Abstract
Nephritis is one of the most severe complications of systemic lupus erythematosus (SLE). One key characteristic of lupus nephritis (LN) is the deposition of immune complexes containing nucleic acids and/or proteins binding to nucleic acids and autoantibodies recognizing these molecules. A variety of cell death processes are implicated in the generation and externalization of modified nuclear autoantigens and in the development of LN. Among these processes, apoptosis, primary and secondary necrosis, NETosis, necroptosis, pyroptosis, and autophagy have been proposed to play roles in tissue damage and immune dysregulation. Cell death occurs in healthy individuals during conditions of homeostasis yet autoimmunity does not develop, at least in part, because of rapid clearance of dying cells. In SLE, accelerated cell death combined with a clearance deficiency may lead to the accumulation and externalization of nuclear autoantigens and to autoantibody production. In addition, specific types of cell death may modify autoantigens and alter their immunogenicity. These modified molecules may then become novel targets of the immune system and promote autoimmune responses in predisposed hosts. In this review, we examine various cell death pathways and discuss how enhanced cell death, impaired clearance, and post-translational modifications of proteins could contribute to the development of lupus nephritis.
Introduction
Systemic lupus erythematosus (SLE) is a heterogeneous autoimmune disorder characterized by the presence of pathogenic autoantibodies, immune complex formation and deposition in various organs, profound innate and adaptive immune dysregulation and inflammation, and a wide range of clinical manifestations including kidney involvement (1, 2). A characteristic of lupus is the production of antibodies (Abs) recognizing nucleic acids and proteins binding to nucleic acids. Among them, synthesis of anti-double-stranded (ds)DNA Abs is considered a hallmark feature of SLE (3, 4).
Lupus glomerulonephritis (LN) is one of the most common and severe complications in SLE and a major cause of morbidity and mortality (5, 6). LN affects predominantly younger individuals and is frequently observed in children (7). Various mechanisms have been proposed in the pathogenesis of this complex lupus complication and both innate and adaptive branches of the immune system appear to contribute to LN (8-11).
Dysregulated cell death and defective clearance of dying cells have been proposed to contribute to autoantigen generation and induction of autoantibodies and other aberrant immune responses in SLE and in LN specifically (12). Indeed, dysregulation in various cell death processes (e.g. apoptosis, primary and secondary necrosis, NETosis, necroptosis, pyroptosis and autophagy) and the response of the immune system to these processes have been implicated in the pathogenesis of LN (12, 13). This review will focus on the putative mechanisms by which various mechanisms of cell death can promote immune dysregulation and renal disease in SLE.
Apoptosis
Apoptosis is a silent form of cell death that is active during both physiological and pathological conditions and plays a critical role in homeostasis of tissues experiencing a high rate of turnover, as observed during embryogenesis and development (14). Apoptosis also plays a key role in the immune system by eliminating autoreactive T cells and B cells during positive and negative selection to prevent autoimmunity (15). Apoptosis can be initiated by ligation of cell surface receptors such as Fas or tumor necrosis factor (TNF) receptor or due to cellular stress (12). Once activated, a series of enzymatic reactions leads to changes in membrane phospholipid expression, DNA fragmentation, post-translational modifications of histones, and membrane blebbing (16). Apoptotic cells express “eat me” signals, which include phosphatidylserine and phosphatidylethanolamine exposure on the membrane outer leaflet (14). Phosphatidylserine can be recognized directly by phagocytic cells expressing scavenger receptors leading to clearance or it can bind to opsonizing agents to enhance phagocytosis. Uptake of apoptotic cells occurs very rapidly and leads to an anti-inflammatory effect with the release of transforming growth factor beta (TGF-β) (17). Various defects in the apoptotic cell death pathway or in clearance of apoptotic material have been implicated in SLE subjects and in mouse models (Table 1) (12).
Table 1.
Cell death genes and autoimmunity
| Gene | Encoded Protein | Cell Death Pathway | Mutant Phenotype |
|---|---|---|---|
| FAS or FASLG | Fas or FasL | Apoptosis | Lymphadenopathy, splenomegaly, autoantibody, hypergammaglobulinemia, glomerulonephritis |
| BCL2 | Bcl-2 | Apoptosis | Prolonged antibody response, ANA, immune complex deposition, renal disease |
| BCL2L11 | Bim | Apoptosis | Splenomegaly, lymphadenopathy, glomerular damage, interglomerular proliferation |
| TNFSF13B | BAFF | Apoptosis | Expansion of mature B cells and effector T cells, anti-DNA, glomerular deposition |
| TNFRSF13B | TACI | Apoptosis | Deleting TACI in B cells prevents BAFF-induced kidney disease but TACI−/− mice develop fatal glomerulonephritis |
| PTEN | PTEN | Apoptosis | Anti-DNA, ANA, glomerular IgG deposition |
| TP53 | P53 | Apoptosis | Glomerulonephritis with depletion of splenic Treg cells |
| TYR03/AXL/MERTK (TAM) | Tyro3, Axl, Mer | Apoptosis | Hyperproliferation of B and T cells, anti-DNA, kidney infiltrates of B and T cells, glomerular IgG deposition |
| SR-A/MARCO | SR-A/MARCO | Apoptosis | Anti-DNA and ANA |
| SAA1 | SAP | Apoptosis and necrosis | Anti-DNA, inefficient degradation of long chromatin, glomerulonephritis |
| PTX3 | PTX3 | Apoptosis | Autoimmune lung disease |
| DNASE1L3 | DNASE1L3 | Apoptosis | Anti-DNA, ANA. splenomegaly, glomerular IgG deposition, glomerulonephritis |
| MBL2 | MBL | Apoptosis and necrosis | Nephritis |
| C1QA | C1q | Apoptosis and necrosis | Decreased survival, autoantibody formation, glomerulonephritis, glomerular apoptotic cell bodies |
| CYBB | NOX2 | NETosis, LAP | Splenomegaly, ANA, proteinuria, renal pathology |
| DNASE1 | DNase 1 | Apoptosis, necrosis and NETosis | ANA, glomerular immune complex deposition, glomerulonephritis |
| CYBB and RUBCN | NOX2 and rubicon | LAP | Proinflammatory cytokines, IFN signature, ANA, anti-DNA, immune complex deposition, renal damage |
| TIM4 | TIM4 | LAP | Anti-DNA, hyper-active lymphocytes, impaired uptake and clearance of dying cells |
One of the earliest reports linking impaired apoptosis to SLE was the identification of mutations in Fas receptor and Fas ligand (FasL) in MRL/lpr-lpr and C3H/HeJ-gld/gld mice, respectively (18-20). Both mice strains develop similar disease phenotypes characterized by hypergammaglobulinemia, autoantibody production, glomerulonephritis, and arthritis (19-21). Mutations in Fas or FasL have been identified in humans that develop autoimmune lymphoproliferative syndrome (ALPS) (21, 22) but the incidence of renal damage in this condition is extremely rare (23). Based on these findings, the role of Fas/FasL in the development of lupus nephritis appears stronger in mouse models of SLE compared to human SLE.
Other apoptotic signaling molecules including B cell lymphoma 2 (Bcl-2), Bim, transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI), B cell-activating factor (BAFF), phosphatase and tensin homolog (PTEN), and p53 have also been linked to lupus nephritis (15). Bcl-2 is an anti-apoptotic protein reported to be elevated in glomeruli and serum in patients with LN, although the significance of this is unclear (24). Immunized transgenic mice overexpressing BCL2 under the control of immunoglobulin heavy chain enhancer exhibit autoantibodies and develop immune complex-glomerulonephritis (25). Bim is a member of the Bcl-2 family that promotes apoptosis and mice with a combined deficiency in Bim and Fas develop a lupus-like disease with renal damage caused by increased infiltration of B cells and macrophages, apoptotic cells and deposition of immune complexes (26). BAFF is a cytokine essential for B cell survival and maturation and SLE patients have elevated circulating BAFF levels that positively correlate with disease activity (27, 28). Mice overexpressing BAFF manifest lupus-like disease characterized by enhanced germinal center formation, splenomegaly, autoantibodies and immune-complex glomerulonephriits (29). In humans, LN patients treated with Belimumab, a BAFF neutralizing antibody, have shown greater reduction in proteinuria, normalization of anti-dsDNA and complement levels, and renal function improvement compared to those that received standard of care therapy (30). One of the key receptors for BAFF that has also been shown to potentially play a role in renal disease in lupus patients is TACI. In a 2010 study examining 73 Chinese SLE patients for expression of BAFF and BAFF receptors, SLE patients had elevated numbers of CD19+TACI+ B cells compared to healthy controls and lupus nephritis patients expressed the highest numbers of these cells (28). Targeting TACI, instead of BAFF, has been shown to protect against autoimmune kidney disease while maintaining the B cell population (31). PTEN is a tumor suppressor protein that negatively regulates the phosphoinositide 3-kinase (PI3K)-AKT pathway and loss of this protein has been shown to promote autoimmunity (32). Mice with a specific deletion of Pten in Foxp3+ regulatory T cells (Treg) lose immune tolerance and develop a systemic autoimmune-like phenotype characterized by immune complex LN. Tumor suppressor p53 protein has been known to play a critical role for the prevention of tumorigenesis but recent evidence has suggested that p53 also has a vital function in inhibiting autoimmunity (33-39). Kawashima et al showed that mice with a specific deletion of p53 in T cells develop inflammatory diseases including glomerulonephritis that is associated with a reduction in splenic Treg cells (40). Moreover, p53 interacted with the FoxP3 promoter driving the expression of this gene and promoted the differentiation of regulatory T cells. Apoptosis is a finely tuned machine that requires proper functioning of both pro-apoptotic and anti-apoptotic proteins. Based on the previous findings, aberrant expression and function of these proteins can result in decreased apoptosis and lead to an autoimmune-like phenotype in both mice and humans likely through the accumulation of autoreactive immune cells. In addition, increased apoptosis has also been observed in SLE and may also contribute to the development of nephritis.
Enhanced apoptosis contributes to development of nephritis
Accelerated apoptosis in SLE can potentially overwhelm the host clearance mechanisms and result in an accumulation of apoptotic debris that can undergo secondary necrosis (12). Secondary necrotic cells lose the integrity of the plasma membrane and release nuclear autoantigens that can lead to immune complex formation, their glomerular deposition, and development of nephritis (14). Apoptosis of kidney cells, defective clearance, and release of nucleosomes may lead to development of nephritis but whether or not increased apoptosis of glomerular cells contributes to renal pathology is controversial (3, 41). Soto et al reported a decrease in apoptotic cells from the glomerulus and tubulointerstitium in LN biopsies compared to control kidneys (42). In addition, renal cells from LN patients had enhanced proliferation without an increase in apoptosis. It is worth noting that the apoptotic cell numbers from control kidneys was higher than apoptotic renal cell numbers from healthy subjects from previous similar studies (42, 43). Faurschou et al found that kidney biopsies from both lupus nephritis patients and healthy controls did not express apoptotic glomerular cells (44). However, one-third of the LN biopsies expressed low levels of apoptotic tubular cells that positively correlated with interstitial inflammation. In lupus-prone NZB/NZW F1 (NZB/W) mice, the appearance of circulating anti-dsDNA antibodies and glomerular deposits did not correlate with the activation of apoptotic pathways in the kidneys, but rather, correlates with the downregulation of Dnase1 gene expression (45). Seredkina et al suggests that severe nephritis may develop due to the decreased expression of DNase1 that leads to an accumulation of chromatin, subsequent immune complex formation and deposition in glomerular membranes. However, Kalaaji et al demonstrated that nucleosomes released from apoptotic intraglomerular cells deposited in glomerulus basement membranes (GBM) and were targeted by nephritogenic lupus antibodies (46, 47). Unlike the results from Seredkina et al, Kalaaji demonstrated intraglomerular cell death that correlates with anti-dsDNA Ab in nephritic NZB/W mice (45, 47). Lupus nephritis patients have been reported to have increased numbers of apoptotic glomerular cells and infiltrating neutrophils compared to healthy controls, in correlation with anti-dsDNA Ab levels, complement consumption, and cell proliferation (41). Takemura et al reported the presence of apoptotic cells in the mesangial area and glomerular capillaries from LN patients and this positively correlates with the expression of Fas antigen (48). Bcl-2 expression was upregulated in mesangial cells and infiltrating leukocytes from LN patients and this correlates with the expansion of glomerular cells and degree of proteinuria. These authors postulated that increased Bcl-2 expression may contribute to the hypercellularity of glomerular cells and prolonged survival of infiltrating leukocytes in LN (48). Increased apoptosis of interstitial inflammatory cells, renal tubular epithelial cells, and glomerular parenchymal cells has been reported in lupus nephritis patients in association with enhanced expression of apoptosis-related proteins Fas, Bax, and caspase-3 (49). In summary, it is debatable whether or not increased apoptosis of glomerular cells is a significant source of circulating and/or tissue nucleosomes promoting glomerulonephritis. It is unclear why there are significant discrepancies when determining if renal cells from LN patients undergo increased apoptosis but one potential explanation could be the type of experimental method used to quantify this process (44, 51).
Accelerated apoptosis has also been observed in SLE for various key immune cells including phagocytes (monocytes, macrophages, neutrophils, and immature dendritic cells) that are critical for clearance (52). Autoreactive T cells in SLE patients induce apoptosis in autologous monocytes through TNF-related ligands (53). In NZB × SWR (SNF1) lupus-prone mice, induction of accelerated macrophage apoptosis using clodronate liposomes results in increases in anti-dsDNA and anti-nucleosome antibody levels and enhanced proteinuria and LN features (54). SLE patients have increased numbers of circulating apoptotic neutrophils compared to healthy control donors and this positively correlates with disease activity and anti-dsDNA levels (55). Serum from SLE patients can induce apoptosis in antigen presenting cells (APCs) and lymphocytes and is associated with complement consumption (56, 57). In summary, SLE patients experience accelerated apoptosis of immune cells that are critical for clearance of apoptotic debris. This can potentially result in inefficient removal of dying cells and chronic exposure of intracellular autoantigens.
Defects in clearance mechanisms contribute to nephritis
Conflicting results have been observed when apoptotic cells are administered to mice with regards to immunogenicity (58, 59). While apoptosis is considered a silent form of cell death, defects in apoptotic cell clearance may result in secondary necrosis and lead to autoimmunity. The apoptotic clearance program is a highly redundant and multi-tiered system and defects in apoptotic cell receptors and bridging molecules have been observed in SLE. The TAM receptor protein tyrosine kinase subfamily includes Mer, Tyro3, and Axl and they detect ligands bound to phosphatidylserine on the membrane of dying cells and are involved in their removal (60). TAM-deficient mice develop an autoimmune-like syndrome with renal immune complex deposition (61, 62). Class A scavenger receptors macrophage receptor with collagenous structure (MARCO) and scavenger receptor A (SR-A) also recognize apoptotic cells and mice deficient in these receptors develop autoantibodies following transfer of apoptotic cells (63). In lupus-prone NZB/W mice and in SLE patients, autoantibodies against SR-A and MARCO are present and detected before onset of disease and may play a putative role in reduced uptake of dying cells (63).
In addition to phosphatidylserine exposure, proper recognition and clearance of apoptotic cells requires their opsonization by serum proteins including C-reactive protein (CRP), serum amyloid protein (SAP), pentraxin-related protein (PTX3), IgM, mannose binding lectin (MBL), and complement C1q (12). CRP and SAP are short pentraxins produced by the liver in response to interleukin 6 (IL-6) and PTX3 is a long pentraxin that is generated from a variety of tissues after TLR stimulation and in response to inflammatory cytokines (64). CRP interacts with polysaccharides and phosphocholine exposed on apoptotic cells and microbes and mediates activation of the classical pathway of complement (65). CRP also opsonizes cells and mediates their removal by interacting with the Fc receptor on phagocytic cells (14). Abnormalities in CRP function and expression have been observed in SLE (66-69). SLE patients produce autoantibodies against CRP and this correlates with disease activity, anti-dsDNA, and LN (66, 67, 69). Genome-wide linkage studies have revealed that CRP maps to a locus associated with SLE and polymorphisms in the CRP locus are associated with development of SLE, autoantibodies and low CRP expression (70). CRP can improve murine lupus and its associated nephritis (71, 72).
SAP is another acute phase reactant and opsonin that is critical for the removal of apoptotic cells (73). SAP binds to DNA and chromatin exposed in apoptotic blebs and solubilizes chromatin released during necrosis (74). As observed with CRP, defects in SAP lead to murine lupus-like disease including glomerulonephritis (75). SLE patients also develop anti-SAP Abs that correlate with disease activity and are reduced with improved clinical outcome (76). Exogenous administration of SAP has a therapeutic effect in mice, including decrease in immune-complex deposition and prevention of LN (77).
PTX3 binds to nuclear antigens exposed on the cell membrane in apoptotic cells (78) and regulates autoimmunity by sequestering cell debris that would otherwise be internalized by APCs. Similar to CRP and SAP, autoantibodies against PTX3 are detected in SLE patients and correlate with disease activity (79). Ptx3-deficient mice that are crossed with Fas-deficient (lpr) C57BL/6 mice develop autoimmune lung disease but no glomerulonephritis (80). In summary, the pentraxins CRP, SAP, and PTX3 play critical roles in the clearance of apoptotic cells and dysregulation in the function of these molecules may promote autoimmunity.
