Abstract
Development of novel imaging probes for cancer diagnosis is critical for early disease detection and management. The past two decades have witnessed a surge in the development and evolution of radiolabeled nanoparticles as a new frontier in personalized cancer nanomedicine. The dynamic synergism of positron emission tomography (PET) and nanotechnology combines the sensitivity and quantitative nature of PET with the multifunctionality and tunability of nanomaterials, which can help overcome certain key challenges in the field. In this review, we discuss the recent advances in radionanomedicine, exemplifying the ability to tailor the physicochemical properties of nanomaterials to achieve optimal in vivo pharmacokinetics and targeted molecular imaging in living subjects. Innovations in development of facile and robust radiolabeling strategies and biomedical applications of such radionanoprobes in cancer theranostics are highlighted. Imminent issues in clinical translation of radiolabeled nanomaterials are also discussed, with emphasis on multidisciplinary efforts needed to quickly move these promising agents from bench to bedside.
Keywords: Positron Emission Tomography, Nanotechnology, Cancer Theranostics, Radiochemistry, Radiolabeled Nanoparticles
Graphical Abstract
1. Positron Emission Tomography in Molecular Imaging
Rapid development in the fields of cancer biology, genomics, proteomics and clinical oncology has revolutionized personalized cancer management through the integration of molecular and physiological information with the anatomic readouts obtained via conventional imaging modalities.[1] Defined as “non-invasive, real-time characterization and measurement of biological processes at the cellular and molecular level within living cells, tissues and intact subjects”, [2] molecular imaging promises enormous potential in the areas of diagnostics, therapy monitoring, drug discovery and development, and understanding nanoscale reactions such as protein-protein interactions and enzymatic conversion.[3, 4] Molecular imaging encompasses different modalities including optical bioluminescence, optical fluorescence (FL), targeted ultrasound, molecular magnetic resonance imaging (MRI) and spectroscopy (MRS), single-photon-emission computed tomography (SPECT), and positron emission tomography (PET).[1] The inherent strengths and limitations of each modality have spurred active development of multimodal systems, e.g. SPECT/CT, PET/CT, optical/CT and PET/MRI for synergistic imaging. Owing to their high detection sensitivity (10−11–10−12 M), quantifiability, limitless depth of penetration, as well as advances in radiotracer development, non-invasive nuclear imaging modalities, SPECT and PET offer tremendous opportunities in early lesion detection, patient screening and stratification, and individualized treatment monitoring and dose optimization.[4, 5]
Since its inception in 1970s,[6] PET has emerged as a clinical modality of choice for staging and restaging of a variety of malignancies. PET requires internal administration of tracer quantities (usually nanomolar) of a radiolabeled pharmaceutical, specific and selective for a target of interest. The fate of the radiolabeled agent in vivo is tracked with a camera which detects two coincident high energy gamma-rays (511 keV) emitted ~180° apart resulting from the annihilation of the emitted positron with a nearby electron.[7] While [18F]fluorodeoxyglucose (FDG) remains the most widely used PET tracer (>95% of all clinical PET scans), PET has adopted different positron emitting radionuclides including Copper-61/64(61/64Cu, t1/2: 3.3 h and 12.7 h), Gallium-66/68 (66/68Ga, t1/2: 9.5 and 1.1 h), Zirconium-89 (89Zr, t1/2: 78.4 h), and Iodine-124 (124I, t1/2: 100.2 h), among many others. FDG is a glucose analog that is selectively taken up by rapidly metabolizing cells, a hallmark of most malignancies, and has been clinically approved for staging of a number of cancers including breast, colorectal, esophageal, head and neck cancers, melanomas and lymphomas.[1] Since glucose metabolism is not specific only to cancer cells, imaging with FDG can be counter-productive in certain cases. This has fueled intense research for development of newer imaging agents such as antibodies and their fragments, small proteins and peptides and other biologically relevant entities, specifically targeted for a molecular event. Nanomaterials that combine different imaging modalities, targeting ligands and therapeutic moieties all in a single vector, have recently emerged as a new frontier in molecularly targeted probes.
2. Nanotechnology in Positron Emission Tomography: An Emerging Paradigm
The introduction of nanotechnology in nuclear imaging (mainly PET and SPECT) has generated much interest in the past decade.[8] Nanomaterials, typically smaller than a few hundred nanometers have emerged as forerunners in nanooncology, for targeted drug delivery, therapy and patient monitoring.[9] The biggest advantage that nanotechnology brings is to bridge the gap between the macroscopic and microscopic worlds, where nanomaterials prove to be the ideal medium for interfacing with the biological systems. Nanoparticles possess novel properties that distinguish them from bulk material: large functional surface area, easily controllable surface chemistry which facilitates binding to small molecule drugs, imaging labels and targeting ligands like antibodies, peptides, nucleic acids, etc. Moreover, their small size (~100– 10,000 times smaller than human cells) allows unique intracellular and extracellular interactions, such as extravasation through endothelial cells and enhanced permeability and retention (EPR) in tumor tissues.[4] Owing to the immense and unique possibilities it offers, nanotechnology has attracted significant investment from the National Institutes of Health (NIH)/National Cancer Institute (NCI) and some nanoparticles have also progressed into clinical trials.[10] For example, gold nanoparticles have found clinical applications towards head and neck cancer, as evidenced by recent completion of two first-in-human phase I clinical trials (AuNP-conjugated tissue necrosis factor (TNF) treatment in solid tumors [11] and AuNP-mediated hyperthermia for therapy of refractory and/or recurrent tumors of the head and neck).[10, 12] In addition, fluorescent silica nanoparticles (also known as C dots) are about to enter phase II clinical trials for lymph node mapping in head and neck melanoma, breast and cervical/uterine cancer patients.[13] Although much work is still required in understanding the long-term toxicities and optimal applications of nanomaterials in human subjects, these studies demonstrate the paradigm-shifting revolution that nanotechnology can bring in advancement of cancer prevention, diagnosis, treatment and management.
The role of nanotechnology in molecular imaging is four-fold. Nanoparticles can act as signal amplifiers, resulting in higher contrast indices and enhanced sensitivity. The large surface area can be functionalized with different targeting moieties, creating a multifunctional nanoplatform for targeted detection of different diseases. A big advantage of using nanoprobes over the traditional biological moieties is the competence for multimodality. Besides radiolabeling for PET, most nanoparticles possess intrinsic properties that can be easily harnessed for other molecular imaging modalities. For example radiolabeled iron oxide nanoparticles (IONPs) simultaneously signal for both MRI and PET; the generated data can potentially overcome the limitations of individual modality. The final advantage lies in the ability to combine both diagnostic and therapeutic capabilities onto the same vector, giving rise to the concept of theranostics.
The union of PET and nanotechnology represents a symbiotic relationship, promising mutual benefits for each. On one hand, the unique physicochemical properties and multivalency of nanomaterials promise unprecedented applications in molecular PET; noninvasive interpretation of biological events, synergistic multimodal imaging (such as PET/CT, PET/MR, and PET/US) and theranostics. On the other hand, PET has emerged as a formidable tool in the biomedical applications of nanomaterials for cancer theranostics. For example, it is widely known that the physicochemical properties of nanoparticles such as size, shape, surface charge and chemistry (PEGylation, ligand conjugation), and composition affect their in vivo biodistribution.[14, 15] Efforts are being undertaken to understand and optimize the factors influencing the pharmacokinetics, intratumoral penetration, tumor bioavailability, and cargo delivery mechanisms of nanoparticulate agents. In this regard, the sensitivity, whole body imaging capability, non-invasive and quantitative nature of PET makes it an excellent choice for accurately exploring the complex biological pathways and in vivo ADME (Absorption, Distribution, Metabolism and Excretion) profiles of nanoparticles, which have been the major roadblocks in clinical translation of nanomaterials. Secondly, PET has been exploited in image-guided therapy and drug delivery applications of nanomaterials, which allow real-time monitoring of the therapeutic outcomes.[16–18] Moreover, nanoparticles radiolabeled with positron emitters can potentially be employed in treatment prognosis and patient selection, aptly demonstrated in a recent study using 89Zr-labeled nanoreporter for Doxil.[19] Co-injection of the nanoreporter with the clinically approved anti-cancer nanodrug Doxil was able to predict the therapeutic outcomes based on the tumor uptake in a murine breast cancer model. While still in preclinical settings, such systems hold promise for clinically relevant cancer management in the future.
3. Development of Radiolabeled Nanoparticles
The radiostability of the isotope: nanoparticle complex is integral to the correct interpretation of in vivo biodistribution data, and thus, development of radiolabeled nanoplatforms is a non-trivial issue and warrants careful thought. Successful design of a radiolabeled nanoprobe involves rational selection of the isotope, the radiolabeling strategy and the nanoplatform; carefully tuned to achieve the highest radiochemical yields and stability, as well as optimal in vivo biodistribution and imaging contrast. Selection of an appropriate radioisotope depends on its imaging characteristics, decay half-life, chelation chemistry and availability. Ideally, isotopes with low positron energy and high β+ branching ratio are favorable for PET imaging. Based on their half-lives, positron emitting isotopes can be either short-lived (e.g. 11C; t1/2 = 20 min, 15O; t1/2 = 2 min, 18F; t1/2 = 109.7min, 68Ga; t1/2 = 67.7 min and so on) or longer-lived (72As; t1/2 = 26 h, 89Zr; t1/2 = 3.2 d and 124I; t1/2 = 4.2 d). Matching the decay half-life with the biological half-life of the tracer is another important aspect, especially for nanomaterials with prolonged circulation in vivo.
Complexation of the radionuclide and the nanoparticle is an important aspect in the development of a successful radiotracer. Ideally, a radiolabeling method must be robust, quick, safe, and highly efficient with minimal effect on the intrinsic pharmacokinetics of the vector.[20] There are five major radiolabeling strategies, each with their advantages and disadvantages; their selection determined by the isotope and nanoparticle chosen for the imaging purpose (Figure 1). Traditional method involves tethering of the radiometals to the nanoplatform via chelators such as 1,4,7-triazacyclononane-1,4,7-triacetic acid (NOTA), 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA), p-isothiocyanatobenzyl-desferrioxamine (Df-Bz-NCS), diethylene triamine pentaacetic acid (DTPA), etc. via specific coordination chemistry. Since the nanoparticle itself is not radiolabeled, potential detachment of the radiometal containing chelator or polymer coating from the nanoparticle in the presence of high protein concentration, or transchelation of the radiometal from the chelator-complex have raised concerns over erroneous interpretation of the imaging results. Moreover, limitations such as specific coordination chemistries, harsh and prolonged reaction conditions, complex purification procedures, and influence on the nanomaterial surface properties and therapeutic loading capacity, have prompted development of intrinsically radiolabeled nanoplatforms.[20]
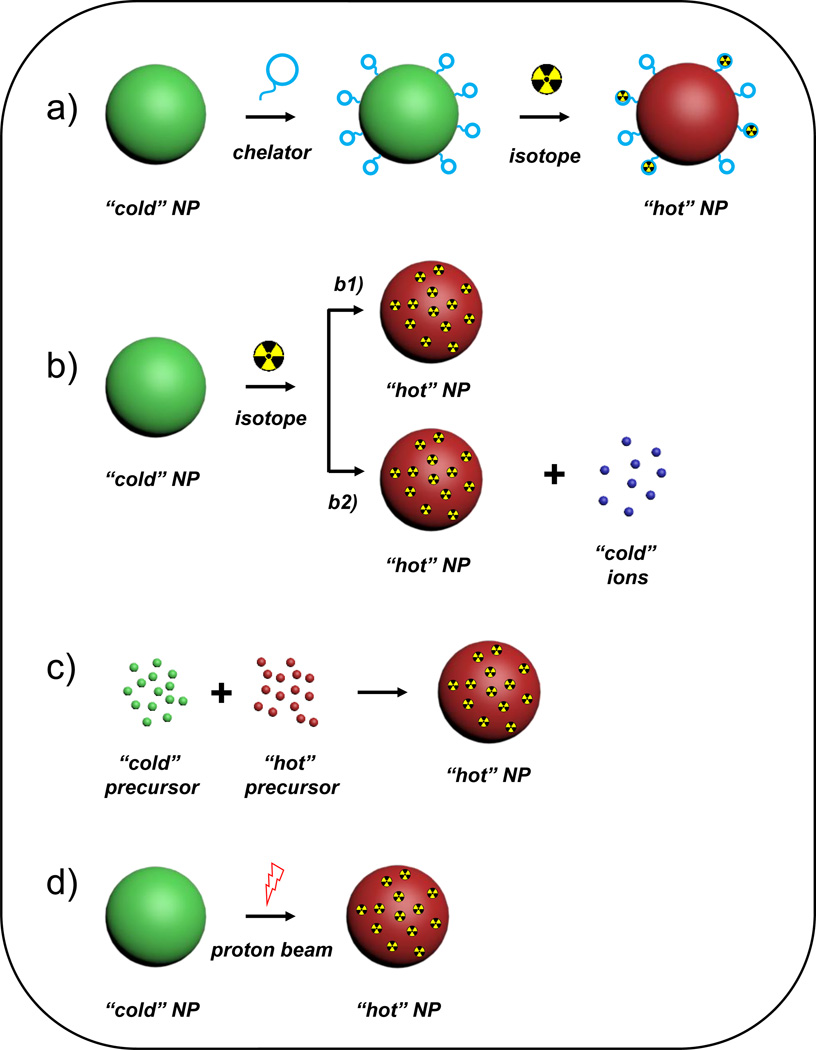
Figure 1.
Schematics depicting five major strategies for radiolabeling nanomaterials. (a) Chelator-mediated complexation, (b) Specific trapping (b1) and ion-exchange (b2). (c) Hot-plus-cold precursor synthesis, (d) Proton beam activation. NP: nanoparticle.
To overcome the limitations of traditional radiolabeling methods, nanoparticles have been uniquely employed for chelator-free or direct incorporation of the radionuclide into the nanoparticle core. Intrinsic radiolabeling strategies can be classified as follows: (1) mixing radioactive and cold precursors during the synthesis of the nanoparticle, (2) specific trapping of certain nuclides into the nanoparticles via coordination bonding, (3) ion-exchange mechanisms, and (4) ion bombardment.[20] Incorporation of trace amounts of radioactive precursor during synthesis results in stable formulations, with high radiochemical yields. However, longer labeling times, potential lattice mismatch between the radionuclide and nanoparticle, prolonged exposure to the personnel and generation of excessive radioactive waste; limit the applicability of the process. Post-synthesis radiolabeling has thus, gained wider acceptance. Specific trapping is a more generalized approach, shown to be highly successful for a number of isotopes (64Cu [21], 89Zr [22, 23], 72As [24] and 69Ge [25], etc.) and nanoplatforms. Ion exchange methods have also been variously applied to radiolabel lanthanide-doped upconversion nanoparticles with 153Sm for SPECT [26], and 18F [27] and 64Cu [28] for PET. Proton beam activation is another promising strategy, applied till date on Al2O3 nanoparticles to produce [18F]-labeled Al2O3. [29, 30] The readers are directed to our earlier review for comprehensive details about each technique. [20]
Lastly, selection of the right nanoplatform is of utmost importance. Factors that influence the choice of the nanomaterial include chemical composition, intrinsic functionality, colloidal stability, hydrodynamic size, surface characteristics, ease of surface modification, addition of imaging and therapeutic moieties and ligands for target recognition, etc. A plethora of nanoplatforms have been designed for PET integrated Theranostics (Table 1). In the following sections, we will discuss the state-of-the-art in nuclear nanomedicine, focusing on the recent advances in the evolution of inorganic radiotracers.
Table 1.
Representative Radiolabeled Theranostic Nanoparticles
| Class | Nanoparticle | Isotope | Therapeutic Arm | Reference |
|---|---|---|---|---|
| Silica | MSN | 64Cu | Drug Delivery | [43–45] |
| 135Ho | Chemotherapy, RT | [312] | ||
| HMSN | 64Cu | Drug Delivery | [55, 313] | |
| Biodegradable MSN | 89Zr | Drug Delivery | [311] | |
| Carbon | SWNT | Na125I | RIT | [82] |
| 225Ac | RIT | [83] | ||
| 89Zr/225Ac | RIT | [80] | ||
| Graphene Oxide | 64Cu | PDT | [85] | |
| Gold | Nanostars | 131I | PTT | [181] |
| Nanorod Vesicles | 64Cu | PTT | [314] | |
| Nanorods | 64Cu | PTT | [21] | |
| Drug Delivery | [202] | |||
| 125I | PTT | [191] | ||
| Nanoshells | 18F | PTT | [315] | |
| Magnetic | SPION | 64Cu | Drug Delivery | [244] |
| 131I | Gene Therapy | [237] | ||
| 111In | Chemotherapy | [234] | ||
| Copper Sulfide | Nanospheres | 64Cu | PTT | [17, 281] |
| RT, PTT | [282, 283] | |||
| Nanodots | 64Cu | PTT | [284] | |
| Porphyrin | Porphylipoprotein | 64Cu | PDT, Drug Delivery | [299] |
| Nanoporphyrin | 64Cu | PDT, PTT, Drug Delivery |
[301] | |
RT = Radiotherapy, RIT = Radioimmunotherapy, PDT = Photodynamic Therapy, PTT = Photothermal Therapy,
4. Radiolabeled Nanomaterials for Cancer Theranostics
4.1. Radioactive Silica Nanoparticles
Silica, “Generally Recognized as Safe” (GRAS) by the Food and Drug Administration (FDA) [31] is among the most biocompatible and well-tolerated inorganic nanomaterials, being endogenous to humans and other animals. The widespread use of silica nanomaterials in multimodal imaging is interesting because unlike other inorganic nanoparticles, these do not possess intrinsic properties to directly serve as contrast or therapeutic agents. However, the well-defined siloxane chemistry for easily tunable size, morphology and porosity, as well as facile surface functionalization, give silica nanomaterials a distinct edge over their counterparts.[32] Their inability to absorb wavelengths in the electromagnetic spectrum and non-interference with the magnetic fields has been utilized in complexing with other functional nanomaterials and drugs to design multifunctional agents (Table 2).[33–35] Moreover, high surface area-to-volume ratio, rigid and stable skeletal network and well-established, scalable synthetic procedures are added advantages, propelling their application as contrast agents in cancer theranostics.
Table 2.
Selected Multi-component Radiolabeled Nanoparticle Systems
| Component 1 | Component 2 | Imaging Modality | Therapeutic Modality |
References |
|---|---|---|---|---|
| Silica | Gold Nanoshell | PET | −/− | [192, 193] |
| PET | PTT | [194] | ||
| Gold Nanosphere | PET | Chemotherapy, PTT | [316] | |
| SPION | Silica | PET/MR | −/− | [34, 35] |
| SPECT/MR | −/− | [238] | ||
| SPECT/MR/FL | Stem Cell Therapy | [236] | ||
| Gold | PET/MR/FL | −/− | [204] | |
| Al(OH)3 | PET/MR | −/− | [248] | |
| Crosslinked Dextran | PET/CT | −/− | [250] | |
| Melanin | PET/MR/PAT/PTI | PTT | [304] | |
| QDs | Phospholipid Micelles | PET/FL | −/− | [112] |
| UCNP | Porphyrin phospholipid |
PET/PA/FL/UCL/CT/ Cerenkov |
−/− | [140] |
| Micelle | PET/UCL | −/− | [139] | |
| Graphene | Gold Nanorod Vesicle | PET/PAT | PT, Drug Delivery | [96] |
| SPION | PET/MR/PAT | −/− | [89] | |
| CuS | Silica | PET | PTT | [33] |
| Ferritin | PET/PAT | PTT | [309] | |
| MoS2 | SPION | PET/PAT/MR | PTT | [286] |
| Bi2Se3 | FeSe2 | PET/CT/MR/PAT | PTT, RT | [287] |
PTI = Photothermal Imaging, PAT = Photoacoustic Tomography
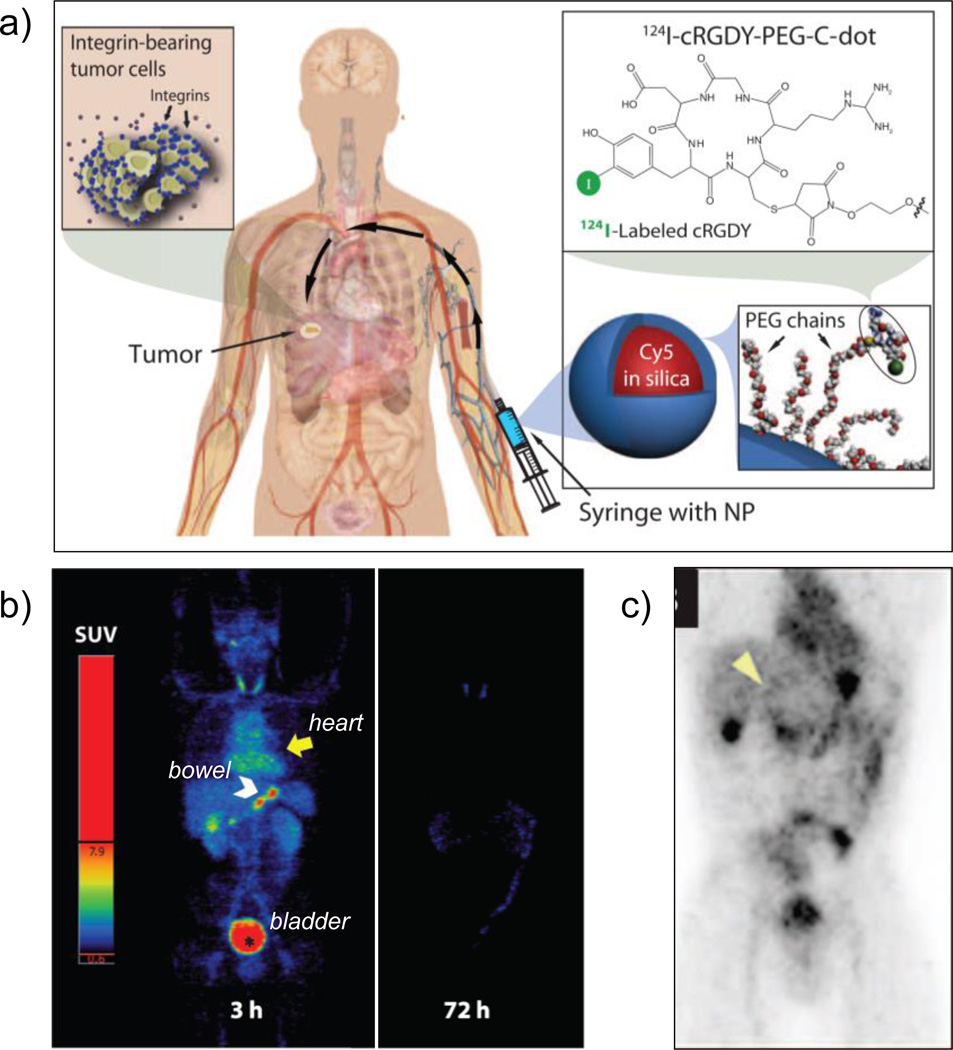
Silica-based ultrasmall (6–7 nm sized) core-shell hybrid nanoparticles (also known as C dots, or Cornell Dots) were approved by the US FDA in 2010 as an investigational new drug (IND), and were reported recently for imaging in patients with metastatic melanoma.[36, 37] Cy5 dye-loaded C dots (<10 nm) were labeled with 124I for PET imaging and conjugated with PEG and cRGDY peptide for detection of integrin-expressing lesions. The tracers were well tolerated, exhibiting good in vivo stability, reproducible pharmacokinetic signatures consistent with renal clearance, and preferential accumulation at the target site (Figure 2). However, several synthetic challenges prompted the group to design a more biologically competent, water-based approach for the preparation of <10 nm fluorescent, core-shell nanoparticles (called C’ dots) with different core compositions and enhanced quantum yields (~0.8; nearly approaching the theoretical brightness limit).[38]
Figure 2.
