Abstract
Metabotropic glutamate receptor 1 (mGluR1) blockade has been shown to decrease impulsive choice, as measured in delay discounting. However, several variables are known to influence an animal’s discounting, including sensitivity to delayed reinforcement and sensitivity to reinforcer magnitude. The goal of this experiment was to determine the effects of mGluR1, as well as mGluR5, antagonism on these parameters. Forty Sprague Dawley rats were trained in delay discounting, in which consistently choosing a small, immediate reward reflects impulsive choice. For half of the rats, the delay to the large reinforcer increased across blocks of trials, whereas the delay decreased across the session for half of the rats. Following training, half of the rats received injections of the mGluR1 antagonist JNJ 16259685 (JNJ; 0, 0.1, 0.3, or 1.0 mg/kg; i.p), and half received injections of the mGluR5 antagonist MPEP (0, 1.0, 3.0, or 10.0 mg/kg; i.p.). Administration of JNJ increased sensitivity to delayed reinforcement (i.e., promoted impulsive choice), regardless of which schedule was used. However, the order in which delays were presented modulated the effects of JNJ on sensitivity to reinforcer magnitude. Specifically, JNJ decreased sensitivity to reinforcer magnitude in rats trained on the descending schedule only. MPEP did not alter sensitivity to reinforcer magnitude or sensitivity to delayed reinforcement. These results show that mGluR1 is an important mediator of impulsive choice, and they provide further evidence that delay order presentation is an important variable that influences drug effects in delay discounting.
Keywords: impulsive choice, delay discounting, sensitivity to delayed reinforcement, sensitivity to reinforcer magnitude, metabotropic glutamate receptor, rat
Impulsive choice is the tendency to choose a small, immediate reward over a large, delayed reward [1] and is often measured using delay-discounting procedures. Recent evidence has implicated the glutamatergic system in impulsive choice, as administration of the glutamate N-methyl-D-aspartate receptor (NMDAr) channel blockers ketamine and memantine increase impulsive choice [2–3], whereas the effects of the NMDAr channel blocker MK-801 have been mixed, as some studies have reported a decrease in impulsive choice [4–5] but one study observing no change in impulsivity [6]. Although some evidence has shown that MK-801 decreases impulsive choice, it is a known psychotomimetic [7] that disrupts learning in animals [8].
Instead of targeting the NMDAr, Group I metabotropic glutamate receptors (mGluRs) are a potential mediator of impulsive choice. To our knowledge, only two studies have focused on the contribution of Group I mGluRs in impulsive choice, with results showing that an mGluR1 antagonist decreases impulsive choice [9], whereas mGluR5 allosteric modulators do not alter impulsive choice [10]. Although previous studies have examined the contribution of Group I mGluRs in discounting, they have not examined the effects of mGluR ligands in mediating sensitivity to reinforcer magnitude (i.e., how much an animal responds for the large reinforcer (LR) when its delivery is immediate; see 6 for a full discussion of what this parameter measures) and sensitivity to delayed reinforcement (i.e., what is typically considered to be impulsive choice), two parameters that influence an animal’s discounting [11]. This analysis is important as we can determine the behavioral mechanisms underlying an animal’s discounting. For example, previous studies have reported that ketamine and memantine increase impulsive choice [2–3]; however, the use of quantitative analyses revealed that these drugs decrease sensitivity to reinforcer magnitude without altering impulsive choice [6]. Thus, the goal of this study was to further characterize the contribution of Group I mGluRs on these parameters in in a delay-discounting procedure. Because the order in which delays are presented can modulate the effects of drugs in discounting [e.g., 12], rats were trained on a task in which the delay to the LR either increased or decreased across the session. Rats received injections of either JNJ 16259685 (JNJ; highly potent and selective mGluR1 antagonist) or MPEP (mGluR5 antagonist).
Forty male Sprague Dawley rats (250–275 g upon arrival in the laboratory) were used. Rats were tested previously in delay discounting and received 12 injections of NMDAr ligands [6]. Rats were individually housed in clear polypropylene cages (51 cm long × 26.5 cm wide × 32 cm high) with metal tops containing food and a water bottle in a room maintained on a 12:12-h cycle. Rats were tested during the light phase and were restricted to approximately 10 g of food each day but had ad libitum access to water. All experimental procedures were carried out according to the Current Guide for the Care and Use of Laboratory Animals (USPHS) under a protocol approved by the Northern Kentucky University Institutional Animal Care and Use Committee.
