Abstract
Formyl peptide receptors (FPRs) are G-protein-coupled receptors that play an important role in the regulation of inflammatory process and cellular dysfunction. In humans, three different isoforms are expressed (FPR1, FPR2 and FPR3). FPR2 appears to be directly involved in the resolution of inflammation (ROI), an active process carried out by specific pro-resolving mediators that modulate specific receptors. Previously, we identified 2-arylacetamido pyridazin-3(2H)-ones as FPR1- or FPR2-selective agonists, as well as a large number of mixed-agonists for the three isoforms. Here, we report a new series of 2-arylacetamido pyridazinones substituted at position 5 and their development as FPR agonists. We also synthesized a new series of 2-oxothiazolones bearing a 4-bromophenylacetamido fragment, which was fundamental for activity in the pyridazinone series. The compounds of most interest were 4a, a potent, mixed FPR agonist recognized by all three isotypes (FPR1 EC50 = 19 nM, FPR2 EC50 = 43 nM, FPR3 EC50 = 40 nM), and 4b, which had potent activity and a preference for FPR2 (EC50 = 13 nM). These novel compounds may represent valuable tools for studying FPR activation and signaling.
Keywords: formyl peptide receptor (FPR), agonist, pyridazin-3(2H)-one, neutrophil, Ca2+ flux
INTRODUCTION
Inflammation is a primary response of the immune system to injury or infection by invading microbial pathogens and is mediated by the action of many cell types with distinct functions [Beutler, 2004]. The critical role of the inflammatory process in health and disease is essential; however, the mechanisms leading to the restoration of homeostasis and resolution of inflammation have only recently been elucidated. These studies have demonstrated that the resolution of inflammation is an active process carried out by specific pro-resolving mediators, such as lipoxin, resolvins, protectins, and maresins, which have anti-inflammatory and pro-resolving activities that are mediated via interactions with specific receptors [Serhan et al., 2002; Serhan and Levy, 2003; Serhan et al., 2011; Serhan et al., 2015]. Among the receptors activated by pro-resolving mediators is formyl peptide receptor 2 (FPR2), which belongs to a group of G-protein coupled receptors [Corminboeuf and Leroy, 2015]. In addition to FPR2, two other isoforms, FPR1 and FPR3, have been identified in humans and exhibit a high level of amino acid homology with FPR2 [Ye et al, 2009]. Despite their high level of sequence homology, the FPRs differ in their ability to bind the prototypic N-formyl peptide N-formyl-methionine-leucine-phenylalanine (fMLF). FPR1 has high affinity receptor for this ligand, FPR2 low-affinity receptor, while FPR3 does not bind fMLF. FPR1 and FPR2 have a similar distribution in a variety of tissues and cells involved in inflammation, including endothelial cells, platelets and immature dendritic cells, monocytes, neutrophils, macrophages, T lymphocytes, and epithelial cells [Ye et al, 2009; Migeotte et al., 2006], whereas FPR3 is expressed in monocytes and dendritic cells [Migeotte et al., 2005; 2006].
The involvement of FPR2 in the resolution of inflammation makes this receptor an attractive target for treating a variety of pathologies, including rheumatoid arthritis, asthma, chronic obstructive pulmonary disease (COPD), cancer, cardiovascular, and Alzheimer's diseases [Mantovani et al., 2008; Libby, 2002; 2015; Bozinovski et al, 2013]. There are only a few reported examples of FPR2-selective ligands [Schepetkin et al, 2014].
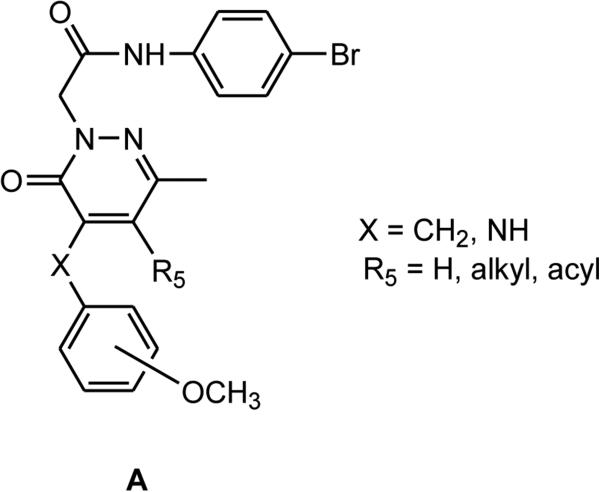
Our research in the field of FPR ligands led to the identification of a large number of pyridazin-3(2H)-one derivatives that were mixed FPR1/FPR2 agonists (Figure 1, general structure A) [Cilibrizzi et al., 2009; 2012; Cilibrizzi et al., 2013; Crocetti et al., 2013; Giovannoni et al., 2013, Vergelli et al. 2016]. Key requirements for activity were the presence of a methyl group at position 6 of the pyridazinone ring, a 4-bromophenylacetamide moiety at N-2, and a benzyl/aniline group at position 4 of the scaffold. In the series of 4-anilino derivatives, we identified some compounds with good potency and with a moderate preference for FPR2 subtype [Vergelli et al., 2016]. In the present paper, we further investigated 4-benzylpyridazinones derivatives as isosteres of the 4-anilino derivatives and examine their structure-activity relationships [Vergelli et al., 2016], as well as several derivatives lacking a benzyl fragment at position 4. We also synthesized and evaluated a series of 2-oxothiazolones by maintaining the 4-bromophenylacetamide side fragment.
Figure 1.
FPR1/FPR2 mixed agonists
MATERIALS and METHODS
Chemistry
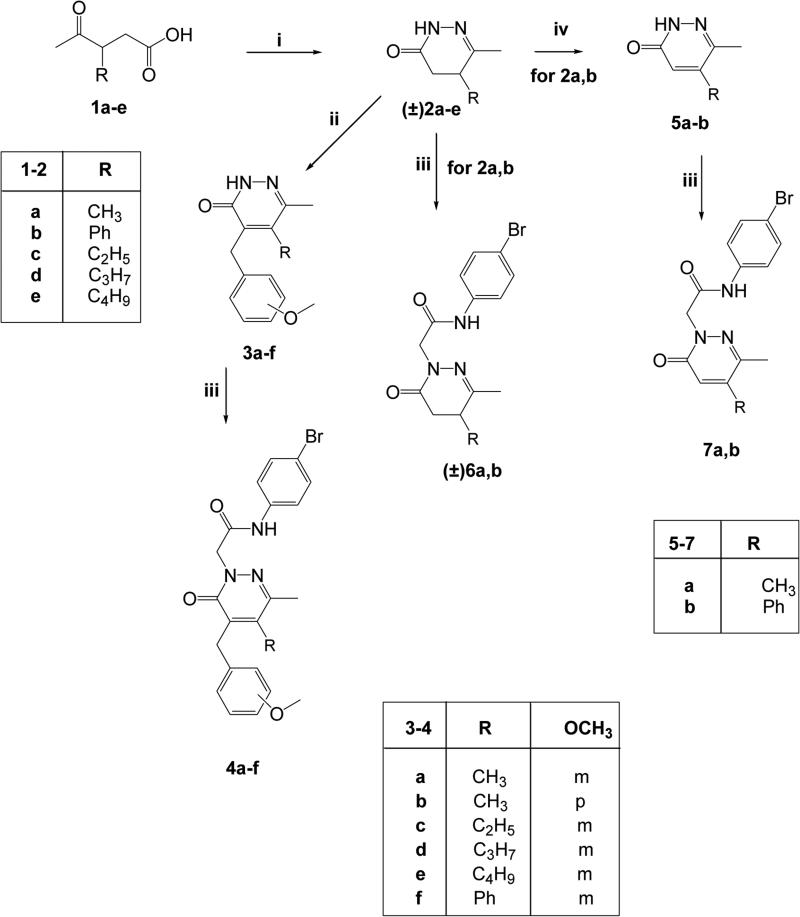
The synthetic pathways followed to obtain the final compounds are shown in Figures 2 and 3, and the structures were confirmed by analytical and spectral data.
Figure 2.
Synthesis of the final compounds 4a-f, (±)6a,b, 7a,b. Reagents and conditions: i) N2H4· H2O, EtOH, reflux, 3h; ii) 3 or 4-methoxybenzaldehyde, KOH 5% (w/v) in anhydrous EtOH, reflux, 7-20 h; iii) N-(4-bromophenyl)-2-chloroacetamide, K2CO3, anhydrous CH3CN, reflux, 5-7 h; iv) Br2/AcOH, reflux. 8h.
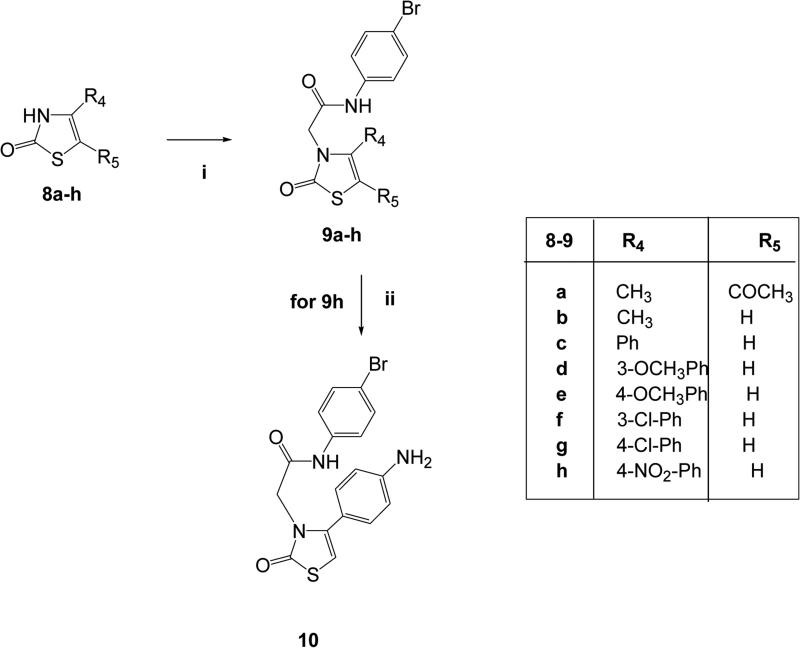
Figure 3.
