SUMMARY
Double-stranded RNA (dsRNA)-specific ribonuclease III (RNase III) proteins are required for RNA maturation and gene regulation. The mechanism of prokaryotic RNase IIIs has been well characterized, but how eukaryotic RNase IIIs (exemplified by Rnt1p, Drosha, and Dicer) work is less clear. Recently, we reported the crystal structure of Rnt1p in complex with RNA, revealing a double-ruler mechanism for substrate selection. Here, we present more structures of Rnt1p, either RNA-free or RNA-bound, featuring two major conformations of the enzyme. Using these structures with existing data, we describe the functional cycle of Rnt1p in five steps, selecting, loading, locking, cleavage, and releasing. We also describe atomic details of the two-Mg2+-ion catalytic mechanism that is applicable to all eukaryotic RNase III enzymes. Overall, our results indicate that substrate selection is achieved independent of cleavage, allowing the recognition of substrates with different structures while preserving the basic mechanism of cleavage.
Keywords: Ribonuclease III, RNase III, Rnt1p, double-stranded RNA, dsRNA, dsRNA processing, RNA processing, X-ray crystallography, small-angle X-ray scattering, SAXS
Graphical abstract
eTOC BLURB
Song et al. describe the functional cycle of a eukaryotic RNase III that processes dsRNA in five steps. They also describe atomic details of the two-Mg2+-ion catalytic mechanism that involves six amino acid side chains, which is applicable to all eukaryotic RNase IIIs.
INTRODUCTION
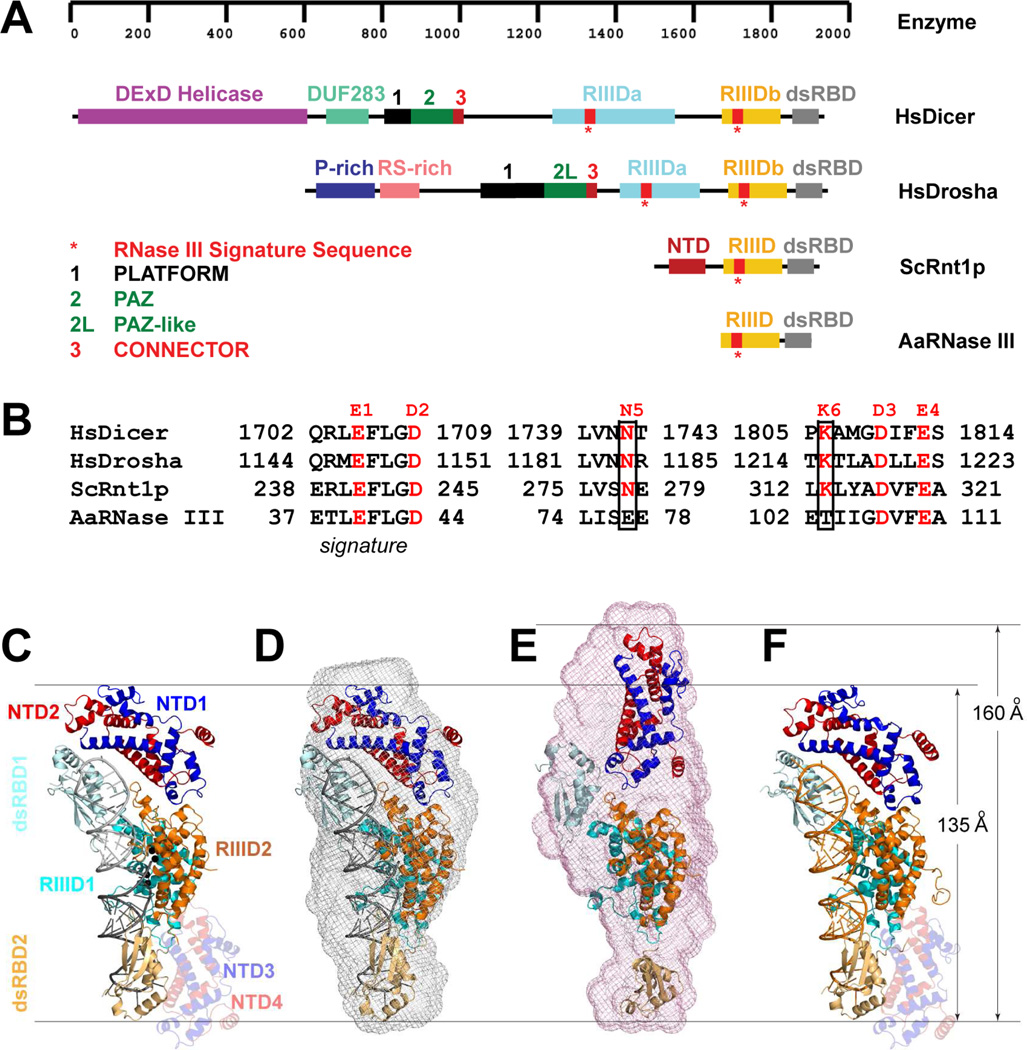
Ribonuclease III (RNase III) represents a growing family of proteins with diversity of form and function. The RNase III proteins are Mg2+ dependent, double-stranded RNA (dsRNA)-specific endoribonucleases that are characterized by a signature sequence in their specialized endonuclease domain (RIIID). The cleavages performed by RNase III generate 5’ phosphoryl and 3’ hydroxyl ends with a 2-nucleotide (2-nt) 3’ overhang in the dsRNA product. RNase IIIs play important roles in the cell, which include RNA processing, post-transcriptional gene expression control, and defense against viral infection (Court, 1993; Robertso et al., 1968; van Rij and Andino, 2006; Wu et al., 2000). In 1968, the founding member of the RNase III family was discovered in Escherichia coli (Robertso et al., 1968). Currently, representative members of the RNase III family also include eukaryotic Rnt1p, Drosha, and Dicer (Figure 1A). A typical bacterial RNase III possesses a single RIIID followed by a dsRNA-binding domain (dsRBD). In addition to RIIID and dsRBD, eukaryotic RNase III possesses an N-terminal extension that contains 150–1,200 amino acid residues. Saccharomyces cerevisiae Rnt1p contains a 155-residue N-terminal domain (NTD), whereas human Dicer and Drosha have much longer N-terminal extensions. In Dicer, the N-terminal extension contains a helicase domain, a DUF283 domain, a platform, a PAZ domain, and a connector. In Drosha, the N-terminal extension contains a P-rich domain, a RS-rich domain, a platform, a PAZ-like domain, and a connector (Figure 1A).
Figure 1.
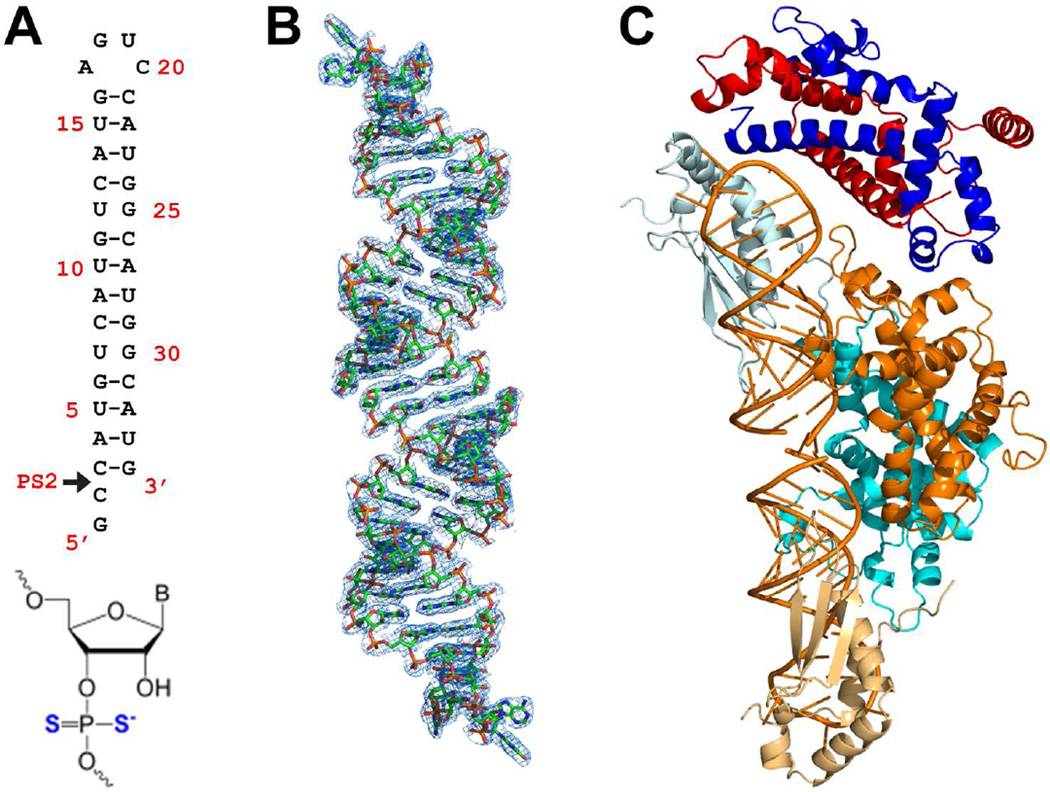
Representative members of the RNase III family and the scope of this study. (A) Domain structures of Homo sapiens Dicer (HsDicer) (Kwon et al., 2016), Homo sapiens Drosha (HsDrosha) (Kwon et al., 2016), Saccharomyces cerevisiae Rnt1p (ScRnt1p) (Liang et al., 2014), and Aquifex aeolicus RNase III (AaRNase III) (Gan et al., 2006). The protein domains are color coded as indicated. (B) Catalytic amino acid residues in the RIIID of HsDicer, HsDrosha, ScRnt1p, and AaRNase III. The N5 and K6, unique for eukaryotes, are boxed. (C) Crystal structure of the Rnt1p post-cleavage complex (PDB entry 4OOG). The structure is illustrated as a ribbon diagram (helices as spirals, strands as arrows, loops as tubes, RNAs as tube-and-stick models, and Mg2+ ions as spheres). The protein domains are color coded. The extra NTD dimer (NTD3/NTD4) is shaded. (D) Crystal structure (PDB entry 4OOG, excluding the extra NTD dimer) docked into the SAXS envelope (this work). (E) Best-fit rigid-body model of apo-Rnt1p (starting structure: PDB entry 4OOG) docked into the SAXS envelope (this work). (F) Crystal structure of Rnt1p in complex with an RNA substrate analog, i.e., the substrate-loaded complex (this work).