MBL is a serum pattern recognition receptor that recognizes and opsonizes carbohydrate moieties present on microorganisms, resulting in complement activation (81). The role of MBL in the lupus pathogenesis is potentially complex as high expression results in complement activation and tissue damage while low levels lead to defective apoptotic cell clearance (82, 83). Variants in the MBL2 gene result in MBL deficiency, predisposition to SLE, and increased risk of LN (84-86). IgM is also involved in the opsonization and clearance of dying cells and low serum IgM levels have been reported in SLE patients (87). Finally, C1q is a member of the classical complement pathway and an opsonin that binds to apoptotic cells and mediates their removal by phagocytic cells (88). Deficiencies in C1q have been linked to SLE (89). SLE patients may have anti-C1q Abs in association with LN, complement consumption, autoantibodies, and disease activity (90, 91). C1q-deficient mice develop immune complex nephritis and accumulation of renal apoptotic bodies (92). Together, these results suggest that recognition and opsonization of apoptotic cells is highly critical for their clearance and prevention of autoimmune disease. Although significant redundancy exists in proteins involved in the clearance of apoptotic cells, the functional loss of just one protein may be sufficient to promote activation of the immune response.
Once phagocytic cells have recognized apoptotic cells, they must be efficiently ingested to prevent immune system activation. Defects in phagocytosis have been observed in lupus (93). SLE patients have an accumulation of apoptotic cells in lymph node germinal centers likely due to a reduction in tingible body macrophages that specialize in the removal of dead cells (93). Defects in the differentiation of myeloid progenitors into macrophages may potentially lead to phagocytosis defects in SLE (60). Macrophages derived from SLE monocytes display impaired uptake of apoptotic material (94, 95). Lupus-prone mouse macrophages have been reported to display impaired lysosomal maturation that can lead to the recycling and accumulation of nuclear antigens to the cell surface that can potentially activate autoreactive lymphocytes (96). Impairments in lysosomal acidification can promote leakage of nuclear contents into the cytosol resulting in activation of the cytosolic sensor for dsDNA and inflammasome protein absent in melanoma 2 (AIM2) and sensor for IgG, tripartite motif-containing protein 21 (TRIM21). Activation of AIM2 results in another form of cell death called pyroptosis and release of IL-1β (mentioned below), while stimulation of TRIM21 can lead to type I IFN production, a phenomenon that promotes immune dysregulation in SLE (97, 98). Together, these results suggest that defective uptake and processing of apoptotic cells in SLE can result in activation of innate and adaptive immune responses.
Apoptosis-associated histone modifications in LN
Apoptosis is characterized by chromatin condensation and DNA fragmentation. Post-translational modifications of histones alter the structure and function of chromatin during apoptosis (99). Most histone modifications occur at the N-terminus and include serine and threonine phosphorylation, lysine acetylation and ubiquitination, lysine and arginine methylation and ADP ribosylation. Histone phosphorylation weakens its interactions with DNA and promotes structural chromatin reorganization (100) and DNA fragmentation (101-104).
Acetylation of lysine residues on histones results in structural chromatin changes leading to an open conformation that activates gene transcription (105). Histone acetylation also promotes increased accessibility to nucleases and DNA fragmentation (99). Addition and removal of acetyl groups on histones is mediated by histone acetyltransferases (HAT) and histone deacetylases (HDAC). HDAC inhibitors delivered at high concentrations induce apoptosis (106, 107). Conversely, hypoacetylated histone H4 has been associated with early apoptosis (108). Histone methylation represses transcription and hypermethylated histone H4 has been linked to apoptosis (109). Ubiquitination targets proteins for proteasomal degradation and promotes protein-protein interactions (12). Histone H2A deubiquitination is also associated with chromatin condensation in apoptosis (110, 111). Poly(ADP-ribosyl)ation is involved in many signaling pathways including DNA repair and apoptosis and is characterized by the addition of poly(ADP-ribose) (PAR) residues by poly(ADP-ribose) polymerases (PARPs) (12). Chemical inhibition of poly(ADP-ribosyl)ation prevents DNA cleavage and cell death, suggesting the critical role of poly(ADP-ribosyl)ation in apoptosis (112-115). In summary, many types of post-translational histone modifications are generated during the apoptotic process. Various post-translational modifications that take place during apoptosis could create neoantigens that become targets for autoantibody formation (12, 116). Indeed, experiments performed by several groups suggest that apoptosis-induced post-translational histone modifications are targets for autoimmune responses in SLE patients and mice (117-120). Plasma from SLE patients and lupus-prone mice contains autoantibodies specific for modified histones that target acetylated residues in H2B and H4, methylated H3, and ubiquitinated H2A. Deposition of histone H3 is observed in kidney sections of MRL/lpr mice and glomerular ubiquitinated histone H2A has been reported in a significant proportion of patients with LN (120, 121). In summary, SLE patients generate autoantibodies targeting modified histones and this could promote immune complex formation and glomerulonephritis. Indeed, modified histones have been reported to be more immunogenic compared to unmodified histones (117, 122). Lupus-prone mice treated with a triacetylated histone H4 peptide have enhanced mortality, proteinuria, and glomerular IgG deposition when compared to mice treated with a nonacetylated histone H4 peptide (117). Bone marrow-derived DCs exposed to acetylated nucleosomes undergo maturation and activate syngenic T cells. Apoptotic microparticles, which expose modified nucleosomes at the cell surface (123-125), are found in both SLE patients and mice (125-127). Apoptotic microparticles from MRL/lpr mice express elevated levels of modified chromatin and induce enhanced maturation of DCs than BALB/c mice (125). DNASE1L3 is a serum enzyme that is critical for the degradation of chromatin in microparticles precluding autoantibody recognition of microparticle DNA and humans and mice lacking DNASE1L3 develop SLE-like disease (127-131). Microparticles from SLE patients express apoptosis-related histone modifications while these were absent in microparticles from healthy individuals (126). Microparticles from SLE patients activate pDCs and myeloid DCs that results in the induction of proinflammatory cytokines and type I IFN and also primes neutrophils for neutrophil extracellular trap (NET) formation (126). Based on this evidence, increased apoptosis-induced histone modifications in SLE may have immunostimulatory roles and contribute to the pathogenesis of LN
Necrosis
In contrast to apoptosis, primary and secondary necrosis are considered inflammatory modalities of cell death. Primary necrosis is often induced by cellular injury caused by ATP depletion, heat-shock or freeze/thaw, toxins, oxidative stress, and other noxious insults (14, 132, 133). Necrosis can also be induced by inhibition of apoptosis combined with blockade of autophagy and/or caspase inhibition (134-138). Apoptotic cells can become secondary necrotic cells after phagocytes fail to clear the dying cells (132, 133). Necrotic cell death is characterized by ROS production, mitochondria hyperpolarization, lysosomal membrane disintegration, cellular and organelle swelling, and plasma membrane rupture (133). Necrotic cell death is considered inflammatory because loss of plasma membrane integrity promote release of autoantigens and damage associated molecular pattern (DAMPs) that serve as chemoattractants for inflammatory cell recruitment (139, 140). To prevent further inflammation, rapid clearance of necrotic cells is critical. Necrotic cells differ from apoptotic cells in the way they are internalized by macrophages (132, 141). While apoptotic cells are ingested by phagocytosis, necrotic cells are cleared by macropinocytosis (132, 141). Both apoptotic and necrotic cells depend on the externalization of phosphatidylserine for phagocyte recognition (142-144). CRP, SAP, C1q, and DNase I are also critical for clearance of necrotic cells (145-147). Unlike apoptosis, where complement binding is a late event, complement binding to necrotic cells occurs early on (146). In contrast to apoptosis, genes required for necrosis have yet to be defined although receptor-interacting serine/threonine-protein kinase 1 and 3 (RIP1 and RIP3) appear to be critical for a type of programmed necrotic cell death called necroptosis (137, 148, 149). Necrosis can be a programmed occurrence in healthy individuals during development and intestinal epithelial cell homeostasis (137, 150, 151). In SLE, accelerated primary necrosis or accelerated apoptosis resulting in secondary necrosis, defective clearance of necrotic cells, and post-translational modifications during necrosis may play an important role in the development of LN.
Accelerated primary or secondary necrosis in LN
As previously mentioned, accelerated apoptosis leading to secondary necrosis has been observed in SLE patients and mice and promotes autoimmunity and renal damage (41, 46, 47, 49, 53-55, 152, 153). Necrosis is frequently observed in subjects with LN and is associated with enhanced serological activity and proteinuria compared to LN patients that do not display necrosis (154). Nucleosomes can induce necrosis in lupus and control lymphocytes both in vitro and in vivo (155). Due to the enhanced secondary necrosis observed in SLE, nucleosomes can accumulate and induce necrosis in neighboring cells, thereby resulting in an amplification loop of nucleosome release and immune dysregulation (155).
Defective clearance of necrotic cells in LN
Due to the inflammatory nature of cell death via necrosis, rapid clearance of dying cells is critical to prevent inflammation propagation and tissue damage (14). Necrotic cell clearance defects have been reported in SLE (132, 156). C1q and MBL are involved in macropinocytosis uptake of both apoptotic and necrotic cells and decreased C1q and MBL, reported in SLE, may lead to impaired macropinocytosis of necrotic cells, immune dysregulation and renal damage (86, 87, 89-91). Defects in expression and activity of CRP and SAP also contribute to defective clearance of necrotic cells (66, 68, 76). Some lupus patients have defective serum DNase I activity (157, 158) and they tend to have higher disease activity, and higher autoantibody levels when compared to SLE patients with normal enzyme activity (158). In mice, DNase I deficiency results in a lupus-like syndrome with glomerular immune complex deposition and glomerulonephritis (157). In summary, defects in the clearance of necrotic cells may potentiate immune dysregulation and organ damage in LN.
Post-translational modifications in necrosis
Little is known about the various post-translational modifications that occur during necrosis and their contribution to the development of LN. During programmed cell death, PARP is inactivated and cleaved by caspase-3 resulting in apoptosis (159). PARP is also cleaved in necrotic cells in a caspase-independent manner (160). Autoantibodies to PARP have been detected in SLE (161, 162) and they do not inhibit its catalytic activity but, rather, prevent caspase-3-mediated cleavage that results in decreased apoptosis (163). Collectively, the decreased clearance of apoptotic cells can result in cells undergoing secondary necrosis and the release of nucleosomes. Additionally, defects in the uptake of primary necrotic cells can also promote autoantigen externalization. Nucleosomes can induce primary necrosis in neighboring cells and this can result in enhanced autoantigen release, inflammation, and epitope spreading. Autoantibodies generated against nuclear components can inhibit PARP cleavage leading to prolonged survival of autoreactive cells that can mount an autoimmune response (163).
NETosis
An additional mechanism by which nuclear autoantigens may be modified and released to the extracellular space is through a specialized form of neutrophil cell death called NETosis (164). In response to microbial stimuli but also to a variety of sterile inflammatory signals (activated platelets, endothelial cells, crystals, autoantibodies, immune complexes and various proinflammatory cytokines), neutrophils can extrude a meshwork of nuclear material bound to neutrophil granular proteins (including LL-37, neutrophil elastase (NE), and myeloperoxidase (MPO)) (165-175). After recognition of a microbial insult, induction of NETosis typically requires hydrogen peroxide production by nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and superoxide dismutase (176). Hydrogen peroxide is consumed by MPO, which mediates the release of NE from azurophilic granules into the cytosol and its translocation to the nucleus (177), cleaving histones and promoting chromatin decondensation (174). Chromatin decondensation is also promoted by the activation and nuclear localization of peptidylarginine deiminase 4 (PAD4), an enzyme that citrullinates histones, leading to disruption in the electrostatic interactions of histones with DNA (178, 179). The nuclear and granular membranes become permeable and this allows for the formation of cytoplasmic complexes of granular proteins and chromatin (180). Once the plasma membrane ruptures, chromatin fibers decorated with granular proteins are extruded from the cell in the form of NETs (165). NETs can also be induced in a NADPH oxidase-independent manner dependent on calcium-activated small conductance potassium (SK) channel member SK3 and mitochondrial ROS (167). In fact, patients with chronic granulomatous disease lack NADPH oxidase activity but can still develop autoimmunity and have the ability to form immunogenic NETs through enhanced mitochondrial ROS production and promotion of interferogenic responses by extrusion of oxidized mitochondrial DNA (181). NETs can also induce IFN-α by activating TLRs on pDCs (182). DNase I is an important enzyme for the degradation of NETs and C1q also plays a key role in opsonizing NETs for clearance by macrophages (183, 184). NETs are considered dual-edged swords in that NET induction and clearance may result in a protective antimicrobial effect but excessive NET formation and inefficient removal could lead to tissue damage and autoantigen modification and externalization (185). Excessive NET formation promoting tissue damage has been proposed in sepsis, psoriasis, diabetes, atherosclerosis, and SLE, among others (175, 186-189). This next section will discuss how enhanced NETosis, defective clearance of NETs, and post-translational modifications of proteins during NETosis may contribute to the development of lupus LN.
Enhanced NETosis in SLE may promote tissue damage
Neutrophils from SLE patients and lupus-prone mice are more prone to form NETs than neutrophils from healthy controls (175, 182, 190, 191). Neutrophils exposed to RNP immune complexes undergo enhanced NETosis through the induction of mitochondrial ROS (181). NET-derived self-DNA complexed with neutrophil-derived antimicrobial peptides such as LL-37 or human neutrophil peptide (HNP) activate pDC TLR9 and induce IFN-α (191). SLE patients generate autoantibodies that bind antimicrobial peptides and this can further enhance NET formation. These findings reveal a putative pathogenic amplification loop in SLE where neutrophils become primed to undergo NETosis after exposure to type I IFNs and other inflammatory stimuli, thereby releasing immunostimulatory nucleic acids that further activate type I IFN production.
SLE patients express a distinct subset of proinflammatory neutrophils, called low-density granulocytes (LDGs) (54, 192, 193) that have a heightened capacity to form NETs (175) and enhanced capacity to synthesize mitochondrial ROS (181). This leads to heightened externalization of immunostimulatory molecules (175, 181). Furthermore, LDG NETs are enriched in oxidized mitochondrial DNA that promotes type I IFN responses in target cells through a cGAS-STING-mediated pathway (181). Analysis of kidney biopsies from patients with LN have revealed the presence of NETs and infiltrating netting neutrophils in the glomeruli (175), which positively correlates with higher levels of circulating autoantibodies and enhanced activity index in kidney biopsies. As such, LDGs’ enhanced ability to spontaneously form NETs leads to enhanced externalization of modified autoantigens and immunostimulatory molecules that may promote vascular and renal damage. NETs (and LL-37 expressed in NETs) can also induce inflammasome activation, pyroptosis, and release of IL-1β and IL-18 in human and murine macrophages, further amplifying NETosis (171).
Enhanced NETosis has also been observed in the NZM2328 and MRL/lpr murine models of lupus (190, 194). Given that PAD4 has been implicated in NET formation, lupus-prone mice have been treated with PAD chemical inhibitors (Cl-amidine or BB-Cl-amidine) and found to have improved vascular function, reduced type I IFN signatures, glomerular NETosis, renal inflammation and immune complex deposition (190, 194). These results suggest that enhanced NETosis in lupus mice promotes endothelial and renal damage and that targeting this cell death pathway may be explored as a potential therapeutic option for the treatment of kidney disease. In addition, inhibition of NETosis through mitochondrial ROS scavengers in vivo in lupus-prone mice also decreased renal inflammation and immune complex deposition (181). Overall, these observations implicate aberrant NET formation in the pathogenesis of LN.
Defective clearance of NETs contributes to renal disease
Previous studies have suggested that SLE patients have an impaired ability to degrade NETs and proposed that this impairment contributes to the development of LN (157, 158, 184, 195). DNase I is the major endonuclease found in circulation involved in degrading NETs and, as mentioned above, impaired activity of this enzyme leads to lupus-like disease in mice and humans (157, 184, 196). The correlation between DNase I deficiency and increased prevalence of LN was also confirmed in humans as SLE subjects with renal involvement were reported to have significantly reduced DNase I activity (157). Impaired DNase I activity in humans can be linked to mutations and polymorphisms in DNase I, the presence of DNase I specific inhibitors and anti-NET antibodies, and binding of C1q, LL-37, and HMGB1 to NETs to prevent degradation (184, 191, 195-201). While a previous phase I study of DNase I in patients with LN revealed no change in serum markers of disease activity (202), it is possible that more effective preparations of the enzyme may prove efficacious in future trials.
Collectively, these data imply that impaired DNase I activity and enhanced externalization of autoantigens and antimicrobial peptides promotes IFN production, inflammasome activation/pyroptosis, autoantibody formation, and prevents degradation of NETs that creates an amplification loop for inflammation and tissue damage.