(a) Schematic of the IND-approved hybrid (PET-optical) imaging nanoparticles (C dots). The core-contains Cy5 dye and the surface was attached with poly(ethylene glycol) (PEG) chains, integring αvβ3-binding cRGDY peptide ligands and 124I radiolabel. (b) Maximum intensity PET projections, 3 and 72 h after intravenous injection of 124I-cRGDY–PEG–C dots. (c) Coronal PET image 4 h p.i., demarcating the peripheral aspect of the tumor (arrowhead) and other major organs of nanoparticle uptake (bladder, gastro-intestina tract, gall bladder and heart). Adapted with permission from [36]. Copyright by American Association for the Advancement of Science.
Besides the ultrasmall C dots, 20–25 nm sized, dye-incorporated dual-modal silica nanoparticles have also shown promising results for sentinel lymph node imaging and clearance kinetics.[39, 40] Despite these encouraging results, the application of silica nanoparticles in theranostics has remained elusive due to the challenges in encapsulating drugs/therapeutics into the nanoparticles. With their tailored porous structure and high surface area, mesoporous silica nanoparticles (MSNs) show significant advantages over traditional drug nanocarriers, resulting in an exponential rise in their biomedical applications since the first report in 2001.[41, 42] Our group first demonstrated CD105-specific, in vivo PET imaging and image-guided doxorubicin delivery with antibody conjugated MSNs in 4T1 breast cancer.[43] Uniform (~ 80 nm) MSNs (synthesized via a soft-template method) were conjugated to TRC105 antibody via PEG linkers and subsequently functionalized with NOTA for 64Cu chelation. Serial PET scanning demonstrated rapid and persistent accumulation of the nanoconjugates at the tumor site (5.9 ± 0.4 %ID/g at 5 h post-injection (p.i.)) which was attributed to both EPR effect and TRC105-mediated binding to CD105 (over expressed in tumor vasculature). Enhanced CD105-targeted delivery of doxorubicin was also demonstrated simultaneously, clearly demonstrating the superiority of surface functionalized MSNs in targeted theranostics. The strategy could be tailored to target different biomarkers and tumor models [44], or to develop multimodal imaging agents.[45] Moreover, the facile silica chemistry has been harnessed for radiolabeling with varied isotopes ranging from very short lived 18F (t1/2 = 109.8 min)[46] to longer lived 89Zr (t1/2 = 72.8 h) [47] via simple one-step reactions with appropriate chelators.
Further improvement in the morphology of silica nanoparticles was achieved by designing hollow MSNs (HMSNs) with a large interstitial cavity and a mesoporous shell.[48, 49] HMSNs with low density and high specific area, showed extraordinarily high drug loading capacity [50] and could integrate various functional nanocrystals for multimodality imaging (MR/upconversion/ultrasound) and therapy.[51–54] We recently reported an HMSN based dual modality PET/near-infrared fluorescence (NIRF) imaging agent by conjugating zwitterionic dye, 800ZW and PET tracer 64Cu.[55] As-synthesized HMSNs could load up to 1129.2 mg doxorubicin per gram of HMSN (3–15 times higher than reported MSNs). Enhanced CD105 specific tumor accumulation (~9.9 %ID/g) was observed after conjugation with TRC105, making surface engineered HMSNs a highly attractive drug delivery nanoplatform for future cancer theranostics.
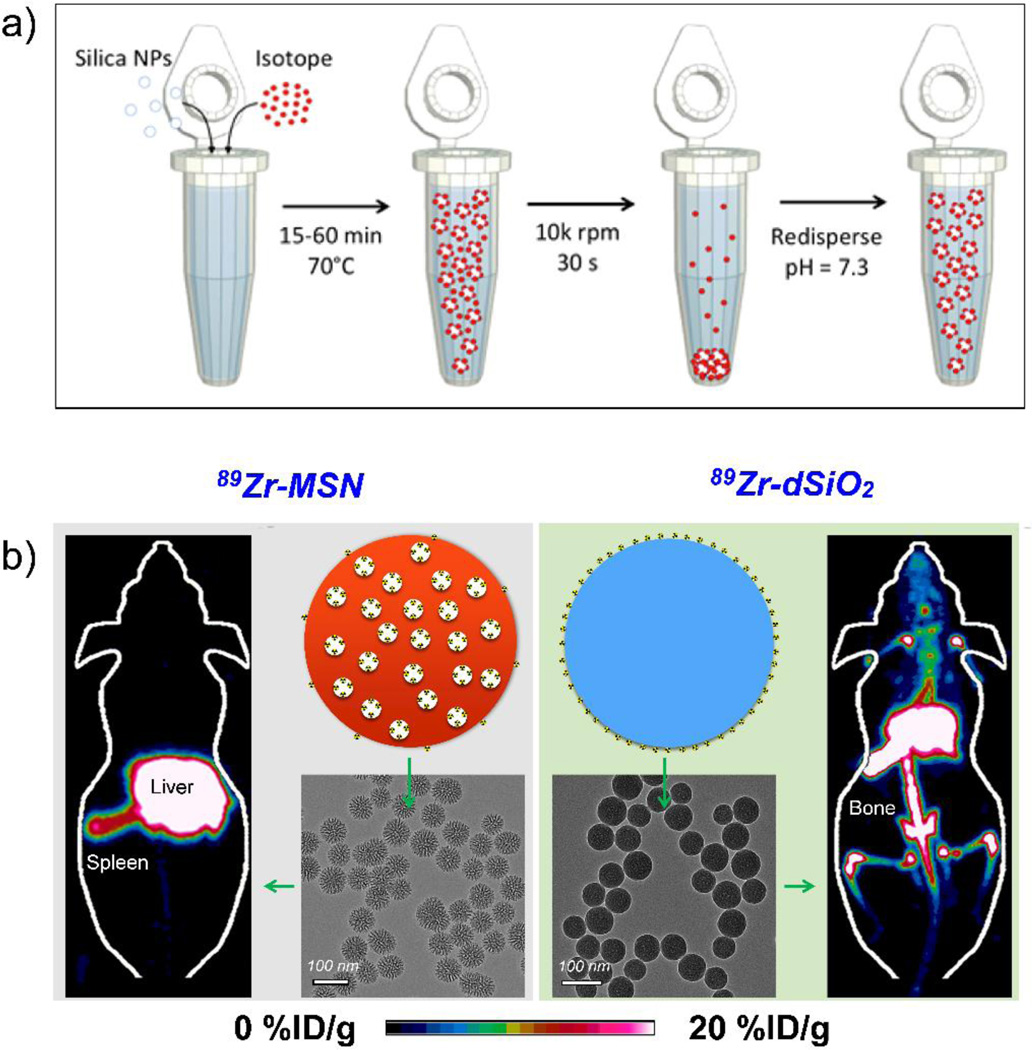
Though chelator-free radiolabeling has been demonstrated with several nanoparticles, most reports suffer from the same specificity issues as traditional chelator-based methods. This problem was recently overcome by the discovery of intrinsic radiolabeling ability of silica nanomaterials. Amorphous dense silica nanoparticles have been shown to serve as general substrates for chelator-free radiolabeling of 22Na [56] and six medically relevant radiometals (68Ga, 64Cu, 89Zr, 90Y, 111In, and 177Lu), with the labeling characteristics depending on the oxophilicity of the radioisotope.[22] Schaffer et al. demonstrated that >99 % labeling yields could be obtained for all isotopes at pH = 7.3, 70 °C and incubation times up to 1 h (when specific activity ~100 Ci/µmol), while stability of the binding correlated with hardness of the radioisotope. However, long-term in vivo radiostability tests performed by our group demonstrated that while dense silica nanoparticles could chelate 89Zr, such binding was weak with the isotope detached from the nanoparticles in vivo and accumulated in the bones (89Zr is a well-known osteophile) within a day of intravenous administration. [23] Systematic studies demonstrated that MSNs could serve as a more reliable platform for radiolabeling oxophilic radiometals with >20-fold higher radiostability than the dense silica nanoparticles, indicating a crucial role of mesochannels in stabilizing 89Zr inside MSNs (Figure 3). This difference in in vivo stability profile was rationalized by the presence of mesochannels, which not only protect the guest 89Zr ions from transmetallation by intrinsic protein chelators in the body, but also provide a higher number of surrounding deprotonated silanol groups (Si-O−) for stronger coordination with the isotope. Although PET imaging is highly sensitive and quantitative for in vivo applications, its spatial resolution (mm level) is significantly lower than that of MRI (typically <500 µm). Taking advantage of the easily tailorable chemistry of silica, Burke et al. synthesized 68Ga radiolabeled silica coated iron oxide nanorods to combine the high sensitivity of PET with MR contrast.[35] As with 89Zr, 68Ga is a hard Lewis acid that coordinates easily and stably with the Lewis base donor atoms like oxygen from the silanol groups. PEG modified nanoconjugates were further used for high sensitivity liver imaging. This facile and robust radiolabeling technique holds important implications for nanomedicine. Firstly, it can allow systematic and long-term tracking of nanoparticle biodistribution, thereby providing accurate, real-time information on their in vivo fate, biodegradation rates and clearance pathways, potential toxicity, drug delivery, and chemotherapeutic efficacy. Secondly, by simply incorporating certain functional groups, MSNs can directly and stably chelate other radioisotopes, for example, 64Cu and 72As, making them a versatile chelation platform. Lastly, labeling with radiotherapeutic isotopes (like 111In, 177Lu, etc.) can open up new possibilities for theranostics with simultaneous radiotherapy and drug delivery.
Figure 3.
(a) Scheme for intrinsic radiolabeling of silica nanoparticles; incubation of the nanoparticles with free isotope at 70°C for 15–60 min, followed by purification by centrifugation. Adapted with permission from [22] (b) Maximum intensity projection PET image, schematic and Transmission electron microscopy (TEM) images of 89Zr-labeled mesoporous silica (89Zr-MSN) (left) and 89Zr-labeled dense silica (89Zr-dSiO2) (right). Absence of radioacitivity from the bones in the case of 89Zr-MSN demonstrates the high in vivo radiostability of these intrinsically radiolabeled nanoparticles when compared with that of 89Zr-dSiO2. Reproduced with permission from [23]. Copyright by American Chemical Society.
4.2. Radioactive Carbon Nanoallotropes
Carbon nanomaterials are lower dimensional carbon allotropes that have garnered great attention in the past few decades, since the discovery of fullerenes in 1985,[57] compounded further by the discoveries of carbon nanotubes (CNTs) [58], graphene [59] carbon dots [60, 61] and nanodiamonds.[62] (Figure 4) The nanomaterials range typically between 1 nm-1 µm, and have been employed as optical imaging and therapeutic agents as well as drug nanocarriers in biomedical applications, owing to their unique electronic structure and readily tunable shapes and sizes.[63] The strong absorption in NIR and far NIR (NIR II) windows (750–1000 nm and 1000–1700 nm, respectively), allows deep tissue imaging with high resolution, enhanced contrast and minimized autofluorescence and photobleaching, leading to widespread applications in optical and photoacoustic imaging, photothermal imaging and therapy. [64–68] In addition, CNTs, graphene and carbon dots exhibit strong and unique G-band peaks in the Raman scattering spectrum, which have been harnessed for multiplexed, multicolor Raman imaging, both in vitro and in vivo.[65, 69, 70] Large surface area and abundance of π electrons has further allowed loading of hydrophobic drugs via π-π interactions for efficient delivery in physiological conditions.[71] The unique properties of carbon nanomaterials have been exploited to develop novel multiplexed, multifunctional nanoprobes consisting of biologics, radionuclides, drugs and optical probes, etc.
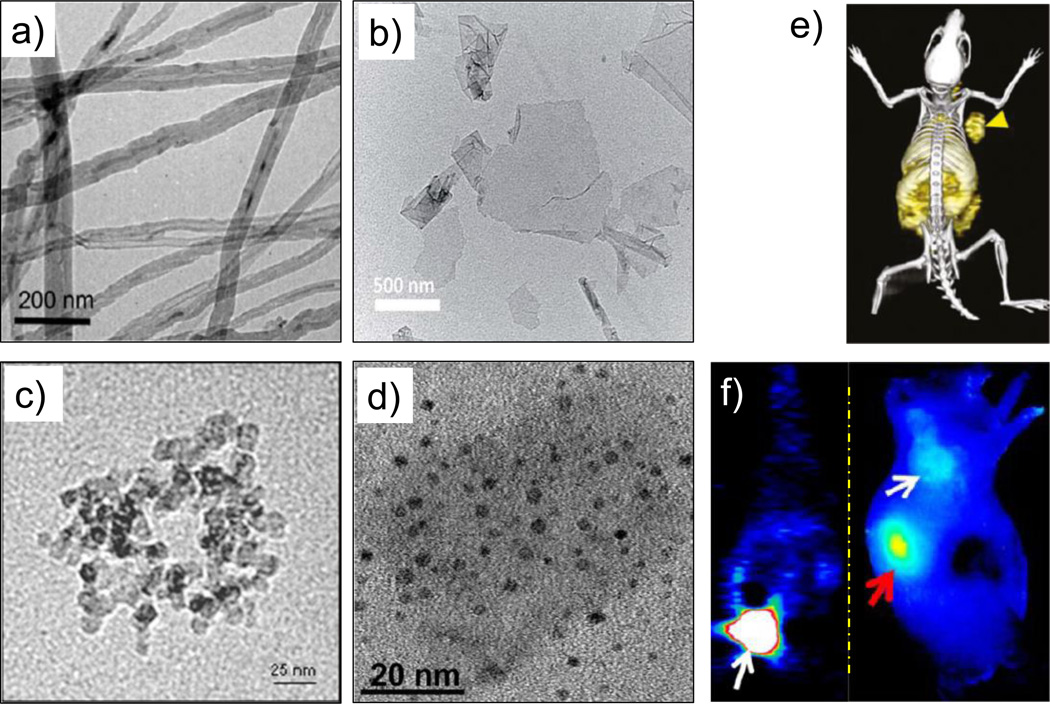
Figure 4.
TEM images of different (radiolabeled) carbon nanomaterials. (a) 14C-labeled multiwalled carbon nanotubes. Adapted with permission from [77]. Copyright by American Chemical Society. (b) Graphene oxide nanosheets. Adapted with permission from [95]. Copyright of the Royal Society of Chemistry. (c) Amine-modified nanodiamonds. Adapted with permission from [62]. (d) 800ZW-conjugated carbon nanodots. Adapted with permission from [98]. (e) In vivo PET/CT image of 64Cu-labeled GO conjugates in 4T1 murine breast tumor-bearing mice. Adapted with permission from [88]. (f) Coronal PET image 1 h p.i. of renal clearable 64Cu-labeled carbon nanodots; bladder indicated by white arrow (left), and NIR fluorescence image of SSC-7 tumor-bearing mice, 2 h p.i. of 800ZW-carbon nanodots; white arrow indicates the tumor and red arrow kidney (right). Adapted with permission from [98]. Copyright by American Chemical Society.
In nuclear medicine, biological properties of CNTs have been explored in vivo via PET or SPECT, using radionuclides like 125I[72], 111I[73], 99mTc[74]. Both single-walled (SWNTs) and multi-walled (MWNTs) nanotubes demonstrated small active molecule-like rapid clearance from systemic blood circulation, effortless transportation through tissues and organs, and rapid renal clearance, with no retention in the RES. However, other biodistribution studies with 86Y-DOTA-SWNT and 111In-DOTA-SWNT reported by McDevitt et al.[75] and [14C]-labeled MWNTs, reported by Georgin et al.[76, 77] demonstrated contradictory results with major accumulation in the RES and much slower hepatobiliary clearance. Liu et al. first demonstrated tumor-targeted PET imaging with 64Cu-DOTA radiolabeled SWNTs, functionalized with phospholipid and PEG to impart longer blood circulation, superior hydrophilicity and reduced RES uptake.[78] Conjugation with cRGDyK peptide conferred integrin αvβ3 specific uptake in U87MG glioblastoma xenografts (~13 %ID/g), attributed to the multivalency effect of SWNTs, which was further confirmed by the unique Raman signatures of the nanoprobes. Similarly, antibody [79, 80] and hyaluronic acid [81] functionalized CNTs have been explored for multimodality cancer theranostics. Furthermore, chelator-free radiolabeling of SWNTs has also been reported, whereby metal halide Na125I was filled in the nanotubes by direct covalent conjugation and capped off to prevent any leakage.[82] In further studies, alpha-emitters like 225Ac3+ [83] and beta-emitters like 64Cu2+ [84] along with Gd3+ ions were stably loaded into the bores of the ultrashort nanotubes by simple sonication, and employed for radioimmunotherapy and PET/MR imaging, respectively. The strategy potentially protects the encapsulated radioisotopes from transmetallation, thereby preventing any leakage and consequent off-target toxicity in vivo. However, it is noteworthy that in both the studies, the radiolabeled CNTs accumulated rapidly in the lungs, possibly due to aggregation, which may influence the actual in vivo radiostability since the nanoparticles are removed quickly from the circulation. Further studies are warranted to establish the legibility and superiority of this strategy, over traditional chelation methods and further evaluate its suitability for longer-circulating biologically active nanotubes for in vivo imaging.
Besides CNTs, graphene is the other nanocarbon allotrope that has gained widespread acceptance in the biomedical community. Single-layered graphene shows ultra-high surface area with every atom exposed on the surface, thereby providing immense opportunities for bioconjugation, drug and gene delivery. The unique electronic and optical properties have been harnessed for phototherapy [85], while the surface itself can be used for growth of various inorganic materials for multimodality imaging and theranostics. [86, 87] While Yang et al. [68] first studied the in vivo biodistribution and tumor ablation ability of PEG-modified, Cy7 dye labeled nanographene sheets in U87MG tumor-bearing mice, the inherent limitations of optical imaging, mainly autofluorescence and tissue depth limitations, prompted the need of radionuclide imaging for a more accurate assessment. Our lab first reported PET imaging of 64Cu-NOTA conjugated, PEGylated graphene oxide (GO), specifically directed to the tumor neovasculature, through targeting the CD105 receptor, as evidenced by the rapid, persistent and CD105 specific uptake in 4T1 metastatic breast tumors.[88] Graphene oxide nanosheets have been widely employed for developing passively [89] and actively targeted agents [90–92], radiotracers for isotopes like 66Ga[93], 125I[94] 111In[95], and theranostic agents [85, 92, 96, 97]. Of note, however, while intrinsic radiolabeling techniques have been applied to CNTs, graphene is yet to benefit from the merits of these novel methodologies.
Despite being around for decades, long-term toxicity concerns have severely impeded the clinical translation of CNTs and graphene; prompting the introduction of other lower dimensional nanocarbons into the biomedical arena. PET imaging with carbon dots [98], nanodiamonds [99] and fullerenes [100] is an emerging field, with initial studies dedicated solely to the assessment of their in vivo biodistribution. For example, 64Cu-DOTA conjugated fluorescent carbon dots were recently synthesized and their in vivo fate was analyzed, following injection via three different routes, i.e. intravenous (i.v.), intramuscular (i.m.) and subcutaneous (s.c.). Although low RES uptake was observed (<1 %ID/g) within 24 h p.i. with renal clearance in all the groups, the rate of clearance from the blood followed the order: i.v. > i.m. > s.c. [98] It is noteworthy that the other ultrasmall carbon nanoallotropes also demonstrate greatly reduced RES accumulation and rapid clearance predominantly through the renal pathway, potentially addressing the toxicity concerns posed by the use of carbon nanomaterials. However, the rapid clearance compromises on the enhanced tumor contrast, characteristic of graphitic carbon tracers (Figure 4). Further optimization of in vivo pharmacokinetics and tumor homing patterns of these emerging members of the nanocarbon family, as well as their application as multimodality and multiplexing agents, is therefore warranted. Given that one of the major hurdles in the clinical translation of traditional carbon nanomaterials is their substantial and prolonged RES retention, carbon dots, nanodiamonds and fullerenes have much to offer.
4.3. Radioactive Fluorescent Nanomaterials
Fluorescence-based imaging methods have attracted great attention in the last two decades because they are sensitive, selective, rich in contrast, convenient, versatile, non-destructive, widely available and cheap.[101] Compared to the traditional organic dyes, which suffer from short fluorescence lifetimes (1–10 ns), inorganic nanoparticles like quantum dots and upconversion nanophosphors (UCNPs), with longer life-times (typically few hundred nanoseconds), improved optical characteristics and multiplexing ability, are better candidates for in vivo optical imaging. For a more comprehensive review of the current state-of-the-art in fluorescence imaging with nanomaterials, readers are directed to these excellent articles.[101, 102] Despite its advantages, optical imaging suffers from limited tissue penetration, autofluorescence and qualitative nature. The combination of fluorescence- and radionuclide-based imaging offers synergistic advantages over each individual modality. In the following sections, we will discuss dual-modality optical/PET imaging with two of the most frequently employed fluorescent agents; quantum dots and UCNPs.
