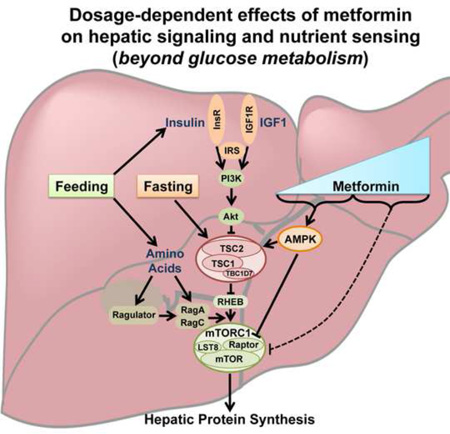
Abstract
Metformin is the most widely prescribed drug for the treatment of type-2 diabetes. However, knowledge of the full effects of metformin on biochemical pathways and processes in its primary target tissue, the liver, is limited. One established effect of metformin is to decrease cellular energy levels. The AMP-activated protein kinase (AMPK) and mechanistic target of rapamycin (mTOR) complex 1 (mTORC1) are key regulators of metabolism that are respectively activated and inhibited in acute response to cellular energy depletion. Here we show that metformin robustly inhibits mTORC1 in mouse liver tissue and primary hepatocytes. Using mouse genetics, we find that at the lowest concentrations of metformin that inhibit hepatic mTORC1 signaling, this inhibition is dependent on AMPK and the tuberous sclerosis complex (TSC) protein complex (TSC complex). Finally, we show that metformin profoundly inhibits hepatocyte protein synthesis in a manner that is largely dependent on its ability to suppress mTORC1 signaling.
Graphical Abstract
INTRODUCTION
Type-2 diabetes is a growing global epidemic affecting approximately 9% of the adult population worldwide (World Health Organization, 2016). The first-line treatment and most prescribed anti-diabetes drug is the biguanide metformin, which lowers blood glucose levels and reduces cardiovascular events in patients with type-2 diabetes (UK Prospective Diabetes Study Group, 1998; Inzucchi et al., 1998). Due to the well-established effects of metformin on hepatic glucose production (Johnson et al., 1993; Perriello et al., 1994) and the high expression level of the metformin transporter OCT1 in hepatocytes (Grundemann et al., 1994; Zhang et al., 1997), the liver is considered to be one of its primary target tissues. However, our understanding of the molecular and cellular targets influencing the physiological effects of metformin is far from complete.
One established direct target of metformin is mitochondrial complex I, which metformin inhibits, thereby decreasing cellular respiration and ATP levels (El-Mir et al., 2000; Owen et al., 2000; Zhou et al., 2001). Among other effects, metformin-induced energy depletion can stimulate the AMP-activated protein kinase (AMPK), and does so prominently in the liver (Zhou et al., 2001; Shaw et al. 2005). Through multiple downstream effectors, AMPK promotes ATP-producing catabolic processes while inhibiting ATP-consuming anabolic processes (Hardie and Ashford, 2014). Notably, compounds that activate AMPK inhibit the mechanistic target of rapamycin complex 1 (mTORC1) (Bolster et al., 2002; Krause et al., 2002; Dubbelhuis and Meijer, 2002), a major driver of anabolic metabolism that is aberrantly activated under conditions of hyperinsulinemia and obesity and is believed to contribute to the development of insulin resistance (Um et al., 2004; Khamzina et al., 2005, Howell and Manning, 2011). However, whether metformin treatment inhibits hepatic mTORC1 signaling, along with the mechanisms and consequences, is unknown.
mTORC1 is a nutrient-sensitive multiprotein complex whose core essential components include the protein kinase mTOR and scaffolding protein Raptor (Laplante and Sabatini, 2012). This protein kinase complex serves to link nutrient and energy signals, both local and systemic, to key anabolic processes that utilize nutrients and energy to produce macromolecules, including proteins, lipids, and nucleotides (Dibble and Manning, 2013; Howell et al., 2013). mTORC1 senses these upstream signals through two systems of small G proteins, the Rag and Rheb GTPases. Intracellular amino acids, and perhaps glucose, signal to mTORC1 through a pathway influencing engagement of the Rag proteins with mTORC1 (Bar-Peled and Sabatini, 2014; Efeyan et al., 2013). A second layer of regulation integrates multiple signals, including growth factor- and insulin-stimulated signaling pathways that influence the nucleotide binding state of Rheb, which in its GTP-bound state is an essential activator of mTORC1. These signals are largely perceived through a protein complex composed of the tuberous sclerosis complex proteins (TSC1 and TSC2) and the protein TBC1D7 (collectively referred to as the TSC complex), which functions as a GTPase-activating protein (GAP) for Rheb, thereby inhibiting the Rheb-dependent activation of mTORC1 (Dibble and Manning, 2013). Thus many signals that turn off mTORC1, such as growth factor withdrawal, do so by promoting Rheb inhibition through the TSC complex. As such, loss of function of any component of the TSC complex leads to sustained mTORC1 activity under conditions that would normally inhibit mTORC1 through this protein complex, such as in the liver during fasting (Sengupta et al., 2010; Yecies et al., 2011).
The biosynthetic processes stimulated by mTORC1, such as protein synthesis, are heavily dependent on ATP, and multiple mechanisms have evolved to ensure mTORC1 is only active under energy charged conditions. Energy stress-mediated activation of AMPK provides one such mechanism of mTORC1 regulation. AMPK can directly phosphorylate TSC2 on S1387, thereby promoting its inhibition of Rheb and mTORC1 (Inoki et al., 2003; Shaw et al., 2004). In parallel, AMPK also exerts a direct inhibitory signal on mTORC1 through the phosphorylation of S722 and S792 on Raptor (Gwinn et al., 2008). Additional mechanisms of mTORC1 inhibition in response to perturbations in cellular energy levels have been suggested in various studies (Dibble and Manning, 2013), including those that are independent of AMPK or the TSC complex (Ben-Sahra et al., 2011; Dennis et al., 2001; Kalender et al., 2010; Kim et al., 2013; Liu et al., 2014; Sofer et al., 2005; Zheng et al., 2011). However, the relative importance of different mechanisms inhibiting mTORC1 is likely influenced by the particular form, degree and duration of energy stress, as well as the cell or tissue type.
