Abstract
Dodecafluoropentane emulsion (DDFPe), an advanced oxygen transport drug, given IV at 90-min intervals maintains viability in the penumbra during cerebral ischemia in the standard rabbit anterior stroke model (STND). This study investigated shortened dosage schedules of DDFPe in nonstandard posterior (NSTND) strokes following occlusions of the posterior cerebral arteries. DDFPe given at shortened schedules of 30 or 60-min injection intervals will reduce neurological deficits, percent stroke volume (%SV), and serum glutamate levels in NSTND ischemic strokes. New Zealand White rabbits (N = 26) were randomly placed into three groups: A (n = 9) controls given saline injections every 60 min, B (n = 9) 2 % DDFPe given IV every 30 min, and C (n = 8) DDFPe every 60 min. Injections began 1 h after embolization. Groups were subdivided into STND and NSTND based on angiographically verified embolization of the cerebral arteries. Neurological assessments and blood samples were done at 0.5–1-h intervals. Rabbits were euthanized at 7 h following embolization. Stained brain slices were measured for %SV. The 30 and 60-min subgroups did not differ and were combined as DDFPe-STND or DDFPe-NSTND groups. In the DDFPe-STND stroke group, the %SV, neurological assessment scores (NAS), and serum glutamate were decreased vs. STND controls (p = 0.0016, 0.008, and 0.016, respectively). In the DDFPe-NSTND stroke group, %SV, NAS, and serum glutamate did not differ statistically compared to NSTND controls (p = 0.82, 0.097, and 0.06, respectively). More frequent dosage schedules provided no additional improvement. In anterior strokes, DDFPe improves recovery but not in the more severe NSTND strokes.
Keywords: Stroke, DDFPe, Rabbit, Ischemia, Tissue plasminogen activator, Neurological assessment score
Introduction
The mortality and morbidity of stroke patients increase the longer that neurons are deprived of oxygen, hence the admonition “time is brain.” Excessive time is a major factor in poor outcomes for stroke patients. The current therapy involving administration of tissue plasminogen activator (tPA) must be initiated within 4.5 h from the onset of the vascular event [1]. Therefore, due to time and other contraindications, IV tPA intervention reaches less than 6 % of stroke patients, and it improves the outcomes in less than 20 % of those treated [2]. Intra-arterial interventions are also time limited and reach even fewer patients. This investigation builds upon previous studies testing the effectiveness of a neuroprotective perfluorocarbon, dodecafluoropentane emulsion (DDFPe). Administration of DDFPe has the potential for increasing the time window for intervention and reducing the debilitating outcomes that stroke patients often encounter [3, 4].
DDFPe benefits stroke patients by supplying oxygen to the penumbra, the salvageable brain tissue that receives dangerously low blood flow due to an occluded vessel, which is most likely sustained via collateral vessels. Perfluorocarbons (PFCs) are effective at solubilizing oxygen, and DDFPe has characteristics that allow it to absorb seven times more oxygen in vitro at human body temperature than others in its class [5]. Its short linear chain length translates into a higher CF3/CF2 ratio than other PFCs, which allows more electronegative pockets for gases to occupy. Its unique abilities are also due to its 29 °C boiling point. It expands into oxygen-rich minute bubbles at 37 °C in vitro, and it is likely that, although microbubbles may not be present due to intra-vascular pressure, a similar trend of DDFPe expansion and enhanced oxygen carrying capacity at 100 times is seen in vivo. Harnessing these traits, this nanodroplet emulsion can be used as a neuroprotectant by oxygenating brain tissue that larger 8 μm red blood cells are not able to reach due to vascular occlusion [5].
To study DDFPe’s neuroprotective capabilities, rabbit models have been chosen to mimic human ischemic stroke scenarios, and multiple studies standardized an angiographic procedure to induce an ischemic stroke [6–10]. Flow-driven plastic spheres injected into the internal carotid artery produce a permanent occlusion downstream in the anterior cerebral artery (ACA) or middle cerebral artery (MCA) yielding a standard (STND) occlusion. If the spheres travel into the posterior cerebral circulation (PCC) or produce a spasm, nonstandard (NSTND) and often severe strokes are produced. These NSTNDs are typically excluded from analysis. Stroke severity has been measured and assessed by variables such as percent stroke volume (%SV), determined by staining with 2, 3, 5-triphenyltetrazolium chloride, and by neurological assessment scores (NAS). In an earlier study, application of 0.3 ml/kg DDFPe in the rabbit stroke model preserved 80 % of the stroke volume compared to controls after 7 h [4]. In other DDFPe studies, different doses were tested, and neuroprotection was maintained for a full 24 h using just a 0.1 ml/kg dose administered every 90 min [3].
