Abstract
Cadmium is an environmental pollutant that has been associated with cardiovascular disease in populations, but the relationship of cadmium with hypertension has been inconsistent. We studied the association between urinary cadmium concentrations, a measure of total body burden, and blood pressure in American Indians, a U.S. population with above national average cadmium burden. Urinary cadmium (Cd) was measured using inductively coupled plasma mass spectrometry, and adjusted for urinary creatinine concentration. Among 3,714 middle-aged American Indian participants of the Strong Heart Study (mean age 56 years, 41% male, 67% ever-smokers, 23% taking anti-hypertensive medications), urinary Cd ranged from 0.01 to 78.48 μg/g creatinine (geometric mean=0.94 μg/g) and it was correlated with smoking pack-year among ever-smokers (r2=0.16, P<0.0001). Participants who were smokers were on average light smokers (mean 10.8 pack-years), and urinary Cd was similarly elevated in light- and never-smokers (geometric means of 0.88 μg/g creatinine for both categories). Log-transformed urinary Cd was significantly associated with higher systolic blood pressure in models adjusted for age, sex, geographic area, body mass index, smoking (ever vs. never, and cumulative pack-years) and kidney function (mean blood pressure difference by lnCd concentration [β]=1.64, P=0.002). These associations were present among light- and never-smokers (β=2.03, P=0.002, n=2,627), although not significant among never-smokers (β=1.22, P=0.18, n=1,260). Cd was also associated with diastolic blood pressure among light- and never-smokers (β=0.94, P=0.004). These findings suggest there is a relationship between cadmium body burden and increased blood pressure in American Indians, a population with increased cardiovascular disease risk.
Keywords: cadmium, blood pressure, toxicity
Hypertension is a common clinical condition contributing substantially to poor health outcomes, including cardiovascular disease (CVD) and mortality1. Hypertension risk varies by age, sex, lifestyle and behaviors, and increasing evidence suggests that blood pressure is influenced by toxic metal environmental pollutants including cadmium (Cd)2–4. Experimental evidence associates Cd to endothelial dysfunction, oxidative stress, atherosclerosis, hypertension, and kidney dysfunction5–9. These data are supported by research demonstrating a relationship between Cd body burden and CVD in populations9–11. However, the epidemiology evidence for association of Cd with hypertension has been inconsistent2, 4, 12–19. Little research has been done in populations with low-to-moderate Cd exposure for associations with increased systolic and diastolic blood pressures. Blood Cd but not urine Cd was associated with increased blood pressure in the National Health and Nutrition Examination Survey (NHANES) II (1976–1988)2 and NHANES 1999–200413. A prospective study in Belgium showed associations of changes in blood Cd levels with diastolic blood pressure among women12. However, a Croatian study showed associations of urinary but not blood Cd with diastolic blood pressure17. These studies varied on the choice of Cd measurement (blood, urine, nails), study design and definition of outcomes. In addition, the relationship between Cd and blood pressure is complicated by the fact that smoking, a major source of Cd exposure in populations, is associated with Cd levels but also independently associated with hypertension20–24.
American Indians suffer disproportionally from hypertension-related morbidity and CVD25, 26. Prior research in American Indian communities from the Strong Heart Study (SHS) has demonstrated an association of Cd burden with incident CVD and mortality10. Middle-aged American Indians recruited from the same communities have higher Cd body burden compared to national averages from individuals 35 year-old or older from the Third National Health and Nutrition Examination Survey (NHANES III) 10, 27. Therefore, American Indians are a population at risk of diseases related to Cd toxicity.
We studied the association of Cd body burden, as measured in urine, with blood pressure and hypertension in SHS American Indians. Urinary Cd represents Cd concentrations in the renal cortex and has a half-life of decades, representing a suitable biomarker for cumulative Cd exposure or body burden28. Because environmental exposures are amenable to public health interventions, this research could inform on the Cd-related burden of hypertension and its complications in this population.