(3,4-dihydro-2H-pyrano[2,3-b]quinolin-7-yl)-(cis-4-methoxycyclohexyl)-methanone (JNJ 16259685) and 2-methyl-6-(phenylethynyl)pyridine hydrochloride (MPEP) were purchased from Tocris Bioscience (Ellisville, MO). JNJ was dissolved in distilled water, and MPEP was dissolved in 0.9% NaCl. Because JNJ (0.3 and 1.0 mg/kg) did not stay in solution, it had to be heated and stirred each day prior to the injection. To get MPEP into solution, it was heated and stirred once. All injections occurred at room temperature at a volume of 1 ml/kg.
Eight operant conditioning chambers (28 × 21 × 21 cm; ENV-008; MED Associates, St. Albans, VT) located inside sound attenuating chambers (ENV-018M; MED Associates) were used. A description of the operant chambers has been detailed previously [6].
After completing the experiment described in [6], half of the rats (n = 20) continued training on the discounting task, in which the delay to the LR increased across blocks of trials. Conversely, for half of the rats (n = 20), the delay to the LR decreased across the session. Rats received injections of either the mGluR1 antagonist JNJ (0, 0.1, 0.3 or 1.0 mg/kg, i.p.; n = 20) or the mGluR5 antagonist MPEP (0, 1.0, 3.0, or 10.0 mg/kg; i.p.; n = 20). Each injection occurred 40 min prior to task performance. The doses and pretreatment times were chosen based on previous work [13].
Omissions were analyzed with a two-way ANOVA, with dose as a within-subjects factor and schedule as a between-subjects factor. A main effect of dose was probed using Dunnett’s post hoc test, and a significant interaction was probed with additional one-way ANOVAs and Dunnett’s post hoc tests, when appropriate.
The proportion of responses for the LR was analyzed with mixed factorial ANOVAs. For baseline data, a three-way ANOVA was used, with delay as a within-subjects factor and drug assignment and schedule as between-subjects factors. Additional two-way or one-way ANOVAs and independent-samples t tests were used to probe significant interactions, when appropriate. To determine if JNJ or MPEP altered responses for the LR, separate three-way ANOVAs were conducted, with delay and dose as within-subjects factors and schedule as a between-subjects factor. A main effect of dose was probed using Dunnett’s post hoc test, and additional two-way or one-way ANOVAs and independent-samples t tests were used to probe significant interactions, when appropriate. For all ANOVA analyses, degrees of freedom were corrected using Greenhouse Geisser estimates of sphericity, if need be.
The exponential discounting function was fit to each subject’s data and is defined by the equation V = Ae−bD, where V is the subjective value of the reinforcer, A is reinforcer magnitude (i.e., responses for the LR when its delivery is immediate), b is the rate of discounting (i.e., impulsive choice), and D is the delay to delivery of the LR. The exponential function was fit to the data via nonlinear mixed effects modeling (NLME) using the NLME tool in the R statistical software package [14], with A and b as free parameters. To determine if baseline A and b parameter estimates differed across the four groups of rats, the NLME models defined schedule and drug assignment as fixed, nominal between-subjects factors, delay as a fixed, continuous within-subject factor, and subject as a random factor. To determine if JNJ or MPEP altered parameter estimates, similar NLME models were used, except that dose was defined as a fixed, nominal within-subjects factor. Separate NLME models were used to analyze each drug (JNJ and MPEP) treatment.
One rat did not respond during the 0-s delay block following JNJ (1.0 mg/kg); therefore, data for this subject were excluded from ANOVA and NLME analyses. Because one rat had 22 omissions (out of a possible 25 free-choice trials) following MPEP (10.0 mg/kg), data were excluded from both analyses. Statistical significance was defined as p < .05 in all cases, with the exception on the independent-samples t tests, in which a Bonferroni correction was used.