Conflicting studies exist regarding how metformin inhibits mTORC1 signaling, with some concluding that the drug acts through AMPK and the TSC complex (Dowling et al., 2007) and others stating that the regulation is entirely independent of this mechanism (Ben-Sahra et al., 2011; Kalender et al., 2010). However, these and other studies have been restricted to cancer cells and mouse embryo fibroblasts and have often used high doses (e.g., ≥10 mM) and durations (e.g., ≥24 hours) of metformin treatment. Surprisingly, the effects of metformin on mTORC1 signaling, including the mechanism of regulation and downstream consequences, have not been studied in the physiological context of the liver – the target organ of metformin treatment for its primary indication, type-2 diabetes. Here we demonstrate that metformin strongly inhibits mTORC1 signaling in mouse liver and primary mouse and human hepatocytes. Using mouse genetics, we reveal a biphasic response to metformin for its suppression of mTORC1. At the lowest doses that inhibit mTORC1, metformin requires AMPK and the TSC complex for this effect, whereas at higher doses AMPK and TSC complex-independent mechanisms are revealed. Finally, we demonstrate that metformin strongly attenuates hepatic protein synthesis through a mechanism requiring its inhibitory effects on mTORC1.
RESULTS AND DISCUSSION
Hepatic mTORC1 signaling is inhibited by metformin, which at the lowest inhibitory dose acts through AMPK
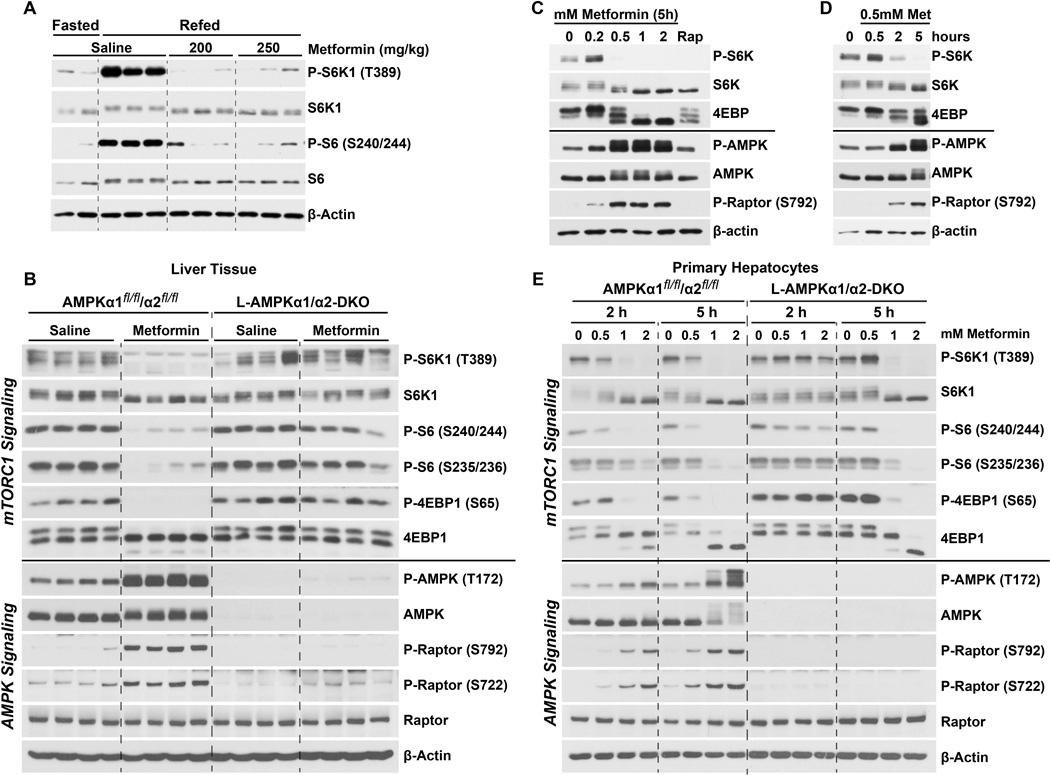
Hepatic mTORC1 signaling is acutely activated by feeding (Figure 1A) (Yecies et al., 2011). Therefore, to determine the pharmacological effects of metformin on mTORC1 activation in the mouse liver, we used an established fasting-refeeding paradigm to obtain consistently elevated levels of mTORC1 activity prior to treatment. Mice were fasted overnight followed by refeeding prior to a 1 h administration of metformin or vehicle. It has been suggested that the standard dose of metformin used in humans for the treatment of type-2 diabetes (~20 mg/kg) is similar to the 250 mg/kg dose typically used in mice, with both giving plasma concentrations in the 10 µM range (Foretz et al., 2014; Memmott et al., 2010). We found that plasma metformin concentrations with dosing of 200 or 250 mg/kg in our experimental paradigm were ~35 µM (Figure S1A), which is consistent with previous studies using the same delivery method and acute treatment (1 to 2 hours) with doses ranging from 125 to 350 mg/kg (Dowling et al., 2016; Memmott et al., 2010; Chandel et al., 2016). In these previous studies, metformin concentrations rapidly decreased after the first hour to levels well below the plasma concentration of 10 µM measured in humans. Thus, the mice in our study experienced a pharmacological range of metformin similar to humans. At both 200 and 250 mg/kg doses of metformin, liver mTORC1 activity was strongly suppressed, as measured by phosphorylation of the mTORC1-specific substrate ribosomal S6 kinase 1 (S6K1) and its downstream target S6 (Figure 1A).
Figure 1. Metformin suppresses hepatic mTORC1 signaling in an AMPK-dependent manner.
(A) Male mice (age 10 weeks) were fasted overnight then left unfed (Fast; n=2) or refed for 6 h, with saline, 200 or 250 mg/kg metformin treatment (n=3 per treatment) for the last 1 h. See Figure S1 for measurements of plasma metformin concentrations.
(B) Female AMPKα1fl/fl/α2fl/fl or L-AMPKα1/α2-DKO mice (age 6 mos) were fasted overnight, refed for 2 h and treated with saline or 250 mg/kg metformin for the last 1 h (n=4 per treatment). See Figure S1 for supporting data with a male cohort age 12 weeks.
(C,D) Primary hepatocytes were treated (C) for 5 h with indicated doses of metformin or 20 nM rapamycin (Rap) or (D) with 0.5 mM metformin for the indicated times.
(E) Primary hepatocytes from AMPKα1fl/fl/α2fl/fl or L-AMPKα1/α2-DKO mice were treated with the indicated concentrations of metformin for 2 or 5 h.