In this study, we are examining the effect DDFPe has on NSTND strokes due to PCC occlusion or a spasm. We hypothesize that DDFPe injection at intervals less than 90 min in the rabbit permanent occlusion model will decrease neurological deficits regardless of nonstandard occlusions.
Methods
Animal Preparation and Embolus Procedure
All animal procedures were approved by the Institutional Care and Use Committee. A more detailed description of experimental procedures has been published [11]. White New Zealand rabbits were used (4.1 ± 0.3 kg, 15 male and 11 female). Anesthesia was initiated by ketamine (30 mg/kg, im) and rompun (3 mg/kg, im) and maintained with mask ventilation using isoflurane at 1–2 %. Butterfly intravenous lines were inserted in an ear vein and artery. Anesthesia depth was determined by corneal reflex, response to paw pad pinch, and with the assistance of heart rate and ECG monitors. A 3F catheter was placed in the femoral artery and guided to the internal carotid artery for angiographic embolization with two (or three if necessary by angiography) insoluble microspheres (700–900 μm diameter in size) (Embosphere® Microspheres, BioSphere Medical, S.A., Roissy, France) (Fig. 1) [4, 11]. To induce an occlusion in the posterior cerebral arteries, we observed a delay of a few seconds before injection of the second microsphere that encouraged more frequent flow direction to the posterior vessels after the anterior vessels were occluded.
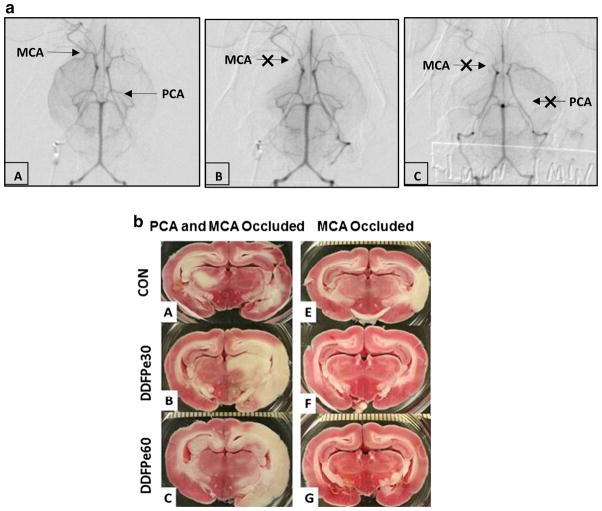
Fig. 1.
a Subtracted internal carotid angiogram basilar view. In panel A, selective injection of the right internal carotid artery (ICA) shows standard anatomy of the circle of Willis. The middle cerebral artery (MCA) and posterior cerebral artery (PCA) are patent. Panel B visualizes occlusion of the proximal right MCA following injection of microspheres. In panel C, occlusion of the proximal right MCA and left PCA is indicated by arrows. b. Representative TTC stained brain sections from animals with PCA and MCA (panels A–C) and MCA only (panels D–F) occlusions following saline control (CON) or DDFPe (DDFPe30 and DDFPe60) treatment
Treatment Groups
Rabbits (N = 26) were randomly placed in groups: A (n = 9) saline IV control, B (n = 9) DDFPe (0.3 ml/kg, 2 % w/v of dodecafluoropentane emulsion, NuvOx Pharma, Tucson, AZ) IV at 60-min intervals, or C (n = 8) DDFPe IV at 30-min intervals (Table 1). Injections, blood draws, and NAS testing began 1 h post embolization. Animals were euthanized at 7 h post embolization. After analysis of angiograms, rabbits in each group were categorized as STND or NSTND based on their occlusion site (Table 2). The NSTND designation was given to animals that had a posterior cerebral artery (PCA) occlusion including any other cerebral arteries.
Table 1.
