Abstract
Humans are walking microbial ecosystems, each harboring a complex microbiome with the genetic potential to produce a vast array of natural products. Recent sequencing data suggest that our microbial inhabitants are critical for maintaining overall health. Shifts in microbial communities have been correlated to a number of diseases including infections, inflammation, cancer, and neurological disorders. Some of these clinically and diagnostically relevant phenotypes are a result of the presence of small molecules, yet we know remarkably little about their contributions to the health of individuals. Here, we review microbe-derived natural products as mediators of human disease.
1. INTRODUCTION
Microbial natural products are integral to human health. They are heavily utilized in medicine as antibiotics, antifungals, immunosuppressants, and anticancer agents.1–3 Advances in sequencing technologies and bioinformatics approaches have revealed an untapped reservoir of natural products in hosts with diverse and biosynthetically rich microbial ecosystems, including humans.3–8 It is estimated that microbial cells outnumber mammalian cells 1.3 to 1 in the human body, with a combined genetic potential that exceeds the number of human genes 100-fold.7, 9, 10 Correlation of shifts in microbiome community structure to a number of diseases including irritable bowel syndrome,11–14 obesity,15, 16 and arthritis,17–19 strongly suggests that the functional structure of host-associated ecosystems is incredibly important for maintaining host health.20 However, it should be noted that correlation is not necessarily indicative of causality.
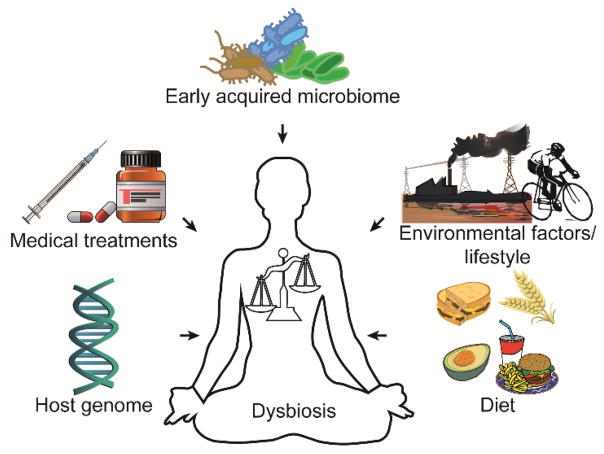
There are two prevailing models for microbial dysbiosis.21 In the first model, a shift in community composition due to infection with an outside agent, overgrowth of a commensal, or treatment with antibiotics leads to a weakened host immune response and opportunistic microbes overgrowing their typical niche. The second model suggests that altered host biology and reduced immune function due to injury, genetic predisposition, or chronic conditions can lead to microbial community compositional changes, microbial overgrowth, and altered immune response. Several factors implicated in microbial dysbiosis are highlighted in Figure 1.22, 23 Both proposed models suggest microbial interactions including metabolic exchange of natural products may be an important factor in continuous microbial community dysbiosis and, by extension, disease. Natural products provide an ecological advantage for the producing microbe by enabling competition for nutritional resources against competing microbes and the host tissue.
Figure 1. Factors Affecting Microbial Dysbiosis.
Microbiome composition can be affected by a number of factors including, but not limited to, genetic predisposition, clinical treatment, microbiome acquired at birth, diet, and other lifestyle factors.
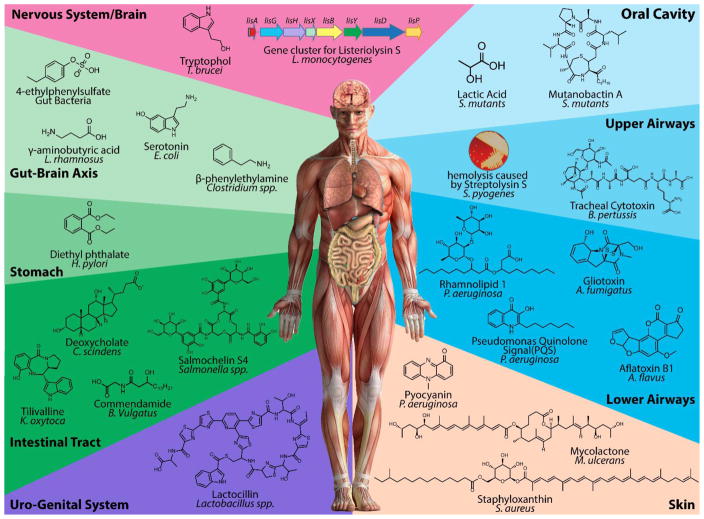
Here, we review the role of select microbe-derived natural products in mediating disease in humans (Figure 2). The comprehensive review of all small molecule virulence factors is beyond the scope of a single article and many high quality reviews have been published demonstrating the importance of quorum sensing,24–26 siderophores,27, 28 short chain fatty acids (SCFAs),29–31 and single metabolites such as (pre-)colibactin.32, 33 Therefore, our emphasis is on brief examples of the role of specific metabolites as mediators of disease for the major human organ systems which may be of interest to the natural products community. We further describe emerging technologies, propose potential strategies, and highlight specific challenges for natural product discovery from the human microbiome.
Figure 2. Microbial-derived Natural Products in Human Disease.
Microbes involved in mediating human disease produce a structurally diverse array of natural products. While we illustrate characterized molecules, many metabolites involved in microbe-driven disease remain structurally unresolved including streptolysin S and listerioslysin S, metabolites recognized for their hemolytic activities.
2. NATURAL PRODUCTS IN HUMAN DISEASE
The association of microbial diseases with the human host was first discovered in the late 17th century when Antony van Leeuwenhoek visualized his own dental plaque using a microscope. However, the concept that microbes cause some diseases was proposed as early as the mid-16th century. This theory was further substantiated by the work of a number of prominent scientists including Louis Pasteur and Robert Koch through the second half of the 19th century. The discovery of penicillin in 1928 by Alexander Fleming ushered in the antibiotic era for treatment of microbial diseases.34 During this time, researchers recognized that human hosts are inhabited by a large number of microbes, yet only a few were identified as causing disease. This observation led to the hypothesis that these microbes were unique and could be classified as pathogenic microbes. Classification of pathogenic microbes was established by a microbe’s expression of determinants of disease, otherwise referred to as virulence factors. While the definition of virulence factors is broad, one category includes natural products and other small molecules with specialized function.35 The small molecule virulence factors of several common pathogenic microbes have been thoroughly studied. However, specialized metabolite production remains uncharacterized for the majority of human-associated microbes. Recent bioinformatics analyses of specialized metabolite biosynthetic gene clusters (BGCs) applied to metagenomics data from the National Institutes of Health (NIH) Human Microbiome Project (HMP) revealed over 3,000 small molecule BGCs belonging to all natural product molecular families, although very few BGCs were widely distributed.5 The number of BGCs detected is likely an underestimate of the true biosynthetic potential of the microbiota due to the stringent search parameters used. While the total number of BGCs detected in human microbiome is smaller than the number of BGCs from other environments,5, 36, 37 the biosynthetic potential of the microbiome suggests that natural products play a critical role in maintaining microbial community structure and the microbiome remains an untapped reservoir for natural products research.
2.1 SKIN
The skin is our largest organ with a surface area between 1.5 and 2 square meters. It is also our most exposed organ, providing the interface between our immune system and the external environment including its associated microbiota. The resident skin microbial communities are highly variable across the body and between individuals, with colonization driven by a number of host and environmental factors.38–40 Most of the approximately 1,000 microbial species inhabiting our skin are harmless or beneficial, although several species have been implicated in dermatological diseases and chronic wounds.21, 41, 42 The fungal genera Malassezia, the bacterium Propionibacterium acnes, and the opportunistic pathogen Staphylococcus aureus have been correlated to dermatological diseases such as seborrheic dermatitis, acne vulgaris, and atopic dermatitis (AD), respectively.21, 41, 42 It has been proposed that Malassezia ssp. produce lipases which release fatty acids, thereby promoting epidermal hyperproliferation and inflammation.43, 44 However, the role of other fungal natural products in skin disease remains largely uninvestigated. Similarly, the bacterial skin commensal P. acnes has been shown to degrade and utilize host lipids resulting in the characteristic inflammation associated with acne vulgaris.45–49 P. acnes strains have been shown to produce propionate, tyramine, histamine, structurally-uncharacterized thiocillins, and putative natural products which have been described in only the most general terms as heat stable neutrophil chemotactic factors.50–54 S. aureus, a well-studied opportunistic pathogen, is associated with the development of AD. However, no specific link between S. aureus virulence factors and AD flare-ups has been identified.55, 56
Opportunistic infections of the skin affect a wide population, often occurring as chronic wounds in diabetic, elderly, and immobile people or from severe burns.57, 58 While the microbes involved in chronic infections do not cause the initial wound, they are associated with slower healing and persistent inflammation. Culture-based analyses revealed that chronic wound infections arise from a patient’s commensal microbiota (skin, gastro-intestinal, or respiratory) or are nosocomial in nature.59–61 Bacteria are the best characterized drivers of most chronic infections, although infections can also occur due to viruses, fungi, and yeast.62 Only a few natural products have been associated with microbes responsible for chronic infections, despite the wide array of microbial species detected in or isolated from chronic wounds.
Of the microbes investigated for their role in chronic wounds, Streptococcus pyogenes, Pseudomonas aeruginosa, S. aureus, and Mycobacterium ulcerans have been partially characterized. S. pyogenes (also referred to as group A streptococci or GAS) is a commensal skin and oral microbe that is implicated in a variety of skin diseases and opportunistic infections.63 One of the key natural products produced by GAS is streptolysin S (SLS), a potent 2.7 kDa cytotoxic peptide of the bacteriocin family of antimicrobial peptides responsible for the lysis of erythrocytes, leukocytes, platelets, and subcellular organelles.64–66 Interestingly, SLS is only cytotoxic in the presence of carrier molecules or when associated with the bacterial cell surface. The SLS BGC was recently identified and homologous BGCs were found in other streptococci species and Gram-positive pathogens including Listeria monocytogenes, Clostridium botulinum, and S. aureus. The structures of SLS and related peptides remain elusive. Compared to S. pyogenes, the specialized metabolite production by P. aeruginosa has been more thoroughly investigated.25, 67–71 However, only the phenazine pyocyanin (1, PYO) has been studied in the context of chronic wounds and was found to impair wound repair mechanisms.72 Molecules produced by S. aureus during skin infections include staphyloxanthin (2)73–75 and the phenol soluble modulins (PSMs) including delta-toxin.76–78 Staphyloxanthin is a carotenoid pigment proposed to scavenge reactive oxygen species, allowing S. aureus to circumvent the immune response.73 PSMs are a family of alpha-helical peptides with concentration-dependent activity.79, 80 At low nanomolar concentrations, several PSMs induce cytokine release, while at micromolar levels they induce neutrophil lysis. PSMs have also been postulated to allow S. aureus to evade immune system detection by reducing dendritic cell mediated differentiation of T-helper cells. Higher concentrations of PSMs by community associated methicillin resistant S. aureus (MRSA) were shown to be critical for neutrophil lysis and full virulence.78 Intriguingly, treatment of S. aureus with antibiotics leads to the production of PSM structural variations via truncation of the peptide and amino acid substitutions.77 Production concentrations of PSMs are much lower from the S. aureus related commensal species Staphylococcus epidermidis, explaining its reduced virulence.81 It has been suggested that some strains of S. epidermidis may inhibit potential pathogens by enhancing production of human antimicrobial peptides.82–84 Lastly, Buruli ulcers are the third most common mycobacteriosis in humans after tuberculosis and lepracy. The mycolactone (3) polyketides (PKs) produced by M. ulcerans have been implicated in ulcer development.85 Mycolactone production has been associated with strain-specific virulence phenotypes.86 The production and biological roles of structurally distinct mycolactones from pathogenic Mycobacterium species have been thoroughly reviewed.87–89 In vivo testing of mycolactones A and B in guinea pigs resulted in lesions similar to the Buruli ulcers developed by humans infected with M. ulcerans. Despite the severity of Buruli ulcers, the extensive skins lesions are not accompanied by pain. The mechanism of painlessness of the severe mycolactone-mediated lesions has recently been characterized at the molecular level.90, 91 Mycolactones induce type 2 angiotensin II based signaling which results in hyperpolarization of neurons and analgesia.

2.2 RESPIRATORY TRACT
The respiratory tract is divided into the upper and lower airways. The upper airways consist of the nose, nasal cavity, mouth, throat (pharynx), and voice box (larynx). The lower airways are composed of the trachea and lungs including the bronchi, bronchioles, and alveoli. A number of respiratory diseases are the result of infection by a single microbial species including pertussis, scarlet fever, strep throat, and diphtheria. Many of these diseases have been successfully prevented through the administration of vaccines. Other microbe-driven diseases include pneumonia, sinusitis, and lung infections of patients suffering from diseases such as cystic fibrosis (CF) or chronic obstructive pulmonary disorder (COPD). Shifts in microbial community structure have been implicated in respiratory tract infections.92, 93 The microbial interactions and cause of community shifts in respiratory diseases remain under-investigated at the molecular level. In general, this is likely due to the poor accessibility of such samples when compared to the gut or the skin.