A dimer of RIIID is required for RNase III activity (Ji, 2008). The RNase IIIs having one RIIID, such as bacterial RNase III (Gan et al., 2006), S. cerevisiae Rnt1p (Liang et al., 2014), and Kluyveromyces polysporus Dcr1 (Weinberg et al., 2011), forms a homodimer. Those containing two RIIIDs, such as human Dicer (MacRae et al., 2006) and Drosha (Kwon et al., 2016), functions as a monomer. For the mechanism of dsRNA cleavage by the RIIID dimer, bacterial RNase III is the best model system because it is small in size (Figure 1A) and is the most comprehensively studied member of the entire family. Thirty-three years after its discovery, crystal structure of the RIIID dimer was reported in 2001, offering the first glimpse at the RNase III’s active site (Blaszczyk et al., 2001; Zamore, 2001). In 2006, crystal structure of RNase III in complex with dsRNA was determined, providing structural insights into the mechanism of RNase III action (Gan et al., 2006). Two years later, a stepwise model for the phosphoryl transfer reaction and product release was described on the basis of additional structures, including that at a catalytic stage immediately after the cleavage of the phosphodiester bond (the post-cleavage complex) (Gan et al., 2008). Two recent reviews are available for updated and comprehensive information on the genetics, function, structure, and mechanism of bacterial RNase III (Court et al., 2013; Nicholson, 2014). On the basis of protein-RNA interactions of prokaryotic RNase III, models with RNA have been proposed for eukaryotic RNase IIIs, shedding light on their mechanisms of action. The model complex of Dicer with RNA explains how Dicer enzymes recognize the 2-nt 3’ overhang of the dsRNA substrate and measure 22 nucleotides up to position the scissile bond over the cleavage site (Gan et al., 2008; MacRae et al., 2006). The model complex of Drosha with RNA explains how Drosha enzymes recognize the last base pair in the basal junction of the primary microRNA substrate and measure 11 nucleotides up to position the scissile bond over the cleavage site (Gan et al., 2008; Kwon et al., 2016). The model complex of a non-canonical Dicer with RNA explains how homodimers of non-canonical Dicer enzymes bind cooperatively along the dsRNA substrate such that the distance between cleavage sites in adjacent dimers is the length of ∼22 nucleotides (Gan et al., 2008; Weinberg et al., 2011). In addition to the N-terminal extension, eukaryotic RNase IIIs differ from bacterial enzymes also by the number of catalytic side chains. Bacterial RNase IIIs has four, whereas eukaryotes have six (Figure 1B). To elucidate the functional roles of the N-terminal extensions and the two additional catalytic side chains of eukaryotic RNase IIIs, Rnt1p is an excellent model system (Figures 1A and 1B). In addition to the small size of its N-terminal extension, Rnt1p is unique in that it specifically recognizes RNA hairpins with conserved NGNN tetraloops under physiological conditions (Lebars et al., 2001). Unlike Dicer and Drosha, however, the substrate selection and measurement mechanisms of Rnt1p cannot be explained by a model complex. Even the structure of its dsRBD in complex with a product RNA failed to show how the guanine nucleotide in the NGNN tetraloop is recognized (Wu et al., 2004). Recently, we determined the structure of Rnt1p post-cleavage complex, showing that a novel RNA-binding motif (RBM) recognizes the guanine nucleotide in the NGNN tetraloop, that the NTD and dsRBD function as two rulers measuring the distance between the tetraloop and the cleavage site, and that the two additional catalytic side chains play important roles in stabilizing the transition state (Liang et al., 2014).
Like yeast Rnt1p, human Dicer also uses its N-terminal extension to ensure accurate processing (Gu et al., 2012). In order to understand how a particular eukaryotic RNase III recognizes a specific structural feature of its substrate while preserving the basic mechanism of cleavage, we further elucidated the structure of Rnt1p at different stages of its functional cycle using crystallography and small-angle X-ray scattering (SAXS). Here, we present new structural data, including the SAXS solution structure of Rnt1p post-cleavage complex (Figure 1D), the SAXS solution structure of apo-Rnt1p (Figure 1E), and the crystal structure of Rnt1p in complex with a non-hydrolyzable RNA substrate analog (Figure 1F). The apo-Rnt1p structure reveals an open conformation of the enzyme when substrate is not bound. The crystal structure represents a substrate-loaded complex, the first one for the entire RNase III family. Together, the data indicate that RNA processing by Rnt1p takes place in five different steps along its functional cycle, each featuring distinct conformational changes. In addition to the functional cycle, we also describe atomic details of the two-Mg2+-ion mechanism that involves six catalytic side chains. Whereas the five-step functional cycle is unique for Rnt1p, the two-Mg2+-ion catalytic mechanism is applicable to all eukaryotic RNase III enzymes, including Drosha and Dicer.
RESULTS
The Post-Cleavage Complex of Rnt1p Has the Same Structure in Crystal and in Solution
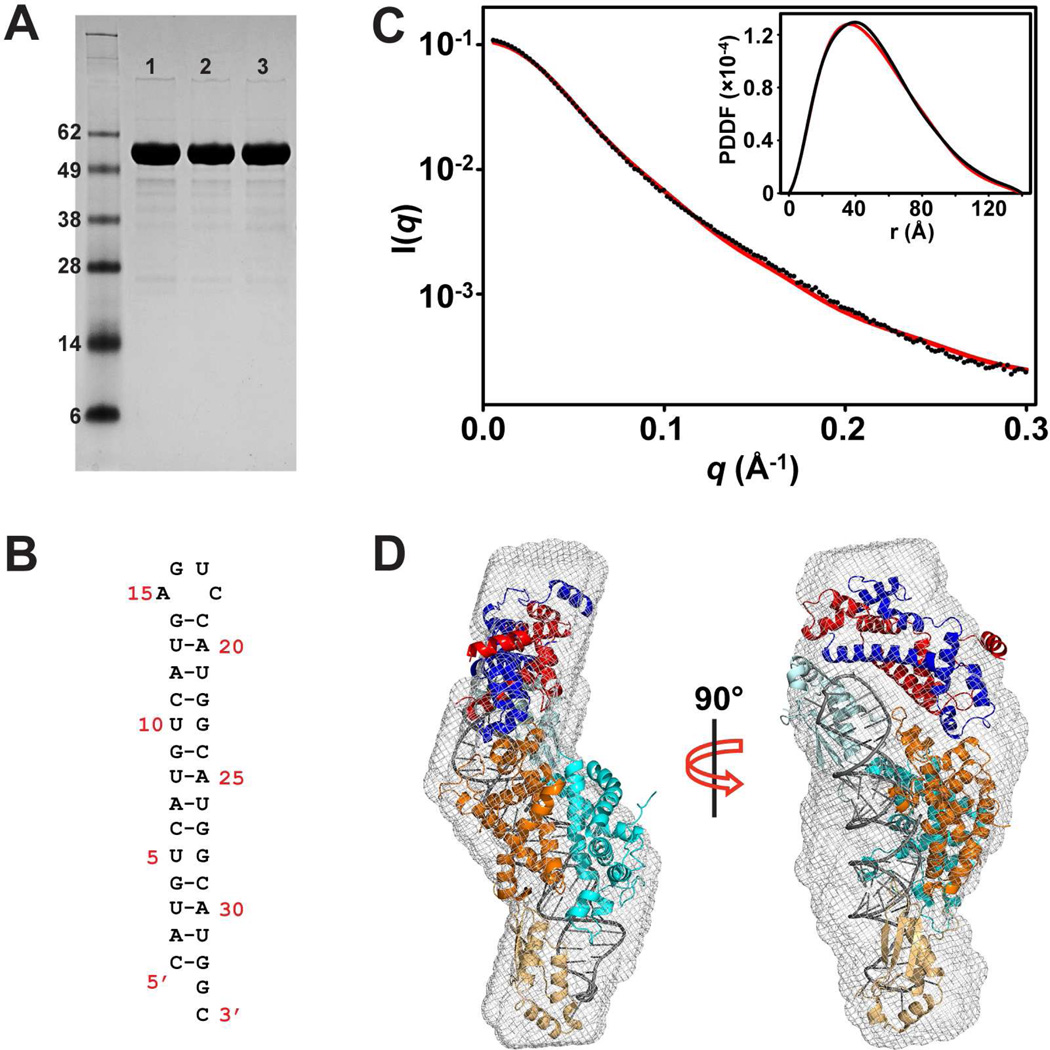
We found previously that during crystallization of the post-cleavage complex (Liang et al., 2014), Rnt1p degraded into two fragments, residues 42–151 containing the NTD and residues 197–457 containing the RIIID and dsRBD. In the crystal lattice, the RIIID-dsRBD dimer is associated with two NTD dimers, creating an imbalanced stoichiometry (Figure 1C). We believed that it is a crystallization artifact because the structural details, especially the protein-RNA interactions, are in excellent agreement with results from both previous studies and experiments designed on the basis of the structure (Liang et al., 2014). Later, we found that only the cleaved form of Rnt1p could be crystallized and that the non-crystallizable Rnt1p was unusually stable in solution. The stability of Rnt1p allowed us to further characterize the protein in solution by SAXS. As shown in Figure 2A, the protein was intact before and after the SAXS experiments. We elucidated the post-cleavage complex first, which contains an Rnt1p dimer and an RNA pseudo-duplex formed by two 34-nt RNA product (Figure 2B). We collected data from the sample after size-exclusion chromatography to ensure that the complex is monodisperse and homogeneous. The experimental scattering profiles, with the scattering intensity I(q) plotted versus momentum transfer q, along with pair-distance distribution function (PDDF) are shown in Figure 2C. The overall structural parameters derived from the SAXS data are summarized in Table 1. The Dmax value (140 ± 5 Å, Table 1) is virtually identical to the longest dimension measured for the post-cleavage complex with the extra NTD dimer excluded (135 Å, Figure 1C).