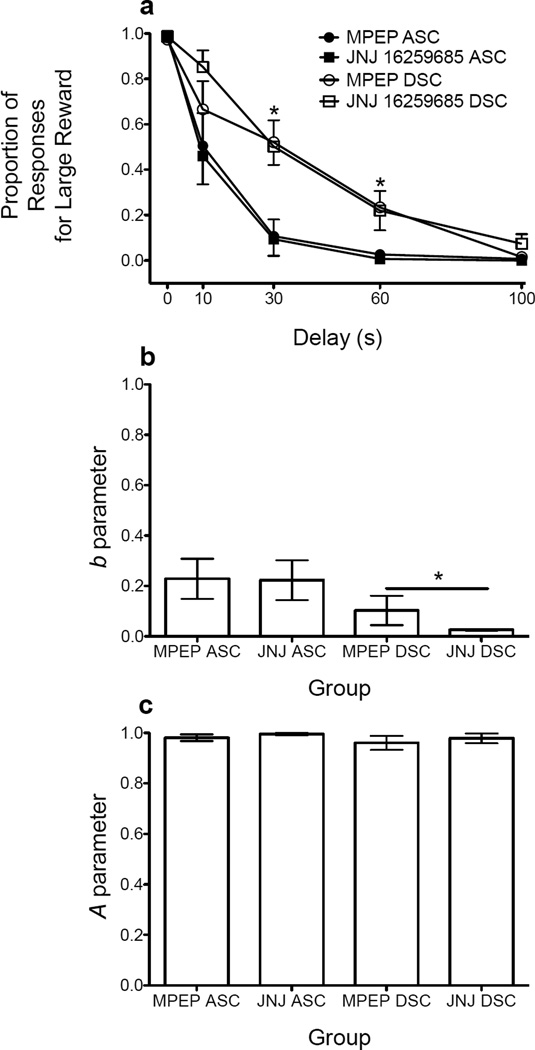
Figure 1 shows baseline data prior to the first injection of JNJ or MPEP. Results of the three-way ANOVA revealed significant main effects of delay (F(2.142, 77.099) = 160.818, p < .01) and schedule (F(1, 36) = 17.974, p < .01), as well as a significant delay × schedule interaction (F(2.142, 77.099) = 7.680, p = .001). Rats trained on the descending schedule responded more for the LR at the 30-s and 60-s delays relative to rats trained on the ascending schedule (t’s ≥ 3.743, p’s < .01; Bonferroni correction; Fig. 1a). Results of the NLME analysis showed that rats trained on the descending schedule were less sensitive to delayed reinforcement compared to rats trained on the ascending schedule (F(3, 153) = 9.529, p < .001; Fig. 1b), although A parameter estimates did not differ across each group of rats (Fig. 1c).
Figure 1.
(a) Mean (± SEM) proportion of responses for the large, delayed reinforcer, (b) mean (± SEM) b parameter estimates, and (c) mean (± SEM) A parameter estimates for each group of rats at the end of baseline. *p < .05, relative to rats trained on the ascending schedule.
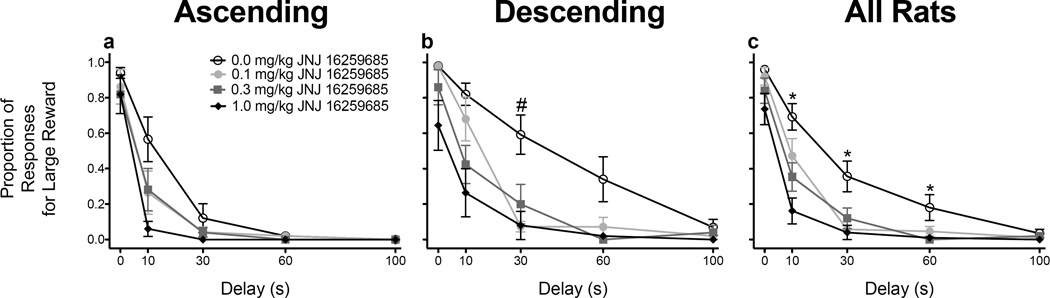
Administration of JNJ or MPEP did not significantly alter omissions (data not shown). Following JNJ administration, a three-way ANOVA revealed main effects of dose (F(3, 51) = 26.931, p < .001), delay (F(2.108, 35.843) = 155.797, p < .001), and schedule (F(1, 17) = 8.650, p = .009), as well as significant dose × schedule (F(3, 51) = 5.025, p = .004), delay × schedule (F(2.108, 35.843) = 3.935, p = .027), and dose × delay (F(5.126, 87.146) = 3.929, p = .003) interactions. Overall, rats responded less for the LR following each dose of JNJ, and rats trained on the ascending schedule (Fig. 2a) responded less for the LR relative to rats trained on the descending schedule (Fig. 2b). Additionally, JNJ (1.0 mg/kg) caused a greater percentage decrease in responding for the LR in rats trained on the descending schedule (64.765%) relative to rats trained on the ascending schedule (46.505%). Furthermore, rats trained on the descending schedule responded more for the LR at the 30-s delay relative to rats trained on the ascending schedule (t(18) = .012; Bonferroni correction). JNJ, regardless of schedule, significantly decreased responding for the LR at the 10-s, 30-s, and 60-s delays (F’s ≥ 5.324, p’s ≤ .022; Fig. 2c).