2.2.1 ORAL CAVITY
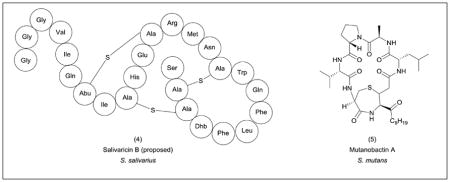
The oral cavity (consisting of the teeth, tongue, cheeks, hard and soft palates, gingival sulcus and tonsils) is inhabited by over 600 bacterial species and 100 fungal species.94–96 Different oral structures and tissues are occupied by distinct microbial communities with community structure affected by diet, dental hygiene, xenobiotics and host genetics.97 Microbes (and in some cases, entire communities) isolated from the oral cavity have been implicated in the development of oral diseases including dental caries, periodontitis, tonsillitis, and many others.98 Sequencing-based correlative studies have also suggested a role for oral bacteria in a number of systemic diseases including cardiovascular disease, stroke, preterm birth, diabetes, and pneumonia.95, 99 Despite efforts to inventory the constituents of the oral microbiome, the role of natural products in the advancement of oral diseases is poorly understood. However, some metabolites have been characterized. For example, the commensal microbe Streptococcus salivarius produces the lantibiotic peptides salivaricin A and B which have been shown to inhibit a black pigmented strain of the genus Prevotella associated with halitosis in vitro.100–102 A proposed structure of salivaricin B (4) has been recently reported.103 In dental caries, it has been postulated that demineralization is caused by increased acidity due to lactic acid production by the commensal species Streptococcus mutans.104 Other microbes, such as Lactobacillus sp., may also be involved in the development of caries through production of similar acids.105 In addition to lactic acid, S. mutans produces a variety of molecules under the microaerophilic conditions it encounters in the oral cavity including formate, acetate, ethanol, and two different mutanobactins.104, 106, 107 Mutanobactin A (5), isolated from S. mutans UA159, is a hybrid polyketide-nonribosomal peptide (PK-NRP) with dual roles. It allows S. mutans to colonize the oral cavity and to compete with the fungal pathogen Candida albicans by preventing its transition from an avirulent yeast-like state to its hyphal form.106, 107 One of the most prevalent oral diseases is periodontal disease which includes the mild and reversible gingivitis and periodontitis, a chronic disease resulting in destruction of both connective and bone tissue. It is widely accepted that the progression of periodontal diseases is the result of the complex interaction between the associated microbial community and host response.108–110 The induction of host response has been attributed to a number of bacterial factors including lipopolysaccharides, proteases and other pathogen-associated molecular patterns, SCFAs, and N-formyl-methionyl-leucyl-phenyalanine type peptides which function as chemo-attractants for leukocytes.111, 112
2.2.2 UPPER AIRWAYS
The measured microflora of the upper respiratory tract is representative of the oral microbiome and includes common genera such as Staphylococcus, Streptococcus, Neisseria, Bacillus, and Corynebacterium, among others.113–115 It is postulated that colonization by opportunistic pathogens including species of Haemophilus, Pseudomonas, Bordetella, and Mycoplasma first occurs in the upper airways and spreads to the lower respiratory tract.116 In terms of natural products, only a handful of small molecules have been implicated in disease progression in the upper airways. In the nasal cavity, lugdunin (6), a NRP antibiotic produced by Staphylococcus lugdunensis, was identified from bioactivity-guided in vitro inhibition of S. aureus and validated in animal models.117 Another example is tracheal cytotoxin (7), a disaccharide-tetrapeptide, considered to be integral to the virulence of Bordetella pertussis, the causative agent of whooping cough.118–123 Tracheal cytotoxin causes extensive damage to ciliated epithelial cells by inducing the host production of interleukin 1 (IL-1) and nitric oxide which leads to diminished ciliary beating, impaired removal of mucus, and the characteristic symptomatic cough.124 Another example is the role of a two-peptide bacteriocin, pneumocin MN, produced by emerging Streptococcus pneumoniae serotypes.125, 126 S. pneumoniae is the most common bacterial agent in community-acquired pneumonias and is divided into more than 90 serotypes. It has been demonstrated that the strains of pneumococci inhabiting the upper airway are constantly in flux, as new strains outcompete existing strains.127 Vaccination with the 7-valent conjugate vaccine consisting of protein-polysaccharide derivatives of the capsule has led to an incredible decrease in invasive pneumococcal disease (IPD) in children.128 At the same time, isolates of non-vaccine serotypes have become more common. It has been proposed that the bacteriocins produced by these serotypes provide a competitive edge against other serotypes.125 The bacteriocin-producing serotypes were subsequently included in the 13-valent vaccine. However, both the structure of pneumocin MN and its mechanism of interaction with the host have not been resolved. Lastly, S. pyogenes, in addition to being an opportunistic skin pathogen, is the causative agent of streptococcal pharyngitis, commonly known as strep throat. Traditional clinical diagnosis of S. pyogenes infection is through its characteristic haemolysis pattern after culturing on blood agar plates; a method first described over a century ago.129 This haemolysis is primarily due to the bacteriocin SLS which is a contributor to S. pyogenes virulence.130, 131

2.2.3 LOWER AIRWAYS
Once thought sterile, the lower respiratory tract is now known to harbor its own microbial community, albeit at much lower abundance than the oral cavity or gut.113, 132 The complex anatomical structure of the lung leads to a surface area of 30 times that of human skin with environmental gradients of oxygen, temperature, and pH.133 The overall complexity of the lung represents a very dynamic, heterogeneous environment that is exposed and inhabited, at least transiently, by microbes from the air, oral cavity, and nasal passages. The characterization and investigation of the lung microbiome suffers from possible contamination of the community from microbes inhabiting the oral cavity. However, statistical methods have been utilized to identify organisms that are enriched in the lung.114, 134, 135 Healthy lungs are generally inhabited by a variety of species including the genera Pseudomonas, Streptococcus, Veillonella, and Prevotella. Clinical features of several lung diseases have been correlated with lung microbiome dysbiosis including asthma,136, 137 COPD,138, 139 and CF.140, 141
While a number of pulmonary pathogens have been identified, only the most prevalent have been characterized at the chemical level. For example, P. aeruginosa has the genetic capacity to produce a suite of virulence factors including homoserine lactones, quinolones, phenazines, rhamnolipids, and siderophores.25, 66–70 The molecules produced by P. aeruginosa have been shown to interact with host tissue by modulating host gene expression, producing free radicals, directly damaging epithelial cells, and scavenging iron.142–145 The detection of the redox active phenazines PYO (1) and phenzine-1-carboxylate (8, PCA), which are involved in anaerobic respiration, have been shown to negatively correlate with lung function.146 Interestingly, a number of P. aeruginosa metabolites have been detected in serum of CF patients, suggesting that they are trafficked via the circulatory system to other organs and potentially cause unknown off-organ effects.147 A recent study compared the repertoire of P. aeruginosa quinolones produced in laboratory cultures to sputum samples from CF patients.148 The predominant culture produced quinolone, pseudomonas quinolone signal (9, PQS), was not detected in sputum samples.

Aspergillus species represent one of the most prevalent fungi responsible for infections of patients with pulmonary disorders. Aspergillus species are known to produce a wide variety of natural products including gliotoxin (10), cephalosporin (11), aflatoxin B1 (12), and lovastatin (13).149, 150 While cephalosporin and lovastatin have been harnessed for medicinal purposes, aflatoxin B and gliotoxin are both toxins. Produced by A. parasiticus and A. flavus, the difuranocoumarin compound aflatoxin B is one of the most potent carcinogens.151–154 Although aflatoxin B has been most extensively studied in liver tumorigenesis, the respiratory system is also a target of this mycotoxin. Studies have shown that aflatoxin B is bioactivated by cytochrome P450 monooxygenases and biotransformed into DNA binding metabolites by cytosolic enzymes. Gliotoxin is produced by A. fumigatus, an opportunistic pathogen affecting patients with pulmonary diseases which often leads to clinical complications.155 Gliotoxin is a member of the epidithiodioxopiperazine family and is critical for A. fumigatus pathogenicity by inducing apoptosis, preventing NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) activation, and inhibiting angiogenesis; a partial molecular mechanism of action has been described.156–158 Despite intensive efforts to elucidate the natural products of Aspergillus species, the impact of these compounds during infections has seen limited attention.

In CF patients, co-occurrence of P. aeruginosa and A. fumigatus correlates with decreased lung function, suggesting that they may interact on the chemical level.159–161 In vitro, A. fumigatus biotransforms the antifungal phenazines produced by P. aeruginosa to induce production of its own siderophores, providing A. fumigatus with an ecological advantage in the competition for environmental metals (Figure 3).162 Furthermore, phenazines were shown to induce morphological changes and formation of reactive oxygen and nitrogen species in A. fumigatus (Figure 3).163, 164 Interestingly, inhibition of A. fumigatus biofilm formation was more pronounced when grown in mixed cultures with CF-associated P. aeruginosa isolates compared to non-CF isolates.165 While these findings have yet to be substantiated in vivo, they indicate that microbe-microbe interactions may significantly alter host-microbe interactions.
Figure 3. In vitro Chemical Interactions of P. aeruginosa and A. fumigatus.
P. aeruginosa produces several redox active phenazines including pyocyanin (PYO), phenazine-1-carboxylic acid (PCA), and 1-hydroxyphenazine (1-HP). A. fumigatus modifies the phenazines by producing dimers and biotransforming PCA to 1-HP. 1-HP is further modified to 1-methoxyphenazine and phenazine-1-sulfate. A. fumigatus uses 1-HP to induce its own triacetylfusarinine C and fusarinine C siderophore production. P. aeruginosa phenazines also lead to the production of reactive oxygen and nitrogen species in A. fumigatus.
Pulmonary tuberculosis is rapidly re-emerging as a global health concern.166 Multi-drug resistant (MDR) strains of Mycobacterium tuberculosis have been identified.167 While treatments for MDR tuberculosis are limited, M. tuberculosis natural products provide promising targets for drug development. For example, the ability of M. tuberculosis to grow inside macrophages has been partially attributed to small molecules.168, 169 The production of the PK-NRP mycobactin siderophores (14),170–172 PK phenolic glycolipids (15),173, 174 and dimycocerosate esters is required for virulence.175–178 Additionally, a cyclopropyl glycolipid modification of the cell wall has been shown to be critical for induction of host hyperinflammation during initial infection.179

2.3 GASTRO-INTESTINAL TRACT
The gastro-intestinal (GI) tract represents the densest microbial ecosystem in the human host.180, 181 Composed of the stomach and intestines, the composition of the microbial community of the GI tract is dependent upon anatomical location with community densities ranging from 103 cells/mL at the outlet of the stomach to approximately 1012 cells/mL in the colon. The members of the GI microbiota play important and beneficial roles in digestion, vitamin biosynthesis, immune regulation, and pathogen exclusion.182 Disease can result when GI microbial community homeostasis is disturbed due to stress, infection, poor nutrition, use of antibiotics, or other external factors.183 A number of studies have correlated compositional shifts in the microbial community of fecal samples to diseases such as obesity, inflammatory bowel disease (IBD), and Crohn’s disease.8, 184, 185 The study of the chemical interchange occurring between the gut microbiota and the host is a rapidly advancing field with a specific focus on understanding the role of the healthy microbiota in managing pathogenic microbes and the modulation of host response and behavior by microbial metabolites.
2.3.1 STOMACH
Like the lower respiratory tract, the human stomach was once thought to be sterile. Due to sequencing efforts, we now know that a number of microbes have adapted to exist in this harsh, acidic environment including Helicobacter, Streptococcus, Staphylococcus, Peptostreptococcus, Veillonella, Bacteroides, Fusobacterium, Bacillus, Lactobacillus, Deinococcus, and Neisseria, among others.186, 187 The contribution to the stomach microbiota from transient colonization by microbiota from the oral and nasal cavities is unknown. The most common and well established example of a stomach microflora is Helicobacter pylori.188 H. pylori has been associated with a number of diseases including gastritis, hypochlorhydria, duodenal ulcers, and gastric cancer.189 The discovery of H. pylori as a disease-causing agent was awarded the Nobel Prize in Medicine in 2005. H. pylori has been postulated to produce several metabolites which interact with the human host including diethyl phthalate (16), which induces monocyte migration, N-nitrosamines that have been suggested to contribute to gastric cancer (although this is under debate), and a peptidoglycan which induces NF-κB activation.190–192, 193 In general, the role of small molecules in the gastric cavity remains virtually unexplored.

2.3.2 INTESTINAL TRACT
The microbiota of the intestinal tract is currently one of the most intense focal points of microbiome research. We now know that more than 1,000 different species are present in human gut with Bacteroidetes and Firmicutes as the most dominant phyla.194 Although each location of the intestine is anatomically distinct, exploration of the intestinal microbiota has not been studied at high spatial resolution. The majority of microbiome community studies have focused on characterizing the community composition of fecal content in relation to diseases of the gut. Information about the microbial constituents of the entire intestine has been extrapolated primarily based on analysis of fecal matter. However, this is analogous to identifying the source of a river by studying the ecology of the banks of its delta. It is clear that a more sophisticated, spatially-resolved study of the intestinal tract is critical for the advancement of this field.
Mounting evidence suggests that the gut microbiota strongly influences human health through both primary and secondary metabolism including the modification of bile acids195–197 and amino acids,198 carbohydrate fermentation to generate SCFAs,29–31, 199 and the production of virulence factors such as siderophores, antimicrobial peptides, and glycolipids.200, 201 SCFAs and siderophores are the classical examples of microbial metabolites involved in microbe-host interactions and have been thoroughly reviewed.27–31 One of the most interesting interactions by intestinal microbiota is the competition for iron. A number of pathogenic microbes including Escherichia coli, Klebsiella pneumoniae, and Salmonella typhimurium produce structurally related siderophores. E. coli-produced enterobactin (17) is quickly rendered ineffective through sequestration by the mammalian protein siderocalin and is partitioned into host lipid bilayers due to its hydrophobicity.28 To circumvent these limitations, certain pathogenic strains of E. coli and Salmonella have adapted NRP siderophore biosynthesis by incorporating genes for glycosylation of the enterobactin structure resulting in the production of the salmochelins (18). In addition to siderophores, these same organisms produce ribosomally-synthesized and post-translationally modified peptides (RiPPs) which have been shown to have antibiotic properties. For example, probiotic strains of E. coli produce microcins which exert antibacterial activity against closely related enteropathogenic species.202, 203 Some of these microcins are modified at the C-terminus with linearized monoglycosylated enterobactin (19) which target siderophore transporters of competing microbes resulting in a “Trojan horse” type antibiotic. 204–206

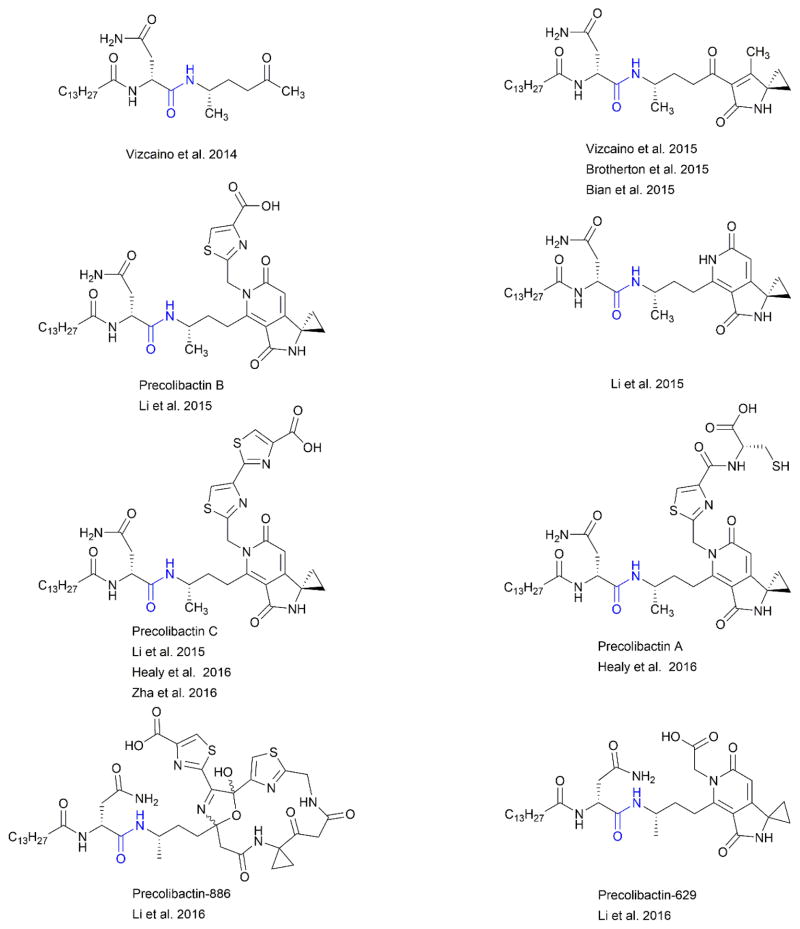
Perhaps most illustrative of the opportunities and challenges associated with characterizing microbiome-derived natural products is the emerging structural elucidation of the metabolite colibactin. Colibactin is a structurally uncharacterized PK-NRP that is thought to arise from a prodrug called precolibactin which has also not been fully structurally elucidated.32, 33, 207 Over ten years ago, the colibactin encoding hybrid PKS-NRPS BGC (clb) was identified owing to the correlation of its presence in E. coli strains to the induction of double stranded DNA breaks in human cell lines.208 This BGC was found to be widely distributed among the B2 phylogenetic group comprised of both commensal and extra-intestinal pathogenic strains. 55 intestinal pathogenic strains of E. coli, 97 extra-intestinal pathogenic strains, and 32 fecal strains from healthy individuals were analyzed by PCR-based analyses for the presence of the clb BGC. Although absent in intestinal strains, clb was identified in 53% and 34% of the extra-intestinal and fecal strains, respectively. The potential link between colibactin and colitis-associated colorectal cancer was first shown in 2012.209 In this study, changes in colonic microbiota of mice models was shown to be associated with inflammation of the colon. This led to the hypothesis that in addition to host-associated inflammation, bacterial factors associated with clb gene locus may be important for development of colitis-associated colorectal cancer. In patient samples, 20.8% of the healthy cohort (non-IBD and non-colorectal cancer volunteers), 40% of patients with IBD, and 66.7% patients with colorectal cancer were shown to harbor E. coli containing clb gene cluster. Thus, it has been proposed that colitis-induced inflammation of the colon creates an environment that promotes carcinogenesis by both host and microbe-associated factors. The intriguing biological activity associated with the presence of the clb BGC spurred interest in characterizing the product of gene cluster by various groups. The elucidation of the structure of colibactin has been hindered by both the complexity of the clb gene cluster and likely instability of its product colibactin. Therefore, extensive efforts have been undertaken to structurally characterize the pro-molecule, resulting in various proposed structures of precolibactin (Figure 4A). Several potentially important structural elements in different proposed precolibactins include an N-acylated-D-asparagine prodrug conferring moiety which is hypothesized to be cleaved from the active colibactin by a periplasmic protease,210, 211 a cyclopropane containing ring system suggested to be the warhead group responsible for crosslinking DNA,212–217 and the presence of a thiazole, thiazolinyl-thiazole (predicted structure), or bithiazole motif possibly involved in non-covalent interactions with DNA.214–217 A recent study described two additional precolibactins, precolibactin-629 and precolibactin-886. Precolibactin-866 results from the incorporation of an aminomalonyl unit which yields a unique macrocycle.218 The discovery of precolibactin-866 led to a proposed biosynthetic scheme as a plausible explanation for the varied chemical framework of isolated precolibactins. In this scheme, the type II thioesterase ClbQ mediates early off-loading of biosynthetic intermediates yielding the described precolibactins.
