Abstract
Background
Glycosaminoglycans (GAGs) are polysaccharides that are distributed on respiratory epithelial cells, endothelial cells, and submucosal glands. Uniquely positioned, certain GAGs exhibit anti-inflammatory properties in respiratory diseases and serve important roles in repairing mucosal surfaces and modulating mucociliary clearance. We hypothesized that topical administration of a synthetic GAG (GM-0111) would prevent sinonasal inflammation in a mouse model of rhinosinusitis (RS).
Methods
To test our hypothesis, C57BL/6 mice were intranasally administered fluorescent GM-0111, and sinonasal tissues were examined for coating and penetration ability. To test therapeutic feasibility, mice (n = 6) were given GM-0111 or hyaluronic acid (HA) (800 μg dose) prior to inducing RS with inflammatory molecule LL-37 (115 μg dose). After 24 h, sinonasal tissues were harvested for histological and biochemical analysis of inflammatory markers (inflammatory cell infiltration, lamina propria (LP) thickening, and neutrophil enzyme myeloperoxidase (MPO)) and cell death.
Results
(1) GM-0111 was observed within sinonasal tissues 1 h and 24 h after intranasal administration, indicating rapid and effective coating and penetration. (2) GM-0111 prevented sinonasal tissues from developing inflammatory changes, with significant reductions in mast cell infiltration (p < 0.05), LP thickening (p < 0.001), and MPO levels (p < 0.01) when compared to tissues treated with LL-37 and those pre-treated with HA. (3) GM-0111 reduced cell death within sinonasal tissues in contrast to LL-37-treated tissues.
Conclusions
We report a new synthetic GAG (GM-0111) that uniformly coats and penetrates into the sinonasal mucosa to prevent sinonasal inflammation and cell death in a mouse model of RS.
Keywords: rhinosinusitis, anti-inflammatory therapeutic, immune response, sulfated glycosaminoglycan, cathelicidin
Introduction
Glycosaminoglycans (GAGs) are highly charged, heterogeneous polysaccharides that are distributed on respiratory epithelial cells, endothelial cells, and submucosal glands and within the extracellular matrix (ECM).[1–5] Hyaluronic acid (HA) and sulfated GAGs (e.g., heparan sulfate, chondroitin sulfate, and heparin) represent the two main classes of GAGs.[1,3] With the exception of HA, which is nonsulfated and only exists as a major component of the ECM, GAGs are attached to cell surfaces and ECM protein cores to form proteoglycans.[2,6,7] Due to their unique position and high anionic character, GAGs modulate several important airway functions, including mucociliary clearance,[4] hydration and water homeostasis,[8,9] inflammation,[2,9,10] angiogenesis,[11] and tissue repair and remodeling of mucosal surfaces.[12] Moreover, accumulating evidence suggests that certain GAGs provide a protective mechanism against injury in asthma,[13,14] bronchitis,[15] chronic obstructive pulmonary disorder (COPD),[2,16,17] allergic rhinitis,[17] and chronic rhinosinusitis (CRS),[18] affording opportunities to develop new therapeutics for upper airway inflammatory diseases.[1]
GM-0111 is a synthetic, sulfated HA derivative (Fig. 1) previously demonstrated to be effective in attenuating inflammatory responses in rosacea,[19] initial cystitis (IC),[20,21] and periodontitis.[22] We therefore hypothesized that GM-0111 could prevent sinonasal inflammation in a mouse model of rhinosinusitis (RS). RS is an inflammatory respiratory disease that affects up to 16% of the United States population and is in dire need of new effective therapies.[23–31] We recently reported that intranasal administration of the inflammatory modulating peptide LL-37 causes profound sinonasal mucosal inflammation in the mouse with similar clinical signs to human RS,[32] as LL-37 causes cell death in the airways [33] and is elevated in sinus epithelial cells [34] and the nasal mucosa cultures of patients with RS.[35,36] Employing this model and based on prior evidence, we hypothesized that GM-0111 would (1) effectively coat and penetrate the sinuses through topical, intranasal delivery and (2) prevent sinonasal inflammation by protecting the sinonasal epithelium against inflammatory cell infiltration and cell death.[21,32].
Figure 1.
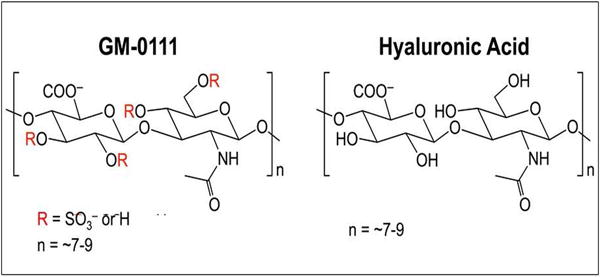
GAGs used in this study.
Materials and Methods
Study Compounds
Hyaluronic acid (HA, ~5.5 kDa) and GM-0111 were supplied by GlycoMira Therapeutics (Salt Lake City, UT) and prepared as previously described.[37] LL-37 is a C-terminal peptide fragment from human cathelicidin with a sequence of LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES and was obtained at >95% purity from the DNA/Peptide Synthesis Core Facility at the University of Utah. All study compounds were dissolved in NanoPure ddH2O or PBS (pH 7.4) and filtered through a sterile 0.22 μm filter before use.
Animals
Male C57BL/6 mice (6–8 weeks old) were purchased from Charles River (Santa Clara, CA) and housed in pathogen-free conditions at the University of Utah Laboratory of Comparative Medicine. Procedures were performed under the regulation of the Institutional Animal Care and Use Committee (IACUC) at the University of Utah (protocol number 14-05006) and according to the Guide for the Care and Use of Laboratory Animals.