4.3.1. Quantum Dots
Quantum dots (QDs) are inorganic fluorophores (<10 nm) with excellent light-emitting properties that can overcome many limitations of traditional fluorophores. Due to their small size (2–10 nm), QDs display unique properties unavailable in either bulk material or individual atoms, such as size- and composition- tunable emission in a narrow, symmetric energy band, large Stokes’ shift (>200 nm), superior brightness and long fluorescence lifetimes, as well as large absorption coefficients across a wide spectrum, stemming from the quantum confinement effects.[103, 104] In addition, resistance to photobleaching and chemical degradation further simplifies the storage and handling requirements.[105] Traditionally, QDs are nearly spherical inorganic semiconductor crystals that consist of a core and shell comprising of elements from heavy metal-containing groups II-IV, IV-VI, or III-V.[106] The core is typically made of cadmium selenide (CdSe) with a ZnS shell. There are many types of QDs commercially available. Some other examples of QD cores include CdTe, PbSe, GaAs, GaN, and InP [107], the quantum yields for which can be drastically enhanced (~90%) by coating with a shell of high-energy bandgap material. For more information about the various types of QDs and their applications, readers are directed to these references.[107–110] As with other nanomaterials, QDs provide a multifunctional nanoplatform for multimodality imaging and therapy, especially suitable for synergistic optical/PET imaging which combines the sensitivity, quantification and limitless tissue penetration of PET with high resolution and specificity of optical methods.[104]
Cai et al. reported dual-functional QD nanoprobes for quantitative assessment of in vivo pharmacokinetics and tumor-targeting efficacy of QDs using both PET and NIRF imaging.[111] RGD peptide-bearing, 64Cu-labeled QD705 achieved greater tumor contrast in U87MG xenografts at a fraction of concentration than that needed for in vivo NIRF imaging, thereby significantly reducing potential toxicity. Several other studies since then, have explored the use of directly/indirectly radiolabeled QDs for tumor-targeted imaging. [112–117] However, poor long-term radiostability in vivo has raised concerns. For example, in several studies, while at earlier time-points the PET and NIRF imaging data were in good agreement, substantial differences could be observed at later time-points, possibly attributed to the detachment of the isotope.[111, 113] Therefore, incorporation of the radionuclide in the QD core has been proposed to eliminate the concerns regarding in vivo stability of the radiolabel relative to the QDs, since it is well documented that QDs do not undergo significant degradation unless subjected to very harsh conditions.[118]
Intrinsically radiolabeled QDs were first reported for SPECT/CT imaging where, 125mTc (t1/2 = 58 d) was incorporated into tumor-targeted Cd125mTe/ZnS QDs during the synthetic process.[119, 120] Another study reported the incorporation of 109Cd (t1/2 = 464 d) into zwitterionic radioactive-QDs for reliable biodistribution studies via gamma counting.[121] While the studies reported high incorporation efficiencies and radiostability, sub-optimal radionuclide characteristics such as long half-lives and low specific activities warrant the use of clinically relevant PET and SPECT isotopes. Sun et al. first synthesized 64Cu-doped CdSe/ZnS QDs via a cation-exchange reaction, with nearly 100% radiolabeling yield and high radiostability (in bovine serum and mouse blood over 48 h).[28] Incorporated 64Cu not only accurately reflected the in vivo biodistribution, but also allowed for efficient Cerenkov resonance energy transfer (CRET) imaging in U87MG glioblastomas. 64Cu-doped QDs exhibited rapid and persistent uptake in the U87MG tumor; ~5% ID/g at 1 h time point, which reached 12.7% ID/g at 17 h p.i. (Figure 5). Currently, CRET is a hot topic in the field of molecular imaging as these imaging agents do not require external excitation sources; thus, CRET overcomes a key limitation of fluorescence imaging. Several researchers have successfully employed radiolabeled QDs for Cerenkov luminescence imaging.[122–125]
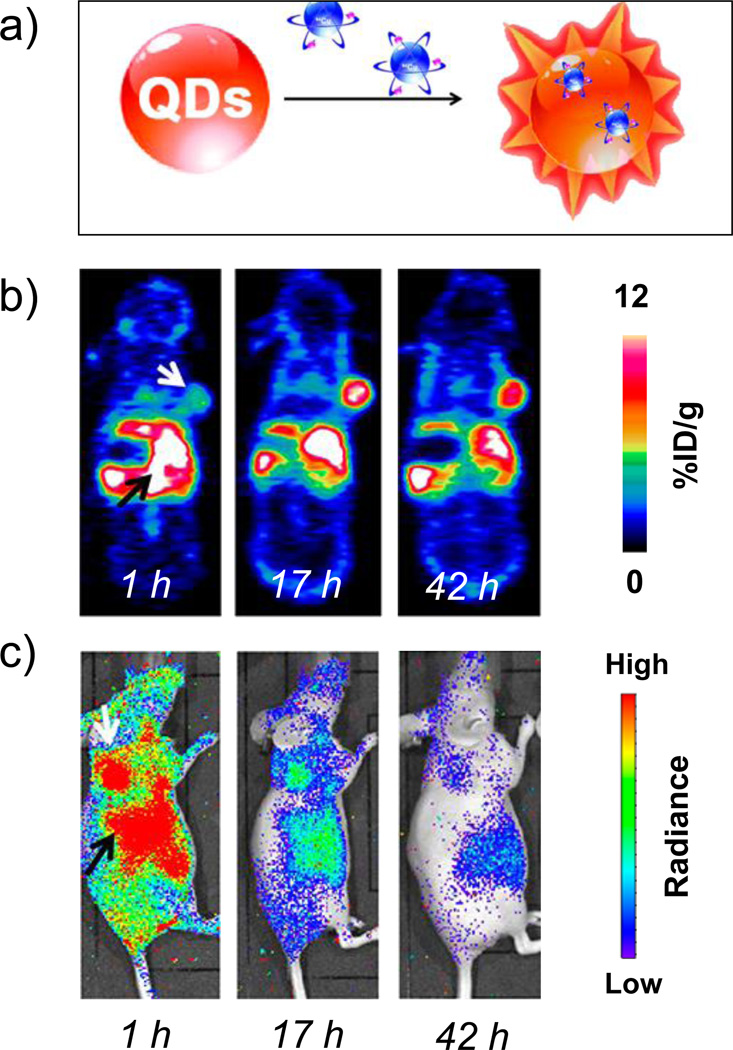
Figure 5.
(a) Schematic of self-illuminating 64Cu-doped quantum dots. Representative whole-body (b) coronal PET images, and (c) luminescence images, of U87MG tumor-bearing mice at 1, 17, and 42 h after intravenous injection of 64Cu-doped QD580. White arrow:tumor area; black arrow:liver area. Adapted with permission from [28]. Copyright by American Chemical Society.
4.3.2. Rare-Earth Upconversion Nanophosphors
Rare-earth UCNPs have recently emerged as promising candidates as a new generation of multifunctional and multimodality nanoplatform for personalized theranostics. Upconversion luminescence (UCL) refers to a unique non-linear process whereby multiple low energy photons (near-infrared) are sequentially absorbed or undergo energy transfers to emit higher energy (visible to ultraviolet) light. [126, 127] The basic structure of UCNPs consists of an inorganic host matrix (fluorides, oxides, heavy halides etc.), a sensitizer (to enhance UCL efficiency; Yb3+) and an emitter (Er3+, Tm3+ and Ho3+ dopant ions). Lanthanide doping into the host material confers UCNPs with several advantages over conventional luminescence probes; namely sharp emission bandwidths, large anti-Stokes shifts, excellent photostability, non-blinking, minimal autofluorescence, and deeper tissue penetration.[126, 128] Structure, fabrication, characteristics and optical imaging applications of UCNPs have recently been reviewed in excellent articles.[126–131] Owing to the well-established synthetic procedures and facile surface chemistries, UCNPs are increasingly being employed for diverse applications such as cell tracking [132], small animal imaging [130], drug delivery [133], photothermal [134], photodynamic [133] and combination therapies. [135, 136]
Multiple functions embedded in a single nanoparticle can play a crucial role in precise disease diagnosis and thus, the use of functionalized UCNPs as multimodal contrast agents is under active exploration.[137] Simple variations in dopant atoms or lattice composition can endow UCNPs with MR, PET, SPECT and CT imaging capabilities, in addition to their intrinsic UCL property.[129] Interestingly, chelator-based PET imaging with UCNPs has been rarely reported, with one study reporting DOTA chelated 68Ga labeled UCNPs for integrin αvβ3 targeted PET imaging in M21 tumor models.[138] In a more recent study, bimodal in vivo imaging was employed to demonstrate hepatobiliary excretion of micelle-encapsulated UCNPs via PET and upconversion luminescence imaging.[139] 64Cu-NOTA-UCNPs displayed high radiochemical purity (~99%) and reasonable serum stability (100 % upto 4 h and ~75% after 24 h). The multiplexing capabilities of UCNPs were very ably demonstrated in a recent exciting study by Rieffel et.al who reported a hexamodal porphyrin-phospholipid-coated UCNP (PoP-UCNP) system, where the exquisite affinity of copper for porphyrins was used for post-labeling of UCNPs with 64Cu by simple incubation (>80% labeling yield).[140] PEGylated PoP-UCNPs (~74 nm) were further employed for in vivo lymphatic mapping (Figure 6). With just two active imaging components, fluorescence, NIR-to-NIR UCL, PET, CT, Cerenkov luminescence, and photoacoustic tomography (PAT) could be performed, exemplifying the feasibility of simple yet higher order multimodality imaging agents. In another noteworthy study, integrin αvβ3 targeted, Er3+/Yb3+ co-doped NaGdF4 UCNPs were reported. UCNPs were radiolabeled with 124I (t1/2 = 4.2 days) using a tyrosine residue of (cRGDyk)2 ligand, thereby creating a clinically relevant trimodal (UCL/MR/PET) agents with synergistically acting imaging capabilities.[141]
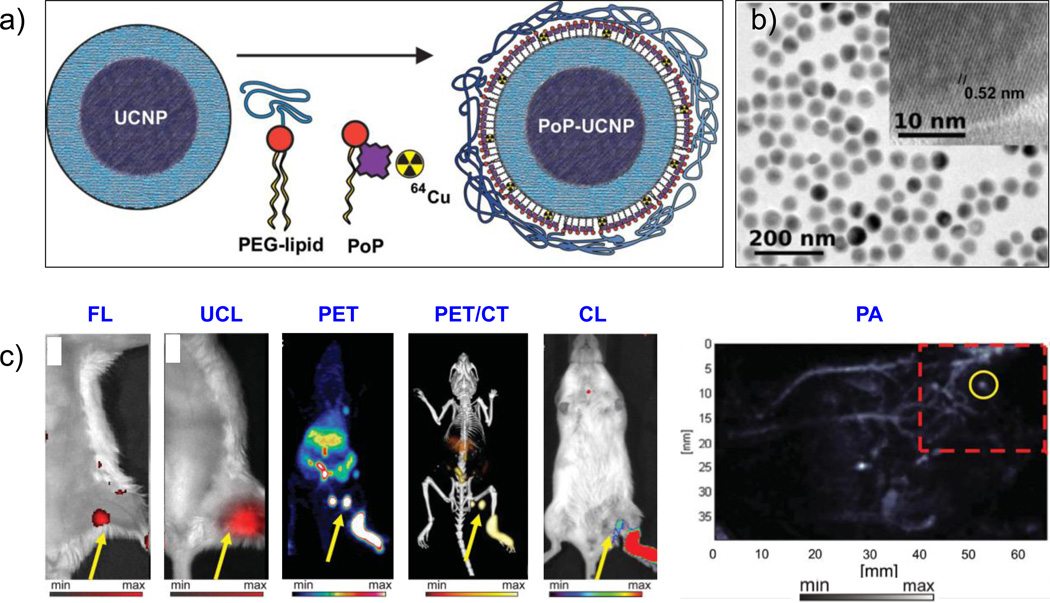
Figure 6.
(a) Schematic diagram of the PoP-UCNP structure. Core-shell UCNPs were transferred to the aqueous phase by lipid coating with PEG-lipid and porphyrin-phospholipid (PoP) followed by seamless intrinsic radiolabeling with 64Cu. (b) TEM image of PoP-UCNP, with the inset showing the crystalline and core-shell nanostructure of UCNP. (c) Hexamodal in vivo lymphatic imaging using PoP-UCNPs in mice via fluorescence imaging (FL), upconversion luminescence (UCL) imaging, PET, PET/CT, Cerenkov luminescence (CL) imaging, and photoaccoustic (PA) imaging. Reproduced with permission from [140]. Copyright by John Wiley and Sons.
Research on intrinsically radiolabeled UCNPs has also been on the rise. For example, SPECT imaging has been achieved by introducing Samarium-153 (153Sm; t1/2 = 46.3 h) ions into the UCNP lattice, either by co-doping during hydrothermal synthesis [142–145] or via cation-exchange based post-labeling method.[26] Notably, chelator-free radiolabeling if UCNPs with PET isotopes has been seldom explored. Although, the incorporation of 18F into the UCNP matrix via specific trapping or cation assisted ligand exchange reactions has been well documented [27, 146–148], the relatively short half-life of 18F (t1/2: 109.7 min) and limited applicability to selected radioisotope: host combinations, warrants development of alternative labeling procedures to fully utilize the vast repertoire of more suitable isotopes.
While inorganic optical imaging agents have been around for more than two decades, several issues have hampered their prospects for clinical translation. Structural complexities, heavy metal compositions, potential toxicity, prolonged in vivo residence, suboptimal stability and clearance kinetics as well as in effective tumor-targeting, have elicited concerns. For instance, newer QDs have been designed from silicon and certain polymers, in an effort to minimize potential toxicities and make them more biocompatible,[149–151] Similarly, efforts are also being concentrated towards developing renal clearable [15] and biodegradable quantum dots [152] and ultrasmall UCNPs [153]. Further challenges, in terms of their synthesis, surface functionalization, stability, in vivo pharmacokinetics and tumor-targeted imaging remain to be conquered. In this regard, synthesizing sub-10 nm UCNPs may not only improve their renal clearance profile and minimize toxicity, but may also aid in enhanced tumor-cell targeting.[154].In addition, cancer theranostics, whereby therapeutic components are combined with the multimodal imaging capacity of QDs and UCNPs into a single vector, also presents a relatively lesser explored, yet promising avenue for research. Further multidisciplinary efforts are required to optimize these nanoplatforms to fully realize their potential in cancer prevention, diagnosis, therapy, patient-stratification and management.
4.4. Radioactive Gold Nanomaterials
Owing to their unique physicochemical properties, varied and tailorable shapes, sizes and chemical compositions, gold nanostructures make excellent candidates for molecular imaging and, potentially, therapy of various diseases, including cancer [155, 156], cardiovascular disease [157–159], viral infections [160–162], and others.[163, 164] Gold nanoparticles (AuNPs) display excellent biocompatibility as gold is relatively inert in biological environments.[165] They also possess unique plasmonic properties that allow researchers to track their biodistribution in vivo.[166] The unique surface plasmon resonance peaks of gold nanostructures can be easily tuned between visible and NIR windows by simply changing the shape and size of AuNPs, which has been variously harnessed for FL, PA and Raman imaging, as well as for photothermal therapy (PTT).[167–171] Furthermore, AuNPs can be easily functionalized with active or passive targeting entities through interactions between gold and thiol-containing compounds.[172, 173] Lastly, many gold-based nanoplatforms have been shown to effectively load large drug payloads with optimal release kinetics for targeted delivery to diseased tissues. [174–177]
The biggest advantage of AuNPs in imaging lies in their multiplexing ability. The inherent optical properties and high X-ray absorption coefficient allows their use as multimodal contrast agents, with widespread applications in optical, MR, CT and radionuclide imaging.[178] For example, sharply branched gold nanostars, with tip-enhanced plasmonic properties have been used in multimodality imaging and phototherapy.[172, 179–182] Interestingly, the shape of gold nanoparticles can significantly impact their biodistribution in vivo, influencing the rate of clearance by the RES organs [183, 184], rate of endocytosis [185, 186], and transport of the nanoparticles through the blood.[187, 188] As such, SPECT and PET imaging have emerged as an important tool for tracking the in vivo pharmacokinetics of AuNPs. AuNPs and nanorods, radiolabeled with 99mTc[189], 125I and 111In[190, 191] have been utilized for targeted SPECT imaging. Xie et al. first reported the biodistribution of Au nanoshell-coated silica cores, radiolabeled with 64Cu via bifunctional chelating agent DOTA, in head and neck squamous cell carcinoma (HNSCC) xenograft-bearing rats.[192] In addition to nanoshells [193, 194], chelator-based PET studies have extensively been carried out for other AuNP morphologies like nanospheres [195–198], hollow nanospheres[199, 200], nanostars[180], nanocages[201], nanorods[202], nanotripods[203], nanodumbells [204], and ultrasmall nanoparticles [205, 206] (Figure 7a–f). In these studies, PET was successfully used to assess the biodistribution and tumor accumulation of radiolabeled nanoplatforms. For example, Cheng et al. reported the synthesis of novel, anisotropic, branched Au-tripods (size <20 nm), functionalized with cRGD peptide for targeting U87MG xenografts in mice via dual modal PAT and PET.[203] At 24 h post-injection, PET imaging revealed ~7.9 %ID/g of RGD-Au-tripods in the U87MG tumors, about 3 times higher than the blocking group. In addition, the authors noted that there were no signs of acute or systemic toxicity. However, toxicity normally does not occur until tissues have been exposed to the gold nanoparticles for 7–30 days, warranting further long-term studies. [207]
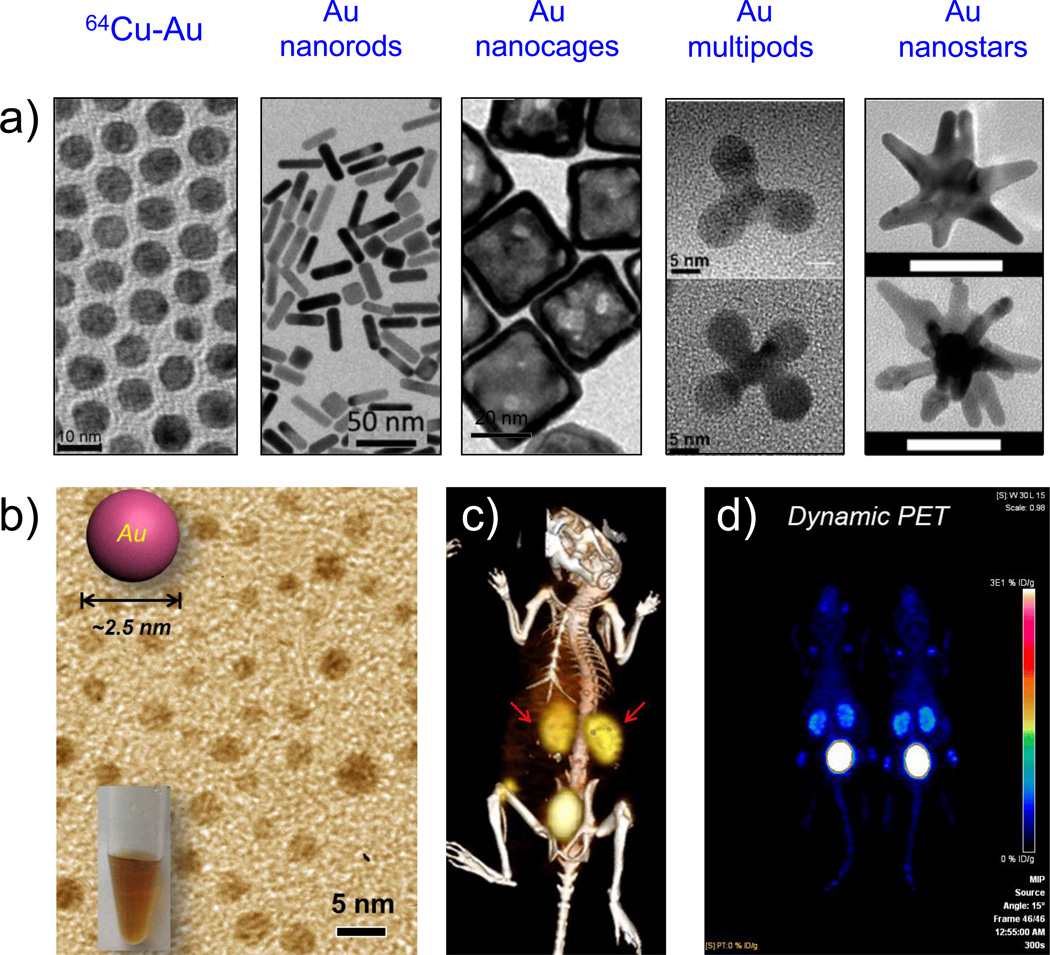
Figure 7.
(a) TEM images of various morphologies of gold nanoparticles. From left to right: Alloyed 64Cu-Au nanoparticles. Adapted with permission from [213]; Copyright by John Wiley and Sons. Au nanorods. Adapted with permission from [212]. Au nanocages. Adapted with permission from [1[201]. Au multipods; upper panel, tripod; lower panel, tetrapod. Adapted with permission from [203]. Copyright by American Chemical Society. Au nanostars. Adapted with permission from [180]. Copyright of Multidisciplinary Digital Publishing Institute. (b) TEM image of 2–3 nm sized renal clearable ultrasmall gold nanoparticles. (c) Representative PET/CT image of mouse injected with 64Cu-NOTA-Au-GSH, 2 h p.i. Kidneys are marked by the red arrow. (d) Selected frame (55 min p.i.) from dynamic PET scanning of 64Cu-NOTA-Au-GSH. Adapted with permission from [206]. Copyright by John Wiley and Sons.
In an effort to improve the radiostability, scientists have intrinsically radiolabeled AuNPs, with the earlier studies incorporating Au-198 (βmax=0.96 MeV; t1/2=2.7 d) and Au-199 (βmax=0.46 MeV; t1/2=3.14 d) for radiotherapy [208, 209], SPECT [210] and radioluminescence imaging.[211, 212] In 2014, Zhao et al. reported the use of 64Cu-alloyed AuNPs, where radioactive 64CuCl2 precursor was mixed with ‘cold’ Au precursors during the synthesis.[213, 214] Similarly, Sun et al. demonstrated post-synthetic chelator-free 64Cu radiolabeling of different Au morphologies by simple hydrazine-mediated reduction of the 64CuCl2 precursor on the surface of the PEGylated nanoparticles.[21] While effective as contrast agents, AuNPs demonstrated high liver and spleen uptake, which is a common limitation of most intravenously administered nanoparticles. To overcome these limitations, Zhao et al. synthesized ultrasmall 64Cu-labeled gold nanoclusters for PET imaging of prostate cancer in a mouse model.[215] The 5 nm sized nanoparticles were PEGylated with 5-kDa PEG and the biodistribution studies revealed significant renal and hepatobiliary excretion, thus showing that some liver and spleen uptake can be avoided by modifying the clearance mechanism of the nanoparticles. It is well known that nanoparticle size can significantly impact its biodistribution in vivo. Smaller, renal clearable nanoparticles are better suited for clinical translation as they are not restricted by toxicity concerns. The kinetics of renal clearable nanoparticles were further analyzed by dynamic PET imaging of ~3 nm sized 64Cu-NOTA-Au-GSH.[206] When compared to previous studies[216], 64Cu-NOTA-Au-GSH demonstrated rapid renal clearance (>75%ID at 24 h post-injection) and drastically reduced hepatic uptake, with an elimination half-life (< 6 min, over 130-times shorter than previously reported for similar nanoparticles), demonstrating potential as imaging agents in models of acute renal failure and other renal diseases (Figure 7g–i). As discussed in this section, radioactive gold nanoparticles make excellent theranostic agents when they are effectively synthesized. Further in-depth pharmacokinetic studies are needed for successful clinical translation in the future.
4.5. Radioactive Magnetic Nanoprobes
Non-invasive MRI is characterized by high spatial resolution (~sub-millimeter level) and exquisite soft tissue contrast.[8] However, the inherent low sensitivity of MRI has spurred rapid development of exogenous contrast agents.[217, 218] Integrated PET/MR imaging forms a powerful modality, combining excellent soft tissue contrast and functional imaging parameters provided by MR with high sensitivity and quantification of radiotracer metabolism provided by PET.[219, 220] Interestingly, even though the idea of simultaneous PET/MR imaging has been around since the 1990s, virtually no dual modality contrast agents were reported until a few years back, for the lack of suitable equipment.[221] With rapid strides in technology, prototype PET/MRI systems have been successfully conceived for small-animal imaging, accelerating the research for novel bimodal magnetic radiotracers.[222, 223] Synchronous PET/MR imaging has the potential to become the imaging modality of choice for various clinical applications such as neurological studies, certain types of cancer, stroke, and the emerging field of stem cell therapy, warranting the development of hybrid PET/MR probes.[8] Depending on the constituents of the contrast agents, magnetic nanoparticles can be categorized into (i) superparamagnetic iron oxide nanoparticles (SPIONs) based T2 contrast agents, and (ii) paramagnetic gadolinium (Gd) or manganese (Mn) based T1 contrast agents. Readers are directed to these excellent reviews for a more comprehensive understanding of the fabrication, surface chemistry and biological applications of magnetic nanoparticles.[224–227]
In recent years, SPIONs have emerged as one of the most promising contrast agents in MRI for disease diagnosis and treatment monitoring. Phenomenal advances have been made in engineering iron oxide based magnetic contrast agents in terms of composition, structure, biocompatibility, relaxivity and contrast effects [217, 228, 229] and several SPION formulations have been approved in the clinic, such as Ferridex I.V.® for liver and spleen imaging, Ferumoxytol® for iron replacement therapy, and Combidex® for imaging lymph node metastases.[230]
SPIONs have been labeled with multiple radioisotopes for both SPECT (99mTc [231, 232], 125I [233], 111I [234, 235], 125I [236] and 131I [237, 238]) and PET.[239] Traditional approach involving chelating agents such as DOTA [240–243], NOTA [204, 244, 245], DTPA [246] and bis(dithiocarbamatebisphosphonate) (DTCPB) [247, 248] for 64Cu and 68Ga, and DFO for 89Zr [249] has been reported in an array of differently designed and surface-functionalized SPIONs. For example, Thorek and coworkers reported a simple 89Zr-labeled version of clinically relevant ferumoxytol with excellent toxicity and clearance profile, for high resolution investigation of lymphatic drainage in murine cancer models.[249] The nanoformulation not only allowed for clear preoperative mapping for nodal resection, but also provided non-invasive intra-operative guidance for tumor staging and therapy planning. Besides radiometals, traditional radioisotopes like 18F[250], 11C[251] and 124I[252] have also been variously used for dual PET/MR imaging in vivo.