Synthesis of the final compounds 9a-h and 10. Reagents and conditions: i) N-(4-bromophenyl)-2-chloroacetamide, K2CO3,anhydrou CH3CN, reflux, 5-7h;ii) 10% Pd/C, anhydrous EtOH, H2, Parr, 30 PSI, 2 h.
In Figure 2, synthesis of the 4-benzylpyridazinones 4a-f and the 4-unsubstituted derivatives of type 6 and 7 is presented. Previously described oxoacids 1a-e [Metz et al., 1980; Holloway et al., 2010; Xu et al., 2005] were reacted with hydrazine hydrate to obtain the corresponding 4,5-dihydropyridazinones (±)2a-e (compounds (±)2a,b,d [Haider and Holzer, 2004; Pinna et al., 1988; Xu et al., 2005]), which were converted into derivatives 3a-f by treatment with the appropriate arylaldehyde in the presence of KOH. Alkylation of 3a-f with commercially available N-(4-bromophenyl)-2-chloroacetamide in anhydrous CH3CN resulted in the final compounds 4a-f. Compounds (±)2a,b [Haider and Holzer, 2004; Pinna et al., 1988] were also key intermediates for the synthesis of 4-unsubstituted derivatives 6 and 7 by direct alkylation with N-(4-bromophenyl)-2-chloroacetamide (compounds 6a,b) or by oxidation with Br2 and acetic acid (compounds 5a,b) [Haider and Holzer, 2004; Coates and McKillop, 1993] and further alkylation (7a,b).
In Figure 3, synthesis of the final oxothiazolones 9a-h and 10 is presented. The previously described compounds 8a-h [Wang et al., 2005; Yarligan et al., 2005; Pihlaja et al. 2002; Isomura et al. 1988; Zhao et al., 2013] were alkylated as shown to obtain the desired 9a-h. The final 9h was then subject to catalytic reduction with Pd/C in a Parr instrument to obtain compound 10.
Experimentals
Reagents and starting materials were obtained from commercial sources. Extracts were dried over Na2SO4, and the solvents were removed under reduced pressure. All reactions were monitored by thin layer chromatography (TLC) using commercial plates pre-coated with Merck silica gel 60 F-254, and visualization was performed by UV fluorescence (λmax = 254 nm) or by staining with iodine or potassium permanganate. Chromatographic separations were performed on a silica gel column using gravity chromatography (Kieselgel 40, 0.063-0.200 mm; Merck), flash chromatography (Kieselgel 40, 0.040-0.063 mm; Merck), or silica gel preparative TLC (Kieselgel 60 F254, 20 × 20 cm, 2 mm). Yields refer to chromatographically and spectroscopically pure compounds, unless otherwise stated. Compounds are named following IUPAC rules, as applied by Beilstein-Institut AutoNom 2000 (4.01.305) or CA Index Name. All melting points were determined on a microscope hot stage Büchi apparatus and are uncorrected. The identity and purity of intermediates and final compounds were determined through NMR analysis and TLC chromatography. 1H NMR, 13C NMR, and NOESY spectra were recorded with Avance 400 instruments (Bruker Biospin Version 002 with SGU). Chemical shifts (δ) are reported in ppm to the nearest 0.01 ppm (for 1H NMR) or 0.1 ppm (for 13C NMR) using the solvent as an internal standard. Coupling constants (J values) of 1H NMR are given in Hz and were calculated using ‘TopSpin 1.3’ software and rounded to the nearest 0.1 Hz. Microanalyses were performed with a Perkin-Elmer 260 elemental analyzer for C, H, and N, and the results were within ± 0.4 % of the theoretical values, unless otherwise stated.
General Procedures for (±)2c and (±)2e
To a solution of appropriate acid 1c and 1e (1.52 mmol) [Holloway et al., 2010] in EtOH (5 mL), hydrazine hydrate (3.04 mmol) was added. The mixture was refluxed for 3 h. After cooling the solvent was evaporated under vacuum and cold water (10 mL) was added. The suspension was extracted with CH2Cl2 (3 × 15 mL), the organic phase was dried over Na2SO4 and evaporated to give the final compounds (±)2c and (±)2e.
(±)5-Ethyl-6-methyl-4,5-dihydropyridazin-3(2H)-one, (±)2c
Yield = 26%; oil. 1H-NMR (CDCl3) δ 0.94 (t, 3H, CH3CH2, J = 7.2 Hz); 1.50-1.65 (m, 2H, CH2CO); 2.00 (s, 3H, CH3C=N); 2.30-2.38 (m, 2H, CH3CH2); 2.47-2.53 (m, 1H, CHCH2CH3); 9.17 (exch br s, 1H, NH). Anal. Calcd for C7H12N2O (140.18): C, 59.98; H, 8.63; N, 19.98; Found: C, 59.85; H, 8.65; N, 19.92.
(±)5-Butyl-6-methyl-4,5-dihydropyridazin-3(2H)-one, (±)2e
Yield = 34%; mp = 63-67 °C (Cyclohexane). 1H-NMR (CDCl3) δ 0.91-0.95 (m, 3H, CH3(CH2)3); 1.32-1.37 (m, 2H, CH3CH2(CH2)2); 1.44-1.50 (m, 2H, CH3CH2CH2CH2); 1.87-1.89 (m, 2H, CH3(CH2)2CH2); 2.07 (s, 3H, CH3C=N); 2.37-2.42 (m, 1H, CH(CH2)3CH3); 2.48-2.60 (m, 2H, CH2CO); 8.24 (exch br s, 1H, NH). Anal. Calcd for C9H16N2O (168.24): C, 64.25; H, 9.59; N, 16.65; Found: C, 64.41; H, 9.58; N, 16.59.
General Procedures for 3a-f
To a solution of the suitable intermediate (±)2a-e ((±)2a,b and (±)2d [Haider and Holzer, 2004; Pinna et al., 1988; Xu et al., 2005]) (1.44 mmol) in 5-9 mL of KOH/absolute EtOH (5% w/v), 3- or 4-methoxybenzaldehyde (1.44-2.16 mmol) was added and the mixture was refluxed under stirring for 7-20 h. After cooling, the mixture was concentrated in vacuo, diluted with ice-cold water (5-10 mL), neutralized with 2N HCl and extracted with CH2Cl2 (3 × 20 mL). Removal of the solvent resulted in crude compounds 3a,b,f, which were purified by flash chromatography using cyclohexane/ethyl acetate 1:3 (for 3a,b) and cyclohexane/ethyl acetate 2:1 (for 3f) as eluents. Compounds 3c-e were recovered as crude precipitate after dilution and neutralization. These final compounds were purified by crystallization from EtOH.
4-(3-Methoxybenzyl)-5,6-dimethylpyridazin-3(2H)-one, 3a
Yield = 58%; mp = 185-186 °C (EtOH). 1H-NMR (CDCl3) δ 2.18 (s, 3H, N=CCH3); 2.28 (s, 3H, CH3); 3.79 (s, 3H, OCH3); 4.03 (s, 2H, CH2); 6.76 (d, 1H, Ar, J = 10.8 Hz); 6.81-6.83 (m, 2H, Ar); 7.20 (t, 1H, Ar, J = 8.0 Hz); 9.98 (exch br s, 1H, NH). Anal. Calcd for C14H16N2O2 (244.29): C, 68.83; H, 6.60; N, 11.47; Found: C, 69.01; H, 6.59; N, 11.50.
4-(4-Methoxybenzyl)-5,6-dimethylpyridazin-3(2H)-one, 3b
Yield = 44%; mp = 150-151 °C (EtOH). 1H-NMR (CDCl3) δ 2.19 (s, 3H, N=CCH3); 2.28 (s, 3H, CH3); 3.79 (s, 3H, OCH3); 3.98 (s, 2H, CH2); 6.82 (d, 2H, Ar, J = 8.4 Hz); 7.21 (d, 2H, Ar, J = 8.4 Hz); 10.33 (exch br s, 1H, NH). Anal. Calcd for C14H16N2O2 (244.29): C, 68.83; H, 6.60; N, 11.47; Found: C, 68.99; H, 6.59; N, 11.49.
5-Ethyl-4-(3-methoxybenzyl)-6-methylpyridazin-3(2H)-one, 3c
Yield = 44%; mp = 168-170 °C (EtOH). 1H-NMR (CDCl3) δ 1.08 (t, 3H, CH3CH2, J = 7.6 Hz); 2.37 (s, 3H, N=CCH3); 2.62 (q, 2H, CH3CH2, J = 7.6 Hz); 3.79 (s, 3H, OCH3); 4.03 (s, 2H, CH2Ph); 6.76 (m, 1H, Ar); 6.82 (m, 2H, Ar); 7.20 (m, 1H, Ar). Anal. Calcd for C15H18N2O2 (258.32): C, 69.74; H, 7.02; N, 10.84; Found: C, 69.91; H, 7.01; N, 10.87.
