ABSTRACT
Tuberculosis in goats is usually diagnosed clinically, at postmortem, or by a positive skin test. However, none of these approaches detects all infected animals. Serology offers an additional tool to identify infected animals missed by current tests. We describe the use of the Enferplex Caprine TB serology test to aid the management of a large dairy goat herd undergoing a tuberculosis breakdown. Initial skin and serology testing showed that IgG antibodies were present in both serum and milk from 100% of skin test-positive animals and in serum and milk from 77.8 and 95.4% of skin test-negative animals, respectively. A good correlation was observed between serum and milk antibody levels. The herd had been vaccinated against Mycobacterium avium subsp. paratuberculosis, but no direct serological cross-reactions were found. Subsequent skin testing revealed 13.7% positive animals, 64.9% of which were antibody positive, while 42.1% of skin test-negative animals were seropositive. Antibody responses remained high 1 month later (57.1% positive), and the herd was slaughtered. Postmortem analysis of 20 skin test-negative goats revealed visible lesions in 6 animals, all of which had antibodies to six Mycobacterium bovis antigens. The results provide indirect evidence that serology testing with serum or milk could be a useful tool in the diagnosis and management of tuberculosis in goats.
KEYWORDS: tuberculosis, antibodies, goats
INTRODUCTION
Tuberculosis (TB) due to infection by Mycobacterium bovis is a major problem in cattle in the United Kingdom, causing huge financial losses, as well as being a significant zoonosis risk. The presence of significant levels of TB in wildlife vectors such as the badger has led to trial culling or vaccination of these animals in high-risk areas such as southwestern England and Wales, respectively. Spillover hosts such as sheep, goats, deer, and alpacas also present a risk of spreading TB (1). Currently, control programs involving cattle are focused on detecting cell-mediated immunity (CMI) through the use of tuberculin skin testing and gamma interferon (IFN-γ) tests. In goats, the single intradermal comparative tuberculin test (SICTT) and the single intradermal test (SIT) are used to detect infection (2–5). Published estimates of SICTT sensitivity range from 42.7 to 83.7%, while those of SIT range from 44.6 to 93.8% (2, 3, 5). However, a recent study adopting bacteriology as the gold standard showed the sensitivity of the SIT by the severe interpretation to be as low as 43.9% and to decrease to 38.8% by the standard interpretation, while the sensitivity of the SICTT ranged between 21.3 and 7%, depending of the interpretation criterion used (6).
Recent studies also showed that when goats are coinfected with Mycobacterium avium subsp. paratuberculosis, the sensitivity and specificity of the TB skin tests appear to be adversely affected, with a substantial proportion of M. bovis-infected animals going undetected (2–4, 7–11). Since skin and serology tests measure different arms of the immune response, detection of specific antibodies has the potential to identify infected animals not revealed by skin tests. Several studies have documented antibody responses in goats and other animals that are negative in CMI tests (2, 12–14).
In previous work, we have described the development of multiplex serology tests by using antigen arrays to detect antibodies to specific M. bovis antigens in several species, including cattle, goats, and alpacas (15–21). The sensitivities and specificities obtained with these serology tests varied with the population under study. In goats confirmed as infected by SICTT, histopathology, and culture, the multiplex test detected 57/60 (95.0%) positive animals in one herd and 120/120 (100%) in a second herd and gave positive signals in a further 4% of SICTT-negative animals (20). The test has been used previously to help manage a goat breakdown herd by identifying infected animals that were not detected by the SICTT (22). In cattle, the multiplex has been shown to detect a substantial proportion of animals with lesions that were negative or inconclusive by the SICTT (17). These studies suggest that the multiplex test does indeed identify infected animals missed by skin tests. However, further work is required to gauge the extent of such detection and its usefulness in aiding the diagnosis of TB in goats and other species.
Here we describe the application of the multiplex serology test to a large dairy goat herd undergoing a TB breakdown in the United Kingdom. Samples from the herd were submitted for antibody testing in order to assist in the diagnosis and management of the TB outbreak. This investigation provided the opportunity to assess further the relative merits of serology versus skin testing and also to examine the use of milk for TB serology in goats.
RESULTS
Skin testing.
A summary of the skin testing done in the purchased and index herds relevant to this study is shown in Table 1. The 183 animals in the purchased herd and 9 animals in the index herd were tested by SICTT on 16 December 2013. One hundred thirty-six reactors and five inconclusive reactors were found in the purchased herd, and one reactor was found in the index herd. Five hundred nine animals in the index herd were skin tested between 16 December 2013 and 6 January 2014, and 17 SICTT reactors were found (Table 1). Further skin tests of 280 animals on 3 February revealed no reactors. However, 14 SICTT reactors out of 494 animals were found following a skin test on 10 March 2014. Finally, 555 animals were tested by SIT on 19 May 2014, and 76 reactors were detected.
TABLE 1.
Summary of skin tests applied to purchased and index herds
| Date | Herd | Skin test | No. tested | Skin test resulta |
|---|---|---|---|---|
| 16 December 2013 | Purchased | SICTT standard | 183 | 136 R, 5 IR, 42 clear |
| 16 December 2013 | Index | SICTT standard | 9 | 1 R, 8 clear |
| 30 December 2013 | Index | SICTT standard | 450 | 15 R, 435 clear |
| 3 January 2014 | Index | SICTT standard | 50 | 1 R, 49 clear |
| 3 February 2013 | Index | SICTT standard | 280 | 280 clear |
| 10 March 2014 | Index | SICTT standard | 494 | 14 R, 480 clear |
| 19 May 2014 | Index | SIT severe | 555 | 76 R, 479 clear |
R, reactor; IR, inconclusive reactor; clear, negative skin test.
The mean skin thicknesses at the purified protein derivative A (PPD-A) and PPD-B injection sites of the 14 reactors in March 2014 were 13.0 ± 6.1 and 23.9 ± 11.1 mm, respectively, with a mean difference of 10.9 ± 6.0 mm (data not shown). Of the 494 animals tested, 22 had a skin thickness elevated above normal, but the PPD-A site values (20.2 ± 5.5 mm) exceeded those of the PPD-B site (12.6 ± 4.0 mm), with a mean difference of −7.7 ± 3.9 mm (data not shown). Of the 22 animals, 21 underwent the SIT in May 2014 and 8 were positive.
Serology results from the index herd relative to skin test results.
In total, 447/494 animals that underwent SICTT on 10 March 2014 were tested for antibody 8 days later. By the two-antigen rule, the multiplex assay determined 351/447 (78.5%) as positive (Table 2). The results of the serology test applied to serum samples taken from 14 SICTT-positive and 433 SICTT-negative animals are shown in Table 2. The multiplex test detected antibodies in all 14 SICTT-positive animals, with all animals giving positive antibody responses to five or six test antigens. Of the SICTT-negative animals, 77.8% were antibody positive (Table 2). The proportion of antibody-positive samples was significantly lower in SICTT-negative animals than in SICTT-positive animals by the two-antigen rule. The proportions of samples positive for MPB70, MPB83, CFP10, and ESAT6 individually were significantly lower in SICTT-negative animals than in SICTT-positive animals (Table 2). When all 447 samples were analyzed (irrespective of the SICTT results), the percentage of positive samples was lower with the CFP10 and ESAT6 antigens than with the MPB70 peptide, PPD-B, MPB70, and MPB83 antigens (Table 2; P < 0.001 for all comparisons between CFP10 or ESAT6 and the other antigens). The antibody levels in samples taken on 18 March 2014 are shown in Table 3. The numbers of relative light units (RLU) were significantly lower in SICTT-negative animals than in SICTT-positive animals for all six antigens (P < 0.0001 in all cases) when numbers of RLU above the individual antigen cutoffs were analyzed.