Figure 2.
Solution structure of Rnt1p post-cleavage complex by SAXS. (A) SDS-PAGE analysis of the Rnt1p protein used in the SAXS experiment. Lane 1 is apo-Rnt1p before the SAXS experiment. Lane 2 is apo-Rnt1p after the SAXS experiment. Lane 3 is the Rnt1p post-cleavage complex after the SAXS experiment. (B) Sequence and secondary structure of the product RNA when self-annealed. (C) Overlay of experimental scattering profiles (black) with back-calculated scattering profiles (red) for the complex. The insert shows the overlay of the structure (red) with experimental (black) PDDFs. (D) Shown in two views, crystal structure of the post-cleavage complex of Rnt1p (PDB entry 4OOG, excluding the extra NTD dimer) in the ab initio SAXS envelope. The protein domains and RNA are color coded as in Figure 1C.
Table 1.
Structural parameters derived from SAXS data
|
Rg (Å) Guinier |
Rg (Å) GNOM |
Dmax (Å) |
MWPred (kDa)a |
MWsaxs (kDa)b |
χ / χ2free | |
|---|---|---|---|---|---|---|
| Post-cleavage | 40.1 ± 0.5 | 40.2 ± 0.2 | 140 ± 5 | 128 | – | 1.01/1.51 |
| Apo-Rnt1p | 46.7 ± 0.8 | 45.9 ± 0.5 | 160 ± 5 | 108 | 106 | 0.34/0.12 |
The MWpred was calculated from the primary sequences of components.
The MWSAXS was calculated using the power-law relationship between a mass parameter, QR, and particle mass, empirically determined on the basis of SAXS data for a particular class of macromolecular particles. Due to the lack of SAXS data, such relationship remains undetermined for protein-nucleic acid complexes (Rambo and Tainer, 2013a).
To illustrate the solution structure of the post-cleavage complex of intact Rnt1p, an ab initio shape envelope was built using the program DAMMIN (Svergun, 1999). As expected, the post-cleavage complex of degraded Rnt1p (Liang et al., 2014) without the extra NTD dimer fits very well into the shape envelope of the post-cleavage complex of intact Rnt1p (Figure 2D). The back-calculated scattering profile for the crystal structure also fits well to the experimental data (χ=1.01, χ2free=1.51) (Figure 2C). Furthermore, the predicted Rg value based on the crystal structure is virtually identical to that derived from the SAXS data (Table 1). As shown, the post-cleavage complex of intact Rnt1p adopts the same conformation, domain arrangement, and protein-RNA interactions as in the crystal lattice, but having the correct stoichiometry as the free NTD dimer is not available to contact the free end of the (RIIID-dsRBD)2-RNA2 complex.
Solution Structure of Apo-Rnt1p Reveals an Open Conformation of the Protein
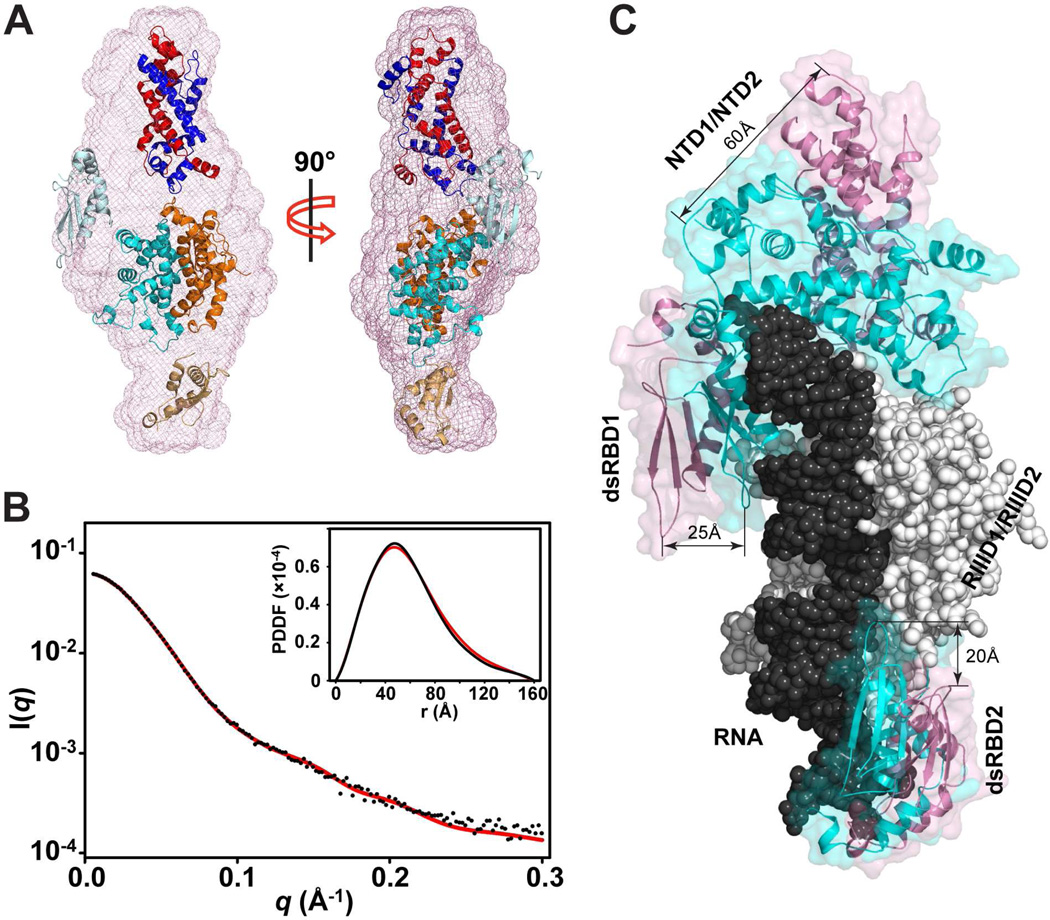
SAXS experiments have been used as a powerful technique to assess the architecture of multi-domain biological molecules (Kakar et al., 2015; Putnam et al., 2007). To better understand the molecular mechanism of RNA processing by Rnt1p, we performed the SAXS analysis of apo-Rnt1p in solution. The overall structural parameters derived from the SAXS data are summarized in Table 1. The estimated molecular weight of apo-Rnt1p (106 kDa) indicates that apo-Rnt1p forms a dimer (108 kDa) in solution. The Dmax value (160 Å) of apo-Rnt1p is, however, 20 Å larger than that of the post-cleavage complex (140 Å, Table 1), indicating that apo-Rnt1p adopts a much extended conformation in solution. In addition, Rg value for apo-Rnt1p is significantly larger than that for the complex (Table 1). These differences could be better visualized by the ab initio shape reconstitution from DAMMIN (Figure 3A). As shown, the envelope of apo-Rnt1p is enlarged as compared to the post-cleavage complex (Figures 1D and 1E). Therefore, apo-Rnt1p adopts an open conformation that is significantly different from that of the complex. The experimental scattering profile and the PDDF for apo-Rnt1p are shown in Figure 3B. The pattern of the profile for apo-Rnt1p (Figure 3B) is also different from that of the post-cleavage complex (Figure 2B).
Figure 3.
Solution structure of apo-Rnt1p by SAXS. (A) Shown in two views, the best-fit rigid-body model of apo-Rnt1p (starting structure: PDB entry 4OOG) in the ab initio envelope. The protein domains are color coded as in Figure 1C. (B) Overlay of experimental scattering profiles (black) with back-calculated scattering profiles (red) for apo-Rnt1p. The insert shows the overlay of the model (red) with experimental (black) PDDFs for apo-Rnt1p. (C) Superimposition of apo-Rnt1p and the post-cleavage complex on the basis of Cα positions in the RIIID dimer. The RIIID dimer and RNA in the complex are shown as atomic sphere models in white and black, respectively. The RIIID dimer in apo-Rnt1p is not shown for clarity. The NTD and dsRBD are illustrated as ribbon diagrams outlined with transparent molecular surfaces in pink for apo-Rnt1p and cyan for the post-cleavage complex.
Domain analysis based on the crystal structure of the post-cleavage complex indicates that apo-Rnt1p could be divided into four rigid bodies: the RIIID dimer, the intertwined NTD dimer, and the two dsRBDs. The RIIID and dsRBD are connected by a 7-residue sequence, whereas the NTD and RIIID are connected by a 46-residue linker. The SAXS-based rigid body refinement of apo-Rnt1p was carried out with Xplor-NIH (Schwieters et al., 2006; Schwieters et al., 2003), during which the relative positions of the NTD dimer and the other three rigid bodies were refined and optimized against the SAXS data. The resulting structure, which fits well to the scattering data, represents the average conformation of apo-Rnt1p in solution (Figure 3A).