Figure 4. Precolibactin Structures.
Immense effort to elucidate both the structure and biosynthetic pathway of colibactin has been taken. At least 10 precolibactins have been structurally characterized. The metabolites shown were chosen to represent the structural variations described.
In contrast to colibactin, the E. coli heat-stable enterotoxins STh (20) and STp (21), 19 and 18-amino acid RiPPs, respectively, have been suggested to protect against colorectal cancer by suppressing proliferation of cancer cells by increasing intracellular cyclic guanosine monophosphate (cGMP) through a guanylyl cyclase C-mediated signaling cascade.219, 220 These enterotoxins interact with the same signaling cascade in intestinal epithelial cells, leading to diarrheal disease. The BGCs for structurally similar peptides have also been identified in K. pneumoniae, Yersinia enterolitica, and Vibrio cholerae.221–223 Other metabolites have also been associated with GI diseases. For example, Klebsiella-mediated, antibiotic-associated hemorrhagic colitis has been linked to the NRP tilivalline (22).224, 225 Tilivalline was first isolated from Klebsiella oxytoca in the early 1980s and was shown to have toxicity against mouse leukemia L1210 cells.226, 227 More recently, the pathological symptoms of tilivalline-mediated damage in the colon were more pronounced in animals treated with antibiotics and infected with K. oxytoca as compared to control animals. This work implicated tilivalline in antibiotic-mediated colitis. Tilivalline has been shown to induce caspase-3-dependent apoptosis in cultured human epithelial cells, which has also been observed in the pathology of antibiotic-associated hemorrhagic colitis.224, 225

A promising application of microbiome research to treat GI disorders is the utilization of fecal transplants for Clostridium difficile infections.228–230 C. difficile infections of the colon are thought to arise from alteration of the commensal gut community by treatment with antibiotics.231, 232 It has been hypothesized that the susceptibility of patients to C. difficile infections post-antibiotic treatment is due to clearance of beneficial microbes capable of preventing C. difficile colonization. Recent studies have shed light on potential mechanisms, including the possible involvement of secondary bile acids produced by commensal microbes.233–235 Through correlative analysis of microbial abundance and resistance to infection in both mice and humans, Clostridium scindens was identified and validated as an inhibitory bacterium of C. difficile.233 This activity was later associated to the presence of bile acid inducible (bai) operon of genes required for secondary bile acid production by C. scindens. While primary bile acids are required for the germination of C. difficile spores, secondary bile acids have been shown to act as growth inhibitors of vegetative cells both in vitro and in mouse models of C. difficile infection.236, 237 It is hypothesized that although the host-derived primary bile acid taurocholate promotes germination of C. difficile spores, the secondary bile acid deoxycholate (23) produced by the commensal bacterium C. scindens inhibits growth of C. difficile vegetative cells, thereby preventing colonization. It is postulated that when commensals such as C. scindens are killed by antibiotic treatment, secondary bile acids are not available to prevent growth of vegetative C. difficile cells which then leads to infection. Further work will be required to validate these findings and explore the use of secondary bile acids as potential therapeutic treatments.
2.3.3 THE GUT-BRAIN AXIS
One of the most intriguing areas of microbiome research is the role of microbial metabolites, particularly from the gut microbiome, in mediating mental health and neurological diseases ranging from clinical depression to autism.238–241 This bidirectional communication system between the gastro-intestinal tract and the central nervous system (CNS) is commonly referred to as the gut-brain axis.238 Amino acid metabolism by gut microbiota generates neurotransmitters such as serotonin (24),242 γ-aminobutyric acid (25)243 and β-phenylethylamine (26).244, 245 In healthy individuals, host metabolism is positively influenced by microbe produced SCFAs which promote energy homeostasis, regulate immune response, and affect peripheral nervous system function. SCFAs have been extensively reviewed.29–31, 246, 247

A number of possible links between the gut microbiota and disease have been identified along the gut-brain axis. Although controversial, autism spectrum disorders (ASD) is among the most notable. ASD is generally characterized by stereotypic behaviors such as impairment of communication and social anxieties. Intriguingly, ASD is often accompanied by various disorders of the GI tract including, most commonly, chronic constipation, diarrhea, and abdominal pain.248–250 The prevalence of GI disorders in people suffering from ASD has been correlated to more severe autism-related behaviors, several of which have been recapitulated in mouse models.251 In ASD-model mice, high serum levels of the small aromatic molecule 4-ethylphenylsulfate (27, 4EPS) were observed when compared to control mice.252 Subsequent injection of 4EPS into healthy mice was sufficient to induce behaviors similar to the ASD-model mice. In pediatric patients suffering from ASD, elevated levels of urinary p-cresol (28) and its conjugate derivative p-cresylsulfate (29), a metabolite structurally related to 4EPS, were measured.253, 254 These metabolites are primarily produced by the gut microbiota through fermentation of amino acids in the large intestine. In the murine models, autism-related behaviors were corrected by treatment with Bacteroides fragilis.252 Mice treated with B. fragilis had similar levels of 4EPS when compared to healthy control mice. This suggests that members of the healthy microbiota, and their metabolites, may potentially be used as probiotic therapies to mitigate autism-related behaviors.

Active research programs are investigating direct links between microbial metabolism in the gut and modulation of the immune system.255 It is anticipated that these research areas will shed light on how the human gut microbiome affects distant organ systems. While we have focused on a specific example of gut metabolites affecting the nervous system, other microbe-derived molecules such as N1,N12-diacetylspermine (30), trimethylamine-N-oxide (31), deoxycholate (23), and indoxyl sulfate (32) have been implicated in colorectal cancer, cardiovascular disease, hepatocellular carcinoma, and chronic kidney disease, respectively.256–259 Untargeted metabolomics of plasma extracts from germ-free and conventional mice suggests that the gut microbiota significantly affects the level of 10% of all metabolites detected in systemic circulation.260 Bioactivity-guided approaches have also been utilized to identify microbial metabolites which may have interesting biological roles in host-microbe interactions. For example, in an effort to identify bacterial effectors which act as environmental stimuli to activate the human transcriptional factor NF-κB, functional metagenomics workflows were applied. Metagenomic DNA was cloned into a E. coli based cosmid library from stool samples of three phenotypically distinct patients including a healthy volunteer, a patient with ulcerative colitis, and a patient with Crohn’s disease.261 The sterile spent culture broth of each clone was screened against a human fluorescent reporter cell line for activation of NF-κB. Of 26 effector genes identified, an effector cosmid encoding for commendamide (33, N-acyl-3-hydroxypalmitoyl-glycine) was recovered from all three patients. Commendamide and its minor analogues (with variation in length and saturation of the acyl chain) are structurally similar to mammalian N-acyl-amides that play a role as signaling molecules through activation of G-protein-coupled receptors (GPCRs). In vitro screening of commendamide for activation of a library of 242 GPCRs revealed activation of GPCR132/G2A receptor. G2A-knock out mice were previously shown to be susceptible to atherosclerosis and developed a late-onset stage autoimmune disorder.262 The functional role of commendamide in affecting host immunity requires further testing in appropriate in vivo models. It is also important to note that microbial metabolite production in organs other than the GI tract may also have effects elsewhere in the body. For instance, secondary metabolites from P. aeruginosa chronic lung infections have been measured in the plasma of CF patients.147 Unraveling the systemic roles of microbial metabolites is an extremely exciting and potentially revolutionary emerging field of research.

2.4 NERVOUS SYSTEM
The CNS is comprised of the brain and spinal cord. The CNS is protected by the blood brain barrier, which is lined by endothelial cells that prevent the passage of microbes and large hydrophilic molecules. However, some parasites and microbes (including Trypanosoma brucei and L. monocytogenes, respectively) express proteins that allow them to cross this barrier which is critical to their infections of the CNS.263–269 Infection of the CNS with T. brucei causes the disease trypanosomiasis. It is hypothesized that tryptophan-derived 3-indole ethanol (34, tryptophol) is responsible for the induction of sleep from which trypanosomiasis derives its common name of sleeping sickness.270–272 Listeriosis is a fatal disease where ingestion of the gram-positive bacterium L. monocytogenes by immunocompromised individuals leads to infection of the CNS, resulting in meningitis and eventual death.273 The hemolytic activity of L. monocytogenes is closely correlated to its pathogenicity. Until recently, the hemolytic activity of L. monocytogenes was wholly attributed to the protein toxin listeriolysin O.274 In silico analysis of the L. monocytogenes genome for the SLS BGC revealed the closely related peptide listeriolysin S.275 However, like SLS, the structure of listeriolysin S remains uncharacterized.

2.5 URO-GENITAL SYSTEM
The uro-genital system consists of the urinary and genital tracts. Like several other organ systems, the urinary tract was assumed to be a sterile environment. However, recent sequencing studies indicate that it harbors a unique microbiota.276–279 While the urinary tract is normally colonized by Lactobacillus and Streptococcus species, a shift in community composition has been implicated in urinary tract infections (UTIs) through correlative analysis. UTIs are primarily associated with infection by the Gram-negative bacterium E. coli, but can also be caused by both Gram-negative and -positive bacteria.280–283 The role of microbial small molecules in most UTIs remains largely uninvestigated. However, siderophore production may be an important role in E. coli driven UTIs.284–287 UTI causing E. coli strains were widely believed to originate from the GI tract. In several studies, researchers compared siderophore production between strains isolated from the urinary tract, fecal samples, and extra-urinary sites.285, 287 Two studies revealed different results for the most abundant siderophores produced by E. coli strains isolated from UTIs. In one study, using phenotypic assays, the proportion of aerobactin (35) producers was significantly higher in UTI and extra-urinary isolates.287 In a second study, mass spectrometry (MS) based analysis showed that both UTI and rectal strains of E. coli produce enterobactin (17) with a preferential expression of yersiniabactin (36) and salmochelin (18) in UTI isolates.285 Therefore, the quantitative contribution of iron acquisition during infection requires further investigation both in vitro and in vivo.

The lower genital tract of both males and females is inhabited by a dynamic microbial community. Normal vaginal flora is dominated by several Lactobacillus species, but community structure varies with the menstrual cycle and sexual activity.288, 289 It is hypothesized that Lactobacillus play a protective role against pathogens. This was recently further supported through the discovery of the thiopeptide antibiotic lactocillin (37).5 Isolated from Lactobacillus gasseri JV-V03, a vaginal isolate, lactocillin was shown to have in vitro antibacterial activity against Gram-positive vaginal pathogens. However, the BGC encoding for lactocillin was not detected in six of the vaginal metagenomes where L. gasseri was the most abundant strain and the production of lactocillin was not observed in vivo. Therefore, publicly available metatranscriptomics data from the oral cavity were analyzed, which suggested that the BGC of lactocillin is transcribed in vivo. Interestingly, the gene cluster family for similar thiopeptides is widespread throughout different anatomical locations on the human body. Although the expression of these peptides in other body habitats remains to be demonstrated, the presence of the lactocillin gene cluster family in different anatomical locations suggests that these natural products may mediate microbiome-related functions including preventing infection. While Lactobacillus may provide protection against microbial invaders, unexpected shifts in the vaginal microbiome may affect diseases such as bacterial vaginosis (BV) by contributing to infections of the upper reproductive tract and complications during pregnancy.290 BV has not been associated with a specific microbe, but instead has been correlated to the diminished presence of Lactobacillus.

3. THE UNTAPPED (RESEARCH) POTENTIAL OF THE MICROBIOTA
The human microbiota is an untapped resource of incredible potential for natural product research.4 The complexity of the chemical interactions between the host and its resident microbiota requires the development of new approaches and tools for use by a truly interdisciplinary community. Every facet of natural product research (including discovery, comparative genomics analyses, unraveling biosynthetic pathways, characterizing biological function, etc.) can be applied to the microbiota and will have the opportunity to impact clinically relevant phenotypes of many acute and chronic diseases affecting human populations. As a result of advances in sequencing technologies, we are beginning to comprehend the microbial and chemical diversities associated with the microbiome as well as the spatial dependencies required for the chemical crosstalk within microbial communities consisting of thousands of species. While we have provided some specific examples of microbial specialized metabolites involved in mediating disease, most molecules and their biosynthetic origins remain uncharacterized. In this section, we briefly describe the challenges and the emerging technologies associated with characterizing natural products from the human microbiota.
3.1 METABOLITE DETECTION & ANNOTATION
Not surprisingly, human microbiome samples are “contaminated” with human metabolites. In microbiome sequencing workflows, human DNA contamination is circumvented by the application of amplification (16S/18S/ITS sequencing) or through the exclusion of all eukaryotic DNA in data post-processing (metagenomics). Unfortunately, these approaches cannot be applied to untargeted metabolomics methods for natural product discovery from the microbiome. In general, current metabolomics workflows applied to human-derived samples focus heavily on the products of primary metabolism, biotransformation of human metabolites and xenobiotics, and well-characterized secondary metabolites including SCFAs.291–295 The detection of microbial natural products in human samples is hindered by the presence of highly abundant host metabolites. Therefore, the development of robust, reproducible methods for metabolite extraction and analyte separation on small sample amounts to improve the detection of microbial metabolites is critical to the success of microbiome-based natural product research. It is important to note that metabolomics, like sequencing workflows, suffers from a lack of standardization of analytical and bioinformatics methods which makes harnessing available data and cross-comparison between datasets extremely difficult. Modern tools and resources for metabolomics analysis were recently reviewed.296
Untargeted methods provide detection of a large number of metabolites, but prioritizing key metabolites is a formidable challenge.297 One method to identify compounds of interest is applying self-organizing maps (SOMs). SOMs aid in the identification of metabolites that differentiate samples using multivariate statistics to pinpoint the most abundant changes between case and control for purification and structural elucidation.298–300 Correlation detection strategies can also be applied to untargeted metabolomics analysis in order to correlate the presence of metabolites with genotypic and phenotypic data, although these methods are not yet widely used in guiding workflows for metabolite characterization.301–305 For example, co-occurrence methods have been utilized to reveal relationships between the microbiome and metabolome of the human intestinal mucosa,306 while correlations of spatial distributions of the skin microbiome and metabolome highlighted the role of skin microbes in the degradation of beauty products.38 However, integration of multi-omics datasets is not trivial and has been primarily utilized for microbial community profiling.307, 308
Metabolomics analysis can also be combined with bioactivity-guided and candidate molecule workflows. Bioactivity-guided screening utilizes a collection of microbes, extracts, or cosmid libraries (generated from metagenomics DNA) to test for in vitro activity against human pathogens or activation of human signaling pathways.117, 261 Identification of a metabolite of interest is achieved by utilizing analytical methods including differential metabolomics,2 imaging mass spectrometry (IMS),162 or by disrupting the biosynthetic genes through random mutagenesis combined with high-throughput phenotypic screening for loss of function.261 Data can be integrated to group molecules of similar bioactivity to generate compound activity maps.309, 310 This method yields previously uncharacterized natural products from complex samples and probable biological functions.