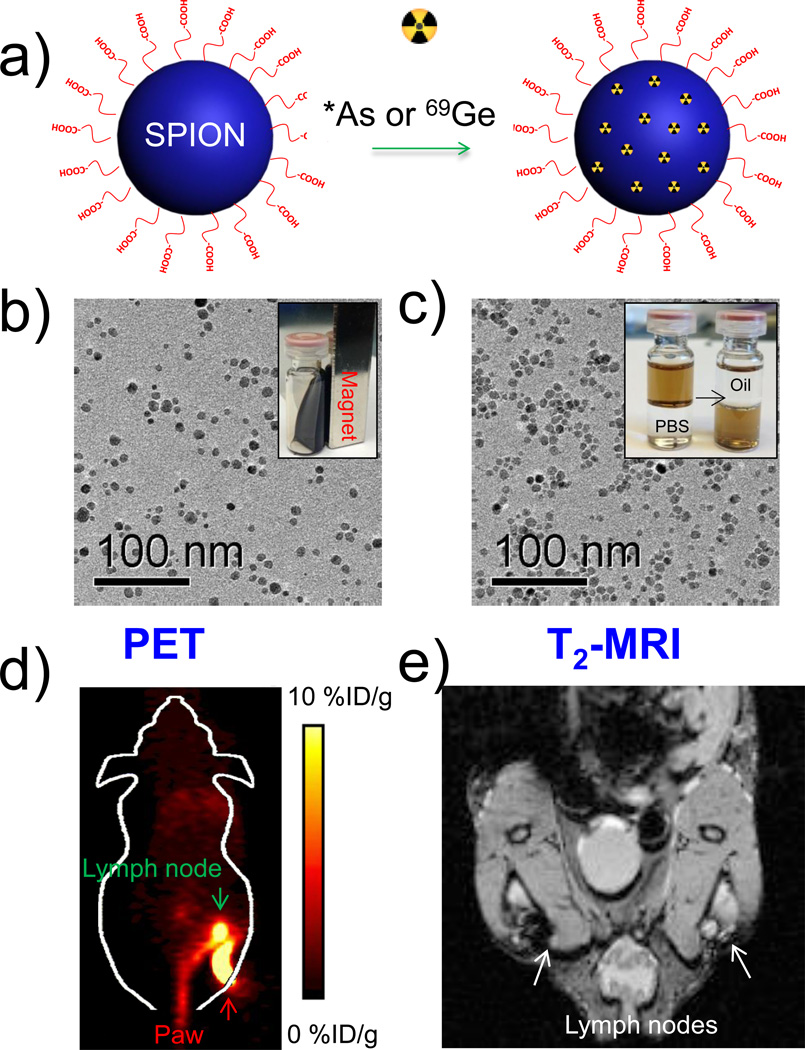
Development of new radioisotopes for PET imaging and lack of chelators thereof has prompted active research in the development of intrinsically radiolabeled SPIONs. Owing to their versatile chemistry, SPIONs have served as perfect hosts for some isotopes, which were difficult to label via traditional chelator-based routes.[253] For example, our group recently demonstrated intrinsic labeling of radioarsenic (*AsIII and *AsV, *=71, 72, 74, 76) and germanium-69 (69Ge; t½ = 39.05 h, 21% β+, Emax = 1205 keV) at the surface of SPIONs.[24, 25] (Figure 8) Rapid and specific labeling with high yields could be achieved simply by mixing water soluble poly(acrylic acid) (PAA)-modified SPIONs with these isotopes. PEGylated radiolabeled SPIONs were further used for in vivo PET/MR imaging and sentinel lymph node mapping. In addition, conventional radiometals, such as 64Cu[254] and 68Ga [255], have also been incorporated into SPIONs to develop novel multimodal tracers For example, heat induced 89Zr binding on ultrasmall magnetite cores (120 °C, pH 8) resulted in a radiochemical yield of ~93% and high stability as determined by in vivo PET/CT and biodistribution studies.[256] 64Cu and 111In radiometals could be similarly labeled indicating that tight metal ion binding to the magnetite crystal surface could likely be due to interaction between the positively charged metal ions and the anionic oxide surface layer.[256] The strategy was also applied to feraheme nanoparticles for monocyte tracking. [253] Furthermore, the technique can potentially be extended to other metal/metal oxide-based nanoparticles to develop novel, clinically translatable, multimodality PET/MR radiotracers.
Figure 8.
(a) Schematic illustration of chelator-free synthesis of *As (Or 69Ge)-SPION. (b) TEM image of oleic acid capped SPIONs (SPIONOA); inset shows the ferrofluidic behavior of SPIONOA. (c) TEM image of poly(acrylic acid)-modified SPIONs (SPIONPAA);inset depicts transfer of the SPIONs from oil phase to water phase. (d) In vivo lymph node PET imaging 2.5 h after subcutaneous injection of *As-SPIONPEG. (e) In vivo MR lymph node mapping 15 h post injection of SPIONPAA into the left footpad of the mouse. Adapted with permission from [24]. Copyright by John Wiley and Sons.
One of the most frequently used contrast agents in clinics (Gd3+ chelates) comprises of paramagnetic complexes (usually Gd3+ and Mn2+) that shorten the longitudinal (T1) relaxation time of water, thus increasing the signal intensity of T1 weighted MR images.[257, 258] To develop more efficient agents with larger number of magnetic centers, Gd/Mn based nanomaterials are being steadily developed.[225, 259] However, the progress of dual modal paramagnetic radiotracers is still slow paced.[260–262] Huang et al. first reported the synthesis of water soluble human serum albumin (HSA)-coated MnO nanoparticles (MONPs), which were subsequently used for dual modality PET/MR imaging in U87MG murine xenografts after 64Cu-DOTA coupling.[260] R1 relaxivity of HSA-MONPs was evaluated to be 1.97 mM−1s−1 which is close but inferior to the clinical standard, Magnevist (5 mM−1s−1). In another noteworthy study, radioactive fluorimagnetic GdVO4:Eu3+ tetragonal ultrathin nanosheets were developed and evaluated for PET, fluorescence and T1-weighted MR imaging in PC-3 prostate tumor bearing mice.[262] The nanoconjugates were labeled with 64Cu via DOTA chelation and integrin α2β1 targeting was achieved via Asp-Gly-Ala (DGEA) peptide conjugation. The R1 value was estimated to be 33.25 mM−1 s−1, approximately 7 times higher than the commercial T1-enhanced MRI agents.
Recent efforts to integrate multiple modalities have led to nanosystems with not only augmented diagnostic capabilities, but also simultaneous drug delivery and therapeutic functionalities. Important capabilities of magnetic nanoparticles for therapy are the external control of magnetic heat generation and magnetic attractive forces for enhanced transportation and targeted movement of nanoparticle-conjugated therapeutic moieties.[263] While theranostic applications of parmagnetic nanomaterials are yet to be explored, reports using SPIONs span over a wide range and include drug delivery [227, 264, 265], gene therapy [266], immunotherapy [267], hyperthermia [268–271] and photodynamic therapy (upon complexation with photosensitizers) [272].
4.6. Other Radioactive Nanomaterials
Search for novel and effective agents with improved imaging and therapeutic outcomes is the primary fuel for innovations in molecular theranostics. In addition to the already established arsenal of nanoradiotracers, numerous other nanomaterials have been engineered and explored for use in cancer theranostics in the past few years. For instance, chalcogenides, especially copper sulfide nanoparticles (CuS NPs) have gained significant attention from the research community as photothermal contrast agents and have been employed for several biomedical applications including PAT [273–275], PTT [276–278], and drug delivery [279]. Similar to many other nanoplatforms discussed in this article, CuS nanoparticles may be synthesized in different shapes and sizes; nanospheres, nanocages, nanoflowers, nanoplates, nanotubes, nanorods, and nanowires[280]. Zhou et al. first developed radiolabeled CuS nanoparticles, where 64Cu was integrated into the CuS matrix, for combined PET imaging and photothermal ablation of tumors.[281] The strategy was extended to develop CuS NPs for tumor-targeted PET imaging-guided photothermal therapy [17], combined radio- and photothermal therapies [282, 283], and ultrasmall CuS nanodots.[284] While addition of radioactive precursor during synthesis ensures high incorporation efficiency, the cumbersome chemistry and handling procedures impede clinical translation.[20] Riedinger et al. recently proposed a post-synthetic mechanism for incorporation of 64Cu(I) radionuclides in covellite nanocrystals (CuS NCs) by reduction with ascorbic acid. High radiochemical yield (~50 %) and radiochemical purity (~99 %) could be obtained by simply mixing the NCs with 64CuCl2 and vitamin C at room temperature.[285] In another report, the affinity of 64Cu for dichalcogenides was exploited by Liu et al. to develop a tetramodal (PET/MR/PAT/PTT) theranostic agent based on self-assembled MoS2 nanosheet and iron oxide nanocomposites.[286] Strong PAT signals and obvious darkening effects in T2 weighted MR images indicated prominent, time dependent passive tumor retention of double-PEGylated nanoconstructs, further corroborated by quantitative PET data (~6%ID/g accumulation in tumors). Complete tumor resection was seen within 14 days upon irradiation with 808 nm laser (0.78 W cm−2, 5 min) 8 h after i.v. injection of MoS2-IO-(d)PEG. Similarly, FeSe2-decorated Bi2Se3 nanosheets were developed by the same group for tetramodal imaging (PET/CT/PAT/MR) and integrated photothermal-radiation therapy.[287] Though preliminary, these studies highlight the promising potential of transitional metal dichalcogenides for multimodal image-guided cancer therapy. In addition, other nanomaterials; copper nanoparticles and nanoclusters [288, 289], oxides of titanium [290], zinc [291], aluminum [29, 30], cerium [292], layered double hydroxides [293], and oxysulfides [294] have also shown promising results as multifunctional theranostic radiotracers (Figure 9a–f).
Figure 9.
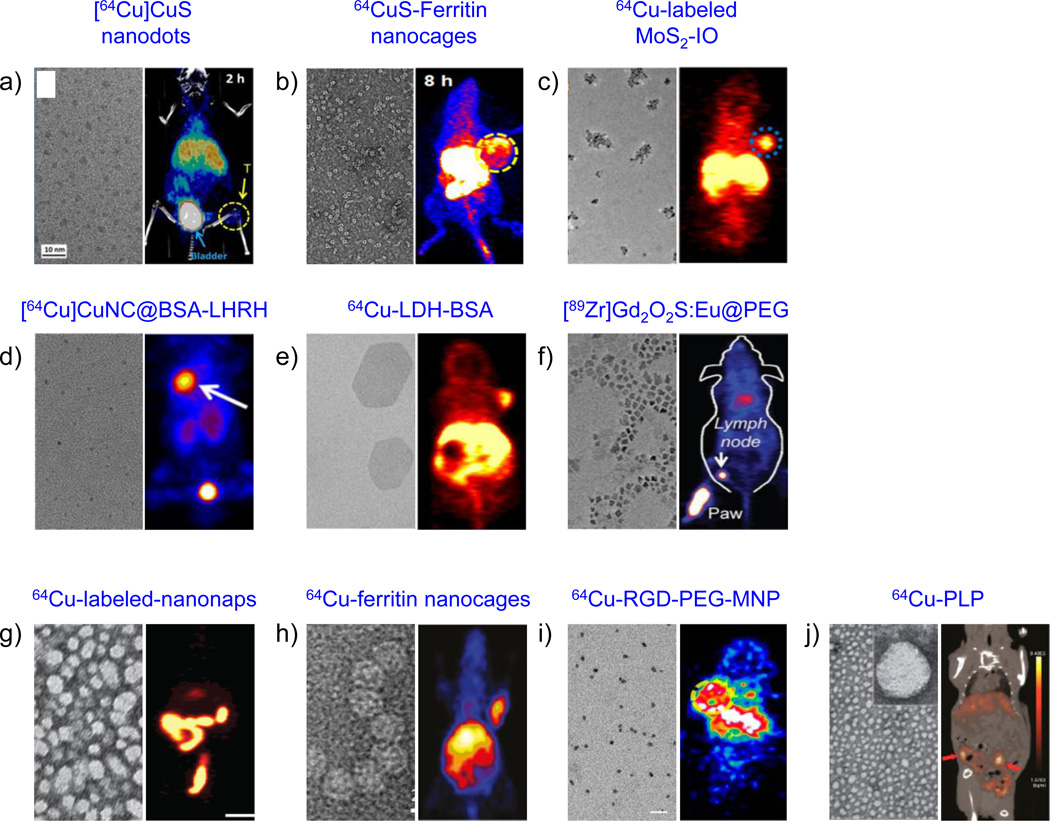
Representative examples of other inorganic (a–f) and organic (g–j) nanomaterials employed for PET-guided cancer theranostics. Left panel: TEM images, right panel: in vivo PET images. (a) Copper sulfide nanodots; PET/CT image depicts 4T1 tumor uptake (yellow arrow) and dominant renal clearance (blue arrow; bladder) after i.v. injection of 5.6 nm sized [64Cu]CuS nanodots. Reproduced with permission from [284]. (b) Biomimetic CuS-Ferritin nanocages. TEM image depicts a dark CuS core inside a ferritin cage and PET image shows U87MG tumors (yellow circle), 8 h after i.v. injection of 64CuS-Ferritin nanocages. Reproduced with permission from [309]. (c) Iron oxide decorated MoS2 nanosheets. TEM image shows double-PEGylated MoS2-IO and PET image shows enhanced EPR-mediated uptake of intrinsically 64Cu-labeled MoS2-IO in 4T1 tumor-bearing mice (blue circle), 24 h p.i. Reproduced with permission from [286]. (d) High resolution TEM image of ultrasmall BSA-coated Cu nanoclusters (CuNCBSA). PET image depicts LHRH peptide-aided uptake of [64Cu]CuNCBSA-LHRH in orthotopic A549 lung tumor bearing mice (white arrow). Reproduced with permission from [288]. Copyright of American Chemical Society. (e) Layered double hydroxide (LDH) nanoparticles. Coronal PET image 16 h p.i. of 64Cu-LDH-BSA shows enhanced 4T1 tumor uptake. Reproduced with permission from [293]. Copyright of Nature Publishing Group. (f) TEM image shows Gd2O2S:Eu nanoparticles before PEGylation. PET-based lymph node mapping shows rapid delineation of sentinel lymph nodes 0.5 h after injection of [89Zr]Gd2O2S:EuPEG nanoparticles. Reproduced with permission from [294]. Copyright of John Wiley and Sons. (g) TEM image of ~20 nm frozen naphthalocyanine micelles (nanonaps) and PET image of 64Cu-labeled-nanonaps, 3 h after oral gavage. Reproduced with permission from [300]. Copyright of Nature Publishing Group. (h) TEM image of hybrid ferritin nanocages, conjugated to RGD targeting moiety and Cy5.5 dye. PET images of cRGDyK targeting of U87MG tumors, 24 h after the administration of ferritin nanoprobes. Reproduced with permission from [306]. (i) TEM image of multimodal PEGylated melanin nanoparticles (MNP; 10.7 nm) and PET images of 64Cu-RGD-PEG-MNP in U87MG tumor-bearing mice, 24 h p.i. Reproduced with permission from [305] (j) TEM shows a core-shell spherical structure of porphylipoprotein (PLP). PET/CT image of mouse with ovarian cancer metastasis after 24 h intravenous injection of 64Cu-PLP (red arrow: tumor). Reproduced with permission from [299]. Copyright of American Chemical Society.
Among the organic nanomaterials, lipid-based nanoparticles, micelles, dendrimers, and polymers are the traditional choices and have been best characterized as PET imaging probes.[295] Porphysomes are a new class of organic, biodegradable nanovesicles, composed of porphyrin and lipid bilayers. The porphyrin component allows direct loading of 64Cu into the tetrapyrrole ring without compromising their intrinsic photothermal properties and in vivo pharmacokinetics.[296, 297] [64Cu]porphysomes demonstrated good in vivo radiostability and selective uptake in orthotopic prostate tumor models. Other porphyrin-based systems have since been utilized for development of multifunctional nanoprobes for a variety of applications.[140, 298–301] Recently, there is an upward trend of employing nanoparticles with intrinsic biological functionalities for theranostic applications.[302, 303] The biomimetic nanoparticles are biocompatible, biodegradable and most importantly, overcome the precursor toxicity issues that plague the clinical translation of current organic and inorganic nanomaterials. One very interesting example is melanin nanoparticles (MNPs) with intrinsic photoacoustic property and natural affinity for metals (64Cu2+ and Fe3+), that could be harnessed for multiple imaging modalities like PET, MR, PAT, photothermal imaging and therapy via PTT, all combined into one nanoplatform.[304, 305] Another notable example of biomimicry applied for cancer theranostics is self-assembled ferritin nanocages with inherent propensity to bind metal ions for PET/MR imaging and load photosensitizers, optical dyes and other therapeutic molecules to develop a multifunctional nanoplatform.[306–308] In a recent study, ferritin nanocages were also utilized as templates for controlled synthesis of ultrasmall [64Cu]CuS nanoparticles for PA/PET-directed photothermal therapy.[309] Engineering of biomimetic nanomaterials is an emerging field that holds immense potential for combating several issues presented by the use of synthetic nanomaterials. While bioinspired systems have shown promising outcomes for a variety of biomedical applications, such approaches remain untapped in the field of nuclear nanomedicine. Systems like membrane-coated nanomaterials, tumor cell and macrophage mimetics, viral vectors and others, have the potential to revolutionize the current state of cancer theranostics, if utilized properly. On the flip side, contemporary biomimetic nanotechnologies lack the exquisite precision and controllability in terms of synthesis, functionalization and characterization that is afforded by inorganic nanoparticles, and which must be addressed before any clinical applications can be envisaged.
5. Conclusion and Future Perspectives
Radiation oncology has emerged as a frontrunner in precision medicine and personalized treatment planning. Integration of nanotechnology into the former promises to bring a paradigm shift in the traditional cancer imaging and therapy regimes. Compared to the currently used biological radiotracers, nanomaterials represent an exciting class of novel molecular probes which can be equipped with various imaging labels, targeting ligands and therapeutic moieties, all on the same vehicle. In this review we presented studies exemplifying our current prowess in developing radiolabeled nanoprobes, and tailoring their structural, physicochemical and surface properties to achieve desired in vivo pharmacokinetics and improved imaging and therapeutic outcomes. However, despite its immense initial promise and enormous investment (of capital and resources) over the last decade, nanomedicine has been conspicuous by its absence in the clinic.
With the growing importance of personalized medicine and need for translational research, there is an urgent need to evaluate nanoparticle-induced long-term toxicities as well as effects on reproductive and fetal health. The quantitative nature, limitless tissue penetration and sensitivity of PET can afford excellent means for long-term tracking of radiolabeled nanoparticles in living subjects. For instance, using 64Cu-DOTA labeled MWNTs, Huang et al. demonstrated that CNTs might induce genetic background-dependent toxic effects on the normal development of the embryo.[310] Larger-sized MWNTs could move across the blood-placenta barrier, restricted fetal development, and induced brain deformity, while SWNTs and smaller MWNTs showed reduced fetotoxicity. This is an excellent example of the importance of robust risk-assessment of potential toxic responses, particularly long-term toxic effects, and individual-dependent toxic responses, for all nanoplatforms before they can be tested in human subjects to image and treat diseases.
Besides toxicity, three key issues require immediate attention; development of targeted nanoparticles with enhanced selectivity to achieve optimal efficacy, improved in vivo pharmacokinetics with minimal accumulation in the RES organs like liver and spleen, and most importantly, complete clearance from the body within a reasonable time. More efforts are required to improve nanoparticle delivery both to and within the tumor. Development of actively targeted nanoparticles, especially for quantification of low expression biomarkers could assist in early disease detection and prevention. Careful selection of the target as well as the targeting ligands (e.g. antibody, peptide, small protein) is necessary for achieving optimal specific binding, and must be determined based on the nanoparticle shape, size, charge, surface properties etc. Detailed studies must be carried out to understand long-term interactions of the nanoparticles with the tumor and especially the major organs of accumulation such as the liver and spleen. Evasion of the mononuclear phagocyte system (MPS) can be achieved by designing ultrasmall globular renally clearable nanoparticles, as discussed before. While such nanoparticles promise better imaging characteristics, the short circulation time, rapid clearance and attenuated tumor accumulation might hinder their therapeutic potential. Biodegradable nanoparticles with chemically unstable structures may prove more useful in this regard. We recently reported a biodegradable silica platform, tuned to carry large payloads of both small and large molecular drugs, circulate longer in the blood, actively target the tumor vasculature for maximum therapeutic effect, and degrade and clear from the body after serving its purpose.[311] However, such results are preliminary; complete and in-depth evaluation of the nanoparticle properties, dosages required to achieve the desired effects, systemic accumulation, tissue and organ distributions, excretion profiles, and long-term effects of nanoparticle administration, is urgently needed for nanomedicine to make any appreciable mark in the field. In addition, considering the important role that PET can play in profiling the biological fate of nanomaterials, development of facile, robust, stable and personnel-friendly radiochemistry is a prerequisite.
Despite the lackluster performance of nanomaterials in clinical translation, nanomedicine continues to be a fascinating area of research with distinct advantages to healthcare, if properly harnessed. Efforts must be made to tailor the medical applications to nanoparticle properties. For example, nanoparticles exhibiting enhanced RES sequestration can be harnessed for immunotherapy and vaccine development. Phototherapeutic nanomaterials can be used to treat solid tumors, where the resulting hyperthermia can enable deeper nanoparticle/drug extravasation into the tumor recesses. Similarly, magnetic nanoparticles can be employed for magnetic field-guided targeted delivery to disease site, to achieve superior control over nanoparticle transport in vivo and enhanced therapeutic indices. Deeper and more rigorous examination of physicochemical and biological properties of the existing repertoire of nanomaterials needs to replace or at least progress in tandem with the current trend of “proof-of-concept” studies. The possibilities are endless, if the scientific community is willing to overhaul the current approach to nanomedicine with a single focus on translation. Concerted multidisciplinary efforts, from scientists, clinicians, funding and regulatory authorities, manufacturing agencies etc. may help realize the true potential of nanotheranostics in early-stage disease detection, treatment efficacy, monitoring of disease progression, regression and recurrence in the future.
Acknowledgments
This work is supported, in part, by the University of Wisconsin- Madison, the National Institutes of Health (NIBIB/NCI 1R01CA169365, P30CA014520, and T32CA009206) and the American Cancer Society (125246-RSG-13-099-01-CCE).