4-(3-Methoxybenzyl)-6-methyl-5-propylpyridazin-3(2H)-one, 3d
Yield = 23%; mp = 134-137 °C (EtOH). 1H-NMR (CDCl3) δ 1.00 (t, 3H, CH3(CH2)2, J = 7.6 Hz); 1.38-1.44 (m, 2H, CH3CH2CH2); 2.33 (s, 3H, N=CCH3); 2.49-2.54 (m, 2H, CH3CH2CH2); 3.79 (s, 3H, OCH3); 4.03 (s, 2H, CH2Ph); 6.75 (m, 1H, Ar); 6.82-6.85 (m, 2H, Ar); 7.19 (t, 1H, Ar, J = 7.2 Hz). Anal. Calcd for C16H20N2O2 (272.34): C, 70.56; H, 7.40; N, 10.29; Found: C, 70.39; H, 7.41; N, 10.23.
5-Butyl-4-(3-methoxybenzyl)-6-methylpyridazin-3(2H)-one, 3e
Yield = 22%; mp = 144-146 °C (EtOH/H2O 1:1). 1H-NMR (CDCl3) δ 0.95 (t, 3H, CH3(CH2)3, J = 7.2 Hz); 1.32-1.46 (m, 4H, CH3CH2CH2CH2); 2.35 (s, 3H, N=CCH3); 2.52-2.57 (m, 2H, CH3CH2CH2CH2); 3.79 (s, 3H, OCH3); 4.02 (s, 2H, CH2Ph); 6.76 (m, 1H, Ar); 6.82 (m, 2H, Ar); 7.19 (t, 1H, Ar, J = 7.6 Hz). Anal. Calcd for C17H22N2O2 (286.37): C, 71.30; H, 7.74; N, 9.78; Found: C, 71.49; H, 7.75; N, 9.81.
4-(3-Methoxybenzyl)-6-methyl-5-phenylpyridazin-3(2H)-one, 3f
Yield = 32%; oil. 1H-NMR (CDCl3) δ 2.14 (s, 3H, N=CCH3); 3.73 (s, 3H, OCH3); 3.80 (s, 2H, CH2); 6.42 (s, 1H, Ar); 6.53 (d, 1H, Ar, J = 10.8 Hz); 6.71 (dd, 1H, Ar, J = 2.4 Hz, J = 5.6 Hz); 7.02-7.17 (m, 3H, Ar); 7.41-7.51 (m, 3H, Ar). Anal. Calcd for C19H18N2O2 (306.36): C, 74.49; H, 5.92; N, 9.14; Found: C,74.68; H, 5.91; N, 9.16.
General Procedures for 4a-f
To a mixture of the appropriate intermediate 3a-f (0.40 mmol) and K2CO3 (0.80-1.20 mmol) in CH3CN (5-10 mL), N-(4-bromophenyl)-2-chloroacetamide (0.40-0.72 mmol) was added and the suspension was refluxed under stirring for 5-7 h. After cooling, the mixture was concentrated in vacuo, ice cold water was (5-10 mL) added and then further stirred for 1 h. The precipitate was recovered by suction and purified by flash chromatography using cyclohexane/ethyl acetate 1:1 (compounds 4a,b,e), cyclohexane/ethyl acetate 1:2 (compounds 4c,d) and cyclohexane/ethyl acetate 2:1 for 4c as eluents.
N-(4-Bromophenyl)-2-[5-(3-methoxybenzyl)-3,4-dimethyl-6-oxopyridazin-1(6H)-yl]acetamide, 4a
Yield = 48%; mp = 123-124 °C (EtOH). 1H-NMR (CDCl3) δ 2.23 (s, 3H, N=CCH3); 2.34 (s, 3H, C=CCH3); 3.76 (s, 3H, OCH3); 4.06 (s, 2H, CH2Ar); 4.94 (s, 2H, NCH2); 6.76 (dd, 1H, Ar, J = 6.4 Hz, J = 1.6 Hz); 6.79-6.82 (m, 2H, Ar); 7.19 (t, 1H, Ar, J = 8.0 Hz); 7.30-7.35 (m, 4H, Ar); 9.31 (exch br s, 1H, NH). 13C-NMR (CDCl3) δ 16.0 (CH3); 20.4 (CH3); 32.1 (CH2); 55.1 (CH3); 57.9 (CH2); 111.3 (CH); 114.7 (CH); 116.3 (C); 120.7 (CH); 121.1 (2 CH); 129.5 (CH); 131.5 (2CH); 137.1 (C); 137.9 (C); 139.4 (C); 140.7 (C); 146.6 (C); 159.8 (C); 161.2 (C); 165.6 (C). Anal. Calcd for C22H22N3O3 (456.33): C, 57.90; H, 4.86; N, 9.21; Found: C, 57.74; H, 4.85; N, 9.24.
N-(4-Bromophenyl)-2-[5-(4-methoxybenzyl)-3,4-dimethyl-6-oxopyridazin-1(6H)-yl]acetamide, 4b
Yield = 58%; mp = 177-178 °C (EtOH). 1H-NMR (CDCl3) δ 2.24 (s, 3H, N=CCH3); 2.33 (s, 3H, C=CCH3); 3.78 (s, 3H, OCH3); 4.02 (s, 2H, CH2Ar); 4.94 (s, 2H, NCH2); 6.80 (d, 2H, Ar, J = 8.4 Hz); 7.16 (d, 2H, Ar, J = 8.4 Hz); 7.30-7.38 (m, 4H, Ar); 9.26 (exch br s, 1H, NH). 13C-NMR (CDCl3) δ 16.0 (CH3); 20.3 (CH3); 31.3 (CH2); 55.2 (CH3); 58.1 (CH2); 114.1 (2 CH); 116.4 (C); 121.2 (2CH); 129.4 (2 CH); 129.6 (C); 131.5 (2 CH); 137.0 (C); 138.5 (C); 140.3 (C); 146.8 (C); 158.3 (C); 161.2 (C); 165.6 (C). Anal. Calcd for C22H22N3O3 (456.33): C, 57.90; H, 4.86; N, 9.21; Found: C, 57.69; H, 4.85; N, 9.18.
N-(4-Bromophenyl)-2-[4-ethyl-5-(3-methoxybenzyl)-3-methyl-6-oxopyridazin-1(6H)-yl]acetamide, 4c
Yield = 39%; mp = 86-88 °C (EtOH). 1H-NMR (CDCl3) δ 1.10 (t, 3H, CH3CH2, J = 7.6 Hz); 2.39 (s, 3H, N=CCH3); 2.63 (q, 2H, CH3CH2, J = 7.6 Hz); 3.75 (s, 3H, OCH3); 4.05 (s, 2H, CH2Ar); 4.93 (s, 2H, CH2CO); 6.77 (m, 3H, Ar); 7.18 (t, 1H, Ar, J = 8.8 Hz); 7.33-7.38 (m, 4H, Ar); 9.31 (exch br s, 1H, NH). Anal. Calcd for C23H24BrN3O3 (470.36): C, 58.73; H, 5.14; N, 8.93; Found: C, 58.89; H, 5.13; N, 8.90.
N-(4-Bromophenyl)-2-[5-(3-methoxybenzyl)-3-methyl-6-oxo-4-propylpyridazin-1(6H)-yl]acetamide, 4d
Yield = 30%; mp = 81-83 °C (EtOH). 1H-NMR (CDCl3) δ 1.04 (t, 3H, CH3(CH2)2, J = 7.2 Hz); 1.42-1.49 (m, 2H, CH3CH2CH2); 2.38 (s, 3H, N=CCH3); 2.54-2.58 (m, 2H, CH3CH2CH2); 3.74 (s, 3H, OCH3); 4.03 (s, 2H, CH2Ar); 4.88 (s, 2H, CH2CO); 6.74-6.78 (m, 3H, Ar); 7.17 (t, 1H, Ar, J = 7.6 Hz); 7.25 (m, 4H, Ar); 9.50 (exch br s, 1H, NH). Anal. Calcd for C24H26BrN3O3 (484.39): C, 59.51; H, 5.41; N, 8.67; Found: C, 59.67; H, 5.40; N, 8.64.
N-(4-Bromophenyl)-2-[4-butyl-5-(3-methoxybenzyl)-3-methyl-6-oxopyridazin-1(6H)-yl]acetamide, 4e
Yield = 57%; mp = 75-77 °C (Cyclohexane). 1H-NMR (CDCl3) δ 0.93 (t, 3H, CH3(CH2)3, J = 7.2 Hz); 1.28-1.45 (m, 4H, CH3CH2CH2CH2); 2.38 (s, 3H, N=CCH3); 2.54-2.58 (m, 2H, CH3CH2CH2CH2); 3.73 (s, 3H, OCH3); 3.99 (s, 2H, CH2Ar); 4.88 (s, 2H, CH2CO); 6.75-6.78 (m, 3H, Ar); 7.17 (t, 1H, Ar, J = 7.6 Hz). 7.25-7.27 (m, 4H, Ar); 9.53 (exch br s, 1H, NH). Anal. Calcd for C25H28BrN3O3 (498.41): C, 60.24; H, 5.66; N, 8.43; Found: C, 60.08; H, 5.66; N, 8.46.