Synthesis of Fluorescently-Labeled GM-0111 (CF647-GM-0111)
N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide (EDC) (5.4 mg, 0.0284 mmol, 6 eq) (Sigma Aldrich, St. Louis, MO) and N-hydroxysuccinimide (NHS; 4.9 mg, 0.0426 mmol, 9 eq) (Sigma Aldrich, St. Louis, MO) in 0.4 mL of ddH2O were added to a solution of GM-0111 (26 mg, 0.00473 mmol, 1 eq) in 2 mL of ddH2O (pH 4.75). The mixture was stirred at room temperature and maintained at pH 4.75 with 3 N NaOH for 15 min. Then, CF647-hydrazide (1 mg, 0.000945 mmol, 0.2 eq) (Biotium, Carlsbad, CA) was added in 0.1 mL of ddH2O. The reaction was stirred overnight, in the dark, at room temperature. The mixture was then dialyzed against 3 L of 100 mM NaCl (2 × 4 h) and against 3 L of ddH2O (1 × 24 h). The dialyzed solution was then lyophilized to afford CF647-GM-0111 as a blue powder in 84% yield.
GM-0111 Coating and Penetration Studies
Mice were placed under isoflurane anesthesia. Prior to treatment with GM-0111, mice were intranasally given a saline rinse (0.9% NaCl, 10 uL per nare, over 10 min). A total of 40 μL CF647-GM-0111 (10 mg/mL, 800 μg dose) was then administered to each nare (10 μL per nare, over 40 min, 10 μL for each 10 min interval), released slowly in drops that hung off the end of a micropipette tip held directly in front of the nares, allowing the mouse to spontaneously breathe in the inoculant. Mice were maintained supine during and following inoculations to facilitate retention in the nasal cavity.[32,38] The animals were observed for 24 h for pain or discomfort and subsequently sacrificed 24 h post-administration for tissue processing and fluorescence imaging using a Nikon A1R confocal microscope.
Study Compound Administration
A total of 6 mice were used in each of the following study groups: saline (no treatment control, n = 6), LL-37 (inflammation control, n = 6), GM-0111+LL-37 (n = 6), and HA+LL-37 (n = 6). Prior to treatment, mice were administered isoflurane anesthesia and intranasally given a saline rinse (0.9% NaCl, 10 uL per nare, 10 min). GM-0111 or HA was then instilled at a dose of 800 μg (10 mg/mL, 40 μL per nare over 40 min, 10 μL for each 10 min interval), immediately followed by a single dose of 320 μM LL-37 (40 μL per nare over 40 min, 10 μL for each 10 min interval, 115 μg total dose).[32] The animals were subsequently sacrificed 24 h post-instillation of LL-37, and sinonasal tissues were processed for histological, immunohistochemical (IHC), and biochemical analyses.
Tissue Collection
Animals were sacrificed 24 h post-compound administration, and tissues were harvested as described. Briefly, heads were bisected in the sagittal plane at the nasal septum, and sinonasal tissues were processed for histological or biochemical as previously reported.[32]
Histology and Inflammatory Marker Scoring
To histologically evaluate the degree of inflammation, hematoxylin and eosin (H&E) staining was performed on sinonasal tissues from all treatment groups. Stained sinonasal tissues were scored for inflammation as previously described using sinonasal tissues from 6 different animals per treatment group and scoring 5 random fields per tissue (n = 30 individual scores). Briefly, inflammation was blindly assessed under light microscopic examination for inflammatory cell infiltration, LP thickening, and secretory cell hyperplasia, using a scoring rubric (degree from 0 to 4) outlined in previous reports and an Olympus BX 43 light microscope equipped with a Cannon EOS T3 digital camera.[32,39,40]
Myeloperoxidase (MPO) Assay
Sinonasal tissues were processed as mentioned, and neutrophil enzyme MPO assays were performed as previously described.[32,41] MPO concentrations in the tissue homogenates were determined using a quantitative sandwich enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s protocol (HK210, Hycult Biotech, The Netherlands). All samples (n = 6) were run in duplicate.
Mast Cell Tryptase IHC and Mast Cell Counting
Histological sections were deparaffinized by xylene, rehydrated with decreasing concentration of alcohol (100%, 95%, and 70%), and prepared for IHC as described previously.[20,32] The number of mast cells infiltrating into the tissue were quantified using a staining protocol for tryptase (rabbit anti-mast cell tryptase (1:800 dilution, Mast Cell Tryptase Antibody, sc-32889, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, U.S.A.); biotinylated secondary antibody (1:2000 dilution, goat anti-rabbit IgG-B, sc-2040, Santa Cruz Biotechnology, Inc., Santa Cruz, CA); 3,3′-diaminobenzidine (DAB) peroxidase substrate). Five randomly selected high-power (400×) fields for each tissue were imaged, and the number of mast cells was quantified.[20,32,41] The final results are presented as mast cell numbers per mm2 sample.
TUNEL Analyses
Sinonasal tissues from all sample groups were collected and processed as described above. Fragmented DNA within cells in the epithelium (Ep) and submucosa (LP) were visualized using a Trevigen®TUNEL (terminal deoxynucleotidyl tranferase dUTP nick end labeling) kit (Trevigen Inc., MD), following the manufacturer’s recommended tissue staining protocol.