REFERENCES
- 1.Weissleder R. Molecular imaging in cancer. Science (New York, N.Y.) 2006;312:1168–1171. doi: 10.1126/science.1125949. [DOI] [PubMed] [Google Scholar]
- 2.Weissleder R, Mahmood U. Molecular imaging. Radiology. 2001;219:316–333. doi: 10.1148/radiology.219.2.r01ma19316. [DOI] [PubMed] [Google Scholar]
- 3.James ML, Gambhir SS. A molecular imaging primer: modalities, imaging agents, and applications. Physiol Rev. 2012;92:897–965. doi: 10.1152/physrev.00049.2010. [DOI] [PubMed] [Google Scholar]
- 4.Cai W, Chen X. Nanoplatforms for targeted molecular imaging in living subjects. Small. 2007;3:1840–1854. doi: 10.1002/smll.200700351. [DOI] [PubMed] [Google Scholar]
- 5.Hong H, Chen F, Zhang Y, Cai W. New radiotracers for imaging of vascular targets in angiogenesis-related diseases. Adv Drug Deliv Rev. 2014;76:2–20. doi: 10.1016/j.addr.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phelps ME, Hoffman EJ, Mullani NA, Ter-Pogossian MM. Application of annihilation coincidence detection to transaxial reconstruction tomography. J Nucl Med. 1975;16:210–224. [PubMed] [Google Scholar]
- 7.Gambhir SS. Molecular imaging of cancer with positron emission tomography. Nat Rev Cancer. 2002;2:683–693. doi: 10.1038/nrc882. [DOI] [PubMed] [Google Scholar]
- 8.Hong H, Zhang Y, Sun J, Cai W. Molecular imaging and therapy of cancer with radiolabeled nanoparticles. Nano Today. 2009;4:399–413. doi: 10.1016/j.nantod.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jokerst JV, Gambhir SS. Molecular imaging with theranostic nanoparticles. Acc Chem Res. 2011;44:1050–1060. doi: 10.1021/ar200106e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El-Sayed IH. Nanotechnology in head and neck cancer: the race is on. Curr Oncol Rep. 2010;12:121–128. doi: 10.1007/s11912-010-0087-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. https://clinicaltrials.gov/ct2/show/NCT00356980. A Phase I rrial of TNF-bound colloidal gold (CYT-6091) by intravenous administration in subjects with advanced solid organ malignancies. [Accessed: April 30, 2016];2006
- 12. https://clinicaltrials.gov/ct2/show/NCT00848042. a pilot study of aurolase(tm) therapy in patients with refractory and/or recurrent tumors of the head and neck. [Accessed: April 30, 2016];2009
- 13. https://clinicaltrials.gov/show/NCT02106598. targeted silica nanoparticles for image-guided intraoperative sentinel lymph node mapping in head and neck melanoma, breast and cervical/uterine cancer patients. [Accessed: April 30, 2016];2014
- 14.Ernsting MJ, Murakami M, Roy A, Li SD. Factors controlling the pharmacokinetics, biodistribution and intratumoral penetration of nanoparticles. J Control Release. 2013;172:782–794. doi: 10.1016/j.jconrel.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, Bawendi MG, Frangioni JV. Renal clearance of quantum dots. Nat Biotechnol. 2007;25:1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chakravarty R, Hong H, Cai W. Positron emission tomography image-guided drug delivery: current status and future perspectives. Mol Pharm. 2014;11:3777–3797. doi: 10.1021/mp500173s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou M, Song SL, Zhao J, Tian M, Li C. Theranostic CuS nanoparticles targeting folate receptors for PET image-guided photothermal therapy. J Mater Chem B. 2015;3:8939–8948. doi: 10.1039/C5TB01866H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pang B, Zhao Y, Luehmann H, Yang X, Detering L, You M, Zhang C, Zhang L, Li ZY, Ren Q, Liu Y, Xia Y. (6)(4)Cu-doped PdCu@Au tripods: A multifunctional nanomaterial for positron emission tomography and image-guided photothermal cancer treatment. ACS Nano. 2016;10:3121–3131. doi: 10.1021/acsnano.5b07968. [DOI] [PubMed] [Google Scholar]
- 19.Perez-Medina C, Abdel-Atti D, Tang J, Zhao Y, Fayad ZA, Lewis JS, Mulder WJ, Reiner T. Nanoreporter PET predicts the efficacy of anti-cancer nanotherapy. Nat Commun. 2016;7 doi: 10.1038/ncomms11838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goel S, Chen F, Ehlerding EB, Cai W. Intrinsically radiolabeled nanoparticles: an emerging paradigm. Small. 2014;10:3825–3830. doi: 10.1002/smll.201401048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun X, Huang X, Yan X, Wang Y, Guo J, Jacobson O, Liu D, Szajek LP, Zhu W, Niu G, Kiesewetter DO, Sun S, Chen X. Chelator-free (64)Cu-integrated gold nanomaterials for positron emission tomography imaging guided photothermal cancer therapy. ACS Nano. 2014;8:8438–8446. doi: 10.1021/nn502950t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaffer TM, Wall MA, Harmsen S, Longo VA, Drain CM, Kircher MF, Grimm J. Silica nanoparticles as substrates for chelator-free labeling of oxophilic radioisotopes. Nano Lett. 2015;15:864–868. doi: 10.1021/nl503522y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen F, Goel S, Valdovinos HF, Luo H, Hernandez R, Barnhart TE, Cai W. In vivo integrity and biological fate of chelator-free Zirconium-89-labeled mesoporous silica nanoparticles. ACS Nano. 2015;9:7950–7959. doi: 10.1021/acsnano.5b00526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen F, Ellison PA, Lewis CM, Hong H, Zhang Y, Shi S, Hernandez R, Meyerand ME, Barnhart TE, Cai W. Chelator-free synthesis of a dual-modality PET/MRI agent. Angew Chem Int Ed Engl. 2013;52:13319–13323. doi: 10.1002/anie.201306306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chakravarty R, Valdovinos HF, Chen F, Lewis CM, Ellison PA, Luo H, Meyerand ME, Nickles RJ, Cai W. Intrinsically Germanium-69-labeled iron oxide nanoparticles: synthesis and in-vivo dual-modality PET/MR imaging. Adv Mater. 2014;26:5119–5123. doi: 10.1002/adma.201401372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun Y, Liu Q, Peng J, Feng W, Zhang Y, Yang P, Li F. Radioisotope post-labeling upconversion nanophosphors for in vivo quantitative tracking. Biomaterials. 2013;34:2289–2295. doi: 10.1016/j.biomaterials.2012.11.047. [DOI] [PubMed] [Google Scholar]
- 27.Liu Q, Sun Y, Li C, Zhou J, Yang T, Zhang X, Yi T, Wu D, Li F. 18F-Labeled magnetic-upconversion nanophosphors via rare-earth cation-assisted ligand assembly. ACS Nano. 2011;5:3146–3157. doi: 10.1021/nn200298y. [DOI] [PubMed] [Google Scholar]
- 28.Sun X, Huang X, Guo J, Zhu W, Ding Y, Niu G, Wang A, Kiesewetter DO, Wang ZL, Sun S, Chen X. Self-illuminating 64Cu-doped CdSe/ZnS nanocrystals for in vivo tumor imaging. J Am Chem Soc. 2014;136:1706–1709. doi: 10.1021/ja410438n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perez-Campana C, Gomez-Vallejo V, Martin A, San Sebastian E, Moya SE, Reese T, Ziolo RF, Llop J. Tracing nanoparticles in vivo: a new general synthesis of positron emitting metal oxide nanoparticles by proton beam activation. The Analyst. 2012;137:4902–4906. doi: 10.1039/c2an35863h. [DOI] [PubMed] [Google Scholar]
- 30.Perez-Campana C, Gomez-Vallejo V, Puigivila M, Martin A, Calvo-Fernandez T, Moya SE, Ziolo RF, Reese T, Llop J. Biodistribution of different sized nanoparticles assessed by positron emission tomography: a general strategy for direct activation of metal oxide particles. ACS Nano. 2013;7:3498–3505. doi: 10.1021/nn400450p. [DOI] [PubMed] [Google Scholar]
- 31. http://www.fda.gov/downloads/food/ingredientspackaginglabeling/gras/noticeinventory/ucm269494 Generally recognized as safe determination for silicon dioxide when added directly and/or indirectly to human food. [Accessed: April 30, 2016];2010
- 32.Vivero-Escoto JL, Huxford-Phillips RC, Lin W. Silica-based nanoprobes for biomedical imaging and theranostic applications. Chem Soc Rev. 2012;41:2673–2685. doi: 10.1039/c2cs15229k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen F, Hong H, Goel S, Graves SA, Orbay H, Ehlerding EB, Shi S, Theuer CP, Nickles RJ, Cai W. In vivo tumor vasculature targeting of CuS@MSN based theranostic nanomedicine. ACS Nano. 2015;9:3926–3934. doi: 10.1021/nn507241v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burke BP, Baghdadi N, Clemente GS, Camus N, Guillou A, Kownacka AE, Domarkas J, Halime Z, Tripier R, Archibald SJ. Final step Gallium-68 radiolabelling of silica-coated iron oxide nanorods as potential PET/MR multimodal imaging agents. Farad Discuss. 2014;175:59–71. doi: 10.1039/c4fd00137k. [DOI] [PubMed] [Google Scholar]
- 35.Burke BP, Baghdadi N, Kownacka AE, Nigam S, Clemente GS, Al-Yassiry MM, Domarkas J, Lorch M, Pickles M, Gibbs P, Tripier R, Cawthorne C, Archibald SJ. Chelator free Gallium-68 radiolabelling of silica coated iron oxide nanorods via surface interactions. Nanoscale. 2015;7:14889–14896. doi: 10.1039/c5nr02753e. [DOI] [PubMed] [Google Scholar]
- 36.Phillips E, Penate-Medina O, Zanzonico PB, Carvajal RD, Mohan P, Ye Y, Humm J, Gonen M, Kalaigian H, Schoder H, Strauss HW, Larson SM, Wiesner U, Bradbury MS. Clinical translation of an ultrasmall inorganic optical-PET imaging nanoparticle probe. Sci Transl Med. 2014;6:260ra149. doi: 10.1126/scitranslmed.3009524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benezra M, Penate-Medina O, Zanzonico PB, Schaer D, Ow H, Burns A, DeStanchina E, Longo V, Herz E, Iyer S, Wolchok J, Larson SM, Wiesner U, Bradbury MS. Multimodal silica nanoparticles are effective cancer-targeted probes in a model of human melanoma. J Clin Invest. 2011;121:2768–2780. doi: 10.1172/JCI45600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma K, Mendoza C, Hanson M, Werner-Zwanziger U, Zwanziger J, Wiesner U. Control of ultrasmall sub-10 nm ligand-functionalized fluorescent core–shell silica nanoparticle growth in water. Chem Mater. 2015;27:4119–4133. [Google Scholar]
- 39.Kumar R, Roy I, Ohulchanskky TY, Vathy LA, Bergey EJ, Sajjad M, Prasad PN. In vivo biodistribution and clearance studies using multimodal organically modified silica nanoparticles. ACS Nano. 2010;4:699–708. doi: 10.1021/nn901146y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang L, Yang X, Dobrucki LW, Chaudhury I, Yin Q, Yao C, Lezmi S, Helferich WG, Fan TM, Cheng J. Aptamer-functionalized, ultra-small, monodisperse silica nanoconjugates for targeted dual-modal imaging of lymph nodes with metastatic tumors. Angew Chem Int Ed Engl. 2012;51:12721–12726. doi: 10.1002/anie.201205271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vallet-Regi M, Rámila A, del Real RP, Pérez-Pariente J. A new property of MCM-41: Drug delivery system. Chem Mater. 2001;13:308–311. [Google Scholar]
- 42.Tang F, Li L, Chen D. Mesoporous silica nanoparticles: synthesis, biocompatibility and drug delivery. Adv Mater. 2012;24:1504–1534. doi: 10.1002/adma.201104763. [DOI] [PubMed] [Google Scholar]
- 43.Chen F, Hong H, Zhang Y, Valdovinos HF, Shi S, Kwon GS, Theuer CP, Barnhart TE, Cai W. In vivo tumor targeting and image-guided drug delivery with antibody-conjugated, radiolabeled mesoporous silica nanoparticles. ACS Nano. 2013;7:9027–9039. doi: 10.1021/nn403617j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goel S, Chen F, Hong H, Valdovinos HF, Hernandez R, Shi S, Barnhart TE, Cai W. VEGF(1)(2)(1)-conjugated mesoporous silica nanoparticle: a tumor targeted drug delivery system. ACS Appl Mater Interfaces. 2014;6:21677–21685. doi: 10.1021/am506849p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen F, Nayak TR, Goel S, Valdovinos HF, Hong H, Theuer CP, Barnhart TE, Cai W. In vivo tumor vasculature targeted PET/NIRF imaging with TRC105(Fab)-conjugated, dual-labeled mesoporous silica nanoparticles. Mol Pharm. 2014;11:4007–4014. doi: 10.1021/mp500306k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee SB, Kim HL, Jeong HJ, Lim ST, Sohn MH, Kim DW. Mesoporous silica nanoparticle pretargeting for PET imaging based on a rapid bioorthogonal reaction in a living body. Angew Chem Int Ed Engl. 2013;52:10549–10552. doi: 10.1002/anie.201304026. [DOI] [PubMed] [Google Scholar]
- 47.Miller L, Winter G, Baur B, Witulla B, Solbach C, Reske S, Linden M. Synthesis, characterization, and biodistribution of multiple 89Zr-labeled pore-expanded mesoporous silica nanoparticles for PET. Nanoscale. 2014;6:4928–4935. doi: 10.1039/c3nr06800e. [DOI] [PubMed] [Google Scholar]
- 48.Qi G, Wang Y, Estevez L, Switzer AK, Duan X, Yang X, Giannelis EP. Facile and Scalable Synthesis of Monodispersed Spherical Capsules with a Mesoporous Shell. Chem Mater. 2010;22:2693–2695. [Google Scholar]
- 49.Lin YS, Wu SH, Tseng CT, Hung Y, Chang C, Mou CY. Synthesis of hollow silica nanospheres with a microemulsion as the template. Chem Commun. 2009;28:3542–3544. doi: 10.1039/b902681a. [DOI] [PubMed] [Google Scholar]
- 50.Li L, Tang F, Liu H, Liu T, Hao N, Chen D, Teng X, He J. In vivo delivery of silica nanorattle encapsulated docetaxel for liver cancer therapy with low toxicity and high efficacy. ACS Nano. 2010;4:6874–6882. doi: 10.1021/nn100918a. [DOI] [PubMed] [Google Scholar]
- 51.Luo Z, Ding X, Hu Y, Wu S, Xiang Y, Zeng Y, Zhang B, Yan H, Zhang H, Zhu L, Liu J, Li J, Cai K, Zhao Y. Engineering a hollow nanocontainer platform with multifunctional molecular machines for tumor-targeted therapy in vitro and in vivo. ACS Nano. 2013;7:10271–10284. doi: 10.1021/nn404676w. [DOI] [PubMed] [Google Scholar]
- 52.Shi S, Chen F, Cai W. Biomedical applications of functionalized hollow mesoporous silica nanoparticles: focusing on molecular imaging. Nanomedicine. 2013;8:2027–2039. doi: 10.2217/nnm.13.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fan W, Shen B, Bu W, Chen F, Zhao K, Zhang S, Zhou L, Peng W, Xiao Q, Xing H, Liu J, Ni D, He Q, Shi J. Rattle-structured multifunctional nanotheranostics for synergetic chemo-/radiotherapy and simultaneous magnetic/luminescent dual-mode imaging. J Am Chem Soc. 2013;135:6494–6503. doi: 10.1021/ja312225b. [DOI] [PubMed] [Google Scholar]
- 54.Chen Y, Chen H, Sun Y, Zheng Y, Zeng D, Li F, Zhang S, Wang X, Zhang K, Ma M, He Q, Zhang L, Shi J. Multifunctional mesoporous composite nanocapsules for highly efficient MRI-guided high-intensity focused ultrasound cancer surgery. Angew Chem Int Ed Engl. 2011;50:12505–12509. doi: 10.1002/anie.201106180. [DOI] [PubMed] [Google Scholar]
- 55.Chen F, Hong H, Shi S, Goel S, Valdovinos HF, Hernandez R, Theuer CP, Barnhart TE, Cai W. Engineering of hollow mesoporous silica nanoparticles for remarkably enhanced tumor active targeting efficacy. Sci Rep. 2014;4 doi: 10.1038/srep05080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Al Faraj A, Alotaibi B, Shaik AP, Shamma KZ, Al Jammaz I, Gerl J. Sodium-22-radiolabeled silica nanoparticles as new radiotracer for biomedical applications: in vivo positron emission tomography imaging, biodistribution, and biocompatibility. Int J Nanomedicine. 2015;10:6293–6302. doi: 10.2147/IJN.S93523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kroto HW, Heath JR, O'Brien SC, Curl RF, Smalley RE. C60: Buckminsterfullerene. Nature. 1985;318:162–163. [Google Scholar]
- 58.Iijima S. Helical microtubules of graphitic carbon. Nature. 1991;354:56–58. [Google Scholar]
- 59.Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA. Electric field effect in atomically thin carbon films. Science. 2004;306:666–669. doi: 10.1126/science.1102896. [DOI] [PubMed] [Google Scholar]
- 60.Xu X, Ray R, Gu Y, Ploehn HJ, Gearheart L, Raker K, Scrivens WA. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J Am Chem Soc. 2004;126:12736–12737. doi: 10.1021/ja040082h. [DOI] [PubMed] [Google Scholar]
- 61.Baker SN, Baker GA. Luminescent carbon nanodots: emergent nanolights. Angew Chem Int Ed Engl. 2010;49:6726–6744. doi: 10.1002/anie.200906623. [DOI] [PubMed] [Google Scholar]
- 62.Mochalin VN, Shenderova O, Ho D, Gogotsi Y. The properties and applications of nanodiamonds. Nat Nanotechnol. 2012;7:11–23. doi: 10.1038/nnano.2011.209. [DOI] [PubMed] [Google Scholar]
- 63.Hong G, Diao S, Antaris AL, Dai H. Carbon Nanomaterials for biological imaging and nanomedicinal therapy. Chem Rev. 2015;115:10816–10906. doi: 10.1021/acs.chemrev.5b00008. [DOI] [PubMed] [Google Scholar]
- 64.Gong H, Peng R, Liu Z. Carbon nanotubes for biomedical imaging: the recent advances. Adv Drug Deliv Rev. 2013;65:1951–1963. doi: 10.1016/j.addr.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 65.Yoo JM, Kang JH, Hong BH. Graphene-based nanomaterials for versatile imaging studies. Chem Soc Rev. 2015;44:4835–4852. doi: 10.1039/c5cs00072f. [DOI] [PubMed] [Google Scholar]
- 66.Welsher K, Liu Z, Sherlock SP, Robinson JT, Chen Z, Daranciang D, Dai H. A route to brightly fluorescent carbon nanotubes for near-infrared imaging in mice. Nat Nanotechnol. 2009;4:773–780. doi: 10.1038/nnano.2009.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim JW, Galanzha EI, Shashkov EV, Moon HM, Zharov VP. Golden carbon nanotubes as multimodal photoacoustic and photothermal high-contrast molecular agents. Nat Nanotechnol. 2009;4:688–694. doi: 10.1038/nnano.2009.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang K, Zhang S, Zhang G, Sun X, Lee ST, Liu Z. Graphene in mice: ultrahigh in vivo tumor uptake and efficient photothermal therapy. Nano Lett. 2010;10:3318–3323. doi: 10.1021/nl100996u. [DOI] [PubMed] [Google Scholar]
- 69.Liu Z, Li X, Tabakman SM, Jiang K, Fan S, Dai H. Multiplexed multicolor Raman imaging of live cells with isotopically modified single walled carbon nanotubes. J Am Chem Soc. 2008;130:13540–13541. doi: 10.1021/ja806242t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Malard LM, Pimenta MA, Dresselhaus G, Dresselhaus MS. Raman spectroscopy in graphene. Phys Rep. 2009;473:51–87. [Google Scholar]
- 71.Shi S, Chen F, Ehlerding EB, Cai W. Surface engineering of graphene-based nanomaterials for biomedical applications. Bioconjug Chem. 2014;25:1609–1619. doi: 10.1021/bc500332c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang H, Wang J, Deng X, Sun H, Shi Z, Gu Z, Liu Y, Zhao Y. Biodistribution of carbon single-wall carbon nanotubes in mice. J Nanosci Nanotechnol. 2004;4:1019–1024. doi: 10.1166/jnn.2004.146. [DOI] [PubMed] [Google Scholar]
- 73.Singh R, Pantarotto D, Lacerda L, Pastorin G, Klumpp C, Prato M, Bianco A, Kostarelos K. Tissue biodistribution and blood clearance rates of intravenously administered carbon nanotube radiotracers. Proc Natl Acad Sci USA. 2006;103:3357–3362. doi: 10.1073/pnas.0509009103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guo J, Zhang X, Li Q, Li W. Biodistribution of functionalized multiwall carbon nanotubes in mice. Nucl Med Biol. 2007;34:579–583. doi: 10.1016/j.nucmedbio.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 75.McDevitt MR, Chattopadhyay D, Jaggi JS, Finn RD, Zanzonico PB, Villa C, Rey D, Mendenhall J, Batt CA, Njardarson JT, Scheinberg DA. PET imaging of soluble yttrium-86-labeled carbon nanotubes in mice. PloS One. 2007;2:e907. doi: 10.1371/journal.pone.0000907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Georgin D, Czarny B, Botquin M, Mayne-L'hermite M, Pinault M, Bouchet-Fabre B, Carriere M, Poncy JL, Chau Q, Maximilien R, Dive V, Taran F. Preparation of (14)C-labeled multiwalled carbon nanotubes for biodistribution investigations. J Am Chem Soc. 2009;131:14658–14659. doi: 10.1021/ja906319z. [DOI] [PubMed] [Google Scholar]
- 77.Czarny B, Georgin D, Berthon F, Plastow G, Pinault M, Patriarche G, Thuleau A, L'Hermite MM, Taran F, Dive V. Carbon nanotube translocation to distant organs after pulmonary exposure: insights from in situ (14)C-radiolabeling and tissue radioimaging. ACS Nano. 2014;8:5715–5724. doi: 10.1021/nn500475u. [DOI] [PubMed] [Google Scholar]
- 78.Liu Z, Cai W, He L, Nakayama N, Chen K, Sun X, Chen X, Dai H. In vivo biodistribution and highly efficient tumour targeting of carbon nanotubes in mice. Nat Nanotechnol. 2007;2:47–52. doi: 10.1038/nnano.2006.170. [DOI] [PubMed] [Google Scholar]
- 79.McDevitt MR, Chattopadhyay D, Kappel BJ, Jaggi JS, Schiffman SR, Antczak C, Njardarson JT, Brentjens R, Scheinberg DA. Tumor targeting with antibody-functionalized, radiolabeled carbon nanotubes. J Nucl Med. 2007;48:1180–1189. doi: 10.2967/jnumed.106.039131. [DOI] [PubMed] [Google Scholar]
- 80.Ruggiero A, Villa CH, Holland JP, Sprinkle SR, May C, Lewis JS, Scheinberg DA, McDevitt MR. Imaging and treating tumor vasculature with targeted radiolabeled carbon nanotubes. Int J Nanomedicine. 2010;5:783–802. doi: 10.2147/IJN.S13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Swierczewska M, Choi KY, Mertz EL, Huang X, Zhang F, Zhu L, Yoon HY, Park JH, Bhirde A, Lee S, Chen X. A facile, one-step nanocarbon functionalization for biomedical applications. Nano Lett. 2012;12:3613–3620. doi: 10.1021/nl301309g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hong SY, Tobias G, Al-Jamal KT, Ballesteros B, Ali-Boucetta H, Lozano-Perez S, Nellist PD, Sim RB, Finucane C, Mather SJ, Green ML, Kostarelos K, Davis BG. Filled and glycosylated carbon nanotubes for in vivo radioemitter localization and imaging. Nat Mater. 2010;9:485–490. doi: 10.1038/nmat2766. [DOI] [PubMed] [Google Scholar]