TABLE 2.
IgG antibody responses in serum samples from index herd in relation to skin test status
| Blood sample datea and skin test status (no. of samples) | % Antibody positiveb |
Total % positivec | |||||
|---|---|---|---|---|---|---|---|
| MPB70 peptide | PPD-B | MPB70 | MPB83 | CFP10 | ESAT6 | ||
| 18 March 2014 | |||||||
| SICTT positive (14) | 100 | 100 | 100 | 100 | 100 | 78.6 | 100 |
| SICTT negative (433) | 78.1 | 81.1 | 71.4d | 62.8e | 45.0f | 9.0f | 77.8d |
| Total (447) | 78.7 | 81.7 | 72.3 | 64.0 | 46.8 | 11.2 | 78.5 |
| 22 May 2014 | |||||||
| SIT positive (74) | 41.9 | 63.5 | 55.4 | 54.1 | 43.2 | 37.8 | 64.9 |
| SIT negative (133) | 27.8d | 31.6f | 36.1d | 30.8d | 17.3f | 24.8 | 42.1e |
| Total (207) | 32.9 | 43.0 | 43.0 | 39.1 | 26.6 | 29.5 | 50.2 |
SICTT was performed in March and blood samples were taken 8 days after PPD-B injection, while SIT was performed in May and blood samples were taken 3 days after PPD-B injection.
Values showing statistically significant differences between skin test-positive and -negative animals are in bold.
Positive, antibodies to two or more antigens.
P < 0.05.
P < 0.001.
P < 0.0001.
TABLE 3.
IgG antibody levels above the cutoff in serum samples from the index herd in relation to skin test status
| Blood sample datea and skin test status | Relative light units × 10−3 (mean ± SD)b |
|||||
|---|---|---|---|---|---|---|
| MPB70 peptide | PPD-B | MPB70 | MPB83 | CFP10 | ESAT6 | |
| 18 March 2014 | ||||||
| SICTT positive | 48.1 ± 7.6 | 46.4 ± 6.1 | 49.4 ± 5.4 | 43.3 ± 8.2 | 40.5 ± 12.6 | 44.2 ± 11.8 |
| SICTT negative | 30.4 ± 13.4c | 24.0 ± 13.6c | 32.7 ± 13.0c | 26.8 ± 11.0c | 24.4 ± 11.7c | 26.1 ± 14.6c |
| 22 May 2014 | ||||||
| SIT positive | 25.8 ± 14.0 | 16.4 +/12.7 | 26.4 ±12.3 | 26.8 ± 11.9 | 25.2 ± 13.8 | 29.3 ± 13.1 |
| SIT negative | 20.8 ± 12.8 | 12.3 ± 7.3 | 21.0 ± 9.5d | 22.4 ± 11.3 | 19.8 ± 8.1 | 24.6 ± 10.9 |
SICTT was performed in March and 447 blood samples were taken 8 days after PPD-B injection, while SIT was performed in May and 207 blood samples were taken 3 days after PPD-B injection. Only RLU values above the cutoff for each antigen were analyzed.
Values showing statistically significant differences between skin test-positive and -negative animals are in bold.
P < 0.0001.
P < 0.05.
SIT was performed on 19 May 2014 with 555 animals. Of these 555 animals, 445 had been tested by SICCT on 10 March 2014 and found to be negative and 99 had been tested by SICCT on 3 February 2014 and found to be negative. The results of the SIT performed on 19 May 2014 showed that 76/555 animals (13.7%) were SIT positive (Table 1). Thirty-nine of these 76 animals had been tested previously for antibody in the 18 March 2014 serum sampling, and 38/39 (97.4%) were positive by the two-antigen rule. Similarly, milk samples from 26 of these 76 animals had also been tested for antibody in March 2014 and 26/26 were positive (100.0%). Serum samples from 207 of these 555 skin-tested animals were taken 3 days after SIT on 22 May 2014, i.e., on the day the skin test was read. The results of testing for antibodies are shown in Table 2. Overall, 104/207 (50.2%) animals tested by SIT were positive for antibodies. Of the 76 SIT-positive animals, serum samples were available from 74 and 48 (64.9%) of these were positive for antibody by the two-antigen rule (Table 2). Of the 133 SIT-negative animals, 56 (42.1%) were antibody positive. The proportions of samples from SIT-negative animals that were antibody positive were significantly lower for all antigens except ESAT6 compared to those from SIT-positive animals. When all 207 samples were analyzed, irrespective of SIT, there were no significant differences in the percentage of positive samples between individual antigens. There was a trend toward lower RLU levels in SIT-negative animals than in SIT-positive animals, but the differences in antibody levels were only significant for MPB70 (Table 3).
No further skin testing was performed after 22 May 2014, but Enferplex results obtained from all 522 serum samples submitted on 23 June 2014 showed 298/522 (57.1%) were positive for antibodies overall by the two-antigen rule (data not shown). Serum from only 1 animal that was SIT positive in May 2014 was submitted for serology in June 2014, precluding any meaningful comparison of antibody responses in SIT-positive and SIT-negative animals.
Effect of skin testing on antibody responses.
In the data above, the sample sets were not identical as some animals had been removed and other animals included for testing and hence the results reflect the prevalence only of the animals submitted for testing on each occasion.
However, data were available from 122 animals that were serially sampled on 22 May (3 days after the SIT) and 23 June (31 days after the May SIT) 2014. Overall, 54/122 (43.4%) samples were positive for IgG antibodies in May by the two-antigen rule, while 80/122 (65.6%) were positive in June (P = 0.0011). These samples were used specifically to assess whether skin testing boosted antibody responses to PPD-B, since that was the antigen used in the SIT. The proportion of samples positive for anti-PPD-B antibodies was significantly higher 31 days after the SIT than 3 days after the SIT (Fig. 1A). The levels of anti-PPD-B antibodies found at 31 days were also significantly higher than those observed at day 3 after the SIT (Fig. 1B). The results suggest that antibody responses to PPD-B are boosted when PPD-B is given 31 days prior to blood sampling.
FIG 1.
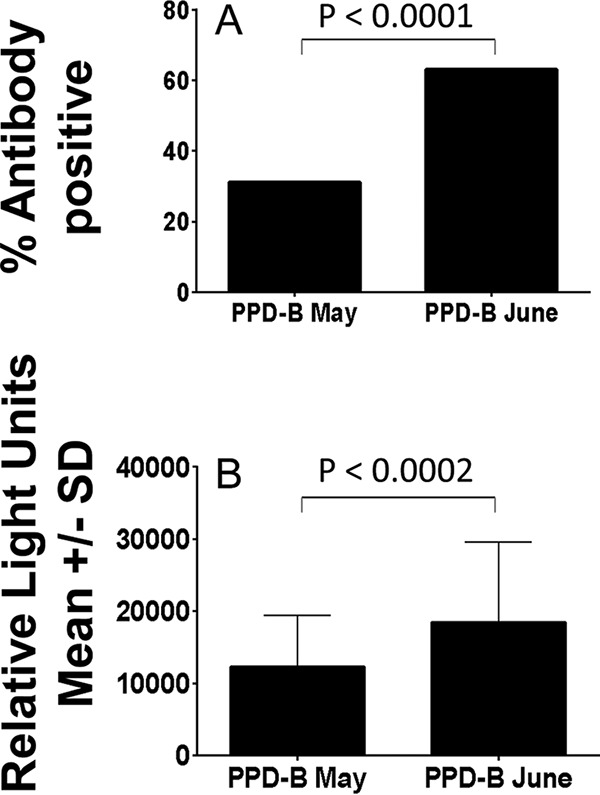
Effect of skin testing on IgG anti-PPD-B antibody responses. Serum samples (n = 122) were taken 3 days (PPD-B May) and 31 days (PPD-B June) after a SIT performed on 19 May 2014, and antibodies were measured. A, proportion of samples positive by the two-antigen rule; B, antibody levels above the cutoff. SD, standard deviation.