METHODS
SHS design and population
The SHS recruited a population based sample of 4,545 unrelated tribal members 45 year or older without regard to disease status from 13 tribal communities in Arizona, Oklahoma and North and South Dakota29. This study uses data from a clinical visit in 1989–91, which is the SHS visit that has measures of urinary cadmium. During the clinical visit, information on demographic characteristics (age, sex, education), lifestyle/behaviors (including smoking initiation, duration and quantity), and medical history were obtained through interviews. Physical exams included anthropometrics (body mass index [BMI], waist and hip girth) and blood pressure. Resting sitting blood pressure was measured in the brachial artery three consecutive times by trained personnel using a calibrated mercury column sphygmomanometer and size-adjusted cuff, and the last two measures were averaged. Participants were instructed to bring all medications taken regularly, including both prescribed and over-the-counter. All medications were categorized according to the American Hospital Formulary Service Pharmacologic-Therapeutic Classification System and summarized by therapeutic class as previously described 30. A fasting blood sample and urine samples were obtained for biomarker measures, which were assayed using standard methods. The SHS/SHFS protocols were approved by the Indian Health Services Institutional Review Board, by Institutional Review Boards of all Institutions and by the Indian communities29, 31. All participants gave informed consent for participation.
From the initial sample, we excluded individuals with kidney failure defined as on dialysis or receiving a transplant, missing covariates or phenotypes, or urinary Cd measures. The final dataset included 3,714 SHS participants for whom urinary Cd measures were available. Urinary cadmium measurements.
Cd was measured in spot urine samples (stored at -80°C) using inductively coupled plasma spectrometry32. The analytic methods and quality control (QC) criteria have been previously described32. The limit of detection was 0.0015 μg/L. In one participant where Cd was below the limit of detection (0.03% of the total sample), the concentration was imputed as the limit of detection divided by the square root of two32. The intra-assay and inter-assay coefficients of variation for Cd in the SHS were 1.3% and 8.7%, respectively. To account for urine dilution, urine Cd concentrations are expressed in μg per g of urine creatinine. Urine creatinine was measured by an alkaline picrate method.
Blood pressure outcomes
The main outcomes assessed in this study are quantitative blood pressure traits of systolic and diastolic blood pressures. For hypertensive individuals taking antihypertensive medications, measured blood pressure is expected to be lower than if not treated. Adjusting for hypertension treatment as a covariate is not recommended, as it has shown to shrink estimated effects and reduce power in simulation studies33. Therefore, we added 10 and 15 mm Hg to measured systolic and diastolic blood pressures, respectively, for individuals reporting taking blood pressure-lowering medications as previously described34, 35. Hypertension was defined by a systolic blood pressure of 140 mm Hg or higher, or a diastolic blood pressure of 90 mm Hg or higher, or use of antihypertensive drugs36.
Covariates and definitions
Urinary Cd levels and blood pressure have been shown to vary by age, sex, BMI and kidney function. Smoking is a source of Cd exposure and a potential confounder. All these covariates were included in models, in addition to variables to account for Cd variation in geographic regions. We also tested other variables including education (less than high-school versus high-school or higher degree) which was not a significant predictor in models. Kidney function was estimated using the estimated glomerular filtration rate (eGFR) derived from the equation developed by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI). The equation is based on serum creatinine, age, sex, and race/ethnicity data37. Chronic kidney disease (CKD) was defined by and an eGFR<60 ml/min/1.72 m2.
Statistical analyses
Urinary Cd-to-creatinine ratio was right-skewed and the data was natural log-transformed (lnCd). Geometric means, medians and percentiles of urinary Cd were also estimated. We first examined the association of lnCd with prevalent systolic and diastolic blood pressure traits in SHS participants using linear regression models. Models were adjusted for age, age2, sex, age-by-sex interactions, BMI and geographical location (Arizona, North and South Dakotas, Oklahoma), and eGFR (continuous). We adjusted for smoking quantity and intensity using a variable for never vs ever users and cumulative smoking dosage (pack-years), and also performed analysis in strata of ever versus never smoking. We also examined the association of lnCd with blood pressure among never smokers and light smokers (<10 pack-years). To examine non-linear effects of Cd on blood pressure, we tested the association with blood pressure and hypertension within quartiles of Cd distribution using the lower quartile as referent. In secondary analyses, we examined the associations among current smokers, and if associations were modified by sex or changed when excluding individuals taking antihypertensive medications, given prior research suggesting confounding effects of anti-hypertensive medications. We also tested Cd associations with hypertension. Statistical tests were two sided with significance set at P<0.05.