Timetable design for interval dosing
| Groups | Animals (n) | Interval schedule (min)* | Treatment group | Total #of doses (n) |
|---|---|---|---|---|
| A | 9 | 60 | CON** | 6 |
| B | 9 | 60 | DDFPe | 6 |
| C | 8 | 30 | DDFPe | 12 |
| 26 |
At embolization (considered as 0 min), countdown would begin and the first injection given 60 min later. Continued series of injections would occur at either 60 or 30 min apart. The brains were to be removed 7 h after stroke induction
CON treatment group A to receive equivalent volume saline injections as groups B and C
Table 2.
After stroke induction, groups A, B, and C were subdivided by stroke location (STND or NSTND) and treatment (CON or DDFPe)
| Groups | (n) | Stroke location* | Treatment group |
|---|---|---|---|
| A | 4 | STND | CON |
| B + C** | 6 | STND | DDFPe |
| A | 5 | NSTND | CON |
| B + C** | 11 | NSTND | DDFPe |
| 26 |
Following stroke induction angiography was used to determine location of stroke area. Anterior or mid circulatory areas of the brain were classified as standard (STND) and posterior or brainstem areas were classified as nonstandard (NSTND)
Groups B and C were combined because of no statistical differences in the variables
Neurological Assessment Score
The NAS used in this study [7, 12] is a composite score comprised of tests of fine and large motor skills, sensory, balance, and reflex measures and utilizes the published wryneck test [13–16]. Scores range from 1 to 18, with scores greater than 5 indicating more severe neurological injury. Assessment was done at 1, 1.5, 3.5, 4.5, 5.5, and 6.5 h following embolization.
Blood Collection for Glutamate Levels
Blood samples were collected via the ear artery (2–3 ml) two times prior to surgery, immediately after occlusion and at 1, 3, and 5 h following embolization. The serum specimens were stored at −8 °C until analysis was performed using the glutamate ELISA kit by Creative Diagnostics® New York, USA.
Percent Infarct Volume Analysis
Following euthanization, the brain was removed, chilled in saline, and sectioned coronally at 4.0-mm intervals using a rabbit brain matrix device (RBM-7000C; ASI instruments Inc.; Warren, MI). The brain sections (n = 9 sections/rabbit brain) were incubated in 1 % 2,3,5-triphenyltetrazolium chloride (TTC) (Sigma-Aldrich: St. Louis, MO) for 35 min at 37 °C and then placed in formalin and digitally imaged and measured using the NIH Image J Software, Version 0.99i. Stroke volume was calculated as a percent of total brain volume [3, 4, 6, 11] by a technician blinded to treatment.
Statistics
Infarct %SV, NAS, and serum glutamate values were compared among experimental groups using the ANOVA test. Comparisons of STND and NSTND control and treatment groups were made using Fisher’s PLSD test for post hoc analysis. Statistical significance was considered to be p < 0.05.
Results
Angiography
Representative angiograms and stained brain sections are shown in Fig. 1a, b, respectively. An angiogram just prior to occlusion with labels on the MCA and PCA is illustrated in panel A of Fig. 1a. Occlusion of the MCA after injection of beads is shown in panel B and occlusion of both MCA and PCA in panel C. No arterial spasms were observed in angiograms of the 26 animals studied. A third sphere was required in one animal when the occlusion sites were not observed during angiography for a 3.8 % occurrence rate.
DDFPe Treatment Groups
Inspection of angiograms was used to classify rabbits as having a STND or NSTND stroke. Rabbits identified as having a STND stroke and treated with DDFPe at 30 or 60 min (n = 3 and 3, respectively) had no statistical differences in %SV, NAS, and serum glutamate levels. These two DDFPe treated STND stroke groups (B and C) were combined (Table 2) and referred to as the DDFPe-STND group (N = 6). Likewise, because there were no statistical differences in the %SV, NAS, or serum glutamate levels between the 30 and 60 min DDFPe-treated NSTND stroke animals (B and C) (n = 6 and 5, respectively), they were also combined as the DDFPe-NSTND group (n = 11). Also, control animals were separated into Con-STND and Con-NSTND (n = 4 and 5, respectively) groups (see Table 2).