N-(4-Bromophenyl)-2-[5-(3-methoxybenzyl)-3-methyl-6-oxo-4-phenylpyridazin-1(6H)-yl]acetamide, 4f
Yield = 24%; oil. 1H-NMR (CDCl3) δ 2.09 (s, 3H, CCH3,); 3.65 (s, 3H, OCH3); 3.87 (s, 2H, CH2Ar); 5.00 (s, 2H, NCH2); 6.38 (s, 1H, Ar); 6.64 (d, 1H, Ar, J = 8.8 Hz); 7.02-7.06 (m, 2H, Ar); 7.19 (d, 1H, Ar, J= 7.6 Hz); 7.42 (m, 8H, Ar); 9.22 (exch br s, 1H, NH). 13C-NMR (CDCl3) δ 21.1 (CH3); 31.1 (CH2); 55.2 (CH3); 58.3 (CH2); 111.4 (CH); 112.1 (CH); 114.7 (CH); 115.3 (CH); 116.4 (C); 120.9 (CH); 121.1 (CH); 125.4 (C); 127.5 (CH); 128.0 (CH); 128.8 (CH); 129.0 (CH); 129.3 (CH); 129.8 (CH); 131.5 (CH); 134.7 (C); 137.0 (C); 138.5 (C); 139.7 (C); 145.2 (C); 145.8 (C); 161.2 (C); 165.5 (C). Anal. Calcd for C27H24N3O3 (518.40): C, 62.56; H, 4.67; N, 8.11; Found: C, 62.74; H, 4.68; N, 8.13.
General Procedures for (±)6a,b and 7a,b
Compounds (±)6a,b and 7a,b were obtained starting from appropriate substrates (±)2a,b [Haider and Holzer, 2004; Pinna et al., 1988] and 5a,b [Haider and Holzer, 2004; Coates and McKillop, 1993] following the same procedure described for 4a-f. The final compounds were purified by column chromatography using cyclohexane/ethyl acetate 1:1 as eluent.
(±)N-(4-Bromophenyl)-2-[3,4-dimethyl-6-oxo-5,6-dihydropyridazin-1(4H)-yl]acetamide, (±)6a
Yield = 36%; mp = 84-85 °C (EtOH). 1H-NMR (CDCl3) δ 1.22 (d, 3H, CHCH3, J = 6.8 Hz); 2.08 (s, 3H, N=CCH3); 2.33-2.40 (m, 1H, CH3CH); 2.64-2.70 (m, 2H, CHCH2); 4.49 (d, 1H, NCH-H, J = 15.6 Hz); 4.58 (d, 1H, NCH-H, J = 15.6 Hz); 7.30-7.40 (m, 4H, Ar); 8.19 (exch br s, 1H, NH). 13C-NMR (CDCl3) δ 15.7 (CH3); 21.1 (CH3); 31.9 (CH); 34.1 (CH2); 53.1 (CH2); 116.8 (C); 121.4 (2 CH); 131.8 (2 CH); 136.8 (C); 158.6 (C); 166.3 (C); 166.5 (C). Anal. Calcd for C14H16BrN3O2 (338.20): C, 49,72; H, 4.77; N, 12.42; Found: C, 49.59; H, 4.75; N, 12.47.
(±)N-(4-Bromophenyl)-2-[3-methyl-6-oxo-4-phenyl-5,6-dihydropyridazin-1(4H)-yl]acetamide, (±)6b
Yield = 62%; oil. 1H-NMR (CDCl3) δ 2.06 (s, 3H, CH3); 2.82 (dd, 1H, CHCH-H, J = 4.8 Hz, J = 11.6 Hz); 2.96 (dd, 1H, CHCH-H, J = 7.6 Hz, J = 9.2 Hz); 3.84 (dd, 1H, CH-Ph, J = 5.2 Hz, J = 2.0 Hz); 4.46 (d, 1H, NCH-H, J = 15.6 Hz); 4.72 (d, 1H, NCH-H, J = 15.6 Hz); 7.17-7.19 (m, 2H, Ar); 7.28-7.34 (m, 3H, Ar); 7.42 (d, 2H, Ar, J = 8.8 Hz) 7.90 (exch br s, 1H, NH). 13C-NMR (CDCl3) δ 22.3 (CH3); 34.4 (CH2); 43.5 (CH); 53.7 (CH2); 116.9 (C); 121.4 (CH); 127.2 (2 CH); 128.1 (CH); 129.2 (CH); 129.5 (2 CH); 131.8 (2 CH); 136.6 (C); 137.1 (C); 155.9 (C); 165.8 (C); 166.4 (C). Anal. Calcd for C19H19BrN3O2 (400.27): C, 57.01; H, 4.53; N, 10.50; Found: C, 57.17; H, 4.52; N, 10.47.
N-(4-Bromophenyl)-2-[3,4-dimethyl-6-oxopyridazin-1(6H)-yl]acetamide, 7a
Yield = 81%; mp = 205-206 °C (EtOH). 1H-NMR (CDCl3) δ 2.23 (s, 3H, N=CCH3); 2.33 (s, 3H, =CCH3); 4.92 (s, 2H, CH2); 6.81 (s, 1H, Ar); 7.39-7.44 (m, 4H, Ar); 9.11 (exch br s, 1H, NH). 13C-NMR (CDCl3) δ 19.0 (CH3); 19.2 (CH3); 57.5 (CH2); 116.0 (C); 121.0 (2CH); 127.4 (CH); 132.0 (2CH); 137.0 (C); 144.8 (C); 161.1 (C); 165.4 (C). Anal. Calcd for C14H14BrN3O2 (336.18): C, 50.02; H, 4.20; N, 12.50; Found: C, 50.16; H, 4.19; N, 12.53.
N-(4-Bromophenyl)-2-[3-methyl-6-oxo-4-phenylpyridazin-1(6H)-yl]acetamide, 7b
Yield = 64%; mp = 199-200 °C (EtOH). 1H-NMR (CDCl3) δ 2.30 (s, 3H, CH3); 5.01 (s, 2H, CH2); 6.91 (s, 1H, Ar); 7.40-7.42 (m, 2H, Ar); 7.43-7.48 (m, 4H, Ar); 7.49-7.5 (m, 3H, Ar); 9.22 (exch br s, 1H, NH). 13C-NMR (CDCl3) δ 20.4 (CH3); 58.0 (CH2); 116.9 (C); 121.4 (CH); 127.9 (CH); 128.0 (2CH); 128.9 (2CH); 129.4 (2CH); 131.8 (2CH); 135.5 (C); 137.0 (C); 145.5 (C); 148.3 (C); 161.0 (C); 165.2 (C). Anal. Calcd for C19H16BrN3O2 (398.25): C, 57.30; H, 4.05; N, 10.55; Found: C, 57.43; H, 4.06; N, 10.58.
General Procedures for 9a-h
Compounds 9a-h were obtained starting from appropriate substrate of type 8 [Wang et al., 2005; Yarligan et al., 2005; Pihlaja et al. 2002; Isomura et al. 1988; Zhao et al., 2013] following the same procedure described for 4a-f. The final compounds were purified by column chromatography using cyclohexane/ethyl acetate 1:1 (for 9a,f,g), cyclohexane/ethyl acetate 1:2 (for 9b), cyclohexane/ethyl acetate 2:1 (for 9d) and CH2Cl2/CH3OH 98:2 (9c,e) as eluents.
2-(5-Acetyl-4-methyl-2-oxo-thiazol-3-yl)-N-(4-bromophenyl)acetamide, 9a
Yield = 19%; mp = 228-230 °C (EtOH). 1H-NMR (CDCl3) δ 2.39 (s, 3H, COCH3); 2.65 (s, 3H, CH3); 4.55 (s, 2H, CH2CO); 7.34-7.44 (m, 4H, Ar); 8.19 (exch br s, 1H, NH). Anal. Calcd for C14H13BrN2O3S (369.23): C, 45.54; H, 3.55; N, 7.59; Found: C, 45.65; H, 3.54; N, 7.60.
N-(4-Bromophenyl)-2-(4-methyl-2-oxo-thiazol-3-yl)acetamide, 9b
Yield = 35%; mp = 204-205 °C (EtOH). 1H-NMR (CDCl3) δ 2.40 (s, 3H, CH3); 4.45 (s, 2H, CH2CO); 5.85 (s, 1H, H5-thiazole); 7.36-7.43 (m, 4H, Ar); 8.43 (exch br s, 1H, NH). Anal. Calcd for C12H11BrN2O2S (327.20): C, 44.05; H, 3.39; N, 8.56; Found: C, 44.17; H, 3.39; N, 8.54.
N-(4-Bromophenyl)-2-(2-oxo-4-phenyl-thiazol-3-yl)acetamide, 9c
Yield = 18%; mp = 181-183 °C (EtOH). 1H-NMR (CDCl3) δ 4.41 (s, 2H, CH2CO); 6.14 (s, 1H, H5-thiazole); 7.32 (s, 4H, Ar); 7.45 (s, 5H, Ar); 8.45 (exch br s, 1H, NH). 13C-NMR (CDCl3) δ 48.8 (CH2); 99.4 (CH); 117.1 (C); 121.3 (CH); 121.4 (CH); 129.9 (CH); 130.3 (CH); 130.8 (C); 131.8 (CH); 136.6 (C); 138.3 (C); 165.1 (2 CO). Anal. Calcd for C17H13BrN2O2S (389.27): C, 52.45; H, 3.37; N, 7.20; Found: C, 52.29; H, 3.36; N, 7.22.