Like the RIIID dimer, which has been observed in both RNA-free and bound states, the NTD dimer is also observed in both states. Unlike the RIIID dimer, however, the NTD dimer is not responsible for cleavage. Instead, it locks the RNA substrate into the correct position for efficient and accurate cleavage (Lamontagne et al., 2000; Liang et al., 2014). Superimposition of apo-Rnt1p and post-cleavage complex reveals that the displacement of the NTD dimer (60 Å) is most dramatic, much greater than the displacement of dsRBD1 (25 Å) and dsRBD2 (20 Å) between the open and closed conformations (Figure 3C). In the open form of Rnt1p, dsRBD has a sufficient amount of freedom and space for initial substrate selection.
The New Crystal Structure of Rnt1p Represents the First Substrate-Loaded Complex for the Entire RNase III Family
To understand how Rnt1p interacts with substrate RNA, we crystallized Rnt1p in complex with a non-cleavable RNA substrate analog that was designed in two steps. First, we removed the 2-nt pGpC overhang from the 3’ end of the RNA in the post-cleavage complex (Figure 2B) and added the pGpC sequence to the 5’ end (Figure 4A). Then, we replaced the pro-Sp and Rp oxygen atoms of the scissile phosphate with sulfur atoms, creating a phosphorodithioate nucleotide at the cleavage site, PS2 (Figure 4A). The presence of sulfur interferes with coordination of Mg2+ to the phosphate oxygen. Therefore, Rnt1p was not able to hydrolyze the phosphorodithioate bond and a snapshot, representing a substrate-loaded complex, was captured. Like the post-cleavage complex, the crystal structure of the substrate-loaded complex also contains two RIIID-dsRBD fragments, an RNA pseudo-duplex, which is formed by two substrate analogs joined by their 2-nt 5’ overhangs (Figure 4B), and two NTD dimers (Figure 1F). Statistics for X-ray diffraction data and final structure are summarized in Table 2. Since the extra NTD dimer is a crystallization artifact, the substrate-loaded structure is presented without it (Figure 4C). We have reported several RNase III structures with a substrate or substrate-mimicking RNA bound (Blaszczyk et al., 2004; Gan et al., 2008; Gan et al., 2005), but none of these previous structures had the RNA properly loaded. This new structure represents the first substrate-loaded complex for the entire RNase III family.
Figure 4.
Crystal structure of the Rnt1p substrate-loaded complex. (A) Sequence and secondary structure of the RNA substrate analog. The arrowhead indicates the position of the phosphorodithioated nucleotide (bottom) at the Rnt1p cleavage site. With the modification, the scissile bond cannot be cleaved by Rnt1p, and therefore, this RNA represents an Rnt1p substrate. (B) Electron density map (net in blue, 2Fo − Fc, contoured at 1σ) for the RNA pseudo-duplex (stick model in atomic colors: N, blue; C, green; O, red; P, orange; S, yellow) formed by two RNA substrate analog molecules joined with their 2-nt 5’ overhangs. (C) The architecture of the Rnt1p substrate-loaded complex. It contains one NTD dimer, one RIIID dimer, two dsRBDs, and two substrate analog RNAs in addition to solvent molecules (not shown). The protein domains are color coded as in Figure 1C and the RNA is in orange. The extra NTD dimer is not shown because it is a crystallization artifact.
Table 2.
Data collection and refinement statistics
| Substrate-loaded Complex | |
|---|---|
| Data collectiona | |
| Space group | P21 |
| Cell dimensions | |
| a,b,c (Å) | 62.0, 164.1, 176.9 |
| α, β, γ (°) | 90, 96.8, 90 |
| Resolution (Å) | 40.00–2.78 (2.90–2.78)b |
| Rpim | 0.118 (0.990) |
| CC1/2 | 0.991 (0.396)c |
| CC* | 0.998 (0.753)c |
| I / σI | 6.79 (0.57) |
| Completeness (%) | 99.3 (96.0) |
| Redundancy | 4.5 (3.8) |
| Refinement | |
| Resolution (Å) | 38.99–2.78 |
| No. reflections | 86,174 |
| Rwork / Rfree | 0.221 / 0.267 |
| No. atoms | |
| Protein | 16,286 |
| Ligand/ion | 2,924 |
| Water | 358 |
| B-factors | |
| Protein | 57.62 |
| Ligand/ion | 53.33 |
| Water | 49.43 |
| R.m.s. deviations | |
| Bond lengths (Å) | 0.011 |
| Bond angles (°) | 1.50 |
One crystal was used for data collection.
Values in parentheses are for highest-resolution shell.
The CC1/2 and CC* values are calculated according to (Karplus and Diederichs, 2012).
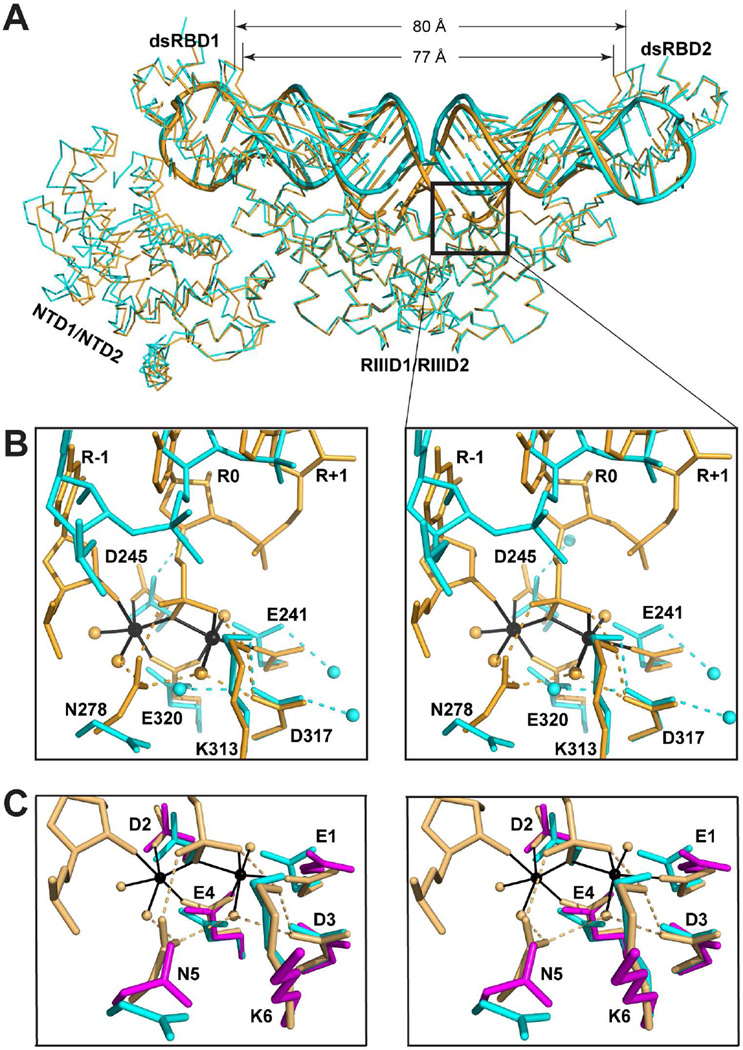
Comparative analysis of the substrate-loaded and post-cleavage structures shows that both the protein and the RNA exhibit significant conformational differences between the two states. Best alignment of the two structures was achieved on the basis of Cα positions in the RIIID dimer with the root-mean-square deviation (RMSD) of 0.52 Å for a total of 306 pairs of Cα positions. Two significant differences are seen. First, while the dsRNA in both structures adopts an A-form conformation, they differ dramatically near the scissile bonds (in box, Figure 5A). Second, subtle but significant overall conformational changes occur to the RNA in concert with the formation of the cleavage assembly as if the duplex is “squeezed” from two ends toward the middle portion by NTD1/NTD2/dsRBD1 on one end and dsRBD2 on the other (Figure 5A). Both the NTD1/NTD2/dsRBD1 and the dsRBD2 shift as rigid bodies. The RMSD for the Cα positions in the NTD1/NTD2/dsRBD1 is 0.52 Å between the two structures, which is identical to that for the RIIID dimer, and the RMSD for the Cα positions in the dsRBD2 is 0.34 Å between the two structures, which is even smaller than that for the RIIID dimer. As expected, the most important protein-RNA interactions for substrate selection are conserved in the post-cleavage and substrate-loaded complexes. The dsRBD1 recognizes the conserved guanine nucleotide in the second position of the AGUC tetraloop with the specialized RNA-binding motif, RBM0, a clamp-shaped pocket formed by eight amino acid residues as previously described (Liang et al., 2014).
Figure 5.
Comparison of crystal structures of yeast Rnt1p and human Drosha. (A) Functionally relevant distortion of Rnt1p-bound RNA. The A-form dsRNA (substrate-loaded, in cyan, this work) is distorted when the scissile bond nucleotides are pulled into the cleavage sites (post-cleavage, in orange, PDB entry 4OOG). The protein is shown as a Cα trace and the RNA as a tube-and-stick model. The box highlights the most distorted portion of RNA, and the distances are between the Cα positions of G409 in the two dsRBDs. (B) Stereoview showing the cleavage site architecture in the substrate-loaded (in cyan, this work) and post-cleavage (in orange, PDB entry 4OOG) structures. Amino acids and nucleotides are shown as stick models. Water molecules and Mg2+ ions are shown as spheres. Hydrogen bonds are indicated with dashed lines. Metal coordination bonds are indicated with solid lines. (C) Stereoview showing the catalytic side chain arrangement in the post-cleavage complex of Rnt1p (in orange, PDB entry 4OOG), the substrate-loaded complex of Rnt1p (in cyan, this work), and the RNA-free structure of human Drosha (in magenta, PDB entry 5B16). RNA and water molecules in the substrate-loaded complex are not shown for clarity.