NETosis-derived post-translational modifications in SLE
Various post-translational modifications of cellular proteins occur during NETosis and the enhanced externalization of these proteins, combined with defective clearance, may promote disruptions in adaptive immunity that further drive SLE pathogenesis (117, 119, 120, 203). SLE NETs contain histone modifications that are also observed during apoptosis (203). SLE NETs express higher levels of acetylated H4-K8, 12, 16, acetylated H2B-K12, and tri-methylated H3-K27 compared to NETs from healthy donors. These modified histones were targeted by autoantibodies in SLE and administration of a triacetylated histone H4 peptide in lupus-prone mice led to increased mortality and renal damage (117, 119, 120). Hypoacetylated H4-K8, K12, K16, and H2B-K12 and hypomethylated H3-K27 were found in unstimulated SLE neutrophils and induction of NETosis resulted in a significant enhancement in histone acetylation and methylation when compared to healthy control neutrophils. These results may indicate that SLE neutrophils are more susceptible to NETosis-induced histone modifications than healthy donor neutrophils (203). Hyperacetylated histones in NETs can upregulate the activation marker CD71 on macrophages (203). These results indicate that many histone modifications that occur during apoptosis are also observed during NETosis.
PAD4 mediates citrullination of histones H1, H2A, H3, and H4 during NETosis (204, 205). Citrullinated histone H1 is present in NETs and autoantibodies specific for citrullinated histone H1 are present in lupus sera (204). Autoantibodies targeting histone H1 appear to be specific for SLE and correlate with disease activity (206). In addition to histones, the antimicrobial peptide LL-37, which is externalized in NETs, was also reported to undergo citrullination in lung tissues from individuals with chronic obstructive pulmonary disorder (COPD) (207). Citrullinated LL-37 has an impaired ability to kill various bacteria (208) and this could potentially be implicated in the enhanced susceptibility of patients with autoimmunity to develop secondary bacterial infections (209). Whether citrullination of other proteins during NETosis impairs distinct immunogenicity or tissue damage potential in SLE remains to be determined. NETosis also results in the translocation of hypopolarized mitochondria to the cell surface that mediates the externalization of oxidized mitochondrial DNA, which is both proinflammatory and interferogenic (181). Collectively, enhanced NETosis and defective clearance of NETs occur in SLE patients and the enhanced externalization of modified autoantigens and antimicrobial peptides contributes to the production of type I IFN and autoantibodies specific for NET components that may promote the development of autoimmunity.
Pyroptosis
Pyroptosis is an inflammatory form of programmed cell death that occurs in response to danger signals and it is triggered by inflammasome activation, resulting in release of IL-1β and IL-18 and in externalization of cellular material (13). The canonical inflammasome is a multi-protein complex found in the cytosol that includes members of the nucleotide-binding domain and leucine-rich repeat-containing (NLR) family and AIM2 and is dependent on caspase 1. The noncanonical inflammasome is a cytosolic LPS sensor that signals through caspase 11. Inflammasome activation is completed in two steps with the first step being recognition of the pathogen-associated molecular pattern (PAMP) or DAMP by TLRs or RLRs, activation of NF-κB, synthesis of pro-IL-1β and pro-IL-18, and upregulation of inflammasome proteins (13). The second step involves the assembly of the inflammasome, conversion of procaspase-1 to caspase 1, and cleavage of pro-IL-1β and pro-IL-18 into their active secreted form that mediates inflammation (210). In addition, caspase 1 also triggers DNA fragmentation, nuclear condensation, lysosome exocytosis, disappearance of organelles, pore formation in plasma membrane, cellular swelling, and disintegration of the plasma membrane, all characteristic of pyroptosis (13). IL-1β signals through the IL-1 receptor and activates NF-κB that results in the production of proinflammatory mediators including cyclooxygenase-2 (COX-2) and IFN-γ (211). IL-18 signals mainly through the p38 MAPK pathway and mediates production of IL-1α, IL-6, and IL-8 (211). Additionally, IL-1β and IL-18 can induce NETosis to amplify the inflammatory response (171, 173). Pyroptosis can also result in the release of HMGB1 and this alarmin may induce inflammation by the production of proinflammatory cytokines, recruitment of immune cells through chemotaxis, and further induction of pyroptosis in macrophages (13, 212). Moreover, HMGB1 forms complexes with and increases the immunogenicity of DNA, histones, and LPS (213-215). Pyroptosis can lead to the release of undigested lysosomal contents that may include microbial products, previously phagocytosed autoantigens, and antimicrobial peptides (13). These factors may induce an inflammatory reaction that possibly could contribute to lupus flares. Pyroptosis also results in the release of intact nuclei that can potentially serve as source of autoantigens for the formation of anti-nuclear antibodies (13, 216, 217). Inflammasome activation also externalizes the adaptor ASC that displays ‘prionoid’-like activity and propagates the inflammatory response after internalization by neighboring cells. Moreover, patients and mice with autoimmune disease generate autoantibodies to ASC specks (218). These studies suggest that pyroptosis results in the externalization of various molecules that can potentiate the inflammatory response and potentially serve as a source of autoantigens.
Because pyroptosis is an inflammatory form of cell death involving release of self-antigens, rapid and efficient clearance of cells is crucial to prevent chronic inflammation and autoimmunity. Similar to apoptosis and necrosis, macrophages undergoing pyroptosis also expose phosphatidylserine on their surface for macrophage uptake (219). Pyroptotic cells release ATP as a “find-me” signal for recruitment of macrophages. Excess externalization of ATP can result in further activation of the NLRP3 inflammasome and lead to release of proinflammatory mediators (220).
NLRP3 is the best characterized inflammasome protein that recognizes various microbes and microbial products but also crystals, ATP and pore-forming toxins (210). Dysregulated NLRP3 inflammasome activation has been implicated in various diseases (221-230) including SLE (171, 231, 232). Given the inflammatory nature of this form of programmed cell death and the externalization of nuclear material, this next section will discuss how accelerated pyroptosis, defective clearance, post-translational modifications during pyroptosis could contribute to the break in tolerance and lead to renal damage in lupus.
Enhanced pyroptosis in lupus nephritis
Numerous studies have reported that enhanced pyroptosis of human and murine macrophages in SLE may contribute to the development of nephritis and other lupus manifestations (171, 231, 232). Kahlenberg et al demonstrated that both LL-37 and NETs containing LL-37 activate the NLRP3 inflammasome in both human and murine macrophages that results in the secretion of IL-1β and IL-18. LL-37 requires P2X7 receptor-induced potassium efflux to activate NLRP3. SLE LDGs with their heightened capacity to form NETs can externalize higher levels of LL-37 that can further activate the inflammasome (175). Importantly, macrophages derived from SLE patients have a lower threshold for activation, greater caspase-1 cleavage, and enhanced production of IL-1β and IL-18 compared to control macrophages once they are exposed to NETs (171). IL-18 can induce NET formation potentially providing a feed-forward inflammatory loop where NETs induce activation of the inflammasome and release of proinflammatory mediators that induce NETosis in neighboring neutrophils.
It has also been shown that the inflammasome is involved in the development of nephritis in murine models of lupus (231-233). Kidneys from MRL/lpr mice have enhanced protein expression of various inflammasome components compared to kidneys from control mice (234). Yuan et al demonstrated that MRL/lpr mice treated with isoflurane had reduced renal NLRP3 inflammasome expression and activation, proteinuria, autoantibodies, and renal inflammatory markers (231). P2X7 receptor plays a critical role in the activation of the NLRP3 inflammasome and release of IL-1β by binding extracellular ATP and inducing pore formation that results in the efflux of intracellular K+ (235). Zhao et al treated MRL/lpr mice and the accelerated model of IFN-adenovirus administration to NZM2328 mice with a P2X7 inhibitor, brilliant blue G (BBG), and observed reduced renal injury and marked improvement in survival compared to untreated mice (232). Together, these data demonstrate P2X7-mediated NLRP3 inflammasome activation is enhanced in both MRL/lpr and NZM2328 AdIFN-α mouse models of glomerulonephritis and targeting P2X7 may ameliorate renal disease.
Mice lacking caspase-1 are protected from developing SLE and LN in the pristane-induced lupus model (233). In humans, microarray analysis from kidney biopsies from LN patients revealed upregulation of inflammasome-associated transcripts when compared to normal kidneys (236). Low levels of serum IL-1 receptor antagonist are associated with renal flares in SLE patients, suggesting that IL-1 signaling may play a pathogenic role in LN (237). Elevated levels of IL-18 have been described in sera and urine of SLE patients (238), particularly in patients with active LN (238, 239). Certain polymorphisms in the IL-18 gene are associated with SLE, increased expression, and development of kidney disease (238-243). Based on these data, heightened expression of IL-1β and IL-18, associated to inflammasome activation and death by pyroptosis, may play pathogenic roles in LN.
Defective clearance of pyroptotic cells
Impaired removal of pyroptotic cells can potentially lead to chronic exposure of potential autoantigens and break of self-tolerance in lupus. Wang et al provided some insight into pyroptotic cell clearance (219). Pyroptotic cells are efficiently internalized by human monocytic THP1-cell-derived macrophages and murine peritoneal macrophages and this process is also dependent on the expression of phosphatidylserine. In addition, pyroptotic cells release ATP as a “find-me” signal for macrophage recruitment. Since pyroptotic cells and apoptotic cells both externalize phosphatidylserine for recognition by phagocytes, defects in phagocytic removal of apoptotic cells could also play a role in the impaired removal of pyroptotic cells. C1q prevents procaspase-1 cleavage and caspase-1-mediated cleavage of pro-IL-1β in human monocyte-derived macrophages suggesting an inhibitory effect on inflammasome activation (244). Although previous literature has not reported the role of C1q in removal of pyroptotic cells, it would be interesting to determine if impaired C1q activity in SLE contributes to the dysregulated inflammasome activity previously mentioned.
At this time, there is not sufficient literature available supporting a link between post-translational modifications during pyroptosis and development of SLE and further work is needed.
Autophagy
Autophagy is a catabolic process that involves the recycling of aged cellular components and proteins into nutrients and amino acids to prolong survival and limit cellular stress (245, 246). Autophagy also plays a critical role in lymphocyte homeostasis and the innate and adaptive immune response (247). Three types of autophagy have been characterized and they include macroautophagy (hereafter called autophagy), microautophagy, and chaperone-mediated autophagy (CMA) (247). Macroautophagy is the best-understood autophagy pathway that involves the enclosure of a targeted portion of the cytoplasm into double-membrane vesicles (autophagosomes) that fuse with lysosomes (autolysosomes) leading to degradation (248). Microautophagy is the engulfment of cytoplasmic material by lysosomes and CMA is the selective uptake of cytosolic proteins into lysosomes that is mediated by chaperone proteins (249, 250). Although autophagy plays a critical role in promoting cellular survival, constitutive activation of this pathway can kill the cell in a process known as type II programmed cell death (247). According to the guidelines established by the Nomenclature Committee on Cell Death, autophagic cell death is cell death that is inhibited by genetic manipulation of at least two components from the autophagy pathway and verified using clonogenicity assays (251). Autophagic cell death is characterized by the presence of autophagosomes/autolysosomes, upregulation of autophagy-related genes (Atg) genes, a compromised plasma membrane, and lack of phagocyte recruitment (248, 252). Autophagic cell death has been shown to be induced by certain cytotoxic agents in the absence of an intact apoptotic pathway, in cancer cells in response to chemotherapy/radiation treatment, by dysregulated RAS oncogenic activity, and IFN-γ among others (138, 251, 253-256). A novel form of autophagic cell death that is dependent on Na+, K+-ATPase pump called autosis has also been described (251).
Although it is relatively unknown how cells dying from autophagy are cleared, a noncanonical form of autophagy dependent on ATG5 and ATG7 called microtubule-associated protein 1 light chain 3 alpha (L3C)-associated phagocytosis (LAP) is essential for efficient degradation of phagocytosed microbes and dead cells (245, 257, 258). LAP is induced after TIM4 recognizes phosphatidylserine on dead cells and this leads to the recruitment of the autophagy machinery (Beclin1, VPS34, and LC3) to the phagosome leading to lysosomal fusion, acidification, and subsequent degradation of the phagocytosed cargo (245, 257). Macrophages lacking ATG7 do not recruit autophagy machinery to the phagosomes and are unable to undergo acidification (257). This results in an inability to degrade the phagocytosed cargo and induction of proinflammatory cytokines. Previous genetic studies have linked polymorphisms in autophagy and LAP genes ATG5 and ATG7, with SLE susceptibility (259-261). Given the critical role of LAP in processing and removing dead cell debris and defective removal of dying cells in SLE, this next section will examine how dysregulated autophagy and impaired LAP contribute to the development of nephritis.
Dysregulated autophagy may contribute to the development of SLE
Although previous literature has not reported the role of autophagic cell death in SLE, studies suggest that autophagy may be dysregulated in SLE (262-266). There are conflicting reports as to whether or not autophagy is defective or active in SLE (262-266). T cells from MRL/lpr and NZB/W mice display enhanced autophagy compared to control mice (266). In addition, autophagic vacuoles were elevated in specific subsets of T cells from SLE patients compared to healthy control and patients with other autoimmune diseases. Basal levels of autophagy are higher in CD4+ T cells from SLE patients compared to healthy donors (262). However, T cells from SLE patients are resistant to autophagy induction (262). T cells from lupus patients also have enhanced expression of genes that negatively regulate the autophagy pathway including α-synuclein. Under serum starvation conditions to induce autophagy, T cells from SLE patients fail to induce autophagy, and instead, form aggregates of α-synuclein that have been shown to potentially serve a pathogenic role in other diseases (265, 267, 268). Collectively, these results suggest that T cells from SLE mice and humans have higher basal levels of autophagy that may potentially result in increased autophagic cell death. In addition, the lack of response to autophagy-inducing stimuli in T cells from SLE patients may result in decreased cell survival and increased apoptosis that can lead to increased autoantigen release (262). B cells from SLE patients and NZB/W mice have activated autophagy and inhibition of autophagy abrogates plasma cell development in mice and humans (264). These results suggest that autophagy may play a vital role in autoantibody formation in SLE. A majority of SLE patients express autoantibodies targeting a small GTPase family inhibitor, D4GDI, and treatment of T cells from healthy donors and SLE patients with α-D4GDI Ab induces autophagy (263). Chronic exposure to autophagy stimuli may lead to enhanced cell death and these authors suggested that repeated exposure to autoantibodies may lead to the selection of a T cell population that is resistant to autophagy induction in SLE.
Defective removal of dead cells by noncanonical autophagy may lead to nephritis
Defects in the LAP pathway have been shown to result in SLE-like disease in mice (258). LAP-deficient mice develop lupus-like disease characterized by increased circulating anti-dsDNA Ab, glomerular deposition of IgG and complement, glomerulonephritis, and IFN signature. LAP-deficient mice injected with apoptotic cells were capable of internalizing the dead cells but were unable to degrade the phagocytosed cargo resulting in induction of proinflammatory cytokines. LAP-sufficient mice generate IL-10 in response to administration of dying cells. TIM-4 deficient mice develop a similar phenotype characterized by the presence anti-dsDNA Ab, hyperactive lymphocytes, and impaired uptake and clearance of apoptotic cells (269). Together, these results suggest that recognition of dying cells by TIM-4 and their clearance by LAP is critical for prevention of autoimmunity.
Post-translational modifications of histones in autophagy
Although previous literature has not examined the role of histone modifications during autophagy and their potential role in breaking tolerance in LN patients, post-translational modifications of histones plays a critical role in regulating autophagy (270). There are many histone modifications during autophagy that also occur during apoptosis but they serve opposing roles. H3K4 trimethylation, H3K9 dimethylation, H3K56 acetylation, and H4K16 acetylation repress autophagy, while H4K20 trimethylation promotes autophagy (270-278). At this time, it has not been reported whether autoantibodies targeting histone modifications that occur during autophagy exist in SLE.
Conclusions
Glomerulonephritis is one of the most common and serious clinical manifestations in SLE patients. Dysregulation in cell death pathways and in clearance of death material may promote enhanced synthesis of modified autoantigens that could promote autoimmunity in SLE. Maintaining homeostasis requires programmed cell death without compromising membrane integrity and their timely removal by scavenger cells. In SLE, accelerated cell death combined with the defective clearance of these dying cells leads to the externalization and accumulation of nuclear and cytoplasmic autoantigens (Figures 1 and 2). In addition, post-translational modification of histones and other proteins increases their immunogenicity and may lead to autoantibody formation, induction of type I IFN responses and tissue damage (Figure 3). Interestingly, the byproducts of one cell death pathway can induce activation of a different cell death pathway in a neighboring cell resulting in an amplification loop that exacerbates disease (Figures 1 and 3). Moving forward, genetic and genomic analyses may further clarify the host’s predisposition to mount dysregulated immune responses to death cells, to promote enhanced cell death or impaired clearance. Given the critical role that inflammatory cell death processes play in the pathogenesis of SLE, therapeutics that inhibit inflammatory forms of cell death, that enhance clearance or that limit certain deleterious posttranslational modifications may prove to be efficacious in the treatment of SLE. Previous groups have successfully inhibited NETosis and reduced disease activity by using molecules that scavenge ROS, inhibit PAD activation, modulate intracellular and extracellular calcium pools, block MPO activation, and disrupt the stabilization of the actin cytoskeleton (170). Given the critical role of DNASE1L3 in the degradation of DNA in circulating apoptotic microparticles and prevention of autoimmunity in mice (126), examining the effect of DNASE1L3 in SLE patients should be explored. Finally, HDAC inhibitors have been shown to modulate renal disease in various mouse models of lupus and should be further investigated in SLE patients (279-281).