Statistical Analysis
All Likert scale and continuous variables were summarized by the mean and standard deviation. Mast cell counts were also summarized by median (IQR) and range. For the inflammatory degree scores (i.e., inflammatory cell infiltration, LP thickening, and secretory cell hyperplasia), five measurements per mouse (n = 6) were made (n = 30). For mast cell counts, we compared LL-37 versus saline using a Wilcoxon rank sum test. MPO and mast cells were evaluated across saline and LL-37 concentrations using an ANOVA trend test, and then all pair-wise comparisons were made, adjusting p-values using Tukey’s method. For all analyses, a p value < 0.05 indicates a statistically significant difference.
Results
GM-0111 Coats and Penetrates the Sinuses
Delivery method and mucosal penetration efficiency have tremendous effects on the efficacy of therapies to reduce inflammation in patients with RS.[42,43] Intranasal saline irrigation has been shown to increase drug delivery in comparison to the low sinus distribution efficiency of other methods, such as nasal sprays and nebulizers.[44–46] We therefore examined the ability of fluorescently labeled GM-0111 to be intranasally delivered in saline to mice. GM-0111 showed rapid, uniform coating and penetration within the sinonasal epithelium (Ep) and submucosa (LP) after 1 h and was even observed 24 h post-instillation, indicating good retention (Fig. 2). Additionally, a thick, homogenous coating was evident at the air-epithelial lining, indicating that GM-0111 might be a promising candidate for preventing sinonasal inflammation by serving as a protective barrier to inflammatory assault.
Figure 2.
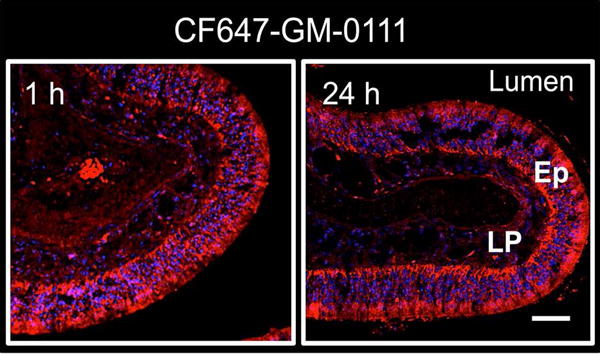
GM-0111 rapidly coats and penetrates the sinuses. Fluorescent confocal images of CF647-GM-0111 coating and distribution within the sinonasal tissues of mice harvested 1 h (left) and 24 h (right) after intranasal delivery in saline. The scale bar represents 2 mm. Lu = lumen; Ep = epithelium; LP = lamina propria.
GM-0111 Prevents Sinonasal Inflammation
Substantial inflammatory changes were observed in the sinonasal tissues from LL-37-treated animals when compared to saline-treated animals. H&E staining of LL-37-treated tissues demonstrated increased swelling of seromucous glands, mucus secretion (arrow), and the presence of polymorphonuclear leukocytes (PMNs) and other inflammatory cell infiltrates (box) into the LP when compared to saline-treated animals (Fig. 3A). Histological scoring in Fig. 3B confirmed significant increases in inflammatory cell infiltration (9-fold, p < 0.001), LP thickening (10-fold, p < 0.001), and secretory cell hyperplasia (26-fold, p < 0.001). In contrast, the sinonasal tissues from mice pre-treated with GM-011 showed similar morphological characteristics to those from the saline-treated control group (Fig. 3A). Significant decreases in inflammatory cell infiltration (2-fold, p < 0.001), LP thickening (2-fold, p < 0.001), and secretory cell hyperplasia (2-fold, p < 0.001) were observed with respect to LL-37-treated tissues, indicating that GM-0111 successfully prevented sinonasal inflammation. HA had a small reductive effect on inflammation, with an average of 0.3-fold decrease in inflammatory changes when compared to LL-37-treated mice (p < 0.01).
Figure 3.
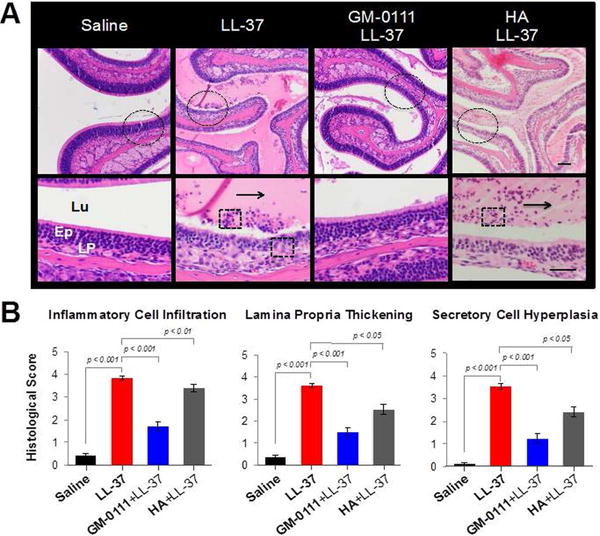
GM-0111 prevents sinonasal inflammation. (A) H&E staining in high- (top panel) and low- (bottom panel, dotted circle from top panel) magnification images show marked inflammatory changes in sinonasal tissues from LL-37-treated and HA-pre-treated mice when compared to saline-treated mice. The sinonasal tissues from mice pre-treated with GM-0111 exhibited similar histology to that of the saline-treated control group. Arrows: mucus; Boxes: inflammatory cell infiltrates. (B) Histological scoring of LL-treated sinonasal tissues demonstrate significant increases in inflammatory cell infiltration, LP thickening, and secretory cell hyperplasia when compared to saline-treated tissues (p < 0.001). Tissues pre-treated with GM-0111 demonstrate significant decreases in these measures when compared to LL-37-treated tissues (p < 0.001) and those pre-treated with HA (p < 0.05). The scale bars represent 2 mm. Lu = lumen; Ep = epithelium; LP = lamina propria.