Orthogonal to bioactivity-guided methods, candidate molecule workflows harness characterized biosynthetic pathways to mine (meta)genomic data to identify putative BCGs and direct metabolite purification workflows.5 The Minimal Information about a Biosynthetic Gene cluster (MIBig) consortia aims to develop a public repository of specialized metabolite BGCs to maintain community knowledge of molecule biosynthesis to aid in the analysis of sequencing data from any microbial system, including humans.311 Growing out of antecedent tools for comparative analysis of genetic information such as BLAST,312 HMMER,313 and algorithms for the detection of specific classes of gene cluster families (many of which were recently reviewed314), ClusterFinder has been successfully used to identify putative gene clusters from microbiome data and led to the characterization of metabolites.36, 315 Part of the antiSMASH platform,316–318 ClusterFinder is a more general probabilistic algorithm used to identify BGCs of broad structural classes ranging from polysaccharides to PKs and peptides. AntiSMASH 3.0 also has algorithms to compare orphan gene clusters to a manually curated database of characterized gene clusters (ClusterBlast) and to predict substrate specificity of active sites with some metabolite structural prediction, thus reducing the manual curation necessary to link metabolites and their BGCs.318–320 Candidate molecule methods can be combined with bioactivity data to correlate the presence of a specific BGC to bioactivity as shown with the identification of the clb and bai gene clusters,32, 233 with other–omics datasets as was performed for transcriptional analysis of the metabolite lactocillin in the oral cavity,5 or with tandem mass spectrometry (MS/MS) in order to link metabolites to gene clusters using peptidogenomics321 and glycogenomics322. Peptidogenomics has recently been automated through the Pep2Path platform.323
One of the major challenges facing natural products researchers is the annotation of metabolites. This often occurs because structural information of characterized metabolites, including high resolution MS data and MS/MS data, is buried within manuscripts and, in a growing number of cases, large supplemental information documents. To bypass these issues and coordinate global metabolite assignments, users can contribute to the Global Natural Products Social Molecular Networking (GNPS, gnps.ucsd.edu).324 GNPS is a public, online platform for analysis of MS/MS data, where similarities in fragmentation patterns function as proxy for structural similarities. This concept can be harnessed by users to rapidly dereplicate their data against spectra generated from characterized metabolites in both public and user-curated MS/MS databases and to generate molecular networks which can be used to visualize the chemical space of samples. Judicious application of the mapping features available in GNPS allows researchers to quickly pinpoint conditions where a metabolite of interest is produced and identify the producing organism. A step-by-step procedure for secondary metabolic pathway targeted metabolomics, including molecular networking, utilized for the elucidation of a preliminary structure of a precolibactin has recently been published.216, 325 Other methods including CSI:FingerID combine fragmentation tree analysis and machine learning to search structural databases such as PubChem and ChemSpider to aid in metabolite identification.326, 327
3.2 CULTIVATION, METABOLITE PRODUCTION & VALIDATION
Despite recent success characterizing single microbiome-derived metabolites such as lugdunin and lactocillin,5, 117 it is likely that many compounds of interest are under-investigated due to limitations of traditional in vitro culturing techniques. Cultivating the uncultivable will allow researchers the opportunity to scale for natural product isolation as well as test interesting hypotheses. A distinct advantage of model systems to induce metabolite production is their inherent reproducibility. However, even if a member of the microbiota can be cultured in a model system, it does not necessitate that the metabolite of interest will be produced. The selective pressures and nutritional availability within a microbiome environment are distinct from the environment of a microbe cultivated in model systems. Therefore, regardless of how similar the community structure may be as measured by sequencing-based analysis, the metabolic output from a model system may not reflect that from the original sample. It is incredibly important to validate the metabolite produced in a culture system is identical to the metabolite originally detected. This validation is most easily accomplished by comparing high resolution MS data, MS/MS spectra, and retention time of the purified compound to the original samples under identical acquisition parameters.
Conventional cultivation of members of the microbiota is identical to the steps used in isolation of microbes from marine and terrestrial environments including agar based culturing of mixed communities to identify single colonies of interest, re-streaking isolates to purity, typing by 16S and archiving.328 The isolation of microbes from the microbiota is enhanced by the development of environment specific media.329 For example, YCFA medium and gut microbiota medium (GMM) have been developed for isolation of human gut microbes.330, 331 Sequencing-based comparisons were used to validate that the isolates recovered had similar phylogenetic diversity as measured for the community.328 In CF research, this type of workflow was coined culture enriched molecular profiling and was used to increase metagenomics coverage, but has been also utilized for the gut and can be easily harnessed for microbial metabolite isolation.332–334 Extensions of conventional cultivation include culturing single cells in multi-well plate format to create personalized microbiota collections,331 parallel cultivation in hundreds of different conditions,335 microscale apparatuses,336–338 culturing with “helper” organisms,339, 340 and using sequencing information to access the nutritional needs of isolates to optimize growth conditions.329 Efforts are also underway to recapitulate entire microbiome communities. A recent review nicely summarizes the available in vivo, in vitro, and ex vivo models under development to investigate the microbiome,341 although newer techniques are continuously being developed.342 While primarily intended to investigate community structure, many of the in vitro systems have the potential to be harnessed for natural products research.
Beyond cultivation, molecular biology methods have the potential to enhance the discovery of microbiome-derived natural products. Any method currently applied to natural product discovery has applicability to the microbiome. For instance, similar to how BGCs have been cloned from environmental DNA, microbiome BGCs can be cloned from metagenomics DNA and expressed in bacterial or fungal hosts.261 Additionally, rational genetic modification to remove, replace, or enhance regulators within BGCs hypothesized to produce metabolites of interest can be used to increase production yields and to circumvent arduous purification processes from complex cultures as was recently illustrated during the characterization of lugdunin.117, 343
4. CONCLUSIONS AND PERSPECTIVES
One of the greatest challenges and opportunities for natural products researchers interested in microbiome work is characterizing the biological roles for microbiome-derived natural products.344 Although there is a great interest in elucidating the roles of such molecules in vivo, in general, members of the microbiota are under-investigated at the chemical level. From a discovery perspective, the human microbiome (including both disease-causing microbes and those that enhance human health) is one of the next frontiers for natural products. Analyses of sequencing data strongly suggest that microbial community dysbiosis may cause a wide range of human diseases including some autoimmune and neurological disorders. With increasing public and industrial interest in probiotic microbes and metabolites, it is apparent that the discoveries of metabolites unique to healthy individuals will lead to new therapeutics for chronic microbe-driven diseases. As the few examples highlighted in this review show, researchers have begun to explore the role of microbial natural products from bacteria in human disease. However, this avenue remains largely unexplored for fungal diseases. The available workflows for microbiome analyses exist on a continuum from simplistic in vitro co-culture to complex in vivo animal models. 191, 331 While each workflow and analysis has limitations which should be considered during experimental set-up and addressed in manuscripts, every method contributes to our understanding of the microbiome. These experiments, together with bioinformatics-based multi-omics and human cell culture-based studies, will elucidate the roles of specific metabolites in biological context. As cultivation techniques, analytical methods, and bioinformatics approaches driven by large datasets improve and become integrated with each other, we as a community can start to unravel the role of microbiome-derived natural products in human health and disease.
Supplementary Material
References
- 1.Carter GT. Nat Prod Rep. 2011;28:1783–1789. doi: 10.1039/c1np00033k. [DOI] [PubMed] [Google Scholar]
- 2.Harvey AL, Edrada-Ebel R, Quinn RJ. Nat Rev Drug Discov. 2015;14:111–129. doi: 10.1038/nrd4510. [DOI] [PubMed] [Google Scholar]
- 3.Newman DJ, Cragg GM. Journal of natural products. 2016;79:629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 4.Challinor VL, Bode HB. Annals of the New York Academy of Sciences. 2015;1354:82–97. doi: 10.1111/nyas.12954. [DOI] [PubMed] [Google Scholar]
- 5.Donia MS, Cimermancic P, Schulze CJ, Wieland Brown LC, Martin J, Mitreva M, Clardy J, Linington RG, Fischbach MA. Cell. 2014;158:1402–1414. doi: 10.1016/j.cell.2014.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Medema MH, Fischbach MA. Nat Chem Biol. 2015;11:639–648. doi: 10.1038/nchembio.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto JM, Hansen T, Le Paslier D, Linneberg A, Nielsen HB, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, Jian M, Zhou Y, Li Y, Zhang X, Qin N, Yang H, Wang J, Brunak S, Dore J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J, Bork P, Ehrlich SD. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharon G, Garg N, Debelius J, Knight R, Dorrestein PC, Mazmanian SK. Cell Metab. 2014;20:719–730. doi: 10.1016/j.cmet.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sender R, Fuchs S, Milo R. Cell. 2016;164:337–340. doi: 10.1016/j.cell.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 10.Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 11.Clarke G, Quigley EM, Cryan JF, Dinan TG. Trends Mol Med. 2009;15:478–489. doi: 10.1016/j.molmed.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Greenblum S, Turnbaugh PJ, Borenstein E. Proc Natl Acad Sci U S A. 2012;109:594–599. doi: 10.1073/pnas.1116053109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kennedy PJ, Cryan JF, Dinan TG, Clarke G. World J Gastroenterol. 2014;20:14105–14125. doi: 10.3748/wjg.v20.i39.14105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Major G, Spiller R. Curr Opin Endocrinol Diabetes Obes. 2014;21:15–21. doi: 10.1097/MED.0000000000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, Griffin NW, Lombard V, Henrissat B, Bain JR, Muehlbauer MJ, Ilkayeva O, Semenkovich CF, Funai K, Hayashi DK, Lyle BJ, Martini MC, Ursell LK, Clemente JC, Van Treuren W, Walters WA, Knight R, Newgard CB, Heath AC, Gordon JI. Science. 2013;341:1241214. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, Egholm M, Henrissat B, Heath AC, Knight R, Gordon JI. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scher JU, Abramson SB. Nat Rev Rheumatol. 2011;7:569–578. doi: 10.1038/nrrheum.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scher JU, Sczesnak A, Longman RS, Segata N, Ubeda C, Bielski C, Rostron T, Cerundolo V, Pamer EG, Abramson SB, Huttenhower C, Littman DR. Elife. 2013;2:e01202. doi: 10.7554/eLife.01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang X, Zhang D, Jia H, Feng Q, Wang D, Liang D, Wu X, Li J, Tang L, Li Y, Lan Z, Chen B, Zhong H, Xie H, Jie Z, Chen W, Tang S, Xu X, Wang X, Cai X, Liu S, Xia Y, Qiao X, Al-Aama JY, Chen H, Wang L, Wu QJ, Zhang F, Zheng W, Zhang M, Luo G, Xue W, Xiao L, Yin Y, Yang H, Wang J, Kristiansen K, Liu L, Li T, Huang Q. Nat Med. 2015;21:895–905. doi: 10.1038/nm.3914. [DOI] [PubMed] [Google Scholar]
- 20.Dethlefsen L, McFall-Ngai M, Relman DA. Nature. 2007;449:811–818. doi: 10.1038/nature06245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams MR, Gallo RL. Curr Allergy Asthma Rep. 2015;15:65. doi: 10.1007/s11882-015-0567-4. [DOI] [PubMed] [Google Scholar]
- 22.Davies J. J Antibiot. 2013;66:361–364. doi: 10.1038/ja.2013.61. [DOI] [PubMed] [Google Scholar]
- 23.Phelan VV, Liu WT, Pogliano K, Dorrestein PC. Nat Chem Biol. 2012;8:26–35. doi: 10.1038/nchembio.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hawver LA, Jung SA, Ng WL. FEMS microbiology reviews. 2016 doi: 10.1093/femsre/fuw014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heeb S, Fletcher MP, Chhabra SR, Diggle SP, Williams P, Camara M. FEMS microbiology reviews. 2011;35:247–274. doi: 10.1111/j.1574-6976.2010.00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wongsuk T, Pumeesat P, Luplertlop N. J Basic Microbiol. 2016;56:440–447. doi: 10.1002/jobm.201500759. [DOI] [PubMed] [Google Scholar]
- 27.Crosa JH, Walsh CT. Microbiol Mol Biol Rev. 2002;66:223–249. doi: 10.1128/MMBR.66.2.223-249.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fischbach MA, Lin H, Liu DR, Walsh CT. Nat Chem Biol. 2006;2:132–138. doi: 10.1038/nchembio771. [DOI] [PubMed] [Google Scholar]
- 29.Koh A, De Vadder F, Kovatcheva-Datchary P, Backhed F. Cell. 2016;165:1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 30.Lee WJ, Hase K. Nat Chem Biol. 2014;10:416–424. doi: 10.1038/nchembio.1535. [DOI] [PubMed] [Google Scholar]