Neurological Assessment Scores
NAS for the DDFPe-STND group (Fig. 2a) was improved compared to the Con-STND group (p < 0.0001). ANOVA showed difference beginning at 1.5 h and extending to 6.5 h at p ≤ 0.014. The DDFPe-STND’s NAS also differed from the DDFPe-NSTND group (p < 0.0001). In contrast, the DDFPe-NSTND group did not differ from controls, the Con-NSTND (p = NS) (Fig. 2a) and Con-STND (p = NS) groups.
Fig. 2.
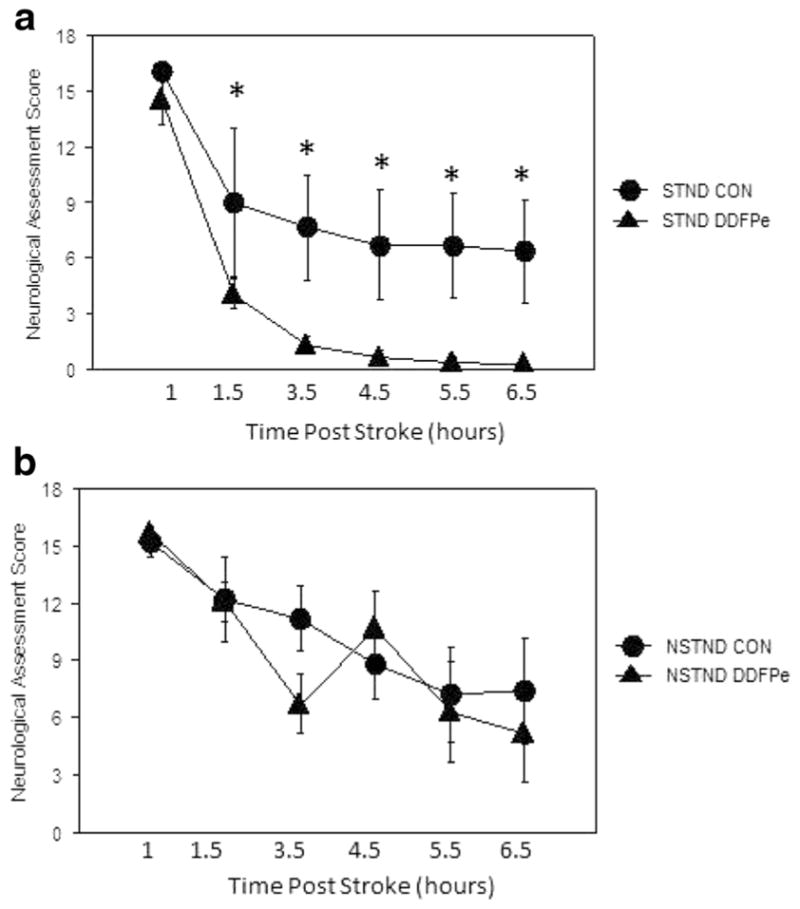
a, b Functional recovery as measured by neurological assessment scores (NAS) from animals with MCA (STND) occlusions and PCA (NSTND) occlusions. Assessment was made at 1, 1.5, 3.5, 4.5, 5.5, and 6.5 h following embolization. a Functional recovery was significantly improved in DDFPe-STND vs. Con-STND animals (p = 0.0063). Post hoc analysis showed significant differences at 1.5–6.5 h. b NAS measurements in NSTND-DDFPe vs. controls were not different at any time point. Mean ± SEM are shown. *Indicates significance at p ≤ 0.01 vs. DDFPe-STND
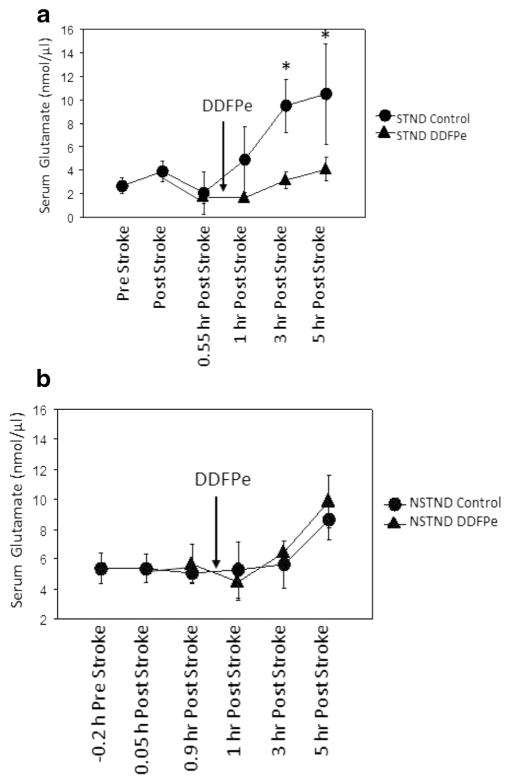
Glutamate Serum Levels
The serum glutamate level for the treated group DDFPe-STND was different from its control group Con-STND (p = 0.018), and ANOVA showed a difference at 3 and 5 h (p = 0.0087 and p = 0.0052, respectively) (Fig. 3a). Overall time points, the treated group DDFPe-STND was also different from the DDPFe-NSTND and Con-NSTND groups (p < 0.001 and p = 0.011, respectively). However, the treated NSTND group did not differ from its control, the Con-NSTND group (p = NS) (Fig. 3a).