GM-0111 Reduces Neutrophil and Mast Cell Counts in the Sinuses
We next quantified the levels of neutrophil enzyme MPO via ELISA (Fig. 4A) and the abundance of mast cells by IHC staining for mast cell biomarker tryptase (Fig. 4B), as these cells are potent mediators of sinonasal inflammation. A significant 17-fold increase in MPO level (p < 0.01) and 4-fold increase in mast cells (p < 0.05) were demonstrated in tissues treated with LL-37 when compared to those treated saline. HA reduced neutrophil and mast cell counts to a small degree, exhibiting approximate 2-fold (p < 0.05) and 0.7-fold decreases in MPO levels and mast cell counts, respectively, when compared to LL-37. Remarkably, sinonasal tissues pre-treated with GM-0111 showed a significant 7-fold reduction in MPO levels (p < 0.01) and 3-fold reduction in mast cells (p < 0.05) with respect to LL-37-treatd tissues. Taken together, these data suggest that GM-0111 is a promising candidate for preventing sinonasal inflammation by blocking neutrophil and mast cell infiltration.
Figure 4.
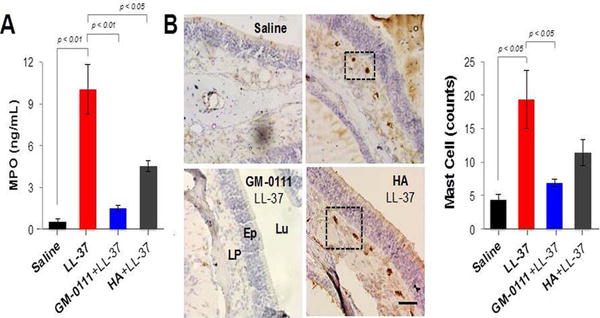
GM-0111 reduces neutrophil and mast cell counts within the sinuses. (A) ELISA quantification of MPO levels demonstrates a significant 17-fold increase in tissues treated with LL-37 when compared to saline groups (p < 0.01). Pre-treatment with GM-0111 resulted in a significant 7-fold (p < 0.01) reduction in MPO, whereas HA had less of an effect (2-fold, p < 0.05) compared to treatment with LL-37. (B) Tryptase IHC (brown stain, boxes) and quantification resulted in a significant 4-fold increase in mast cell counts in tissues treated with LL-37 compared to saline groups (p < 0.05). Pre-treatment with GM-0111 demonstrated in a significant 3-fold (p < 0.05) reduction in mast cells, whereas HA had less of an effect (0.7-fold) compared to treatment with LL-37. Scale bar represents 2 mm. HA = hyaluronic acid; ELISA = enzyme-linked immunosorbent assay; MPO = myeloperoxidase; Lu = lumen; epithelium; LP = lamina propria.
GM-0111 Prevents Cell Death within the Sinuses
We verified whether LL-37 induces cell death in our model using qualitative analysis of fragmented DNA in sinonasal tissues via terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL, Fig. 5). Tissues from mice harvested 24 h after being pre-treated with GM-0111, followed by LL-37, as well as after being treated with LL-37 alone, were stained with TUNEL and compared to saline-treated tissues. Noticeable decreases in TUNEL signal were detected in the LP of sinonasal tissues pre-treated with GM-0111 compared to LL-37-treated tissues, indicating that LL-37 induces cell death and that GM-0111 prevents it.
Figure 5.
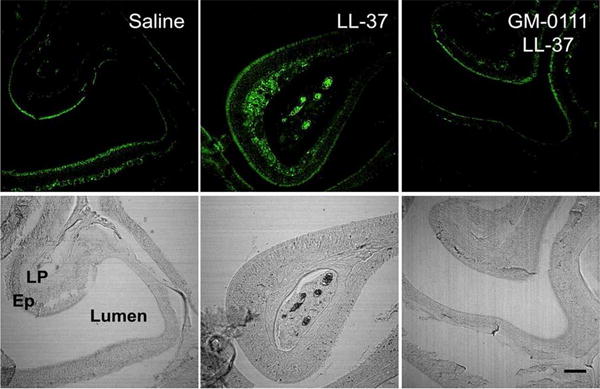
GM-0111 prevents cell death in the sinuses. Sinonasal tissues were subjected to TUNEL staining (top panels) and referenced to their corresponding bright field images (bottom panels). TUNEL = terminal deoxynucleotidyl transferase dUTP nick end labeling; Lu = lumen; Ep = epithelium; LP = lamina propria.
