Abstract
Coronary artery disease (CAD) has been reported to be a major cause of death worldwide. Current treatment methods include atherectomy, coronary angioplasty (as a percutaneous coronary intervention), and coronary artery bypass. Among them, the insertion of stents into the coronary artery is one of the commonly used methods for CAD, although the formation of in-stent restenosis (ISR) is a major drawback, demanding improvement in stent technology. Stents can be improved using the delivery of DNA, siRNA, and miRNA rather than anti-inflammatory/anti-thrombotic drugs. In particular, genes that could interfere with the development of plaque around infected regions are conjugated on the stent surface to inhibit neointimal formation. Despite their potential benefits, it is necessary to explore the various properties of gene-eluting stents. Furthermore, multifunctional electronic stents that can be used as a biosensor and deliver drug- or gene-based on physiological condition will be a very promising way to the successful treatment of ISR. In this review, we have discussed the molecular mechanism of restenosis, the use of drug- and gene-eluting stents, and the possible roles that these stents have in the prevention and treatment of coronary restenosis. Further, we have explained how multifunctional electronic stents could be used as a biosensor and deliver drugs based on physiological conditions.
Keywords: Biosensing Techniques, Coronary Artery Disease, Coronary Restenosis, DNA, Drug-Eluting Stents
INTRODUCTION
Coronary artery disease (CAD) is one of the leading causes of death worldwide.1 According to the WHO, 17.5 million people died because of CAD in 2008.2 The major cause for CAD is atherosclerosis; focal manifestations that hinder blood flow because of lesions in critical areas of the vasculature. Some clinical procedures to treat atherosclerosis include percutaneous transluminal coronary angioplasty (PTCA), coronary artery bypass graft (CABG), and stenting.3
In 1964, Dotter and Judkins were the first to attempt angioplasty by implanting first percutaneous coilspring graft in a dog's femoral artery.4 Later, Andreas Gruntzing developed a polyvinyl chloride balloon fitted, double-lumen catheter in 1975 and performed the first angioplasty on human in 1977. Since then there have been many advancements in percutaneous coronary intervention (PCI), as an efficient and frequently performed invasive procedure despite restenosis.5 In the 1980s, an invasive method involving balloon angioplasty was superseded by the development of coronary stents.6 Coronary stenting along with aggressive antithrombotic and antiplatelet therapies became the most significant treatments, with improved angiographic and clinical outcomes.5 Different types of stents were developed for coronary interventions, such as bare metal stents (BMS), drug-eluting stents (DES) with metals, DES with biodegradable polymers, DES without a polymer coating, bifurcation stents, and self-expanding stents.7 During stent implantation, the target vessel is often injured, and the endothelial cells are denudated.8 Although stenting has become popular and accepted as a safer strategy because it is less invasive than that of other angioplasty procedures, postprocedural in-stent restenosis (ISR) remains a drawback.5 Implantation of coronary stents reduces the rate of restenosis due to balloon angioplasty from ~30-60% to 16-44% by the advent of BMS and then development of DES further reduces it by up to <15% depending on the lesion.12 BMS has also been associated with undesirable effects such as inflammation, thrombogenesis, and hyper-proliferation of vascular smooth muscle cells, resulting in ISR.9 Even though the occurrence of ISR has not been completely inhibited by the drugs, the possibility of ISR is lessened by the development of DES.10 The negative clinical outcomes of all treatment procedures necessitate the urgent need for the development of an alternate procedure for coronary restenosis. As intramuscular gene delivery is regarded as a reliable approach to treat coronary diseases,11 the occurrence of ISR could be controlled by delivering nucleic acids that could down regulate the proliferation of endothelial cells. In this review, we will discuss the mechanisms of coronary restenosis and ISR. We have mainly concentrated on gene-eluting stents which minimizes the occurrence of ISR with a brief introduction on DES. Furthermore, we have explained recent multifunctional electronic stents that could be used as biosensors and drug delivery methods based on physiological conditions.