Comparison of multiplex test results with serum and milk samples.
Paired samples of serum and milk were available from a total of 275 animals sampled on18 March 2014, and these were assayed in the multiplex test (Table 4). Paired milk and serum samples were available for 12 of the 14 SICTT-positive animals from this sampling point. All 12 SICTT-positive animals tested had IgG antibodies in serum and milk (100%). Of the serum and milk samples from SICTT-negative animals, 95.4 and 95.1% were positive, respectively (Table 4), while 93.5% of the paired serum and milk samples were positive (data not shown). Antibody responses to CFP10 and ESAT6 in both milk and serum samples were significantly lower in SICTT-negative animals than in SICTT-positive animals (Table 4). High correlations were observed between the signals obtained with serum and milk (Spearman's correlation coefficient [r] range, 0.79 to 0.94) that were highly statistically significant for all six antigens (Fig. 2). Comparing the results obtained with serum versus milk on the basis of the two-antigen rule, a kappa statistic of 0.651 was observed overall, indicating substantial agreement between the two sets of results.
TABLE 4.
Comparison of IgG antibody responses in paired serum and milk samples in relation to skin test status in March 2014
| Samplea and skin test status (no. of samples) | % Antibody positiveb |
Total % positivec | |||||
|---|---|---|---|---|---|---|---|
| MPB70 peptide | PPD-B | MPB70 | MPB83 | CFP10 | ESAT6 | ||
| Serum | |||||||
| SICTT positive (12) | 100 | 100 | 100 | 100 | 100 | 75.0 | 100 |
| SICTT negative (263) | 90.9 | 95.1 | 89.7 | 80.2 | 55.5d | 10.7e | 95.4 |
| Total (275) | 91.3 | 95.3 | 90.2 | 81.1 | 57.5 | 14.2 | 95.6 |
| Milk | |||||||
| SICTT positive (12) | 100 | 100 | 100 | 100 | 100 | 83.3 | 100 |
| SICTT negative (263) | 93.2 | 93.9 | 92.0 | 89.0 | 75.7d | 16.0e | 95.1 |
| Total (275) | 93.5 | 94.2 | 92.4 | 89.5 | 76.7 | 18.9 | 95.3 |
Paired serum and milk samples were taken on 18 March 2014.
Values showing statistically significant differences between skin test-positive and -negative animals are in bold.
Positive, antibodies to two or more antigens.
P < 0.05.
P < 0.0001.
FIG 2.
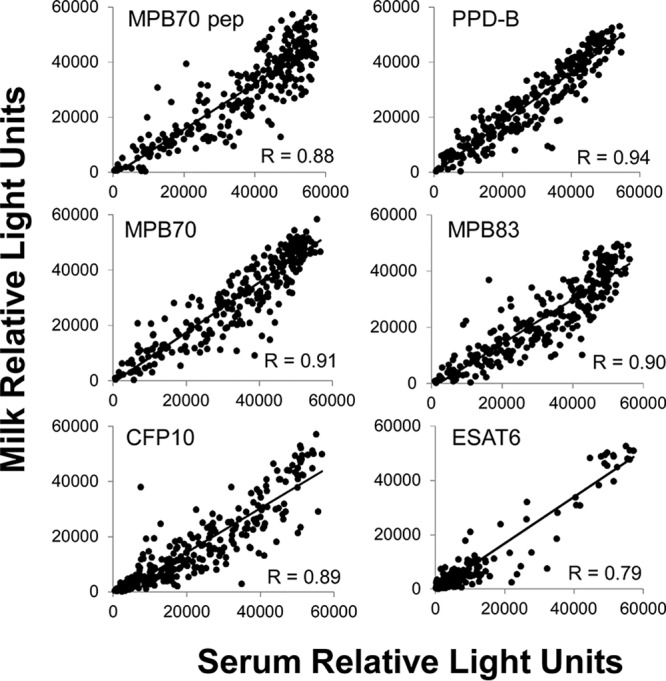
Correlation between observed RLU signals of IgG antibodies against individual M. bovis antigens in serum and milk samples from the index herd. R, Spearman's rank correlation coefficient. P < 0.0001 for all comparisons. pep, peptide.
Effect of M. avium subsp. paratuberculosis vaccination on Enferplex Caprine TB serology.
The goats in the index herd had been vaccinated against M. avium subsp. paratuberculosis. To assess whether M. avium subsp. paratuberculosis vaccination could have had any effect on the multiplex TB test results, serum samples obtained from goats in Norway (which has officially TB-free [OTF] status) that had (n = 74) or had not (n = 18) been vaccinated against M. avium subsp. paratuberculosis were tested. The results are shown in Fig. 3. No samples registered as positive for TB antibodies by the two-antigen rule, regardless of the animals being M. avium subsp. paratuberculosis vaccinated or not. The results show that there was no direct cross-reaction between M. avium subsp. paratuberculosis antibodies and the M. bovis antigens used in the multiplex test.
FIG 3.
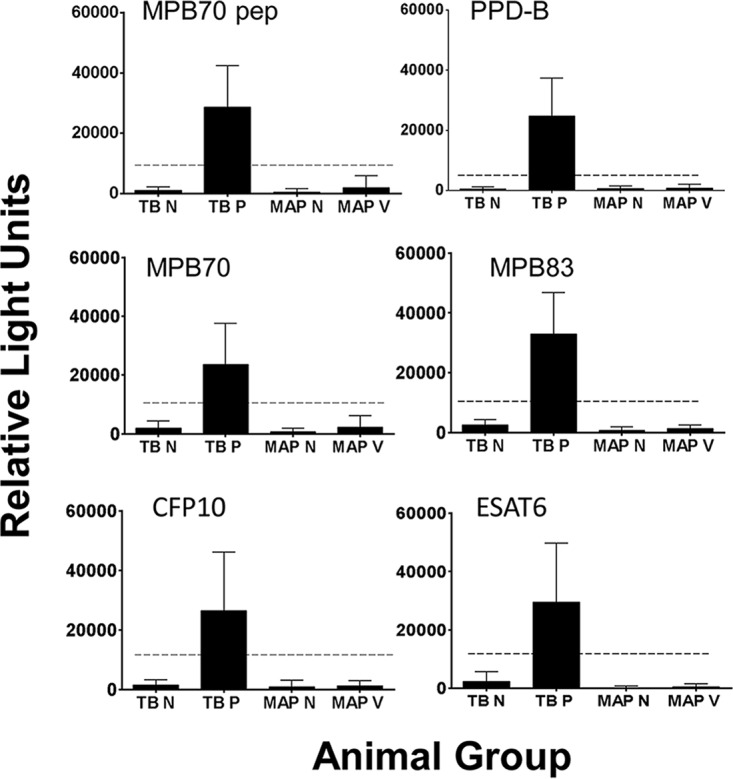
Effect of M. avium subsp. paratuberculosis vaccination on the Enferplex Caprine TB serology test. Serum samples from OTF Norwegian goats that had (MAP V; n = 74) or had not (MAP N; n = 18) been vaccinated against M. avium subsp. paratuberculosis were analyzed in the TB test, along with positive (TB P; n = 34) and negative (TB N; n = 73) TB control samples. The dotted line indicates the cutoff for each antigen. pep, peptide.
Relationship between the number of antigens recognized and antibody responses in the multiplex test.