Discussion
GM-0111 is a synthetic GAG that possesses the structural backbone of HA and is chemically sulfated to obtain the potent anti-inflammatory properties of highly sulfated GAGs, such as heparin.[37] Heparin has exhibited anti-inflammatory activity in the sinonasal mucosa by inhibiting mast cell activation.[15] However, due to its strong anti-coagulant activity,[49] heparin causes epistaxis,[50] making it less than ideal for patients with RS-associated diseases. By contrast, GM-0111 has very low anti-coagulant activity (< 0.5 U/mg).[37] HA has also exhibited anti-inflammatory properties in upper airway inflammatory diseases.[12,16] One pilot study reported the reduction of nasal symptoms in patients with CRS using a dosing regimen of HA alone and in combination with nasal corticosteroids.[18] A major disadvantage of using HA as a therapeutic, however, is that it is easily degraded by endogenous enzymes, which may decrease its activity.[51] Unlike HA, GM-0111 is resistant to enzymatic degradation by hyaluronidases and weakly inhibits chondroitinases and heparinases, making its in vivo stability optimal for therapeutic effect (unpublished data). Moreover, GM-0111 is safe and homogenous in structure, avoiding the risk of potential pro-inflammatory mediator contamination, which is common when isolating animal-derived heparin and HA.[52,53]
The desirable structural and biological properties of GM-0111 were indeed manifested in this current study, as GM-0111 demonstrated superior anti-inflammatory activity to that of HA in preventing sinonasal inflammation in vivo. Based on our data and the evidence supporting the anti-inflammatory and protective roles of GAGs in airway mucosal diseases, we suggest that GM-0111 prevents sinonasal inflammation through multiple mechanisms of action. GM-0111 reduces mast cell and neutrophil infiltration within the sinonasal epithelium and submucosa, most likely by protecting the epithelium from cell death and the release of pro-inflammatory signaling molecules, such as leukocyte elastase, selectins, damage-associated molecular pattern (DAMP) molecules, and pattern recognition receptors (PRRs).[54,55] This mechanism is consistent to that of other GAG analogs (e.g., heparin and pentosan polysulfate) used to treat ulcerative colitis and IC by acting as urothelial GAG layer replenishing agents and providing barriers to further damage by cytotoxic molecules.[56,57] The rapid, deep, and lasting penetration of GM-0111 into the sinonasal tissues suggests that GM-0111 could be providing a protective barrier against inflammatory assault.[33,56]
Prior investigations have attributed the increased expression of LL-37 mRNA and protein in both upper airway epithelial cell cultures and in the human nasal mucosa of patients with RS[34–36] to increased cell death and pro-inflammatory signaling[33,48] through modulating the production of cytokines, chemokines, ATP, and reactive oxygen species.[48,58–61] We therefore postulate that LL-37 acts through similar mechanisms to induce the observed inflammatory changes, triggered by cell death, within the sinonasal epithelium and mucosa. With respect to how GM-0111 might be inhibiting the induction of sinonasal inflammation in this model, it is plausible that GM-0111 may be sequestering cationic LL-37, neutralizing toxic mediators and protecting against cell death through electrostatic interactions due to its highly anionic character. Having a higher degree of sulfation and more negative charge than HA, GM-0111 most likely interacts more strongly with cationic LL-37, leading to the observed reductions in inflammatory changes and cell death.
Based on our results and the above discussion, we suggest that treatment with GM-0111 prevents sinonasal inflammation by serving as a protective barrier against inflammatory assault and by neutralizing toxic mediators. Both of these mechanisms favor inhibition of inflammatory cell infiltration, recruitment of mast cells to the epithelium, and the prevention of cell death (Fig. 6).
Figure 6.
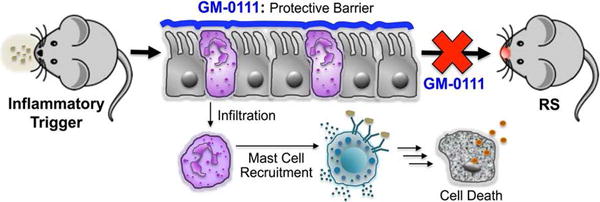
GM-0111 prevents mice from developing RS by coating the sinonasal epithelium and providing a protective barrier against inflammatory cell infiltration into the submucosa and subsequent mast cell recruitment and cell death, which contribute to the initiation, onset, and sustenance of RS-associated diseases.
Although there is a significant advantage of using murine models to study sinonasal physiology, there are inherent limitations that should be considered, including but not limited to, differing anatomic sinonasal structure and immune response.[62,63] It is plausible that LL-37-induced inflammation in the mouse may not accurately reflect the multifactorial physiology of the human condition. However, understanding the early underpinnings of the inflammatory response in the sinonasal cavity will enable us to design strategies to prevent these changes from occurring. Future work aims to expand the development of GM-0111 to both prevent and treat persistent and sustained inflammation in a chronic inflammatory model of sinonasal inflammation. We are also continuing studies that will help us determine which death pathways are being activated by LL-37 and inhibited by GM-0111, as well as the key molecular targets involved in initiating inflammation and cell death within the sinuses.
Conclusions
GM-0111, a synthetic GAG, prevents inflammation and cell death within the sinuses in vivo, demonstrated by significant reductions in inflammatory cell infiltration, LP thickening, secretory hyperplasia, MPO levels, and TUNEL staining. This work provides an opportunity to foundationally develop GM-0111 as an effective, translational therapy for preventing and treating inflammation in the upper airways, with a specific focus of RS-associated inflammation.
Acknowledgments
We thank Drs. Won Yong Lee and Justin Savage at GlycoMira Therapeutics for providing scientific and technical advice regarding animal work, data interpretation, and histological imaging and examination of sinonasal tissues. The authors thank Dr. Angela Presson at the University of Utah for performing statistical analyses of the groups studied and Dr. Mausam Kalita for help with analysis of HA and GM-0111.
Financial disclosures: This work is supported by grants from the University of Utah Program in Personalized Health and the National Center for Advancing Translational Sciences of the National Institute of Health under Award Number KL2TR001065 (JAA) and from the National Institute of Allergy and Infectious Diseases under Award Number 1R43AI126987 (AP, JAA, GDP). Dr. Alt is a consultant for Medtronic ENT (Jacksonville, FL., USA) and Spirox, which are not affiliated with this research.