To determine the mechanism of metformin’s inhibition of hepatic mTORC1 signaling, we compared the response of mice with tamoxifen-inducible, liver-specific deletion of both isoforms of the AMPK catalytic subunit, AMPKα1 and AMPKα2, to littermate controls. Control mice (AMPKα1fl/fl/α2fl/fl) or those lacking AMPK (L-AMPKα1/α2-DKO) were fasted overnight, refed for 2 h and treated with saline or metformin (250 mg/kg) for the last hour. In control mice, metformin treatment strongly induced hepatic AMPK activation, as scored by the activating phosphorylation on AMPK-T172 and the AMPK-dependent phosphorylation sites on Raptor, S722 and S792 (Figure 1B). As above, metformin inhibited multiple markers of mTORC1 signaling in the livers of control mice, including phosphorylation of S6K1, S6 and eukaryotic initiation factor 4E (eIF4E)-binding protein 1 (4E-BP1), as indicated by phosphorylation of 4E-BP1-S65 and corresponding mobility shifts. However, the L-AMPKα1/α2-DKO mice exhibited sustained liver mTORC1 signaling upon metformin treatment, with a corresponding loss of induction of AMPK and Raptor phosphorylation (Figure 1B). It should be noted that some dampening of mTORC1 activity can be detected in metformin-treated L-AMPKα1/α2-DKO livers, as observed in lighter immunoblot exposures for phosphorylation of S6K1, which is the direct substrate most sensitive to mTORC1 inhibition (Kang et al., 2013), and mobility shifting of 4E-BP1 (Figure S1B). However, mTORC1 signaling in AMPK-deficient livers is consistently resistant to metformin relative to controls.
Primary hepatocytes were isolated to determine whether the effects of metformin on liver mTORC1 signaling were cell autonomous, and to more closely examine the dose and time-dependence of the response. While the underlying cause remains a point of debate, it has been established that cells grown in culture, under supraphysiological levels of nutrients and oxygen, require much higher concentrations of metformin to elicit responses similar to those seen in vivo (He and Wondisford, 2015; Dowling et al., 2016; Memmott et al., 2010; Chandel et al., 2016). To this end, neither AMPK activation nor mTORC1 inhibition was observed in hepatocyte cultures treated with metformin doses of 0.2 mM or less. However, 0.5 mM metformin resulted in robust activation of AMPK at 2 h, which was concomitant with attenuation of mTORC1 signaling (Figure 1C,D). Next, primary hepatocytes from control and L-AMPKα1/α2-DKO mice were isolated and compared for their response to this range of doses and durations of metformin treatment. Hepatocytes from control mice responded to metformin in a dose-dependent manner, with all markers of mTORC1 activity decreasing as AMPK activity increased (Figure 1E). Similar to the in vivo observations, primary hepatocytes isolated from L-AMPK α1/α2-DKO mice were resistant to metformin-induced suppression of mTORC1 at all doses after 2 h treatment. However, at 5 h, L-AMPKα1/α2-DKO hepatocytes remained resistant to metformin for suppression of mTORC1 signaling only at lower doses, with mTORC1 being fully inhibited at higher doses of metformin, even in the absence of AMPK (Figure 1E). These data demonstrate that at the lowest doses and durations of metformin that inhibit mTORC1 signaling in hepatocytes, the compound acts through AMPK activation. However, AMPK-independent mechanisms of metformin action on mTORC1 also exist, which are revealed at higher doses and prolonged treatment.
Lower doses of metformin inhibit hepatic mTORC1 signaling in a manner that is dependent on the TSC complex
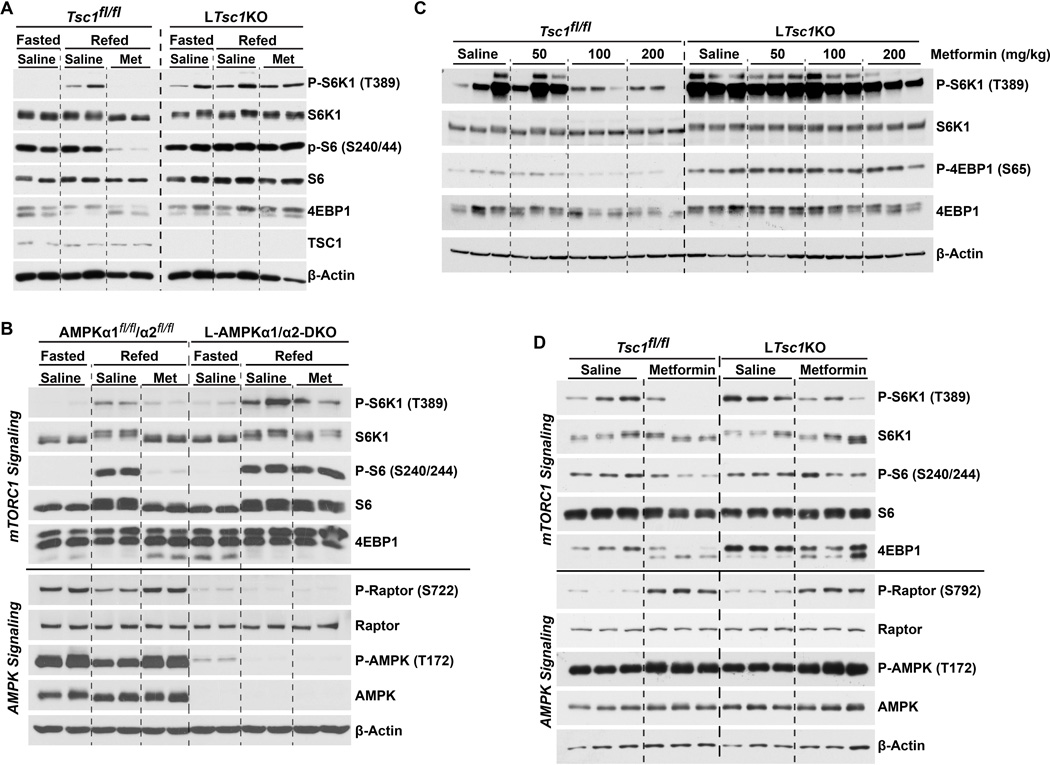
One established mechanism whereby AMPK can inhibit mTORC1 is through activation of the TSC complex (Inoki et al., 2003). To directly address a potential role for the TSC complex in the metformin-mediated inhibition of mTORC1 in the liver, we utilized LTsc1KO mice with hepatocyte-specific deletion of Tsc1, encoding an essential core component of the complex. As previously demonstrated (Yecies et al., 2011), the LTsc1KO mice displayed sustained hepatic mTORC1 signaling under fasting conditions, at levels similar to refed littermate Tsc1fl/fl controls (Figure 2A). Interestingly, in this cohort of male mice, metformin (200 mg/kg) acutely suppressed mTORC1 signaling in livers of control mice, but mTORC1 activity was largely resistant to metformin in the livers of LTsc1KO mice (Figure 2A). Unlike the TSC complex, AMPK is not required for suppression of mTORC1 signaling in the fasted state (Figure 2B), but L-AMPKα1/α2-DKO livers again showed sustained mTORC1 signaling with metformin treatment. Comparison of a cohort of female mice treated with increasing doses of metformin revealed suppression of mTORC1 signaling at doses as low as 100 mg/kg in controls, while the LTsc1KO levels showed no reduction in mTORC1 signaling at this dose and only a modest reduction at 200 mg/kg (Figure 2C). An additional experiment treating a female cohort with 250 mg/kg further demonstrated that mTORC1 signaling was resistant to metformin in the livers of LTsc1KO mice, but a partial decrease was observed (Figure 2D). Importantly, the LTsc1KO mice responded similarly to control mice with respect to metformin-induced activation of AMPK and its phosphorylation of Raptor. This result is consistent with the TSC complex functioning downstream of AMPK in the suppression of mTORC1 signaling by metformin, with Raptor phosphorylation providing an additional mechanism of mTORC1 inhibition in the absence of the TSC complex.