RESULTS
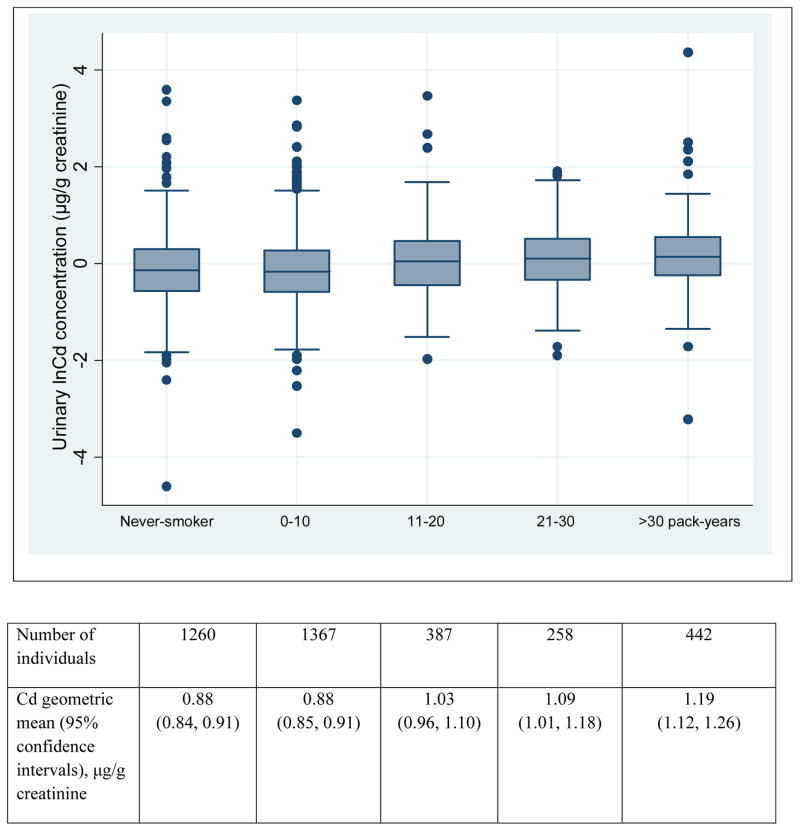
Among 3,714 SHS participants the mean age was 56 years, 41% were men and 67% were ever-smokers (Table 1). Thirty-eight percent of participants had hypertension, and 23% were on an anti-hypertensive medication. Urinary Cd ranged from 0.01 to 78.48 μg/g (geometric mean=0.94 μg/g) with a higher average among ever-smokers and current smokers than never-smokers. Urinary Cd was correlated with smoking pack-year among ever-smokers (r2=0.16, P<0.0001). On average, SHS participants were light smokers (mean 10.8 pack-years), but Cd levels were also elevated among light or never-smokers (Table 1, Figure 1). Ten percent (N=370) participants had an eGFR <60 ml/min/1.72 m2 but the mean urinary Cd concentration was similar among these individuals and those with an eGFR≥60 ml/min/1.72 m2.
Table 1.
Baseline characteristics of Strong Heart Study participants
| Characteristics | Strong Heart Study (n=3,714) |
|---|---|
| Mean age, years | 56.2 (8.0) |
| Men, % | 40.6 |
| Education < 12 years | 47.4 |
| Mean systolic blood pressure, mm Hg | 127.2 (19.3) |
| Mean diastolic blood pressure, mm Hg | 76.8 (10.2) |
| Mean body mass index, kg/m2 | 30.9 (6.3) |
| Hypertension, % | 38.4 |
| Hypertension treatment, % | 23.1 |
| Ever smoker, % | 67.0 |
| Mean smoking, pack-years | 10.8 (18.2) |
| Mean eGFR, ml/min/1.73m2 | 82.5 (21.7) |
| Urinary Cd overall, μg/g creatinine* | 0.94 (0.92, 0.96) |
| Urinary Cd ever-smokers, μg/g creatinine* | 0.97 (0.95, 0.998) |
| Urinary Cd current-smokers, μg/g creatinine* | 1.14 (1.010, 1.18) |
| Urinary Cd never-smokers, μg/g creatinine* | 0.88 (0.84, 0.91) |
Numbers are mean (standard deviation) unless stated. eGFR, estimated glomerular filtration rate.
geometric mean and 95% confidence intervals
Figure 1.
Urinary Cd concentrations by smoking heaviness (pack-years) in the SHS.
In cross-sectional analysis, urinary lnCd was significantly associated with higher systolic blood pressure in models adjusted for age, sex, geographic area, BMI and smoking (ever vs. never, and cumulative pack-years) (β representing the mean blood pressure difference by unit of lnCd concentration=1.11, standard error [SE]=0.54, P=0.037, n=3,714) (Table 2, Model 2). These estimates were stronger when further adjusting for kidney function (β=1.64, SE=0.54, P=0.002, Table 2, Model 3) or when excluding individuals taking antihypertensive medications (β=1.57, SE=0.54, P=0.004) (Table 2). The interaction by sex was not significant (P=0.82). The association of urinary lnCd with blood pressure among current smokers was not significant in fully adjusted models (Table 2).