Footnotes
Potential Conflicts of Interest: None
Presented at the American Rhinologic Society – San Diego, CA, USA September 16–17, 2016
References
- 1.Souza-Fernandes AB, Pelosi P, Rocco PRM. Bench-to-bedside review: the role of glycosaminoglycans in respiratory disease. Crit Care Lond Engl. 2006;10:237. doi: 10.1186/cc5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Negrini D, Passi A, Moriondo A. The role of proteoglycans in pulmonary edema development. Intensive Care Med. 2008;34:610–8. doi: 10.1007/s00134-007-0962-y. [DOI] [PubMed] [Google Scholar]
- 3.Scott JE. Supramolecular organization of extracellular matrix glycosaminoglycans, in vitro and in the tissues. FASEB J Off Publ Fed Am Soc Exp Biol. 1992;6:2639–45. [PubMed] [Google Scholar]
- 4.Manzanares D, Monzon M-E, Savani RC, Salathe M. Apical oxidative hyaluronan degradation stimulates airway ciliary beating via RHAMM and RON. Am J Respir Cell Mol Biol. 2007;37:160–8. doi: 10.1165/rcmb.2006-0413OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Handel TM, Johnson Z, Crown SE, Lau EK, Proudfoot AE. Regulation of protein function by glycosaminoglycans–as exemplified by chemokines. Annu Rev Biochem. 2005;74:385–410. doi: 10.1146/annurev.biochem.72.121801.161747. [DOI] [PubMed] [Google Scholar]
- 6.Hardingham TE, Fosang AJ. Proteoglycans: many forms and many functions. FASEB J Off Publ Fed Am Soc Exp Biol. 1992;6:861–70. [PubMed] [Google Scholar]
- 7.Iozzo RV, Schaefer L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol J Int Soc Matrix Biol. 2015;42:11–55. doi: 10.1016/j.matbio.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tammi MI, Day AJ, Turley EA. Hyaluronan and homeostasis: a balancing act. J Biol Chem. 2002;277:4581–4. doi: 10.1074/jbc.R100037200. [DOI] [PubMed] [Google Scholar]
- 9.Gerdin B, Hällgren R. Dynamic role of hyaluronan (HYA) in connective tissue activation and inflammation. J Intern Med. 1997;242:49–55. doi: 10.1046/j.1365-2796.1997.00173.x. [DOI] [PubMed] [Google Scholar]
- 10.Manuele C, Paola V, Antonio M, Giuseppe O, Lorenzo S, Valentina G, et al. Hyaluronic acid and upper airway inflammation in pediatric population: A systematic review. Int J Pediatr Otorhinolaryngol. 2016;85:22–6. doi: 10.1016/j.ijporl.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 11.Bhakuni T, Ali MF, Ahmad I, Bano S, Ansari S, Jairajpuri MA. Role of heparin and non heparin binding serpins in coagulation and angiogenesis: A complex interplay. Arch Biochem Biophys. 2016;604:128–42. doi: 10.1016/j.abb.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 12.Turino GM, Cantor JO. Hyaluronan in Respiratory Injury and Repair. Am J Respir Crit Care Med. 2003;167:1169–75. doi: 10.1164/rccm.200205-449PP. [DOI] [PubMed] [Google Scholar]
- 13.Monagle K, Ryan A, Hepponstall M, Mertyn E, Monagle P, Ignjatovic V, et al. Inhalational use of antithrombotics in humans: Review of the literature. Thromb Res. 2015;136:1059–66. doi: 10.1016/j.thromres.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Ahmed T, Garrigo J, Danta I. Preventing bronchoconstriction in exercise-induced asthma with inhaled heparin. N Engl J Med. 1993;329:90–5. doi: 10.1056/NEJM199307083290204. [DOI] [PubMed] [Google Scholar]
- 15.Zeng D, Prosperini G, Russo C, Spicuzza L, Cacciola RR, Di Maria GU, et al. Heparin attenuates symptoms and mast cell degranulation induced by AMP nasal provocation. J Allergy Clin Immunol. 2004;114:316–20. doi: 10.1016/j.jaci.2004.05.026. [DOI] [PubMed] [Google Scholar]
- 16.Bray BA. The Role of Hyaluronan in the Pulmonary Alveolus. J Theor Biol. 2001;210:121–30. doi: 10.1006/jtbi.2001.2305. [DOI] [PubMed] [Google Scholar]
- 17.Suleimani YMA, Dong Y, Walker MJA. Differential responses to various classes of drugs in a model of allergic rhinitis in guinea pigs. Pulm Pharmacol Ther. 2008;21:340–8. doi: 10.1016/j.pupt.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Cassandro E, Chiarella G, Cavaliere M, Sequino G, Cassandro C, Prasad SC, et al. Hyaluronan in the Treatment of Chronic Rhinosinusitis with Nasal Polyposis. Indian J Otolaryngol Head Neck Surg. 2015;67:299–307. doi: 10.1007/s12070-014-0766-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang J, Xu X, Rao NV, Argyle B, McCoard L, Rusho WJ, et al. Novel sulfated polysaccharides disrupt cathelicidins, inhibit RAGE and reduce cutaneous inflammation in a mouse model of rosacea. PloS One. 2011;6:e16658. doi: 10.1371/journal.pone.0016658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oottamasathien S, Jia W, McCoard L, Slack S, Zhang J, Skardal A, et al. A murine model of inflammatory bladder disease: cathelicidin peptide induced bladder inflammation and treatment with sulfated polysaccharides. J Urol. 2011;186:1684–92. doi: 10.1016/j.juro.2011.03.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee WY, Savage JR, Zhang J, Jia W, Oottamasathien S, Prestwich GD. Prevention of anti-microbial peptide LL-37-induced apoptosis and ATP release in the urinary bladder by a modified glycosaminoglycan. PloS One. 2013;8:e77854. doi: 10.1371/journal.pone.0077854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Savage JR, Pulsipher A, Rao NV, Kennedy TP, Prestwich GD, Ryan ME, et al. A Modified Glycosaminoglycan, GM-0111, Inhibits Molecular Signaling Involved in Periodontitis. PloS One. 2016;11:e0157310. doi: 10.1371/journal.pone.0157310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orlandi RR, Kingdom TT, Hwang PH, Smith TL, Alt JA, Baroody FM, et al. International Consensus Statement on Allergy and Rhinology: Rhinosinusitis. Int Forum Allergy Rhinol. 2016;6(Suppl 1):S22–209. doi: 10.1002/alr.21695. [DOI] [PubMed] [Google Scholar]