Figure 2.
Mean (± SEM) proportion of responses for the large reinforcer following each dose of JNJ 16259685 in rats trained on the ascending (n = 10; a) and descending (n = 9; b) schedules, as well as averaged across schedules (n = 19; c). *p < .05, relative to each dose of JNJ 16259685. #p < .05, relative to rats trained on the ascending schedule (averaged across dose).
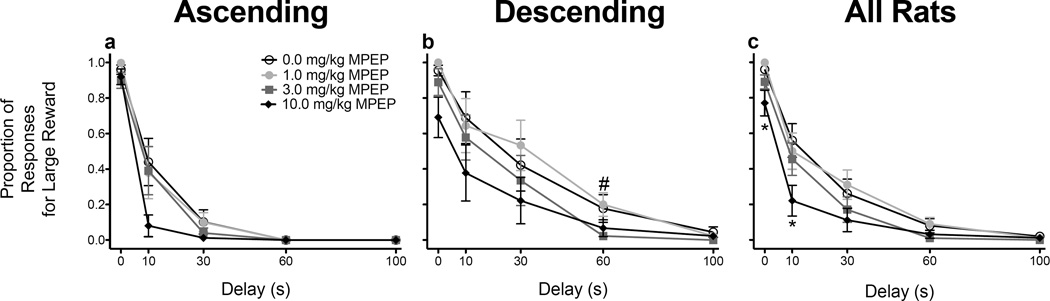
Following MPEP administration, a three-way ANOVA revealed main effects of dose (F(1.992, 31.872) = 8.590, p = .001) and delay (F(1.831, 29.299) = 94.566, p <.001), as well as significant delay × schedule (1.831, 29.299) = 3.727, p = .04) and dose × delay (F(5.215, 83.441) = 3.065, p = .013) interactions. Overall, MPEP (3.0 and 10.0 mg/kg) decreased responding for the LR. Rats trained on the ascending schedule (Fig. 3a) responded less for the LR at the 60-s delay relative to rats trained on the descending schedule (Fig. 3b; t(17) = .007; Bonferroni correction). MPEP (10.0 mg/kg), regardless of schedule, significantly decreased responding for the LR at the 0-s and 10-s delays (F’s ≥ 6.245, p’s ≤ .008; Fig 3c).
Figure 3.
Mean (± SEM) proportion of responses for the large, delayed reinforcer following each dose of MPEP in rats trained on the ascending (n = 10; a) and descending (n = 9; b) schedules, as well as averaged across schedules (n = 19; c). *p < .05, relative to vehicle. #p < .05, relative to rats trained on the ascending schedule (averaged across dose).