Table 2.
Association of urinary Cd with systolic and diastolic blood pressure in the Strong Heart Study
| Trait/Models | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
|
|
||||||
| Mean blood pressure difference by lnCd levels (SE) | P | Mean blood pressure difference by lnCd levels (SE) | P | Mean blood pressure difference by lnCd levels (SE) | P | |
| Overall sample (n=3714) | ||||||
| Systolic blood pressure | −0.01 (0.52) | 0.98 | 1.11 (0.54) | 0.037 | 1.64 (0.54) | 0.002 |
| Diastolic blood pressure | −0.62 (0.27) | 0.02 | 0.05 (0.28) | 0.85 | 0.30 (0.28) | 0.29 |
| Current smokers (n=1,272)* | ||||||
| Systolic blood pressure | −0.77 (0.93) | 0.41 | 0.84 (0.96) | 0.38 | 1.38 (0.97) | 0.16 |
| Diastolic blood pressure | −1.13 (0.51) | 0.03 | −0.16 (0.53) | 0.77 | 0.16 (0.53) | 0.77 |
| Light- and never-smokers (n=2,627) | ||||||
| Systolic blood pressure | 1.01 (0.63) | 0.11 | 1.57 (0.64) | 0.01 | 2.03 (0.64) | 0.002 |
| Diastolic blood pressure | 0.37 (0.32) | 0.25 | 0.75 (0.33) | 0.02 | 0.94 (0.33) | 0.004 |
| Never smokers (n=1260)** | ||||||
| Systolic blood pressure | 0.57 (0.91) | 0.63 | 0.85 (0.91) | 0.93 | 1.22 (0.91) | 0.18 |
| Diastolic blood pressure | 0.32 (0.46) | 0.70 | 0.51 (0.46) | 0.27 | 0.66 (0.46) | 0.15 |
| Among individuals not taking blood pressure medications (n=2,856) | ||||||
| Systolic blood pressure | 0.35 (0.52) | 0.50 | 1.41 (0.53) | 0.008 | 1.57 (0.54) | 0.004 |
| Diastolic blood pressure | −0.55 (0.28) | 0.05 | 0.05 (0.29) | 0.86 | 0.16 (0.29) | 0.58 |
Model 1, adjusted for age, sex, geographic area; Model 2, additionally adjusted for BMI and smoking (ever vs. never, and cumulative pack-years); Model 3, Model 2 with additional adjustments for eGFR;
same models except for not adjusting for ever smoking;
same models except for no adjustments for ever smoking or pack-years.
Among never-smokers, the adjusted estimates of effect for lnCd levels on blood pressure were consistent with that observed among smokers, although not significant (β=1.22, P=0.18, Table 2, Model 3). Because never smokers and light smokers (≤10 pack-years) had similar low urinary Cd concentrations (Figure 1), we also performed a sensitivity analysis combining never-smokers and light smokers, for which lnCd association with systolic blood pressure showed even stronger estimates (β=2.03, P=0.002, Table 2, Model 3). These findings suggest that a relationship exists between Cd and blood pressure even when there is minimal confounding by smoking.
The association of lnCd with diastolic blood pressure was concordant in direction as compared to systolic blood pressure, although it was not statistically significant (Table 2, Models 2 and 3). However, there was a significant association of urinary Cd concentration with diastolic blood pressure among never-smokers and light-smokers (P=0.004, Table 2, Model 3).