In the post-cleavage complex of Rnt1p, the scissile bond nucleotides are pulled into the active center to form the cleavage assembly (in orange, Figure 5B) (Liang et al., 2014). In contrast, these nucleotides do not interact with any of the six catalytic side chains in the substrate-loaded complex (in cyan, Figure 5B). Previously, we compared the cleavage site architecture of the Rnt1p post-cleavage complex with RNA-free structures of Dicers from K. polysporus, G. intestinalis, and Homo sapiens, which showed substantial conformational differences of N5 and K6 side chains between the two states (Liang et al., 2014). The recent RNA-free structure of H. sapiens Drosha (Kwon et al., 2016) allowed us to compare the cleavage site architecture of Rnt1p with human Drosha, showing also substantial conformational changes of N5 and K6 side chains (Figure 5C). Interestingly, however, the K6 side chain of Rnt1p exhibits almost identical conformations in the substrate-loaded (in cyan, Figure 5C) and post-cleavage (in orange, Figure 5C) structures. Is it due to the presence of substrate RNA? An apo-Rnt1p structure at atomic resolution is warranted for an answer.
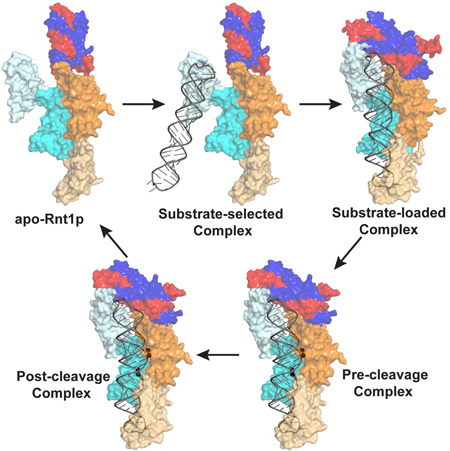
The Functional Cycle of Rnt1p Consists of Five Consecutive Steps
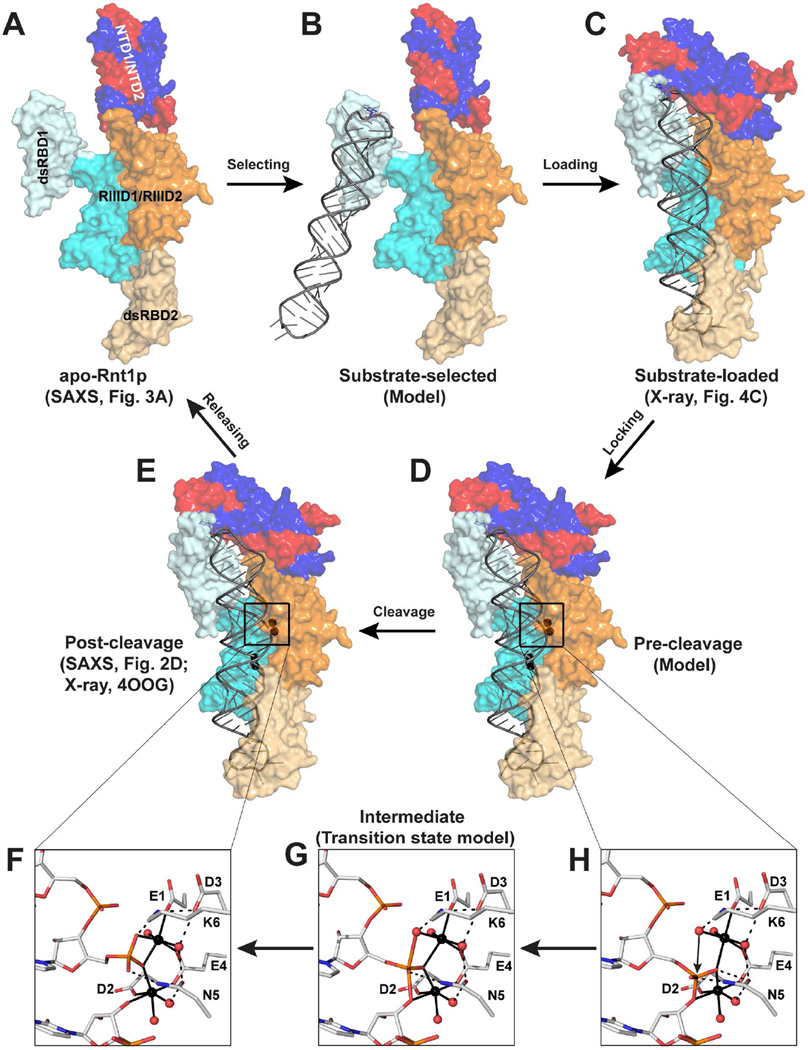
Like other members of the RNase III family, Rnt1p processes dsRNA by a single RNA cleavage event on each RNA strand to generate products with the 2-nt 3’ overhang. Unlike other RNase IIIs, however, Rnt1p mainly selects hairpin RNA substrates that are capped by a tetraloop bearing a guanine nucleotide in the second position (Gua16, Figure 2B). On the basis of the SAXS solution structure of apo-Rnt1p (Figure 6A) and the crystal structure of the substrate-loaded complex (Figure 6C), we predict the structure of a substrate-selected complex by including an RNA substrate via aligning the dsRBD1 in the substrate-loaded structure together with the RNA onto the dsRBD1 in the apo-Rnt1p structure. The resulting model represents a substrate-selected complex (Figure 6B) between apo-Rnt1p (Figure 6A) and substrate-loaded complex (Figure 6C). The Rnt1p pre-cleavage complex (Figure 6D), containing all elements for the chemical transition state of phosphoryl transfer, can be derived from the crystal structure of the post-cleavage complex (Figure 6E) (Liang et al., 2014) as described below.
Figure 6.
The functional cycle of Rnt1p and two-Mg2+-ion mechanism of eukaryotic RNase IIIs. (A) The SAXS structure of apo-Rnt1p shows that the ligand-free protein adopts an open conformation in solution (this work). (B) Model of substrate-selected complex, in which the dsRBD of Rnt1p recognizes a stem-loop RNA capped with an NGNN tetraloop. (C) The crystal structure of the substrate-loaded complex shows that the RNA substrate is properly loaded into the catalytic valley where the stem is held in place and the NTD dimer moves in to contact the tetraloop (this work). (D) Model of the pre-cleavage complex, illustrating that the active cleavage assembly is formed in the presence of two Mg2+ ions at each active site (this work). (E) The crystal structure of the post-cleavage complex (PDB entry 4OOG), showing (F) the architectural arrangement of the cleavage assembly immediately after the cleavage of the phosphodiester bond. (G) Model of the pentavalent phosphorus intermediate (transition state) of Rnt1p-catalyzed phosphoryl transfer reaction. (H) Model of the pre-cleavage complex, illustrating the roles played by each of the six catalytic side chains and two Mg2+ ions in the catalysis of eukaryotic RNase III enzymes. In panels F-H, amino acid and nucleotide residues are shown as stick models and Mg2+ ions and water oxygens as spheres in atomic color scheme (C, grey; N, blue; O, red; P, orange; S, yellow; and Mg, black). Metal coordination bonds are indicated with solid lines and hydrogen bonds with dashed lines.
All phosphoryl transfer reactions in DNA and RNA have been suggested to involve a pentacovalent intermediate and inversion of the stereo configuration at the phosphorus (Burgers and Eckstein, 1979; Yang et al., 2006). The post-cleavage complex structure (Figures 6E and 6F) is a snapshot of the protein-product complex immediately after the cleavage of the scissile bond. It offers an opportunity to derive the arrangement of the reaction intermediate at the transition state (intermediate, Figure 6G) and the catalytic site arrangement immediately before the transition state (pre-cleavage, Figures 6H and 6D). Previously, such intermediate and pre-cleavage complexes were derived for bacterial RNase III based on the crystal structure of the post-cleavage complex, visualizing the two-Mg2+-ion mechanism of phosphoryl transfer facilitated by four catalytic side chains (Gan et al., 2008).
Together, the three structures (Figures 6A, 6C, and 6E) and two structure-derived models (Figures 6B and 6D) represent five distinct states (substrate-free, substrate-selected, substrate-loaded, pre-cleavage, and post-cleavage) and five consecutive steps (selecting, loading, locking, cleavage, and releasing) of dsRNA processing by Rnt1p. In vivo, apo-Rnt1p exists as a dimer in the open conformation (Figure 6A). Similar to bacterial RNase III, it first binds an RNA substrate with one of its two dsRBDs. Unlike bacterial RNase III, it only selects a hairpin substrate by first recognizing the guanine nucleotide in the second position of the capping loop (Figure 6B). Then, the substrate will be properly loaded when the NTD dimer closes in and interacts with both the substrate and the dsRBD (Figure 6C). In the presence of Mg2+ ions and water molecules, each RNA strand will be locked into one cleavage site to form the pre-cleavage complex (Figure 6D). The hydrolysis of the two phosphodiester bonds will convert the pre-cleavage into post-cleavage complex (Figure 6E). Then, the products will depart from the post-cleavage complex and Rnt1p returns to its substrate-free form, which concludes one functional cycle of Rnt1p.
DISCUSSION
The SAXS Data Provide Accurate Structural Information
Despite the resolution limit of SAXS, we applied rigorous metrics and analyzed SAXS data in light of the crystal structures of Rnt1p and thus were able to obtain accurate structural information of Rnt1p in solution (Putnam et al., 2007; Rambo and Tainer, 2013b). Comparison of the ab initio shape envelope of the post-cleavage complex with the crystal structure revealed that the complex assumes the same conformation in solution. Similarly, comparison of the envelope of apo-Rnt1p with the crystal structure revealed that the protein adopts an open conformation in the substrate-free form, which was derived by rigid body modeling against the scattering profiles. The PDDFs of SAXS solution structures of apo-Rnt1p and post-cleavage complex are in good agreement with those calculated from the experimental data.