- 31.Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. Adv Immunol. 2014;121:91–119. doi: 10.1016/B978-0-12-800100-4.00003-9. [DOI] [PubMed] [Google Scholar]
- 32.Balskus EP. Nat Prod Rep. 2015;32:1534–1540. doi: 10.1039/c5np00091b. [DOI] [PubMed] [Google Scholar]
- 33.Bode HB. Angewandte Chemie. 2015;54:10408–10411. doi: 10.1002/anie.201505341. [DOI] [PubMed] [Google Scholar]
- 34.Fleming A. Bull World Health Organ. 2001;79:780–790. [PMC free article] [PubMed] [Google Scholar]
- 35.Clatworthy AE, Pierson E, Hung DT. Nat Chem Biol. 2007;3:541–548. doi: 10.1038/nchembio.2007.24. [DOI] [PubMed] [Google Scholar]
- 36.Cimermancic P, Medema MH, Claesen J, Kurita K, Wieland Brown LC, Mavrommatis K, Pati A, Godfrey PA, Koehrsen M, Clardy J, Birren BW, Takano E, Sali A, Linington RG, Fischbach MA. Cell. 2014;158:412–421. doi: 10.1016/j.cell.2014.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, Gordon JI, Relman DA, Fraser-Liggett CM, Nelson KE. Science. 2006;312:1355–1359. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bouslimani A, Porto C, Rath CM, Wang M, Guo Y, Gonzalez A, Berg-Lyon D, Ackermann G, Moeller Christensen GJ, Nakatsuji T, Zhang L, Borkowski AW, Meehan MJ, Dorrestein K, Gallo RL, Bandeira N, Knight R, Alexandrov T, Dorrestein PC. Proc Natl Acad Sci U S A. 2015;112:E2120–2129. doi: 10.1073/pnas.1424409112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fierer N, Hamady M, Lauber CL, Knight R. Proc Natl Acad Sci U S A. 2008;105:17994–17999. doi: 10.1073/pnas.0807920105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grice EA, Kong HH, Conlan S, Deming CB, Davis J, Young AC, Bouffard GG, Blakesley RW, Murray PR, Green ED, Turner ML, Segre JA. Science. 2009;324:1190–1192. doi: 10.1126/science.1171700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cogen AL, Nizet V, Gallo RL. Br J Dermatol. 2008;158:442–455. doi: 10.1111/j.1365-2133.2008.08437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Findley K, Grice EA. PLoS Pathog. 2014;10:e1004436. doi: 10.1371/journal.ppat.1004436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakabayashi A, Sei Y, Guillot J. Medical mycology. 2000;38:337–341. doi: 10.1080/mmy.38.5.337.341. [DOI] [PubMed] [Google Scholar]
- 44.Saunders CW, Scheynius A, Heitman J. PLoS Pathog. 2012;8:e1002701. doi: 10.1371/journal.ppat.1002701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Falcocchio S, Ruiz C, Pastor FIJ, Saso L, Diaz P. J Mol Catal B-Enzym. 2006;40:132–137. [Google Scholar]
- 46.Burkhart CN, Burkhart CG. Int J Dermatol. 2003;42:925–927. doi: 10.1111/j.1365-4632.2003.01588.x. [DOI] [PubMed] [Google Scholar]
- 47.Gribbon EM, Cunliffe WJ, Holland KT. J Gen Microbiol. 1993;139:1745–1751. doi: 10.1099/00221287-139-8-1745. [DOI] [PubMed] [Google Scholar]
- 48.Jappe U. Acta Derm Venereol. 2003;83:241–248. doi: 10.1080/00015550310016463. [DOI] [PubMed] [Google Scholar]
- 49.Coenye T, Peeters E, Nelis HJ. Res Microbiol. 2007;158:386–392. doi: 10.1016/j.resmic.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 50.Achermann Y, Goldstein EJ, Coenye T, Shirtliff ME. Clin Microbiol Rev. 2014;27:419–440. doi: 10.1128/CMR.00092-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Allaker RP, Greenman J, Osborne RH. Microbios. 1986;48:165–172. [PubMed] [Google Scholar]
- 52.Puhvel SM, Sakamoto M. J Invest Dermatol. 1978;71:324–329. doi: 10.1111/1523-1747.ep12529815. [DOI] [PubMed] [Google Scholar]
- 53.Shu M, Wang Y, Yu J, Kuo S, Coda A, Jiang Y, Gallo RL, Huang CM. PloS one. 2013;8:e55380. doi: 10.1371/journal.pone.0055380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wieland Brown LC, Acker MG, Clardy J, Walsh CT, Fischbach MA. Proc Natl Acad Sci U S A. 2009;106:2549–2553. doi: 10.1073/pnas.0900008106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gong JQ, Lin L, Lin T, Hao F, Zeng FQ, Bi ZG, Yi D, Zhao B. Br J Dermatol. 2006;155:680–687. doi: 10.1111/j.1365-2133.2006.07410.x. [DOI] [PubMed] [Google Scholar]
- 56.Hauser C, Wuethrich B, Matter L, Wilhelm JA, Sonnabend W, Schopfer K. Dermatologica. 1985;170:35–39. [PubMed] [Google Scholar]
- 57.Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, Gottrup F, Gurtner GC, Longaker MT. Wound Repair Regen. 2009;17:763–771. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Church D, Elsayed S, Reid O, Winston B, Lindsay R. Clin Microbiol Rev. 2006;19:403–434. doi: 10.1128/CMR.19.2.403-434.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Erol S, Altoparlak U, Akcay MN, Celebi F, Parlak M. Burns. 2004;30:357–361. doi: 10.1016/j.burns.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 60.Ramzy PI, Herndon DN, Wolf SE, Irtun O, Barret JP, Ramirez RJ, Heggers JP. Arch Surg. 1998;133:1275–1280. doi: 10.1001/archsurg.133.12.1275. [DOI] [PubMed] [Google Scholar]
- 61.Wurtz R, Karajovic M, Dacumos E, Jovanovic B, Hanumadass M. Burns. 1995;21:181–184. doi: 10.1016/0305-4179(95)80005-9. [DOI] [PubMed] [Google Scholar]
- 62.Trent JT, Federman D, Kirsner RS. Ostomy Wound Manage. 2001;47:28–34. [PubMed] [Google Scholar]
- 63.Johansson L, Thulin P, Low DE, Norrby-Teglund A. Clin Infect Dis. 2010;51:58–65. doi: 10.1086/653116. [DOI] [PubMed] [Google Scholar]
- 64.Molloy EM, Cotter PD, Hill C, Mitchell DA, Ross RP. Nat Rev Microbiol. 2011;9:670–681. doi: 10.1038/nrmicro2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee SW, Mitchell DA, Markley AL, Hensler ME, Gonzalez D, Wohlrab A, Dorrestein PC, Nizet V, Dixon JE. Proc Natl Acad Sci U S A. 2008;105:5879–5884. doi: 10.1073/pnas.0801338105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Datta V, Myskowski SM, Kwinn LA, Chiem DN, Varki N, Kansal RG, Kotb M, Nizet V. Mol Microbiol. 2005;56:681–695. doi: 10.1111/j.1365-2958.2005.04583.x. [DOI] [PubMed] [Google Scholar]
- 67.Jimenez PN, Koch G, Thompson JA, Xavier KB, Cool RH, Quax WJ. Microbiol Mol Biol Rev. 2012;76:46–65. doi: 10.1128/MMBR.05007-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McGrath S, Wade DS, Pesci EC. FEMS microbiology letters. 2004;230:27–34. doi: 10.1016/S0378-1097(03)00849-8. [DOI] [PubMed] [Google Scholar]
- 69.Huse H, Whiteley M. Chem Rev. 2011;111:152–159. doi: 10.1021/cr100063u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Soberon-Chavez G, Lepine F, Deziel E. Applied microbiology and biotechnology. 2005;68:718–725. doi: 10.1007/s00253-005-0150-3. [DOI] [PubMed] [Google Scholar]
- 71.Mentel M, Ahuja EG, Mavrodi DV, Breinbauer R, Thomashow LS, Blankenfeldt W. Chembiochem : a European journal of chemical biology. 2009;10:2295–2304. doi: 10.1002/cbic.200900323. [DOI] [PubMed] [Google Scholar]
- 72.Muller M, Li Z, Maitz PK. Burns. 2009;35:500–508. doi: 10.1016/j.burns.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 73.Liu GY, Essex A, Buchanan JT, Datta V, Hoffman HM, Bastian JF, Fierer J, Nizet V. J Exp Med. 2005;202:209–215. doi: 10.1084/jem.20050846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pelz A, Wieland KP, Putzbach K, Hentschel P, Albert K, Gotz F. J Biol Chem. 2005;280:32493–32498. doi: 10.1074/jbc.M505070200. [DOI] [PubMed] [Google Scholar]
- 75.Wieland B, Feil C, Gloria-Maercker E, Thumm G, Lechner M, Bravo JM, Poralla K, Gotz F. J Bacteriol. 1994;176:7719–7726. doi: 10.1128/jb.176.24.7719-7726.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gonzalez DJ, Okumura CY, Hollands A, Kersten R, Akong-Moore K, Pence MA, Malone CL, Derieux J, Moore BS, Horswill AR, Dixon JE, Dorrestein PC, Nizet V. J Biol Chem. 2012;287:13889–13898. doi: 10.1074/jbc.M112.349860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gonzalez DJ, Vuong L, Gonzalez IS, Keller N, McGrosso D, Hwang JH, Hung J, Zinkernagel A, Dixon JE, Dorrestein PC, Nizet V. Mol Cell Proteomics. 2014;13:1262–1272. doi: 10.1074/mcp.M113.031336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang R, Braughton KR, Kretschmer D, Bach TH, Queck SY, Li M, Kennedy AD, Dorward DW, Klebanoff SJ, Peschel A, DeLeo FR, Otto M. Nat Med. 2007;13:1510–1514. doi: 10.1038/nm1656. [DOI] [PubMed] [Google Scholar]
- 79.Kretschmer D, Gleske AK, Rautenberg M, Wang R, Koberle M, Bohn E, Schoneberg T, Rabiet MJ, Boulay F, Klebanoff SJ, van Kessel KA, van Strijp JA, Otto M, Peschel A. Cell Host Microbe. 2010;7:463–473. doi: 10.1016/j.chom.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rautenberg M, Joo HS, Otto M, Peschel A. Faseb J. 2011;25:1254–1263. doi: 10.1096/fj.10-175208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cheung GY, Rigby K, Wang R, Queck SY, Braughton KR, Whitney AR, Teintze M, DeLeo FR, Otto M. PLoS Pathog. 2010;6:e1001133. doi: 10.1371/journal.ppat.1001133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cogen AL, Yamasaki K, Sanchez KM, Dorschner RA, Lai Y, MacLeod DT, Torpey JW, Otto M, Nizet V, Kim JE, Gallo RL. J Invest Dermatol. 2010;130:192–200. doi: 10.1038/jid.2009.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lai Y, Cogen AL, Radek KA, Park HJ, Macleod DT, Leichtle A, Ryan AF, Di Nardo A, Gallo RL. J Invest Dermatol. 2010;130:2211–2221. doi: 10.1038/jid.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gallo RL, Nakatsuji T. Firmocidin, an antimicrobial molecule produced by staphylococcus epidermidis. US20150290209. US Patent Appl. 2015
- 85.George KM, Chatterjee D, Gunawardana G, Welty D, Hayman J, Lee R, Small PL. Science. 1999;283:854–857. doi: 10.1126/science.283.5403.854. [DOI] [PubMed] [Google Scholar]
- 86.Mve-Obiang A, Lee RE, Portaels F, Small PL. Infection and immunity. 2003;71:774–783. doi: 10.1128/IAI.71.2.774-783.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chany AC, Tresse C, Casarotto V, Blanchard N. Nat Prod Rep. 2013;30:1527–1567. doi: 10.1039/c3np70068b. [DOI] [PubMed] [Google Scholar]
- 88.Hong H, Demangel C, Pidot SJ, Leadlay PF, Stinear T. Nat Prod Rep. 2008;25:447–454. doi: 10.1039/b803101k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kishi Y. Proc Natl Acad Sci U S A. 2011;108:6703–6708. doi: 10.1073/pnas.1015252108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.En J, Goto M, Nakanaga K, Higashi M, Ishii N, Saito H, Yonezawa S, Hamada H, Small PL. Infect Immun. 2008;76:2002–2007. doi: 10.1128/IAI.01588-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Marion E, Song OR, Christophe T, Babonneau J, Fenistein D, Eyer J, Letournel F, Henrion D, Clere N, Paille V, Guerineau NC, Saint Andre JP, Gersbach P, Altmann KH, Stinear TP, Comoglio Y, Sandoz G, Preisser L, Delneste Y, Yeramian E, Marsollier L, Brodin P. Cell. 2014;157:1565–1576. doi: 10.1016/j.cell.2014.04.040. [DOI] [PubMed] [Google Scholar]
- 92.Dickson RP, Erb-Downward JR, Huffnagle GB. Expert Rev Respir Med. 2013;7:245–257. doi: 10.1586/ers.13.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Friaza V, la Horra C, Rodriguez-Dominguez MJ, Martin-Juan J, Canton R, Calderon EJ, Del Campo R. J Microbiol Methods. 2010;82:98–101. doi: 10.1016/j.mimet.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 94.Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. J Clin Microbiol. 2005;43:5721–5732. doi: 10.1128/JCM.43.11.5721-5732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, Yu WH, Lakshmanan A, Wade WG. J Bacteriol. 2010;192:5002–5017. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ghannoum MA, Jurevic RJ, Mukherjee PK, Cui F, Sikaroodi M, Naqvi A, Gillevet PM. PLoS Pathog. 2010;6:e1000713. doi: 10.1371/journal.ppat.1000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xu X, He J, Xue J, Wang Y, Li K, Zhang K, Guo Q, Liu X, Zhou Y, Cheng L, Li M, Li Y, Li Y, Shi W, Zhou X. Environmental microbiology. 2015;17:699–710. doi: 10.1111/1462-2920.12502. [DOI] [PubMed] [Google Scholar]
- 98.Duran-Pinedo AE, Frias-Lopez J. Microbes Infect. 2015;17:505–516. doi: 10.1016/j.micinf.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wade WG. Pharmacol Res. 2013;69:137–143. doi: 10.1016/j.phrs.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 100.Burton JP, Chilcott CN, Moore CJ, Speiser G, Tagg JR. J Appl Microbiol. 2006;100:754–764. doi: 10.1111/j.1365-2672.2006.02837.x. [DOI] [PubMed] [Google Scholar]
- 101.Ross KF, Ronson CW, Tagg JR. Appl Environ Microbiol. 1993;59:2014–2021. doi: 10.1128/aem.59.7.2014-2021.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Upton M, Tagg JR, Wescombe P, Jenkinson HF. J Bacteriol. 2001;183:3931–3938. doi: 10.1128/JB.183.13.3931-3938.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Barbour A, Tagg J, Abou-Zied OK, Philip K. Sci Rep. 2016;6:31749. doi: 10.1038/srep31749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Abbe K, Takahashi S, Yamada T. J Bacteriol. 1982;152:175–182. doi: 10.1128/jb.152.1.175-182.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Badet MC, Richard B, Dorignac G. J Appl Microbiol. 2001;90:1015–1018. doi: 10.1046/j.1365-2672.2001.01340.x. [DOI] [PubMed] [Google Scholar]
- 106.Joyner PM, Liu J, Zhang Z, Merritt J, Qi F, Cichewicz RH. Org Biomol Chem. 2010;8:5486–5489. doi: 10.1039/c0ob00579g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Takahashi N, Iwami Y, Yamada T. Oral Microbiol Immunol. 1991;6:299–304. doi: 10.1111/j.1399-302x.1991.tb00497.x. [DOI] [PubMed] [Google Scholar]
- 108.Berezow AB, Darveau RP. Periodontol. 2000;201155:36–47. doi: 10.1111/j.1600-0757.2010.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Duran-Pinedo AE, Chen T, Teles R, Starr JR, Wang X, Krishnan K, Frias-Lopez J. Isme J. 2014;8:1659–1672. doi: 10.1038/ismej.2014.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Khan SA, Kong EF, Meiller TF, Jabra-Rizk MA. PLoS Pathog. 2015;11:e1004952. doi: 10.1371/journal.ppat.1004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schiffmann E, Corcoran BA, Wahl SM. Proc Natl Acad Sci U S A. 1975;72:1059–1062. doi: 10.1073/pnas.72.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Takeuchi O, Akira S. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 113.Boutin S, Graeber SY, Weitnauer M, Panitz J, Stahl M, Clausznitzer D, Kaderali L, Einarsson G, Tunney MM, Elborn JS, Mall MA, Dalpke AH. PloS one. 2015;10:e0116029. doi: 10.1371/journal.pone.0116029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Charlson ES, Bittinger K, Haas AR, Fitzgerald AS, Frank I, Yadav A, Bushman FD, Collman RG. Am J Respir Crit Care Med. 2011;184:957–963. doi: 10.1164/rccm.201104-0655OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dickson RP, Huffnagle GB. PLoS Pathog. 2015;11:e1004923. doi: 10.1371/journal.ppat.1004923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Siegel SJ, Weiser JN. Annu Rev Microbiol. 2015;69:425–444. doi: 10.1146/annurev-micro-091014-104209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zipperer A, Konnerth MC, Laux C, Berscheid A, Janek D, Weidenmaier C, Burian M, Schilling NA, Slavetinsky C, Marschal M, Willmann M, Kalbacher H, Schittek B, Brotz-Oesterhelt H, Grond S, Peschel A, Krismer B. Nature. 2016;535:511–516. doi: 10.1038/nature18634. [DOI] [PubMed] [Google Scholar]