Fig. 3.
a, b. Serum glutamate levels in STND and NSTND animals. A nonlinear line graph details serum collection at 0.2 h prior, 0.05, and 0.9 h post stroke. DDFPe was initiated at 1 h (arrow) and serum collected at 1, 3, and 5 h. a In the DDFPe-STND group, serum glutamate was statistically lower compared to Con-STND at 3 and 5 h (p = 0.0087 and p = 0.0052, respectively) and vs. both NSTND treatment groups (p ≤ 0.01). b DDFPe-NSTND was not different compared to Con-NSTND. Arrows indicate when DDFPe administration began. Mean ± SEM are shown. *Indicates significance vs. Con-STND at 3 and 5 h
Percent Stroke Volume
%SV for the DDFPe-STND group (Fig. 1a and Fig. 4) was improved compared to all other groups: DDFPe-NSTND (p = 0.001), Con-STND (p = 0.016), and Con-NSTND (p = 0.01) (Table 3). In contrast, the DDFPe-NSTND group did not differ from controls Con-NSTND (p = NS) and Con-STND (p = NS).
Fig. 4.
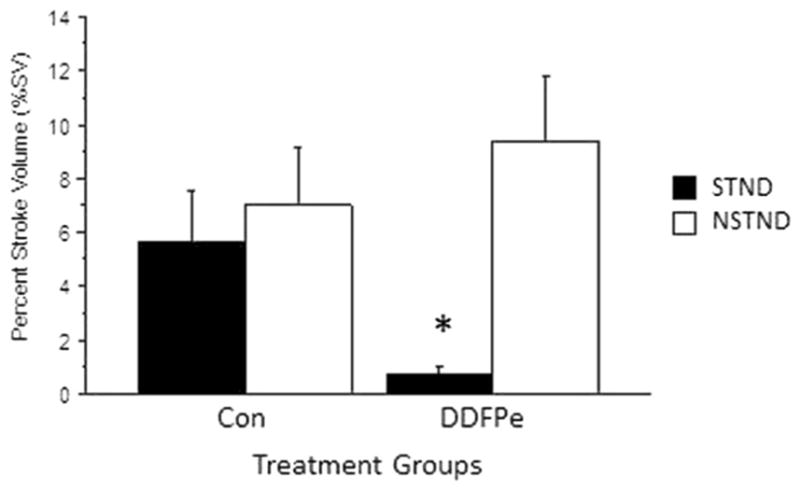
%SV for the DDFPe-STND group was improved compared to Con-STND (p = 0.016), DDFPe-NSTND (p = 0.001), and Con-NSTND (p = 0.01). In contrast, the DDFPe-NSTND group did not differ from controls Con-NSTND (p = NS) and Con-STND (p = NS). Mean ± SEM are shown. *Indicates significance at p ≤ 0.02 vs. other treatment groups
Table 3.