- 24.Caulley L, Thavorn K, Rudmik L, Cameron C, Kilty SJ. Direct costs of adult chronic rhinosinusitis by using 4 methods of estimation: Results of the US Medical Expenditure Panel Survey. J Allergy Clin Immunol. 2015;136:1517–22. doi: 10.1016/j.jaci.2015.08.037. [DOI] [PubMed] [Google Scholar]
- 25.Smith KA, Orlandi RR, Rudmik L. Cost of adult chronic rhinosinusitis: A systematic review. The Laryngoscope. 2015;125:1547–56. doi: 10.1002/lary.25180. [DOI] [PubMed] [Google Scholar]
- 26.Suh JD, Kennedy DW. Treatment options for chronic rhinosinusitis. Proc Am Thorac Soc. 2011;8:132–40. doi: 10.1513/pats.201003-028RN. [DOI] [PubMed] [Google Scholar]
- 27.Meltzer EO, Hamilos DL. Rhinosinusitis diagnosis and management for the clinician: a synopsis of recent consensus guidelines. Mayo Clin Proc. 2011;86:427–43. doi: 10.4065/mcp.2010.0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bachert C, Pawankar R, Zhang L, Bunnag C, Fokkens WJ, Hamilos DL, et al. ICON: chronic rhinosinusitis. World Allergy Organ J. 2014;7:25. doi: 10.1186/1939-4551-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinol Suppl. 2012:3. preceding table of contents, 1–298. [PubMed] [Google Scholar]
- 30.Ferguson BJ, Otto BA, Pant H. When surgery, antibiotics, and steroids fail to resolve chronic rhinosinusitis. Immunol Allergy Clin North Am. 2009;29:719–32. doi: 10.1016/j.iac.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 31.Cain RB, Lal D. Update on the management of chronic rhinosinusitis. Infect Drug Resist. 2013;6:1–14. doi: 10.2147/IDR.S26134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alt JA, Qin X, Pulsipher A, Orb Q, Orlandi RR, Zhang J, et al. Topical cathelicidin (LL-37) an innate immune peptide induces acute olfactory epithelium inflammation in a mouse model. Int Forum Allergy Rhinol. 2015;5:1141–50. doi: 10.1002/alr.21634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lau YE, Bowdish DME, Cosseau C, Hancock REW, Davidson DJ. Apoptosis of airway epithelial cells: human serum sensitive induction by the cathelicidin LL-37. Am J Respir Cell Mol Biol. 2006;34:399–409. doi: 10.1165/rcmb.2005-0170OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen P-H, Fang S-Y. The expression of human antimicrobial peptide LL-37 in the human nasal mucosa. Am J Rhinol. 2004;18:381–5. [PubMed] [Google Scholar]
- 35.Nell MJ, Tjabringa GS, Vonk MJ, Hiemstra PS, Grote JJ. Bacterial products increase expression of the human cathelicidin hCAP-18/LL-37 in cultured human sinus epithelial cells. FEMS Immunol Med Microbiol. 2004;42:225–31. doi: 10.1016/j.femsim.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 36.Ooi EH, Wormald P-J, Carney AS, James CL, Tan LW. Human cathelicidin antimicrobial peptide is up-regulated in the eosinophilic mucus subgroup of chronic rhinosinusitis patients. Am J Rhinol. 2007;21:395–401. doi: 10.2500/ajr.2007.21.3048. [DOI] [PubMed] [Google Scholar]
- 37.Prestwich GD, Oottamasathien S, Kennedy TP. US20130209531 Applications of partially and fully sulfated hyaluronan. 2013
- 38.Visweswaraiah A, Novotny LA, Hjemdahl-Monsen EJ, Bakaletz LO, Thanavala Y. Tracking the tissue distribution of marker dye following intranasal delivery in mice and chinchillas: a multifactorial analysis of parameters affecting nasal retention. Vaccine. 2002;20:3209–20. doi: 10.1016/s0264-410x(02)00247-5. [DOI] [PubMed] [Google Scholar]
- 39.Wilson KF, McMains KC, Orlandi RR. The association between allergy and chronic rhinosinusitis with and without nasal polyps: an evidence-based review with recommendations. Int Forum Allergy Rhinol. 2014;4:93–103. doi: 10.1002/alr.21258. [DOI] [PubMed] [Google Scholar]
- 40.Kim DW, Khalmuratova R, Hur DG, Jeon S-Y, Kim S-W, Shin H-W, et al. Staphylococcus aureus enterotoxin B contributes to induction of nasal polypoid lesions in an allergic rhinosinusitis murine model. Am J Rhinol Allergy. 2011;25:e255–261. doi: 10.2500/ajra.2011.25.3727. [DOI] [PubMed] [Google Scholar]