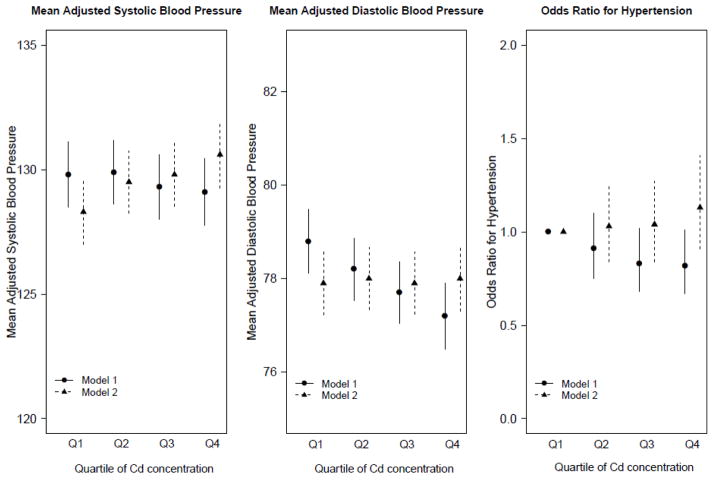
In adjusted models, systolic blood pressure increased across quartiles of Cd concentrations (P=0.029), while diastolic blood pressure was unchanged (Figure 2, supplementary Table 1). In secondary analysis, lnCd was not significantly associated with hypertension (Table 3) but there was a trend for increased odds of hypertension in the upper quartile of Cd distribution compared to the lower quartile (Figure 2, Supplementary Table 2).
Figure 2.
Mean systolic and diastolic blood pressure values and oods ratios of hypertension by quartile of Cd concentrations. The corresponding values of Cd for each quartiles are: Q1: < 62 μg/g; Q2: 0.62 to 0.93 μg/g; Q3: 0.94 to 1.45 μg/g; Q4: > 1.45 μg/g.
Table 3.
Association of urinary Cd with hypertension
| Trait/Models | Model 1 | Model 2 | |
|---|---|---|---|
|
| |||
| n cases/total | Odds ratio (95% CI) | Odds ratio (95% CI) | |
| Hypertension | 1,429/3,714 | 0.91 (0.82, 1.02) | 1.08 (0.96, 1.22) |
Model 1, minimally adjusted model for age, sex and geographic region; Model 2, adjusted for age, sex, geographic area, BMI, smoking (ever vs. never, and cumulative pack-years) and eGFR. Urinary cadmium/creatinine was log-transformed for analyses, see text.
DISCUSSION
Adolescent and young adult SHS American Indians have a high prevalence of hypertension (15%) and pre-hypertension (35%), which has been attributed to obesity and metabolic conditions38. This population has above national average Cd body burden and is at risk for Cd-related health conditions. The main contribution of our study is the association findings between urinary Cd concentrations and elevated systolic blood pressure among middle-aged American Indians. These associations were present when adjusting for smoking exposure, and among individuals who were light- or never-smokers for increased systolic and diastolic blood pressures. Importantly, as tobacco smoke is a source for Cd, we did observe a correlation between urinary Cd and smoking pack-year among ever-smokers (r2=0.16, P<0.0001). A prior study has also shown a stronger association with blood pressure among never-smokers compared to ever-smokers13, suggesting that the effect of Cd on blood pressure is unrelated to other smoking toxicants.
The potential toxic role of Cd as a risk factor for hypertension has not been highlighted in clinical guidelines for blood pressure36, 39. Prior population studies have shown inconsistent associations between Cd and blood pressure for low-to-moderate Cd exposure2, 4, 12–19. Some of these inconsistencies can be explained by differences in populations, study design, measurements of Cd (blood vs. urine) and potential sources of Cd. In NHANES III, the urinary Cd geometric mean was 0.36 μg/g for individuals 35 years or older27 compared to 0.88 μg/g among never-smokers American Indians in our study. In addition, most smokers in our study were light-smokers (mean 8.8 to 10.8 pack-years). These findings suggest other sources of Cd exposure in this population (e.g. food or water) and a potential role for Cd source on these outcomes, for example, by exposing to other toxins or risk factors that augment Cd toxicity. The sources of Cd and these additional risk factors will need to be further evaluated in this population.
Urinary Cd concentrations have decreased between 1988 and 2008 in the U.S., in parallel with declining smoking rates and changes in exposure to tobacco smoke27. However, it is unknown if Cd exposure is decreasing in American Indians. Urine levels of Cd correlate with increased renal cortex Cd concentration, a major site of Cd accumulation in the body40, and thus reflect total body burden. Cd has been associated with kidney dysfunction2 but we did not find differences in urinary Cd concentrations among individuals with and without CKD in our study. However, adjusting for kidney function strengthened the associations between Cd and systolic blood pressure (P=0.002) highlighting the importance of adjusting for physiologic functionality.