Effects of shortened 0.3 ml/kg DDFPe interval dosing on percent stroke volume (%SV) 7 h after stroke induction
| (n) | Stroke location | Treatment group | SV ± SEM (%) | p vs. STND DDFPe | p vs. NSTND DDFPe |
|---|---|---|---|---|---|
| 4 | STND | CON | 5.6 ± 2.0 | 0.016* | NS |
| 6 | STND | DDFPe | 0.7 ± 0.3 | – | 0.001* |
| 5 | NSTND | CON | 7.0 ± 2.2 | 0.01* | NS |
| 11 | NSTND | DDFPe | 9.4 ± 2.4 | 0.001* | – |
| 26 |
p ≤ 0.05 is significant. Mean ± SEM are presented
Discussion
Approximately 20 % of ischemic strokes involve the posterior circulation, yet these scenarios are underrepresented in experimental protocols [11, 17, 18]. Purposeful occlusion of the posterior circulation followed by treatment has been reported in a few studies [19, 20]. Instead, the standard MCA occlusion model is used more often (Fig. 1a) for reasons of mechanical reproducibility, reversibility, and appropriate modeling of human ischemic strokes. Previous studies with occlusions producing standard strokes have shown the efficacy of DDFPe in the anterior territory as a neuroprotectant in rabbits and rats [3, 4]. To determine the efficacy of DDFPe in NSTND strokes, rabbit models which include PCA occlusions were analyzed in this study. In addition to %SV and NAS, serum glutamate samples provided functional and cellular injury information during the first 6.5 h of recovery.
Following anterior strokes in the STND group, the NAS measurements provided continuous significant measurable improvements in recovery with DDFPe therapy (Fig. 2a). Previous findings in three rabbit embolic models showed a strong positive correlation between increased NAS score and %SV [11]. Embolic spheres, as used in the current and some previous studies rather than homologous clots, produced high NAS values of 3.2 ± 0.8 after 24 h of recovery which have been attributed to sphere insolubility [11, 12]. The NAS in the early acute period following stroke in STND controls in this study averaged higher at an NAS = 6. Therapy provided in the early recovery period provided continuous and measurable functional improvement.
The hypothesis of this study was that DDFPe treatment or increased frequency of treatment will reduce percent stroke volume (%SV), neurological deficits, and serum glutamate levels in NSTND strokes as it does with STND strokes. Remarkably, there was not a statistical difference in results between the 30 and 60-min groups of DDFPe treatment, and they were combined into subgroups STND and NSTND strokes. Both 30 and 60-min treatments began at 1 h after the initiation of embolism-induced ischemic stroke. Beginning DDFPe therapy at 1 h after stroke onset is an attainable goal for first responders in human patients [21]. Previously [3], DDFPe given at 1, 2, or 3 h post stroke event showed equally good efficacy for %SV reduction.
The dose of DDFPe was the same as previously reported, 0.3 ml/kg [3, 22], but the interval for administering DDFPe was either 30 or 60 min, shorter than the 90 min used in previous studies. We predicted that a shorter dosage interval would supply more oxygen and carry away more carbon dioxide and, thus, be beneficial in the NSTND stroke model. We found that the STND model responded in the same manner as when using the longer interval, i.e., the DDFPe-treated group was significantly improved compared to its control group in NAS, serum glutamate level, and %SV (Figs. 2–4). In contrast, the NSTND model of ischemic stroke did not respond to DDFPe treatment in an appreciable manner compared to its control group in any of the three metrics. It is possible that DDFPe might have provided a small benefit on NSTND strokes if the sample sizes were larger in the treatment groups.
Percent stroke volume and neurological deficit testing (NAS) are well recognized metrics of recovery following stroke. Another potential metric for determining stroke debilitation in the rabbit stroke model is serum glutamate. High serum glutamate levels have been associated with increased neuronal damage and are an indicator of severe cerebral ischemia [23, 24]. A clinical study found an increase in serum glutamate levels up to 40 % following stroke in patients at 6 h compared to nonstroke control patients [23]. Ischemic brain cells once depolarized fail to repolarize due to an insufficient energy supply and the uptake of glutamate decreases. This excess glutamate spills into the vascular system where circulating levels can be measured [23, 24]. Beginning at 3 h post embolism in this study STND controls showed glutamate elevation (Fig. 3a). Starting at this time, DDFPe treatment was shown to be effective in STND animals by maintaining a normal serum glutamate level which was decreased compared to controls. However, DDFPe treatment did not significantly affect serum glutamate levels in NSTND stroke model animals. Perhaps, this is due to a higher vulnerability of the brainstem to ischemia or a lack of collateral blood vessels from which DDFPe supplies oxygen to the ischemic region. Circulating glutamate levels have promise as a rapidly available biomarker for cerebral ischemia.