Figure 2. Mice with liver-specific disruption of the TSC complex display metformin-resistant mTORC1 signaling.
(A) Male TSC1fl/fl or LTsc1KO mice (age 10 weeks) were fasted overnight then refed for 6 h and treated with saline or metformin for the last 1 h (n=2 per condition).
(B) Male AMPKα1fl/fl/α2fl/fl or L-AMPKα1/α2-DKO (age 5 mos) mice were treated as in (A).
(C) Female TSC1fl/fl or LTsc1KO mice (age 12 week) were treated as in (A) with saline, 50, 100, or 200 mg/kg metformin (n=3 per condition).
(D) Female mice (age 14 weeks) treated as in (A) with saline or 250 mg/kg metformin (n=3 per condition).
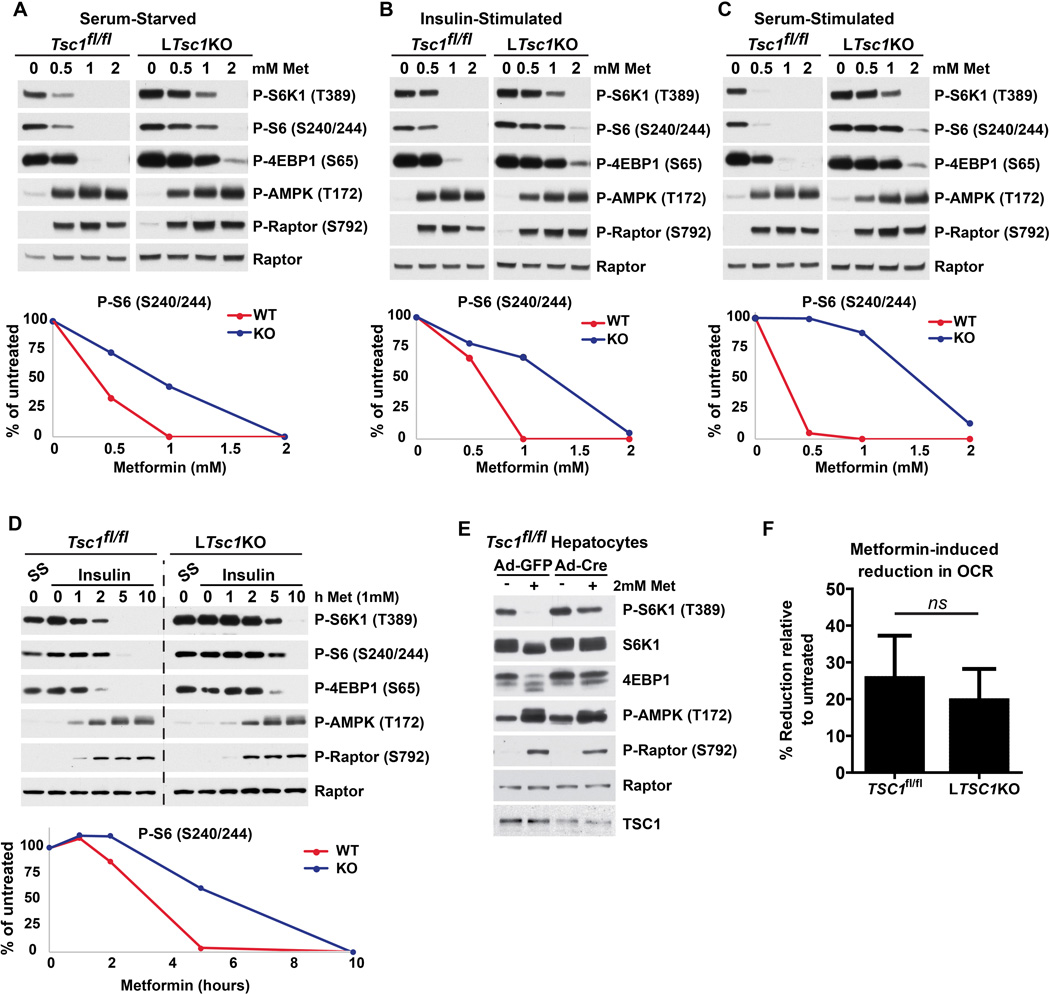
To more closely investigate potential dose-dependent effects of metformin on mTORC1 signaling in a cell-autonomous manner, we compared the response of primary hepatocytes isolated from control or LTsc1KO mice. To control for variations in response stemming from culture conditions, these comparisons were performed under serum-starved (Figure 3A), insulin-stimulated (Figure 3B), and serum-stimulated (Figure 3C) conditions. Although the pharmacodynamics varied somewhat, all three conditions showed similar results. Primary hepatocytes from control mice responded to metformin in a dose-dependent manner, with mTORC1 activity decreasing concomitantly with increased AMPK activity (Figure 3A–C). Similar to the in vivo findings and results with L-AMPK α1/α2-DKO hepatocytes, mTORC1 signaling was resistant to metformin in hepatocytes derived from LTsc1KO mice at lower doses of metformin (0.5–1 mM). However, at 2 mM metformin mTORC1 was inhibited in both control and LTsc1KO hepatocytes, supporting the existence of a TSC complex-independent mechanism at higher concentrations (Figure 3A–C). The effects of metformin on hepatocyte mTORC1 signaling were also time-dependent, with shorter durations (<5 h at 1 mM) having very little effect in LTsc1KO hepatocytes compared to controls, while at 10 h of treatment mTORC1 was inhibited even in the absence of the TSC complex (Figure 3D). These data indicate that AMPK and the TSC complex are required to inhibit hepatic mTORC1 signaling acutely and at the lowest effective inhibitory doses of metformin, while more potent treatments engage additional mechanisms that bypass this first line of response.