Serum samples (n = 447) taken on 18 March 2014 were analyzed, and animals were grouped according to the numbers of antigens recognized (Table 5). Animals recognizing two antigens and judged as seropositive by the two-antigen rule generally recognized both PPD-B and MPB70 peptide. Animals recognizing further antigens generally recognized the same additional antigen as the number of antigens recognized increased, the progression being MPB70, MPB83, CFP10, and finally ESAT6 for those animals recognizing all six antigens. The mean RLU signal level for antibody to each antigen also increased with the number of antigens recognized, particularly for MPB70 peptide, PPD-B, MPB70, and MPB83 (Fig. 4). An increase in the RLU signal level was also noted for CFP10 and ESAT6, but only once at least four antigens were recognized. The results indicate that RLU signal levels and the proportions of the March 2014 samples positive for individual antigens tend to increase as the number of antigens recognized increased. Similar antigen response patterns were observed in the March milk samples and in the serum samples obtained in May and June 2014, but with the MPB70 responses being slightly more prominent than the MPB70 peptide responses in the May serum samples (data not shown).
TABLE 5.
Antigen-specific IgG antibody responses in animals sampled in March 2014 grouped by numbers of antigens recognized
| No. of antigens recognized | No. of serum samplesa | % Antibody positiveb |
|||||
|---|---|---|---|---|---|---|---|
| PPD-B | MPB 70 peptide | MPB70 | MPB83 | CFP10 | ESAT6 | ||
| 0 | 49 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 47 | 38.3 | 36.2 | 2.1 | 8.5 | 12.8 | 2.1 |
| 2 | 31 | 87.1c | 61.3d | 22.6 | 9.7 | 19.4 | 0 |
| 3 | 41 | 100d | 90.2d | 87.8c | 12.2 | 9.8 | 0 |
| 4 | 89 | 100 | 100d | 100d | 94.4c | 4.5 | 1.1 |
| 5 | 143 | 100 | 100 | 100 | 100d | 100c | 0.7 |
| 6 | 47 | 100 | 100 | 100 | 100 | 100 | 100c |
Total n = 447.
Progressive increases in the proportions of samples positive for antibody as the number of antigens recognized increases are in bold.
P < 0.0001 (compared to the preceding number of antigens recognized).
P < 0.05 (compared to the preceding number of antigens recognized).
FIG 4.
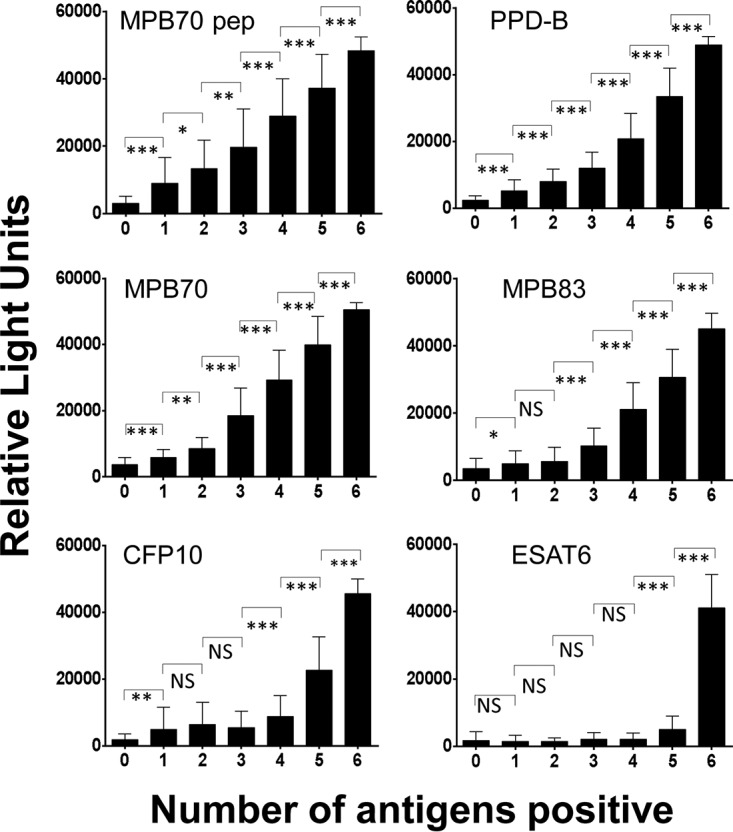
Relationship between the number of antigens recognized and the IgG antibody signal. Serum samples taken on 18 March 2014 (n = 447) were analyzed for the number of antigens recognized by antibody and the RLU signal obtained for each antigen individually. *, P < 0.01; **, P < 0.001; ***, P < 0.0001; NS, not significant; pep, peptide.
Postmortem analyses.
Postmortem examinations (by the Animal and Plant Health Agency [APHA]) of the skin test reactors from the index and purchased herds (16 December 2013) showed that 122/158 (77.2%) had visible lesions (VL) in total. This was broken down as follows between the purchased and index herd reactors, 111 VL in the purchased herd (111/141; 78.7%) and 11 VL in the index herd (11/17; 64.7%).
M. bovis was isolated from 5/5 animals that underwent bacterial culture, i.e., 3/3 purchased animals and 2/2 index animals (postmortem on 17 December 2013). In January 2014, 42 purchased goats and 8 index goats deemed to be dangerous close contacts were sent to APHA for postmortem examination and 9 showed VL, i.e., 7 VL in purchased animals and 2 VL in index animals.
Of the 14 animals that were positive by both SICTT and antibody tests in March 2014, 4 showed tuberculous lesions in the chest (n = 3) and udder (n = 1) at postmortem. Two animals had acid-fast bacteria by histology. The remaining 10 animals showed no VL. Following the identification of positive serological responses in the majority of the SICTT-negative animals in March 2014, 8 SICTT-negative, antibody-positive animals underwent postmortem examination. Four of these animals were selected at random from 47 animals positive for six antigens in serum and milk. These four animals showed VL that were confirmed as positive by histology; two of the four animals were also positive for acid-fast bacteria. The other four animals were selected at random from 141 animals positive for five antigens in serum and milk. None of these animals showed VL. One further animal that was SICTT positive and reactive to five antigens showed VL at postmortem. Five milk samples (four positive for six antigens, one negative for all six antigens) were Ziehl-Neelsen stained, and two of the antibody-positive animals were positive for acid-fast bacteria, i.e., one animal with lesions and one without lesions.
On slaughter of the entire herd in September 2014, a further 12 animals were selected from the index herd for postmortem examination. Two of these 12 were determined to have lesions suspicious of TB, and a further 4 were found to have lesions, but they were not typical of TB. No acid-fast bacteria were detected in any of the 12 animals. Serum and milk samples taken from these 12 animals in March 2014 and also in June 2014 were all Enferplex Caprine TB test positive for six antigens.
DISCUSSION
In this study, we examined the results of tuberculin testing and serology in an M. avium subsp. paratuberculosis-vaccinated goat herd undergoing a TB breakdown. It has been shown in some studies that the SICTT and SIT are inefficient in goats and that M. avium subsp. paratuberculosis vaccination can result in decreased skin test sensitivity or specificity (4, 5, 23). Examination of the skin responses of the M. avium subsp. paratuberculosis-vaccinated goats described here to avian PPD in the SICTT did not show significantly elevated responses overall. Only in 22/494 SICTT-negative animals were the PPD-A responses elevated and exceeded the PPD-B responses in March 2014. These results suggest that the relatively low SICTT skin reactivity in the index herd is unlikely to be due to masking by high PPD-A responses. Low sensitivities of SICTT and SIT present a problem for the accurate diagnosis of TB in goats, irrespective of M. avium subsp. paratuberculosis vaccination. Serological testing has been shown in a few studies to detect infection that CMI tests have missed (2, 17, 20), though the usefulness of applying such antibody tests in the management of TB infection has not been investigated to any great extent.