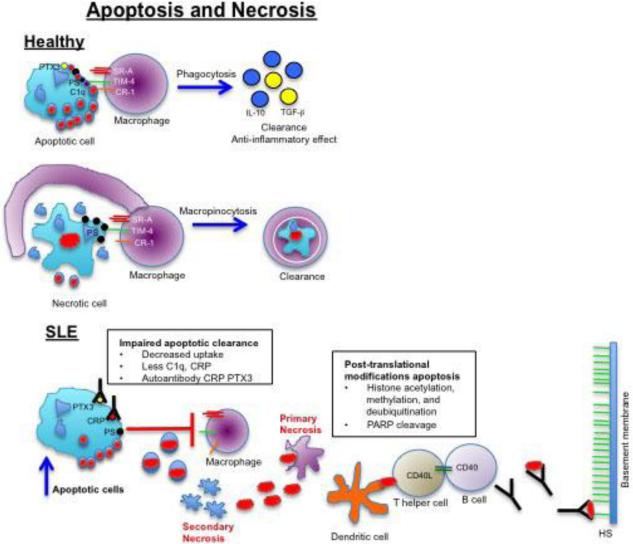
Figure 1. Potential role of apoptosis and necrosis in the development of lupus nephritis.
In healthy patients, apoptotic cells are phagocytosed by macrophages after recognition of phosphatidylserine (PS) on the outer membrane leading to clearance and induction of anti-inflammatory mediators. Necrotic cells also expose PS on their membrane and are internalized by macrophages using macropinocytosis. In certain SLE patients, increased apoptosis and defective clearance of dying cells leads to secondary necrosis and release of nucleosomes (red oval structures). Circulating nucleosomes can induce primary necrosis in neighboring cells and can be internalized and presented by dendritic cells to autoreactive helper T cells that mediate autoantibody production by autoreactive B cells. Autoantibodies can form immune complexes with nucleosomes and bind to heparan sulfate (HS) on the glomerular basement membrane to induce glomerulonephritis.
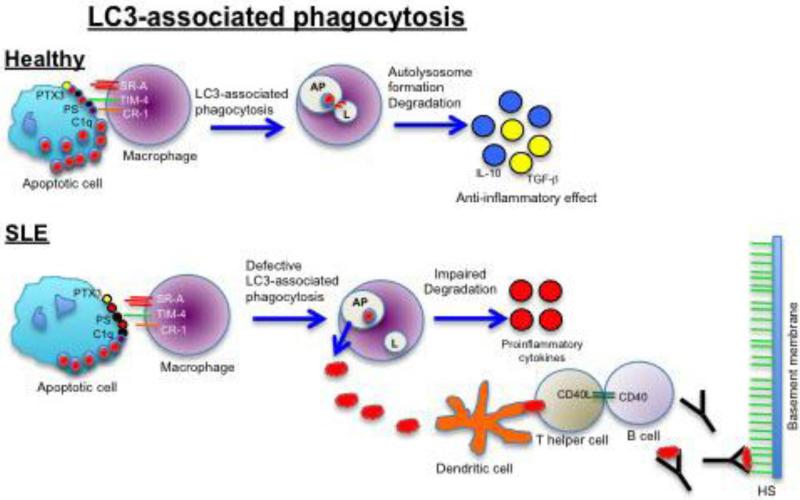
Figure 2. Putative role of LC3-associated phagocytosis (LAP) in the development of lupus nephritis.
In healthy patients, engagement of the TIM-4 receptor leads to activation of LAP, recruitment of autophagy machinery (LC3) to autophagosomes (AP), fusion with lysosomes (L) and degradation of intracellular contents. In SLE patients, LAP can be defective leading to internalization of dying cells but impaired clearance that results in the release of nucleosomes and induction of proinflammatory cytokines, autoantibody formation, and kidney damage.
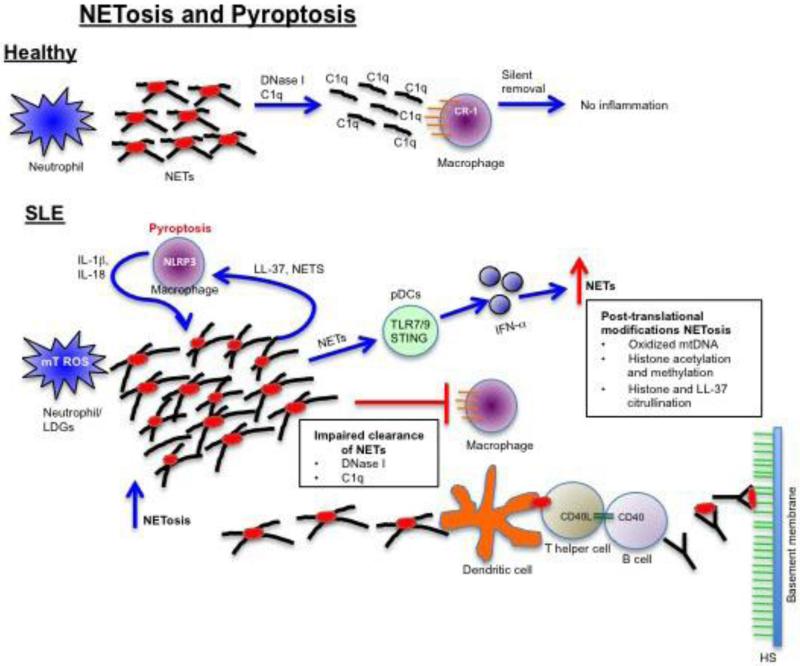
Figure 3. Putative role of NETosis and pyroptosis in the development of lupus nephritis.
In healthy patients, NETs generated in response to sterile/microbial stimuli become opsonized by C1q and processed by DNase I leading to clearance by macrophages in the absence of inflammation. In SLE, neutrophils or low-density granulocytes (LDGs) have enhanced spontaneous NET formation that is driven by mitochondrial ROS. Due to DNase I or C1q deficiency, macrophages are unable to clear the NETs leading to activation of plasmacytoid dendritic cells (pDCs) and type I IFN, induction of pyroptosis in macrophages, autoantibody formation specific for modified NET proteins, and immune complex deposition in the glomerular basement membrane.
Highlights.
-
*
An imbalance in cell death and cell death clearance may promote autoantigen modification and availability to activate the innate and adaptive immune systems.
-
*
Byproducts of one cell death pathway can induce other cell death mechanisms in adjacent cells.
-
*
Aberrant cell death pathways have been implicated in the development of lupus nephritis.
List of Abbreviations
- AIM2
absent in melanoma 2
- APC
antigen presenting cell
- Atg
autophagy-related gene
- BAFF
B cell-activating factor
- Bcl-2
B cell lymphoma 2
- CRP
C-reactive protein
- DAMP
damage-associated molecular pattern
- dsDNA
double stranded deoxyribonucleic acid
- H2B-K12
histone H2B at lysine 12
- HAT
histone acetyltransferase
- HDAC
histone deacetylase
- HMGB1
high mobility group box 1 protein
- IL-1•
interleukin 1 beta
- IFN
interferon
- LAP
LC3 (light chain 3)-associated phagocytosis
- LL-37
cathelicidin
- LN
lupus glomerulonephritis
- MARCO
macrophage receptor with collagenous structure
- MBL
mannose binding lectin
- MPO
myeloperoxidase
- NADPH
nicotinamide adenine dinucleotide phosphate
- NE
neutrophil elastase
- NET
neutrophil extracellular trap
- NLR
nucleotide-binding domain and leucine-rich repeat-containing
- PAD4
peptidylarginine deiminase 4
- PARP
poly(ADP-ribose) polymerase
- pDC
plasmacytoid dendritic cell
- PTEN
phosphatase and tensin homolog
- PTX3
pentraxin-related protein
- RIP1
receptor-interacting serine/threonine-protein kinase 1
- ROS
reactive oxygen species
- SAP
serum amyloid protein
- SLE
systemic lupus erythematosus
- SR-A
scavenger receptor A
- TACI
cyclophilin ligand interactor
- TLR
toll-like receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cojocaru M, Cojocaru IM, Silosi I, Vrabie CD. Manifestations of systemic lupus erythematosus. Maedica (Buchar) 2011;6(4):330–6. [PMC free article] [PubMed] [Google Scholar]
- 2.Pickering MC, Botto M, Taylor PR, Lachmann PJ, Walport MJ. Systemic lupus erythematosus, complement deficiency, and apoptosis. Adv Immunol. 2000;76:227–324. doi: 10.1016/s0065-2776(01)76021-x. [DOI] [PubMed] [Google Scholar]
- 3.Fenton K. The effect of cell death in the initiation of lupus nephritis. Clin Exp Immunol. 2015;179(1):11–6. doi: 10.1111/cei.12417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pisetsky DS. Anti-DNA antibodies--quintessential biomarkers of SLE. Nat Rev Rheumatol. 2016;12(2):102–10. doi: 10.1038/nrrheum.2015.151. [DOI] [PubMed] [Google Scholar]
- 5.Saxena R, Mahajan T, Mohan C. Lupus nephritis: current update. Arthritis research & therapy. 2011;13(5):240. doi: 10.1186/ar3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Appel GB RJ, D'Agatis V. Secondary glomerular disease. In: Brenner B, editor. Brenner and Rector's The Kidney. 8 ed. Saunders Elsevier; Philadelphia, PA: 2007. pp. 1067–148. [Google Scholar]
- 7.Mak A, Mok CC, Chu WP, To CH, Wong SN, Au TC. Renal damage in systemic lupus erythematosus: a comparative analysis of different age groups. Lupus. 2007;16(1):28–34. doi: 10.1177/0961203306074469. [DOI] [PubMed] [Google Scholar]
- 8.Davidson A, Aranow C. Pathogenesis and treatment of systemic lupus erythematosus nephritis. Curr Opin Rheumatol. 2006;18(5):468–75. doi: 10.1097/01.bor.0000240356.45550.13. [DOI] [PubMed] [Google Scholar]
- 9.Kanta H, Mohan C. Three checkpoints in lupus development: central tolerance in adaptive immunity, peripheral amplification by innate immunity and end-organ inflammation. Genes Immun. 2009;10(5):390–6. doi: 10.1038/gene.2009.6. [DOI] [PubMed] [Google Scholar]
- 10.Lech M, Anders HJ. The pathogenesis of lupus nephritis. J Am Soc Nephrol. 2013;24(9):1357–66. doi: 10.1681/ASN.2013010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sterner RM, Hartono SP, Grande JP. The Pathogenesis of Lupus Nephritis. J Clin Cell Immunol. 2014;5(2) doi: 10.4172/2155-9899.1000205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pieterse E, van der Vlag J. Breaking immunological tolerance in systemic lupus erythematosus. Front Immunol. 2014;5:164. doi: 10.3389/fimmu.2014.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magna M, Pisetsky DS. The Role of Cell Death in the Pathogenesis of SLE: Is Pyroptosis the Missing Link? Scand J Immunol. 2015;82(3):218–24. doi: 10.1111/sji.12335. [DOI] [PubMed] [Google Scholar]
- 14.Munoz LE, Gaipl US, Franz S, Sheriff A, Voll RE, Kalden JR, et al. SLE--a disease of clearance deficiency? Rheumatology (Oxford) 2005;44(9):1101–7. doi: 10.1093/rheumatology/keh693. [DOI] [PubMed] [Google Scholar]
- 15.Dieker JW, van der Vlag J, Berden JH. Deranged removal of apoptotic cells: its role in the genesis of lupus. Nephrol Dial Transplant. 2004;19(2):282–5. doi: 10.1093/ndt/gfg485. [DOI] [PubMed] [Google Scholar]
- 16.Saraste A, Pulkki K. Morphologic and biochemical hallmarks of apoptosis. Cardiovasc Res. 2000;45(3):528–37. doi: 10.1016/s0008-6363(99)00384-3. [DOI] [PubMed] [Google Scholar]
- 17.Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature. 1997;390(6658):350–1. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 18.Lynch DH, Watson ML, Alderson MR, Baum PR, Miller RE, Tough T, et al. The mouse Fas-ligand gene is mutated in gld mice and is part of a TNF family gene cluster. Immunity. 1994;1(2):131–6. doi: 10.1016/1074-7613(94)90106-6. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi T, Tanaka M, Brannan CI, Jenkins NA, Copeland NG, Suda T, et al. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell. 1994;76(6):969–76. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356(6367):314–7. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 21.Fisher GH, Rosenberg FJ, Straus SE, Dale JK, Middleton LA, Lin AY, et al. Dominant interfering Fas gene mutations impair apoptosis in a human autoimmune lymphoproliferative syndrome. Cell. 1995;81(6):935–46. doi: 10.1016/0092-8674(95)90013-6. [DOI] [PubMed] [Google Scholar]
- 22.Rieux-Laucat F, Le Deist F, Hivroz C, Roberts IA, Debatin KM, Fischer A, et al. Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science. 1995;268(5215):1347–9. doi: 10.1126/science.7539157. [DOI] [PubMed] [Google Scholar]
- 23.Wu J, Wilson J, He J, Xiang L, Schur PH, Mountz JD. Fas ligand mutation in a patient with systemic lupus erythematosus and lymphoproliferative disease. J Clin Invest. 1996;98(5):1107–13. doi: 10.1172/JCI118892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fathi NA, Hussein MR, Hassan HI, Mosad E, Galal H, Afifi NA. Glomerular expression and elevated serum Bcl-2 and Fas proteins in lupus nephritis: preliminary findings. Clin Exp Immunol. 2006;146(2):339–43. doi: 10.1111/j.1365-2249.2006.03219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strasser A, Whittingham S, Vaux DL, Bath ML, Adams JM, Cory S, et al. Enforced BCL2 expression in B-lymphoid cells prolongs antibody responses and elicits autoimmune disease. Proc Natl Acad Sci U S A. 1991;88(19):8661–5. doi: 10.1073/pnas.88.19.8661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hutcheson J, Perlman H. Loss of Bim results in abnormal accumulation of mature CD4-CD8-CD44-CD25- thymocytes. Immunobiology. 2007;212(8):629–36. doi: 10.1016/j.imbio.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mackay F, Tangye SG. The role of the BAFF/APRIL system in B cell homeostasis and lymphoid cancers. Curr Opin Pharmacol. 2004;4(4):347–54. doi: 10.1016/j.coph.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 28.Zhao LD, Li Y, Smith MF, Jr., Wang JS, Zhang W, Tang FL, et al. Expressions of BAFF/BAFF receptors and their correlation with disease activity in Chinese SLE patients. Lupus. 2010;19(13):1534–49. doi: 10.1177/0961203310375268. [DOI] [PubMed] [Google Scholar]
- 29.Mackay F, Woodcock SA, Lawton P, Ambrose C, Baetscher M, Schneider P, et al. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999;190(11):1697–710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dooley MA, Houssiau F, Aranow C, D'Cruz DP, Askanase A, Roth DA, et al. Effect of belimumab treatment on renal outcomes: results from the phase 3 belimumab clinical trials in patients with SLE. Lupus. 2013;22(1):63–72. doi: 10.1177/0961203312465781. [DOI] [PubMed] [Google Scholar]
- 31.Figgett WA, Deliyanti D, Fairfax KA, Quah PS, Wilkinson-Berka JL, Mackay F. Deleting the BAFF receptor TACI protects against systemic lupus erythematosus without extensive reduction of B cell numbers. J Autoimmun. 2015;61:9–16. doi: 10.1016/j.jaut.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 32.Shrestha S, Yang K, Guy C, Vogel P, Neale G, Chi H. Treg cells require the phosphatase PTEN to restrain TH1 and TFH cell responses. Nat Immunol. 2015;16(2):178–87. doi: 10.1038/ni.3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Firestein GS, Echeverri F, Yeo M, Zvaifler NJ, Green DR. Somatic mutations in the p53 tumor suppressor gene in rheumatoid arthritis synovium. Proc Natl Acad Sci U S A. 1997;94(20):10895–900. doi: 10.1073/pnas.94.20.10895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hara T, Ogawa F, Muroi E, Komura K, Takenaka M, Hasegawa M, et al. Anti-p53 autoantibody in systemic sclerosis: association with limited cutaneous systemic sclerosis. J Rheumatol. 2008;35(3):451–7. [PubMed] [Google Scholar]
- 35.Herkel J, Mimran A, Erez N, Kam N, Lohse AW, Marker-Hermann E, et al. Autoimmunity to the p53 protein is a feature of systemic lupus erythematosus (SLE) related to anti-DNA antibodies. J Autoimmun. 2001;17(1):63–9. doi: 10.1006/jaut.2001.0518. [DOI] [PubMed] [Google Scholar]
- 36.Leech M, Xue JR, Dacumos A, Hall P, Santos L, Yang Y, et al. The tumour suppressor gene p53 modulates the severity of antigen-induced arthritis and the systemic immune response. Clin Exp Immunol. 2008;152(2):345–53. doi: 10.1111/j.1365-2249.2008.03629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okuda Y, Okuda M, Bernard CC. Regulatory role of p53 in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2003;135(1-2):29–37. doi: 10.1016/s0165-5728(02)00428-9. [DOI] [PubMed] [Google Scholar]
- 38.Simelyte E, Rosengren S, Boyle DL, Corr M, Green DR, Firestein GS. Regulation of arthritis by p53: critical role of adaptive immunity. Arthritis Rheum. 2005;52(6):1876–84. doi: 10.1002/art.21099. [DOI] [PubMed] [Google Scholar]
- 39.Zheng SJ, Lamhamedi-Cherradi SE, Wang P, Xu L, Chen YH. Tumor suppressor p53 inhibits autoimmune inflammation and macrophage function. Diabetes. 2005;54(5):1423–8. doi: 10.2337/diabetes.54.5.1423. [DOI] [PubMed] [Google Scholar]
- 40.Kawashima H, Takatori H, Suzuki K, Iwata A, Yokota M, Suto A, et al. Tumor suppressor p53 inhibits systemic autoimmune diseases by inducing regulatory T cells. J Immunol. 2013;191(7):3614–23. doi: 10.4049/jimmunol.1300509. [DOI] [PubMed] [Google Scholar]
- 41.Makino H, Sugiyama H, Yamasaki Y, Maeshima Y, Wada J, Kashihara N. Glomerular cell apoptosis in human lupus nephritis. Virchows Arch. 2003;443(1):67–77. doi: 10.1007/s00428-003-0827-x. [DOI] [PubMed] [Google Scholar]
- 42.Soto H, Mosquera J, Rodriguez-Iturbe B, Henriquez La Roche C, Pinto A. Apoptosis in proliferative glomerulonephritis: decreased apoptosis expression in lupus nephritis. Nephrol Dial Transplant. 1997;12(2):273–80. doi: 10.1093/ndt/12.2.273. [DOI] [PubMed] [Google Scholar]
- 43.Szabolcs MJW,L, Buttyan R, D'Agati V. Apoptosis in human renal biopsies (Abstract). J Am Soc Nephrol. 1994;5:844. [Google Scholar]
- 44.Faurschou M, Penkowa M, Andersen CB, Starklint H, Jacobsen S. Renal cell apoptosis in human lupus nephritis: a histological study. Lupus. 2009;18(11):994–9. doi: 10.1177/0961203309106175. [DOI] [PubMed] [Google Scholar]
- 45.Seredkina N, Zykova SN, Rekvig OP. Progression of murine lupus nephritis is linked to acquired renal Dnase1 deficiency and not to up-regulated apoptosis. Am J Pathol. 2009;175(1):97–106. doi: 10.2353/ajpath.2009.080943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kalaaji M, Fenton KA, Mortensen ES, Olsen R, Sturfelt G, Alm P, et al. Glomerular apoptotic nucleosomes are central target structures for nephritogenic antibodies in human SLE nephritis. Kidney Int. 2007;71(7):664–72. doi: 10.1038/sj.ki.5002133. [DOI] [PubMed] [Google Scholar]
- 47.Kalaaji M, Mortensen E, Jorgensen L, Olsen R, Rekvig OP. Nephritogenic lupus antibodies recognize glomerular basement membrane-associated chromatin fragments released from apoptotic intraglomerular cells. Am J Pathol. 2006;168(6):1779–92. doi: 10.2353/ajpath.2006.051329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takemura T, Murakami K, Miyazato H, Yagi K, Yoshioka K. Expression of Fas antigen and Bcl-2 in human glomerulonephritis. Kidney Int. 1995;48(6):1886–92. doi: 10.1038/ki.1995.487. [DOI] [PubMed] [Google Scholar]