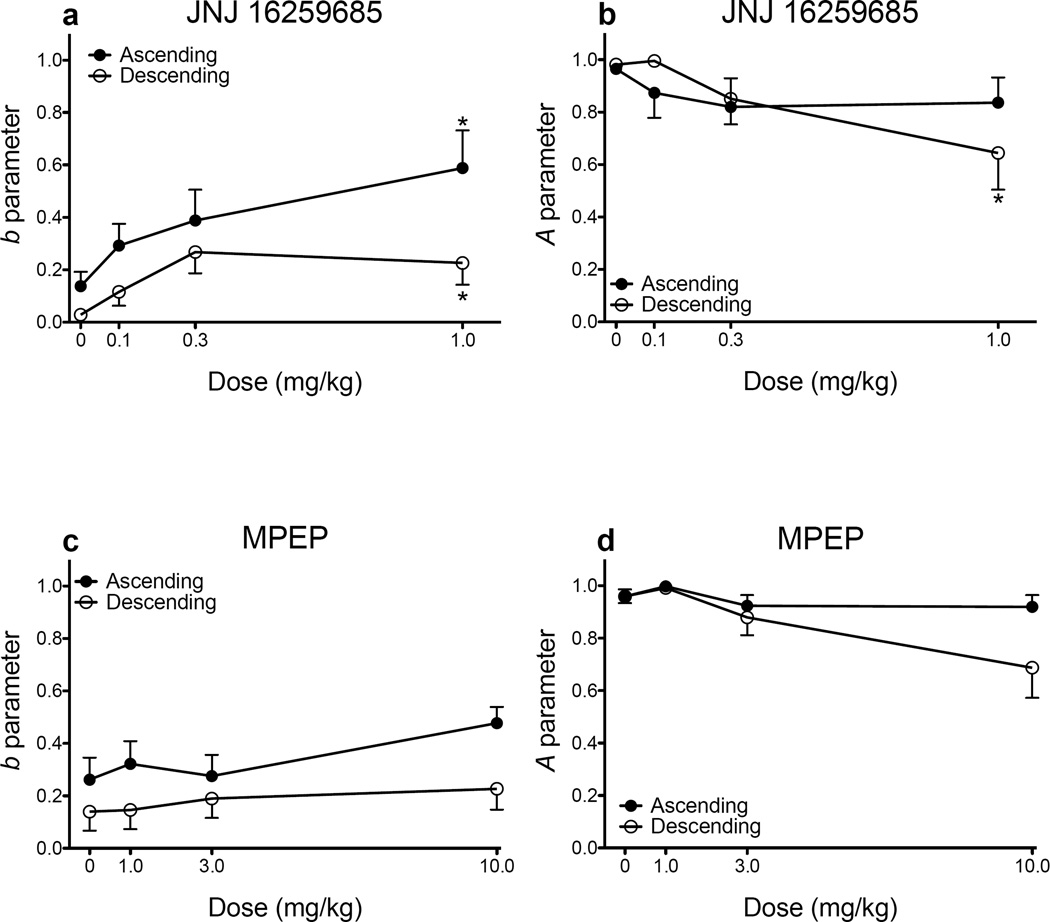
Figure 4 shows parameter estimates derived from the exponential discounting function. JNJ significantly increased sensitivity to delayed reinforcement (i.e., increased impulsive choice), regardless of which schedule was used (main effect of dose: F(3, 359) = 3.211, p = .023; Fig. 4a). However, the type of schedule used modulated the effects of JNJ on sensitivity to reinforcer magnitude (dose × schedule interaction: F(3, 359) = 3.676, p = .012; Fig. 4b). Specifically, JNJ (1.0 mg/kg) significantly decreased sensitivity to reinforcer magnitude when rats were trained on the descending schedule, but JNJ did not alter A parameter estimates in rats trained on the ascending schedule. In contrast to JNJ, MPEP did not affect sensitivity to delayed reinforcement (Fig. 4c) or sensitivity to reinforcer magnitude, although there was a trend for a dose × schedule interaction (F(3, 359) = 2.341, p = .073; Fig. 4d).
Figure 4.
Mean (± SEM) b parameter estimates (a) and mean (± SEM) A parameter estimates (b) following administration of JNJ 16259685 (ascending: n = 10; descending: n = 9). Mean (± SEM) b parameter estimates (c) and mean (± SEM) A parameter estimates (d) following administration of MPEP (ascending: n = 10; descending: n = 9). *p < .05, relative to vehicle.