- 83.Matson ML, Villa CH, Ananta JS, Law JJ, Scheinberg DA, Wilson LJ. Encapsulation of alpha-Particle-Emitting 225Ac3+ Ions Within Carbon Nanotubes. J Nucl Med. 2015;56:897–900. doi: 10.2967/jnumed.115.158311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cisneros BT, Law JJ, Matson ML, Azhdarinia A, Sevick-Muraca EM, Wilson LJ. Stable confinement of positron emission tomography and magnetic resonance agents within carbon nanotubes for bimodal imaging. Nanomedicine (Lond) 2014;9:2499–2509. doi: 10.2217/nnm.14.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rong P, Yang K, Srivastan A, Kiesewetter DO, Yue X, Wang F, Nie L, Bhirde A, Wang Z, Liu Z, Niu G, Wang W, Chen X. Photosensitizer loaded nano-graphene for multimodality imaging guided tumor photodynamic therapy. Theranostics. 2014;4:229–239. doi: 10.7150/thno.8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang K, Feng L, Shi X, Liu Z. Nano-graphene in biomedicine: theranostic applications. Chem Soc Rev. 2013;42:530–547. doi: 10.1039/c2cs35342c. [DOI] [PubMed] [Google Scholar]
- 87.Yang K, Feng L, Hong H, Cai W, Liu Z. Preparation and functionalization of graphene nanocomposites for biomedical applications. Nat Protoc. 2013;8:2392–2403. doi: 10.1038/nprot.2013.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hong H, Yang K, Zhang Y, Engle JW, Feng L, Yang Y, Nayak TR, Goel S, Bean J, Theuer CP, Barnhart TE, Liu Z, Cai W. In vivo targeting and imaging of tumor vasculature with radiolabeled, antibody-conjugated nanographene. ACS Nano. 2012;6:2361–2370. doi: 10.1021/nn204625e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xu C, Shi S, Feng L, Chen F, Graves SA, Ehlerding EB, Goel S, Sun H, England CG, Nickles RJ, Liu Z, Wang T, Cai W. Long circulating reduced graphene oxide-iron oxide nanoparticles for efficient tumor targeting and multimodality imaging. Nanoscale. 2016;25:25. doi: 10.1039/c5nr09193d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shi S, Yang K, Hong H, Chen F, Valdovinos HF, Goel S, Barnhart TE, Liu Z, Cai W. VEGFR targeting leads to significantly enhanced tumor uptake of nanographene oxide in vivo. Biomaterials. 2015;39:39–46. doi: 10.1016/j.biomaterials.2014.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shi S, Yang K, Hong H, Valdovinos HF, Nayak TR, Zhang Y, Theuer CP, Barnhart TE, Liu Z, Cai W. Tumor vasculature targeting and imaging in living mice with reduced graphene oxide. Biomaterials. 2013;34:3002–3009. doi: 10.1016/j.biomaterials.2013.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cornelissen B, Able S, Kersemans V, Waghorn PA, Myhra S, Jurkshat K, Crossley A, Vallis KA. Nanographene oxide-based radioimmunoconstructs for in vivo targeting and SPECT imaging of HER2-positive tumors. Biomaterials. 2013;34:1146–1154. doi: 10.1016/j.biomaterials.2012.10.054. [DOI] [PubMed] [Google Scholar]
- 93.Hong H, Zhang Y, Engle JW, Nayak TR, Theuer CP, Nickles RJ, Barnhart TE, Cai W. In vivo targeting and positron emission tomography imaging of tumor vasculature with (66)Ga-labeled nano-graphene. Biomaterials. 2012;33:4147–4156. doi: 10.1016/j.biomaterials.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang K, Wan J, Zhang S, Zhang Y, Lee ST, Liu Z. In vivo pharmacokinetics, long-term biodistribution, and toxicology of PEGylated graphene in mice. ACS Nano. 2011;5:516–522. doi: 10.1021/nn1024303. [DOI] [PubMed] [Google Scholar]
- 95.Jasim DA, Menard-Moyon C, Begin D, Bianco A, Kostarelos K. Tissue distribution and urinary excretion of intravenously administered chemically functionalized graphene oxide sheets. Chem Sci. 2015;6:3952–3964. doi: 10.1039/c5sc00114e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Song J, Yang X, Jacobson O, Lin L, Huang P, Niu G, Ma Q, Chen X. Sequential drug release and enhanced photothermal and photoacoustic effect of hybrid reduced graphene oxide-loaded ultrasmall gold nanorod vesicles for cancer therapy. ACS Nano. 2015;9:9199–9209. doi: 10.1021/acsnano.5b03804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen L, Zhong X, Yi X, Huang M, Ning P, Liu T, Ge C, Chai Z, Liu Z, Yang K. Radionuclide (131)I labeled reduced graphene oxide for nuclear imaging guided combined radio- and photothermal therapy of cancer. Biomaterials. 2015;66:21–28. doi: 10.1016/j.biomaterials.2015.06.043. [DOI] [PubMed] [Google Scholar]
- 98.Huang X, Zhang F, Zhu L, Choi KY, Guo N, Guo J, Tackett K, Anilkumar P, Liu G, Quan Q, Choi HS, Niu G, Sun YP, Lee S, Chen X. Effect of injection routes on the biodistribution, clearance, and tumor uptake of carbon dots. ACS Nano. 2013;7:5684–5693. doi: 10.1021/nn401911k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rojas S, Gispert JD, Martin R, Abad S, Menchon C, Pareto D, Victor VM, Alvaro M, Garcia H, Herance JR. Biodistribution of amino-functionalized diamond nanoparticles. In vivo studies based on 18F radionuclide emission. ACS Nano. 2011;5:5552–5559. doi: 10.1021/nn200986z. [DOI] [PubMed] [Google Scholar]
- 100.Li J, Yang W, Cui R, Wang D, Chang Y, Gu W, Yin W, Bai X, Chen K, Xia L, Geng H, Xing G. Metabolizer in vivo of fullerenes and metallofullerenes by positron emission tomography. Nanotechnology. 2016;27:155101. doi: 10.1088/0957-4484/27/15/155101. [DOI] [PubMed] [Google Scholar]
- 101.Chen M, Yin M. Design and development of fluorescent nanostructures for bioimaging. Prog Polym Sci. 2014;39:365–395. [Google Scholar]
- 102.Wolfbeis OS. An overview of nanoparticles commonly used in fluorescent bioimaging. Chem Soc Rev. 2015;44:4743–4768. doi: 10.1039/c4cs00392f. [DOI] [PubMed] [Google Scholar]
- 103.Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambhir SS, Weiss S. Quantum dots for live cells, in vivo imaging, and diagnostics. Science. 2005;307:538–544. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang SP, Goel S, W C. In vivo molecular imaging with quantum dots: toward multimodality and theranostics. In: Prokopovich P, editor. Biological and pharmaceutical applications of nanomaterials. 2015. pp. 319–346. CRC Press. [Google Scholar]
- 105.Resch-Genger U, Grabolle M, Cavaliere-Jaricot S, Nitschke R, Nann T. Quantum dots versus organic dyes as fluorescent labels. Nat Methods. 2008;5:763–775. doi: 10.1038/nmeth.1248. [DOI] [PubMed] [Google Scholar]
- 106.Smith AM, Nie S. Semiconductor nanocrystals: structure, properties, and band gap engineering. Acc Chem Res. 2010;43:190–200. doi: 10.1021/ar9001069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jin S, Hu YX, Gu ZJ, Liu L, Wu HC. Application of quantum dots in biological imaging. J Nanomater. 2011;2011:13. [Google Scholar]
- 108.Huang HC, Barua S, Sharma G, Dey SK, Rege K. Inorganic nanoparticles for cancer imaging and therapy. J Control Release. 2011;155:344–357. doi: 10.1016/j.jconrel.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 109.Medintz IL, Uyeda HT, Goldman ER, Mattoussi H. Quantum dot bioconjugates for imaging, labelling and sensing. Nat Mater. 2005;4:435–446. doi: 10.1038/nmat1390. [DOI] [PubMed] [Google Scholar]
- 110.Robe A, Pic E, Lassalle HP, Bezdetnaya L, Guillemin F, Marchal F. Quantum dots in axillary lymph node mapping: biodistribution study in healthy mice. BMC Cancer. 2008;8:111. doi: 10.1186/1471-2407-8-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cai W, Chen K, Li ZB, Gambhir SS, Chen X. Dual-function probe for PET and near-infrared fluorescence imaging of tumor vasculature. J Nucl Med. 2007;48:1862–1870. doi: 10.2967/jnumed.107.043216. [DOI] [PubMed] [Google Scholar]
- 112.Duconge F, Pons T, Pestourie C, Herin L, Theze B, Gombert K, Mahler B, Hinnen F, Kuhnast B, Dolle F, Dubertret B, Tavitian B. Fluorine-18-labeled phospholipid quantum dot micelles for in vivo multimodal imaging from whole body to cellular scales. Bioconjug Chem. 2008;19:1921–1926. doi: 10.1021/bc800179j. [DOI] [PubMed] [Google Scholar]
- 113.Chen K, Li ZB, Wang H, Cai W, Chen X. Dual-modality optical and positron emission tomography imaging of vascular endothelial growth factor receptor on tumor vasculature using quantum dots. Eur J Nucl Med Mol Imaging. 2008;35:2235–2244. doi: 10.1007/s00259-008-0860-8. [DOI] [PubMed] [Google Scholar]
- 114.Tu C, Ma X, House A, Kauzlarich SM, Louie AY. PET imaging and biodistribution of silicon quantum dots in mice. ACS Med Chem Lett. 2011;2:285–288. doi: 10.1021/ml1002844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hu K, Wang H, Tang G, Huang T, Tang X, Liang X, Yao S, Nie D. In vivo cancer dual-targeting and dual-modality imaging with functionalized quantum dots. J Nucl Med. 2015;56:1278–1284. doi: 10.2967/jnumed.115.158873. [DOI] [PubMed] [Google Scholar]
- 116.Schipper ML, Iyer G, Koh AL, Cheng Z, Ebenstein Y, Aharoni A, Keren S, Bentolila LA, Li J, Rao J, Chen X, Banin U, Wu AM, Sinclair R, Weiss S, Gambhir SS. Particle size, surface coating, and PEGylation influence the biodistribution of quantum dots in living mice. Small. 2009;5:126–134. doi: 10.1002/smll.200800003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schipper ML, Cheng Z, Lee SW, Bentolila LA, Iyer G, Rao J, Chen X, Wu AM, Weiss S, Gambhir SS. microPET-based biodistribution of quantum dots in living mice. J Nucl Med. 2007;48:1511–1518. doi: 10.2967/jnumed.107.040071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cai W, Hong H. In a "nutshell": intrinsically radio-labeled quantum dots. Am J Nucl Med Mol Imaging. 2012;2:136–140. [PMC free article] [PubMed] [Google Scholar]
- 119.Woodward JD, Kennel SJ, Mirzadeh S, Dai S, Wall JS, Richey T, Avenell J, Rondinone AJ. In vivo SPECT/CT imaging and biodistribution using radioactive Cd125mTe/ZnS nanoparticles. Nanotechnology. 2007;18:175103. [Google Scholar]
- 120.Kennel SJ, Woodward JD, Rondinone AJ, Wall J, Huang Y, Mirzadeh S. The fate of MAb-targeted Cd(125m)Te/ZnS nanoparticles in vivo. Nucl Med Biol. 2008;35:501–514. doi: 10.1016/j.nucmedbio.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 121.Sun M, Hoffman D, Sundaresan G, Yang L, Lamichhane N, Zweit J. Synthesis and characterization of intrinsically radiolabeled quantum dots for bimodal detection. Am J Nucl Med Mol Imaging. 2012;2:122–135. [PMC free article] [PubMed] [Google Scholar]
- 122.Guo W, Sun X, Jacobson O, Yan X, Min K, Srivatsan A, Niu G, Kiesewetter DO, Chang J, Chen X. Intrinsically radioactive [64Cu]CuInS/ZnS quantum dots for PET and optical imaging: improved radiochemical stability and controllable Cerenkov luminescence. ACS Nano. 2015;9:488–495. doi: 10.1021/nn505660r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li J, Dobrucki LW, Marjanovic M, Chaney EJ, Suslick KS, Boppart SA. Enhancement and wavelength-shifted emission of Cerenkov luminescence using multifunctional microspheres. Phys Med Biol. 2015;60:727–739. doi: 10.1088/0031-9155/60/2/727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Boschi F, Spinelli AE. Quantum dots excitation using pure beta minus radioisotopes emitting Cerenkov radiation. RSC Adv. 2012;2:11049–11052. [Google Scholar]
- 125.Dothager RS, Goiffon RJ, Jackson E, Harpstrite S, Piwnica-Worms D. Cerenkov radiation energy transfer (CRET) imaging: a novel method for optical imaging of PET isotopes in biological systems. PloS One. 2010;5:0013300. doi: 10.1371/journal.pone.0013300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shen J, Zhao L, Han G. Lanthanide-doped upconverting luminescent nanoparticle platforms for optical imaging-guided drug delivery and therapy. Adv Drug Deliv Rev. 2013;65:744–755. doi: 10.1016/j.addr.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 127.Gu Z, Yan L, Tian G, Li S, Chai Z, Zhao Y. Recent advances in design and fabrication of upconversion nanoparticles and their safe theranostic applications. Adv Mater. 2013;25:3758–3779. doi: 10.1002/adma.201301197. [DOI] [PubMed] [Google Scholar]
- 128.Nam J, Won N, Bang J, Jin H, Park J, Jung S, Park Y, Kim S. Surface engineering of inorganic nanoparticles for imaging and therapy. Adv Drug Deliv Rev. 2013;65:622–648. doi: 10.1016/j.addr.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 129.Park YI, Lee KT, Suh YD, Hyeon T. Upconverting nanoparticles: a versatile platform for wide-field two-photon microscopy and multi-modal in vivo imaging. Chem Soc Rev. 2015;44:1302–1317. doi: 10.1039/c4cs00173g. [DOI] [PubMed] [Google Scholar]
- 130.Zhou J, Liu Z, Li F. Upconversion nanophosphors for small-animal imaging. Chem Soc Rev. 2012;41:1323–1349. doi: 10.1039/c1cs15187h. [DOI] [PubMed] [Google Scholar]
- 131.Chen G, Qiu H, Prasad PN, Chen X. Upconversion nanoparticles: design, nanochemistry, and applications in theranostics. Chem Rev. 2014;114:5161–5214. doi: 10.1021/cr400425h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Janowski M, Bulte JW, Walczak P. Personalized nanomedicine advancements for stem cell tracking. Adv Drug Deliv Rev. 2012;64:1488–1507. doi: 10.1016/j.addr.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang C, Cheng L, Liu Z. Upconversion nanoparticles for photodynamic therapy and other cancer therapeutics. Theranostics. 2013;3:317–330. doi: 10.7150/thno.5284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liu F, He X, Lei Z, Liu L, Zhang J, You H, Zhang H, Wang Z. Facile preparation of doxorubicin-loaded upconversion@polydopamine nanoplatforms for simultaneous in vivo multimodality imaging and chemophotothermal synergistic therapy. Adv Healthc Mater. 2015;4:559–568. doi: 10.1002/adhm.201400676. [DOI] [PubMed] [Google Scholar]
- 135.Chen Q, Wang C, Cheng L, He W, Cheng Z, Liu Z. Protein modified upconversion nanoparticles for imaging-guided combined photothermal and photodynamic therapy. Biomaterials. 2014;35:2915–2923. doi: 10.1016/j.biomaterials.2013.12.046. [DOI] [PubMed] [Google Scholar]
- 136.Yuan Y, Min Y, Hu Q, Xing B, Liu B. NIR photoregulated chemo- and photodynamic cancer therapy based on conjugated polyelectrolyte-drug conjugate encapsulated upconversion nanoparticles. Nanoscale. 2014;6:11259–11272. doi: 10.1039/c4nr03302g. [DOI] [PubMed] [Google Scholar]
- 137.Chen F, Bu W, Cai W, Shi J. Functionalized upconversion nanoparticles: versatile nanoplatforms for translational research. Curr Mol Med. 2013;13:1613–1632. doi: 10.2174/1566524013666131111122133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gallo J, Alam IS, Jin J, Gu YJ, Aboagye EO, Wong WT, Long NJ. PET imaging with multimodal upconversion nanoparticles. Dalton Trans. 2014;43:5535–5545. doi: 10.1039/c3dt53095g. [DOI] [PubMed] [Google Scholar]
- 139.Seo HJ, Nam SH, Im HJ, Park JY, Lee JY, Yoo B, Lee YS, Jeong JM, Hyeon T, Kim JW, Lee JS, Jang IJ, Cho JY, Hwang do W, Suh YD, Lee DS. Rapid hepatobiliary excretion of micelle-encapsulated/radiolabeled upconverting nanoparticles as an integrated form. Sci Rep. 2015;5:15685. doi: 10.1038/srep15685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rieffel J, Chen F, Kim J, Chen G, Shao W, Shao S, Chitgupi U, Hernandez R, Graves SA, Nickles RJ, Prasad PN, Kim C, Cai W, Lovell JF. Hexamodal imaging with porphyrin-phospholipid-coated upconversion nanoparticles. Adv Mater. 2015;27:1785–1790. doi: 10.1002/adma.201404739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lee J, Lee TS, Ryu J, Hong S, Kang M, Im K, Kang JH, Lim SM, Park S, Song R. RGD peptide-conjugated multimodal NaGdF4:Yb3+/Er3+ nanophosphors for upconversion luminescence, MR, and PET imaging of tumor angiogenesis. J Nucl Med. 2013;54:96–103. doi: 10.2967/jnumed.112.108043. [DOI] [PubMed] [Google Scholar]
- 142.Cao T, Yang Y, Sun Y, Wu Y, Gao Y, Feng W, Li F. Biodistribution of sub-10 nm PEG-modified radioactive/upconversion nanoparticles. Biomaterials. 2013;34:7127–7134. doi: 10.1016/j.biomaterials.2013.05.028. [DOI] [PubMed] [Google Scholar]
- 143.Peng J, Sun Y, Zhao L, Wu Y, Feng W, Gao Y, Li F. Polyphosphoric acid capping radioactive/upconverting NaLuF4:Yb,Tm,153Sm nanoparticles for blood pool imaging in vivo. Biomaterials. 2013;34:9535–9544. doi: 10.1016/j.biomaterials.2013.07.098. [DOI] [PubMed] [Google Scholar]
- 144.Sun Y, Zhu X, Peng J, Li F. Core-shell lanthanide upconversion nanophosphors as four-modal probes for tumor angiogenesis imaging. ACS Nano. 2013;7:11290–11300. doi: 10.1021/nn405082y. [DOI] [PubMed] [Google Scholar]
- 145.Yang Y, Sun Y, Cao T, Peng J, Liu Y, Wu Y, Feng W, Zhang Y, Li F. Hydrothermal synthesis of NaLuF4:153Sm,Yb,Tm nanoparticles and their application in dual-modality upconversion luminescence and SPECT bioimaging. Biomaterials. 2013;34:774–783. doi: 10.1016/j.biomaterials.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 146.Sun Y, Yu M, Liang S, Zhang Y, Li C, Mou T, Yang W, Zhang X, Li B, Huang C, Li F. Fluorine-18 labeled rare-earth nanoparticles for positron emission tomography (PET) imaging of sentinel lymph node. Biomaterials. 2011;32:2999–3007. doi: 10.1016/j.biomaterials.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 147.Zhou J, Yu M, Sun Y, Zhang X, Zhu X, Wu Z, Wu D, Li F. Fluorine-18-labeled Gd3+/Yb3+/Er3+ co-doped NaYF4 nanophosphors for multimodality PET/MR/UCL imaging. Biomaterials. 2011;32:1148–1156. doi: 10.1016/j.biomaterials.2010.09.071. [DOI] [PubMed] [Google Scholar]
- 148.Liu Q, Chen M, Sun Y, Chen G, Yang T, Gao Y, Zhang X, Li F. Multifunctional rare-earth self-assembled nanosystem for tri-modal upconversion luminescence /fluorescence /positron emission tomography imaging. Biomaterials. 2011;32:8243–8253. doi: 10.1016/j.biomaterials.2011.07.053. [DOI] [PubMed] [Google Scholar]
- 149.Wu JZ, Dai J, Shao YB, Sun YC. One-step synthesis of fluorescent silicon quantum dots (Si-QDs) and their application for cell imaging. RSC Adv. 2015;5:83581–83587. [Google Scholar]
- 150.Erogbogbo F, Yong KT, Roy I, Xu G, Prasad PN, Swihart MT. Biocompatible luminescent silicon quantum dots for imaging of cancer cells. ACS Nano. 2008;2:873–878. doi: 10.1021/nn700319z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Erogbogbo F, Tien CA, Chang CW, Yong KT, Law WC, Ding H, Roy I, Swihart MT, Prasad PN. Bioconjugation of luminescent silicon quantum dots for selective uptake by cancer cells. Bioconjug Chem. 2011;22:1081–1088. doi: 10.1021/bc100552p. [DOI] [PubMed] [Google Scholar]
- 152.Zhao F, Li Z, Wang L, Hu C, Zhang Z, Li C, Qu L. Supramolecular quantum dots as biodegradable nano-probes for upconversion-enabled bioimaging. Chem Commun. 2015;51:13201–13204. doi: 10.1039/c5cc04831a. [DOI] [PubMed] [Google Scholar]
- 153.Gargas DJ, Chan EM, Ostrowski AD, Aloni S, Altoe MV, Barnard ES, Sanii B, Urban JJ, Milliron DJ, Cohen BE, Schuck PJ. Engineering bright sub-10-nm upconverting nanocrystals for single-molecule imaging. Nat Nanotechnol. 2014;9:300–305. doi: 10.1038/nnano.2014.29. [DOI] [PubMed] [Google Scholar]
- 154.Yang D, Dai Y, Liu J, Zhou Y, Chen Y, Li C, Ma P, Lin J. Ultra-small BaGdF5-based upconversion nanoparticles as drug carriers and multimodal imaging probes. Biomaterials. 2014;35:2011–2023. doi: 10.1016/j.biomaterials.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 155.Huang HC, Ramos J, Grandhi TSP, Potta T, Rege K. Gold Nanoparticles in cancer imaging and therapeutics. Nano Life. 2010;01:289–307. [Google Scholar]
- 156.Jiao PF, Zhou HY, Chen LX, Yan B. Cancer-targeting multifunctionalized gold nanoparticles in imaging and therapy. Curr Med Chem. 2011;18:2086–2102. doi: 10.2174/092986711795656199. [DOI] [PubMed] [Google Scholar]
- 157.Deb S, Ghosh K, Shetty SD. Nanoimaging in cardiovascular diseases: Current state of the art. Indian J Med Res. 2015;141:285–298. doi: 10.4103/0971-5916.156557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Spivak MY, Bubnov RV, Yemets IM, Lazarenko LM, Tymoshok NO, Ulberg ZR. Gold nanoparticles - the theranostic challenge for PPPM: nanocardiology application. EPMA J. 2013;4:18. doi: 10.1186/1878-5085-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Stendahl JC, Sinusas AJ. Nanoparticles for cardiovascular imaging and therapeutic delivery, Part 2: Radiolabeled probes. J Nucl Med. 2015;56:1637–1641. doi: 10.2967/jnumed.115.164145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wan XY, Zheng LL, Gao PF, Yang XX, Li CM, Li YF, Huang CZ. Real-time light scattering tracking of gold nanoparticles- bioconjugated respiratory syncytial virus infecting HEP-2 cells. Sci Rep. 2014;4:4529. doi: 10.1038/srep04529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang T, Zhang Z, Gao D, Li F, Wei H, Liang X, Cui Z, Zhang XE. Encapsulation of gold nanoparticles by simian virus 40 capsids. Nanoscale. 2011;3:4275–4282. doi: 10.1039/c1nr10568j. [DOI] [PubMed] [Google Scholar]