N-(4-Bromophenyl)-2-[4-(3-methoxyphenyl)-2-oxothiazol-3-yl]-acetamide, 9d
Yield = 25%; mp = 162-165 °C (EtOH). 1H-NMR (CDCl3) δ 3.83 (s, 3H, CH3); 4.39 (s, 2H, CH2CO); 6.15 (s, 1H, H5-thiazole); 7.01 (s, 3H, Ar); 7.42 (s, 5H, Ar); 8.63 (exch br s, 1H, NH). 13C-NMR (CDCl3) δ 48.8 (CH2); 55.5 (CH3); 99.3 (CH); 114.3 (CH); 116.0 (CH); 116.9 (CH); 121.2 (CH); 121.4 (CH); 130.2 (CH); 131.5 (C); 131.7 (CH); 136.7 (C); 138.3 (C); 159.9 (C); 165.2 (CO); 174.2 (CO). Anal. Calcd for C18H15BrN2O3S (419.29): C, 51.56; H, 3.61; N, 6.68; Found: C, 51.43; H, 3.60; N, 6.72.
N-(4-Bromophenyl)-2-[4-(4-methoxyphenyl)-2-oxothiazol-3-yl]acetamide, 9e
Yield = 15%; mp = 210-213 °C (EtOH). 1H-NMR (CDCl3) δ 3.84 (s, 3H, CH3); 4.37 (s, 2H, CH2CO); 6.07 (s, 1H, H5-thiazole); 6.96 (d, 2H, Ar, J = 8.8 Hz); 7.38 (m, 6H, Ar); 8.79 (exch br s, 1H, NH). 13C-NMR (CDCl3) δ 48.7 (CH2); 55.3 (CH3); 100.1 (CH); 114.4 (CH); 115.8 (CH); 117.1 (CH); 121.7 (CH); 121.3 (CH); 129.8 (CH); 131.2 (C); 132.1 (CH); 135.9 (C); 138.4 (C); 160.1 (C); 164.2 (CO); 173. (CO). Anal. Calcd for C18H15BrN2O3S (419.29): C, 51.56; H, 3.61; N, 6.68; Found: C, 51.61; H, 3.61; N, 6.65.
N-(4-Bromophenyl)-2-[4-(3-chlorophenyl)-2-oxothiazol-3-yl]acetamide, 9f
Yield = 16%; mp = 203-204 °C (EtOH). 1H-NMR (CDCl3) δ 4.37 (s, 2H, CH2CO); 6.18 (s, 1H, H5-thiazole); 7.36-7.46 (m, 8H, Ar); 8.59 (exch br s, 1H, NH). Anal. Calcd for C17H12BrClN2O2S (423.71): C, 48.19; H, 2.85; N, 6.61; Found: C, 48.35; H, 2.84; N, 6.62.
N-(4-Bromophenyl)-2-[4-(4-chlorophenyl)-2-oxothiazol-3-yl]acetamide, 9g
Yield = 18%; mp = 230-232 °C (EtOH). 1H-NMR (CDCl3) δ 4.36 (s, 2H, CH2CO); 6.14 (s, 1H, H5-thiazole); 7.37-7.45 (m, 8H, Ar); 8.73 (exch br s, 1H, NH). Anal. Calcd for C17H12BrClN2O2S (423.71): C, 48.19; H, 2.85; N, 6.61; Found: C, 48.03; H, 2.85; N, 6.63.
N-(4-Bromophenyl)-2-[4-(4-nitrophenyl)-2-oxothiazol-3-yl]acetamide, 9h
Yield = 21%; mp = 162-164 °C (EtOH). 1H-NMR (CDCl3) δ 4.38 (s, 2H, CH2CO); 6.30 (s, 1H, H5-thiazole); 7.41 (s, 4H, Ar); 7.72 (d, 2H, Ar, J = 8.8 Hz); 8.83 (d, 2H, Ar, J = 8.8); 8.68 (exch br s, 1H, NH). Anal. Calcd for C17H12BrN3O4S (434.26): C, 47.02; H, 2.79; N, 9.68; Found: C, 47.16; H, 2.78; N, 9.71.
2-[4-(4-Aminophenyl)-2-oxothiazol-3-yl]-N-(4-bromophenyl)acetamide, 10
Compound 9h (0.11 mmol) was subject to catalytic reduction with 10% Pd/C (0.05 mmol) in EtOH (20 mL) for 2 h in a Parr instrument (30 PSI). The catalyst was filtered off, and the solvent was evaporated under vacuum, affording the final compound, which was purified by column chromatography using cyclohexane/ethyl acetate 1:1 as eluent. Yield = 27%; mp = 188-189 °C (EtOH). 1H-NMR (CDCl3) δ 4.32 (s, 2H, CH2CO); 5.41 (exch br s, 2H, NH2); 6.21 (s, 1H, H5-thyazole); 6.53 (m, 2H, Ar); 7.01 (m, 2H, Ar); 7.45 (s, 4H, Ar); 10.28 (exch br s, 1H, NH). Anal. Calcd for C17H14BrN3O2S (404.28): C, 50.50; H, 3.49; N, 10.39; Found: C, 50.61; H, 3.48; N, 10.37.
Cell Culture
Human promyelocytic leukemia HL60 cells stably transfected with FPR1 (FPR1-HL60), FPR2 (FPR2-HL60), or FPR3 (FPR3-HL60) were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum, 10 mM HEPES, 100 μg/ml streptomycin, 100 U/ml penicillin, and G418 (1 mg/mL), as previously described [Giovannoni et al., 2013]. Rat basophilic leukemia (RBL-2H3) cells transfected with mouse Fpr1 (Fpr1-RBL) or mouse Fpr2 (Fpr2-RBL) were cultured in DMEM supplemented with 20% (v/v) FBS, 10 mM HEPES, 100 μg/ml streptomycin, 100 U/ml penicillin, and G418 (250 μg/ml). Wild-type HL60 and RBL-2H3 cells were cultured under the same conditions, but without G418.
Isolation of Human Neutrophils
Blood was collected from healthy donors in accordance with a protocol approved by the Institutional Review Board at Montana State University. Neutrophils were purified from the blood using dextran sedimentation, followed by Histopaque 1077 gradient separation and hypotonic lysis of red blood cells, as previously described [Schepetkin et al., 2014b]. Isolated neutrophils were washed twice and resuspended in HBSS without Ca2+ and Mg2+ (HBSS−). Neutrophil preparations were routinely > 95% pure, as determined by light microscopy, and greater than 98 % viable, as determined by trypan blue exclusion.
Isolation of Murine Neutrophils
Murine bone marrow neutrophils were isolated from bone marrow leukocyte preparations, as described previously [Schepetkin et al., 2014]. Briefly, bone marrow leukocytes were flushed from tibias and femurs of BALB/c mice with HBSS, filtered through a 70 μm nylon cell strainer (BD Biosciences, Franklin Lakes, NJ) to remove cell clumps and bone particles, and resuspended in HBSS at 106 cells/ml. Bone marrow leukocytes were resuspended in 3 ml of 45% Percoll solution and layered on top of a Percoll gradient consisting of 2 ml each of 50, 55, 62, and 81% Percoll solutions in a conical 15-ml polypropylene tube. The gradient was centrifuged at 1600g for 30 min at 10°C, and the cell band located between the 61 and 81% Percoll layers was collected. The cells were washed, layered on top of 3 ml of Histopaque 1119, and centrifuged at 1600g for 30 min at 10°C to remove contaminating red blood cells. The purified neutrophils were collected, washed, and resuspended in HBSS. All animal use was conducted in accordance with a protocol approved by the Institutional Animal Care and Use Committee at Montana State University.
Ca2+ Mobilization Assay
Changes in intracellular Ca2+ were measured with a FlexStation II scanning fluorometer using a FLIPR 3 calcium assay kit (Molecular Devices, Sunnyvale, CA) for human and murine neutrophils, as well as HL60 and RBL cells, as described previously [Schepetkin et al., 2013]. All active compounds were evaluated in wild-type HL60 and RBL cells to verify that the agonists are inactive in nontransfected cells. Neutrophils or HL60 and RBL cells, suspended in HBSS− containing 10 mM HEPES, were loaded with Fluo-4 AM dye (Invitrogen; 1.25 μg/mL final concentration) and incubated for 30 min in the dark at 37 °C. After dye loading, the cells were washed with HBSS− containing 10 mM HEPES, resuspended in HBSS containing 10 mM HEPES and Ca2+ and Mg2+ (HBSS+), and aliquotted into the wells of a flat-bottomed, half-area-well black microtiter plates (2 × 105 cells/well). The compound of interest was added from a source plate containing dilutions of test compounds in HBSS+, and changes in fluorescence were monitored (λex = 485 nm, λem = 538 nm) every 5 s for 240 s at room temperature after automated addition of compounds. Maximum change in fluorescence, expressed in arbitrary units over baseline, was used to determine agonist response. Responses were normalized to the response induced by 5 nM fMLF (Sigma Chemical Co., St. Louis, MO) for FPR1 HL60 cells and human neutrophils, 5 nM WKYMVm (Calbiochem, San Diego, CA) for FPR2 HL60 cells, 10 nM WKYMVM (Tocris Bioscience) for FPR3-HL60 cells, and 10 nM WKYMVm for mouse neutrophils, mFpr1-RBL, and mFpr2-RBL cells, which were assigned a value of 100%. Curve fitting (5-6 points) and calculation of median effective concentration values (EC50 values) were performed using nonlinear regression analysis of the concentration-response curves generated using Prism 5 (GraphPad Software, Inc., San Diego, CA).