- 118.Chang CI, Chelliah Y, Borek D, Mengin-Lecreulx D, Deisenhofer J. Science. 2006;311:1761–1764. doi: 10.1126/science.1123056. [DOI] [PubMed] [Google Scholar]
- 119.Luker KE, Tyler AN, Marshall GR, Goldman WE. Mol Microbiol. 1995;16:733–743. doi: 10.1111/j.1365-2958.1995.tb02434.x. [DOI] [PubMed] [Google Scholar]
- 120.Luker KE, Collier JL, Kolodziej EW, Marshall GR, Goldman WE. Proc Natl Acad Sci U S A. 1993;90:2365–2369. doi: 10.1073/pnas.90.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cookson BT, Cho HL, Herwaldt LA, Goldman WE. Infection and immunity. 1989;57:2223–2229. doi: 10.1128/iai.57.7.2223-2229.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cookson BT, Tyler AN, Goldman WE. Biochemistry. 1989;28:1744–1749. doi: 10.1021/bi00430a048. [DOI] [PubMed] [Google Scholar]
- 123.Goldman WE, Klapper DG, Baseman JB. Infection and immunity. 1982;36:782–794. doi: 10.1128/iai.36.2.782-794.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Heiss LN, Lancaster JR, Jr, Corbett JA, Goldman WE. Proc Natl Acad Sci U S A. 1994;91:267–270. doi: 10.1073/pnas.91.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dawid S, Roche AM, Weiser JN. Infection and immunity. 2007;75:443–451. doi: 10.1128/IAI.01775-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dawid S, Sebert ME, Weiser JN. J Bacteriol. 2009;191:1509–1518. doi: 10.1128/JB.01213-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Garcia-Rodriguez JA, Fresnadillo Martinez MJ. J Antimicrob Chemother. 2002;50(Suppl S2):59–73. doi: 10.1093/jac/dkf506. [DOI] [PubMed] [Google Scholar]
- 128.Whitney CG, Farley MM, Hadler J, Harrison LH, Bennett NM, Lynfield R, Reingold A, Cieslak PR, Pilishvili T, Jackson D, Facklam RR, Jorgensen JH, Schuchat A. N Engl J Med. 2003;348:1737–1746. doi: 10.1056/NEJMoa022823. [DOI] [PubMed] [Google Scholar]
- 129.Bisno AL, Gerber MA, Gwaltney JM, Jr, Kaplan EL, Schwartz RH. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2002;35:113–125. doi: 10.1086/340949. [DOI] [PubMed] [Google Scholar]
- 130.Herbert D, Todd EW. British Journal of Experimental Pathology. 1944;25:242–254. [Google Scholar]
- 131.Yoshino M, Murayama SY, Sunaoshi K, Wajima T, Takahashi M, Masaki J, Kurokawa I, Ubukata K. J Clin Microbiol. 2010;48:635–638. doi: 10.1128/JCM.01362-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Beck JM, Young VB, Huffnagle GB. Transl Res. 2012;160:258–266. doi: 10.1016/j.trsl.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hasleton PS. J Anat. 1972;112:391–400. [PMC free article] [PubMed] [Google Scholar]
- 134.Charlson ES, Diamond JM, Bittinger K, Fitzgerald AS, Yadav A, Haas AR, Bushman FD, Collman RG. Am J Respir Crit Care Med. 2012;186:536–545. doi: 10.1164/rccm.201204-0693OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Morris A, Beck JM, Schloss PD, Campbell TB, Crothers K, Curtis JL, Flores SC, Fontenot AP, Ghedin E, Huang L, Jablonski K, Kleerup E, Lynch SV, Sodergren E, Twigg H, Young VB, Bassis CM, Venkataraman A, Schmidt TM, Weinstock GM, HIVM, Lung P. Am J Respir Crit Care Med. 2013;187:1067–1075. doi: 10.1164/rccm.201210-1913OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Huang YJ, Nariya S, Harris JM, Lynch SV, Choy DF, Arron JR, Boushey H. J Allergy Clin Immunol. 2015;136:874–884. doi: 10.1016/j.jaci.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Riiser A. Allergy Asthma Clin Immunol. 2015;11:35. doi: 10.1186/s13223-015-0102-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang Z, Bafadhel M, Haldar K, Spivak A, Mayhew D, Miller BE, Tal-Singer R, Johnston SL, Ramsheh MY, Barer MR, Brightling CE, Brown JR. The European respiratory journal. 2016;47:1082–1092. doi: 10.1183/13993003.01406-2015. [DOI] [PubMed] [Google Scholar]
- 139.Dy R, Sethi S. Curr Opin Pulm Med. 2016;22:196–202. doi: 10.1097/MCP.0000000000000268. [DOI] [PubMed] [Google Scholar]
- 140.Huang YJ, LiPuma JJ. Clin Chest Med. 2016;37:59–67. doi: 10.1016/j.ccm.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Caverly LJ, Zhao J, LiPuma JJ. Pediatr Pulmonol. 2015;50(Suppl 40):S31–38. doi: 10.1002/ppul.23243. [DOI] [PubMed] [Google Scholar]
- 142.Liu YC, Chan KG, Chang CY. Front Microbiol. 2015;6:1226. doi: 10.3389/fmicb.2015.01226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Beare PA, For RJ, Martin LW, Lamont IL. Mol Microbiol. 2003;47:195–207. doi: 10.1046/j.1365-2958.2003.03288.x. [DOI] [PubMed] [Google Scholar]
- 144.Price-Whelan A, Dietrich LE, Newman DK. Nat Chem Biol. 2006;2:71–78. doi: 10.1038/nchembio764. [DOI] [PubMed] [Google Scholar]
- 145.Zulianello L, Canard C, Kohler T, Caille D, Lacroix JS, Meda P. Infection and immunity. 2006;74:3134–3147. doi: 10.1128/IAI.01772-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hunter RC, Klepac-Ceraj V, Lorenzi MM, Grotzinger H, Martin TR, Newman DK. American journal of respiratory cell and molecular biology. 2012;47:738–745. doi: 10.1165/rcmb.2012-0088OC. [DOI] [PubMed] [Google Scholar]
- 147.Barr HL, Halliday N, Camara M, Barrett DA, Williams P, Forrester DL, Simms R, Smyth AR, Honeybourne D, Whitehouse JL, Nash EF, Dewar J, Clayton A, Knox AJ, Fogarty AW. The European respiratory journal. 2015;46:1046–1054. doi: 10.1183/09031936.00225214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Quinn RA, Phelan VV, Whiteson KL, Garg N, Bailey BA, Lim YW, Conrad DJ, Dorrestein PC, Rohwer FL. Isme J. 2015 doi: 10.1038/ismej.2015.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bok JW, Hoffmeister D, Maggio-Hall LA, Murillo R, Glasner JD, Keller NP. Chem Biol. 2006;13:31–37. doi: 10.1016/j.chembiol.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 150.Frisvad JC, Larsen TO. Front Microbiol. 2015;6:1485. doi: 10.3389/fmicb.2015.01485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Donnelly PJ, Stewart RK, Ali SL, Conlan AA, Reid KR, Petsikas D, Massey TE. Carcinogenesis. 1996;17:2487–2494. doi: 10.1093/carcin/17.11.2487. [DOI] [PubMed] [Google Scholar]
- 152.Eaton DL, Gallagher EP. Annu Rev Pharmacol Toxicol. 1994;34:135–172. doi: 10.1146/annurev.pa.34.040194.001031. [DOI] [PubMed] [Google Scholar]
- 153.Massey TE, Smith GB, Tam AS. Exp Lung Res. 2000;26:673–683. doi: 10.1080/01902140150216756. [DOI] [PubMed] [Google Scholar]
- 154.Singh R, Hsieh DP. Arch Biochem Biophys. 1977;178:285–292. doi: 10.1016/0003-9861(77)90193-x. [DOI] [PubMed] [Google Scholar]
- 155.Dolan SK, O’Keeffe G, Jones GW, Doyle S. Trends Microbiol. 2015;23:419–428. doi: 10.1016/j.tim.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 156.Sugui JA, Pardo J, Chang YC, Zarember KA, Nardone G, Galvez EM, Mullbacher A, Gallin JI, Simon MM, Kwon-Chung KJ. Eukaryot Cell. 2007;6:1562–1569. doi: 10.1128/EC.00141-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Choi HS, Shim JS, Kim JA, Kang SW, Kwon HJ. Biochemical and biophysical research communications. 2007;359:523–528. doi: 10.1016/j.bbrc.2007.05.139. [DOI] [PubMed] [Google Scholar]
- 158.Scharf DH, Heinekamp T, Remme N, Hortschansky P, Brakhage AA, Hertweck C. Applied microbiology and biotechnology. 2012;93:467–472. doi: 10.1007/s00253-011-3689-1. [DOI] [PubMed] [Google Scholar]
- 159.Amin R, Dupuis A, Aaron SD, Ratjen F. Chest. 2010;137:171–176. doi: 10.1378/chest.09-1103. [DOI] [PubMed] [Google Scholar]
- 160.Leclair LW, Hogan DA. Medical mycology. 2010;48(Suppl 1):S125–132. doi: 10.3109/13693786.2010.521522. [DOI] [PubMed] [Google Scholar]
- 161.Shoseyov D, Brownlee KG, Conway SP, Kerem E. Chest. 2006;130:222–226. doi: 10.1378/chest.130.1.222. [DOI] [PubMed] [Google Scholar]
- 162.Moree WJ, Phelan VV, Wu CH, Bandeira N, Cornett DS, Duggan BM, Dorrestein PC. Proc Natl Acad Sci U S A. 2012;109:13811–13816. doi: 10.1073/pnas.1206855109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Briard B, Bomme P, Lechner BE, Mislin GL, Lair V, Prevost MC, Latge JP, Haas H, Beauvais A. Scientific reports. 2015;5:8220. doi: 10.1038/srep08220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zheng H, Kim J, Liew M, Yan JK, Herrera O, Bok JW, Kelleher NL, Keller NP, Wang Y. Current biology : CB. 2015;25:29–37. doi: 10.1016/j.cub.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ferreira JA, Penner JC, Moss RB, Haagensen JA, Clemons KV, Spormann AM, Nazik H, Cohen K, Banaei N, Carolino E, Stevens DA. PloS one. 2015;10:e0134692. doi: 10.1371/journal.pone.0134692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zumla A, Raviglione M, Hafner R, von Reyn CF. N Engl J Med. 2013;368:745–755. doi: 10.1056/NEJMra1200894. [DOI] [PubMed] [Google Scholar]
- 167.Velayati AA, Masjedi MR, Farnia P, Tabarsi P, Ghanavi J, Ziazarifi AH, Hoffner SE. Chest. 2009;136:420–425. doi: 10.1378/chest.08-2427. [DOI] [PubMed] [Google Scholar]
- 168.Lechartier B, Rybniker J, Zumla A, Cole ST. EMBO Mol Med. 2014;6:158–168. doi: 10.1002/emmm.201201772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon SV, Eiglmeier K, Gas S, Barry CE, 3rd, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail MA, Rajandream MA, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Squares S, Sulston JE, Taylor K, Whitehead S, Barrell BG. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 170.De Voss JJ, Rutter K, Schroeder BG, Su H, Zhu Y, Barry CE., 3rd Proc Natl Acad Sci U S A. 2000;97:1252–1257. doi: 10.1073/pnas.97.3.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Quadri LE, Sello J, Keating TA, Weinreb PH, Walsh CT. Chem Biol. 1998;5:631–645. doi: 10.1016/s1074-5521(98)90291-5. [DOI] [PubMed] [Google Scholar]
- 172.Brown KA, Ratledge C. Biochim Biophys Acta. 1975;385:207–220. doi: 10.1016/0304-4165(75)90349-9. [DOI] [PubMed] [Google Scholar]
- 173.Ferreras JA, Stirrett KL, Lu X, Ryu JS, Soll CE, Tan DS, Quadri LE. Chem Biol. 2008;15:51–61. doi: 10.1016/j.chembiol.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Pang JM, Layre E, Sweet L, Sherrid A, Moody DB, Ojha A, Sherman DR. J Bacteriol. 2012;194:715–721. doi: 10.1128/JB.06304-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Azad AK, Sirakova TD, Fernandes ND, Kolattukudy PE. J Biol Chem. 1997;272:16741–16745. doi: 10.1074/jbc.272.27.16741. [DOI] [PubMed] [Google Scholar]
- 176.Reed MB, Domenech P, Manca C, Su H, Barczak AK, Kreiswirth BN, Kaplan G, Barry CE., 3rd Nature. 2004;431:84–87. doi: 10.1038/nature02837. [DOI] [PubMed] [Google Scholar]
- 177.Trivedi OA, Arora P, Vats A, Ansari MZ, Tickoo R, Sridharan V, Mohanty D, Gokhale RS. Mol Cell. 2005;17:631–643. doi: 10.1016/j.molcel.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 178.Tsenova L, Ellison E, Harbacheuski R, Moreira AL, Kurepina N, Reed MB, Mathema B, Barry CE, 3rd, Kaplan G. J Infect Dis. 2005;192:98–106. doi: 10.1086/430614. [DOI] [PubMed] [Google Scholar]
- 179.Rao V, Fujiwara N, Porcelli SA, Glickman MS. J Exp Med. 2005;201:535–543. doi: 10.1084/jem.20041668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Blumberg R, Powrie F. Sci Transl Med. 2012;4:137rv137. doi: 10.1126/scitranslmed.3004184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Shreiner AB, Kao JY, Young VB. Curr Opin Gastroenterol. 2015;31:69–75. doi: 10.1097/MOG.0000000000000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Faith JJ, Guruge JL, Charbonneau M, Subramanian S, Seedorf H, Goodman AL, Clemente JC, Knight R, Heath AC, Leibel RL, Rosenbaum M, Gordon JI. Science. 2013;341:1237439. doi: 10.1126/science.1237439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Dorrestein PC, Mazmanian SK, Knight R. Immunity. 2014;40:824–832. doi: 10.1016/j.immuni.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Palm NW, de Zoete MR, Cullen TW, Barry NA, Stefanowski J, Hao L, Degnan PH, Hu J, Peter I, Zhang W, Ruggiero E, Cho JH, Goodman AL, Flavell RA. Cell. 2014;158:1000–1010. doi: 10.1016/j.cell.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Andersson AF, Lindberg M, Jakobsson H, Backhed F, Nyren P, Engstrand L. PloS one. 2008;3:e2836. doi: 10.1371/journal.pone.0002836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bik EM, Eckburg PB, Gill SR, Nelson KE, Purdom EA, Francois F, Perez-Perez G, Blaser MJ, Relman DA. Proc Natl Acad Sci U S A. 2006;103:732–737. doi: 10.1073/pnas.0506655103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kato S, Fujimura S, Kimura K, Nishio T, Hamada S, Minoura T, Oda M. Digestive diseases and sciences. 2006;51:641–646. doi: 10.1007/s10620-006-3185-0. [DOI] [PubMed] [Google Scholar]
- 189.Kusters JG, van Vliet AH, Kuipers EJ. Clin Microbiol Rev. 2006;19:449–490. doi: 10.1128/CMR.00054-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Keire DA, Anton P, Faull KF, Ruth E, Walsh JH, Chew P, Quisimoro D, Territo M, Reeve JR., Jr J Biol Chem. 2001;276:48847–48853. doi: 10.1074/jbc.M109811200. [DOI] [PubMed] [Google Scholar]
- 191.Sheh A, Fox JG. Gut Microbes. 2013;4:505–531. doi: 10.4161/gmic.26205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Viala J, Chaput C, Boneca IG, Cardona A, Girardin SE, Moran AP, Athman R, Memet S, Huerre MR, Coyle AJ, DiStefano PS, Sansonetti PJ, Labigne A, Bertin J, Philpott DJ, Ferrero RL. Nat Immunol. 2004;5:1166–1174. doi: 10.1038/ni1131. [DOI] [PubMed] [Google Scholar]
- 193.Vermeer IT, Gerrits MM, Moonen EJ, Engels LG, Dallinga JW, Kleinjans JC, van Maanen JM, Kuipers EJ, Kusters JG. Helicobacter. 2002;7:163–169. doi: 10.1046/j.1523-5378.2002.00076.x. [DOI] [PubMed] [Google Scholar]