- 162.Lee JH, Oh BK, Choi JW. Development of a HIV-1 virus detection system based on nanotechnology. Sensors (Basel) 2015;15:9915–9927. doi: 10.3390/s150509915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dykman L, Khlebtsov N. Gold nanoparticles in biomedical applications: recent advances and perspectives. Chem Soc Rev. 2012;41:2256–2282. doi: 10.1039/c1cs15166e. [DOI] [PubMed] [Google Scholar]
- 164.Daniel MC, Astruc D. Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev. 2004;104:293–346. doi: 10.1021/cr030698+. [DOI] [PubMed] [Google Scholar]
- 165.Zhang X. Gold nanoparticles: Recent advances in the biomedical applications. Cell Biochem Biophys. 2015 doi: 10.1007/s12013-015-0529-4. [DOI] [PubMed] [Google Scholar]
- 166.Lai SF, Ko BH, Chien CC, Chang CJ, Yang SM, Chen HH, Petibois C, Hueng DY, Ka SM, Chen A, Margaritondo G, Hwu Y. Gold nanoparticles as multimodality imaging agents for brain gliomas. J Nanobiotechnology. 2015;13:85. doi: 10.1186/s12951-015-0140-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Huang X, El-Sayed MA. Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy. J Adv Res. 2010;1:13–28. [Google Scholar]
- 168.Jain S, Hirst DG, O'Sullivan JM. Gold nanoparticles as novel agents for cancer therapy. Br J Radiol. 2012;85:101–113. doi: 10.1259/bjr/59448833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Huang X, Jain PK, El-Sayed IH, El-Sayed MA. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med Sci. 2008;23:217–228. doi: 10.1007/s10103-007-0470-x. [DOI] [PubMed] [Google Scholar]
- 170.Young JK, Figueroa ER, Drezek RA. Tunable nanostructures as photothermal theranostic agents. Ann Biomed Eng. 2012;40:438–459. doi: 10.1007/s10439-011-0472-5. [DOI] [PubMed] [Google Scholar]
- 171.Bazak R, Houri M, El Achy S, Kamel S, Refaat T. Cancer active targeting by nanoparticles: a comprehensive review of literature. J Cancer Res Clin Oncol. 2015;141:769–784. doi: 10.1007/s00432-014-1767-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Liu Y, Yuan H, Fales AM, Register JK, Vo-Dinh T. Multifunctional gold nanostars for molecular imaging and cancer therapy. Front Chem. 2015;3:51. doi: 10.3389/fchem.2015.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv Drug Deliv Rev. 2014;66:2–25. doi: 10.1016/j.addr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kim B, Han G, Toley BJ, Kim CK, Rotello VM, Forbes NS. Tuning payload delivery in tumour cylindroids using gold nanoparticles. Nat Nanotechnol. 2010;5:465–472. doi: 10.1038/nnano.2010.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Pissuwan D, Niidome T, Cortie MB. The forthcoming applications of gold nanoparticles in drug and gene delivery systems. J Control Release. 2011;149:65–71. doi: 10.1016/j.jconrel.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 176.Ajnai G, Chiu A, Kan T, Cheng C-C, Tsai T-H, Chang J. Trends of gold nanoparticle-based drug delivery system in cancer therapy. J Exp Clin Med. 2014;6:172–178. [Google Scholar]
- 177.Han G, Ghosh P, Rotello VM. Functionalized gold nanoparticles for drug delivery. Nanomedicine (Lond) 2007;2:113–123. doi: 10.2217/17435889.2.1.113. [DOI] [PubMed] [Google Scholar]
- 178.Xing Y, Zhao J, Conti PS, Chen K. Radiolabeled nanoparticles for multimodality tumor imaging. Theranostics. 2014;4:290–306. doi: 10.7150/thno.7341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Li W, Sun X, Wang Y, Niu G, Chen X, Qian Z, Nie L. In vivo quantitative photoacoustic microscopy of gold nanostar kinetics in mouse organs. Biomed Opt Express. 2014;5:2679–2685. doi: 10.1364/BOE.5.002679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Liu Y, Yuan H, Kersey FR, Register JK, Parrott MC, Vo-Dinh T. Plasmonic gold nanostars for multi-modality sensing and diagnostics. Sensors (Basel) 2015;15:3706–3720. doi: 10.3390/s150203706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Liu Y, Ashton JR, Moding EJ, Yuan H, Register JK, Fales AM, Choi J, Whitley MJ, Zhao X, Qi Y, Ma Y, Vaidyanathan G, Zalutsky MR, Kirsch DG, Badea CT, Vo-Dinh T. A plasmonic gold nanostar theranostic probe for in vivo tumor imaging and photothermal therapy. Theranostics. 2015;5:946–960. doi: 10.7150/thno.11974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Pallavicini P, Bernhard C, Chirico G, Dacarro G, Denat F, Dona A, Milanese C, Taglietti A. Gold nanostars co-coated with the Cu(II) complex of a tetraazamacrocyclic ligand. Dalton Trans. 2015;44:5652–5661. doi: 10.1039/c4dt03042g. [DOI] [PubMed] [Google Scholar]
- 183.Peiris PM, Bauer L, Toy R, Tran E, Pansky J, Doolittle E, Schmidt E, Hayden E, Mayer A, Keri RA, Griswold MA, Karathanasis E. Enhanced delivery of chemotherapy to tumors using a multicomponent nanochain with radio-frequency-tunable drug release. ACS Nano. 2012;6:4157–4168. doi: 10.1021/nn300652p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Geng Y, Dalhaimer P, Cai S, Tsai R, Tewari M, Minko T, Discher DE. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat Nanotechnol. 2007;2:249–255. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhang S, Gao H, Bao G. Physical Principles of Nanoparticle Cellular Endocytosis. ACS Nano. 2015;9:8655–8671. doi: 10.1021/acsnano.5b03184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Oh N, Park JH. Endocytosis and exocytosis of nanoparticles in mammalian cells. Int J Nanomedicine. 2014;9(Suppl 1):51–63. doi: 10.2147/IJN.S26592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Decuzzi P, Causa F, Ferrari M, Netti PA. The effective dispersion of nanovectors within the tumor microvasculature. Ann Biomed Eng. 2006;34:633–641. doi: 10.1007/s10439-005-9072-6. [DOI] [PubMed] [Google Scholar]
- 188.Toy R, Hayden E, Camann A, Berman Z, Vicente P, Tran E, Meyers J, Pansky J, Peiris PM, Wu H, Exner A, Wilson D, Ghaghada KB, Karathanasis E. Multimodal in vivo imaging exposes the voyage of nanoparticles in tumor microcirculation. ACS Nano. 2013;7:3118–3129. doi: 10.1021/nn3053439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Morales-Avila E, Ferro-Flores G, Ocampo-García BE, De León-Rodríguez LM, Santos-Cuevas CL, García-Becerra R, Medina LA, Gómez-Oliván L. Multimeric system of 99mTc-labeled gold nanoparticles conjugated to c[RGDfK(C)] for molecular imaging of tumor α(v)β(3) expression. Bioconjug Chem. 2011;22:913–922. doi: 10.1021/bc100551s. [DOI] [PubMed] [Google Scholar]
- 190.Black KC, Akers WJ, Sudlow G, Xu B, Laforest R, Achilefu S. Dual-radiolabeled nanoparticle SPECT probes for bioimaging. Nanoscale. 2015;7:440–444. doi: 10.1039/c4nr05269b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Jang B, Park S, Kang SH, Kim JK, Kim SK, Kim IH, Choi Y. Gold nanorods for target selective SPECT/CT imaging and photothermal therapy in vivo. Quant Imaging Med Surg. 2012;2:1–11. doi: 10.3978/.issn.2223-4292.2012.01.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Xie H, Wang ZJ, Bao A, Goins B, Phillips WT. In vivo PET imaging and biodistribution of radiolabeled gold nanoshells in rats with tumor xenografts. Int J Pharm. 2010;395:324–330. doi: 10.1016/j.ijpharm.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 193.Xie H, Goins B, Bao A, Wang ZJ, Phillips WT. Effect of intratumoral administration on biodistribution of 64Cu-labeled nanoshells. Int J Nanomedicine. 2012;7:2227–2238. doi: 10.2147/IJN.S30699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Xie H, Diagaradjane P, Deorukhkar AA, Goins B, Bao A, Phillips WT, Wang Z, Schwartz J, Krishnan S. Integrin alphavbeta3-targeted gold nanoshells augment tumor vasculature-specific imaging and therapy. Int J Nanomedicine. 2011;6:259–269. doi: 10.2147/IJN.S15479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Bhatnagar P, Li Z, Choi Y, Guo J, Li F, Lee DY, Figliola M, Huls H, Lee DA, Zal T, Li KC, Cooper LJ. Imaging of genetically engineered T cells by PET using gold nanoparticles complexed to Copper-64. Integr Biol (Camb) 2013;5:231–238. doi: 10.1039/c2ib20093g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Karmani L, Bouchat V, Bouzin C, Leveque P, Labar D, Bol A, Deumer G, Marega R, Bonifazi D, Haufroid V, Michiels C, Gregoire V, Feron O, Lucas S, Vander Borght T, Gallez B. (89)Zr-labeled anti-endoglin antibody-targeted gold nanoparticles for imaging cancer: implications for future cancer therapy. Nanomedicine (London) 2014;9:1923–1937. doi: 10.2217/nnm.13.185. [DOI] [PubMed] [Google Scholar]
- 197.Karmani L, Labar D, Valembois V, Bouchat V, Nagaswaran PG, Bol A, Gillart J, Leveque P, Bouzin C, Bonifazi D, Michiels C, Feron O, Gregoire V, Lucas S, Vander Borght T, Gallez B. Antibody-functionalized nanoparticles for imaging cancer: influence of conjugation to gold nanoparticles on the biodistribution of 89Zr-labeled cetuximab in mice. Contrast Media Mol Imaging. 2013;8:402–408. doi: 10.1002/cmmi.1539. [DOI] [PubMed] [Google Scholar]
- 198.Zhang Z, Liu Y, Jarreau C, Welch MJ, Taylor JS. Nucleic Acid-directed Self-assembly of multifunctional gold nanoparticle imaging agents. Biomater Sci. 2013;1:1055–1064. doi: 10.1039/C3BM60070J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Tian M, Lu W, Zhang R, Xiong C, Ensor J, Nazario J, Jackson J, Shaw C, Dixon KA, Miller J, Wright K, Li C, Gupta S. Tumor uptake of hollow gold nanospheres after intravenous and intra-arterial injection: PET/CT study in a rabbit VX2 liver cancer model. Mol Imaging Biol. 2013;15:614–624. doi: 10.1007/s11307-013-0635-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Li H, Diaz L, Lee D, Cui L, Liang X, Cheng Y. In vivo imaging of T cells loaded with gold nanoparticles: a pilot study. Radiol Med. 2014;119:269–276. doi: 10.1007/s11547-013-0335-2. [DOI] [PubMed] [Google Scholar]
- 201.Wang Y, Liu Y, Luehmann H, Xia X, Brown P, Jarreau C, Welch M, Xia Y. Evaluating the pharmacokinetics and in vivo cancer targeting capability of Au nanocages by positron emission tomography imaging. ACS Nano. 2012;6:5880–5888. doi: 10.1021/nn300464r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Xiao Y, Hong H, Matson VZ, Javadi A, Xu W, Yang Y, Zhang Y, Engle JW, Nickles RJ, Cai W, Steeber DA, Gong S. Gold Nanorods Conjugated with Doxorubicin and cRGD for Combined Anticancer Drug Delivery and PET Imaging. Theranostics. 2012;2:757–768. doi: 10.7150/thno.4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Cheng K, Kothapalli SR, Liu H, Koh AL, Jokerst JV, Jiang H, Yang M, Li J, Levi J, Wu JC, Gambhir SS, Cheng Z. Construction and validation of nano gold tripods for molecular imaging of living subjects. J Am Chem Soc. 2014;136:3560–3571. doi: 10.1021/ja412001e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Yang M, Cheng K, Qi S, Liu H, Jiang Y, Jiang H, Li J, Chen K, Zhang H, Cheng Z. Affibody modified and radiolabeled gold-iron oxide hetero-nanostructures for tumor PET, optical and MR imaging. Biomaterials. 2013;34:2796–2806. doi: 10.1016/j.biomaterials.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Frigell J, Garcia I, Gomez-Vallejo V, Llop J, Penades S. 68Ga-labeled gold glyconanoparticles for exploring blood-brain barrier permeability: preparation, biodistribution studies, and improved brain uptake via neuropeptide conjugation. J Am Chem Soc. 2014;136:449–457. doi: 10.1021/ja411096m. [DOI] [PubMed] [Google Scholar]
- 206.Chen F, Goel S, Hernandez R, Graves SA, Shi S, Nickles RJ, Cai W. Dynamic positron emission tomography imaging of renal clearable gold nanoparticles. Small. 2016;12:2775–2782. doi: 10.1002/smll.201600194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Abdelhalim MA. Alterations in gold nanoparticle levels are size dependent, with the smaller ones inducing the most toxic effects and related to the time of exposure of the gold nanoparticles. West Indian Med J. 2015;65 doi: 10.7727/wimj.2014.350. [DOI] [PubMed] [Google Scholar]
- 208.Roy K, Lahiri S. A green method for synthesis of radioactive gold nanoparticles. Green Chem. 2006;8:1063–1066. [Google Scholar]
- 209.Chanda N, Kattumuri V, Shukla R, Zambre A, Katti K, Upendran A, Kulkarni RR, Kan P, Fent GM, Casteel SW, Smith CJ, Boote E, Robertson JD, Cutler C, Lever JR, Katti KV, Kannan R. Bombesin functionalized gold nanoparticles show in vitro and in vivo cancer receptor specificity. Proc Natl Acad Sci USA. 2010;107:8760–8765. doi: 10.1073/pnas.1002143107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Zhou C, Hao G, Thomas P, Liu J, Yu M, Sun S, Oz OK, Sun X, Zheng J. Near-infrared emitting radioactive gold nanoparticles with molecular pharmacokinetics. Angew Chem Int Ed Engl. 2012;51:10118–10122. doi: 10.1002/anie.201203031. [DOI] [PubMed] [Google Scholar]
- 211.Wang Y, Liu Y, Luehmann H, Xia X, Wan D, Cutler C, Xia Y. Radioluminescent gold nanocages with controlled radioactivity for real-time in vivo imaging. Nano Lett. 2013;13:581–585. doi: 10.1021/nl304111v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Black KC, Wang Y, Luehmann HP, Cai X, Xing W, Pang B, Zhao Y, Cutler CS, Wang LV, Liu Y, Xia Y. Radioactive 198Au-doped nanostructures with different shapes for in vivo analyses of their biodistribution, tumor uptake, and intratumoral distribution. ACS Nano. 2014;8:4385–4394. doi: 10.1021/nn406258m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zhao Y, Sultan D, Detering L, Cho S, Sun G, Pierce R, Wooley KL, Liu Y. Copper-64-alloyed gold nanoparticles for cancer imaging: improved radiolabel stability and diagnostic accuracy. Angew Chem Int Ed Engl. 2014;53:156–159. doi: 10.1002/anie.201308494. [DOI] [PubMed] [Google Scholar]
- 214.Hu H, Huang P, Weiss OJ, Yan X, Yue X, Zhang MG, Tang Y, Nie L, Ma Y, Niu G, Wu K, Chen X. PET and NIR optical imaging using self-illuminating (64)Cu-doped chelator-free gold nanoclusters. Biomaterials. 2014;35:9868–9876. doi: 10.1016/j.biomaterials.2014.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Zhao Y, Sultan D, Detering L, Luehmann H, Liu Y. Facile synthesis, pharmacokinetic and systemic clearance evaluation, and positron emission tomography cancer imaging of (6)(4)Cu-Au alloy nanoclusters. Nanoscale. 2014;6:13501–13509. doi: 10.1039/c4nr04569f. [DOI] [PubMed] [Google Scholar]
- 216.Liu J, Yu M, Zhou C, Yang S, Ning X, Zheng J. Passive tumor targeting of renal-clearable luminescent gold nanoparticles: long tumor retention and fast normal tissue clearance. J Am Chem Soc. 2013;135:4978–4981. doi: 10.1021/ja401612x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.McCarthy JR, Weissleder R. Multifunctional magnetic nanoparticles for targeted imaging and therapy. Adv Drug Deliv Rev. 2008;60:1241–1251. doi: 10.1016/j.addr.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ohgushi M, Nagayama K, Wada A. Dextran-magnetite: A new relaxation reagent and its application to T2 measurements in gel systems. J Magn Reson (1969) 1978;29:599–601. [Google Scholar]
- 219.Quick HH. Integrated PET/MR. J Magn Reson Imaging. 2014;39:243–258. doi: 10.1002/jmri.24523. [DOI] [PubMed] [Google Scholar]
- 220.Wehrl HF, Sauter AW, Divine MR, Pichler BJ. Combined PET/MR: a technology becomes mature. J Nucl Med. 2015;56:165–168. doi: 10.2967/jnumed.114.150318. [DOI] [PubMed] [Google Scholar]
- 221.Bouziotis P, Psimadas D, Tsotakos T, Stamopoulos D, Tsoukalas C. Radiolabeled iron oxide nanoparticles as dual-modality SPECT/MRI and PET/MRI agents. Curr Top Med Chem. 2012;12:2694–2702. doi: 10.2174/1568026611212230007. [DOI] [PubMed] [Google Scholar]
- 222.Shao Y, Cherry SR, Farahani K, Meadors K, Siegel S, Silverman RW, Marsden PK. Simultaneous PET and MR imaging. Phys Med Biol. 1997;42:1965–1970. doi: 10.1088/0031-9155/42/10/010. [DOI] [PubMed] [Google Scholar]
- 223.Higuchi T, Nekolla SG, Jankaukas A, Weber AW, Huisman MC, Reder S, Ziegler SI, Schwaiger M, Bengel FM. Characterization of normal and infarcted rat myocardium using a combination of small-animal PET and clinical MRI. J Nucl Med. 2007;48:288–294. [PubMed] [Google Scholar]
- 224.Laurent S, Forge D, Port M, Roch A, Robic C, Vander Elst L, Muller RN. Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem Rev. 2008;108:2064–2110. doi: 10.1021/cr068445e. [DOI] [PubMed] [Google Scholar]
- 225.Kim J, Piao Y, Hyeon T. Multifunctional nanostructured materials for multimodal imaging, and simultaneous imaging and therapy. Chem Soc Rev. 2009;38:372–390. doi: 10.1039/b709883a. [DOI] [PubMed] [Google Scholar]
- 226.Shubayev VI, Pisanic TR, 2nd, Jin S. Magnetic nanoparticles for theragnostics. Adv Drug Deliv Rev. 2009;61:467–477. doi: 10.1016/j.addr.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Sun C, Lee JS, Zhang M. Magnetic nanoparticles in MR imaging and drug delivery. Adv Drug Deliv Rev. 2008;60:1252–1265. doi: 10.1016/j.addr.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Lee N, Hyeon T. Designed synthesis of uniformly sized iron oxide nanoparticles for efficient magnetic resonance imaging contrast agents. Chem Soc Rev. 2012;41:2575–2589. doi: 10.1039/c1cs15248c. [DOI] [PubMed] [Google Scholar]
- 229.De M, Chou SS, Joshi HM, Dravid VP. Hybrid magnetic nanostructures (MNS) for magnetic resonance imaging applications. Adv Drug Deliv Rev. 2011;63:1282–1299. doi: 10.1016/j.addr.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Kievit FM, Zhang M. Surface engineering of iron oxide nanoparticles for targeted cancer therapy. Acc Chem Res. 2011;44:853–862. doi: 10.1021/ar2000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Kitamura N, Kosuda S, Araki K, Tomifuji M, Mizokami D, Shiotani A, Shinmoto H, Fujii H, Ichihara K. Comparison of animal studies between interstitial magnetic resonance lymphography and radiocolloid SPECT/CT lymphoscintigraphy in the head and neck region. Ann Nucl Med. 2012;26:281–285. doi: 10.1007/s12149-011-0565-0. [DOI] [PubMed] [Google Scholar]
- 232.Torres Martin de Rosales R, Tavare R, Glaria A, Varma G, Protti A, Blower PJ. ((9)(9)m)Tc-bisphosphonate-iron oxide nanoparticle conjugates for dual-modality biomedical imaging. Bioconjug Chem. 2011;22:455–465. doi: 10.1021/bc100483k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Patel N, Duffy BA, Badar A, Lythgoe MF, Arstad E. Bimodal imaging of inflammation with SPECT/CT and MRI using Iodine-125 labeled VCAM-1 targeting microparticle conjugates. Bioconjug Chem. 2015;26:1542–1549. doi: 10.1021/acs.bioconjchem.5b00380. [DOI] [PubMed] [Google Scholar]
- 234.Zolata H, Abbasi Davani F, Afarideh H. Synthesis, characterization and theranostic evaluation of Indium-111 labeled multifunctional superparamagnetic iron oxide nanoparticles. Nucl Med Biol. 2015;42:164–170. doi: 10.1016/j.nucmedbio.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 235.Wang H, Kumar R, Nagesha D, Duclos RI, Jr, Sridhar S, Gatley SJ. Integrity of (111)In-radiolabeled superparamagnetic iron oxide nanoparticles in the mouse. Nucl Med Biol. 2015;42:65–70. doi: 10.1016/j.nucmedbio.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 236.Tang Y, Zhang C, Wang J, Lin X, Zhang L, Yang Y, Wang Y, Zhang Z, Bulte JW, Yang GY. MRI/SPECT/Fluorescent Tri-modal probe for evaluating the homing and therapeutic efficacy of transplanted mesenchymal stem cells in a rat ischemic stroke model. Adv Funct Mater. 2015;25:1024–1034. doi: 10.1002/adfm.201402930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Chen J, Zhu S, Tong L, Li J, Chen F, Han Y, Zhao M, Xiong W. Superparamagnetic iron oxide nanoparticles mediated (131)I-hVEGF siRNA inhibits hepatocellular carcinoma tumor growth in nude mice. BMC Cancer. 2014;14:1–8. doi: 10.1186/1471-2407-14-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Park SI, Kwon BJ, Park JH, Jung H, Yu KH. Synthesis and characterization of 3-[131I]iodo-L-tyrosine grafted Fe3O4@SiO2 nanocomposite for single photon emission computed tomography (SPECT) and magnetic resonance imaging (MRI) J Nanosci Nanotechnol. 2011;11:1818–1821. doi: 10.1166/jnn.2011.3416. [DOI] [PubMed] [Google Scholar]
- 239.Ai F, Ferreira CA, Chen F, Cai W. Engineering of radiolabeled iron oxide nanoparticles for dual-modality imaging. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015;22:619–630. doi: 10.1002/wnan.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Glaus C, Rossin R, Welch MJ, Bao G. In vivo evaluation of (64)Cu-labeled magnetic nanoparticles as a dual-modality PET/MR imaging agent. Bioconjug Chem. 2010;21:715–722. doi: 10.1021/bc900511j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Jarrett BR, Gustafsson B, Kukis DL, Louie AY. Synthesis of 64Cu-labeled magnetic nanoparticles for multimodal imaging. Bioconjug Chem. 2008;19:1496–1504. doi: 10.1021/bc800108v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Lee HY, Li Z, Chen K, Hsu AR, Xu C, Xie J, Sun S, Chen X. PET/MRI dual-modality tumor imaging using arginine-glycine-aspartic (RGD)-conjugated radiolabeled iron oxide nanoparticles. J Nucl Med. 2008;49:1371–1379. doi: 10.2967/jnumed.108.051243. [DOI] [PubMed] [Google Scholar]