Here, we examined antibody responses in the index herd in March 2014 and found a very high seroprevalence (>77%) in animals that were SICTT negative. The conventional expectation from the literature on TB in cattle and other species is that skin responses to tuberculin would precede any development of antibody responses, with the latter only appearing in the late stages of the infection, when lesions are at an advanced stage (14, 24). However, several experimental studies performed over the last decade have shown that antibody responses can develop from as early as 2 to 6 weeks postinfection (15, 25–29), suggesting that antibody responses are not confined to the late stages of disease. Thirty-eight of the 39 animals that were SICTT negative and antibody positive in March 2014 went on to become SIT positive in May 2014. Similar results were obtained with 26 milk samples taken at the same time. These data show that antibodies can appear in advance of skin reactivity.
The reasons for the high seroprevalence in SICTT/SIT-negative animals observed in this study are not known. It is possible that the use of six M. bovis-specific antigens in the multiplex test rather than the one or two antigens usually used in the tests in the literature provided a significant increase in sensitivity. The high antibody levels observed may also have been partly a consequence of high infection pressure arising from the purchased goats, over 60% of which had VL at postmortem examination. Although not proven, it is likely that many of these animals were capable of transmitting infection to the index herd.
Many studies have reported an anamnestic antibody response following skin testing in cattle and other species, including goats (2, 27, 30–35). Our data show that in 122 animals that received SIT in May 2014, the proportions that were anti-PPD-B antibody positive and the absolute levels of anti-PPD-B antibody 31 days after PPD-B injection were significantly higher than those observed 3 days after PPD-B injection, suggesting that some boosting had taken place 31 days following PPD-B administration. The results suggest that optimal PPD-B boosting might improve serodiagnosis in goats.
The data also show that the proportion of animals showing a positive antibody response by the two-antigen rule was higher in SICTT- or SIT-positive animals than in SICTT- or SIT-negative animals, respectively. Similar results have been described for cattle (13, 31). In the present study, this effect was observed for four of six antigens following SICTT. The absolute levels of antibodies to all six antigens were also significantly higher in SICTT-positive animals than in SICTT-negative animals. In contrast, although a similar trend was observed, the differences in antibody levels following the SIT were only significant for MPB70. The reason(s) for these results is not clear. Both TH1 and TH2 subsets of T helper cells are known to be able to help previously activated B cells make antibody (36), and perhaps the presence of both subsets in skin test-positive animals allows higher antibody responses to develop than in skin test-negative animals, where the TH2 subset probably dominates. It should be noted that serum samples were taken at different time points after tuberculin administration in March (8 days after the SICTT) and May (3 days after the SIT) and that the SICTT includes administration of PPD-A as well as PPD-B. These factors may have played a role in determining antibody responses. Also, the number of SICTT-positive animals in this study was relatively low compared to the number of SICTT-negative animals, and this observation requires confirmation with larger sets of data on skin test-positive goats.
One finding from this work that may have some diagnostic and prognostic value is the trend observed in the antigens recognized. The numbers of antigens recognized increased in what appeared to be a pattern. The same antigens were recognized initially, and as the number of other antigens recognized rose, so did the proportion positive and the RLU signal level for each antigen. Studies of experimental TB infection in nonhuman primates have shown that the number of antigens recognized increases with time and that increasing antibody levels and an increasing number of antigens recognized correlated with the development and progression of lesions (37). Although the present study looked at a population of animals at a fixed time point in a cross-sectional study, it is tempting to speculate that the results of this assay represent a progression of stages of antigen recognition. The animals start by recognizing two antigens (PPD-B and MPB70 peptide) and then sequentially develop additional and increasing antibody responses to MPB70, MPB83, CFP10, and ESAT6. In the fully developed state, the antigen recognition repertoire could indicate a high likelihood that the animals have lesions. These animals potentially would be the ones most likely to transmit infection and to be anergic in CMI responses, thereby being epidemiologically very important but poorly detectable by the skin test. In this study, the animals with VL at postmortem examination were positive for six antigens, whereas those without VL were positive for five antigens. This observation is consistent with the above premise. However, the antigen reactivity patterns observed may have been affected by preceding skin tests and not necessarily reflect the evolution of natural antibody responses in infected goats that were not skin tested. Further investigation is required to establish whether there is a relationship between the number of antigens recognized and lesion development in goats with and without prior skin testing.
It could be argued that the high levels and prevalence of antibodies described here were due to cross-reactions between M. avium subsp. paratuberculosis antibodies induced by M. avium subsp. paratuberculosis vaccination and M. bovis antigens in the multiplex test. However, analysis of serum samples from Norwegian goats that were or were not vaccinated against M. avium subsp. paratuberculosis showed no cross-reaction with any of the M. bovis antigens, including PPD-B, used in the Enferplex Caprine TB test, an observation consistent with the absence of genes for MPB70, MPB83, CFP10, and ESAT6 in the M. avium subsp. paratuberculosis genome (38) and other work where PPD-A and PPD-B cross-reactions have been examined in antibody tests (30).
Despite this, we cannot rule out other possible effects of M. avium subsp. paratuberculosis vaccination on the antibody results obtained in this study. For example, a linked recognition type of mechanism observed in vaccine studies of hepatitis B virus, influenza virus, and human immunodeficiency virus (39–43) may have given rise to enhanced responses to M. bovis antigens. M. bovis and M. avium subsp. paratuberculosis possess many of the same proteins, and cross-reactions at the T cell level could, through this mechanism, provide extra T cell help to B cells specific for M. bovis antigens, particularly membrane-bound antigens rather than secreted antigens such as CFP10 and ESAT6. Administration of PPD-A during SICTT could provide a further antibody boost through such T cell help. Whether such a mechanism operates in M. avium subsp. paratuberculosis-vaccinated animals requires further investigation.
It is possible that M. avium subsp. paratuberculosis vaccination also played a role in the extent of pathology observed in this herd. M. avium subsp. paratuberculosis vaccination has been shown to provide a degree of protection against M. bovis in goats, causing reductions in lesion volumes and bacterial loads, particularly in lymph nodes (23). Such effects could have reduced the identification of VL at postmortem examination. However, further work on natural TB infections in M. avium subsp. paratuberculosis-vaccinated animals is required to determine the likelihood that such protection affects lesion detection.
Six of 20 antibody-positive, SICTT-negative goats chosen at random from 47 animals positive for six antigens in the present study that underwent postmortem analysis had VL compatible with a diagnosis of TB. This proportion is similar to that observed in SICCT-positive animals, where 4/14 showed VL. However, the number of animals that underwent postmortem examination in this study overall is small and no antibody-nonreactive animals were examined pathologically. It was therefore not possible to ascertain the positive and negative predictive values of the serology test. Also, the presence of antibody provides only indirect evidence of TB infection in skin test-negative goats that did not undergo postmortem examination. Nevertheless, a large number of studies have documented correlations between antibodies and TB lesions and/or bacterial loads in cattle and other species (13, 14, 17, 28, 31, 33, 34, 37, 44, 45). The Enferplex test has previously been shown to detect infected cattle with lesions that were missed by the SICTT (17). These studies thus present supporting evidence that antibodies generally act as a surrogate marker of infection.
The results of this study show that there is a significant level of agreement between the antibody results from serum and milk samples taken at the same time point. Antibodies to TB antigens have been demonstrated in milk from cattle (33). Previously, the use of the Enferplex Caprine TB test was reported for serum (20) and this is the first report of its potential use for goat milk samples. Further work is warranted to establish the utility of using milk for diagnostic purposes in goats.