Our study is limited to cross-sectional Cd-blood pressure associations, although urinary Cd is considered a biomarker of lifetime body burden as compared to blood Cd concentrations28. We used a constant to account for blood pressure lowering medications, but our sensitivity analysis excluding treated individuals did not show substantial changes of findings. Cd was associated with small increases in blood pressure and not with hypertension in our study. However, at population level, these small increases in blood pressure have shown to have a large impact on CVD events1 and our ultimate goal is to focus on population prevention measures. We are currently examining these associations using prospective data. Future studies should focus on the interplay of environmental and genetic factors in the Cd-blood pressure associations. For example, genome wide association studies have identified associations of the SLC39A8 gene with hypertension34. This gene encodes a zinc transporter and main carrier of Cd into cells in humans. A recent study has shown the contribution of genetic variability to arsenic-associated longitudinal increases in blood pressure41. These and our findings suggest a potential causal role of Cd and other metals in the occurrence of hypertension.
In summary, we identified significant associations between urinary Cd and increased blood pressure in American Indians, independent of smoking exposure. These findings suggest a role for low to moderate Cd burden in increased blood pressure. If confirmed in other studies, our findings may have implications for public health promotion and policies in relation to exposure to Cd and potentially to other toxic metals.
Supplementary Material
| What is known about topic | Cd is an environmental pollutant implicated in reproductive, cancer and cardiovascular disease health outcomes, but the association with hypertension has been inconsistent. The main source of Cd exposure is through smoking, which is preventable. |
| What this study adds | This study identified a relationship between long-term Cd body burden, as measured in urine, with increased blood pressure in American Indians, a population with above national average Cd burden and increased cardiovascular disease risk. The associations were independent of smoking, suggesting other sources of Cd exposure. These findings have implications for public health promotion and policies in relation to exposure to Cd and potentially to other toxic metals. |
Acknowledgments
This research is supported by the NHLBI HL123677-02 to NF and the NIEHS training grant (ES007141-32) to PB. MTP was supported by the Strategic Action for Research in Health sciences [CP12/03080], which is an initiatives from Carlos III Health Institute Madrid and the Spanish Ministry of Economy and Competitiveness and are co-funded with European Funds for Regional Development (FEDER).
Footnotes
CONFLICT OF INTEREST/DISCLOSURES. none
References
- 1.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–13. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 2.Whittemore AS, DiCiccio Y, Provenzano G. Urinary cadmium and blood pressure: results from the NHANES II survey. Environ Health Perspect. 1991;91:133–40. doi: 10.1289/ehp.9191133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang J, Liu M, Parvez F, Wang B, Wu F, Eunus M, et al. Association between Arsenic Exposure from Drinking Water and Longitudinal Change in Blood Pressure among HEALS Cohort Participants. Environ Health Perspect. 2015;123(8):806–12. doi: 10.1289/ehp.1409004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Telisman S, Jurasovic J, Pizent A, Cvitkovic P. Blood pressure in relation to biomarkers of lead, cadmium, copper, zinc, and selenium in men without occupational exposure to metals. Environ Res. 2001;87(2):57–68. doi: 10.1006/enrs.2001.4292. [DOI] [PubMed] [Google Scholar]
- 5.Wolf MB, Baynes JW. Cadmium and mercury cause an oxidative stress-induced endothelial dysfunction. Biometals. 2007;20(1):73–81. doi: 10.1007/s10534-006-9016-0. [DOI] [PubMed] [Google Scholar]
- 6.Messner B, Knoflach M, Seubert A, Ritsch A, Pfaller K, Henderson B, et al. Cadmium is a novel and independent risk factor for early atherosclerosis mechanisms and in vivo relevance. Arterioscler Thromb Vasc Biol. 2009;29(9):1392–8. doi: 10.1161/ATVBAHA.109.190082. [DOI] [PubMed] [Google Scholar]