- 243.Xie J, Chen K, Huang J, Lee S, Wang J, Gao J, Li X, Chen X. PET/NIRF/MRI triple functional iron oxide nanoparticles. Biomaterials. 2010;31:3016–3022. doi: 10.1016/j.biomaterials.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Yang X, Hong H, Grailer JJ, Rowland IJ, Javadi A, Hurley SA, Xiao Y, Yang Y, Zhang Y, Nickles RJ, Cai W, Steeber DA, Gong S. cRGD-functionalized, DOX-conjugated, and (6)(4)Cu-labeled superparamagnetic iron oxide nanoparticles for targeted anticancer drug delivery and PET/MR imaging. Biomaterials. 2011;32:4151–4160. doi: 10.1016/j.biomaterials.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Kim SM, Chae MK, Yim MS, Jeong IH, Cho J, Lee C, Ryu EK. Hybrid PET/MR imaging of tumors using an oleanolic acid-conjugated nanoparticle. Biomaterials. 2013;34:8114–8121. doi: 10.1016/j.biomaterials.2013.07.078. [DOI] [PubMed] [Google Scholar]
- 246.Nahrendorf M, Zhang H, Hembrador S, Panizzi P, Sosnovik DE, Aikawa E, Libby P, Swirski FK, Weissleder R. Nanoparticle PET-CT imaging of macrophages in inflammatory atherosclerosis. Circulation. 2008;117:379–387. doi: 10.1161/CIRCULATIONAHA.107.741181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Torres Martin de Rosales R, Tavare R, Paul RL, Jauregui-Osoro M, Protti A, Glaria A, Varma G, Szanda I, Blower PJ. Synthesis of 64Cu(II)-bis(dithiocarbamatebisphosphonate) and its conjugation with superparamagnetic iron oxide nanoparticles: in vivo evaluation as dual-modality PET-MRI agent. Angew Chem Int Ed Engl. 2011;50:5509–5513. doi: 10.1002/anie.201007894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Cui X, Belo S, Kruger D, Yan Y, de Rosales RT, Jauregui-Osoro M, Ye H, Su S, Mathe D, Kovacs N, Horvath I, Semjeni M, Sunassee K, Szigeti K, Green MA, Blower PJ. Aluminium hydroxide stabilised MnFe2O4 and Fe3O4 nanoparticles as dual-modality contrasts agent for MRI and PET imaging. Biomaterials. 2014;35:5840–5846. doi: 10.1016/j.biomaterials.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Thorek DL, Ulmert D, Diop NF, Lupu ME, Doran MG, Huang R, Abou DS, Larson SM, Grimm J. Non-invasive mapping of deep-tissue lymph nodes in live animals using a multimodal PET/MRI nanoparticle. Nat Commun. 2014;5 doi: 10.1038/ncomms4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Devaraj NK, Keliher EJ, Thurber GM, Nahrendorf M, Weissleder R. 18F labeled nanoparticles for in vivo PET-CT imaging. Bioconjug Chem. 2009;20:397–401. doi: 10.1021/bc8004649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Sharma R, Xu Y, Kim SW, Schueller MJ, Alexoff D, Smith SD, Wang W, Schlyer D. Carbon-11 radiolabeling of iron-oxide nanoparticles for dual-modality PET/MR imaging. Nanoscale. 2013;5:7476–7483. doi: 10.1039/c3nr02519e. [DOI] [PubMed] [Google Scholar]
- 252.Choi JS, Park JC, Nah H, Woo S, Oh J, Kim KM, Cheon GJ, Chang Y, Yoo J, Cheon J. A hybrid nanoparticle probe for dual-modality positron emission tomography and magnetic resonance imaging. Angew Chem Int Ed Engl. 2008;47:6259–6262. doi: 10.1002/anie.200801369. [DOI] [PubMed] [Google Scholar]
- 253.Hoffman D, Sun M, Yang L, McDonagh PR, Corwin F, Sundaresan G, Wang L, Vijayaragavan V, Thadigiri C, Lamichhane N, Zweit J. Intrinsically radiolabelled [(59)Fe]-SPIONs for dual MRI/radionuclide detection. Am J Nucl Med Mol Imaging. 2014;4:548–560. [PMC free article] [PubMed] [Google Scholar]
- 254.Wong RM, Gilbert DA, Liu K, Louie AY. Rapid size-controlled synthesis of dextran-coated, 64Cu-doped iron oxide nanoparticles. ACS Nano. 2012;6:3461–3467. doi: 10.1021/nn300494k. [DOI] [PubMed] [Google Scholar]
- 255.Madru R, Tran TA, Axelsson J, Ingvar C, Bibic A, Stahlberg F, Knutsson L, Strand SE. (68)Ga-labeled superparamagnetic iron oxide nanoparticles (SPIONs) for multi-modality PET/MR/Cherenkov luminescence imaging of sentinel lymph nodes. Am J Nucl Med Mol Imaging. 2013;4:60–69. [PMC free article] [PubMed] [Google Scholar]
- 256.Boros E, Bowen AM, Josephson L, Vasdev N, Holland JP. Chelate-free metal ion binding and heat-induced radiolabeling of iron oxide nanoparticles. Chem Sci. 2015;6:225–236. doi: 10.1039/c4sc02778g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Pan D, Schmieder AH, Wickline SA, Lanza GM. Manganese-based MRI contrast agents: past, present and future. Tetrahedron. 2011;67:8431–8444. doi: 10.1016/j.tet.2011.07.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Burtea C, Laurent S, Vander Elst L, Muller RN. Contrast agents: magnetic resonance. In: Semmler W, Schwaiger M, editors. Molecular imaging I. Springer; 2008. pp. 135–165. [DOI] [PubMed] [Google Scholar]
- 259.Zhen Z, Xie J. Development of manganese-based nanoparticles as contrast probes for magnetic resonance imaging. Theranostics. 2012;2:45–54. doi: 10.7150/thno.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Huang J, Xie J, Chen K, Bu L, Lee S, Cheng Z, Li X, Chen X. HSA coated MnO nanoparticles with prominent MRI contrast for tumor imaging. Chem Commun. 2010;46:6684–6686. doi: 10.1039/c0cc01041c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Patel D, Kell A, Simard B, Xiang B, Lin HY, Tian G. The cell labeling efficacy, cytotoxicity and relaxivity of copper-activated MRI/PET imaging contrast agents. Biomaterials. 2011;32:1167–1176. doi: 10.1016/j.biomaterials.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 262.Hu H, Li D, Liu S, Wang M, Moats R, Conti PS, Li Z. Integrin alpha2beta1 targeted GdVO4:Eu ultrathin nanosheet for multimodal PET/MR imaging. Biomaterials. 2014;35:8649–8658. doi: 10.1016/j.biomaterials.2014.06.059. [DOI] [PubMed] [Google Scholar]
- 263.Yoo D, Lee JH, Shin TH, Cheon J. Theranostic magnetic nanoparticles. Acc Chem Res. 2011;44:863–874. doi: 10.1021/ar200085c. [DOI] [PubMed] [Google Scholar]
- 264.Laurent S, Saei AA, Behzadi S, Panahifar A, Mahmoudi M. Superparamagnetic iron oxide nanoparticles for delivery of therapeutic agents: opportunities and challenges. Expert Opin Drug Deliv. 2014;11:1449–1470. doi: 10.1517/17425247.2014.924501. [DOI] [PubMed] [Google Scholar]
- 265.Veiseh O, Gunn JW, Zhang M. Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Adv Drug Deliv Rev. 2010;62:284–304. doi: 10.1016/j.addr.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Li C, Li L, Keates AC. Targeting cancer gene therapy with magnetic nanoparticles. Oncotarget. 2012;3:365–370. doi: 10.18632/oncotarget.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Cho NH, Cheong TC, Min JH, Wu JH, Lee SJ, Kim D, Yang JS, Kim S, Kim YK, Seong SY. A multifunctional core-shell nanoparticle for dendritic cell-based cancer immunotherapy. Nat Nanotechnol. 2011;6:675–682. doi: 10.1038/nnano.2011.149. [DOI] [PubMed] [Google Scholar]
- 268.Cherukuri P, Glazer ES, Curley SA. Targeted hyperthermia using metal nanoparticles. Adv Drug Deliv Rev. 2010;62:339–345. doi: 10.1016/j.addr.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Kumar CS, Mohammad F. Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery. Adv Drug Deliv Rev. 2011;63:789–808. doi: 10.1016/j.addr.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Salunkhe AB, Khot VM, Pawar SH. Magnetic hyperthermia with magnetic nanoparticles: a status review. Curr Top Med Chem. 2014;14:572–594. doi: 10.2174/1568026614666140118203550. [DOI] [PubMed] [Google Scholar]
- 271.Thiesen B, Jordan A. Clinical applications of magnetic nanoparticles for hyperthermia. Int J Hyperthermia. 2008;24:467–474. doi: 10.1080/02656730802104757. [DOI] [PubMed] [Google Scholar]
- 272.Tassa C, Shaw SY, Weissleder R. Dextran-coated iron oxide nanoparticles: a versatile platform for targeted molecular imaging, molecular diagnostics, and therapy. Acc Chem Res. 2011;44:842–852. doi: 10.1021/ar200084x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Ku G, Zhou M, Song S, Huang Q, Hazle J, Li C. Copper sulfide nanoparticles as a new class of photoacoustic contrast agent for deep tissue imaging at 1064 nm. ACS Nano. 2012;6:7489–7496. doi: 10.1021/nn302782y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Gao D, Zhang P, Liu C, Chen C, Gao G, Wu Y, Sheng Z, Song L, Cai L. Compact chelator-free Ni-integrated CuS nanoparticles with tunable near-infrared absorption and enhanced relaxivity for in vivo dual-modal photoacoustic/MR imaging. Nanoscale. 2015;7:17631–17636. doi: 10.1039/c5nr05237h. [DOI] [PubMed] [Google Scholar]
- 275.Liu R, Jing L, Peng D, Li Y, Tian J, Dai Z. Manganese (II) chelate functionalized copper sulfide nanoparticles for efficient magnetic resonance/photoacoustic dual-modal imaging guided photothermal therapy. Theranostics. 2015;5:1144–1153. doi: 10.7150/thno.11754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Li Y, Lu W, Huang Q, Huang M, Li C, Chen W. Copper sulfide nanoparticles for photothermal ablation of tumor cells. Nanomedicine (Lond) 2010;5:1161–1171. doi: 10.2217/nnm.10.85. [DOI] [PubMed] [Google Scholar]
- 277.Tian Q, Jiang F, Zou R, Liu Q, Chen Z, Zhu M, Yang S, Wang J, Wang J, Hu J. Hydrophilic Cu9S5 nanocrystals: a photothermal agent with a 25.7% heat conversion efficiency for photothermal ablation of cancer cells in vivo. ACS nano. 2011;5:9761–9771. doi: 10.1021/nn203293t. [DOI] [PubMed] [Google Scholar]
- 278.Tian Q, Tang M, Sun Y, Zou R, Chen Z, Zhu M, Yang S, Wang J, Wang J, Hu J. Hydrophilic flower-like CuS superstructures as an efficient 980 nm laser-driven photothermal agent for ablation of cancer cells. Adv Mater. 2011;23:3542–3547. doi: 10.1002/adma.201101295. [DOI] [PubMed] [Google Scholar]
- 279.Ramadan S, Guo L, Li Y, Yan B, Lu W. Hollow copper sulfide nanoparticle-mediated transdermal drug delivery. Small. 2012;8:3143–3150. doi: 10.1002/smll.201200783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Goel S, Chen F, Cai W. Synthesis and biomedical applications of copper sulfide nanoparticles: from sensors to theranostics. Small. 2014;10:631–645. doi: 10.1002/smll.201301174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Zhou M, Zhang R, Huang M, Lu W, Song S, Melancon MP, Tian M, Liang D, Li C. A chelator-free multifunctional [64Cu]CuS nanoparticle platform for simultaneous micro-PET/CT imaging and photothermal ablation therapy. J Am Chem Soc. 2010;132:15351–15358. doi: 10.1021/ja106855m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Zhou M, Chen Y, Adachi M, Wen X, Erwin B, Mawlawi O, Lai SY, Li C. Single agent nanoparticle for radiotherapy and radio-photothermal therapy in anaplastic thyroid cancer. Biomaterials. 2015;57:41–49. doi: 10.1016/j.biomaterials.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Zhou M, Zhao J, Tian M, Song S, Zhang R, Gupta S, Tan D, Shen H, Ferrari M, Li C. Radio-photothermal therapy mediated by a single compartment nanoplatform depletes tumor initiating cells and reduces lung metastasis in the orthotopic 4T1 breast tumor model. Nanoscale. 2015;7:19438–19447. doi: 10.1039/c5nr04587h. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 284.Zhou M, Li J, Liang S, Sood AK, Liang D, Li C. CuS Nanodots with Ultrahigh Efficient Renal Clearance for Positron Emission Tomography Imaging and Image-Guided Photothermal Therapy. ACS Nano. 2015;9:7085–7096. doi: 10.1021/acsnano.5b02635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Riedinger A, Avellini T, Curcio A, Asti M, Xie Y, Tu R, Marras S, Lorenzoni A, Rubagotti S, Iori M, Capponi PC, Versari A, Manna L, Seregni E, Pellegrino T. Post-synthesis incorporation of (6)(4)Cu in CuS nanocrystals to radiolabel photothermal probes: A feasible approach for clinics. J Am Chem Soc. 2015;137:15145–15151. doi: 10.1021/jacs.5b07973. [DOI] [PubMed] [Google Scholar]
- 286.Liu T, Shi S, Liang C, Shen S, Cheng L, Wang C, Song X, Goel S, Barnhart TE, Cai W, Liu Z. Iron oxide decorated MoS2 nanosheets with double PEGylation for chelator-free radiolabeling and multimodal imaging guided photothermal therapy. ACS Nano. 2015;9:950–960. doi: 10.1021/nn506757x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Cheng L, Shen S, Shi S, Yi Y, Wang X, Song G, Yang K, Liu G, Barnhart TE, Cai W, Liu Z. FeSe2-Decorated Bi2Se3 nanosheets fabricated via cation exchange for chelator-Free Cu-labeling and multimodal image-guided photothermal-radiation therapy. Adv Funct Mater. 2016;26:2185–2197. doi: 10.1002/adfm.201504810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Gao F, Cai P, Yang W, Xue J, Gao L, Liu R, Wang Y, Zhao Y, He X, Zhao L, Huang G, Wu F, Zhao Y, Chai Z, Gao X. Ultrasmall [(64)Cu]Cu nanoclusters for targeting orthotopic lung tumors using accurate positron emission tomography imaging. ACS Nano. 2015;9:4976–4986. doi: 10.1021/nn507130k. [DOI] [PubMed] [Google Scholar]
- 289.Yang S, Sun S, Zhou C, Hao G, Liu J, Ramezani S, Yu M, Sun X, Zheng J. Renal clearance and degradation of glutathione-coated copper nanoparticles. Bioconjug Chem. 2015;26:511–519. doi: 10.1021/acs.bioconjchem.5b00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Kotagiri N, Sudlow GP, Akers WJ, Achilefu S. Breaking the depth dependency of phototherapy with Cerenkov radiation and low-radiance-responsive nanophotosensitizers. Nat Nanotechnol. 2015;10:370–379. doi: 10.1038/nnano.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Hong H, Shi J, Yang Y, Zhang Y, Engle JW, Nickles RJ, Wang X, Cai W. Cancer-targeted optical imaging with fluorescent zinc oxide nanowires. Nano Lett. 2011;11:3744–3750. doi: 10.1021/nl201782m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Rojas S, Gispert JD, Abad S, Buaki-Sogo M, Victor VM, Garcia H, Herance JR. In vivo biodistribution of amino-functionalized ceria nanoparticles in rats using positron emission tomography. Mol Pharm. 2012;9:3543–3550. doi: 10.1021/mp300382n. [DOI] [PubMed] [Google Scholar]
- 293.Shi S, Fliss BC, Gu Z, Zhu Y, Hong H, Valdovinos HF, Hernandez R, Goel S, Luo H, Chen F, Barnhart TE, Nickles RJ, Xu ZP, Cai W. Chelator-Free labeling of layered double hydroxide nanoparticles for in vivo pet imaging. Sci Rep. 2015;5 doi: 10.1038/srep16930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Zhan Y, Ai F, Chen F, Valdovinos HF, Orbay H, Sun H, Liang J, Barnhart TE, Tian J, Cai W. Intrinsically Zirconium-89 labeled Gd2O2S:Eu nanoprobes for in vivo positron emission tomography and gamma-ray-induced radioluminescence imaging. Small. 2016;23:201600594. doi: 10.1002/smll.201600594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Srivatsan A, Chen X. Recent advances in nanoparticle-based nuclear imaging of cancers. Adv Cancer Res. 2014;124:83–129. doi: 10.1016/B978-0-12-411638-2.00003-3. [DOI] [PubMed] [Google Scholar]
- 296.Liu TW, MacDonald TD, Shi J, Wilson BC, Zheng G. Intrinsically Copper-64-labeled organic nanoparticles as radiotracers. Angew Chem Int Ed Engl. 2012;51:13128–13131. doi: 10.1002/anie.201206939. [DOI] [PubMed] [Google Scholar]
- 297.Liu TW, Macdonald TD, Jin CS, Gold JM, Bristow RG, Wilson BC, Zheng G. Inherently multimodal nanoparticle-driven tracking and real-time delineation of orthotopic prostate tumors and micrometastases. ACS Nano. 2013;7:4221–4232. doi: 10.1021/nn400669r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Huang H, Hernandez R, Geng J, Sun H, Song W, Chen F, Graves SA, Nickles RJ, Cheng C, Cai W, Lovell JF. A porphyrin-PEG polymer with rapid renal clearance. Biomaterials. 2016;76:25–32. doi: 10.1016/j.biomaterials.2015.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Cui L, Lin Q, Jin CS, Jiang W, Huang H, Ding L, Muhanna N, Irish JC, Wang F, Chen J, Zheng G. A PEGylation-free biomimetic porphyrin nanoplatform for personalized cancer theranostics. ACS Nano. 2015;9:4484–4495. doi: 10.1021/acsnano.5b01077. [DOI] [PubMed] [Google Scholar]
- 300.Zhang Y, Jeon M, Rich LJ, Hong H, Geng J, Zhang Y, Shi S, Barnhart TE, Alexandridis P, Huizinga JD, Seshadri M, Cai W, Kim C, Lovell JF. Non-invasive multimodal functional imaging of the intestine with frozen micellar naphthalocyanines. Nat Nanotechnol. 2014;9:631–638. doi: 10.1038/nnano.2014.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Li Y, Lin TY, Luo Y, Liu Q, Xiao W, Guo W, Lac D, Zhang H, Feng C, Wachsmann-Hogiu S, Walton JH, Cherry SR, Rowland DJ, Kukis D, Pan C, Lam KS. A smart and versatile theranostic nanomedicine platform based on nanoporphyrin. Nat Commun. 2014;5:4712. doi: 10.1038/ncomms5712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Perez-Medina C, Tang J, Abdel-Atti D, Hogstad B, Merad M, Fisher EA, Fayad ZA, Lewis JS, Mulder WJ, Reiner T. PET imaging of tumor-associated macrophages with 89Zr-labeled high-density lipoprotein nanoparticles. J Nucl Med. 2015;56:1272–1277. doi: 10.2967/jnumed.115.158956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Zhang P, Chen Y, Zeng Y, Shen C, Li R, Guo Z, Li S, Zheng Q, Chu C, Wang Z, Zheng Z, Tian R, Ge S, Zhang X, Xia NS, Liu G, Chen X. Virus-mimetic nanovesicles as a versatile antigen-delivery system. Proc Natl Acad Sci USA. 2015;112:E6129–E6138. doi: 10.1073/pnas.1505799112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Lin J, Wang M, Hu H, Yang X, Wen B, Wang Z, Jacobson O, Song J, Zhang G, Niu G, Huang P, Chen X. Multimodal-imaging-guided cancer phototherapy by versatile biomimetic theranostics with UV and gamma-irradiation protection. Adv Mater. 2016;28:3273–3279. doi: 10.1002/adma.201505700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Fan Q, Cheng K, Hu X, Ma X, Zhang R, Yang M, Lu X, Xing L, Huang W, Gambhir SS, Cheng Z. Transferring biomarker into molecular probe: melanin nanoparticle as a naturally active platform for multimodality imaging. J Am Chem Soc. 2014;136:15185–15194. doi: 10.1021/ja505412p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Lin X, Xie J, Niu G, Zhang F, Gao H, Yang M, Quan Q, Aronova MA, Zhang G, Lee S, Leapman R, Chen X. Chimeric ferritin nanocages for multiple function loading and multimodal imaging. Nano Lett. 2011;11:814–819. doi: 10.1021/nl104141g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Huang P, Rong P, Jin A, Yan X, Zhang MG, Lin J, Hu H, Wang Z, Yue X, Li W, Niu G, Zeng W, Wang W, Zhou K, Chen X. Dye-loaded ferritin nanocages for multimodal imaging and photothermal therapy. Adv Mater. 2014;26:6401–6408. doi: 10.1002/adma.201400914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Ji T, Zhao Y, Wang J, Zheng X, Tian Y, Zhao Y, Nie G. Tumor fibroblast specific activation of a hybrid ferritin nanocage-based optical probe for tumor microenvironment imaging. Small. 2013;9:2427–2431. doi: 10.1002/smll.201300600. [DOI] [PubMed] [Google Scholar]
- 309.Wang Z, Huang P, Jacobson O, Wang Z, Liu Y, Lin L, Lin J, Lu N, Zhang H, Tian R, Niu G, Liu G, Chen X. Biomineralization-inspired synthesis of copper sulfide-ferritin nanocages as cancer theranostics. ACS Nano. 2016;10:3453–3460. doi: 10.1021/acsnano.5b07521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Huang X, Zhang F, Sun X, Choi KY, Niu G, Zhang G, Guo J, Lee S, Chen X. The genotype-dependent influence of functionalized multiwalled carbon nanotubes on fetal development. Biomaterials. 2014;35:856–865. doi: 10.1016/j.biomaterials.2013.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Goel S, Chen F, Luan S, Valdovinos HF, Shi S, Graves SA, Ai F, Barnhart TE, Theuer CP, Cai W. Engineering intrinsically zirconium-89 radiolabeled self-destructing mesoporous silica nanostructures for in vivo biodistribution and tumor targeting studies. Advanced Science. 2016 doi: 10.1002/advs.201600122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Munaweera I, Koneru B, Shi Y, Di Pasqua AJ, Balkus J, Kenneth J. Chemoradiotherapeutic wrinkled mesoporous silica nanoparticles for use in cancer therapy. APL Materials. 2014;2 113315-1-13. [Google Scholar]
- 313.Chakravarty R, Goel S, Hong H, Chen F, Valdovinos HF, Hernandez R, Barnhart TE, Cai W. Hollow mesoporous silica nanoparticles for tumor vasculature targeting and PET image-guided drug delivery. Nanomedicine. 2015;10:1233–1246. doi: 10.2217/nnm.14.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Song J, Yang X, Jacobson O, Huang P, Sun X, Lin L, Yan X, Niu G, Ma Q, Chen X. Ultrasmall gold nanorod vesicles with enhanced tumor accumulation and fast excretion from the body for cancer therapy. Adv Mater. 2015;27:4910–4917. doi: 10.1002/adma.201502486. [DOI] [PubMed] [Google Scholar]
- 315.Lu W, Xiong C, Zhang G, Huang Q, Zhang R, Zhang JZ, Li C. Targeted photothermal ablation of murine melanomas with melanocyte-stimulating hormone analog-conjugated hollow gold nanospheres. Clin Cancer Res. 2009;15:876–886. doi: 10.1158/1078-0432.CCR-08-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Cheng B, He H, Huang T, Berr SS, He J, Fan D, Zhang J, Xu P. Gold nanosphere gated mesoporous silica nanoparticle responsive to near-infrared light and redox potential as a theranostic platform for cancer therapy. J Biomed Nanotechnol. 2016;12:435–449. doi: 10.1166/jbn.2016.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]