MECHANISM OF RESTENOSIS AND ISR
Arterial restenosis has been a major drawback of coronary angioplasty for the past two decades and is a multifactorial healing response because of injury caused during transluminal coronary revascularization that involves several mechanisms.12 The occurrence of restenosis and ISR due to angioplasty or stenting follows a similar process which includes Elastic recoil, Thrombus organization, Remodeling and Resolution of inflammation.13 Over stretching is caused within an hour after balloon deflation/stenting, elastic recoil is the phenomenon occurring due to overstretching of lumen at stent implanted area, which causes a loss of luminal area of about 4%. However, compared to angioplasty, stenting has reduced elastic recoil significantly. One of the major factors responsible for ISR is neointimal formation which occurs three months after the procedure.14 Fibrin and platelet association with neointimal accumulation and neovascularization at the site of ISR signifies the role of thrombus formation in ISR promotion. Thrombus organization is caused due to damage/tearing in the endothelial layer which results in medial dissection induction and exposure to intimal components such as collagen, fibronectin, etc, leading to deposition and aggregation of platelets.15 Neointimal formation increases with little change for the first six months and reduces gradually from the sixth month to three years. Though cell proliferation is considered to be a chronic factor for neointimal formation, it does not correlate with medial disruption or with other factors such as the movement of the primary plaque. Remodeling results in lumen loss of about 40% at the restenosis lesions.16
PATHOPHYSIOLOGY OF RESTENOSIS AND ISR
Healthy blood vessels consist of three primary layers such as the tunica intima, tunica media, and tunica adventitia. The tunica intima is the innermost layer that is in contact with blood flowing through the artery. The middle layer or tunica media is composed primarily of SMC. The tunica adventitia is the outermost layer comprising collagen, and this layer is responsible for the structure and elasticity of blood vessels.17 Removal of an atherosclerotic plaque results in platelet activation, adhesion, and aggregation. Activated platelets then release various cytokines, growth factors, and chemokines, thereby triggering SMC proliferation, leukocyte recruitment, and coagulation cascade activation. SMCs dedifferentiate because of a phenotypic modification, migrate into the intima, and later form the neointima.18 The process of restenosis is similar to that of a wound healing as T cells and a few B cells are also involved in restenosis.
Although the occurrence of restenosis is highly minimized by stents, the risk of ISR remains a threat, while considering long-term success.12 Unlike restenosis, ISR is mainly caused by neointimal formation alone.19 The occurrence of ISR could be foretold by the characteristics of the vessels and lesions, such as size, location, calcification, occlusion, and tortuosity.20 Based on the length of restenosis with respect to stent length, ISR has been relegated into four types. They have been defined as follows: [1] focal ISR ≤10 mm, [2] diffuse (ISR >10 mm; remains within the stent),21 [3] proliferative (ISR >10 mm; lesion extends outside the stent), and [4] occlusive. ISR with greater severity is categorized as an aggressive ISR.22 There are a few variables that are known to increase the risk of ISR, such as diabetes, previous restenosis history, and certain genetic factors.23 Patients who have a positive allergic patch-test reaction to the stent components are also prone to ISR.24
The growth factors and cytokines involved in the restenosis process are important for the treatment of restenosis. Smooth muscle cell proliferation involves the action of fibroblast growth factor (FGF-2), platelet-derived growth factor (PDGF) A & B, transforming growth factor β (TGF-β), and insulin like growth factor-1 (IGF-1) that are produced by the smooth muscle cells themselves.25 In addition, vascular endothelial growth factor (VEGF); monocyte chemoattractant protein-1 (MCP-1); interleukin (Il)-1, 6, and 8; adenosine diphosphate; thrombin; serotonin; and thromboxane A2 are also involved in endothelialization and other processes involved in restenosis.25 The β-integrin molecule, Mac-1 (CD11b/CD18) , which is responsible for the recruitment of monocytes, also plays a role in restenosis,26 and several studies have shown that ISR could be controlled by regulating growth factors.27
Recent research has shown a higher expression of a large number of well-known genes in ISR tissue at the time of re-vascularization in patients with ISR.28 These genes could be targeted for the suppression of neointimal formation using gene-based methodology, thereby reducing the risk of ISR.29 Gene therapy for ISR is comparatively more effective than drug therapy.