Cell Migration Assay
Neutrophils were suspended in HBSS+ containing 2% (v/v) fetal bovine serum (FBS) (2×106 cells/mL), and cell migration was analyzed in 96-well ChemoTx chemotaxis chambers (Neuroprobe, Gaithersburg, MD), as previously described [Schepetkin et al., 2014b]. Briefly, lower wells were loaded with 30 μL of HBSS+ containing 2% (v/v) FBS and the indicated concentrations of test compound, DMSO (negative control), or 1 nM fMLF as a positive control. Neutrophils were added to the upper wells and allowed to migrate through the 5.0 μm pore polycarbonate membrane filter for 60 min at 37 °C and 5% CO2. The number of migrated cells was determined by measuring ATP in lysates of transmigrated cells using a luminescence-based assay (CellTiter-Glo; Promega, Madison, WI), and luminescence measurements were converted to absolute cell numbers by comparison of the values with standard curves obtained with known numbers of neutrophils. The results are expressed as percentage of negative control and were calculated as follows: (number of cells migrating in response to test compounds/spontaneous migration in response to control medium)×100. EC50 values were determined by nonlinear regression analysis of the concentration-response curves generated using Prism 5 software.
RESULTS AND DISCUSSION
The newly synthesized compounds were evaluated for their ability to induce intracellular Ca2+ flux in human neutrophils and HL60 cells transfected with FPR1, FPR2 and FPR3 (Tables 1-3). Compounds were also evaluated in mouse neutrophils and RBL-2H3 cells transfected with mouse FPR1 and FPR2. To verify receptor specificity, these compounds were also evaluated, as applicable, in wild-type non-transfected HL60 and RBL cells and were found to be inactive.
Table 1.
Activity of 4-benzyl-5-substituted pyridazinones 4a-f in human neutrophils and FPR-transfected HL60 cell
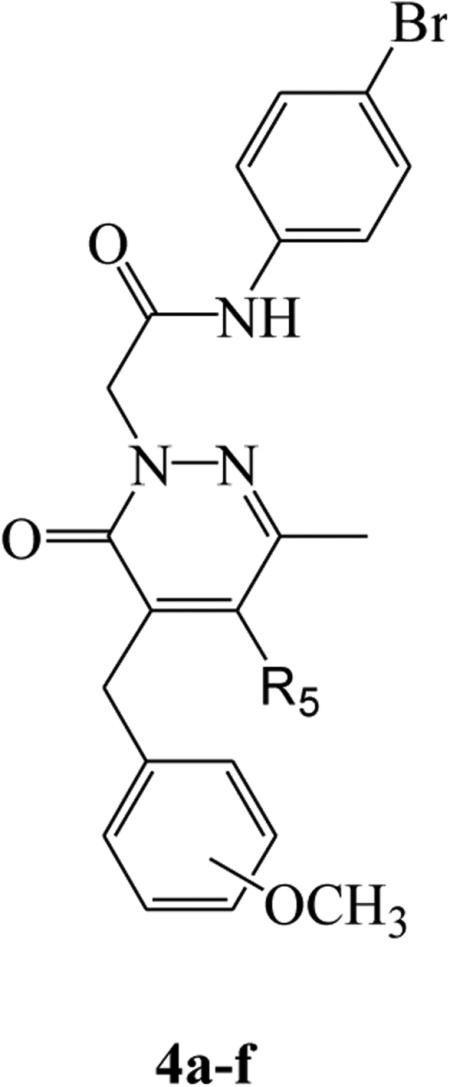
| ||||||
|---|---|---|---|---|---|---|
| Compd | R5 | OCH3 | Ca2+ flux | |||
| human neutrophils | FPR1-HL60 | FPR2-HL60 | FPR3-HL60 | |||
| EC50 (μM) and efficacy (%)[a] | ||||||
| 4a | CH3 | m | 0.006 ± 0.002 (150) | 0.019 ± 0.005 (85) | 0.043 ± 0.016 (80) | 0.040 ± 0.011 (165) |
| 4b | CH3 | p | 0.27 ± 0.025 (140) | 0.9 ± 0.033 (60) | 0.013 ± 0.003 (95) | 0.13 ± 0.032 (100) |
| 4c | C2H5 | m | 1.2 ± 0.3 (100) | 3.2 ± 0.6 (120) | 1.9 ± 0.41 (100) | 4.1 ± 1.7 (100) |
| 4d | C3H5 | m | 3.2 ± 1.2 (70) | 2.2 ± 0.5 (115) | 4.6 ± 1.3 (90) | 3.4 ± 0.76 (65) |
| 4e | C4H9 | m | 2.7 ± 0.7 (25) | N.A.[b] | 15.7 ± 4.2 (90) | N.A. |
| 4f | C6H5 | m | 5.4 ± 0.26 (100) | 2.2 ± 0.62 (160) | N.A. | N.A. |
EC50 values represent the average of means from three independent experiments and were determined by nonlinear regression analysis of the concentration-response curves (5-6 points) generated using GraphPad Prism 5 with 95% confidential interval (p < 0.05). Efficacy is expressed as % of the response induced by 5 nM fMLF (FPR1), 5 nM WKYMVm (FPR2), or 10 nM WKYMVM (FPR3).
N.A., no activity (no response was observed during the first 2 min after addition of compounds under investigation) considering the limits of efficacy > 20% and EC50 < 50 μM.
Table 3.
Activity of 9a-h, 10 in human neutrophils and FPR-transfected HL60 cells
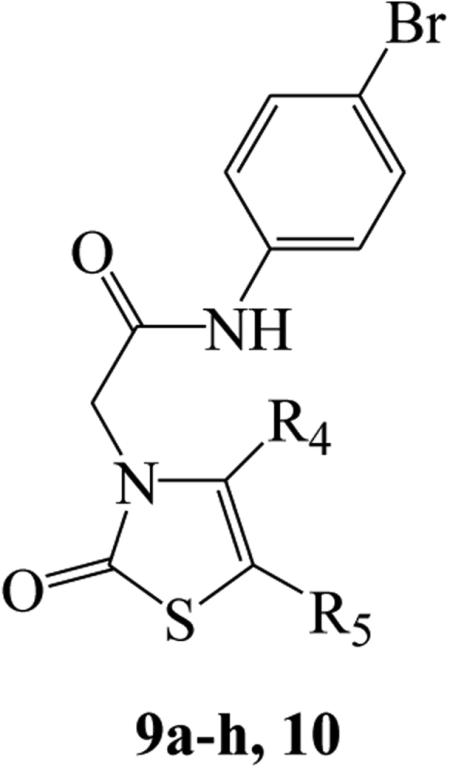
| ||||||
|---|---|---|---|---|---|---|
| Compd | R4 | R5 | Ca2+ flux | |||
| human neutrophils | FPR1-HL60 | FPR2-HL60 | FPR3-HL60 | |||
| EC50 (μM) and efficacy (%)[a] | ||||||
| 9a | CH3 | COCH3 | 12.2 ± 2.5(55) | 8.9 ± 1.9 (75) | 5.8 ± 1.4 (60) | N.A.[b] |
| 9b | CH3 | H | 10.7 ± 2.3 (55) | 12.4 ± 2.6 (70) | 4.1 ± 1.1 (80) | 27.8 ± 3.2 (85) |
| 9c | Ph | H | 6.0 ± 1.5 (95) | 1.8 ± 0.6 (100) | 2.1 ± 0.6 (95) | 19.7 ± 2.4 (135) |
| 9d | 3-OCH3Ph | H | 1.3 ± 0.3 (125) | 0.28 ± 0.08 (90) | 0.23 ± 0.04 (120) | 5.1 ± 1.7 (100) |
| 9e | 4-OCH3Ph | H | 7.4 ± 2.3 (110) | 2.6 ± 0.6 (110) | 1.8 ± 0.16 (100) | 36.1 ± 3.3 (85) |
| 9f | 3-ClPh | H | 11.1 ± 2.8 (140) | 6.0 ± 1.7 (75) | 3.0 ± 0.7 (110) | 32.2 ± 3.6 (75) |
| 9g | 4-ClPh | H | 12.2 ± 3.1 (125) | 2.8 ± 0.7 (75) | 2.4 ± 0.5 (75) | N.A. |
| 9h | 4-NO2Ph | H | 1.5 ± 0.4 (95) | 9.1 ± 1.4 (60) | N.A. | N.A. |
| 10 | 4-NH2Ph | H | 36.1 ± 3.9 (65) | 34.9 ± 4.2 (80) | 14.7 ± 3.7 (65) | N.A. |
EC50 values represent the average of means from three independent experiments and were determined by nonlinear regression analysis of the concentration-response curves (5-6 points) generated using GraphPad Prism 5 with 95% confidential interval (p < 0.05). Efficacy is expressed as % of the response induced by 5 nM fMLF (FPR1-HL60), 5 nM WKYMVm (FPR2-HL60), or 10 nM WKYMVM (FPR3-HL60).
N.A., no activity (no response was observed during first 2 min after addition of compounds under investigation) considering the limits of efficacy > 20% and EC50 < 50 μM.