Figure 3. Low dose metformin suppresses hepatocyte mTORC1 signaling in a TSC complex-dependent manner.
(A–D) Primary hepatocytes from TSC1fl/fl or LTsc1KO mice were serum-starved overnight and treated (A–C) for 5 h with increasing doses of metformin together with (A) no stimulation, (B) 100 nM insulin, or (C) 5% FBS, or (D) with a time course of treatment with 1 mM metformin following a 15-min pretreatment with 100 nM insulin. Quantitation of P-S6 band intensities via densitometry is shown in graphical format below each panel.
(E) Primary hepatocytes from TSC1fl/fl mice were infected with adenovirus expressing GFP or Cre 36 h prior to treatment with 2 mM metformin for 5 h.
(F) Oxygen consumption rates (OCR) of primary hepatocytes from TSC1fl/fl or LTsc1KO mice were measured for 1 h in the presence of 1 mM metformin and are graphed as the mean ± SEM OCR as a percent of untreated samples measured in parallel (n=4 experiments).
We observed similar results in hepatocytes with acute deletion of Tsc1. We isolated Tsc1fl/fl hepatocytes and treated them with either adenovirus expressing GFP or Cre to delete Tsc1 in an isogenic setting. Here, even partial loss of TSC1 sustained mTORC1 signaling after metformin treatment (Figure 3E). One possible explanation for the apparent higher degree of metformin resistance for mTORC1 regulation in these hepatocytes with acute deletion of Tsc1 is that adaptive mechanisms to cope with sustained mTORC1 signaling might be induced in the LTsc1KO livers. Importantly, in this experiment and the others presented (Figures 3A–D), the induction of AMPK activity by metformin was similar between Tsc1-deleted and control hepatocytes. Consistent with the similarity in AMPK activation, there was no significant difference in the effects of metformin on oxygen consumption rates between control and LTsc1KO hepatocytes (Figure 3F). Therefore, hepatocytes lacking the TSC complex remain responsive to metformin but lose the inhibitory signal from low-dose metformin to mTORC1.
Metformin inhibits protein synthesis in hepatocytes through the TSC complex
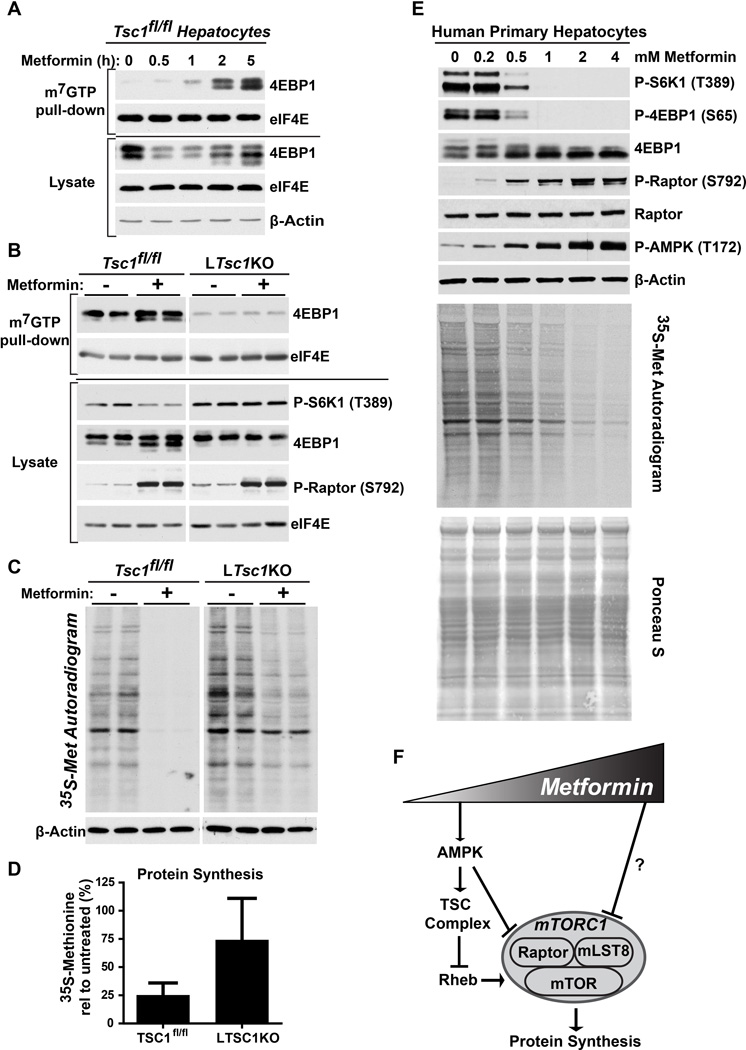
Aside from the inhibition of hepatic gluconeogenesis, the physiological effects of metformin in the liver are poorly understood. The acute induction of cap-dependent translation and, ultimately, a global increase in protein synthesis are key functions of mTORC1 signaling. In cancer cells, metformin has been found to have similar effects to mTOR inhibitors for the suppression of protein synthesis (Larsson et al., 2012). Thus, we analyzed the effects of metformin on events controlling cap-dependent translation initiation and protein synthesis in hepatocytes. The 5’-end of mRNAs is capped with a 7-methylguanosine (m7GTP) moiety that is bound by the translation initiation protein eIF4E, which nucleates a translation initiation complex. When mTORC1 signaling is inhibited, its direct downstream target 4E-BP1 is dephosphorylated and able to bind to eIF4E at the m7GTP cap, thereby blocking translation initiation. Consistent with its suppression of mTORC1 signaling in hepatocytes, metformin treatment induced binding of 4E-BP1 to eIF4E on m7GTP-agrose beads, which mimic the mRNA 5’-cap structure (Figure 4A). Importantly, this effect was dependent on the TSC complex and inhibition of mTORC1, as metformin failed to induce association of 4EBP1 with m7GTP-bound eIF4E in LTsc1KO hepatocytes (Figure 4B). This result is consistent with sustained phosphorylation of 4EBP1 and other markers of mTORC1 activity upon metformin treatment of these cells.