The finding that JNJ increased impulsive choice in the absence of motor impairment is inconsistent with a previous report showing a decrease in delay discounting following administration of the mGluR1 antagonist JNJ 16567083 [9]. The discounting procedure used in each experiment was similar, although there were some methodological differences, such as the delays to the LR (0–100 s vs. 0–60 s) and the response requirement (FR 10 vs. FR 1) [see 6 for a discussion as to why our lab used an FR 10]. It is unlikely that procedural differences accounted for the discrepancy across studies, as the rate of discounting across each study appears to be comparable. Another factor that could account for the discrepant results is the type of animal tested in each experiment (Sprague Dawley vs. Wistar). Differential drug effects have been observed across rat strains. For example, administration of methylphenidate decreases impulsive choice in Wistar-Kyoto rats but has no effect on Sprague Dawley and Spontaneously Hypertensive rats [15]. Furthermore, Wistar rats are more sensitive to the locomotor stimulant effects of amphetamine relative to Sprague Dawley rats [16]. Finally, rats in the current study previously received injections of NMDAr ligands, whereas rats tested in [9] were drug naïve. This differential drug history may have altered how rats in the current study responded to mGluR1 antagonism. Although there are discrepancies across experiments, our work, in conjunction with Sukhotina et al. [9], indicate mGluR1 as an important mediator of impulsive choice.
Despite the discrepancy across studies, one novel finding is that the order in which delays are presented modulates the effects of JNJ on sensitivity to reinforcer magnitude, but not sensitivity to delayed reinforcement. Similar to the current results, a previous report found that administration of either amphetamine or methylphenidate differentially alters discounting in rats trained on an ascending or descending schedule [12]. Amphetamine/methylphenidate did not alter responding for the LR at 0 s in rats trained on the ascending schedule, but these drugs promoted responding for this reward alternative at larger delays [12]. Conversely, rats trained on the descending schedule were less likely to choose the LR, even at 0 s. Tanno et al. [12] argued that the discrepant results were due to increased perseveration. In the current study, this explanation does not account for the differential findings observed across schedules following JNJ administration, as rats trained on both schedules responded less for the LR. One potential explanation for the observed findings is that rats trained on the descending schedule were satiated by time they got to the 0-s delay block (the last block in this schedule). However, this explanation also does not provide a complete explanation for the discrepant results, as rats trained on the descending schedule earned fewer pellets during the first block of trials (i.e., 100-s delay: 8.800 ± 1.200) following JNJ (1.0 mg/kg) compared to when they received vehicle (15.000 ± 1.080). If satiation were occurring, one would expect rats to earn fewer pellets as the session progressed, but not at the beginning of the session.
MPEP decreased responding for the LR at the 0-s and 10-s delays. However, quantitative analyses showed that MPEP did not alter impulsive choice. Instead, MPEP tended to decrease sensitivity to reinforcer magnitude, although this was observed primarily in rats trained on the descending schedule. Although using the exponential model allows us to determine the behavioral mechanisms being altered in discounting, one limitation concerning the use of this analysis needs to be acknowledged. Because A parameter estimates tend to be at a ceiling, observing increases in this parameter can be difficult. However, this limitation did not impact the results of the study, as JNJ and MPEP decreased A parameter estimates. To prevent the ceiling effect observed with A parameter estimates, future studies can use a concurrent-chains procedure, in which animals cannot respond exclusively for the LR during any block of trials [see 17 for a discussion of this procedure]. Overall, the current results, in conjunction with previous results [10], show that mGluR5 receptors do not mediate sensitivity to reinforcer magnitude or sensitivity to delayed reinforcement.
In conclusion, this study suggests that mGluR1 is an important mediator of impulse-control disorders. Furthermore, the current results add to a growing literature showing that the order in which delays are presented can modulate the effects of drugs on discounting [e.g., 12]. When determining the underlying neurochemical processes involved in discounting, one needs to take into consideration the behavioral mechanisms of discounting, as well as the order in which delays to the LR are presented.