In the 4-benzylpyridazinone series (Table 1), the introduction of a methyl at C-5 was associated with high FPR agonist activity (compounds 4a,b) leading to some of themost potent mixed agonists. For example, 4a activated all three FPR subtypes with good potency (FPR1 EC50 = 19 nM, FPR2 EC50 = 43 nM, FPR3 EC50 = 40 nM). Although 4b was an FPR1 and FPR3 agonist with submicromolar activity (EC50 = 0.9 and 0.13 μM, respectively), it exhibited a preference for FPR2 (EC50 = 13 nM).These results are consistent with our previous studies [Cilibrizzi et al., 2009], which showed that when OCH3 was shifted from the meta to the para position of the benzyl at C-4, the selectivity of a given ligand shifted from mixed FPR1/FPR2 to FPR2-specific.
Elongation of the aliphatic chain (compounds 4c-e) was detrimental for activity and led to mixed agonists with EC50 values in the micromolar range, with the exception of the 3-butyl derivative 4e, which was a weak but selective FPR2 agonist (EC50 = 15.7 μM). Surprisingly, the 5-phenyl derivative 4f exhibited FPR1 selectivity (EC50 = 2.2 μM, Table 1).
As shown in Table 2 elimination of the substituent at position 4 and introduction of CH3 or C6H5 at C-5 (products of type 6 and 7) led to compounds endowed with micromolar activity and a slight preference for FPR2. The unsaturation at C-4/C-5 of the heterocyclic ring (7a,b) appeared to be associated with increased agonist activity and selectivity (generally submicromolar range) relative to the 4,5-dihydro analogues, (±)6a,b. As compounds (±)6a,b were mixtures of isomers, it is possible that one of the isomers could be more active than the other, reducing overall specific activity or even interfering with binding of the active isomer.
Table 2.
Activity of C-5 substituted pyridazinones (±)-6a-b and 7a-b in human neutrophils and FPR-transfected HL60 cells
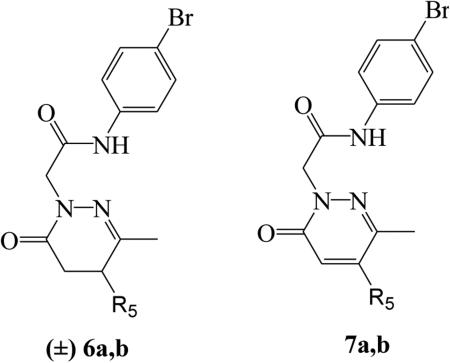
| |||||
|---|---|---|---|---|---|
| Compd | R5 | Ca2+ flux | |||
| human neutrophils | FPR1-HL60 | FPR2-HL60 | FPR3-HL60 | ||
| EC50 (μM) and efficacy (%)[a] | |||||
| (±)-6a | CH3 | 13.0 ± 1.8 (115) | 13.0 ± 4.6 (60) | 2.6 ± 0.89 (120) | 11.8 ± 3.0 (150) |
| (±)-6b | C6H5 | 1.4 ± 0.38 (75) | 4.1 ± 1.6 (90) | 0.63 ± 0.17 (110) | 0.49 ± 0.14 (120) |
| 7a | CH3 | 2.0 ± 0.29 (130) | 5.7 ± 2.1 (110) | 0.51 ± 0.19 (90) | N.A.[b] |
| 7b | C6H5 | 0.20 ± 0.056 (110) | N.A. | 0.15 ± 0.052 (115) | 0.97 ± 0.23 (95) |
EC50 values represent the average of means from three independent experiments and were determined by nonlinear regression analysis of the concentration-response curves (5-6 points) generated using GraphPad Prism 5 with 95% confidential interval (p < 0.05). Efficacy is expressed as % of the response induced by 5 nM fMLF (FPR1-HL60), 5 nM WKYMVm (FPR2-HL60), or 10 nM WKYMVM (FPR3-HL60).
N.A., no activity (no response was observed during first 2 min after addition of compounds under investigation) considering the limits of efficacy > 20% and EC50 < 50 μM.
Table 3 reports the activities of the 2-oxothiazole derivatives, 9a-h and 10 that exhibited EC50 values in the micromolar range for the three FPR isoforms, with the exception of compound 9d, which had EC50 values of 0.28 μM and 0.23 μM for FPR1 and FPR2, respectively. No appreciable FPR2 selectivity was observed for this series, although they did have a lower affinity for FPR3 (EC50 ~ 20-fold higher than for FPR1 and FPR2).
The most active derivatives (4a,b and 7b) were also evaluated for their chemoattractant activity toward human neutrophils (Table 4). As expected for FPR agonists, they stimulated neutrophil migrations with EC50 values that correlated well with their ability to induce human neutrophil intracellular Ca2+ flux.
Table 4.
Chemoattractant activity of selected pyridazinones in human neutrophils
| Compd | EC50 (μM)[a] |
|---|---|
| 4a | 0.27 ± 0.06 |
| 4b | 0.51 ± 0.11 |
| 7b | 2.1 ± 0.53 |
The data are presented as the mean ± SD of three independent experiments with cells from different donors, in which median effective concentration values (EC50) were determined by nonlinear regression analysis of the concentration-response curves (5-6 points) generated using GraphPad Prism 5 with 95% confidential interval (p < 0.05).
All of the synthesized compounds were also evaluated in mouse neutrophils and RBL cells transfected with mouse Fpr1 or Fpr2 (Table 5). The majority of tested compounds had no agonist effects at mouse neutrophils or RBL cells transfected with mouse Fpr; however, four compounds were active in mouse neutrophils and three had activity in either Fpr1 or Fpr2 RBL cells. As shown in Table 5, compounds 4f, (±)6,b, and 7a,b all activated Ca2+ flux in mouse neutrophils with low micromolar activity. From the literature, it is well known that there are differences in affinity between FPR1 and Fpr1 for fMLF and other FPR agonists/antagonists [He et al., 2000] and these receptors have only 72% sequence similarities (Dahlgren et al., 2016). However, it is difficult to explain the complete inactivity of the most potent human FPR agonists of this series (4a and 4b) towards mouse neutrophils or mouse Fpr-transfected RBL cells. Comparison of the results between human FPR-transfected HL60 cells and mouse Fpr1/Fpr2-transfected RBL cells was also difficult to interpret. For example, (±)6a was active for all human FPR and mouse Fpr subtypes tested, whereas 4f was inactive in FPR2-HL60 cells but had an EC50 value of 28.8 μM for mouse Fpr2. Likewise, (±)6a was active for all human FPR subtypes but only activated mFpr1 and not mouse neutrophils or mFpr2. Thus, the difference in human and mouse formyl peptide receptor responses to various agonists are clearly evident and will need to be considered when using agonists in mouse models involving Fpr function.
Table 5.
Activity of selected pyridazinones in mouse neutrophils and mouse Fpr-transfected RBL cells
| Compd | Ca2+ flux | ||
|---|---|---|---|
| mouse neutrophils | mFpr1-RBL | mFpr2-RBL | |
| EC50 (μM) and efficacy (%)[a] | |||
| 4a | N.A.[b] | N.A. | N.A. |
| 4b | N.A. | N.A. | N.A. |
| 4f | 24.3 ± 4.8 (95) | N.A. | 28.8 ± 5.2 (180) |
| (±)-6a | N.A. | 25.1 ± 4.5 (70) | N.A |
| (±)-6b | 14.2 ± 3.4 (160) | 2.3 ± 0.7 (60) | 3.5 ± 1.1 (220) |
| 7a | 21.7 ± 5.2 (70) | N.A. | N.A. |
| 7b | 15.7 ± 4.1 (75) | N.A. | N.A |
EC50 values represent the average of means from three independent experiments and were determined by nonlinear regression analysis of the concentration-response curves (5-6 points) generated using GraphPad Prism 5 with 95% confidential interval (p < 0.05). Efficacy is expressed as % of the response induced by 10 nM WKYMVm.
N.A., no activity (no response was observed during first 2 min after addition of the compounds under investigation)
In conclusion, we have identified a new series of FPRs agonists with EC50 values in the nanomolar to low micromolar range. The majority of compounds were mixed FPRs agonists, but with a slight preference for FPR2. Compounds 4a and 4b are notable for their high potency. In particular, compound 4a is a mixed FPR agonist with EC50 values of 19 nM (FPR1), 43 nM (FPR2), and 40 nM (FPR3). Similarly interesting is compound 4b, which had considerable potency, but exhibited a preference for FPR2 (EC50 = 13 nM) and could represent a novel lead compound for further chemical manipulation and studies.
Acknowledgments
Funding: This work was supported in part by National Institutes of Health IDeA Program COBRE grant GM110732 (MTQ) and grant AI033503 (RDY), an equipment grant from the M.J. Murdock Charitable Trust (MTQ), a USDA National Institute of Food and Agriculture Hatch project (MTQ), Montana University System Research Initiative 51040-MUSRI2015-03, and the Montana State University Agricultural Experiment Station (MTQ).
Footnotes
CONFLICT OF INTEREST
The authors declare to have no financial/commercial conflict of interest.