- 49.Cui JH, Qiao Q, Guo Y, Zhang YQ, Cheng H, He FR, et al. Increased apoptosis and expression of FasL, Bax and caspase-3 in human lupus nephritis class II and IV. J Nephrol. 2012;25(2):255–61. doi: 10.5301/JN.2011.8451. [DOI] [PubMed] [Google Scholar]
- 50.Soto HM, Parra G, Rodriguez-Itrube B. Circulating levels of cytokines in poststreptococcal glomerulonephritis. Clin Nephrol. 1997;47(1):6–12. [PubMed] [Google Scholar]
- 51.Watanabe M, Hitomi M, van der Wee K, Rothenberg F, Fisher SA, Zucker R, et al. The pros and cons of apoptosis assays for use in the study of cells, tissues, and organs. Microsc Microanal. 2002;8(5):375–91. doi: 10.1017/S1431927602010346. [DOI] [PubMed] [Google Scholar]
- 52.CA J. The immune system in health and disease. Immunobiology. Current Biology Publications; New York: 1999. pp. 339–58.pp. 433–4. [Google Scholar]
- 53.Kaplan MJ, Lewis EE, Shelden EA, Somers E, Pavlic R, McCune WJ, et al. The apoptotic ligands TRAIL, TWEAK, and Fas ligand mediate monocyte death induced by autologous lupus T cells. J Immunol. 2002;169(10):6020–9. doi: 10.4049/jimmunol.169.10.6020. [DOI] [PubMed] [Google Scholar]
- 54.Denny MF, Chandaroy P, Killen PD, Caricchio R, Lewis EE, Richardson BC, et al. Accelerated macrophage apoptosis induces autoantibody formation and organ damage in systemic lupus erythematosus. J Immunol. 2006;176(4):2095–104. doi: 10.4049/jimmunol.176.4.2095. [DOI] [PubMed] [Google Scholar]
- 55.Courtney PA, Crockard AD, Williamson K, Irvine AE, Kennedy RJ, Bell AL. Increased apoptotic peripheral blood neutrophils in systemic lupus erythematosus: relations with disease activity, antibodies to double stranded DNA, and neutropenia. Ann Rheum Dis. 1999;58(5):309–14. doi: 10.1136/ard.58.5.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bengtsson AA, Sturfelt G, Gullstrand B, Truedsson L. Induction of apoptosis in monocytes and lymphocytes by serum from patients with systemic lupus erythematosus - an additional mechanism to increased autoantigen load? Clin Exp Immunol. 2004;135(3):535–43. doi: 10.1111/j.1365-2249.2003.02386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ren Y, Tang J, Mok MY, Chan AW, Wu A, Lau CS. Increased apoptotic neutrophils and macrophages and impaired macrophage phagocytic clearance of apoptotic neutrophils in systemic lupus erythematosus. Arthritis Rheum. 2003;48(10):2888–97. doi: 10.1002/art.11237. [DOI] [PubMed] [Google Scholar]
- 58.Bondanza A, Zimmermann VS, Dell'Antonio G, Cin ED, Balestrieri G, Tincani A, et al. Requirement of dying cells and environmental adjuvants for the induction of autoimmunity. Arthritis Rheum. 2004;50(5):1549–60. doi: 10.1002/art.20187. [DOI] [PubMed] [Google Scholar]
- 59.Mevorach D, Zhou JL, Song X, Elkon KB. Systemic exposure to irradiated apoptotic cells induces autoantibody production. J Exp Med. 1998;188(2):387–92. doi: 10.1084/jem.188.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Munoz LE, Lauber K, Schiller M, Manfredi AA, Herrmann M. The role of defective clearance of apoptotic cells in systemic autoimmunity. Nat Rev Rheumatol. 2010;6(5):280–9. doi: 10.1038/nrrheum.2010.46. [DOI] [PubMed] [Google Scholar]
- 61.Lemke G, Burstyn-Cohen T. TAM receptors and the clearance of apoptotic cells. Ann N Y Acad Sci. 2010;1209:23–9. doi: 10.1111/j.1749-6632.2010.05744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lu Q, Lemke G. Homeostatic regulation of the immune system by receptor tyrosine kinases of the Tyro 3 family. Science. 2001;293(5528):306–11. doi: 10.1126/science.1061663. [DOI] [PubMed] [Google Scholar]
- 63.Wermeling F, Chen Y, Pikkarainen T, Scheynius A, Winqvist O, Izui S, et al. Class A scavenger receptors regulate tolerance against apoptotic cells, and autoantibodies against these receptors are predictive of systemic lupus. J Exp Med. 2007;204(10):2259–65. doi: 10.1084/jem.20070600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Garlanda C, Bottazzi B, Bastone A, Mantovani A. Pentraxins at the crossroads between innate immunity, inflammation, matrix deposition, and female fertility. Annu Rev Immunol. 2005;23:337–66. doi: 10.1146/annurev.immunol.23.021704.115756. [DOI] [PubMed] [Google Scholar]
- 65.Volanakis JE. Human C-reactive protein: expression, structure, and function. Mol Immunol. 2001;38(2-3):189–97. doi: 10.1016/s0161-5890(01)00042-6. [DOI] [PubMed] [Google Scholar]
- 66.Bell SA, Faust H, Schmid A, Meurer M. Autoantibodies to C-reactive protein (CRP) and other acute-phase proteins in systemic autoimmune diseases. Clin Exp Immunol. 1998;113(3):327–32. doi: 10.1046/j.1365-2249.1998.00655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Figueredo MA, Rodriguez A, Ruiz-Yague M, Romero M, Fernandez-Cruz A, Gomez-de la Concha E, et al. Autoantibodies against C-reactive protein: clinical associations in systemic lupus erythematosus and primary antiphospholipid syndrome. J Rheumatol. 2006;33(10):1980–6. [PubMed] [Google Scholar]
- 68.Pereira Da Silva JA, Elkon KB, Hughes GR, Dyck RF, Pepys MB. C-reactive protein levels in systemic lupus erythematosus: a classification criterion? Arthritis Rheum. 1980;23(6):770–1. doi: 10.1002/art.1780230609. [DOI] [PubMed] [Google Scholar]
- 69.Sjowall C, Bengtsson AA, Sturfelt G, Skogh T. Serum levels of autoantibodies against monomeric C-reactive protein are correlated with disease activity in systemic lupus erythematosus. Arthritis research & therapy. 2004;6(2):R87–94. doi: 10.1186/ar1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Russell AI, Cunninghame Graham DS, Shepherd C, Roberton CA, Whittaker J, Meeks J, et al. Polymorphism at the C-reactive protein locus influences gene expression and predisposes to systemic lupus erythematosus. Hum Mol Genet. 2004;13(1):137–47. doi: 10.1093/hmg/ddh021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rodriguez W, Mold C, Marnell LL, Hutt J, Silverman GJ, Tran D, et al. Prevention and reversal of nephritis in MRL/lpr mice with a single injection of C-reactive protein. Arthritis Rheum. 2006;54(1):325–35. doi: 10.1002/art.21556. [DOI] [PubMed] [Google Scholar]
- 72.Szalai AJ, Weaver CT, McCrory MA, van Ginkel FW, Reiman RM, Kearney JF, et al. Delayed lupus onset in (NZB x NZW)F1 mice expressing a human C-reactive protein transgene. Arthritis Rheum. 2003;48(6):1602–11. doi: 10.1002/art.11026. [DOI] [PubMed] [Google Scholar]
- 73.Bharadwaj D, Mold C, Markham E, Du Clos TW. Serum amyloid P component binds to Fc gamma receptors and opsonizes particles for phagocytosis. J Immunol. 2001;166(11):6735–41. doi: 10.4049/jimmunol.166.11.6735. [DOI] [PubMed] [Google Scholar]
- 74.Gillmore JD, Hutchinson WL, Herbert J, Bybee A, Mitchell DA, Hasserjian RP, et al. Autoimmunity and glomerulonephritis in mice with targeted deletion of the serum amyloid P component gene: SAP deficiency or strain combination? Immunology. 2004;112(2):255–64. doi: 10.1111/j.1365-2567.2004.01860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bickerstaff MC, Botto M, Hutchinson WL, Herbert J, Tennent GA, Bybee A, et al. Serum amyloid P component controls chromatin degradation and prevents antinuclear autoimmunity. Nat Med. 1999;5(6):694–7. doi: 10.1038/9544. [DOI] [PubMed] [Google Scholar]
- 76.Zandman-Goddard G, Blank M, Langevitz P, Slutsky L, Pras M, Levy Y, et al. Anti-serum amyloid component P antibodies in patients with systemic lupus erythematosus correlate with disease activity. Ann Rheum Dis. 2005;64(12):1698–702. doi: 10.1136/ard.2005.035832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang W, Wu J, Qiao B, Xu W, Xiong S. Amelioration of lupus nephritis by serum amyloid P component gene therapy with distinct mechanisms varied from different stage of the disease. PLoS One. 2011;6(7):e22659. doi: 10.1371/journal.pone.0022659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rovere P, Peri G, Fazzini F, Bottazzi B, Doni A, Bondanza A, et al. The long pentraxin PTX3 binds to apoptotic cells and regulates their clearance by antigen-presenting dendritic cells. Blood. 2000;96(13):4300–6. [PubMed] [Google Scholar]
- 79.Augusto JF, Onno C, Blanchard S, Dubuquoi S, Mantovani A, Chevailler A, et al. Detection of anti-PTX3 autoantibodies in systemic lupus erythematosus. Rheumatology (Oxford) 2009;48(4):442–4. doi: 10.1093/rheumatology/ken507. [DOI] [PubMed] [Google Scholar]
- 80.Lech M, Rommele C, Kulkarni OP, Susanti HE, Migliorini A, Garlanda C, et al. Lack of the long pentraxin PTX3 promotes autoimmune lung disease but not glomerulonephritis in murine systemic lupus erythematosus. PLoS One. 2011;6(5):e20118. doi: 10.1371/journal.pone.0020118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pradhan V, Surve P, Ghosh K. Mannose binding lectin (MBL) in autoimmunity and its role in systemic lupus erythematosus (SLE). J Assoc Physicians India. 2010;58:688–90. [PubMed] [Google Scholar]
- 82.Panda AK, Parida JR, Tripathy R, Pattanaik SS, Ravindran B, Das BK. Low producer MBL genotypes are associated with susceptibility to systemic lupus erythematosus in Odisha, India. Hum Immunol. 2013;74(1):114–9. doi: 10.1016/j.humimm.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 83.Stuart LM, Takahashi K, Shi L, Savill J, Ezekowitz RA. Mannose-binding lectin-deficient mice display defective apoptotic cell clearance but no autoimmune phenotype. J Immunol. 2005;174(6):3220–6. doi: 10.4049/jimmunol.174.6.3220. [DOI] [PubMed] [Google Scholar]
- 84.Jakab L, Laki J, Sallai K, Temesszentandrasi G, Pozsonyi T, Kalabay L, et al. Association between early onset and organ manifestations of systemic lupus erythematosus (SLE) and a down-regulating promoter polymorphism in the MBL2 gene. Clin Immunol. 2007;125(3):230–6. doi: 10.1016/j.clim.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 85.Sandrin-Garcia P, Brandao LA, Coelho AV, Guimaraes RL, Pancoto JA, Segat L, et al. Mannose binding lectin gene (MBL2) functional polymorphisms are associated with systemic lupus erythematosus in southern Brazilians. Hum Immunol. 2011;72(6):516–21. doi: 10.1016/j.humimm.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 86.Tanha N, Troelsen L, From Hermansen ML, Kjaer L, Faurschou M, Garred P, et al. MBL2 gene variants coding for mannose-binding lectin deficiency are associated with increased risk of nephritis in Danish patients with systemic lupus erythematosus. Lupus. 2014;23(11):1105–11. doi: 10.1177/0961203314536478. [DOI] [PubMed] [Google Scholar]
- 87.Saiki O, Saeki Y, Tanaka T, Doi S, Hara H, Negoro S, et al. Development of selective IgM deficiency in systemic lupus erythematosus patients with disease of long duration. Arthritis Rheum. 1987;30(11):1289–92. doi: 10.1002/art.1780301112. [DOI] [PubMed] [Google Scholar]
- 88.Nauta AJ, Castellano G, Xu W, Woltman AM, Borrias MC, Daha MR, et al. Opsonization with C1q and mannose-binding lectin targets apoptotic cells to dendritic cells. J Immunol. 2004;173(5):3044–50. doi: 10.4049/jimmunol.173.5.3044. [DOI] [PubMed] [Google Scholar]
- 89.Kirschfink M, Petry F, Khirwadkar K, Wigand R, Kaltwasser JP, Loos M. Complete functional C1q deficiency associated with systemic lupus erythematosus (SLE). Clin Exp Immunol. 1993;94(2):267–72. doi: 10.1111/j.1365-2249.1993.tb03442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Siegert C, Daha M, Westedt ML, van der Voort E, Breedveld F. IgG autoantibodies against C1q are correlated with nephritis, hypocomplementemia, and dsDNA antibodies in systemic lupus erythematosus. J Rheumatol. 1991;18(2):230–4. [PubMed] [Google Scholar]
- 91.Trendelenburg M, Lopez-Trascasa M, Potlukova E, Moll S, Regenass S, Fremeaux-Bacchi V, et al. High prevalence of anti-C1q antibodies in biopsy-proven active lupus nephritis. Nephrol Dial Transplant. 2006;21(11):3115–21. doi: 10.1093/ndt/gfl436. [DOI] [PubMed] [Google Scholar]
- 92.Botto M, Dell'Agnola C, Bygrave AE, Thompson EM, Cook HT, Petry F, et al. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat Genet. 1998;19(1):56–9. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- 93.Baumann I, Kolowos W, Voll RE, Manger B, Gaipl U, Neuhuber WL, et al. Impaired uptake of apoptotic cells into tingible body macrophages in germinal centers of patients with systemic lupus erythematosus. Arthritis Rheum. 2002;46(1):191–201. doi: 10.1002/1529-0131(200201)46:1<191::AID-ART10027>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 94.Herrmann M, Voll RE, Zoller OM, Hagenhofer M, Ponner BB, Kalden JR. Impaired phagocytosis of apoptotic cell material by monocyte-derived macrophages from patients with systemic lupus erythematosus. Arthritis Rheum. 1998;41(7):1241–50. doi: 10.1002/1529-0131(199807)41:7<1241::AID-ART15>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 95.Tas SW, Quartier P, Botto M, Fossati-Jimack L. Macrophages from patients with SLE and rheumatoid arthritis have defective adhesion in vitro, while only SLE macrophages have impaired uptake of apoptotic cells. Ann Rheum Dis. 2006;65(2):216–21. doi: 10.1136/ard.2005.037143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Monteith AJ, Kang S, Scott E, Hillman K, Rajfur Z, Jacobson K, et al. Defects in lysosomal maturation facilitate the activation of innate sensors in systemic lupus erythematosus. Proc Natl Acad Sci U S A. 2016;113(15):E2142–51. doi: 10.1073/pnas.1513943113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Roberts TL, Idris A, Dunn JA, Kelly GM, Burnton CM, Hodgson S, et al. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science. 2009;323(5917):1057–60. doi: 10.1126/science.1169841. [DOI] [PubMed] [Google Scholar]
- 98.Yang K, Shi HX, Liu XY, Shan YF, Wei B, Chen S, et al. TRIM21 is essential to sustain IFN regulatory factor 3 activation during antiviral response. J Immunol. 2009;182(6):3782–92. doi: 10.4049/jimmunol.0803126. [DOI] [PubMed] [Google Scholar]
- 99.Fullgrabe J, Hajji N, Joseph B. Cracking the death code: apoptosis-related histone modifications. Cell Death Differ. 2010;17(8):1238–43. doi: 10.1038/cdd.2010.58. [DOI] [PubMed] [Google Scholar]
- 100.Kaplan LJ, Bauer R, Morrison E, Langan TA, Fasman GD. The structure of chromatin reconstituted with phosphorylated H1. Circular dichroism and thermal denaturation studies. J Biol Chem. 1984;259(14):8777–85. [PubMed] [Google Scholar]
- 101.Ajiro K. Histone H2B phosphorylation in mammalian apoptotic cells. An association with DNA fragmentation. J Biol Chem. 2000;275(1):439–43. doi: 10.1074/jbc.275.1.439. [DOI] [PubMed] [Google Scholar]