- 194.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Nature. 2012;489:220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Begley M, Gahan CG, Hill C. FEMS microbiology reviews. 2005;29:625–651. doi: 10.1016/j.femsre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 196.Gerard P. Pathogens. 2013;3:14–24. doi: 10.3390/pathogens3010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Rath CM, Alexandrov T, Higginbottom SK, Song J, Milla ME, Fischbach MA, Sonnenburg JL, Dorrestein PC. Analytical chemistry. 2012;84:9259–9267. doi: 10.1021/ac302039u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Neis EP, Dejong CH, Rensen SS. Nutrients. 2015;7:2930–2946. doi: 10.3390/nu7042930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Clemente JC, Ursell LK, Parfrey LW, Knight R. Cell. 2012;148:1258–1270. doi: 10.1016/j.cell.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Donia MS, Fischbach MA. Science. 2015;349:1254766. doi: 10.1126/science.1254766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.McCranie EK, Bachmann BO. Nat Prod Rep. 2014;31:1026–1042. doi: 10.1039/c3np70128j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Patzer SI, Baquero MR, Bravo D, Moreno F, Hantke K. Microbiology. 2003;149:2557–2570. doi: 10.1099/mic.0.26396-0. [DOI] [PubMed] [Google Scholar]
- 203.Zschuttig A, Zimmermann K, Blom J, Goesmann A, Pohlmann C, Gunzer F. PloS one. 2012;7:e33351. doi: 10.1371/journal.pone.0033351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Nolan EM, Fischbach MA, Koglin A, Walsh CT. Journal of the American Chemical Society. 2007;129:14336–14347. doi: 10.1021/ja074650f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Strahsburger E, Baeza M, Monasterio O, Lagos R. Antimicrobial agents and chemotherapy. 2005;49:3083–3086. doi: 10.1128/AAC.49.7.3083-3086.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Thomas X, Destoumieux-Garzon D, Peduzzi J, Afonso C, Blond A, Birlirakis N, Goulard C, Dubost L, Thai R, Tabet JC, Rebuffat S. J Biol Chem. 2004;279:28233–28242. doi: 10.1074/jbc.M400228200. [DOI] [PubMed] [Google Scholar]
- 207.Secher T, Brehin C, Oswald E. Am J Physiol Gastrointest Liver Physiol. 2016;311:G123–129. doi: 10.1152/ajpgi.00091.2016. [DOI] [PubMed] [Google Scholar]
- 208.Nougayrede JP, Homburg S, Taieb F, Boury M, Brzuszkiewicz E, Gottschalk G, Buchrieser C, Hacker J, Dobrindt U, Oswald E. Science. 2006;313:848–851. doi: 10.1126/science.1127059. [DOI] [PubMed] [Google Scholar]
- 209.Arthur JC, Perez-Chanona E, Muhlbauer M, Tomkovich S, Uronis JM, Fan TJ, Campbell BJ, Abujamel T, Dogan B, Rogers AB, Rhodes JM, Stintzi A, Simpson KW, Hansen JJ, Keku TO, Fodor AA, Jobin C. Science. 2012;338:120–123. doi: 10.1126/science.1224820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Bian X, Fu J, Plaza A, Herrmann J, Pistorius D, Stewart AF, Zhang Y, Muller R. Chembiochem : a European journal of chemical biology. 2013;14:1194–1197. doi: 10.1002/cbic.201300208. [DOI] [PubMed] [Google Scholar]
- 211.Brotherton CA, Balskus EP. Journal of the American Chemical Society. 2013;135:3359–3362. doi: 10.1021/ja312154m. [DOI] [PubMed] [Google Scholar]
- 212.Bian X, Plaza A, Zhang Y, Muller R. Chemical Science. 2015;6:3154–3160. doi: 10.1039/c5sc00101c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Brotherton CA, Wilson M, Byrd G, Balskus EP. Organic letters. 2015;17:1545–1548. doi: 10.1021/acs.orglett.5b00432. [DOI] [PubMed] [Google Scholar]
- 214.Healy AR, Vizcaino MI, Crawford JM, Herzon SB. Journal of the American Chemical Society. 2016;138:5426–5432. doi: 10.1021/jacs.6b02276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Li ZR, Li Y, Lai JY, Tang J, Wang B, Lu L, Zhu G, Wu X, Xu Y, Qian PY. Chembiochem : a European journal of chemical biology. 2015;16:1715–1719. doi: 10.1002/cbic.201500239. [DOI] [PubMed] [Google Scholar]
- 216.Vizcaino MI, Crawford JM. Nat Chem. 2015;7:411–417. doi: 10.1038/nchem.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zha L, Wilson MR, Brotherton CA, Balskus EP. ACS chemical biology. 2016;11:1287–1295. doi: 10.1021/acschembio.6b00014. [DOI] [PubMed] [Google Scholar]
- 218.Li ZR, Li J, Gu JP, Lai JY, Duggan BM, Zhang WP, Li ZL, Li YX, Tong RB, Xu Y, Lin DH, Moore BS, Qian PY. Nat Chem Biol. 2016 doi: 10.1038/nchembio.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Weiglmeier PR, Rosch P, Berkner H. Toxins (Basel) 2010;2:2213–2229. doi: 10.3390/toxins2092213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Yoshimura S, Ikemura H, Watanabe H, Aimoto S, Shimonishi Y, Hara S, Takeda T, Miwatani T, Takeda Y. FEBS Lett. 1985;181:138–142. doi: 10.1016/0014-5793(85)81129-7. [DOI] [PubMed] [Google Scholar]
- 221.Klipstein FA, Engert RF, Houghten RA. Infection and immunity. 1983;42:838–841. doi: 10.1128/iai.42.2.838-841.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Takao T, Tominaga N, Shimonishi Y, Hara S, Inoue T, Miyama A. Biochemical and biophysical research communications. 1984;125:845–851. doi: 10.1016/0006-291x(84)91360-3. [DOI] [PubMed] [Google Scholar]
- 223.Takeda T, Peina Y, Ogawa A, Dohi S, Abe H, Nair GB, Pal SC. FEMS microbiology letters. 1991;64:23–27. doi: 10.1016/0378-1097(91)90203-m. [DOI] [PubMed] [Google Scholar]
- 224.Schneditz G, Rentner J, Roier S, Pletz J, Herzog KA, Bucker R, Troeger H, Schild S, Weber H, Breinbauer R, Gorkiewicz G, Hogenauer C, Zechner EL. Proc Natl Acad Sci U S A. 2014;111:13181–13186. doi: 10.1073/pnas.1403274111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Darby A, Lertpiriyapong K, Sarkar U, Seneviratne U, Park DS, Gamazon ER, Batchelder C, Cheung C, Buckley EM, Taylor NS, Shen Z, Tannenbaum SR, Wishnok JS, Fox JG. PloS one. 2014;9:e100542. doi: 10.1371/journal.pone.0100542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Mohr N, Budzikiewicz H. Tetrahedron. 1982;38:147–152. [Google Scholar]
- 227.Shioiri T, Aoyama T, Yamagami N, Shimizu N, Mori N, Kohda K. Anti-cancer drug design. 1995;10:167–176. [PubMed] [Google Scholar]
- 228.Hamilton MJ, Weingarden AR, Sadowsky MJ, Khoruts A. The American journal of gastroenterology. 2012;107:761–767. doi: 10.1038/ajg.2011.482. [DOI] [PubMed] [Google Scholar]
- 229.Rohlke F, Surawicz CM, Stollman N. Journal of clinical gastroenterology. 2010;44:567–570. doi: 10.1097/MCG.0b013e3181dadb10. [DOI] [PubMed] [Google Scholar]
- 230.Yoon SS, Brandt LJ. Journal of clinical gastroenterology. 2010;44:562–566. doi: 10.1097/MCG.0b013e3181dac035. [DOI] [PubMed] [Google Scholar]
- 231.Bien J, Palagani V, Bozko P. Therap Adv Gastroenterol. 2013;6:53–68. doi: 10.1177/1756283X12454590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Buffie CG, Jarchum I, Equinda M, Lipuma L, Gobourne A, Viale A, Ubeda C, Xavier J, Pamer EG. Infect Immun. 2012;80:62–73. doi: 10.1128/IAI.05496-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Buffie CG, Bucci V, Stein RR, McKenney PT, Ling L, Gobourne A, No D, Liu H, Kinnebrew M, Viale A, Littmann E, van den Brink MR, Jenq RR, Taur Y, Sander C, Cross JR, Toussaint NC, Xavier JB, Pamer EG. Nature. 2015;517:205–208. doi: 10.1038/nature13828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Francis MB, Allen CA, Shrestha R, Sorg JA. PLoS Pathog. 2013;9:e1003356. doi: 10.1371/journal.ppat.1003356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Sorg JA, Sonenshein AL. J Bacteriol. 2010;192:4983–4990. doi: 10.1128/JB.00610-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Sorg JA, Sonenshein AL. J Bacteriol. 2008;190:2505–2512. doi: 10.1128/JB.01765-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Wilson KH. J Clin Microbiol. 1983;18:1017–1019. doi: 10.1128/jcm.18.4.1017-1019.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Collins SM, Surette M, Bercik P. Nat Rev Microbiol. 2012;10:735–742. doi: 10.1038/nrmicro2876. [DOI] [PubMed] [Google Scholar]
- 239.Sampson TR, Mazmanian SK. Cell Host Microbe. 2015;17:565–576. doi: 10.1016/j.chom.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Moos WH, Faller DV, Harpp DN, Kanara I, Pernokas J, Powers WR, Steliou K. Biores Open Access. 2016;5:137–145. doi: 10.1089/biores.2016.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Li Q, Zhou JM. Neuroscience. 2016;324:131–139. doi: 10.1016/j.neuroscience.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 242.Berger M, Gray JA, Roth BL. Annu Rev Med. 2009;60:355–366. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Proc Natl Acad Sci U S A. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Irsfeld M, Spadafore M, Pruess BM. Webmedcentral. 2013;4 [PMC free article] [PubMed] [Google Scholar]
- 245.Wall R, Cryan JF, Ross RP, Fitzgerald GF, Dinan TG, Stanton C. Adv Exp Med Biol. 2014;817:221–239. doi: 10.1007/978-1-4939-0897-4_10. [DOI] [PubMed] [Google Scholar]
- 246.Brestoff JR, Artis D. Nat Immunol. 2013;14:676–684. doi: 10.1038/ni.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Thorburn AN, Macia L, Mackay CR. Immunity. 2014;40:833–842. doi: 10.1016/j.immuni.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 248.Adams JB, Johansen LJ, Powell LD, Quig D, Rubin RA. BMC Gastroenterol. 2011;11:22. doi: 10.1186/1471-230X-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Coury DL, Ashwood P, Fasano A, Fuchs G, Geraghty M, Kaul A, Mawe G, Patterson P, Jones NE. Pediatrics. 2012;130(Suppl 2):S160–168. doi: 10.1542/peds.2012-0900N. [DOI] [PubMed] [Google Scholar]
- 250.Hsiao EY. Harv Rev Psychiatry. 2014;22:104–111. doi: 10.1097/HRP.0000000000000029. [DOI] [PubMed] [Google Scholar]
- 251.Hulbert SW, Jiang YH. Neuroscience. 2016;321:3–23. doi: 10.1016/j.neuroscience.2015.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, Codelli JA, Chow J, Reisman SE, Petrosino JF, Patterson PH, Mazmanian SK. Cell. 2013;155:1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Altieri L, Neri C, Sacco R, Curatolo P, Benvenuto A, Muratori F, Santocchi E, Bravaccio C, Lenti C, Saccani M, Rigardetto R, Gandione M, Urbani A, Persico AM. Biomarkers. 2011;16:252–260. doi: 10.3109/1354750X.2010.548010. [DOI] [PubMed] [Google Scholar]
- 254.Persico AM, Napolioni V. Neurotoxicol Teratol. 2013;36:82–90. doi: 10.1016/j.ntt.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 255.Belkaid Y, Hand TW. Cell. 2014;157:121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, Iwakura Y, Oshima K, Morita H, Hattori M, Honda K, Ishikawa Y, Hara E, Ohtani N. Nature. 2013;499:97–101. doi: 10.1038/nature12347. [DOI] [PubMed] [Google Scholar]
- 257.Kuwata G, Hiramatsu K, Samejima K, Iwasaki K, Takahashi K, Koizumi K, Horiguchi S, Moriya SS, Kobayashi M, Kawakita M. J Cancer Res Clin Oncol. 2013;139:925–932. doi: 10.1007/s00432-013-1405-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Wu IW, Hsu KH, Lee CC, Sun CY, Hsu HJ, Tsai CJ, Tzen CY, Wang YC, Lin CY, Wu MS. Nephrol Dial Transplant. 2011;26:938–947. doi: 10.1093/ndt/gfq580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, Siuzdak G. Proc Natl Acad Sci U S A. 2009;106:3698–3703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Cohen LJ, Kang HS, Chu J, Huang YH, Gordon EA, Reddy BV, Ternei MA, Craig JW, Brady SF. Proc Natl Acad Sci U S A. 2015;112:E4825–4834. doi: 10.1073/pnas.1508737112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Le LQ, Kabarowski JH, Weng Z, Satterthwaite AB, Harvill ET, Jensen ER, Miller JF, Witte ON. Immunity. 2001;14:561–571. doi: 10.1016/s1074-7613(01)00145-5. [DOI] [PubMed] [Google Scholar]
- 263.Barrett MP, Burchmore RJ, Stich A, Lazzari JO, Frasch AC, Cazzulo JJ, Krishna S. Lancet. 2003;362:1469–1480. doi: 10.1016/S0140-6736(03)14694-6. [DOI] [PubMed] [Google Scholar]
- 264.Huet G, Richet C, Demeyer D, Bisiau H, Soudan B, Tetaert D, Han KK, Degand P. Biochim Biophys Acta. 1992;1138:213–221. doi: 10.1016/0925-4439(92)90040-t. [DOI] [PubMed] [Google Scholar]
- 265.Mengaud J, Ohayon H, Gounon P, Mege RM, Cossart P. Cell. 1996;84:923–932. doi: 10.1016/s0092-8674(00)81070-3. [DOI] [PubMed] [Google Scholar]
- 266.Kristensson K, Nygard M, Bertini G, Bentivoglio M. Prog Neurobiol. 2010;91:152–171. doi: 10.1016/j.pneurobio.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 267.Masocha W, Kristensson K. Virulence. 2012;3:202–212. doi: 10.4161/viru.19178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Doran KS, Banerjee A, Disson O, Lecuit M. Cold Spring Harb Perspect Med. 2013:3. doi: 10.1101/cshperspect.a010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Lecuit M. Clin Microbiol Infect. 2005;11:430–436. doi: 10.1111/j.1469-0691.2005.01146.x. [DOI] [PubMed] [Google Scholar]
- 270.Brun R, Blum J, Chappuis F, Burri C. Lancet. 2010;375:148–159. doi: 10.1016/S0140-6736(09)60829-1. [DOI] [PubMed] [Google Scholar]
- 271.Cornford EM, Bocash WD, Braun LD, Crane PD, Oldendorf WH, MacInnis AJ. J Clin Invest. 1979;63:1241–1248. doi: 10.1172/JCI109419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Seed JR, Seed TM, Sechelski J. Comparative biochemistry and physiology. C: Comparative pharmacology. 1978;60:175–185. doi: 10.1016/0306-4492(78)90091-6. [DOI] [PubMed] [Google Scholar]
- 273.Vazquez-Boland JA, Kuhn M, Berche P, Chakraborty T, Dominguez-Bernal G, Goebel W, Gonzalez-Zorn B, Wehland J, Kreft J. Clin Microbiol Rev. 2001;14:584–640. doi: 10.1128/CMR.14.3.584-640.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Geoffroy C, Gaillard JL, Alouf JE, Berche P. Infection and immunity. 1987;55:1641–1646. doi: 10.1128/iai.55.7.1641-1646.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Cotter PD, Draper LA, Lawton EM, Daly KM, Groeger DS, Casey PG, Ross RP, Hill C. PLoS Pathog. 2008;4:e1000144. doi: 10.1371/journal.ppat.1000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Ronald A. The American Journal of Medicine. 2002;113:14–19. doi: 10.1016/s0002-9343(02)01055-0. [DOI] [PubMed] [Google Scholar]
- 277.Hilt EE, McKinley K, Pearce MM, Rosenfeld AB, Zilliox MJ, Mueller ER, Brubaker L, Gai X, Wolfe AJ, Schreckenberger PC. J Clin Microbiol. 2014;52:871–876. doi: 10.1128/JCM.02876-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Nelson DE, Van Der Pol B, Dong Q, Revanna KV, Fan B, Easwaran S, Sodergren E, Weinstock GM, Diao L, Fortenberry JD. PloS one. 2010;5:e14116. doi: 10.1371/journal.pone.0014116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Wolfe AJ, Toh E, Shibata N, Rong R, Kenton K, Fitzgerald M, Mueller ER, Schreckenberger P, Dong Q, Nelson DE, Brubaker L. J Clin Microbiol. 2012;50:1376–1383. doi: 10.1128/JCM.05852-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Stamm WE. N Engl J Med. 2001;345:1055–1057. doi: 10.1056/NEJM200110043451409. [DOI] [PubMed] [Google Scholar]
- 281.Hooton TM. N Engl J Med. 2012;366:1028–1037. doi: 10.1056/NEJMcp1104429. [DOI] [PubMed] [Google Scholar]
- 282.Nielubowicz GR, Mobley HL. Nat Rev Urol. 2010;7:430–441. doi: 10.1038/nrurol.2010.101. [DOI] [PubMed] [Google Scholar]
