Abstract
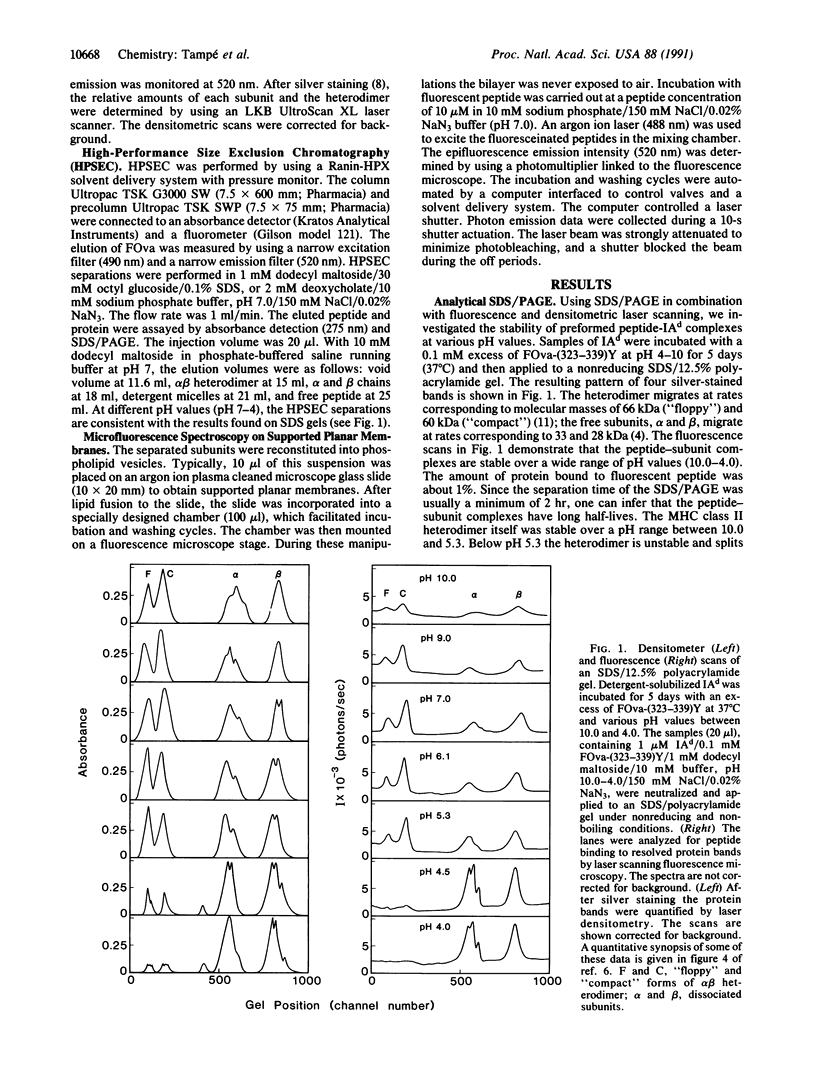
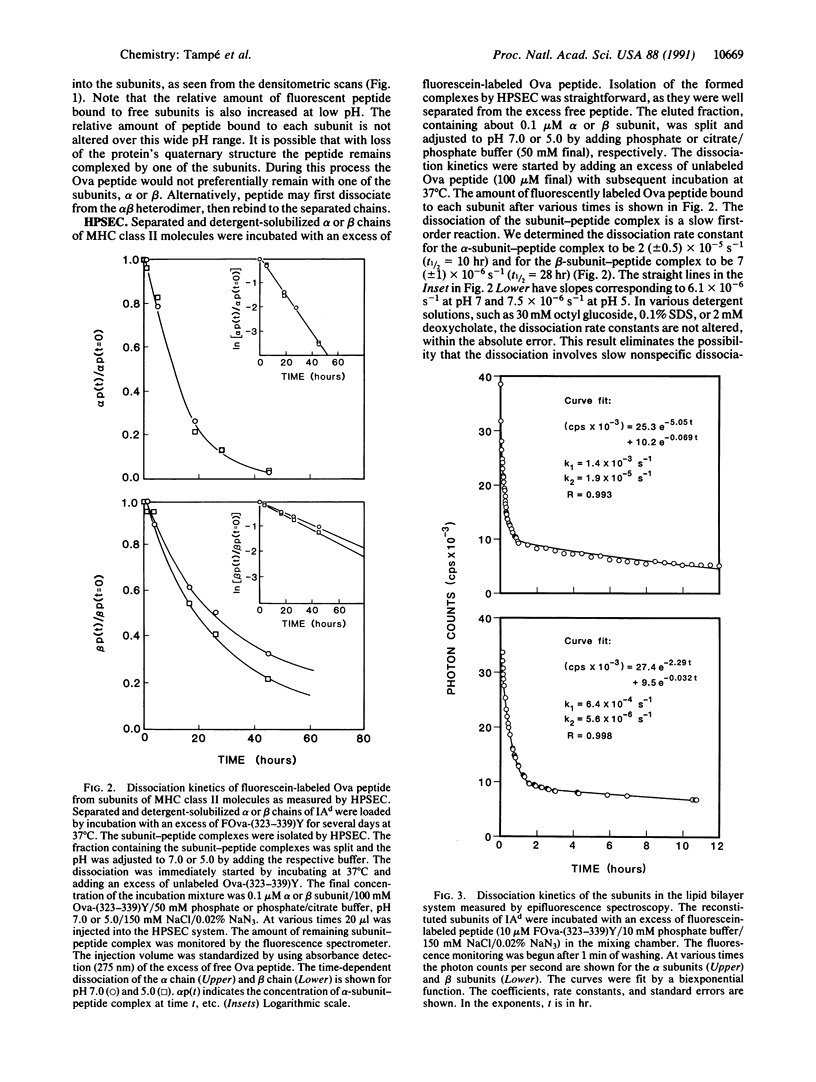
Major histocompatibility complex (MHC) class II molecules are heterodimers formed by noncovalent linkage of alpha and beta chains. It has been shown that the subunits of the MHC class II molecules IAd and IEk bind antigenic peptides as well as antigenic peptides labeled with fluorescent probes. Laser scanning fluorescence microscopy on SDS/polyacrylamide gels demonstrates that the subunit-peptide complexes of IAd are stable over a wide pH range. Below pH 5.3 the heterodimer of IAd dissociates into the free chains, which still bind antigenic peptides such as the 18-amino acid peptide obtained by a tyrosine addition to a chicken ovalbumin peptide, Ova-(323-339)Y. The stability of preformed subunit complexes with fluorescein-labeled Ova-(323-339)Y was investigated by using high-performance size exclusion chromatography and epifluorescence microscopy. Each subunit forms a long-lived complex, both in detergent solutions and in reconstituted lipid bilayers. At 37 degrees C and pH 7.0 the dissociation half-time of the beta-subunit-peptide complex was determined to be 28 hr and that of the alpha-subunit-peptide complex was 10 hr. In contrast to the dissociation of the peptide from the IAd heterodimer, the half-times for dissociation of the peptide from the separate chains are not decreased at pH 5.0.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987 Oct 8;329(6139):506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. The foreign antigen binding site and T cell recognition regions of class I histocompatibility antigens. Nature. 1987 Oct 8;329(6139):512–518. doi: 10.1038/329512a0. [DOI] [PubMed] [Google Scholar]