- 102.Cheung WL, Ajiro K, Samejima K, Kloc M, Cheung P, Mizzen CA, et al. Apoptotic phosphorylation of histone H2B is mediated by mammalian sterile twenty kinase. Cell. 2003;113(4):507–17. doi: 10.1016/s0092-8674(03)00355-6. [DOI] [PubMed] [Google Scholar]
- 103.Lee E, Nakatsuma A, Hiraoka R, Ishikawa E, Enomoto R, Yamauchi A. Involvement of histone phosphorylation in thymocyte apoptosis by protein phosphatase inhibitors. IUBMB Life. 1999;48(1):79–83. doi: 10.1080/713803462. [DOI] [PubMed] [Google Scholar]
- 104.Waring P, Khan T, Sjaarda A. Apoptosis induced by gliotoxin is preceded by phosphorylation of histone H3 and enhanced sensitivity of chromatin to nuclease digestion. J Biol Chem. 1997;272(29):17929–36. doi: 10.1074/jbc.272.29.17929. [DOI] [PubMed] [Google Scholar]
- 105.Verdin E, Ott M. 50 years of protein acetylation: from gene regulation to epigenetics, metabolism and beyond. Nat Rev Mol Cell Biol. 2015;16(4):258–64. doi: 10.1038/nrm3931. [DOI] [PubMed] [Google Scholar]
- 106.McBain JA, Eastman A, Nobel CS, Mueller GC. Apoptotic death in adenocarcinoma cell lines induced by butyrate and other histone deacetylase inhibitors. Biochem Pharmacol. 1997;53(9):1357–68. doi: 10.1016/s0006-2952(96)00904-5. [DOI] [PubMed] [Google Scholar]
- 107.Medina V, Edmonds B, Young GP, James R, Appleton S, Zalewski PD. Induction of caspase-3 protease activity and apoptosis by butyrate and trichostatin A (inhibitors of histone deacetylase): dependence on protein synthesis and synergy with a mitochondrial/cytochrome c-dependent pathway. Cancer Res. 1997;57(17):3697–707. [PubMed] [Google Scholar]
- 108.Boix-Chornet M, Fraga MF, Villar-Garea A, Caballero R, Espada J, Nunez A, et al. Release of hypoacetylated and trimethylated histone H4 is an epigenetic marker of early apoptosis. J Biol Chem. 2006;281(19):13540–7. doi: 10.1074/jbc.M601136200. [DOI] [PubMed] [Google Scholar]
- 109.Cheng MF, Lee CH, Hsia KT, Huang GS, Lee HS. Methylation of histone H3 lysine 27 associated with apoptosis in osteosarcoma cells induced by staurosporine. Histol Histopathol. 2009;24(9):1105–11. doi: 10.14670/HH-24.1105. [DOI] [PubMed] [Google Scholar]
- 110.Marushige Y, Marushige K. Disappearance of ubiquitinated histone H2A during chromatin condensation in TGF beta 1-induced apoptosis. Anticancer Res. 1995;15(2):267–72. [PubMed] [Google Scholar]
- 111.Tanimoto Y, Onishi Y, Hashimoto S, Kizaki H. Peptidyl aldehyde inhibitors of proteasome induce apoptosis rapidly in mouse lymphoma RVC cells. J Biochem. 1997;121(3):542–9. doi: 10.1093/oxfordjournals.jbchem.a021620. [DOI] [PubMed] [Google Scholar]
- 112.Manome Y, Datta R, Fine HA. Early response gene induction following DNA damage in astrocytoma cell lines. Biochem Pharmacol. 1993;45(8):1677–84. doi: 10.1016/0006-2952(93)90309-k. [DOI] [PubMed] [Google Scholar]
- 113.Schraufstatter IU, Hyslop PA, Hinshaw DB, Spragg RG, Sklar LA, Cochrane CG. Hydrogen peroxide-induced injury of cells and its prevention by inhibitors of poly(ADP-ribose) polymerase. Proc Natl Acad Sci U S A. 1986;83(13):4908–12. doi: 10.1073/pnas.83.13.4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tanizawa A, Kubota M, Hashimoto H, Shimizu T, Takimoto T, Kitoh T, et al. VP-16-induced nucleotide pool changes and poly(ADP-ribose) synthesis: the role of VP-16 in interphase death. Exp Cell Res. 1989;185(1):237–46. doi: 10.1016/0014-4827(89)90052-9. [DOI] [PubMed] [Google Scholar]
- 115.Th'ng JP. Histone modifications and apoptosis: cause or consequence? Biochem Cell Biol. 2001;79(3):305–11. [PubMed] [Google Scholar]
- 116.Cline AM, Radic MZ. Apoptosis, subcellular particles, and autoimmunity. Clin Immunol. 2004;112(2):175–82. doi: 10.1016/j.clim.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 117.Dieker JW, Fransen JH, van Bavel CC, Briand JP, Jacobs CW, Muller S, et al. Apoptosis-induced acetylation of histones is pathogenic in systemic lupus erythematosus. Arthritis Rheum. 2007;56(6):1921–33. doi: 10.1002/art.22646. [DOI] [PubMed] [Google Scholar]
- 118.Plaue S, Muller S, van Regenmortel MH. A branched, synthetic octapeptide of ubiquitinated histone H2A as target of autoantibodies. J Exp Med. 1989;169(5):1607–17. doi: 10.1084/jem.169.5.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.van Bavel CC, Dieker J, Muller S, Briand JP, Monestier M, Berden JH, et al. Apoptosis-associated acetylation on histone H2B is an epitope for lupus autoantibodies. Mol Immunol. 2009;47(2-3):511–6. doi: 10.1016/j.molimm.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 120.van Bavel CC, Dieker JW, Kroeze Y, Tamboer WP, Voll R, Muller S, et al. Apoptosis-induced histone H3 methylation is targeted by autoantibodies in systemic lupus erythematosus. Ann Rheum Dis. 2011;70(1):201–7. doi: 10.1136/ard.2010.129320. [DOI] [PubMed] [Google Scholar]
- 121.Stockl F, Muller S, Batsford S, Schmiedeke T, Waldherr R, Andrassy K, et al. A role for histones and ubiquitin in lupus nephritis? Clin Nephrol. 1994;41(1):10–7. [PubMed] [Google Scholar]
- 122.Leung YT, Shi L, Maurer K, Song L, Zhang Z, Petri M, et al. Interferon regulatory factor 1 and histone H4 acetylation in systemic lupus erythematosus. Epigenetics. 2015;10(3):191–9. doi: 10.1080/15592294.2015.1009764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Radic M, Marion T, Monestier M. Nucleosomes are exposed at the cell surface in apoptosis. J Immunol. 2004;172(11):6692–700. doi: 10.4049/jimmunol.172.11.6692. [DOI] [PubMed] [Google Scholar]
- 124.Rosen A, Casciola-Rosen L. Autoantigens as substrates for apoptotic proteases: implications for the pathogenesis of systemic autoimmune disease. Cell Death Differ. 1999;6(1):6–12. doi: 10.1038/sj.cdd.4400460. [DOI] [PubMed] [Google Scholar]
- 125.Dieker J, Hilbrands L, Thielen A, Dijkman H, Berden JH, van der Vlag J. Enhanced activation of dendritic cells by autologous apoptotic microvesicles in MRL/lpr mice. Arthritis research & therapy. 2015;17:103. doi: 10.1186/s13075-015-0617-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dieker J, Tel J, Pieterse E, Thielen A, Rother N, Bakker M, et al. Circulating Apoptotic Microparticles in Systemic Lupus Erythematosus Patients Drive the Activation of Dendritic Cell Subsets and Prime Neutrophils for NETosis. Arthritis Rheumatol. 2016;68(2):462–72. doi: 10.1002/art.39417. [DOI] [PubMed] [Google Scholar]
- 127.Sisirak V, Sally B, D'Agati V, Martinez-Ortiz W, Ozcakar ZB, David J, et al. Digestion of Chromatin in Apoptotic Cell Microparticles Prevents Autoimmunity. Cell. 2016;166(1):88–101. doi: 10.1016/j.cell.2016.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Al-Mayouf SM, Sunker A, Abdwani R, Abrawi SA, Almurshedi F, Alhashmi N, et al. Loss-of-function variant in DNASE1L3 causes a familial form of systemic lupus erythematosus. Nat Genet. 2011;43(12):1186–8. doi: 10.1038/ng.975. [DOI] [PubMed] [Google Scholar]
- 129.Mayes MD, Bossini-Castillo L, Gorlova O, Martin JE, Zhou X, Chen WV, et al. Immunochip analysis identifies multiple susceptibility loci for systemic sclerosis. Am J Hum Genet. 2014;94(1):47–61. doi: 10.1016/j.ajhg.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ozcakar ZB, Foster J, 2nd, Diaz-Horta O, Kasapcopur O, Fan YS, Yalcinkaya F, et al. DNASE1L3 mutations in hypocomplementemic urticarial vasculitis syndrome. Arthritis Rheum. 2013;65(8):2183–9. doi: 10.1002/art.38010. [DOI] [PubMed] [Google Scholar]
- 131.Zochling J, Newell F, Charlesworth JC, Leo P, Stankovich J, Cortes A, et al. An Immunochip-based interrogation of scleroderma susceptibility variants identifies a novel association at DNASE1L3. Arthritis research & therapy. 2014;16(5):438. doi: 10.1186/s13075-014-0438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Krysko DV, Denecker G, Festjens N, Gabriels S, Parthoens E, D'Herde K, et al. Macrophages use different internalization mechanisms to clear apoptotic and necrotic cells. Cell Death Differ. 2006;13(12):2011–22. doi: 10.1038/sj.cdd.4401900. [DOI] [PubMed] [Google Scholar]
- 133.Vanden Berghe T, Vanlangenakker N, Parthoens E, Deckers W, Devos M, Festjens N, et al. Necroptosis, necrosis and secondary necrosis converge on similar cellular disintegration features. Cell Death Differ. 2010;17(6):922–30. doi: 10.1038/cdd.2009.184. [DOI] [PubMed] [Google Scholar]
- 134.Cande C, Cohen I, Daugas E, Ravagnan L, Larochette N, Zamzami N, et al. Apoptosis-inducing factor (AIF): a novel caspase-independent death effector released from mitochondria. Biochimie. 2002;84(2-3):215–22. doi: 10.1016/s0300-9084(02)01374-3. [DOI] [PubMed] [Google Scholar]
- 135.Chautan M, Chazal G, Cecconi F, Gruss P, Golstein P. Interdigital cell death can occur through a necrotic and caspase-independent pathway. Curr Biol. 1999;9(17):967–70. doi: 10.1016/s0960-9822(99)80425-4. [DOI] [PubMed] [Google Scholar]
- 136.Degenhardt K, Mathew R, Beaudoin B, Bray K, Anderson D, Chen G, et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell. 2006;10(1):51–64. doi: 10.1016/j.ccr.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Golstein P, Kroemer G. Redundant cell death mechanisms as relics and backups. Cell Death Differ. 2005;12(Suppl 2):1490–6. doi: 10.1038/sj.cdd.4401607. [DOI] [PubMed] [Google Scholar]
- 138.Shimizu S, Kanaseki T, Mizushima N, Mizuta T, Arakawa-Kobayashi S, Thompson CB, et al. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat Cell Biol. 2004;6(12):1221–8. doi: 10.1038/ncb1192. [DOI] [PubMed] [Google Scholar]
- 139.Cavassani KA, Ishii M, Wen H, Schaller MA, Lincoln PM, Lukacs NW, et al. TLR3 is an endogenous sensor of tissue necrosis during acute inflammatory events. J Exp Med. 2008;205(11):2609–21. doi: 10.1084/jem.20081370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, et al. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15(11):1318–21. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Krysko DV, Brouckaert G, Kalai M, Vandenabeele P, D'Herde K. Mechanisms of internalization of apoptotic and necrotic L929 cells by a macrophage cell line studied by electron microscopy. J Morphol. 2003;258(3):336–45. doi: 10.1002/jmor.10161. [DOI] [PubMed] [Google Scholar]
- 142.Brouckaert G, Kalai M, Krysko DV, Saelens X, Vercammen D, Ndlovu MN, et al. Phagocytosis of necrotic cells by macrophages is phosphatidylserine dependent and does not induce inflammatory cytokine production. Mol Biol Cell. 2004;15(3):1089–100. doi: 10.1091/mbc.E03-09-0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hirt UA, Leist M. Rapid, noninflammatory and PS-dependent phagocytic clearance of necrotic cells. Cell Death Differ. 2003;10(10):1156–64. doi: 10.1038/sj.cdd.4401286. [DOI] [PubMed] [Google Scholar]
- 144.Krysko O, De Ridder L, Cornelissen M. Phosphatidylserine exposure during early primary necrosis (oncosis) in JB6 cells as evidenced by immunogold labeling technique. Apoptosis. 2004;9(4):495–500. doi: 10.1023/B:APPT.0000031452.75162.75. [DOI] [PubMed] [Google Scholar]
- 145.Breathnach SM, Kofler H, Sepp N, Ashworth J, Woodrow D, Pepys MB, et al. Serum amyloid P component binds to cell nuclei in vitro and to in vivo deposits of extracellular chromatin in systemic lupus erythematosus. J Exp Med. 1989;170(4):1433–8. doi: 10.1084/jem.170.4.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gaipl US, Kuenkele S, Voll RE, Beyer TD, Kolowos W, Heyder P, et al. Complement binding is an early feature of necrotic and a rather late event during apoptotic cell death. Cell Death Differ. 2001;8(4):327–34. doi: 10.1038/sj.cdd.4400826. [DOI] [PubMed] [Google Scholar]
- 147.Hack CE, Wolbink GJ, Schalkwijk C, Speijer H, Hermens WT, van den Bosch H. A role for secretory phospholipase A2 and C-reactive protein in the removal of injured cells. Immunol Today. 1997;18(3):111–5. doi: 10.1016/s0167-5699(97)01002-5. [DOI] [PubMed] [Google Scholar]
- 148.Degterev A, Hitomi J, Germscheid M, Ch'en IL, Korkina O, Teng X, et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4(5):313–21. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Feng S, Yang Y, Mei Y, Ma L, Zhu DE, Hoti N, et al. Cleavage of RIP3 inactivates its caspase-independent apoptosis pathway by removal of kinase domain. Cell Signal. 2007;19(10):2056–67. doi: 10.1016/j.cellsig.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 150.Barkla DH, Gibson PR. The fate of epithelial cells in the human large intestine. Pathology. 1999;31(3):230–8. doi: 10.1080/003130299105043. [DOI] [PubMed] [Google Scholar]
- 151.Roach HI, Clarke NM. Physiological cell death of chondrocytes in vivo is not confined to apoptosis. New observations on the mammalian growth plate. J Bone Joint Surg Br. 2000;82(4):601–13. doi: 10.1302/0301-620x.82b4.9846. [DOI] [PubMed] [Google Scholar]
- 152.Bell DA, Morrison B. The spontaneous apoptotic cell death of normal human lymphocytes in vitro: the release of, and immunoproliferative response to, nucleosomes in vitro. Clin Immunol Immunopathol. 1991;60(1):13–26. doi: 10.1016/0090-1229(91)90108-m. [DOI] [PubMed] [Google Scholar]
- 153.Souliotis VL, Sfikakis PP. Increased DNA double-strand breaks and enhanced apoptosis in patients with lupus nephritis. Lupus. 2015;24(8):804–15. doi: 10.1177/0961203314565413. [DOI] [PubMed] [Google Scholar]
- 154.Makino H, Hayashi Y, Yamasaki Y, Shikata K, Kashihara N, Kira S, et al. Clinical significance of necrosis in lupus nephritis. Intern Med. 1994;33(8):461–5. doi: 10.2169/internalmedicine.33.461. [DOI] [PubMed] [Google Scholar]
- 155.Decker P, Wolburg H, Rammensee HG. Nucleosomes induce lymphocyte necrosis. Eur J Immunol. 2003;33(7):1978–87. doi: 10.1002/eji.200323703. [DOI] [PubMed] [Google Scholar]
- 156.Ogden CA, deCathelineau A, Hoffmann PR, Bratton D, Ghebrehiwet B, Fadok VA, et al. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J Exp Med. 2001;194(6):781–95. doi: 10.1084/jem.194.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Napirei M, Karsunky H, Zevnik B, Stephan H, Mannherz HG, Moroy T. Features of systemic lupus erythematosus in Dnase1-deficient mice. Nat Genet. 2000;25(2):177–81. doi: 10.1038/76032. [DOI] [PubMed] [Google Scholar]
- 158.Skiljevic D, Jeremic I, Nikolic M, Andrejevic S, Sefik-Bukilica M, Stojimirovic B, et al. Serum DNase I activity in systemic lupus erythematosus: correlation with immunoserological markers, the disease activity and organ involvement. Clin Chem Lab Med. 2013;51(5):1083–91. doi: 10.1515/cclm-2012-0521. [DOI] [PubMed] [Google Scholar]
- 159.Kaufmann SH, Desnoyers S, Ottaviano Y, Davidson NE, Poirier GG. Specific proteolytic cleavage of poly(ADP-ribose) polymerase: an early marker of chemotherapy-induced apoptosis. Cancer Res. 1993;53(17):3976–85. [PubMed] [Google Scholar]
- 160.Casiano CA, Ochs RL, Tan EM. Distinct cleavage products of nuclear proteins in apoptosis and necrosis revealed by autoantibody probes. Cell Death Differ. 1998;5(2):183–90. doi: 10.1038/sj.cdd.4400336. [DOI] [PubMed] [Google Scholar]