- 7.Varoni MV, Palomba D, Gianorso S, Anania V. Cadmium as an environmental factor of hypertension in animals: new perspectives on mechanisms. Vet Res Commun. 2003;27(Suppl 1):807–10. doi: 10.1023/b:verc.0000014277.06785.6f. [DOI] [PubMed] [Google Scholar]
- 8.Schroeder HA, Vinton WH., Jr Hypertension induced in rats by small doses of cadmium. Am J Physiol. 1962;202:515–8. doi: 10.1152/ajplegacy.1962.202.3.515. [DOI] [PubMed] [Google Scholar]
- 9.Solenkova NV, Newman JD, Berger JS, Thurston G, Hochman JS, Lamas GA. Metal pollutants and cardiovascular disease: mechanisms and consequences of exposure. Am Heart J. 2014;168(6):812–22. doi: 10.1016/j.ahj.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tellez-Plaza M, Guallar E, Howard BV, Umans JG, Francesconi KA, Goessler W, et al. Cadmium exposure and incident cardiovascular disease. Epidemiology. 2013;24(3):421–9. doi: 10.1097/EDE.0b013e31828b0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Myong JP, Kim HR, Jang TW, Lee HE, Koo JW. Association between blood cadmium levels and 10-year coronary heart disease risk in the general Korean population: the Korean National Health and Nutrition Examination Survey 2008–2010. PloS one. 2014;9(11):e111909. doi: 10.1371/journal.pone.0111909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Staessen JA, Kuznetsova T, Roels HA, Emelianov D, Fagard R. Exposure to cadmium and conventional and ambulatory blood pressures in a prospective population study. Public Health and Environmental Exposure to Cadmium Study Group. Am J Hypertens. 2000;13(2):146–56. doi: 10.1016/s0895-7061(99)00187-9. [DOI] [PubMed] [Google Scholar]
- 13.Tellez-Plaza M, Navas-Acien A, Crainiceanu CM, Guallar E. Cadmium exposure and hypertension in the 1999–2004 National Health and Nutrition Examination Survey (NHANES) Environ Health Perspect. 2008;116(1):51–6. doi: 10.1289/ehp.10764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eum KD, Lee MS, Paek D. Cadmium in blood and hypertension. Sci Total Environ. 2008;407(1):147–53. doi: 10.1016/j.scitotenv.2008.08.037. [DOI] [PubMed] [Google Scholar]
- 15.Vivoli G, Bergomi M, Borella P, Fantuzzi G, Caselgrandi E. Cadmium in blood, urine and hair related to human hypertension. J Trace Elem Electrolytes Health Dis. 1989;3(3):139–45. [PubMed] [Google Scholar]
- 16.Kelishadi R, Askarieh A, Motlagh ME, Tajadini M, Heshmat R, Ardalan G, et al. Association of Blood Cadmium Level with Cardiometabolic Risk Factors and Liver Enzymes in a Nationally Representative Sample of Adolescents: The CASPIAN-III Study. J Environ Public Health. 2013;2013:142856. doi: 10.1155/2013/142856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caciari T, Sancini A, Tomei F, Antetomaso L, Tomei G, Scala B, et al. Cadmium blood/urine levels and blood pressure in workers occupationally exposed to urban stressor. Ann Ig. 2012;24(5):417–28. [PubMed] [Google Scholar]
- 18.Lee BK, Kim Y. Association of blood cadmium with hypertension in the Korean general population: analysis of the 2008–2010 Korean National Health and Nutrition Examination Survey data. Am J Ind Med. 2012;55(11):1060–7. doi: 10.1002/ajim.22078. [DOI] [PubMed] [Google Scholar]
- 19.Gallagher CM, Meliker JR. Blood and urine cadmium, blood pressure, and hypertension: a systematic review and meta-analysis. Environ Health Perspect. 2010;118(12):1676–84. doi: 10.1289/ehp.1002077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Primatesta P, Falaschetti E, Gupta S, Marmot MG, Poulter NR. Association between smoking and blood pressure: evidence from the health survey for England. Hypertension. 2001;37(2):187–93. doi: 10.1161/01.hyp.37.2.187. [DOI] [PubMed] [Google Scholar]
- 21.Kim SJ, Lee J, Jee SH, Nam CM, Chun K, Park IS, et al. Cardiovascular risk factors for incident hypertension in the prehypertensive population. Epidemiol Health. 2010;32:e2010003. doi: 10.4178/epih/e2010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niskanen L, Laaksonen DE, Nyyssonen K, Punnonen K, Valkonen VP, Fuentes R, et al. Inflammation, abdominal obesity, and smoking as predictors of hypertension. Hypertension. 2004;44(6):859–65. doi: 10.1161/01.HYP.0000146691.51307.84. [DOI] [PubMed] [Google Scholar]
- 23.Halperin RO, Gaziano JM, Sesso HD. Smoking and the risk of incident hypertension in middle-aged and older men. Am J Hypertens. 2008;21(2):148–52. doi: 10.1038/ajh.2007.36. [DOI] [PubMed] [Google Scholar]