STENT COATING
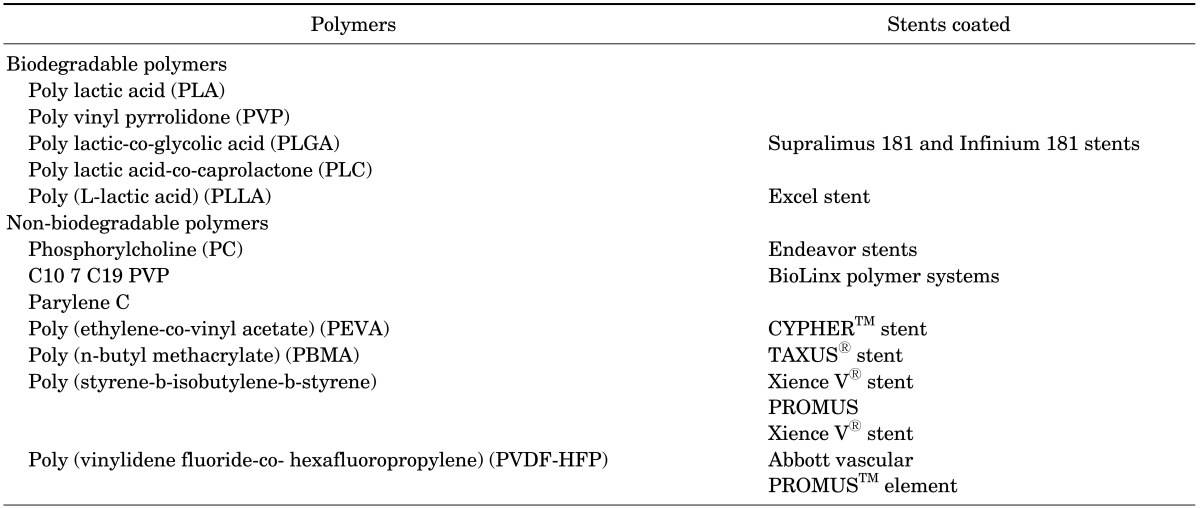
Drug- and gene-eluting stents require a foundation on the stent's surface to incorporate the therapeutic moiety. Mostly natural and synthetic polymers have been used as reservoirs for the delivery of drugs/genes. However, the choice of polymer coating completely depends on the biocompatibility, sterility, and immunogenicity of the polymers.30 Polymers used for stent coating can be categorized as either biodegradable or non-biodegradable polymers, as shown in Table 1. As for biodegradable stents, they are designed to provide temporary structural support for the vessel wall, and they are completely biodegradable. They are usually composed of biodegradable polymers such as polycarbonate and PLA, which can be metabolized in 12 to 18 months. The characteristics of the representative biodegradable polymers used for stent coating are discussed in more detail in the following section.
TABLE 1. Lists of polymers used for stent coating30.
HYALURONIC ACID (HA)
HA is a highly cyto-compatible and cellular matrix–compatible polymer. Hyaluronidase can degrade HA efficiently, and the degraded product could induce the production of ECM and formation of blood capillaries.31 The proliferation and migration of endothelial cells could be enhanced by HA, whereas the proliferation of SMCs was knocked down through the delivery of Akt siRNA using HA nanoparticles.32
PLGA
PLGA is an FDA-approved biodegradable polymer that has been used in the medical sciences field. PLGA has the capability of releasing hydrophobic drugs and genes at a controlled rate.33 It has been reported that DNA could form a complex with PLGA nanoparticles that could be efficiently transfected, and it was expressed only in the stent-coated region, not in an adjacent stent region or a distal organ.34
PLLA
PLLA is the most studied polymer that degrades over time. Its biodegradability helps to override poor biocompatibility and inflammatory response. PLLA was used as a coating in biolimus A9-, paclitaxel-, or sirolimus-eluting stents. It was reported to be effective and safe in both short-term and mid-term treatments.21 The Igaki-Tamai stent was made of a PLLA nanofilament with a zigzag helical design.8 The PLLA stent is biodegradable, and its degraded products are safe, whereas long-term follow-up is required to validate its efficacy.