- 161.Kanai Y, Kawaminami Y, Miwa M, Matsushima T, Sugimura T. Naturally-occurring antibodies to poly(ADP-ribose) in patients with systemic lupus erythematosus. Nature. 1977;265(5590):175–7. doi: 10.1038/265175a0. [DOI] [PubMed] [Google Scholar]
- 162.Okolie EE, Shall S. The significance of antibodies to poly(adenosine diphosphate-ribose) in systemic lupus erythematosus. Clin Exp Immunol. 1979;36(1):151–64. [PMC free article] [PubMed] [Google Scholar]
- 163.Decker P, Isenberg D, Muller S. Inhibition of caspase-3-mediated poly(ADP-ribose) polymerase (PARP) apoptotic cleavage by human PARP autoantibodies and effect on cells undergoing apoptosis. J Biol Chem. 2000;275(12):9043–6. doi: 10.1074/jbc.275.12.9043. [DOI] [PubMed] [Google Scholar]
- 164.Knight JS, Carmona-Rivera C, Kaplan MJ. Proteins derived from neutrophil extracellular traps may serve as self-antigens and mediate organ damage in autoimmune diseases. Front Immunol. 2012;3:380. doi: 10.3389/fimmu.2012.00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–5. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 166.Caudrillier A, Kessenbrock K, Gilliss BM, Nguyen JX, Marques MB, Monestier M, et al. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J Clin Invest. 2012;122(7):2661–71. doi: 10.1172/JCI61303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Douda DN, Khan MA, Grasemann H, Palaniyar N. SK3 channel and mitochondrial ROS mediate NADPH oxidase-independent NETosis induced by calcium influx. Proc Natl Acad Sci U S A. 2015;112(9):2817–22. doi: 10.1073/pnas.1414055112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gupta AK, Hasler P, Holzgreve W, Gebhardt S, Hahn S. Induction of neutrophil extracellular DNA lattices by placental microparticles and IL-8 and their presence in preeclampsia. Hum Immunol. 2005;66(11):1146–54. doi: 10.1016/j.humimm.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 169.Gupta AK, Joshi MB, Philippova M, Erne P, Hasler P, Hahn S, et al. Activated endothelial cells induce neutrophil extracellular traps and are susceptible to NETosis-mediated cell death. FEBS Lett. 2010;584(14):3193–7. doi: 10.1016/j.febslet.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 170.Gupta S, Kaplan MJ. The role of neutrophils and NETosis in autoimmune and renal diseases. Nat Rev Nephrol. 2016 doi: 10.1038/nrneph.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kahlenberg JM, Carmona-Rivera C, Smith CK, Kaplan MJ. Neutrophil extracellular trap-associated protein activation of the NLRP3 inflammasome is enhanced in lupus macrophages. J Immunol. 2013;190(3):1217–26. doi: 10.4049/jimmunol.1202388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS, et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med. 2013;5(178):178ra40. doi: 10.1126/scitranslmed.3005580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mitroulis I, Kambas K, Chrysanthopoulou A, Skendros P, Apostolidou E, Kourtzelis I, et al. Neutrophil extracellular trap formation is associated with IL-1beta and autophagy-related signaling in gout. PLoS One. 2011;6(12):e29318. doi: 10.1371/journal.pone.0029318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol. 2010;191(3):677–91. doi: 10.1083/jcb.201006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Villanueva E, Yalavarthi S, Berthier CC, Hodgin JB, Khandpur R, Lin AM, et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol. 2011;187(1):538–52. doi: 10.4049/jimmunol.1100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Palmer LJ, Cooper PR, Ling MR, Wright HJ, Huissoon A, Chapple IL. Hypochlorous acid regulates neutrophil extracellular trap release in humans. Clin Exp Immunol. 2012;167(2):261–8. doi: 10.1111/j.1365-2249.2011.04518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Metzler KD, Goosmann C, Lubojemska A, Zychlinsky A, Papayannopoulos V. A myeloperoxidase-containing complex regulates neutrophil elastase release and actin dynamics during NETosis. Cell Rep. 2014;8(3):883–96. doi: 10.1016/j.celrep.2014.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Li P, Li M, Lindberg MR, Kennett MJ, Xiong N, Wang Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J Exp Med. 2010;207(9):1853–62. doi: 10.1084/jem.20100239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wang Y, Li M, Stadler S, Correll S, Li P, Wang D, et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol. 2009;184(2):205–13. doi: 10.1083/jcb.200806072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176(2):231–41. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lood C, Blanco LP, Purmalek MM, Carmona-Rivera C, De Ravin SS, Smith CK, et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat Med. 2016;22(2):146–53. doi: 10.1038/nm.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Garcia-Romo GS, Caielli S, Vega B, Connolly J, Allantaz F, Xu Z, et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med. 2011;3(73):73ra20. doi: 10.1126/scitranslmed.3001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Farrera C, Fadeel B. Macrophage clearance of neutrophil extracellular traps is a silent process. J Immunol. 2013;191(5):2647–56. doi: 10.4049/jimmunol.1300436. [DOI] [PubMed] [Google Scholar]
- 184.Hakkim A, Furnrohr BG, Amann K, Laube B, Abed UA, Brinkmann V, et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci U S A. 2010;107(21):9813–8. doi: 10.1073/pnas.0909927107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Saffarzadeh M, Preissner KT. Fighting against the dark side of neutrophil extracellular traps in disease: manoeuvres for host protection. Curr Opin Hematol. 2013;20(1):3–9. doi: 10.1097/MOH.0b013e32835a0025. [DOI] [PubMed] [Google Scholar]
- 186.Gao X, Hao S, Yan H, Ding W, Li K, Li J. Neutrophil extracellular traps contribute to the intestine damage in endotoxemic rats. J Surg Res. 2015;195(1):211–8. doi: 10.1016/j.jss.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 187.Lin AM, Rubin CJ, Khandpur R, Wang JY, Riblett M, Yalavarthi S, et al. Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. J Immunol. 2011;187(1):490–500. doi: 10.4049/jimmunol.1100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Megens RT, Vijayan S, Lievens D, Doring Y, van Zandvoort MA, Grommes J, et al. Presence of luminal neutrophil extracellular traps in atherosclerosis. Thromb Haemost. 2012;107(3):597–8. doi: 10.1160/TH11-09-0650. [DOI] [PubMed] [Google Scholar]
- 189.Wong SL, Demers M, Martinod K, Gallant M, Wang Y, Goldfine AB, et al. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat Med. 2015;21(7):815–9. doi: 10.1038/nm.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Knight JS, Subramanian V, O'Dell AA, Yalavarthi S, Zhao W, Smith CK, et al. Peptidylarginine deiminase inhibition disrupts NET formation and protects against kidney, skin and vascular disease in lupus-prone MRL/lpr mice. Ann Rheum Dis. 2015;74(12):2199–206. doi: 10.1136/annrheumdis-2014-205365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J, et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med. 2011;3(73):73ra19. doi: 10.1126/scitranslmed.3001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bennett L, Palucka AK, Arce E, Cantrell V, Borvak J, Banchereau J, et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197(6):711–23. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hacbarth E, Kajdacsy-Balla A. Low density neutrophils in patients with systemic lupus erythematosus, rheumatoid arthritis, and acute rheumatic fever. Arthritis Rheum. 1986;29(11):1334–42. doi: 10.1002/art.1780291105. [DOI] [PubMed] [Google Scholar]
- 194.Knight JS, Zhao W, Luo W, Subramanian V, O'Dell AA, Yalavarthi S, et al. Peptidylarginine deiminase inhibition is immunomodulatory and vasculoprotective in murine lupus. J Clin Invest. 2013;123(7):2981–93. doi: 10.1172/JCI67390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Leffler J, Martin M, Gullstrand B, Tyden H, Lood C, Truedsson L, et al. Neutrophil extracellular traps that are not degraded in systemic lupus erythematosus activate complement exacerbating the disease. J Immunol. 2012;188(7):3522–31. doi: 10.4049/jimmunol.1102404. [DOI] [PubMed] [Google Scholar]
- 196.Bodano A, Gonzalez A, Ferreiros-Vidal I, Balada E, Ordi J, Carreira P, et al. Association of a non-synonymous single-nucleotide polymorphism of DNASEI with SLE susceptibility. Rheumatology (Oxford) 2006;45(7):819–23. doi: 10.1093/rheumatology/kel019. [DOI] [PubMed] [Google Scholar]
- 197.Emlen W, Ansari R, Burdick G. DNA-anti-DNA immune complexes. Antibody protection of a discrete DNA fragment from DNase digestion in vitro. J Clin Invest. 1984;74(1):185–90. doi: 10.1172/JCI111400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Puccetti A, Madaio MP, Bellese G, Migliorini P. Anti-DNA antibodies bind to DNase I. J Exp Med. 1995;181(5):1797–804. doi: 10.1084/jem.181.5.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Shin HD, Park BL, Kim LH, Lee HS, Kim TY, Bae SC. Common DNase I polymorphism associated with autoantibody production among systemic lupus erythematosus patients. Hum Mol Genet. 2004;13(20):2343–50. doi: 10.1093/hmg/ddh275. [DOI] [PubMed] [Google Scholar]
- 200.Yasutomo K, Horiuchi T, Kagami S, Tsukamoto H, Hashimura C, Urushihara M, et al. Mutation of DNASE1 in people with systemic lupus erythematosus. Nat Genet. 2001;28(4):313–4. doi: 10.1038/91070. [DOI] [PubMed] [Google Scholar]
- 201.Yeh TM, Chang HC, Liang CC, Wu JJ, Liu MF. Deoxyribonuclease-inhibitory antibodies in systemic lupus erythematosus. J Biomed Sci. 2003;10(5):544–51. doi: 10.1159/000072382. [DOI] [PubMed] [Google Scholar]
- 202.Davis JC, Jr., Manzi S, Yarboro C, Rairie J, McInnes I, Averthelyi D, et al. Recombinant human Dnase I (rhDNase) in patients with lupus nephritis. Lupus. 1999;8(1):68–76. doi: 10.1191/096120399678847380. [DOI] [PubMed] [Google Scholar]
- 203.Pieterse E, Hofstra J, Berden J, Herrmann M, Dieker J, van der Vlag J. Acetylated histones contribute to the immunostimulatory potential of neutrophil extracellular traps in systemic lupus erythematosus. Clin Exp Immunol. 2015;179(1):68–74. doi: 10.1111/cei.12359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Dwivedi N, Neeli I, Schall N, Wan H, Desiderio DM, Csernok E, et al. Deimination of linker histones links neutrophil extracellular trap release with autoantibodies in systemic autoimmunity. FASEB J. 2014;28(7):2840–51. doi: 10.1096/fj.13-247254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Nakashima K, Hagiwara T, Yamada M. Nuclear localization of peptidylarginine deiminase V and histone deimination in granulocytes. J Biol Chem. 2002;277(51):49562–8. doi: 10.1074/jbc.M208795200. [DOI] [PubMed] [Google Scholar]
- 206.Schett G, Smole J, Zimmermann C, Hiesberger H, Hoefler E, Fournel S, et al. The autoimmune response to chromatin antigens in systemic lupus erythematosus: autoantibodies against histone H1 are a highly specific marker for SLE associated with increased disease activity. Lupus. 2002;11(11):704–15. doi: 10.1191/0961203302lu247oa. [DOI] [PubMed] [Google Scholar]
- 207.Kilsgard O, Andersson P, Malmsten M, Nordin SL, Linge HM, Eliasson M, et al. Peptidylarginine deiminases present in the airways during tobacco smoking and inflammation can citrullinate the host defense peptide LL-37, resulting in altered activities. Am J Respir Cell Mol Biol. 2012;46(2):240–8. doi: 10.1165/rcmb.2010-0500OC. [DOI] [PubMed] [Google Scholar]
- 208.Larrick JW, Hirata M, Balint RF, Lee J, Zhong J, Wright SC. Human CAP18: a novel antimicrobial lipopolysaccharide-binding protein. Infect Immun. 1995;63(4):1291–7. doi: 10.1128/iai.63.4.1291-1297.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Danza A, Ruiz-Irastorza G. Infection risk in systemic lupus erythematosus patients: susceptibility factors and preventive strategies. Lupus. 2013;22(12):1286–94. doi: 10.1177/0961203313493032. [DOI] [PubMed] [Google Scholar]
- 210.Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157(5):1013–22. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 211.Lee JK, Kim SH, Lewis EC, Azam T, Reznikov LL, Dinarello CA. Differences in signaling pathways by IL-1beta and IL-18. Proc Natl Acad Sci U S A. 2004;101(23):8815–20. doi: 10.1073/pnas.0402800101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Xu J, Jiang Y, Wang J, Shi X, Liu Q, Liu Z, et al. Macrophage endocytosis of high-mobility group box 1 triggers pyroptosis. Cell Death Differ. 2014;21(8):1229–39. doi: 10.1038/cdd.2014.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Tian J, Avalos AM, Mao SY, Chen B, Senthil K, Wu H, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8(5):487–96. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 214.Urbonaviciute V, Furnrohr BG, Meister S, Munoz L, Heyder P, De Marchis F, et al. Induction of inflammatory and immune responses by HMGB1-nucleosome complexes: implications for the pathogenesis of SLE. J Exp Med. 2008;205(13):3007–18. doi: 10.1084/jem.20081165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wahamaa H, Schierbeck H, Hreggvidsdottir HS, Palmblad K, Aveberger AC, Andersson U, et al. High mobility group box protein 1 in complex with lipopolysaccharide or IL-1 promotes an increased inflammatory phenotype in synovial fibroblasts. Arthritis research & therapy. 2011;13(4):R136. doi: 10.1186/ar3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Watson PR, Gautier AV, Paulin SM, Bland AP, Jones PW, Wallis TS. Salmonella enterica serovars Typhimurium and Dublin can lyse macrophages by a mechanism distinct from apoptosis. Infect Immun. 2000;68(6):3744–7. doi: 10.1128/iai.68.6.3744-3747.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zychlinsky A, Prevost MC, Sansonetti PJ. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358(6382):167–9. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]
- 218.Franklin BS, Bossaller L, De Nardo D, Ratter JM, Stutz A, Engels G, et al. The adaptor ASC has extracellular and 'prionoid' activities that propagate inflammation. Nat Immunol. 2014;15(8):727–37. doi: 10.1038/ni.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Wang Q, Imamura R, Motani K, Kushiyama H, Nagata S, Suda T. Pyroptotic cells externalize eat-me and release find-me signals and are efficiently engulfed by macrophages. Int Immunol. 2013;25(6):363–72. doi: 10.1093/intimm/dxs161. [DOI] [PubMed] [Google Scholar]