REFERENCES
- Beutler B. Innate immunity: an overview. Mol Immunol. 2004;40:845–859. doi: 10.1016/j.molimm.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Bozinovski S, Anthony D, Anderson GA, Irving LB, Levy BD, Vlahos R. Treating neutrophilic inflammation in COPD by treating ALX/FPR2 resolution pathways. Pharmacol Ther. 2013;140:280–289. doi: 10.1016/j.pharmthera.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Cilibrizzi A, Crocetti L, Giovannoni MP, Graziano A, Vergelli C, Bartolucci G, Soldani G, Quinn MT, Schepetkin IA, Faggi C. Synthesis, HPLC enantioresolutio and X-ray analysis of a new series of C5-methyl pyridazines as N-formyl peptide receptor (FPR) agonists. Chirality. 2013;25:400–408. doi: 10.1002/chir.22162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cilibrizzi A, Quinn MT, Kirpotina LN, Schepetkin IA, Holderness J, Ye RD, Rabiet MJ, Biancalani C, Cesari N, Graziano A, Vergelli C, Pieretti S, Dal Piaz V, Giovannoni MP. 6-Methyl-2,4-disubstituted pyridazin-3(2H)-ones: a novel class of small-molecule agonists for formyl peptide receptors. J Med Chem. 2009;52:5044–5057. doi: 10.1021/jm900592h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cilibrizzi A, Schepetkin IA, Bartolucci G, Crocetti L, Dal Piaz V, Giovannoni MP, Graziano A, Kirpotina LN, Quinn MT, Vergelli C. Synthesis, enantioresolution, and activity profile of chiral 6-methyl-2,4-disubstituted pyridazin-3(2H)-ones as potent N-formyl peptide receptor agonists. Bioorg Med Chem. 2012;20:3781–3792. doi: 10.1016/j.bmc.2012.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates WJ, McKillop A. One-pot preparation of 6-substituted 3(2H)-pyridazinones from ketones. Synthesis. 1993;3:334–342. [Google Scholar]
- Corminboeuf O, Leroy X. FPR2/ALXR agonists and the resolution of inflammation. J Med Chem. 2015;58:537–559. doi: 10.1021/jm501051x. [DOI] [PubMed] [Google Scholar]
- Crocetti L, Vergelli C, Cilibrizzi A, Graziano A, Khlebnikov AI, Kirpotina LN, Schepetkin IA, Quinn MT, Giovannoni MP. Synthesis and pharmacological evaluation of new pyridazin-based thioderivatives as formyl peptide receptor (FPR) agonists. Drug Dev Res. 2013;74:259–271. [Google Scholar]
- Dahlgren C, Gabl M, Holdfeldt A, Winther M, Forsman H. Basic characteristics of the neutrophil receptors that recognize formylated peptides, a danger-associated molecular pattern generated by bacteria and mitochondria. Biochem Pharmacol. 2016;114:22–39. doi: 10.1016/j.bcp.2016.04.014. [DOI] [PubMed] [Google Scholar]
- Giovannoni MP, Schepetkin IA, Cilibrizzi A, Crocetti L, Khlebnikov AI, Dahlgren C, Graziano A, Dal Piaz V, Kirpotina LN, Zerbinati S, Vergelli C, Quinn MT. Further studies on 2-arylacetamide pyridazin-3(2H)-ones: design, synthesis and evaluation of 4,6-disubstituted analogs as formyl peptide receptors (FPRs) agonists. Eur J Med Chem. 2013;64:512–528. doi: 10.1016/j.ejmech.2013.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider N, Holzer W. Product class 8: pyridazines. Sci Synth. 2004;16:125–249. [Google Scholar]
- He R, Tan L, Browning DD, Wang JM, Ye DR. The synthetic peptide Trp-Lys-Tyr-Met-Val-D-Met is a potent chemotactic agonist for mouse formyl peptide receptor. 2000;165:4598–4605. doi: 10.4049/jimmunol.165.8.4598. [DOI] [PubMed] [Google Scholar]
- Holloway CA, Muratore ME, Storer RI, Dixon DJ. Direct enantioselective bronsted acid catalyzed N-acyliminium cyclization cascades of tryptamines and ketoacids. Org Lett. 2010;12:4720–4723. doi: 10.1021/ol101651t. [DOI] [PubMed] [Google Scholar]
- Isomura Y, Sakamoto S, Yoshida M, Abe T. Preparation of 4-aryl-2(3H)-thiazolone derivative as drug for bone disorders. Jpn Kokai Tokkyo Koho JP. 1988:63112572 A. [Google Scholar]
- Libby P. Atherosclerosis: the new view. Sci Am. 2002;286:46–52. doi: 10.1038/scientificamerican0502-46. [DOI] [PubMed] [Google Scholar]
- Libby P. Fanning the flames: inflammation in cardiovascular diseases. Cardiov Res. 2015;107:307–309. doi: 10.1093/cvr/cvv188. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- Metz G, Schwenker G. Intramolecular cyclocondensation of 4- and 5-oxocarboxylic acids to five a membered ring systems. Synthesis. 1980;5:394–397. [Google Scholar]
- Migeotte I, Communi D, Parmentier M. Formyl peptide receptors: a promiscuous sub family of G protein-coupled receptors controlling immune responses. Cytokine Grow Factor Rev. 2006;17:501–519. doi: 10.1016/j.cytogfr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Migeotte I, Riboldi E, Franssen JD, Gregoire F, Loison C, Wittamer V, Detheux M, Robberecht P, Costagliola S, Vassart G, Sozzani S, Parmentier M, Communi D. Identification and characterization of an endogenous chemotactic ligand specific for FPRL2. J Exp Med. 2005;201:83–93. doi: 10.1084/jem.20041277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihlaja K, Ovcharenko V, Kohlemainen E, Laihia K, Fabian Walter MF, Dehne H, Perjessy A, Kleist M, Teller J, Šustekovà Z. A correlative IR, MS, 1H, 13C and 15N NMR and theoretical study of 4-arylthiazol-2(3H)-ones. J Chem Soc Perkin Trans 2. 2002;2:329–336. [Google Scholar]
- Pinna GA, Curzu MM, Barlocco D, Cignarella G, Cavalletti E, Germini M, Berger K. Synthesis and pharmacological study of 5-aryl-6-methyl-4,5-dihydropyridazin-3(2H)-ones and related 5-aryl-6-methylpyridazin-3(2H)-ones. Farmaco. 1988;43:539–549. [PubMed] [Google Scholar]
- Serhan CN, Chiang N, Dalli J. The resolution code of acute inflammation: novel pro-resolving lipid mediators in resolution. Semin Immunol. 2015;27:200–215. doi: 10.1016/j.smim.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter pro-inflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Krishnamoorthy S, Recchiuti A, Chiang N. Novel anti-inflammatory-pro-resolving mediators and their receptors. Curr Top Med Chem. 2011;11:629–647. doi: 10.2174/1568026611109060629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Levy B. Novel pathways and endogenous mediators in anti-inflammation and resolution. Chem Immunol Allergy. 2003;83:115–145. doi: 10.1159/000071558. [DOI] [PubMed] [Google Scholar]
- Schepetkin AI, Khlebnikov AI, Giovannoni MP, Kirpotina LN, Cilibrizzi A, Quinn MT. Development of small molecule non-peptide formyl peptide receptor (FPR) ligands and molecular modeling of their recognition. Curr Med Chem. 2014a;21:1478–1504. doi: 10.2174/0929867321666131218095521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepetkin AI, Kirpotina LN, Khlebnikov AI, Leopoldo M, Lucente E, Lacivita E, De Giorgio P, Quinn MT. 3-(1H-indol-3-yl)-2-[3-(4-nitrophenyl)ureido]propanamide enantiomers with human formyl-peptide receptor agonist activity: molecular modeling of chiral recognition by FPR2. Biochem. Pharmacol. 2013;85:404–416. doi: 10.1016/j.bcp.2012.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepetkin IA, Kirpotina LN, Khlebnikov AI, Cheng N, Ye RD, Quinn MT. Antagonism of human formyl peptide receptor 1 (FPR1) by chromones and related isoflavones. Biochem Pharmacol. 2014b;92:627–641. doi: 10.1016/j.bcp.2014.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergelli C, Schepetkin IA, Ciciani G, Cilibrizzi A, Crocetti L, Giovannoni MP, Guerrini G, Iacovone A, Kirpotina LN, Khlebnikov AI, Ye RD, Quinn MT. 2-Arylacetamido-4-phenylamino-5-substituted pyridazinones as formyl peptide receptors agonists. Bioorg Med Chem. 2016;24:2530–2543. doi: 10.1016/j.bmc.2016.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Wood G, Duncan KW, Meades C, Gibson D, McLachlan JC, Perry A, Blake D, Zheleva DI, Fisher P. Preparation of pyrimidinylthiazolone compounds as protein kinase inhibitors. 2005 PCT Int Appl WO 2005042525.
- Xu Y, Han B, Xie L, Maynard G. Preparation of imidazopyridazines, triazolopyridazines and related benzodiazepine receptor ligands. 2005. PCT Int Appl WO2005080355. [Google Scholar]
- Yarligan S, Ogretir C, Csizmadia IG, Acikkalpe E, Bierber H, Arsian T. An ab initio study on protonation of some substituted thiazole derivates. J Mol Struct Theochem. 2005;715:199–203. [Google Scholar]
- Ye RD, Boulay F, Wang JM, Dahlgren C, Gerard C, Parmentier M, Serhan CN, Murphy PM. International union of basic and clinical pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol Rev. 2009;61:119–161. doi: 10.1124/pr.109.001578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Cao D, Chen T, Whang YMZ, Xu Y, Chen WWX, Lin Y, Du Z, Xiong B, Li J, Xu C, Zhang N, He J, Shen J. Fragment-based drug discovery of 2-thiazolidinones as inhibiors of the histone reader BRD4 bromodomain. J Med Chem. 2013;56:3833–3851. doi: 10.1021/jm301793a. [DOI] [PubMed] [Google Scholar]