Figure 4. Metformin inhibits protein synthesis in hepatocytes through the TSC complex.
(A) Primary hepatocytes from TSC1fl/fl mice were treated with 1 mM metformin for the indicated times, and mRNA 5’-cap-binding complexes were isolated from cell lysates with m7GTP-agarose and analyzed by immunoblotting.
(B) Primary hepatocytes from TSC1fl/fl and LTsc1KO mice were treated with 1 mM metformin for 5 h and analyzed as in (A).
(C and D) Effects of metformin on hepatocyte protein synthesis. Primary hepatocytes from TSC1fl/fl and LTsc1KO mice were pretreated with insulin for 20 min followed by treatment in the presence or absence of 1 mM metformin for 5 h, with a pulse label of [35S]-methionine for the final 20 min. A representative autoradiogram from 5 independent experiments is shown. (D) Individual lanes from autoradiograms were quantified by densitometry and are graphed as mean ± SEM % incorporation relative to untreated cells (n=5 independent experiments). *P < 0.05 by Student’s two-tailed t-test.
(E) Primary human hepatocytes were treated with the indicated doses of metformin for 5 h and, for assaying protein synthesis, were radiolabeled as in (C). Signaling was assessed by immunoblots, protein synthesis by autoradiogram, and total protein for this assay by Ponceau S staining.
(F) Model of dose-dependent, differential regulation of mTORC1 in response to increasing metformin concentrations.
To more directly measure the relative effects of metformin on active protein synthesis in control and LTsc1KO hepatocytes, the acute incorporation of 35S-methionine into protein over 20 minutes was assessed. Metformin treatment had profound inhibitory effects on protein synthesis in control hepatocytes, resulting in a 75% decrease (Figure 4C,D). However, LTsc1KO hepatocytes, with sustained mTORC1 signaling, displayed a more modest reduction in protein synthesis of about 25% in response to metformin. Primary human hepatocytes responded remarkably similar to mouse hepatocytes, with doses of metformin greater than 0.2 mM activating AMPK with a corresponding decrease in mTORC1 signaling, accompanied by a dose-dependent decrease in protein synthesis (Figure 4E). Thus, metformin has strong inhibitory effects on hepatocyte protein synthesis, in large part, through AMPK and TSC complex-dependent inhibition of mTORC1 signaling.
Conclusions
This study demonstrates that metformin is a potent inhibitor of mTORC1 signaling and its control of protein synthesis in the liver. Like other biological effects of metformin (Foretz et al., 2014; He and Wondisford, 2015), the mechanisms influencing hepatic mTORC1 activity vary with dose. Our data reveal a biphasic response to metformin, where low doses inhibit mTORC1 through AMPK and the TSC complex and higher doses act through alternative mechanisms (Figure 4F). Importantly, even at higher doses of metformin, the acute inhibition of mTORC1 requires AMPK and the TSC complex, indicating that these proteins represent the primary signaling mechanism involved. While loss of either AMPK or the TSC complex from the liver result in qualitatively similar responses, AMPK-deficient livers are somewhat more resistant to metformin for mTORC1 inhibition. This is consistent with AMPK suppressing mTORC1 activation through both the TSC complex (Inoki et al., 2003) and phosphorylation of Raptor (Gwinn et al., 2008). The delayed induction of AMPK- and TSC complex-independent mechanisms revealed by prolonged treatment with metformin, suggests that the alternative regulation might involve induction of transcriptional control mechanisms. In addition to providing mechanistic insights into the effects of metformin on key pathways and functions in the liver, our findings regarding the differential dose-dependent effects of metformin uncover a likely explanation for the discrepancy between previous studies with conflicting conclusions regarding the mechanism of mTORC1 inhibition by metformin. In subsequent studies, it will be important to determine the physiological consequences of hepatic mTORC1 inhibition for the influence of pharmacological doses of metformin on systemic metabolism.
EXPERIMENTAL PROCEDURES
See Supplemental Experimental Procedures for extended methods.
Mouse Studies
Tsc1fl/fl and LTsc1KO mice on a C57BL/6J background were described previously (Yecies et al., 2011), with LTsc1KO mice generated from crosses between Tsc1fl/fl and Alb-Cre Tsc1fl/+ mice, through the albumin-Cre transgene (Postic and Magnuson, 2000; Kwiatkowski et al., 2002). Homozygous AMPKα1fl/fl and AMPKα2fl/fl mice previously described (Hasenour et al., 2014) on an FVB genetic background were bred with or without albumin-CreERT2 expression (Imai et al., 2000) were crossed to generate wild type controls (AMPKα1fl/fl/α2fl/fl) or inducible liver-specific deletion of both catalytic subunits of AMPK (L-AMPKα1/α2-DKO). The final cohorts were generated by tamoxifen (1 mg) treatment every other day for a total of 3 i.p. injections. Experiments, or primary hepatocyte isolation, were carried out approximately 2 weeks post-tamoxifen injection. Detailed methods are provided in Supplemental Experimental Procedures.
Primary Hepatocytes
Primary mouse hepatocytes were isolated as previously described (Yecies et al., 2011), and cryopreserved human hepatocytes were obtained from ThermoFisher (Lot # Hu8150). Oxygen consumption rates (OCR) were measured on an XF24 Seahorse extracellular flux analyzer (Seahorse Bioscience, 100867-100). Detailed methods are provided in Supplemental Experimental Procedures.
Protein Extraction, Immunoblotting, and Protein Synthesis Assays
Upon euthanization, liver tissue was harvested immediately and flash frozen in liquid nitrogen. 50–100 mg pieces of frozen liver tissue were dounce-homogenized in lysis buffer. Detailed methods regarding cell and tissue lysis, immunoblotting, antibodies used, and protein synthesis assays are provided in Supplemental Experimental Procedures
Supplementary Material
Acknowledgments
We thank Benoit Viollet and David Kwiatkowski for genetic mouse models and Daniel Garcia, Liliana Vera, and Amanda Hutchins for technical assistance. This work was supported, in part, by a Senior Scholar in Aging Award from the Ellison Medical Foundation (B.D.M.), NIH grants F32-DK095508 (J.J.H.), F30-DK112604 (M.J.K.), R01-DK080425 (R.J.S.), and P01-CA120964 (R.J.S. and B.D.M.), and a fellowship from the George E. Hewitt Foundation for Medical Research (K.H.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL INFORMATION
Supplemental Information includes Supplemental Experimental Procedures and one figure.