Specialized dsRBD of Rnt1p Can Also Function as a Normal dsRBD
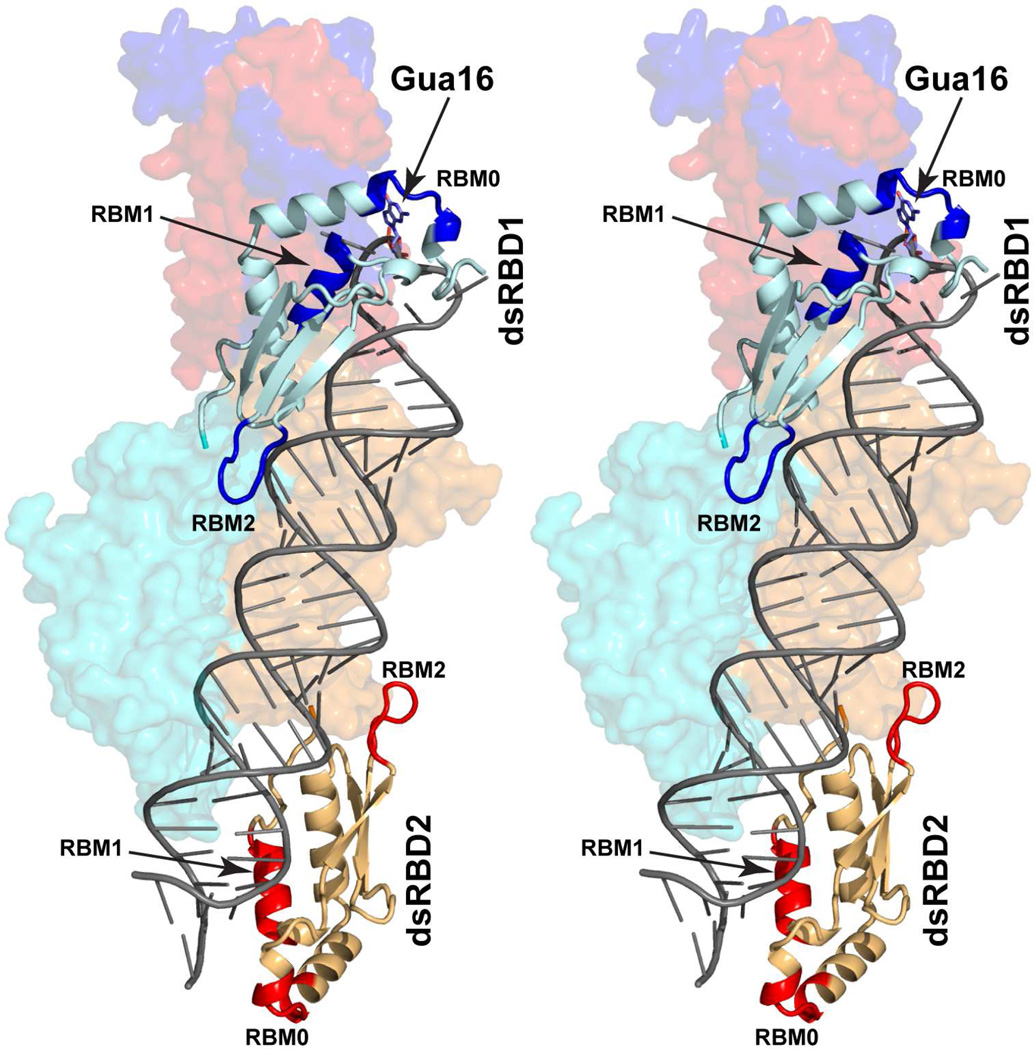
The dsRBD of bacterial RNase III contains two RNA-binding motifs, RBM1 that recognizes four O2’ hydroxyls of substrate RNA and RBM2 that projects itself into the minor groove of bound RNA (Gan et al., 2006). The dsRBD of Rnt1p is specialized by forming the additional RBM0, which specifically recognizes Gua16 in the second position of the 4-nt capping loop of substrate RNA (Liang et al., 2014). Each substrate has one Gua16, and therefore, one dsRBD (as shown, dsRBD1) is sufficient for substrate selection (Lamontagne et al., 2004) (Figure 6B). When the substrate is loaded, the other dsRBD (dsRBD2) binds to the stem of the substrate RNA with its RBM1 and RBM2, without using the RBM0. A model for the substrate-loaded complex containing a stem-loop RNA substrate is shown in Figure 7, illustrating that both dsRBD1 and dsRBD2 bind to the RNA stem with respective RBMs 1 and 2, while only dsRBD1 recognizes the tetraloop with RBM0. The model also shows that when RBM0 is not used, it does not interfere with substrate binding such that the specialized dsRBD of Rnt1p functions just as a normal dsRBD. The binding mode of Rnt1p’s dsRBD without recognizing Gua16 was seen in two structures of dsRBD in complex with stem-loop RNA (Wang et al., 2011; Wu et al., 2004).
Figure 7.
Model for Rnt1p substrate-loaded complex, containing a stem-loop RNA substrate, in stereo. The model was built on the basis of the crystal structure of Rnt1p substrate-loaded complex (this work) in three steps. First, the tetraloop that contacts dsRBD2 was removed; second, the stem of the remaining stem-loop RNA was extended by addition of three basepairs; and third, the stem-loop RNA (upper) and the RNA duplex (lower) are connected. This model shows different RNA-binding modes of the two dsRBDs. The dsRBD1 recognizes Gua16 in the tetraloop of substrate RNA with RBM0 and also binds to the RNA stem with RBMs 1 and 2. In contrast, the dsRBD2 only binds to the RNA stem with RBMs 1 and 2. The model also shows that when RBM0 is not in use, it does not interfere with dsRNA-binding function of dsRBD. The RIIID dimer (in cyan and orange) and NTD dimer (in red and blue) are illustrated as molecular surfaces. The dsRBDs (in pale-cyan and light-orange) are shown as ribbon diagrams (helices as spirals, strands as arrows, and loops as tubes). The RNA substrate is represented with a tube-and-stick model. The RBMs in dsRBD1 are colored in blue and those in dsRBD2 in red. For clarity, the NTD dimer and the RIIID dimer are not labeled
There are two dsRBDs in the homodimer of small RNase III proteins that contain one RIIID in their sequences. Previously, we showed that bacterial RNase III selects a substrate with one of the two dsRBDs in the homodimer of the enzyme (Court et al., 2013; Gan et al., 2005). Here, we show that a eukaryotic RNase III selects a substrate also with one of the two dsRBDs in the homodimer of the protein (Figure 6B). Following the initial substrate selection by one dsRBD, the other dsRBD helps secure the substrate for cleavage by the RIIID dimer. Each of Drosha and Dicer, however, contains an intramolecular RIIID dimer followed by one dsRBD (Figure 1A). Although the single dsRBD is sufficient for initial substrate selection, it must not be enough for substrate binding and cleavage. Therefore, both Drosha and Dicer function with partner proteins. Drosha forms the microRNA Microprocessor complex with DGCR8 that contains two dsRBDs (Wostenberg et al., 2010). Dicer functions with TRBP and PACT, each of which contains three dsRBDs (Haase et al., 2005; Kok et al., 2007; Lee et al., 2006). Structures of functional complexes of Drosha and Dicer are critical for our understanding the detailed mechanisms of their action.
The Two-Mg2+-Ion Catalytic Mechanism of Rnt1p Is Applicable to All Eukaryotic RNase III Enzymes
The center of RNase III machinery is the RIIID dimer featured by a catalytic valley in which two cleavage sites form the dsRNA-processing center. Each RNA strand of the bound substrate aligns at a cleavage site, and the two sites are spaced such that the cleavage of both stands creates the 2-nt 3’ overhangs on the product ends (Gan et al., 2006; Ji, 2008). Each cleavage site of eukaryotic RNase III requires six catalytic side chains, among which the general organization of four (E1, D2, D3 and E4) is preserved from bacteria to human, whereas the involvement of the other two (N5 and K6) is unique for eukaryotes (Figure 1B).
The substrate-loaded complex of Rnt1p represents a stage before forming the pre-cleavage complex in the functional cycle of Rnt1p (Figure 6). As aforementioned, the cleavage site assembly in the post-cleavage complex represents a snapshot of the catalytic stage immediately after the hydrolysis of the scissile bond (Liang et al., 2014). On the basis of the post-cleavage structure (Figure 6F), the cleavage site assembly at the transition (Figure 6G) and pre-cleavage (Figure 6H) states can be reliably derived.
At the pre-cleavage state (Figure 6H), one Mg2+ ion activates the nucleophile OH− by lowering the pKa of a water molecule that is located 3.0 Å away from the 5’ phosphorus of the scissile bond. The catalytic residues N5 and K6, unique for eukaryotic RNase III enzymes, may also play important roles at this stage. On the one hand, N5 will change from outward to inward conformation to form direct hydrogen bonds with the 5’ phosphate group. On the other hand, the positively charged ε-amino group of K6 stabilizes and orients the nucleophile OH− for the in-line attack on the 5’ phosphorus, leading to the trigonal-bipyramidal pentavalent phosphorane intermediate (Figure 6G). At the transition state, N5 and K6 form hydrogen bonds with the pentavalent phosphorane group and stabilize the additional incipient negative charge in the reaction intermediate. In the end, the phosphodiester bond is broken, resulting in the post-cleavage complex (Figure 6F). This two-Mg2+-ion mechanism of scissile bond cleavage, facilitated by six catalytic side chains, is applicable to all eukaryotic RNase III enzymes, including Drosha and Dicer.
