012;122(11):4145–4159. doi: 10.1172/JCI64240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee RJ, Kofonow JM, Rosen PL, Siebert AP, et al. Bitter and sweet taste receptors regulate human upper respiratory innate immunity. The Journal of Clinical Investigation. 2014;124(3):1393–1405. doi: 10.1172/JCI72094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 4.Lai Y, Gallo RL. AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends in Immunology. 2009;30:131–141. doi: 10.1016/j.it.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nature Review Microbiology. 2005;3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 6.Yeaman MR, Yount NY. Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev. 2003;55:27–55. doi: 10.1124/pr.55.1.2. [DOI] [PubMed] [Google Scholar]
- 7.Schittek B, Hipfel R, Sauer B, et al. Dermcidin: a novel human antibiotic peptide secreted by sweat glands. Nature Immunology. 2001;2:1133–1137. doi: 10.1038/ni732. [DOI] [PubMed] [Google Scholar]
- 8.Stewart GD, Skipworth RJ, Ross JA, et al. The dermcidin gene in cancer: role in cachexia, carcinogenesis and tumour cell survival. Curr Opin Clin Nutr Metab Care. 2008;11:208–213. doi: 10.1097/MCO.0b013e3282fb7b8d. [DOI] [PubMed] [Google Scholar]
- 9.Porter D, Weremowicz S, Chin K, et al. A neural survival factor is a candidate oncogene in breast cancer. Proc Natl Acad Sci USA. 2003;100:10931–10936. doi: 10.1073/pnas.1932980100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rieg S, Seeber S, Steffen H, et al. Generation of multiple stable dermcidin-derived antimicrobial peptides in sweat of different body sites. Journal of Investigative Dermatology. 2006;126:354–365. doi: 10.1038/sj.jid.5700041. [DOI] [PubMed] [Google Scholar]
- 11.Shiteck B. The multiple facets of Dermcidin in cell survival and host defense. Journal of Innate Immunity. 2012;4:349–360. doi: 10.1159/000336844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sagawa K, Kimura A, Saito Y, et al. Production and characterization of a monoclonal antibody for sweat-specific protein and its application for sweat identification. Int J Legal Med. 2003;117:90–95. doi: 10.1007/s00414-002-0341-8. [DOI] [PubMed] [Google Scholar]
- 13.Minami Y, Uede K, Sagawa K, et al. Immunohistochemical staining of cutaneous tumours with G-81, a monoclonal antibody to dermcidin. British Journal of Dermatology. 2004;151:165–169. doi: 10.1111/j.1365-2133.2004.06079.x. [DOI] [PubMed] [Google Scholar]
- 14.Rieg S, Garbe C, Sauer B, et al. Dermcidin is constitutively produced by eccrine sweat glands and is not induced in epidermal cells under inflammatory skin conditions. British Journal of Dermatology. 2004;151:534–539. doi: 10.1111/j.1365-2133.2004.06081.x. [DOI] [PubMed] [Google Scholar]
- 15.You J, Fitzgerald A, Cozzi PJ, et al. Post-translation modification of proteins in tears. Electrophoresis. 2010;31:1853–1861. doi: 10.1002/elps.200900755. [DOI] [PubMed] [Google Scholar]
- 16.Shaw JL, Smith CR, Diamandis EP. Proteomic analysis of human cervicovaginal fluid. J Proteome Res. 2007;6:2859–2865. doi: 10.1021/pr0701658. [DOI] [PubMed] [Google Scholar]
- 17.Sakurada K, Akutsu T, Fukushima H, et al. Detection of dermcidin for sweat identification by real-time RT-PCR and ELISA. Forensic Science International. 2010;194:80–84. doi: 10.1016/j.forsciint.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 18.McIntosh RS, Cade JE, Al-Abed M, Shanmuganathan V, et al. The spectrum of antimicrobial peptide expression at the ocular surface. Investigative Ophthalmology and Visual Sciences. 2005;46:1379–1385. doi: 10.1167/iovs.04-0607. [DOI] [PubMed] [Google Scholar]