AUTHOR CONTRIBUTIONS
J.J.H. and K.H. guided and performed most experiments, with assistance from M.T., G.T., D.V.R, and G.H.. M.J.K. and A.S. measured plasma metformin via LC-MS. J.J.H., K.H., R.J.S., and B.D.M. conceived and designed the project, which was supervised by R.J.S. and B.D.M..
REFERENCES
- Bar-Peled L, Sabatini DM. Regulation of mTORC1 by amino acids. Trends in cell biology. 2014;24:400–406. doi: 10.1016/j.tcb.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Sahra I, Regazzetti C, Robert G, Laurent K, Le Marchand-Brustel Y, Auberger P, Tanti J-FF, Giorgetti-Peraldi S, Bost F. Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer research. 2011;71:4366–4372. doi: 10.1158/0008-5472.CAN-10-1769. [DOI] [PubMed] [Google Scholar]
- Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J. Biol. Chem. 2002;277:23977–23980. doi: 10.1074/jbc.C200171200. [DOI] [PubMed] [Google Scholar]
- Chandel NS, Avizonis D, Reczek CR, Weinberg SE, Menz S, Neuhaus R, Christian S, Haegebarth A, Algire C, Pollak M. Are Metformin Doses Used in Murine Cancer Models Clinically Relevant? Cell Metab. 2016;23:569–570. doi: 10.1016/j.cmet.2016.03.010. [DOI] [PubMed] [Google Scholar]
- Dennis PB, Jaeshke A, Saitoh M, Fowler B, Kozma SC, Thomas G. Mammalian TOR: a homeostatic ATP sensor. Science. 2001;294:1102–1105. doi: 10.1126/science.1063518. [DOI] [PubMed] [Google Scholar]
- Dibble CC, Manning BD. Signal integration by mTORC1 coordinates nutrient input with biosynthetic output. Nature cell biology. 2013;15:555–564. doi: 10.1038/ncb2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling RJO, Zakikhani M, Fantus IG, Pollak M, Sonenberg N. Metformin Inhibits Mammalian Target of Rapamycin Dependent Translation Initiation in Breast Cancer Cells. Cancer Res. 2007;67:10804–10812. doi: 10.1158/0008-5472.CAN-07-2310. [DOI] [PubMed] [Google Scholar]
- Dowling RJ, Lam S, Bassi C, Mouaaz S, Aman A, Kiyota T, Al-Awar R, Goodwin PJ, Stambolic V. Metformin Pharmacokinetics in Mouse Tumors: Implications for Human Therapy. Cell Metab. 2016;23:567–568. doi: 10.1016/j.cmet.2016.03.006. [DOI] [PubMed] [Google Scholar]
- Dubbelhuis PF, Meijer AJ. Hepatic amino acid-dependent signaling is under the control of AMP-dependent protein kinase. FEBS Lett. 2002;521:39–42. doi: 10.1016/s0014-5793(02)02815-6. [DOI] [PubMed] [Google Scholar]
- Efeyan A, Zoncu R, Chang S, Gumper I, Snitkin H, Wolfson RL, Kirak O, Sabatini DD, Sabatini DM. Regulation of mTORC1 by the Rag GTPases is necessary for neonatal autophagy and survival. Nature. 2013;493:679–683. doi: 10.1038/nature11745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Mir MY, Nogueira V, Fontaine E, Averet N, Rigoulet M, Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem. 2000;275:223–228. doi: 10.1074/jbc.275.1.223. [DOI] [PubMed] [Google Scholar]
- Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B. Metformin: from mechanisms of action to therapies. Cell Metab. 2014;20:953–966. doi: 10.1016/j.cmet.2014.09.018. [DOI] [PubMed] [Google Scholar]
- Grundemann D, Gorboulev V, Gambaryan S, Veyhl M, Koepsell H. Drug excretion mediated by a new prototype of polyspecific transporter. Nature. 1994;372:549–552. doi: 10.1038/372549a0. [DOI] [PubMed] [Google Scholar]
- Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK Phosphorylation of Raptor Mediates a Metabolic Checkpoint. Molecular Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG, Ashford ML. AMPK: regulating energy balance at the cellular and whole body levels. Physiology (Bethesda) 2014;29:99–107. doi: 10.1152/physiol.00050.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenour CM, Ridley DE, Hughey CC, James FD, Donahue EP, Shearer J, Viollet B, Foretz M, Wasserman DH. 5-Aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside (AICAR) effect on glucose production, but not energy metabolism, is independent of hepatic AMPK in vivo. J Biol Chem. 2014;289:5950–5959. doi: 10.1074/jbc.M113.528232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Wondisford FE. Metformin action: concentrations matter. Cell Metab. 2015;21:159–162. doi: 10.1016/j.cmet.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Howell J, Ricoult S, Ben-Sahra I, Manning B. A growing role for mTOR in promoting anabolic metabolism. Biochemical Society transactions. 2013;41:906–912. doi: 10.1042/BST20130041. [DOI] [PubMed] [Google Scholar]
- Howell JJ, Manning BD. mTOR couples cellular nutrient sensing to organismal metabolic homeostasis. Trends in endocrinology and metabolism: TEM. 2011;22:94–102. doi: 10.1016/j.tem.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai T, Chambon P, Metzger D. Inducible site-specific somatic mutagenesis in mouse hepatocytes. Genesis. 2000;26:147–148. doi: 10.1002/(sici)1526-968x(200002)26:2<147::aid-gene15>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Inoki K, Zhu T, Guan K-L. TSC2 Mediates Cellular Energy Response to Control Cell Growth and Survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- Inzucchi SE, Maggs DG, Spollett GR, Page SL, Rife FS, Walton V, Shulman GI. Efficacy and metabolic effects of metformin and troglitazone in type II diabetes mellitus. N. Engl. J. Med. 1998;338:867–872. doi: 10.1056/NEJM199803263381303. [DOI] [PubMed] [Google Scholar]
- Johnson AB, Webster JM, Sum CF, Heseltine L, Argyraki M, Cooper BG, Taylor R. The impact of metformin therapy on hepatic glucose production and skeletal muscle glycogen synthase activity in overweight type II diabetic patients. Metabolism. 1993;42:1217–1222. doi: 10.1016/0026-0495(93)90284-u. [DOI] [PubMed] [Google Scholar]