In conclusion, in this outbreak, a large proportion of the animals that were negative by skin tests developed antibody responses that were measurable in both serum and milk. Postmortem analysis of a small number of skin test-negative, antibody-positive animals demonstrated the presence of lesions consistent with a diagnosis of TB in some of them. The serology results provide indirect evidence that the skin testing carried out missed infected animals in this population. Antibody testing of milk could provide an easy means of detecting seropositive animals to facilitate management of the infection. Application of the Enferplex Caprine TB test to serum or milk could thus provide a very useful adjunct to skin testing of goats with TB.
MATERIALS AND METHODS
Index and purchased animals.
The index herd was an established dairy herd of over 500 adult goats that had been vaccinated against M. avium subsp. paratuberculosis. In April 2013, 204 adult goats from another dairy farm were purchased and added to the index herd. Seventeen of the purchased animals died over the next 8 months, 11 from a variety of diseases and 6 from unknown causes. In November 2013, over 30 animals showed signs of being unwell and several were losing weight with reduced milk production. Two of the purchased animals were sent to the APHA for postmortem examination on 11 December 2013 and found to have TB with VL in the lungs, chest, liver, mammary gland, udder, and cranial lymph nodes. M. bovis spoligotype 25 was isolated from both animals. A further two animals from the purchased herd and one animal from the index herd were subjected to postmortem examination by APHA on 17 December 2013, and all three showed VL consistent with TB in the lungs, chest, or trachea. The remaining animals in the purchased herd were investigated further by tuberculin testing as described below.
Samples.
With permission from the Department for Environment, Food and Rural Affairs, serology was performed on the index herd at various time points over the next 7 months. All blood sampling was performed by veterinary surgeons, with the farm staff collecting the milk samples. Internal ethical reviews of the project were carried out by RAFT Solutions Ltd., Enfer Scientific, and MV Diagnostics Ltd., and approval was obtained. Sampling was performed in March 2014, 8 days after SICTT. Further blood samples taken in May 2014 (3 days after SIT) and June (31 days after May SIT) were submitted for testing.
As controls for Enferplex Caprine TB testing, positive and negative reference serum samples were obtained from goats in Ireland with confirmed TB (n = 34) or with no evidence or history of TB in their source herd (n = 73) as reported previously (20). The positive samples came from animals that were all SICTT reactors, the majority of which had VL. Serum samples were also obtained from goats in Norway, which is an OTF country, that had (n = 74) or had not (n = 18) been vaccinated against M. avium subsp. paratuberculosis.
Skin tests.
The SICTT was performed by intradermal injection of avian and bovine PPD-A and PPD-B, respectively, into defined sites on the neck. The test was read 72 h later by comparing the increase in skin fold thickness in millimeters at each injection site. For the standard interpretation, an increase in skin thickness of ≥4 mm at the PPD-B site compared to the PPD-A site was deemed to be a positive result. For the severe interpretation, an increase in skin thickness of ≥2 mm was deemed to be a positive result. The SIT was performed by injecting bovine PPD at a single site and reading the result 72 h later. Using the severe interpretation, a skin thickness ≥2 mm greater than the preinjection skin thickness was deemed to be a positive result.
Antibody tests.
The Enferplex Caprine TB test (Enfer Scientific, Naas, Ireland) was used in this study to detect TB antibodies. A panel of four recombinant M. bovis proteins—MPB70, ESAT6 (Lionex GmbH, Germany), MPB83 (GenScript, USA), and CFP10 (Fusion Antibodies, Belfast)—along with soluble PPD-B (Prionics AG) and a synthetic MPB70 peptide (Genosphere Biotechnologies, France) were used as antigens. Each individual antigen was optimized for printing conditions and concentration with defined panels of samples. Briefly, the antigens were deposited in a multiplex planar array as individual 50-nl spots into wells of 96-well black polyethylene microtiter plates with the BioDot AD6000 aspirate/dispense platform. Plates were blocked, stabilized, dried, and stored at 2 to 8°C until use. The assay was carried out as previously described (20), with the following optimization for serum and defatted milk samples from goats. Serum samples were diluted 1:500 and milk samples were diluted 1:10 into sample dilution buffer (Enfer Buffer P; Enfer Scientific) and mixed before 50 μl was added per well. The plates were incubated at 25°C for 60 min with agitation (900 rpm). The plates were washed six times with 1× Enfer wash buffer (Enfer Scientific) and aspirated. The detection antibody was prepared and added as previously described (20). The plates were incubated at 25°C for 30 min with agitation (900 rpm). The plates were washed as described above, and 50 μl of prepared chemiluminescent substrate (50:50 dilution of substrate and diluent; Clifmar Associates, United Kingdom) was added per well. RLU were captured (45-s exposure) immediately, with a Quansys Biosciences imaging system, and data were extracted with Quansys Q view software (v 1.5.4.7). The results were defined with bespoke Enferplex software with Macro, based on individual antigen cutoffs. The latter were set with known positive and negative serum reference samples. No milk samples were available to optimize fully the cutoffs for milk samples, and these were based on those set with known serum samples. A positive result was determined when a sample displayed signals above the cutoffs for at least two antigens (two-antigen rule). This rule gives high specificity when screening herds of cattle likely to be TB free (15). Antibody levels were determined by analyzing numbers of RLU that were above the cutoff set for each antigen.
The relative sensitivity and specificity of the multiplex test were assessed here with serum samples from goats known to be positive (n = 34) or negative (n = 73) from a previous study (20). The previous study used an antigen set slightly different from that used in the present study, which substituted ESAT6 for Rv3616c and included PPD-B. The current assay was determined to have a relative sensitivity of 94.1% and a specificity of 100% compared to the data from the previous study.
Postmortem.
Animals sent for slaughter initially underwent postmortem examination for the presence of VL characteristic of TB. An extended postmortem examination of some animals was carried out that involved histological analyses and staining of tissues with the Ziehl-Neelsen stain by APHA, Starcross, United Kingdom. Cultures for M. bovis were set up for selected animals to confirm a diagnosis of TB. Most of the animals from the index herd were slaughtered without postmortem examination.
Statistics.
Nonparametric statistical analyses were performed with the GraphPad Prism 6.04 statistical program. The Mann-Whitney rank test, Spearman's rank correlation test, Fisher's exact test, and the Kappa agreement test were used.
ACKNOWLEDGMENTS
We thank Liv Solverod, Mastitis Laboratory, TINE, Molde, Norway, for providing serum samples from goats that were or were not vaccinated against M. avium subsp. paratuberculosis. The postmortem analyses were performed by the APHA, Starcross, Devon, United Kingdom.
This research received no specific grant from any funding agency in the public or not-for-profit sectors. The work was funded internally by each participant involved. Enfer Scientific, Synergy Farm Health, and MV Diagnostics Ltd. declare commercial interests in the Enferplex Caprine TB serology test.