POLYZENE-F
Polyzene-F is a biocompatible polymer that has anti-inflammatory, pro-healing, and bacterial resistance properties. Coating the stent with Polyzene-F reduced the risk of thrombosis by ensuring low surface thrombogenecity.35 Studies on Polyzene-F coated stents have so far demonstrated favorable efficacy and safety.36
DRUG ELUTING STENTS (DES)
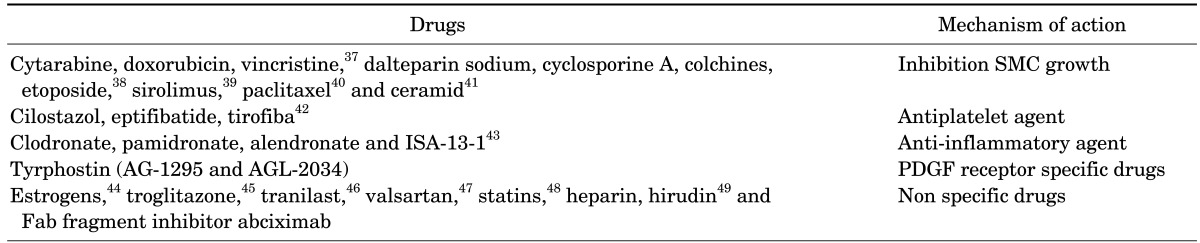
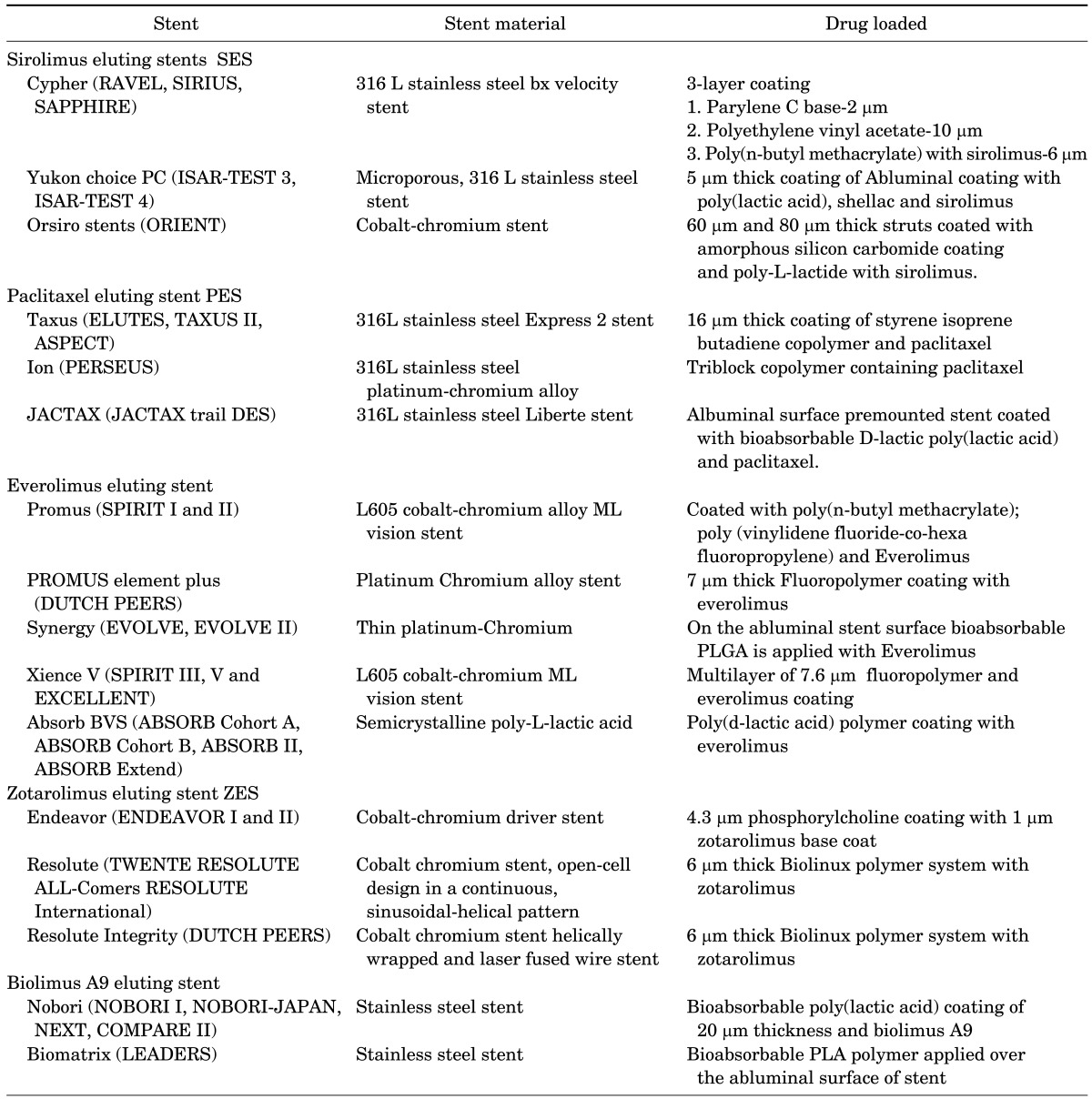
A DES treats restenosis and ISR by delivering anti-inflammatory, anti-proliferative, anti-thrombogenic, and immunosuppressant drugs, as well as certain inhibitors to the site of injury. Drugs delivered using these methods interfere with more than one pathway in the restenosis mechanism. Drugs that are used in DES are listed in Table 2. Based on advancements in stent coating and enhancement in drug elution profile DES, they were categorized as first, second and third generation DES. The brief summary of three generations of DES is given in Table 3.