- Kalender A, Selvaraj A, Kim SY, Gulati P, Brûlé S, Viollet B, Kemp BE, Bardeesy N, Dennis P, Schlager JJ, et al. Metformin, Independent of AMPK, Inhibits mTORC1 in a Rag GTPase-Dependent Manner. Cell Metabolism. 2010;11:390–401. doi: 10.1016/j.cmet.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SA, Pacold ME, Cervantes CL, Lim D, Lou HJ, Ottina K, Gray NS, Turk BE, Yaffe MB, Sabatini DM. mTORC1 phosphorylation sites encode their sensitivity to starvation and rapamycin. Science. 2013;341:1236566. doi: 10.1126/science.1236566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khamzina L, Veilleux A, Bergeron S, Marette A. Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: possible involvement in obesity-linked insulin resistance. Endocrinology. 2005;146:1473–1481. doi: 10.1210/en.2004-0921. [DOI] [PubMed] [Google Scholar]
- Kim SG, Hoffman GR, Poulogiannis G, Buel GR, Jang YJ, Lee KW, Kim B-YY, Erikson RL, Cantley LC, Choo AY, et al. Metabolic stress controls mTORC1 lysosomal localization and dimerization by regulating the TTT-RUVBL1/2 complex. Molecular Cell. 2013;49:172–185. doi: 10.1016/j.molcel.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause U, Bertrand L, Hue L. Control of p70 ribosomal protein S6 kinase and acetyl-CoA carboxylase by AMP-activated protein kinase and protein phosphatases in isolated hepatocytes. Eur J Biochem. 2002;269:3751–3759. doi: 10.1046/j.1432-1033.2002.03074.x. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski DJ, Zhang H, Bandura JL, Heiberger KM, Glogauer M, el-Hashemite N, Onda H. A mouse model of TSC1 reveals sex-dependent lethality from liver hemangiomas, and up-regulation of p70S6 kinase activity in Tsc1 null cells. Human Molecular Genetics. 2002;11:525–534. doi: 10.1093/hmg/11.5.525. [DOI] [PubMed] [Google Scholar]
- Laplante M, Sabatini D. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson O, Morita M, Topisirovic I, Alain T, Blouin MJ, Pollak M, Sonenberg N. Distinct perturbation of the translatome by the antidiabetic drug metformin. Proc Natl Acad Sci U S A. 2012;109:8977–8982. doi: 10.1073/pnas.1201689109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Chhipa RR, Pooya S, Wortman M, Yachyshin S, Chow LM, Kumar A, Zhou X, Sun Y, Quinn B, et al. Discrete mechanisms of mTOR and cell cycle regulation by AMPK agonists independent of AMPK. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:44. doi: 10.1073/pnas.1311121111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memmott RM, Mercado JR, Maier CR, Kawabata S, Fox SD, Dennis PA. Metformin prevents tobacco carcinogen--induced lung tumorigenesis. Cancer prevention research (Philadelphia, Pa.) 2010;3:1066–1076. doi: 10.1158/1940-6207.CAPR-10-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization, W.H. Global Report on Diabetes. 2016 [Google Scholar]
- Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000;348(Pt 3):607–614. [PMC free article] [PubMed] [Google Scholar]
- Perriello G, Misericordia P, Volpi E, Santucci A, Santucci C, Ferrannini E, Ventura MM, Santeusanio F, Brunetti P, Bolli GB. Acute antihyperglycemic mechanisms of metformin in NIDDM. Evidence for suppression of lipid oxidation and hepatic glucose production. Diabetes. 1994;43:920–928. doi: 10.2337/diab.43.7.920. [DOI] [PubMed] [Google Scholar]
- Postic C, Magnuson MA. DNA excision in liver by an albumin-Cre transgene occurs progressively with age. Genesis. 2000;26:149–150. doi: 10.1002/(sici)1526-968x(200002)26:2<149::aid-gene16>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Sengupta S, Peterson TR, Laplante M, Oh S, Sabatini DM. mTORC1 controls fasting-induced ketogenesis and its modulation by ageing. Nature. 2010;468:1100–1104. doi: 10.1038/nature09584. [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Bardeesy N, Manning BD, Lopez L, Kosmatka M, DePinho RA, Cantley LC. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell. 2004;6:91–99. doi: 10.1016/j.ccr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Lamia KA, Vasquez D, Koo S-H, Bardeesy N, DePinho RA, Montminy M, Cantley LC. The Kinase LKB1 Mediates Glucose Homeostasis in Liver and Therapeutic Effects of Metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofer A, Lei K, Johannessen CM, Ellisen LW. Regulation of mTOR and cell growth in response to energy stress by REDD1. Mol Cell Biol. 2005;25:5834–5845. doi: 10.1128/MCB.25.14.5834-5845.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UK Prospective Diabetes Study Group, U. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) The Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J, et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- Yecies Jessica L, Zhang Hui H, Menon S, Liu S, Yecies D, Lipovsky Alex I, Gorgun C, Kwiatkowski David J, Hotamisligil Gökhan S, Lee C-H, et al. Akt Stimulates Hepatic SREBP1c and Lipogenesis through Parallel mTORC1-Dependent and Independent Pathways. Cell Metabolism. 2011;14:21–32. doi: 10.1016/j.cmet.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Dresser MJ, Gray AT, Yost SC, Terashita S, Giacomini KM. Cloning and Functional Expression of a Human Liver Organic Cation Transporter. Molecular Pharmacology. 1997;51:913–921. doi: 10.1124/mol.51.6.913. [DOI] [PubMed] [Google Scholar]
- Zheng M, Wang Y-H, Wu X-N, Wu S-Q, Lu B-J, Dong M-Q, Zhang H, Sun P, Lin S-C, Guan K-L, et al. Inactivation of Rheb by PRAK-mediated phosphorylation is essential for energy-depletion-induced suppression of mTORC1. Nat Cell Biol. 2011;13:263–272. doi: 10.1038/ncb2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, et al. Role of AMP-activated protein kinase in mechanism of metformin action. The Journal of clinical investigation. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.