REFERENCES
- 1.Broughan JM, Downs SH, Crawshaw TR, Upton PA, Brewer J, Clifton-Hadley RS. 2013. Mycobacterium bovis infections in domesticated non-bovine mammalian species. Part 1: review of epidemiology and laboratory submissions in Great Britain 2004–2010. Vet J 198:339–345. doi: 10.1016/j.tvjl.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Gutiérrez M, Tellechea J, Marin JFG. 1998. Evaluation of cellular and serological diagnostic tests for the detection of Mycobacterium bovis-infected goats. Vet Microbiol 62:281–290. doi: 10.1016/S0378-1135(98)00217-X. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez J, de Juan L, Bezos J, Romero B, Saez JL, Gordejo FJR, Briones V, Moreno MA, Mateos A, Domınguez L, Aranaz A. 2008. Interference of paratuberculosis with the diagnosis of tuberculosis in a goat flock with a natural mixed infection. Vet Microbiol 128:72–80. doi: 10.1016/j.vetmic.2007.08.034. [DOI] [PubMed] [Google Scholar]
- 4.Chartier C, Mercier P, Pellet M-P, Vialard J. 2012. Effect of an inactivated paratuberculosis vaccine on the intradermal testing of goats for tuberculosis. Vet J 191:360–363. doi: 10.1016/j.tvjl.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Bezos J, Álvarez J, Romero B, Aranaz A, de Juan L. 2012. Tuberculosis in goats: assessment of current in vivo cell-mediated and antibody-based diagnostic assays. Vet J 191:161–165. doi: 10.1016/j.tvjl.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Bezos J, Marqués S, Álvarez J, Casal C, Romero B, Grau A, Mínguez O, Domínguez L. 2014. Evaluation of single and comparative intradermal tuberculin tests for tuberculosis eradication in caprine flocks in Castilla y León (Spain). Res Vet Sci 96:39–46. doi: 10.1016/j.rvsc.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Bezos J, de Juan L, Romero B, Álvarez J, Mazzucchelli JF, Mateos A, Domınguez L, Aranaz A. 2010. Experimental infection with Mycobacterium caprae in goats and evaluation of immunological status in tuberculosis and paratuberculosis co-infected animals. Vet Immunol Immunopathol 133:269–275. doi: 10.1016/j.vetimm.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 8.Liébana E, Aranaz A, Urquía JJ, Mateos A, Domínguez L. 1998. Evaluation of the gamma-interferon assay for eradication of tuberculosis in a goat herd. Aust Vet J 76:50–53. doi: 10.1111/j.1751-0813.1998.tb15686.x. [DOI] [PubMed] [Google Scholar]
- 9.Bezos J, Álvarez J, de Juan L, Romero B, Rodríguez S, Castellanos E, Saéz-Llorente JL, Mateos A, Domínguez L, Aranaz A. 2011. Factors influencing the performance of an interferon-γ assay for the diagnosis of tuberculosis in goats. Vet J 190:131–135. doi: 10.1016/j.tvjl.2010.09.026. [DOI] [PubMed] [Google Scholar]
- 10.Bezos J, Álvarez J, Mínguez O, Marqués S, Martín O, Vigo V, Pieltain C, Romero B, Rodríguez S, Casal C, Mateos A, Domínguez L, Juan L. 2012. Evaluation of specificity of tuberculosis diagnostic assays in caprine flocks under different epidemiological situations. Res Vet Sci 93:636–640. doi: 10.1016/j.rvsc.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Bezos J, Casal C, Romero B, Schroeder B, Hardegger R, Raeber AJ, López L, Rueda P, Domínguez L. 2014. Current ante-mortem techniques for diagnosis of bovine tuberculosis. Res Vet Sci 97:S44–S52. doi: 10.1016/j.rvsc.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Plackett P, Ripper J, Corner LA, Small K, de Witte K, Melville L, Hides S, Wood PR. 1989. An ELISA for the detection of anergic tuberculous cattle. Aust Vet J 66:15–19. doi: 10.1111/j.1751-0813.1989.tb09706.x. [DOI] [PubMed] [Google Scholar]
- 13.Lilenbaum W, Ribeiro ER, Souza GN, Moreira EC, Fonseca LS, Ferreira MAS, Schettini J. 1999. Evaluation of an ELISA-PPD for the diagnosis of bovine tuberculosis in field trials in Brazil. Res Vet Sci 66:191–195. doi: 10.1053/rvsc.1998.0229. [DOI] [PubMed] [Google Scholar]
- 14.de la Rua-Domenech R, Goodchild AT, Vordermeier HM, Hewinson RG, Christiansen KH, Clifton-Hadley RS. 2006. Ante mortem diagnosis of tuberculosis in cattle: a review of the tuberculin tests, γ-interferon assay and other ancillary diagnostic techniques. Res Vet Sci 81:190–210. doi: 10.1016/j.rvsc.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Whelan C, Shuralev E, O'Keeffe G, Hyland P, Kwok HF, Snoddy P, O'Brien A, Connolly M, Quinn P, Groll M, Watterson T, Call S, Kenny K, Duignan A, Hamilton MJ, Buddle BM, Johnston JA, Davis WC, Olwill SA, Clarke J. 2008. Multiplex immunoassay for serological diagnosis of Mycobacterium bovis infection in cattle. Clin Vaccine Immunol 15:1834–1838. doi: 10.1128/CVI.00238-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whelan C, Whelan AO, Shuralev E, Kwok HF, Hewinson G, Clarke J, Vordermeier HM. 2010. Performance of the Enferplex TB assay with cattle in Great Britain and assessment of its suitability as a test to distinguish infected and vaccinated animals. Clin Vaccine Immunol 17:813–817. doi: 10.1128/CVI.00489-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whelan C, Shuralev E, Kwok HF, Kenny K, Duignan A, Good M, Davis WC, Clarke J. 2011. Use of a multiplex enzyme-linked immunosorbent assay to detect a subpopulation of Mycobacterium bovis-infected animals deemed negative or inconclusive by the single intradermal comparative tuberculin skin test. J Vet Diagn Invest 23:499–503. doi: 10.1177/1040638711403410. [DOI] [PubMed] [Google Scholar]
- 18.Clegg TA, Duignan A, Whelan C, Gormley E, Good M, Clarke J, Toft N, More SJ. 2011. Using latent class analysis to estimate the test characteristics of the γ-interferon test, the single intradermal comparative tuberculin test and a multiplex immunoassay under Irish conditions. Vet Microbiol 151:68–76. doi: 10.1016/j.vetmic.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 19.Rhodes S, Holder T, Clifford D, Dexter I, Brewer J, Smith N, Waring L, Crawshaw T, Gillgan S, Lyashchenko K, Lawrence J, Clarke J, de la Rua-Domenech R, Vordermeier M. 2012. Evaluation of gamma interferon and antibody tuberculosis tests in alpacas. Clin Vaccine Immunol 19:1677–1683. doi: 10.1128/CVI.00405-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shuralev E, Quinn P, Doyle M, Anthony Duignan A, Kwok HF, Bezos J, Olwill SA, Gormley E, Aranaz A, Good M, Davis WC, Clarke J, Whelan C. 2012. Application of the Enfer chemiluminescent multiplex ELISA system for the detection of Mycobacterium bovis infection in goats. Vet Microbiol 154:292–297. doi: 10.1016/j.vetmic.2011.07.028. [DOI] [PubMed] [Google Scholar]
- 21.Aznar I., Frankena K., More SJ., Whelan C., Martin W., Gormley E., Corner LAL, Murphy D., De Jong MCM. 2014. Optimising and evaluating the characteristics of a multiple antigen ELISA for detection of Mycobacterium bovis infection in a badger vaccine field trial. PLoS One 9:e100139. doi: 10.1371/journal.pone.0100139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shanahan A, Good M, Duignan A, Curtin T, More SJ. 2011. Tuberculosis in goats on a farm in Ireland: epidemiological investigation and control. Vet Rec 168:485–490. doi: 10.1136/vr.c6880. [DOI] [PubMed] [Google Scholar]