- 220.Chen K, Zhang J, Zhang W, Zhang J, Yang J, Li K, et al. ATP-P2X4 signaling mediates NLRP3 inflammasome activation: a novel pathway of diabetic nephropathy. Int J Biochem Cell Biol. 2013;45(5):932–43. doi: 10.1016/j.biocel.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 221.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464(7293):1357–61. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Feldmann J, Prieur AM, Quartier P, Berquin P, Certain S, Cortis E, et al. Chronic infantile neurological cutaneous and articular syndrome is caused by mutations in CIAS1, a gene highly expressed in polymorphonuclear cells and chondrocytes. Am J Hum Genet. 2002;71(1):198–203. doi: 10.1086/341357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9(8):857–65. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, et al. NLRP3 is activated in Alzheimer's disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493(7434):674–8. doi: 10.1038/nature11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat Genet. 2001;29(3):301–5. doi: 10.1038/ng756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Marneros AG. NLRP3 inflammasome blockade inhibits VEGF-A-induced age-related macular degeneration. Cell Rep. 2013;4(5):945–58. doi: 10.1016/j.celrep.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440(7081):237–41. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 228.Masters SL, Dunne A, Subramanian SL, Hull RL, Tannahill GM, Sharp FA, et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1beta in type 2 diabetes. Nat Immunol. 2010;11(10):897–904. doi: 10.1038/ni.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Tarallo V, Hirano Y, Gelfand BD, Dridi S, Kerur N, Kim Y, et al. DICER1 loss and Alu RNA induce age-related macular degeneration via the NLRP3 inflammasome and MyD88. Cell. 2012;149(4):847–59. doi: 10.1016/j.cell.2012.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17(2):179–88. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Yuan Y, Liu Z. Isoflurane attenuates murine lupus nephritis by inhibiting NLRP3 inflammasome activation. Int J Clin Exp Med. 2015;8(10):17730–8. [PMC free article] [PubMed] [Google Scholar]
- 232.Zhao J, Wang H, Dai C, Wang H, Zhang H, Huang Y, et al. P2X7 blockade attenuates murine lupus nephritis by inhibiting activation of the NLRP3/ASC/caspase 1 pathway. Arthritis Rheum. 2013;65(12):3176–85. doi: 10.1002/art.38174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kahlenberg JM, Yalavarthi S, Zhao W, Hodgin JB, Reed TJ, Tsuji NM, et al. An essential role of caspase 1 in the induction of murine lupus and its associated vascular damage. Arthritis Rheumatol. 2014;66(1):152–62. doi: 10.1002/art.38225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Pisitkun P, Ha HL, Wang H, Claudio E, Tivy CC, Zhou H, et al. Interleukin-17 cytokines are critical in development of fatal lupus glomerulonephritis. Immunity. 2012;37(6):1104–15. doi: 10.1016/j.immuni.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, et al. The P2X7 receptor: a key player in IL-1 processing and release. J Immunol. 2006;176(7):3877–83. doi: 10.4049/jimmunol.176.7.3877. [DOI] [PubMed] [Google Scholar]
- 236.Kahlenberg JM, Thacker SG, Berthier CC, Cohen CD, Kretzler M, Kaplan MJ. Inflammasome activation of IL-18 results in endothelial progenitor cell dysfunction in systemic lupus erythematosus. J Immunol. 2011;187(11):6143–56. doi: 10.4049/jimmunol.1101284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Sturfelt G, Roux-Lombard P, Wollheim FA, Dayer JM. Low levels of interleukin-1 receptor antagonist coincide with kidney involvement in systemic lupus erythematosus. Br J Rheumatol. 1997;36(12):1283–9. doi: 10.1093/rheumatology/36.12.1283. [DOI] [PubMed] [Google Scholar]
- 238.Migliorini P, Anzilotti C, Pratesi F, Quattroni P, Bargagna M, Dinarello CA, et al. Serum and urinary levels of IL-18 and its inhibitor IL-18BP in systemic lupus erythematosus. Eur Cytokine Netw. 2010;21(4):264–71. doi: 10.1684/ecn.2010.0210. [DOI] [PubMed] [Google Scholar]
- 239.Novick D, Elbirt D, Miller G, Dinarello CA, Rubinstein M, Sthoeger ZM. High circulating levels of free interleukin-18 in patients with active SLE in the presence of elevated levels of interleukin-18 binding protein. J Autoimmun. 2010;34(2):121–6. doi: 10.1016/j.jaut.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 240.Chen DY, Chen YM, Chen HH, Hsieh CW, Lin CC, Lan JL. Functional association of interleukin 18 gene -607 (C/A) promoter polymorphisms with disease course in Chinese patients with adult-onset Still's disease. J Rheumatol. 2009;36(10):2284–9. doi: 10.3899/jrheum.090316. [DOI] [PubMed] [Google Scholar]
- 241.Lin YJ, Wan L, Lee CC, Huang CM, Tsai Y, Tsai CH, et al. Disease association of the interleukin-18 promoter polymorphisms in Taiwan Chinese systemic lupus erythematosus patients. Genes Immun. 2007;8(4):302–7. doi: 10.1038/sj.gene.6364387. [DOI] [PubMed] [Google Scholar]
- 242.Sanchez E, Palomino-Morales RJ, Ortego-Centeno N, Jimenez-Alonso J, Gonzalez-Gay MA, Lopez-Nevot MA, et al. Identification of a new putative functional IL18 gene variant through an association study in systemic lupus erythematosus. Hum Mol Genet. 2009;18(19):3739–48. doi: 10.1093/hmg/ddp301. [DOI] [PubMed] [Google Scholar]
- 243.Warchol T, Lianeri M, Wudarski M, Lacki JK, Jagodzinski PP. IL-18 105 A>C polymorphism contributes to renal manifestations in patients with SLE. Rheumatol Int. 2009;30(2):187–91. doi: 10.1007/s00296-009-0934-3. [DOI] [PubMed] [Google Scholar]
- 244.Benoit ME, Clarke EV, Morgado P, Fraser DA, Tenner AJ. Complement protein C1q directs macrophage polarization and limits inflammasome activity during the uptake of apoptotic cells. J Immunol. 2012;188(11):5682–93. doi: 10.4049/jimmunol.1103760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Sanjuan MA, Green DR. Eating for good health: linking autophagy and phagocytosis in host defense. Autophagy. 2008;4(5):607–11. doi: 10.4161/auto.6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Yu L, Strandberg L, Lenardo MJ. The selectivity of autophagy and its role in cell death and survival. Autophagy. 2008;4(5):567–73. doi: 10.4161/auto.5902. [DOI] [PubMed] [Google Scholar]
- 247.Wang L, Law HK. The Role of Autophagy in Lupus Nephritis. Int J Mol Sci. 2015;16(10):25154–67. doi: 10.3390/ijms161025154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Tsujimoto Y, Shimizu S. Another way to die: autophagic programmed cell death. Cell Death Differ. 2005;12(Suppl 2):1528–34. doi: 10.1038/sj.cdd.4401777. [DOI] [PubMed] [Google Scholar]
- 249.Bejarano E, Cuervo AM. Chaperone-mediated autophagy. Proc Am Thorac Soc. 2010;7(1):29–39. doi: 10.1513/pats.200909-102JS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Mijaljica D, Prescott M, Devenish RJ. Microautophagy in mammalian cells: revisiting a 40-year-old conundrum. Autophagy. 2011;7(7):673–82. doi: 10.4161/auto.7.7.14733. [DOI] [PubMed] [Google Scholar]
- 251.Liu Y, Levine B. Autosis and autophagic cell death: the dark side of autophagy. Cell Death Differ. 2015;22(3):367–76. doi: 10.1038/cdd.2014.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Das G, Shravage BV, Baehrecke EH. Regulation and function of autophagy during cell survival and cell death. Cold Spring Harb Perspect Biol. 2012;4(6) doi: 10.1101/cshperspect.a008813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Elgendy M, Sheridan C, Brumatti G, Martin SJ. Oncogenic Ras-induced expression of Noxa and Beclin-1 promotes autophagic cell death and limits clonogenic survival. Mol Cell. 2011;42(1):23–35. doi: 10.1016/j.molcel.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 254.Pyo JO, Jang MH, Kwon YK, Lee HJ, Jun JI, Woo HN, et al. Essential roles of Atg5 and FADD in autophagic cell death: dissection of autophagic cell death into vacuole formation and cell death. J Biol Chem. 2005;280(21):20722–9. doi: 10.1074/jbc.M413934200. [DOI] [PubMed] [Google Scholar]
- 255.Sharma K, Le N, Alotaibi M, Gewirtz DA. Cytotoxic autophagy in cancer therapy. Int J Mol Sci. 2014;15(6):10034–51. doi: 10.3390/ijms150610034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Yu L, Lenardo MJ, Baehrecke EH. Autophagy and caspases: a new cell death program. Cell Cycle. 2004;3(9):1124–6. [PubMed] [Google Scholar]
- 257.Martinez J, Almendinger J, Oberst A, Ness R, Dillon CP, Fitzgerald P, et al. Microtubule-associated protein 1 light chain 3 alpha (LC3)-associated phagocytosis is required for the efficient clearance of dead cells. Proc Natl Acad Sci U S A. 2011;108(42):17396–401. doi: 10.1073/pnas.1113421108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Martinez J, Cunha LD, Park S, Yang M, Lu Q, Orchard R, et al. Noncanonical autophagy inhibits the autoinflammatory, lupus-like response to dying cells. Nature. 2016;533(7601):115–9. doi: 10.1038/nature17950. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 259.Gateva V, Sandling JK, Hom G, Taylor KE, Chung SA, Sun X, et al. A large-scale replication study identifies TNIP1, PRDM1, JAZF1, UHRF1BP1 and IL10 as risk loci for systemic lupus erythematosus. Nat Genet. 2009;41(11):1228–33. doi: 10.1038/ng.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.International Consortium for Systemic Lupus Erythematosus G. Harley JB, Alarcon-Riquelme ME, Criswell LA, Jacob CO, Kimberly RP, et al. Genome-wide association scan in women with systemic lupus erythematosus identifies susceptibility variants in ITGAM, PXK, KIAA1542 and other loci. Nat Genet. 2008;40(2):204–10. doi: 10.1038/ng.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Zhou XJ, Lu XL, Lv JC, Yang HZ, Qin LX, Zhao MH, et al. Genetic association of PRDM1-ATG5 intergenic region and autophagy with systemic lupus erythematosus in a Chinese population. Ann Rheum Dis. 2011;70(7):1330–7. doi: 10.1136/ard.2010.140111. [DOI] [PubMed] [Google Scholar]
- 262.Alessandri C, Barbati C, Vacirca D, Piscopo P, Confaloni A, Sanchez M, et al. T lymphocytes from patients with systemic lupus erythematosus are resistant to induction of autophagy. FASEB J. 2012;26(11):4722–32. doi: 10.1096/fj.12-206060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Barbati C, Alessandri C, Vomero M, Vona R, Colasanti T, Vacirca D, et al. Autoantibodies specific to D4GDI modulate Rho GTPase mediated cytoskeleton remodeling and induce autophagy in T lymphocytes. J Autoimmun. 2015;58:78–89. doi: 10.1016/j.jaut.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 264.Clarke AJ, Ellinghaus U, Cortini A, Stranks A, Simon AK, Botto M, et al. Autophagy is activated in systemic lupus erythematosus and required for plasmablast development. Ann Rheum Dis. 2015;74(5):912–20. doi: 10.1136/annrheumdis-2013-204343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Colasanti T, Vomero M, Alessandri C, Barbati C, Maselli A, Camperio C, et al. Role of alpha-synuclein in autophagy modulation of primary human T lymphocytes. Cell Death Dis. 2014;5:e1265. doi: 10.1038/cddis.2014.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Gros F, Arnold J, Page N, Decossas M, Korganow AS, Martin T, et al. Macroautophagy is deregulated in murine and human lupus T lymphocytes. Autophagy. 2012;8(7):1113–23. doi: 10.4161/auto.20275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Kalia LV, Kalia SK, McLean PJ, Lozano AM, Lang AE. alpha-Synuclein oligomers and clinical implications for Parkinson disease. Ann Neurol. 2013;73(2):155–69. doi: 10.1002/ana.23746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Vekrellis K, Xilouri M, Emmanouilidou E, Rideout HJ, Stefanis L. Pathological roles of alpha-synuclein in neurological disorders. Lancet Neurol. 2011;10(11):1015–25. doi: 10.1016/S1474-4422(11)70213-7. [DOI] [PubMed] [Google Scholar]
- 269.Rodriguez-Manzanet R, Sanjuan MA, Wu HY, Quintana FJ, Xiao S, Anderson AC, et al. T and B cell hyperactivity and autoimmunity associated with niche-specific defects in apoptotic body clearance in TIM-4-deficient mice. Proc Natl Acad Sci U S A. 2010;107(19):8706–11. doi: 10.1073/pnas.0910359107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Fullgrabe J, Heldring N, Hermanson O, Joseph B. Cracking the survival code: autophagy-related histone modifications. Autophagy. 2014;10(4):556–61. doi: 10.4161/auto.27280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Artal-Martinez de Narvajas A, Gomez TS, Zhang JS, Mann AO, Taoda Y, Gorman JA, et al. Epigenetic regulation of autophagy by the methyltransferase G9a. Mol Cell Biol. 2013;33(20):3983–93. doi: 10.1128/MCB.00813-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Chen H, Fan M, Pfeffer LM, Laribee RN. The histone H3 lysine 56 acetylation pathway is regulated by target of rapamycin (TOR) signaling and functions directly in ribosomal RNA biogenesis. Nucleic Acids Res. 2012;40(14):6534–46. doi: 10.1093/nar/gks345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Das C, Lucia MS, Hansen KC, Tyler JK. CBP/p300-mediated acetylation of histone H3 on lysine 56. Nature. 2009;459(7243):113–7. doi: 10.1038/nature07861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Fullgrabe J, Lynch-Day MA, Heldring N, Li W, Struijk RB, Ma Q, et al. The histone H4 lysine 16 acetyltransferase hMOF regulates the outcome of autophagy. Nature. 2013;500(7463):468–71. doi: 10.1038/nature12313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Jin Q, Yu LR, Wang L, Zhang Z, Kasper LH, Lee JE, et al. Distinct roles of GCN5/PCAF-mediated H3K9ac and CBP/p300-mediated H3K18/27ac in nuclear receptor transactivation. EMBO J. 2011;30(2):249–62. doi: 10.1038/emboj.2010.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Lee IH, Finkel T. Regulation of autophagy by the p300 acetyltransferase. J Biol Chem. 2009;284(10):6322–8. doi: 10.1074/jbc.M807135200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Sierra J, Yoshida T, Joazeiro CA, Jones KA. The APC tumor suppressor counteracts beta-catenin activation and H3K4 methylation at Wnt target genes. Genes Dev. 2006;20(5):586–600. doi: 10.1101/gad.1385806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Wend P, Fang L, Zhu Q, Schipper JH, Loddenkemper C, Kosel F, et al. Wnt/beta-catenin signalling induces MLL to create epigenetic changes in salivary gland tumours. EMBO J. 2013;32(14):1977–89. doi: 10.1038/emboj.2013.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Mishra N, Reilly CM, Brown DR, Ruiz P, Gilkeson GS. Histone deacetylase inhibitors modulate renal disease in the MRL-lpr/lpr mouse. J Clin Invest. 2003;111(4):539–52. doi: 10.1172/JCI16153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Reilly CM, Mishra N, Miller JM, Joshi D, Ruiz P, Richon VM, et al. Modulation of renal disease in MRL/lpr mice by suberoylanilide hydroxamic acid. J Immunol. 2004;173(6):4171–8. doi: 10.4049/jimmunol.173.6.4171. [DOI] [PubMed] [Google Scholar]
- 281.Reilly CM, Thomas M, Gogal R, Jr., Olgun S, Santo A, Sodhi R, et al. The histone deacetylase inhibitor trichostatin A upregulates regulatory T cells and modulates autoimmunity in NZB/W F1 mice. J Autoimmun. 2008;31(2):123–30. doi: 10.1016/j.jaut.2008.04.020. [DOI] [PubMed] [Google Scholar]