A Single Cleavage Mechanism Accommodates Different Substrate Specificity
Eukaryotic RNase IIIs differ dramatically in protein size and domain structure (Figure 1A). They also differ in substrate specificity and partner protein dependence. Rnt1p has the smallest N-terminal extension and the most strict sequence specificity. Therefore, it is the best model system for eukaryotic RNase IIIs. The results indicate that Rnt1p form two major conformations, an open conformation (Figures 6A and 6B) and a closed conformation (Figures 6C, 6D, and 6E). In both forms, the NTD forms an intertwined dimer that positions itself at one end of the RIIID dimer. Also in both forms, one dsRBD is located near the same end as the NTD dimer while the other dsRBD is located at the opposite end (Figure 6A). In the open form, the NTD dimer and the two dsRBDs are wide open (Figures 3C, 6A, and 6B), allowing substrate selection and loading. In the closed form, the NTD dimer and the two dsRBDs are closed, facilitating substrate locking and cleavage (Figures 3C, 6C, 6D, and 6E). Overall, these results indicate that substrate recognition is achieved independent of cleavage, permitting the recognition of substrates with different structures while preserving the basic mechanism of cleavage.
EXPERIMENTAL PROCEDURES
Crystallization, Data Collection, and Structure Determination
The Rnt1p protein was overexpressed and purified as described (Liang et al., 2014). The RNA substrate analog (Figure 4A) was commercially synthesized by SeNA Research, Inc. (Marietta, GA). The substrate-loaded complex was prepared by incubating the purified protein with the RNA at a molar ratio of 1:1.5 at 32 °C for 30 min. Crystals were grown in the reservoir solution containing 0.2 M di-ammonium citrate and 20% (v/v) polyethylene glycol (PEG) 3350 by sitting drop vapor diffusion at 20 °C. For data collection, the crystals were soaked in reservoir solution containing 20% (v/v) ethylene glycol and flash-frozen in liquid nitrogen. The X-ray (λ = 1.0 Å) diffraction data were collected at −173 °C using a Rayonix MX3000HS detector at the 22-ID beamline of SER-CAT of Advanced Photon Source (APS), Argonne National Laboratory. The X-ray data was processed using program HKL3000 (HKL Research, Inc). The structure was solved by molecular replacement using the PHASER program (McCoy, 2007) embedded in the PHENIX suite (Echols et al., 2012) with the post-cleavage structure of Rnt1p (PDB entry 4OOG) (Liang et al., 2014) as a search model. Programs Coot (Emsley and Cowtan, 2004) and Phenix.refine (Afonine et al., 2012) were used for model building and structure refinement. The final structure was evaluated by the validation server of wwPDB and did not show any Ramachandran outliers. Data collection and structure statistics are summarized in Table 2. Coordinates and structure factors have been deposited in the Protein Data Bank under accession code PDB 5T16.
Small-Angle X-Ray Scattering Analysis
The Rnt1p protein was prepared as described (Liang et al., 2014). The RNA product (Figure 2B) was purchased from GE Dhamacon, Inc. (Lafayette, CO). The SAXS data were recorded at the APS beamline 12-ID-B, using a PILATUS 1M detector (Dectris) for small-angle and a PILATUS 100k detector (Dectris) for wide-angle X-ray scattering. The wavelength λ of X-ray radiation was 0.8856 Å and the momentum transfer q was recorded in a range of 0.006–2.8Å−1 (q=(4π/λ)sinθ, where 2θ is the scattering angle). Two samples, apo-Rnt1p and post-cleavage complex, were individually loaded onto a Superdex 200 column (GE Healthcare) before data collection. The column was pre-equilibrated in SAXS buffer (200 mM NaCl, 25 mM MgCl2, and 2 mM β-mercaptoethanol in 25 mM Tris at pH 7.4), and the peak fractions were collected and concentrated to 3 mg/ml. The scattering profiles of each sample were measured in four solute concentrations (8-, 4-, 2-fold dilution and stock solution) to remove the scattering contribution due to inter-particle interactions and to extrapolate the data to infinite dilution. Thirty two-dimensional images were recorded for each buffer or sample solution using a flow cell, with the exposure time of 1–2 seconds per image to minimize radiation damage and to obtain good signal-to-noise ratio. The images were reduced on site to one-dimensional scattering profiles using the Matlab software package.
Analysis of the SAXS data was performed using IGOR-Pro (WaveMetrics) and ATS AS suite (Petoukhov et al., 2012). The forward scattering intensity I(0) and the radius of gyration (Rg) were calculated from the data of infinite dilution at low q values in the range of qR g< 1.3, using the Guinier approximation in PRIMUS (Konarev et al., 2003). These parameters were also estimated from the scattering profile with a broader q range of 0.006–0.30 Å− using the indirect Fourier transform method implemented in the program GNOM (Svergun, 1992), along with the pair distance distribution function (PDDF) and the maximum dimension of the protein (Dmax). The molecular weight was calculated by SAXS MoW method (Fischer et al., 2010), which is independent of protein concentration. Low resolution ab initio shape envelopes were calculated using DAMMIN (Svergun, 1999), which generates models represented by an ensemble of densely packed beads, using scattering profiles within the q range of 0.006–0.30 Å−1. A total of 20 independent models were created and averaged by DAMAVER (Volkov and Svergun, 2003), superimposed by SUPCOMB (Kozin and Svergun, 2001) based on the normalized spatial discrepancy criteria, and filtered using DAMFILT to generate the final model.
For the fitting of the structures, the missing terminal portions (residues 1–41 and 458–472) and inter-domain linkers (residue 152–196) are added and treated as random polypeptide chains. For apo-Rnt1p, the rigid body modeling was performed using the Xplor-NIH package (Schwieters et al., 2006; Schwieters et al., 2003). The starting structure was prepared from the crystal structure of Rnt1p post-cleavage complex. The NTD dimer, RIIID dimer, and two dsRBDs were defined as four rigid bodies, and the relative position and orientation of these rigid bodies were optimized, resulting in models that best fit into the experimental scattering data in the range of scattering vectors up to q = 0.30 Å−1. The theoretical scattering intensity of the atomic structure models was calculated and fitted to the experimental scattering intensity using CRYSOL (Svergun et al., 1995), and the model-data agreement parameter, χ2free, was calculated using Scatter (Rambo and Tainer, 2013a).
HIGHLIGHTS.
Rnt1p, the yeast RNase III enzyme, has two major conformations
The functional cycle of Rnt1p can be described in five steps
The two-Mg2+-ion mechanism of Rnt1p is applicable to all eukaryotic RNase IIIs
Substrate selection by RNase IIIs is achieved independent of cleavage
Acknowledgments
We thank Dr. Xiaobing Zuo for assistance with the SAXS experiments and Drs. Sherif Abou Elela, Donald Court, and Alexander Wlodawer for critical reading of the manuscript and insightful discussions. X-ray diffraction data were collected at the SER-CAT beamlines of the Advanced Photon Source (APS), Argonne National Laboratory (ANL). SAXS data were collected at the APS beamline 12-ID-B, ANL. Use of the APS was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under contract No. DE-AC02-06CH11357 and under the Partner User Proposal PUP No. 22978. This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ACCESSION NUMBER
The coordinates and structure factors of Rnt1p in complex with non-hydrolyzable RNA substrate analog have been deposited in the PDB (http://www/rcsb.org/pdb/) under the accession code 5T16.
AUTHOR CONTRIBUTIONS
H.S., X.F., Y.-X.W., and X.J. conceived the project. H.S., L.J., and G.X.S. prepared and characterized the protein samples. X.F. and H.S. performed the X-ray scattering experiments. H.S. performed the X-ray diffraction experiments. Y.-X.W. and X.J. supervised the research. H.S. and X.J. interpreted the structures and wrote the manuscript with inputs from all authors.