TABLE 2. Drugs used for treating restenosis and ISR.
TABLE 3. First, second and third generation DES based on the drug loaded50.
Advancement in the field of nanotechnology created a new road for the improvement of DES as a controlled drug delivery system. Today, a DES is fabricated with a polymer coating that serves as a reservoir for therapeutic agents, and drugs are delivered using nanoformulations. The two strategies using nanoparticle DES involve an anti-restenosis strategy and a pro-healing strategy. The former strategy involves the inhibition of SMC proliferation by delivering anti-proliferative agents or anti-inflammatory agents to enable the inhibition of the formation of neointimal lesions. The latter strategy facilitates the re-endothelialization of Imatinib scaffolding that mimics the extracellular matrix environment.30
NANOPARTICLE DES
The therapeutic drugs that are delivered using nanoparticles as a carrier for treating restenosis and ISR are discussed in this section.
PDGF RECEPTOR INHIBITORS
PDGF receptors have a crucial role in the proliferation of endothelial cells and inhibition of these receptors could prevent restenosis. In 1996, Banai et al.37 designed AG-1295-impregnated PLA nanoparticles for intravascular delivery. AG1295 is a potent PDGF blocker; it can either inactivate or block the migration and proliferation of SMCs. They reported that 50% of neointimal formation was inhibited in porcine femoral arteries.
In addition, Imatinib mesylate, a PDGF receptor tyrosine kinase inhibitor, could also prevent SMC proliferation by inhibiting the phosphorylation of PDGF receptor β. Imatinib mesylate-loaded bioabsorbable polymeric nanoparticles were coated on a stent and implanted into the coronary artery of a pig model. Masuda et al.38 reported that imatinib mesylate inhibited 50% of neointimal formation and that mitogen-activated protein kinase activity was hampered. However, it had no effect on inflammation or re-endothelialization.
SIROLIMUS (RAPAMYCIN)
Sirolimus is an immunosuppressive and anti-proliferative agent29; it was the first drug used on cardiovascular stents from the Limus family of drugs. It is a macrocyclic lactone capable of binding to FKBP12 as a cytosolic protein, and it blocks cell cycle progression at the G1 phase by blocking the activation of mTOR.39 The inhibition of mTOR activity suppresses cytokine-derived T-cell proliferation by exerting its immunosuppressant ability.
Luderer et al.40 first evaluated the effects of sirolimus-loaded nanoparticles on restenosis. They developed biodegradable 250-nm sirolimus-loaded poly (D,L-lactide) (PDLLA) nanoparticles to prevent restenosis. They used 20-percent sirolimus-loaded PDLLA nanoparticles with their evident biphasic release kinetics with a 5-h initial burst release, and a 30-day sustained release was obtained. This system prevented restenosis by inhibiting SMC proliferation, although no enhancement in endothelialization was observed.
A post-hoc drug delivery system to coat an already implanted stent was designed by Räthel et al.41; they have coupled rapamycin with superparamagnetic nanoparticles (MNPs) and confirmed the anti-proliferative properties of the drug. Drug-containing MNPs were loaded into magnetic microbubbles and were deposited at the stent struts with the help of an external magnet. The products were released by applying an ultrasound, and dose-dependent inhibition of cell proliferation was observed in the MNP-treated SMC cells. They have reported that it is a promising strategy for coating an already implanted stent by magnetizing the stent in the circulating fluids using an external magnetic field.