- 41.Lane AP, Turner J, May L, Reed R. A genetic model of chronic rhinosinusitis-associated olfactory inflammation reveals reversible functional impairment and dramatic neuroepithelial reorganization. J Neurosci Off J Soc Neurosci. 2010;30:2324–9. doi: 10.1523/JNEUROSCI.4507-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liang J, Lane AP. Topical Drug Delivery for Chronic Rhinosinusitis. Curr Otorhinolaryngol Rep. 2013;1:51–60. doi: 10.1007/s40136-012-0003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Snidvongs K, Kalish L, Sacks R, Sivasubramaniam R, Cope D, Harvey RJ. Sinus surgery and delivery method influence the effectiveness of topical corticosteroids for chronic rhinosinusitis: systematic review and meta-analysis. Am J Rhinol Allergy. 2013;27:221–33. doi: 10.2500/ajra.2013.27.3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Snidvongs K, Chaowanapanja P, Aeumjaturapat S, Chusakul S, Praweswararat P. Does nasal irrigation enter paranasal sinuses in chronic rhinosinusitis? Am J Rhinol. 2008;22:483–6. doi: 10.2500/ajr.2008.22.3221. [DOI] [PubMed] [Google Scholar]
- 45.Kosugi EM, Moussalem GF, Simões JC, Souza R de P e SF de, Chen VG, Saraceni Neto P, et al. Topical therapy with high-volume budesonide nasal irrigations in difficult-to-treat chronic rhinosinusitis. Braz J Otorhinolaryngol. 2016;82:191–7. doi: 10.1016/j.bjorl.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hyo N, Takano H, Hyo Y. Particle deposition efficiency of therapeutic aerosols in the human maxillary sinus. Rhinology. 1989;27:17–26. [PubMed] [Google Scholar]
- 47.Yamasaki K, Di Nardo A, Bardan A, Murakami M, Ohtake T, Coda A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007;13:975–80. doi: 10.1038/nm1616. [DOI] [PubMed] [Google Scholar]
- 48.Mader JS, Mookherjee N, Hancock REW, Bleackley RC. The human host defense peptide LL-37 induces apoptosis in a calpain- and apoptosis-inducing factor-dependent manner involving Bax activity. Mol Cancer Res MCR. 2009;7:689–702. doi: 10.1158/1541-7786.MCR-08-0274. [DOI] [PubMed] [Google Scholar]
- 49.Björk I, Lindahl U. Mechanism of the anticoagulant action of heparin. Mol Cell Biochem. 48:161–82. doi: 10.1007/BF00421226. [DOI] [PubMed] [Google Scholar]
- 50.Dang CH, Durkalski VL, Nappi JM. Evaluation of Treatment with Direct Thrombin Inhibitors in Patients with Heparin-Induced Thrombocytopenia. Pharmacotherapy. 2006;4:461–468. doi: 10.1592/phco.26.4.461. [DOI] [PubMed] [Google Scholar]
- 51.Petrey AC, de la Motte CA. Hyaluronan, a Crucial Regulator of Inflammation. Front Immunol. 2014;11:101. doi: 10.3389/fimmu.2014.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chess EK, Bairstow S, Donovan S, Havel K, Hu P, Johnson RJ, et al. Case study: contamination of heparin with oversulfated chondroitin sulfate. Handb Exp Pharmacol. 2012:99–125. doi: 10.1007/978-3-642-23056-1_6. [DOI] [PubMed] [Google Scholar]
- 53.Filion MC, Phillips NC. Pro-inflammatory activity od contaminating DNA in hyaluronan preparations. Woodhead Publishing; 2002. pp. 429–434. [Google Scholar]
- 54.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 55.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–20. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 56.Madersbacher H, van Ophoven A, van Kerrebroeck PEVA. GAG layer replenishment therapy for chronic forms of cystitis with intravesical glycosaminoglycans–a review. Neurourol Urodyn. 2013;32:9–18. doi: 10.1002/nau.22256. [DOI] [PubMed] [Google Scholar]
- 57.Russell AL. Glycoaminoglycan (GAG) deficiency in protective barrier as an underlying, primary cause of ulcerative colitis, Crohn’s disease interstitial cystitis and possibly Reiter’s syndrome. Med Hypotheses. 1999;52:297–301. doi: 10.1054/mehy.1997.0652. [DOI] [PubMed] [Google Scholar]
- 58.Zheng Y, Niyonsaba F, Ushio H, Nagaoka I, Ikeda S, Okumura K, et al. Cathelicidin LL-37 induces the generation of reactive oxygen species and release of human alpha-defensins from neutrophils. Br J Dermatol. 2007;157:1124–31. doi: 10.1111/j.1365-2133.2007.08196.x. [DOI] [PubMed] [Google Scholar]
- 59.Tjabringa GS, Ninaber DK, Drijfhout JW, Rabe KF, Hiemstra PS. Human cathelicidin LL-37 is a chemoattractant for eosinophils and neutrophils that acts via formyl-peptide receptors. Int Arch Allergy Immunol. 2006;140:103–12. doi: 10.1159/000092305. [DOI] [PubMed] [Google Scholar]
- 60.Yang D, Chertov O, Oppenheim JJ. The role of mammalian antimicrobial peptides and proteins in awakening of innate host defenses and adaptive immunity. Cell Mol Life Sci CMLS. 2001;58:978–89. doi: 10.1007/PL00000914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Urb M, Sheppard DC. The role of mast cells in the defence against pathogens. PLoS Pathog. 2012;8:e1002619. doi: 10.1371/journal.ppat.1002619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jacob A, Chole RA. Survey anatomy of the paranasal sinuses in the normal mouse. The Laryngoscope. 2006;116:558–63. doi: 10.1097/01.MLG.0000202085.23454.2F. [DOI] [PubMed] [Google Scholar]
- 63.Harkema JR, Carey SA, Wagner JG, Dintzis SM, Liggitt D. Chapter 6 - Nose, Sinus, Pharynx, and Larynx. Comp Anat Histol. 2012:71–94. [Google Scholar]