- 19.Lai Y, Chen B, Shi J, Palmer JN, et al. Inflammation-mediated upregulation of centrosomal protein 110, a negative modulator of ciliogenesis, in patients with chronic rhinosinusitis. The Journal of allergy and clinical immunology. 2011;128(6):1207–1215. doi: 10.1016/j.jaci.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Hatten KM, Palmer JN, Lee RJ, et al. Corticosteroid use does not alter nasal mucous glucose in chronic rhinosinusitis. Otolaryngology—Head and Neck Surgery. 2015;152(6):1140–1144. doi: 10.1177/0194599815577567. [DOI] [PubMed] [Google Scholar]
- 21.Villenave R, Touzelet O, Thavagnanam S, Sarlang S, et al. Cytopathogenesis of Sendai Virus in Well-Differentiated Primary Pediatric Bronchial Epithelial Cells. Journal of Virology. 2010;84(22):11718–11728. doi: 10.1128/JVI.00798-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dimova S, Brewster ME, Noppe M, et al. The use of human nasal in vitro cell systems during drug discovery and development. Toxicol In Vitro. 2005;19:107–122. doi: 10.1016/j.tiv.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Cozens AL, Yezzi MJ, Kunzelmann K, et al. CFTR expression and chloride secretion in polarized immortal human bronchial epithelial cells. American Journal of Respirator Cellular and Molecular Biology. 1994;10:38–47. doi: 10.1165/ajrcmb.10.1.7507342. [DOI] [PubMed] [Google Scholar]
- 24.Gruenert DC, Finkbeiner WE, Widdicombe JH. Culture and transformation of human airway epithelial cells. American Journal of Physiology. 1995;268:L347–L360. doi: 10.1152/ajplung.1995.268.3.L347. [DOI] [PubMed] [Google Scholar]
- 25.Bals R. Epithelial antimicrobial peptides in host defense against infection. Respir Res I. 2000:141–150. doi: 10.1186/rr25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lai YP, Peng YF, Zuo Y, Li J, Huang J, Wang LF, Wu ZR. Functional and structural characterization of recombinant dermcidin-1L, a human antimicrobial peptide. Biochem Biophys Res Commun. 2005;328:243–250. doi: 10.1016/j.bbrc.2004.12.143. [DOI] [PubMed] [Google Scholar]
- 27.Vuong C, Voyich JM, Fischer ER, Braughton KR, Whitney AR, DeLeo FR, Otto M. Polysaccharide intercellular adhesin (PIA) protects Staphylococcus epidermidis against major components of the human innate immune system. Cell Microbiology. 2004;6:269–275. doi: 10.1046/j.1462-5822.2004.00367.x. [DOI] [PubMed] [Google Scholar]
- 28.Paulmann M, Arnold T, Linke D, Ozdirekcan S, et al. Structure-activity analysis of the dermcidin-derived peptide DCD 1L, an anionic antimicrobial peptide present in human sweat. Journal of Biological Chemistry. 2012;287:8434–8443. doi: 10.1074/jbc.M111.332270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murakami M, Ohtake T, Dorschner RA, Schittek B, Garbe C, Gallo RL. Cathelicidin anti-microbial peptide expression in sweat, an innate defense system for the skin. J Invest Dermatol. 2002;119:1090–1095. doi: 10.1046/j.1523-1747.2002.19507.x. [DOI] [PubMed] [Google Scholar]
- 30.Baechle D, Flad T, Cansier A, Steffen H, Schittek B, et al. Cathepsin D is present in human eccrine sweat and involved in the postsecretory processing of the antimicrobial peptide DCD-1L. Journal of Biological Chemistry. 2006;281:5406–5415. doi: 10.1074/jbc.M504670200. [DOI] [PubMed] [Google Scholar]
- 31.Niyonsaba F, Suzuki A, Ushio H, Nagaoka I, Ogawa H, Okumura K. The human antimicrobial peptide dermcidin activates normal human keratinocytes. British Journal of Dermatology. 2009;160:243–249. doi: 10.1111/j.1365-2133.2008.08925.x. [DOI] [PubMed] [Google Scholar]