Acknowledgments
The current study was supported by NIH grant P20GM103436, as well as a Northern Kentucky University Faculty Project Grant and Northern Kentucky University College of Arts and Sciences Professional Development Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ainslie G. Specious reward: a behavioral theory of impulsiveness and impulse control. Psychol. Bull. 1975;82:463–496. doi: 10.1037/h0076860. [DOI] [PubMed] [Google Scholar]
- 2.Cottone P, Iemolo A, Narayan AR, Kwak J, Momaney D, Sabino V. The uncompetitive NMDA receptor antagonists ketamine and memantine preferentially increase the choice for a small, immediate reward in low-impulsive rats. Psychopharmacology. 2013;226:127–138. doi: 10.1007/s00213-012-2898-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Floresco SB, Tse MT, Ghods-Sharifi S. Dopaminergic and glutamatergic regulation of effort- and delay-based decision making. Neuropsychopharmacology. 2008;33:1966–1979. doi: 10.1038/sj.npp.1301565. [DOI] [PubMed] [Google Scholar]
- 4.Higgins GA, Silenieks LB, MacMillan C, Sevo J, Zeeb FD, Thevarkunnel S. Enhanced attention and impulsive action following NMDA receptor GluN2B-selective antagonist pretreatment. Behav. Brain Res. 2016;311:1–14. doi: 10.1016/j.bbr.2016.05.025. [DOI] [PubMed] [Google Scholar]
- 5.Yates JR, Batten SR, Bardo MT, Beckmann JS. Role of ionotropic glutamate receptors in delay and probability discounting in the rat. Psychopharmacology. 2015;232:1187–1196. doi: 10.1007/s00213-014-3747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yates JR, Gunkel BT, Rogers KK, Hughes MN, Prior NA. Effects of N-methyl-D-aspartate receptor ligands on sensitivity to reinforcer magnitude and delayed reinforcement in a delay-discounting procedure. Psychopharmacology. doi: 10.1007/s00213-016-4469-5. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim AL, Taylor DA, Malone DT. Consequences of early life MK-801 administration: long-term behavioural effects and relevance to schizophrenia research. Behav. Brain Res. 2012;227:276–286. doi: 10.1016/j.bbr.2011.10.052. [DOI] [PubMed] [Google Scholar]
- 8.Harder JA, Aboobaker AA, Hodgetts TC, Ridley RM. Learning impairments induced by glutamate blockade using dizocilpine (MK-801) in monkeys. Br. J. Pharmacol. 1998;125:1013–1018. doi: 10.1038/sj.bjp.0702178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sukhotina IA, Dravolina OA, Novitskaya Y, Zvartau EE, Danysz W, Bespalov AY. Effects of mGlu1 receptor blockade on working memory, time estimation, and impulsivity in rats. Psychopharmacology. 2008;196:211–220. doi: 10.1007/s00213-007-0953-2. [DOI] [PubMed] [Google Scholar]
- 10.Isherwood SN, Pekcec A, Nicholson JR, Robbins TW, Dalley JW. Dissociable effects of mGluR5 allosteric modulation on distinct forms of impulsivity in rats: interactions with NMDA receptor antagonism. Psychopharmacology. 2015;232:3327–3344. doi: 10.1007/s00213-015-3984-0. [DOI] [PubMed] [Google Scholar]
- 11.Ho M-Y, Mobini S, Chian T-J, Bradshaw CM, Szabadi E. Theory and method in the quantitative analysis of “impulsive choice” behaviour: implications for psychopharmacology. Psychopharmacology. 1999;146:362–372. doi: 10.1007/pl00005482. [DOI] [PubMed] [Google Scholar]
- 12.Tanno T, Maguire DR, Hensen C, France CP. Effects of amphetamine and methylphenidate on delay discounting in rats: Interactions with order of delay presentation. Psychopharmacology. 2014;231:85–95. doi: 10.1007/s00213-013-3209-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Besheer J, Faccidomo S, Grondin JJ, Hodge CW. Regulation of motivation to self-administer ethanol by mGluR5 in alcohol-preferring (P) rats. Alcohol Clin. Exp. Res. 2008;32:209–221. doi: 10.1111/j.1530-0277.2007.00570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinheiro J, Bates D, DebRoy S, Sarkar D. Linear and nonlinear mixed effects models. Vienna, Austria: R Foundation for Statistical Computing; 2007. pp. 1–89. [Google Scholar]
- 15.Wooters TE, Bardo MT. Methylphenidate and fluphenazine, but not amphetamine, differentially affect impulsive choice in Spontaneously Hypertensive, Wistar-Kyoto and Sprague-Dawley rats. Brain Res. 2011;1396:43–53. doi: 10.1016/j.brainres.2011.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDermott C, Kelly JP. Comparison of the behavioural pharmacology of the Lister-Hooded with 2 commonly utilised albino rat strains. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32:1816–1823. doi: 10.1016/j.pnpbp.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Aparicio CF, Elcoro M, Alonso-Alvarez B. A long-term study of the impulsive choices of Lewis and Fischer 344 rats. Learn. Behav. 2015;43:251–271. doi: 10.3758/s13420-015-0177-y. [DOI] [PubMed] [Google Scholar]