REFERENCES
- Afonine PV, Grosse-Kunstleve RW, Echols N, Headd JJ, Moriarty NW, Mustyakimov M, Terwilliger TC, Urzhumtsev A, Zwart PH, Adams PD. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. D. 2012;68:352–367. doi: 10.1107/S0907444912001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaszczyk J, Gan J, Tropea JE, Court DL, Waugh DS, Ji X. Noncatalytic assembly of ribonuclease III with double-stranded RNA. Structure (Camb) 2004;12:457–466. doi: 10.1016/j.str.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Blaszczyk J, Tropea JE, Bubunenko M, Routzahn KM, Waugh DS, Court DL, Ji X. Crystallographic and modeling studies of RNase III suggest a mechanism for double-stranded RNA cleavage. Structure. 2001;9:1225–1236. doi: 10.1016/s0969-2126(01)00685-2. [DOI] [PubMed] [Google Scholar]
- Burgers PM, Eckstein F. Stereochemistry of internucleotide bond formation by polynucleotide phosphorylase from Micrococcus luteus . Biochemistry. 1979;18:450–454. doi: 10.1021/bi00570a010. [DOI] [PubMed] [Google Scholar]
- Court DL. RNA processing and degradation by RNase III. In: Belasco JG, Brawerman G, editors. Control of Messenger RNA Stability. New York: Academic Press; 1993. pp. 71–116. [Google Scholar]
- Court DL, Gan J, Liang Y-H, Shaw GX, Tropea JE, Costantino N, Waugh DS, Ji X. RNase III: Genetics and Function; Structure and Mechanism. Annu. Rev. Genet. 2013;47:405–431. doi: 10.1146/annurev-genet-110711-155618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echols N, Grosse-Kunstleve RW, Afonine PV, Bunkoczi G, Chen VB, Headd JJ, McCoy AJ, Moriarty NW, Read RJ, Richardson DC, et al. Graphical tools for macromolecular crystallography in PHENIX. J. Appl. Crystallogr. 2012;45:581–586. doi: 10.1107/S0021889812017293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- Fischer H, Neto MD, Napolitano HB, Polikarpov I, Craievich AF. Determination of the molecular weight of proteins in solution from a single small-angle X-ray scattering measurement on a relative scale. J. Appl. Crystallogr. 2010;43:101–109. [Google Scholar]
- Gan J, Shaw G, Tropea JE, Waugh DS, Court DL, Ji X. A stepwise model for double-stranded RNA processing by ribonuclease III. Mol. Microbiol. 2008;67:143–154. doi: 10.1111/j.1365-2958.2007.06032.x. [DOI] [PubMed] [Google Scholar]
- Gan J, Tropea JE, Austin BP, Court DL, Waugh DS, Ji X. Intermediate states of ribonuclease III in complex with double-stranded RNA. Structure (Camb) 2005;13:1435–1442. doi: 10.1016/j.str.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Gan J, Tropea JE, Austin BP, Court DL, Waugh DS, Ji X. Structural insight into the mechanism of double-stranded RNA processing by ribonuclease III. Cell. 2006;124:355–366. doi: 10.1016/j.cell.2005.11.034. [DOI] [PubMed] [Google Scholar]
- Gu S, Jin L, Zhang Y, Huang Y, Zhang F, Valdmanis PN, Kay MA. The loop position of shRNAs and pre-miRNAs is critical for the accuracy of dicer processing in vivo. Cell. 2012;151:900–911. doi: 10.1016/j.cell.2012.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase AD, Jaskiewicz L, Zhang H, Laine S, Sack R, Gatignol A, Filipowicz W. TRBP, a regulator of cellular PKR and HIV-1 virus expression, interacts with Dicer and functions in RNA silencing. EMBO reports. 2005;6:961–967. doi: 10.1038/sj.embor.7400509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji X. The mechanism of RNase III action: How Dicer dices. Curr. Top. Microbiol. Immunol. 2008;320:99–116. doi: 10.1007/978-3-540-75157-1_5. [DOI] [PubMed] [Google Scholar]
- Kakar S, Fang XY, Lubkowska L, Zhou YN, Shaw GX, Wang YX, Jin DJ, Kashlev M, Ji XH. Allosteric Activation of Bacterial Swi2/Snf2 (Switch/Sucrose Non-fermentable) Protein RapA by RNA Polymerase BIOCHEMICAL AND STRUCTURAL STUDIES. J. Biol. Chem. 2015;290:23656–23669. doi: 10.1074/jbc.M114.618801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karplus PA, Diederichs K. Linking crystallographic model and data quality. Science. 2012;336:1030–1033. doi: 10.1126/science.1218231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok KH, Ng MH, Ching YP, Jin DY. Human TRBP and PACT directly interact with each other and associate with dicer to facilitate the production of small interfering RNA. J. Biol. Chem. 2007;282:17649–17657. doi: 10.1074/jbc.M611768200. [DOI] [PubMed] [Google Scholar]
- Konarev PV, Volkov VV, Sokolova AV, Koch MHJ, Svergun DI. PRIMUS: a Windows PC-based system for small-angle scattering data analysis. J. Appl. Crystallogr. 2003;36:1277–1282. [Google Scholar]
- Kozin MB, Svergun DI. Automated matching of high- and low-resolution structural models. J. Appl. Crystallogr. 2001;34:33–41. [Google Scholar]
- Kwon SC, Nguyen TA, Choi YG, Jo MH, Hohng S, Kim VN, Woo JS. Structure of Human DROSHA. Cell. 2016;164:81–90. doi: 10.1016/j.cell.2015.12.019. [DOI] [PubMed] [Google Scholar]
- Lamontagne B, Hannoush RN, Damha MJ, Abou Elela S. Molecular requirements for duplex recognition and cleavage by eukaryotic RNase III: discovery of an RNA-dependent DNA cleavage activity of yeast Rnt1p. J. Mol. Biol. 2004;338:401–418. doi: 10.1016/j.jmb.2004.02.059. [DOI] [PubMed] [Google Scholar]
- Lamontagne B, Tremblay A, Abou Elela S. The N-terminal domain that distinguishes yeast from bacterial RNase III contains a dimerization signal required for efficient double-stranded RNA cleavage. Mol. Cell Biol. 2000;20:1104–1115. doi: 10.1128/mcb.20.4.1104-1115.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebars I, Lamontagne B, Yoshizawa S, Aboul-Elela S, Fourmy D. Solution structure of conserved AGNN tetraloops: insights into Rnt1p RNA processing. EMBO J. 2001;20:7250–7258. doi: 10.1093/emboj/20.24.7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Hur I, Park SY, Kim YK, Suh MR, Kim VN. The role of PACT in the RNA silencing pathway. EMBO J. 2006;25:522–532. doi: 10.1038/sj.emboj.7600942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang YH, Lavoie M, Comeau MA, Abou Elela S, Ji X. Structure of a eukaryotic RNase III postcleavage complex reveals a double-ruler mechanism for substrate selection. Mol. Cell. 2014;54:431–444. doi: 10.1016/j.molcel.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRae IJ, Zhou K, Li F, Repic A, Brooks AN, Cande WZ, Adams PD, Doudna JA. Structural basis for double-stranded RNA processing by Dicer. Science. 2006;311:195–198. doi: 10.1126/science.1121638. [DOI] [PubMed] [Google Scholar]
- McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. Phaser crystallographic software. J. Appl. Crystallogr. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson AW. Ribonuclease III mechanisms of double-stranded RNA cleavage. Wiley interdisciplinary reviews. RNA. 2014;5:31–48. doi: 10.1002/wrna.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petoukhov MV, Franke D, Shkumatov AV, Tria G, Kikhney AG, Gajda M, Gorba C, Mertens HDT, Konarev PV, Svergun DI. New developments in the ATSAS program package for small-angle scattering data analysis. J. Appl. Crystallogr. 2012;45:342–350. doi: 10.1107/S0021889812007662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam CD, Hammel M, Hura GL, Tainer JA. X-ray solution scattering (SAXS) combined with crystallography and computation: defining accurate macromolecular structures, conformations and assemblies in solution. Q. Rev. Biophys. 2007;40:191–285. doi: 10.1017/S0033583507004635. [DOI] [PubMed] [Google Scholar]
- Rambo RP, Tainer JA. Accurate assessment of mass, models and resolution by small-angle scattering. Nature. 2013a;496:477–481. doi: 10.1038/nature12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambo RP, Tainer JA. Super-Resolution in Solution X-Ray Scattering and Its Applications to Structural Systems Biology. Annu. Rev. Biophys. 2013b;42:415–441. doi: 10.1146/annurev-biophys-083012-130301. [DOI] [PubMed] [Google Scholar]
- Robertso HD, Webster RE, Zinder ND. Purification and Properties of Ribonuclease 3 from Escherichia Coli. J. Biol. Chem. 1968;243:82. [PubMed] [Google Scholar]
- Schwieters CD, Kuszewski JJ, Clore GM. Using Xplor-NIH for NMR molecular structure determination. Progr. NMR Spectroscopy. 2006;48:47–62. [Google Scholar]
- Schwieters CD, Kuszewski JJ, Tjandra N, Clore GM. The Xplor-NIH NMR molecular structure determination package. J. Magn. Reson. 2003;160:65–73. doi: 10.1016/s1090-7807(02)00014-9. [DOI] [PubMed] [Google Scholar]
- Svergun D, Barberato C, Koch MHJ. CRYSOL - A program to evaluate x-ray solution scattering of biological macromolecules from atomic coordinates. J. Appl. Crystallogr. 1995;28:768–773. [Google Scholar]
- Svergun DI. Determination of the regularization parameter in indirect-transform methods using perceptual criteria. J. Appl. Crystallogr. 1992;25:495–503. [Google Scholar]
- Svergun DI. Restoring low resolution structure of biological macromolecules from solution scattering using simulated annealing. Biophys. J. 1999;76:2879–2886. doi: 10.1016/S0006-3495(99)77443-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rij RP, Andino R. The silent treatment: RNAi as a defense against virus infection in mammals. Trends Biotechnol. 2006;24:186–193. doi: 10.1016/j.tibtech.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Volkov VV, Svergun DI. Uniqueness of ab initio shape determination in small-angle scattering. J. Appl. Crystallogr. 2003;36:860–864. doi: 10.1107/S0021889809000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Hartman E, Roy K, Chanfreau G, Feigon J. Structure of a yeast RNase III dsRBD complex with a noncanonical RNA substrate provides new insights into binding specificity of dsRBDs. Structure. 2011;19:999–1010. doi: 10.1016/j.str.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg DE, Nakanishi K, Patel DJ, Bartel DP. The inside-out mechanism of Dicers from budding yeasts. Cell. 2011;146:262–276. doi: 10.1016/j.cell.2011.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wostenberg C, Quarles KA, Showalter SA. Dynamic origins of differential RNA binding function in two dsRBDs from the miRNA “microprocessor” complex. Biochemistry. 2010;49:10728–10736. doi: 10.1021/bi1015716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Henras A, Chanfreau G, Feigon J. Structural basis for recognition of the AGNN tetraloop RNA fold by the double-stranded RNA-binding domain of Rnt1p RNase III. Proc. Natl. Acad. Sci. USA. 2004;101:8307–8312. doi: 10.1073/pnas.0402627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Xu H, Miraglia LJ, Crooke ST. Human RNase III is a 160-kDa protein involved in preribosomal RNA processing. J. Biol. Chem. 2000;275:36957–36965. doi: 10.1074/jbc.M005494200. [DOI] [PubMed] [Google Scholar]
- Yang W, Lee JY, Nowotny M. Making and breaking nucleic acids: two-Mg2+-ion catalysis and substrate specificity. Mol. Cell. 2006;22:5–13. doi: 10.1016/j.molcel.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Zamore PD. Thirty-three years later, a glimpse at the ribonuclease III active site. Mol. Cell. 2001;8:1158–1160. doi: 10.1016/s1097-2765(01)00418-x. [DOI] [PubMed] [Google Scholar]