Limitations
The small sample size may be inadequate to reveal small differences in the NSTND rabbits. But major stroke reductions were well displayed in STND rabbits with these numbers.
Conclusion
DDFPe treatment for anterior strokes improves recovery and reduces stroke volume and peripheral markers of ischemia. More frequent DDFPe dosage schedules did not improve outcomes in the more severe posterior ischemic stroke model. Serum glutamate as a peripheral biomarker provided prompt information on early stroke damage and illustrated DDFPe therapeutic efficacy in STND strokes as did multiple NAS values. Considering that DDFPe maintains neuronal viability during embolic events in the anterior circulation, the possibility of extending the treatment window for reperfusion in human studies should be explored.
Acknowledgments
Sources of Funding
This project was supported by the Fund to Cure Stroke, a grant fund of the University of Arkansas for Medical Sciences Foundation (to author AB) and by the NIGMS IDeA Award P30 GM110702 (sponsoring author AB).
Footnotes
Compliance with Ethical Standards
All animal procedures were approved by the Institutional Care and Use Committee.
Conflict of Interest
A patent has been applied for the use of dodecafluoropentane for stroke therapy by authors WCC and RDS.
Author Contributions Statement
AB conceived the study and designed the data collection. AB obtained research funding. MCA, JL, KC, and AB collected the data. MCA, RDS, WCC, and AB provided statistical advice on study design and analyzed the data. MCA, KC, WCC, and AB drafted the manuscript, and all authors contributed to its revision.
References
- 1.del Zoppo G, Saver J, ECJ, et al. Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator. A science advisory from the American Heart Association/American Stroke Association. Stroke. 2009;40:2945–2948. doi: 10.1161/STROKEAHA.109.192535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circ. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 3.Woods SD, Skinner RD, Ricca AM, Brown AT, et al. Progress in dodecafluoropentane emulsion as a neuroprotective agent in a rabbit stroke model. Mol Neurobiol. 2013;48:363–367. doi: 10.1007/s12035-013-8495-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Culp WC, Woods SD, Skinner RD, Brown AT, et al. Dodecafluoropentane emulsion decreases infarct volume in a rabbit ischemic stroke model. JVIR. 2012;23:116–121. doi: 10.1016/j.jvir.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson JL, Dolezal MC, Kerschen A, Matsunaga TO, et al. In vitro comparison of dodecafluoropentane (DDFP) perfluorodecalin (PFD), and perfluoroctylbromide (PFOB) in the facilitation of oxygen exchange. Artif Cell Blood. 2009;37:156–162. doi: 10.1080/10731190903043192. [DOI] [PubMed] [Google Scholar]
- 6.Culp WC, Flores R, Brown AT, Lowery JD, et al. Successful microbubble sonothrombolysis without tissue plasminogen activator in a rabbit model of acute ischemic stroke. Stroke. 2011;42:2280–2285. doi: 10.1161/STROKEAHA.110.607150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown AT, Skinner RD, Flores R, Hennings L, et al. Stroke location and brain function in an embolic rabbit stroke model. JVIR. 2010;21:903–909. doi: 10.1016/j.jvir.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flores R, Lowery J, Skinner RD, Roberson PK, et al. Transcutaneous therapeutic ultrasound reduces infarct size in a rabbit model of acute insoluble ischemic stroke. J Exp Stroke Transl Med. 2011;4:1–7. SFES 1939-067X/10. [Google Scholar]