- 24.Bowman TS, Gaziano JM, Buring JE, Sesso HD. A prospective study of cigarette smoking and risk of incident hypertension in women. J Am Coll Cardiol. 2007;50(21):2085–92. doi: 10.1016/j.jacc.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 25.Welty TK, Lee ET, Yeh J, Cowan LD, Go O, Fabsitz RR, et al. Cardiovascular disease risk factors among American Indians. The Strong Heart Study. Am J Epidemiol. 1995;142(3):269–87. doi: 10.1093/oxfordjournals.aje.a117633. [DOI] [PubMed] [Google Scholar]
- 26.Lee ET, Cowan LD, Welty TK, Sievers M, Howard WJ, Oopik A, et al. All-cause mortality and cardiovascular disease mortality in three American Indian populations, aged 45–74 years, 1984–1988. The Strong Heart Study. Am J Epidemiol. 1998;147(11):995–1008. doi: 10.1093/oxfordjournals.aje.a009406. [DOI] [PubMed] [Google Scholar]
- 27.Tellez-Plaza M, Navas-Acien A, Caldwell KL, Menke A, Muntner P, Guallar E. Reduction in cadmium exposure in the United States population, 1988–2008: the contribution of declining smoking rates. Environ Health Perspect. 2012;120(2):204–9. doi: 10.1289/ehp.1104020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jarup L, Akesson A. Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol. 2009;238(3):201–8. doi: 10.1016/j.taap.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 29.Lee ET, Welty TK, Fabsitz R, Cowan LD, Le NA, Oopik AJ, et al. The Strong Heart Study. A study of cardiovascular disease in American Indians: design and methods. Am J Epidemiol. 1990;132(6):1141–55. doi: 10.1093/oxfordjournals.aje.a115757. [DOI] [PubMed] [Google Scholar]
- 30.Hayslett JA, Eichner JE, Yeh JL, Wang W, Henderson J, Devereux RB, et al. Hypertension treatment patterns in American Indians: the strong heart study. Am J Hypertens. 2001;14(9 Pt 1):950–6. doi: 10.1016/s0895-7061(01)02146-x. [DOI] [PubMed] [Google Scholar]
- 31.North KE, Williams JT, Welty TK, Best LG, Lee ET, Fabsitz RR, et al. Evidence for joint action of genes on diabetes status and CVD risk factors in American Indians: the strong heart family study. Int J Obes Relat Metab Disord. 2003;27(4):491–7. doi: 10.1038/sj.ijo.0802261. [DOI] [PubMed] [Google Scholar]
- 32.Scheer J, Findenig S, Goessler W, Francesconi KA, Howard B, Umans JG, et al. Arsenic species and selected metals in human urine: validation of HPLC/ICPMS and ICPMS procedures for a long-term population-based epidemiological study. Anal Methods. 2012;4(2):406–413. doi: 10.1039/C2AY05638K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tobin MD, Sheehan NA, Scurrah KJ, Burton PR. Adjusting for treatment effects in studies of quantitative traits: antihypertensive therapy and systolic blood pressure. Stat Med. 2005;24(19):2911–35. doi: 10.1002/sim.2165. [DOI] [PubMed] [Google Scholar]
- 34.Ehret GB, Munroe PB, Rice KM, Bochud M, Johnson AD, Chasman DI, et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011;478(7367):103–9. doi: 10.1038/nature10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levy D, Ehret GB, Rice K, Verwoert GC, Launer LJ, Dehghan A, et al. Genome-wide association study of blood pressure and hypertension. Nat Genet. 2009;41(6):677–87. doi: 10.1038/ng.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA : the journal of the American Medical Association. 2003;289(19):2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 37.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drukteinis JS, Roman MJ, Fabsitz RR, Lee ET, Best LG, Russell M, et al. Cardiac and systemic hemodynamic characteristics of hypertension and prehypertension in adolescents and young adults: the Strong Heart Study. Circulation. 2007;115(2):221–7. doi: 10.1161/CIRCULATIONAHA.106.668921. [DOI] [PubMed] [Google Scholar]
- 39.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) Jama. 2014;311(5):507–20. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 40.Prozialeck WC, Edwards JR. Mechanisms of cadmium-induced proximal tubule injury: new insights with implications for biomonitoring and therapeutic interventions. J Pharmacol Exp Ther. 2012;343(1):2–12. doi: 10.1124/jpet.110.166769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farzan SF, Karagas MR, Jiang J, Wu F, Liu M, Newman JD, et al. Gene-arsenic interaction in longitudinal changes of blood pressure: Findings from the Health Effects of Arsenic Longitudinal Study (HEALS) in Bangladesh. Toxicol Appl Pharmacol. 2015;288(1):95–105. doi: 10.1016/j.taap.2015.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.