- 283.Kline KA, Lewis AL. Microbiol Spectr. 2016;4 doi: 10.1128/microbiolspec.UTI-0012-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Demir M, Kaleli I. Clin Microbiol Infect. 2004;10:1011–1014. doi: 10.1111/j.1469-0691.2004.01001.x. [DOI] [PubMed] [Google Scholar]
- 285.Henderson JP, Crowley JR, Pinkner JS, Walker JN, Tsukayama P, Stamm WE, Hooton TM, Hultgren SJ. PLoS Pathog. 2009;5:e1000305. doi: 10.1371/journal.ppat.1000305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Raymond KN, Dertz EA, Kim SS. Proc Natl Acad Sci U S A. 2003;100:3584–3588. doi: 10.1073/pnas.0630018100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Vagrali MA. Indian journal of pathology & microbiology. 2009;52:126–127. doi: 10.4103/0377-4929.44988. [DOI] [PubMed] [Google Scholar]
- 288.Gajer P, Brotman RM, Bai G, Sakamoto J, Schutte UM, Zhong X, Koenig SS, Fu L, Ma ZS, Zhou X, Abdo Z, Forney LJ, Ravel J. Sci Transl Med. 2012;4:132ra152. doi: 10.1126/scitranslmed.3003605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO, Brotman RM, Davis CC, Ault K, Peralta L, Forney LJ. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4680–4687. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Marrazzo JM. Anaerobe. 2011;17:186–190. doi: 10.1016/j.anaerobe.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Sridharan GV, Choi K, Klemashevich C, Wu C, Prabakaran D, Pan LB, Steinmeyer S, Mueller C, Yousofshahi M, Alaniz RC, Lee K, Jayaraman A. Nat Commun. 2014;5:5492. doi: 10.1038/ncomms6492. [DOI] [PubMed] [Google Scholar]
- 292.Ursell LK, Haiser HJ, Van Treuren W, Garg N, Reddivari L, Vanamala J, Dorrestein PC, Turnbaugh PJ, Knight R. Gastroenterology. 2014;146:1470–1476. doi: 10.1053/j.gastro.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Clarke G, Stilling RM, Kennedy PJ, Stanton C, Cryan JF, Dinan TG. Mol Endocrinol. 2014;28:1221–1238. doi: 10.1210/me.2014-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Gilbert JA, Quinn RA, Debelius J, Xu ZZ, Morton J, Garg N, Jansson JK, Dorrestein PC, Knight R. Nature. 2016;535:94–103. doi: 10.1038/nature18850. [DOI] [PubMed] [Google Scholar]
- 295.Wishart DS. Nat Rev Drug Discov. 2016;15:473–484. doi: 10.1038/nrd.2016.32. [DOI] [PubMed] [Google Scholar]
- 296.Misra BB, van der Hooft JJ. Electrophoresis. 2016;37:86–110. doi: 10.1002/elps.201500417. [DOI] [PubMed] [Google Scholar]
- 297.Covington BC, McLean JA, Bachmann BO. Nat Prod Rep. 2016 doi: 10.1039/c6np00048g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Derewacz DK, Covington BC, McLean JA, Bachmann BO. ACS chemical biology. 2015;10:1998–2006. doi: 10.1021/acschembio.5b00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Goodwin CR, Covington BC, Derewacz DK, McNees CR, Wikswo JP, McLean JA, Bachmann BO. Chem Biol. 2015;22:661–670. doi: 10.1016/j.chembiol.2015.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Goodwin CR, Sherrod SD, Marasco CC, Bachmann BO, Schramm-Sapyta N, Wikswo JP, McLean JA. Analytical chemistry. 2014;86:6563–6571. doi: 10.1021/ac5010794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Kelder T, Stroeve JH, Bijlsma S, Radonjic M, Roeselers G. Nutr Diabetes. 2014;4:e122. doi: 10.1038/nutd.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Faust K, Sathirapongsasuti JF, Izard J, Segata N, Gevers D, Raes J, Huttenhower C. PLoS computational biology. 2012;8:e1002606. doi: 10.1371/journal.pcbi.1002606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Faust K, Raes J. Nat Rev Microbiol. 2012;10:538–550. doi: 10.1038/nrmicro2832. [DOI] [PubMed] [Google Scholar]
- 304.Lozupone C, Faust K, Raes J, Faith JJ, Frank DN, Zaneveld J, Gordon JI, Knight R. Genome Res. 2012;22:1974–1984. doi: 10.1101/gr.138198.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Weiss S, Van Treuren W, Lozupone C, Faust K, Friedman J, Deng Y, Xia LC, Xu ZZ, Ursell L, Alm EJ, Birmingham A, Cram JA, Fuhrman JA, Raes J, Sun F, Zhou J, Knight R. Isme J. 2016;10:1669–1681. doi: 10.1038/ismej.2015.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.McHardy IH, Goudarzi M, Tong M, Ruegger PM, Schwager E, Weger JR, Graeber TG, Sonnenburg JL, Horvath S, Huttenhower C, McGovern DP, Fornace AJ, Jr, Borneman J, Braun J. Microbiome. 2013;1:17. doi: 10.1186/2049-2618-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Ritchie MD, Holzinger ER, Li R, Pendergrass SA, Kim D. Nat Rev Genet. 2015;16:85–97. doi: 10.1038/nrg3868. [DOI] [PubMed] [Google Scholar]
- 308.Franzosa EA, Hsu T, Sirota-Madi A, Shafquat A, Abu-Ali G, Morgan XC, Huttenhower C. Nat Rev Microbiol. 2015;13:360–372. doi: 10.1038/nrmicro3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Kurita KL, Glassey E, Linington RG. Proc Natl Acad Sci U S A. 2015;112:11999–12004. doi: 10.1073/pnas.1507743112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Kurita KL, Linington RG. Journal of natural products. 2015;78:587–596. doi: 10.1021/acs.jnatprod.5b00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Medema MH, Kottmann R, Yilmaz P, Cummings M, Biggins JB, Blin K, de Bruijn I, Chooi YH, Claesen J, Coates RC, Cruz-Morales P, Duddela S, Dusterhus S, Edwards DJ, Fewer DP, Garg N, Geiger C, Gomez-Escribano JP, Greule A, Hadjithomas M, Haines AS, Helfrich EJ, Hillwig ML, Ishida K, Jones AC, Jones CS, Jungmann K, Kegler C, Kim HU, Kotter P, Krug D, Masschelein J, Melnik AV, Mantovani SM, Monroe EA, Moore M, Moss N, Nutzmann HW, Pan G, Pati A, Petras D, Reen FJ, Rosconi F, Rui Z, Tian Z, Tobias NJ, Tsunematsu Y, Wiemann P, Wyckoff E, Yan X, Yim G, Yu F, Xie Y, Aigle B, Apel AK, Balibar CJ, Balskus EP, Barona-Gomez F, Bechthold A, Bode HB, Borriss R, Brady SF, Brakhage AA, Caffrey P, Cheng YQ, Clardy J, Cox RJ, De Mot R, Donadio S, Donia MS, van der Donk WA, Dorrestein PC, Doyle S, Driessen AJ, Ehling-Schulz M, Entian KD, Fischbach MA, Gerwick L, Gerwick WH, Gross H, Gust B, Hertweck C, Hofte M, Jensen SE, Ju J, Katz L, Kaysser L, Klassen JL, Keller NP, Kormanec J, Kuipers OP, Kuzuyama T, Kyrpides NC, Kwon HJ, Lautru S, Lavigne R, Lee CY, Linquan B, Liu X, Liu W, Luzhetskyy A, Mahmud T, Mast Y, Mendez C, Metsa-Ketela M, Micklefield J, Mitchell DA, Moore BS, Moreira LM, Muller R, Neilan BA, Nett M, Nielsen J, O’Gara F, Oikawa H, Osbourn A, Osburne MS, Ostash B, Payne SM, Pernodet JL, Petricek M, Piel J, Ploux O, Raaijmakers JM, Salas JA, Schmitt EK, Scott B, Seipke RF, Shen B, Sherman DH, Sivonen K, Smanski MJ, Sosio M, Stegmann E, Sussmuth RD, Tahlan K, Thomas CM, Tang Y, Truman AW, Viaud M, Walton JD, Walsh CT, Weber T, van Wezel GP, Wilkinson B, Willey JM, Wohlleben W, Wright GD, Ziemert N, Zhang C, Zotchev SB, Breitling R, Takano E, Glockner FO. Nat Chem Biol. 2015;11:625–631. doi: 10.1038/nchembio.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 313.Finn RD, Clements J, Eddy SR. Nucleic Acids Res. 2011;39:W29–37. doi: 10.1093/nar/gkr367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Hufsky F, Scheubert K, Bocker S. Nat Prod Rep. 2014;31:807–817. doi: 10.1039/c3np70101h. [DOI] [PubMed] [Google Scholar]
- 315.Chu J, Vila-Farres X, Inoyama D, Ternei M, Cohen LJ, Gordon EA, Reddy BV, Charlop-Powers Z, Zebroski HA, Gallardo-Macias R, Jaskowski M, Satish S, Park S, Perlin DS, Freundlich JS, Brady SF. Nat Chem Biol. 2016 doi: 10.1038/nchembio.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Blin K, Medema MH, Kazempour D, Fischbach MA, Breitling R, Takano E, Weber T. Nucleic Acids Res. 2013;41:W204–212. doi: 10.1093/nar/gkt449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Medema MH, Blin K, Cimermancic P, de Jager V, Zakrzewski P, Fischbach MA, Weber T, Takano E, Breitling R. Nucleic Acids Res. 2011;39:W339–346. doi: 10.1093/nar/gkr466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Weber T, Blin K, Duddela S, Krug D, Kim HU, Bruccoleri R, Lee SY, Fischbach MA, Muller R, Wohlleben W, Breitling R, Takano E, Medema MH. Nucleic Acids Res. 2015;43:W237–243. doi: 10.1093/nar/gkv437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Rausch C, Weber T, Kohlbacher O, Wohlleben W, Huson DH. Nucleic Acids Res. 2005;33:5799–5808. doi: 10.1093/nar/gki885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Roettig M, Medema MH, Blin K, Weber T, Rausch C, Kohlbacher O. Nucleic Acids Res. 2011;39:W362–367. doi: 10.1093/nar/gkr323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Kersten RD, Yang YL, Xu Y, Cimermancic P, Nam SJ, Fenical W, Fischbach MA, Moore BS, Dorrestein PC. Nat Chem Biol. 2011;7:794–802. doi: 10.1038/nchembio.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Kersten RD, Ziemert N, Gonzalez DJ, Duggan BM, Nizet V, Dorrestein PC, Moore BS. Proc Natl Acad Sci U S A. 2013;110:E4407–4416. doi: 10.1073/pnas.1315492110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Medema MH, Paalvast Y, Nguyen DD, Melnik A, Dorrestein PC, Takano E, Breitling R. PLoS computational biology. 2014;10:e1003822. doi: 10.1371/journal.pcbi.1003822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Wang M, Carver JJ, Phelan VV, Sanchez LM, Garg N, Peng Y, Nguyen DD, Watrous J, Kapono CA, Luzzatto-Knaan T, Porto C, Bouslimani A, Melnik AV, Meehan MJ, Liu WT, Crusemann M, Boudreau PD, Esquenazi E, Sandoval-Calderon M, Kersten RD, Pace LA, Quinn RA, Duncan KR, Hsu CC, Floros DJ, Gavilan RG, Kleigrewe K, Northen T, Dutton RJ, Parrot D, Carlson EE, Aigle B, Michelsen CF, Jelsbak L, Sohlenkamp C, Pevzner P, Edlund A, McLean J, Piel J, Murphy BT, Gerwick L, Liaw CC, Yang YL, Humpf HU, Maansson M, Keyzers RA, Sims AC, Johnson AR, Sidebottom AM, Sedio BE, Klitgaard A, Larson CB, Boya PC, Torres-Mendoza D, Gonzalez DJ, Silva DB, Marques LM, Demarque DP, Pociute E, O’Neill EC, Briand E, Helfrich EJ, Granatosky EA, Glukhov E, Ryffel F, Houson H, Mohimani H, Kharbush JJ, Zeng Y, Vorholt JA, Kurita KL, Charusanti P, McPhail KL, Nielsen KF, Vuong L, Elfeki M, Traxler MF, Engene N, Koyama N, Vining OB, Baric R, Silva RR, Mascuch SJ, Tomasi S, Jenkins S, Macherla V, Hoffman T, Agarwal V, Williams PG, Dai J, Neupane R, Gurr J, Rodriguez AM, Lamsa A, Zhang C, Dorrestein K, Duggan BM, Almaliti J, Allard PM, Phapale P, Nothias LF, Alexandrov T, Litaudon M, Wolfender JL, Kyle JE, Metz TO, Peryea T, Nguyen DT, VanLeer D, Shinn P, Jadhav A, Muller R, Waters KM, Shi W, Liu X, Zhang L, Knight R, Jensen PR, Palsson BO, Pogliano K, Linington RG, Gutierrez M, Lopes NP, Gerwick WH, Moore BS, Dorrestein PC, Bandeira N. Nat Biotechnol. 2016;34:828–837. doi: 10.1038/nbt.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Trautman EP, Crawford JM. Current topics in medicinal chemistry. 2016;16:1705–1716. doi: 10.2174/1568026616666151012111046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Duhrkop K, Shen H, Meusel M, Rousu J, Bocker S. Proc Natl Acad Sci U S A. 2015;112:12580–12585. doi: 10.1073/pnas.1509788112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Heinonen M, Shen H, Zamboni N, Rousu J. Bioinformatics. 2012;28:2333–2341. doi: 10.1093/bioinformatics/bts437. [DOI] [PubMed] [Google Scholar]
- 328.Browne HP, Forster SC, Anonye BO, Kumar N, Neville BA, Stares MD, Goulding D, Lawley TD. Nature. 2016;533:543–546. doi: 10.1038/nature17645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Oberhardt MA, Zarecki R, Gronow S, Lang E, Klenk HP, Gophna U, Ruppin E. Nat Commun. 2015;6:8493. doi: 10.1038/ncomms9493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Duncan SH, Hold GL, Harmsen HJ, Stewart CS, Flint HJ. Int J Syst Evol Microbiol. 2002;52:2141–2146. doi: 10.1099/00207713-52-6-2141. [DOI] [PubMed] [Google Scholar]
- 331.Goodman AL, Kallstrom G, Faith JJ, Reyes A, Moore A, Dantas G, Gordon JI. Proc Natl Acad Sci U S A. 2011;108:6252–6257. doi: 10.1073/pnas.1102938108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Sibley CD, Grinwis ME, Field TR, Eshaghurshan CS, Faria MM, Dowd SE, Parkins MD, Rabin HR, Surette MG. PloS one. 2011;6:e22702. doi: 10.1371/journal.pone.0022702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Stearns JC, Davidson CJ, McKeon S, Whelan FJ, Fontes ME, Schryvers AB, Bowdish DM, Kellner JD, Surette MG. Isme J. 2015;9:1268. doi: 10.1038/ismej.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Lau JT, Whelan FJ, Herath I, Lee CH, Collins SM, Bercik P, Surette MG. Genome Med. 2016;8:72. doi: 10.1186/s13073-016-0327-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Lagier JC, Armougom F, Million M, Hugon P, Pagnier I, Robert C, Bittar F, Fournous G, Gimenez G, Maraninchi M, Trape JF, Koonin EV, La Scola B, Raoult D. Clin Microbiol Infec. 2012;18:1185–1193. doi: 10.1111/1469-0691.12023. [DOI] [PubMed] [Google Scholar]
- 336.Zengler K, Toledo G, Rappe M, Elkins J, Mathur EJ, Short JM, Keller M. Proc Natl Acad Sci U S A. 2002;99:15681–15686. doi: 10.1073/pnas.252630999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Ling LL, Schneider T, Peoples AJ, Spoering AL, Engels I, Conlon BP, Mueller A, Schaberle TF, Hughes DE, Epstein S, Jones M, Lazarides L, Steadman VA, Cohen DR, Felix CR, Fetterman KA, Millett WP, Nitti AG, Zullo AM, Chen C, Lewis K. Nature. 2015;517:455–459. doi: 10.1038/nature14098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Barkal LJ, Theberge AB, Guo CJ, Spraker J, Rappert L, Berthier J, Brakke KA, Wang CCC, Beebe DJ, Keller NP, Berthier E. Nature Communications. 2016;7 doi: 10.1038/ncomms10610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.D’Onofrio A, Crawford JM, Stewart EJ, Witt K, Gavrish E, Epstein S, Clardy J, Lewis K. Chem Biol. 2010;17:254–264. doi: 10.1016/j.chembiol.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Bertrand S, Bohni N, Schnee S, Schumpp O, Gindro K, Wolfender JL. Biotechnol Adv. 2014;32:1180–1204. doi: 10.1016/j.biotechadv.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 341.Fritz JV, Desai MS, Shah P, Schneider JG, Wilmes P. Microbiome. 2013;1:14. doi: 10.1186/2049-2618-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Shah P, Fritz JV, Glaab E, Desai MS, Greenhalgh K, Frachet A, Niegowska M, Estes M, Jager C, Seguin-Devaux C, Zenhausern F, Wilmes P. Nat Commun. 2016;7:11535. doi: 10.1038/ncomms11535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Rutledge PJ, Challis GL. Nat Rev Microbiol. 2015;13:509–523. doi: 10.1038/nrmicro3496. [DOI] [PubMed] [Google Scholar]
- 344.Koppel N, Balskus EP. Cell Chem Biol. 2016;23:18–30. doi: 10.1016/j.chembiol.2015.12.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.