- 9.Brown AT, Flores R, Hamilton E, Roberson PK, et al. Microbubbles improve sonothrombolysis in vitro and decrease hemorrhage in vivo in a rabbit stroke model. Investig Radiol. 2011;46:202–207. doi: 10.1097/RLI.0b013e318200757a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flores R, Hennings LJ, Lowery JD, Brown AT, et al. Microbubble-augmented ultrasound sonothrombolysis decreases intracranial hemorrhage in a rabbit model of acute ischemic stroke. Investig Radiol. 2011;46:419–424. doi: 10.1097/RLI.0b013e31820e143a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Culp WC, Woods SD, Brown AT, Lowery JD, et al. Three variations in rabbit angiographic stroke models. J Neurosci Methods. 2012;212:322–328. doi: 10.1016/j.jneumeth.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown A, Woods S, Skinner R, Hatton J, et al. Neurological assessment scores in rabbit embolic stroke models. Open Neurol J. 2013;7:38–43. doi: 10.2174/1874205X01307010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao BQ, Suzuki Y, Kondo K, Kawano K, et al. Cerebral hemorrhage due to heparin limits its neuroprotective effects: studies in a rabbit model of photothrombotic middle cerebral artery occlusion. Brain Res. 2001;902:30–39. doi: 10.1016/s0006-8993(01)02285-5. [DOI] [PubMed] [Google Scholar]
- 14.Zhao BQ, Suzuki Y, Kondo K, Kawano K, et al. A novel MCA occlusion model of photothrombotic ischemia with cyclic flow reductions: development of cerebral hemorrhage induced by heparin. Brain Res Protocol. 2002;9:85–92. doi: 10.1016/s1385-299x(01)00124-6. [DOI] [PubMed] [Google Scholar]
- 15.Zhao BQ, Suzuki Y, Kondo K, Ikeda Y, et al. Combination of a free radical scavenger and heparin reduces cerebral hemorrhage after heparin treatment in a rabbit middle cerebral artery occlusion model. Stroke. 2001;32:2157–2163. doi: 10.1161/hs0901.095640. [DOI] [PubMed] [Google Scholar]
- 16.Chen ZZ, Jiang XD, Zhang LL, Shang JH, et al. Beneficial effect of autologous transplantation of bone marrow stromal cells and endothelial progenitor cells on cerebral ischemia in rabbits. Neurosci Lett. 2008;445:36–41. doi: 10.1016/j.neulet.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 17.Mehndiratta M, Pandey S, Nayak R, Alam A. Posterior circulation ischemic stroke—clinical characteristics, risk factors, and subtypes in a north Indian population—a prospective study. Neurohospitalist. 2012;2:46–50. doi: 10.1177/1941874412438902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dorňák T, Kral M, Hazlinger M, Herzog R, et al. Posterior vs. anterior circulation infarction: demography, outcomes, and frequency of hemorrhage after thrombolysis. Int J of Stroke. 2015;10:1224–1228. doi: 10.1111/ijs.12626. [DOI] [PubMed] [Google Scholar]
- 19.Boulos AS, Deshaies EM, Dalfino JC, Feustel PJ, et al. Tamoxifen as an effective neuroprotectant in an endovascular canine model of stroke. J Neurosurg. 2011;114:1117–1126. doi: 10.3171/2010.8.JNS09352. [DOI] [PubMed] [Google Scholar]
- 20.Ohishi H, Nishijima M, Ogawa A, Yoshimoto T, et al. Protective effect of mannitol in cerebral infarction—CT findings and physiological observation in experimental cerebral infarction in dogs. No Shinkei Geka. 1984;12:153–158. [PubMed] [Google Scholar]
- 21.Saver JL, Starkman S, Eckstein M, Stratton SJ, et al. Prehospital use of magnesium sulfate as neuroprotection in acute stroke. N Engl J Med. 2015;372:528–536. doi: 10.1056/NEJMoa1408827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Culp WC, Brown AT, Lowery JD, Arthur MC, et al. Dodecafluoropentane emulsion extends window for tPA therapy in a rabbit stroke model. Mol Neurobiol. 2015;52:979–984. doi: 10.1007/s12035-015-9243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aliprandi A, Longoni M, Stanzani L, Tremolizzo L, et al. Increased plasma glutamate in stroke patients might be linked to altered platelet release and uptake. J Cereb Blood Flow Metab. 2005;25:513–519. doi: 10.1038/sj.jcbfm.9600039. [DOI] [PubMed] [Google Scholar]
- 24.Godino M, Lizasoain I, Sanchez-Prieto J. Amelioration of ischemic brain damage by peritoneal dialysis. J Clin Invest. 2013;123:4359–4363. doi: 10.1172/JCI67284. [DOI] [PMC free article] [PubMed] [Google Scholar]