- 23.Pérez de Val B, Nofrarías M, López-Soria S, Garrido JM, Vordermeier HM, Villarreal-Ramos B, Martín M, Puentes E, Juste RA, Domingo M. 2012. Effects of vaccination against paratuberculosis on tuberculosis in goats: diagnostic interferences and cross-protection. BMC Vet Res 8:191–201. doi: 10.1186/1746-6148-8-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pollock JM, Neill SD. 2002. Mycobacterium bovis infection and tuberculosis in cattle. Vet J 163:115–127. doi: 10.1053/tvjl.2001.0655. [DOI] [PubMed] [Google Scholar]
- 25.Thom M, Morgan JH, Hope JC, Villarreal-Ramos B, Martin M, Howard CJ. 2004. The effect of repeated tuberculin skin testing of cattle on immune responses and disease following experimental infection with Mycobacterium bovis. Vet Immunol Immunopathol 102:399–412. doi: 10.1016/j.vetimm.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 26.Koo HC, Park YH, Ahn J, Waters WR, Palmer MV, Hamilton MJ, Barrington G, Mosaad AA, Park KT, Jung WK, Hwang IY, Cho SN, Shin SJ, Davis WC. 2005. Use of rMPB70 protein and ESAT-6 peptide as antigens for comparison of the enzyme-linked immunosorbent, immunochromatographic, and latex bead agglutination assays for serodiagnosis of bovine tuberculosis. J Clin Microbiol 43:4498–4506. doi: 10.1128/JCM.43.9.4498-4506.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waters WR, Palmer MV, Thacker TC, Bannantine JP, Vordermeier HM, Hewinson RG, Greenwald R, Esfandiari J, McNair J, Pollock JM, Andersen P, Lyashchenko KP. 2006. Early antibody responses to experimental Mycobacterium bovis infection of cattle. Clin Vaccine Immunol 13:648–654. doi: 10.1128/CVI.00061-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Welsh MD, Cunningham RT, Corbett DM, Girvin RM, McNair J, Skuce RA, Bryson DG, Pollock JM. 2005. Influence of pathological progression on the balance between cellular and humoral immune responses in bovine tuberculosis. Immunology 114:101–111. doi: 10.1111/j.1365-2567.2004.02003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waters WR, Whelan AO, Lyashchenko KP, Greenwald R, Palmer MV, Harris BN, Hewinson RG, Vordermeier HM. 2010. Immune responses in cattle inoculated with Mycobacterium bovis, Mycobacterium tuberculosis, or Mycobacterium kansasii. Clin Vaccine Immunol 17:247–252. doi: 10.1128/CVI.00442-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thom ML, Hope JC, McAulay M, Villarreal-Ramos B, Coffey TJ, Stephens S, HM, Vordermeier HM, Howard CJ. 2006. The effect of tuberculin testing on the development of cell-mediated immune responses during Mycobacterium bovis infection. Vet Immunol Immunopathol 114:25–36. doi: 10.1016/j.vetimm.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Lightbody KA, Skuce RA, Neill SD, Pollock JM. 1998. Mycobacterial antigen-specific antibody responses in bovine tuberculosis: an ELISA with potential to confirm disease status. Vet Rec 142:295–300. doi: 10.1136/vr.142.12.295. [DOI] [PubMed] [Google Scholar]
- 32.Lightbody KA, McNair J, Neill SD, Pollock JM. 2000. IgG isotype antibody responses to epitopes of the Mycobacterium bovis protein MPB70. in immunised and in tuberculin skin test-reactor cattle. Vet Microbiol 75:177–188. doi: 10.1016/S0378-1135(00)00215-7. [DOI] [PubMed] [Google Scholar]
- 33.Waters WR, Buddle BM, Vordermeier HM, Gormley E, Palmer MV, Thacker TC, Bannantine JP, Stabel JR, Linscott R, Martel E, Milian F, Foshaug W, Lawrence JC. 2011. Development and evaluation of an enzyme-linked immunosorbent assay for use in the detection of bovine tuberculosis in cattle. Clin Vaccine Immunol 18:1882–1888. doi: 10.1128/CVI.05343-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Casal C, Díez-Guerrier A, Álvarez J, Rodriguez-Campos S, Mateos A, Linscott R, Martel E, Lawrence JC, Whelan C, Clarke J, O'Brien A, Domínguez L, Aranaz A. 2014. Strategic use of serology for the diagnosis of bovine tuberculosis after intradermal skin testing. Vet Microbiol 170:342–351. doi: 10.1016/j.vetmic.2014.02.036. [DOI] [PubMed] [Google Scholar]
- 35.Waters WR, Palmer MV, Stafne MR, Bass KE, Maggioli MF, Thacker TC, Linscott R, Lawrence JC, Nelson JT, Esfandiari J, Greenwald R, Lyashchenko KP. 2015. Effects of serial skin testing with purified protein derivative on the level and quality of antibodies to complex and defined antigens in Mycobacterium bovis-infected cattle. Clin Vaccine Immunol 22:641–649. doi: 10.1128/CVI.00119-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abbas AK, Burstein HJ, Bogen SA. 1993. Determinants of helper T cell-dependent antibody production. Semin Immunol 5:441–447. doi: 10.1006/smim.1993.1050. [DOI] [PubMed] [Google Scholar]
- 37.Kunnath-Velayudhan S, Davidow AL, Wang H-L, Molina DM, Huynh VT, Salamon H, Pine R, Michel G, Perkins MD, Xiaowu L, Felgner PL, Flynn JL, Catanzaro A, Gennaro ML. 2012. Proteome-scale antibody responses and outcome of Mycobacterium tuberculosis infection in nonhuman primates and in tuberculosis patients. J Infect Dis 206:697–705. doi: 10.1093/infdis/jis421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li L, Bannantine JP, Zhang Q, Amonsin A, May BJ, Alt D, Banerji N, Kanjilal S, Kapur V. 2005. The complete genome sequence of Mycobacterium avium subspecies paratuberculosis. Proc Natl Acad Sci U S A 102:12344–12349. doi: 10.1073/pnas.0505662102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Russell SM, Liew FY. 1979. T cells primed by influenza virion internal components can cooperate in the antibody response to haemagglutinin. Nature 280:147–148. doi: 10.1038/280147a0. [DOI] [PubMed] [Google Scholar]
- 40.Scherle PA, Gerhard W. 1986. Functional-analysis of influenza-specific helper T cell clones in vivo. T cells specific for internal viral proteins provide cognate help for B cell responses to hemagglutinin. J Exp Med 164:1114–1128. doi: 10.1084/jem.164.4.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scherle PA, Gerhard W. 1988. Differential ability of B cells specific for external vs internal influenza virus proteins to respond to help from influenza virus-specific T-cell clones in vivo. Proc Natl Acad Sci U S A 85:4446–4450. doi: 10.1073/pnas.85.12.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Milich DR, Mclachlan A, Thornton GB, Hughes JL. 1987. Antibody production to the nucleocapsid and envelope of the hepatitis B virus primed by a single synthetic T-cell site. Nature 329:547–549. doi: 10.1038/329547a0. [DOI] [PubMed] [Google Scholar]
- 43.Nabi G, Bonsmann MSG, Tenbusch M, Gardt O, Barouch DH, Temchura V, Überla K. 2013. GagPol-specific CD4+ T cells increase the antibody response to Env by intrastructural help. Retrovirology 10:117–126. doi: 10.1186/1742-4690-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lyashchenko K, Whelan AO, Greenwald R, Pollock JM, Andersen P, Hewinson RG, Vordermeier HM. 2004. Association of tuberculin-boosted antibody responses with pathology and cell-mediated immunity in cattle vaccinated with Mycobacterium bovis BCG and infected with M. bovis. Infect Immun 72:2462–2467. doi: 10.1128/IAI.72.5.2462-2467.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kunnath-Velayudhana S, Salamon H, Wanga H-Y, Davidow AL, Molina DM, Huynh VT, Cirillo DM, Michel G, Talbot EA, Perkins MD, Felgner PL, Liang X, Gennaro ML. 2010. Dynamic antibody responses to the Mycobacterium tuberculosis proteome. Proc Natl Acad Sci U S A 107:14703–14708. doi: 10.1073/pnas.1009080107. [DOI] [PMC free article] [PubMed] [Google Scholar]