- Buus S., Sette A., Colon S. M., Jenis D. M., Grey H. M. Isolation and characterization of antigen-Ia complexes involved in T cell recognition. Cell. 1986 Dec 26;47(6):1071–1077. doi: 10.1016/0092-8674(86)90822-6. [DOI] [PubMed] [Google Scholar]
- Dornmair K., Rothenhäusler B., McConnell H. M. Structural intermediates in the reactions of antigenic peptides with MHC molecules. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):409–416. doi: 10.1101/sqb.1989.054.01.050. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Rothenhäusler B., Dornmair K., McConnell H. M. Specific binding of antigenic peptides to separate alpha and beta chains of class II molecules of the major histocompatibility complex. Proc Natl Acad Sci U S A. 1990 Jan;87(1):352–354. doi: 10.1073/pnas.87.1.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. H. T-lymphocyte recognition of antigen in association with gene products of the major histocompatibility complex. Annu Rev Immunol. 1985;3:237–261. doi: 10.1146/annurev.iy.03.040185.001321. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Tampé R., Clark B. R., McConnell H. M. Energy transfer between two peptides bound to one MHC class II molecule. Science. 1991 Oct 4;254(5028):87–89. doi: 10.1126/science.1656526. [DOI] [PubMed] [Google Scholar]
- Tampé R., McConnell H. M. Kinetics of antigenic peptide binding to the class II major histocompatibility molecule I-Ad. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4661–4665. doi: 10.1073/pnas.88.11.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno M., Tanford C., Reynolds J. A. Phospholipid vesicle formation using nonionic detergents with low monomer solubility. Kinetic factors determine vesicle size and permeability. Biochemistry. 1984 Jun 19;23(13):3070–3076. doi: 10.1021/bi00308a034. [DOI] [PubMed] [Google Scholar]