PACLITAXEL
As a non-Limus-family-related drug, paclitaxel has been widely used because it has an effect on the stabilization of microtubules, thus resulting in the inhibition of cell division at the G0/G1 and G2/M phases.42 Paclitaxel is known to influence the motility, morphology, and migration of cells between organelles. Bhargava et al.43 designed a novel cobalt-chromium stent coated with paclitaxel-loaded porous carbon-carbon nanoparticles, and they evaluated it in a porcine coronary artery. Interestingly, both the porous carbon-carbon nanoparticles coating the cobalt-chromium stent that was loaded with low and medium paclitaxel doses exhibited significant effects on endothelialization, neointimal formation, thrombosis, and inflammatory responses, as compared with the CYPHER stent, which is an FDA-approved sirolimus-eluting stent (SES). Moreover, polymeric stents have no significant effect on endothelialization or neointimal formation, as compared with non-polymeric stents.
Chorny et al.44 used magnetic nanoparticles for the local delivery of paclitaxel. They hypothesized that release kinetics for encapsulated drugs could be significantly altered by simultaneously using magnetic targeting through induced magnetization with a uniform field. They observed significant inhibition of SMC proliferation with paclitaxel-loaded MNPs, as compared with those cells cultured with non-magnetic drug-loaded NPs, although they showed a significant localization of MNOs that were delivered locally to stented arteries using uniform field-controlled targeting, as compared with non-magnetic nanoparticles. Thus, they proved their hypothesis using uniform field-controlled targeting and MNPs, by delivering the drug in a site-specific manner, and demonstrated a promising strategy for preventing ISR.
PITAVASTATIN (PS)
PS is an HMG-CoA reductase inhibitor and is one of the most potent drugs among the statins that significantly affects SMC proliferation. Tsukie et al.45 designed a PS-eluting stent. It attenuates ISR, similar to that of SES. Interestingly, endothelial healing was not delayed by PS-eluting stents, unlike SES, suggesting that further development is necessitated to improve the safety and usefulness of the system.
S-NITROGLUTATHIONE (SN)
SN, a platelet selective donor and potential antithrombotic nitric oxide donor, is considered to be a restenosis-preventing agent because of its selective platelet inhibition properties. Most drugs that treat cardiovascular ailments interfere with the nitric oxide pathway because nitric oxide is a multifunctional molecule that could regulate thrombus formation and blood flow.46 Acharya et al.47 studied the effect of polymer coatings on SN-eluting stents. They reported on a stent loaded with SN (0.5% w/w) that had an optimal concentration of PCL coating 17.5% w/v for prolonged delivery of SN. They have also reported that PCL was a suitable carrier for SN than PLGA and poly (ethylene glycol) (PEG), although further optimization of coating methods would result in enhanced drug-release kinetics for PCL-coated stents.
GENE-ELUTING STENTS (GES)
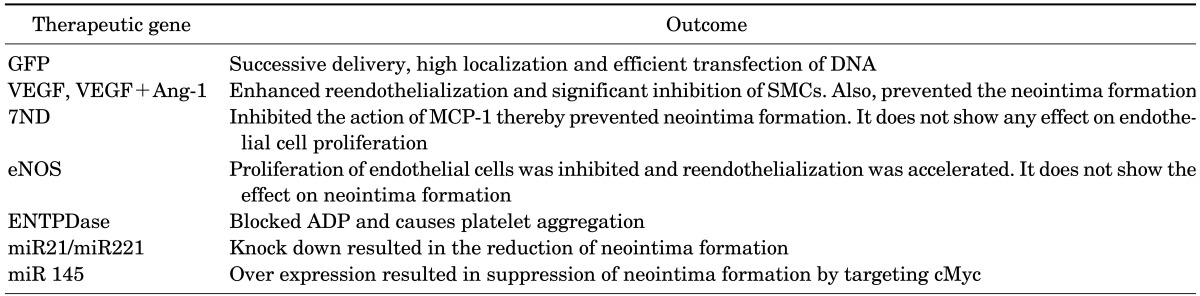
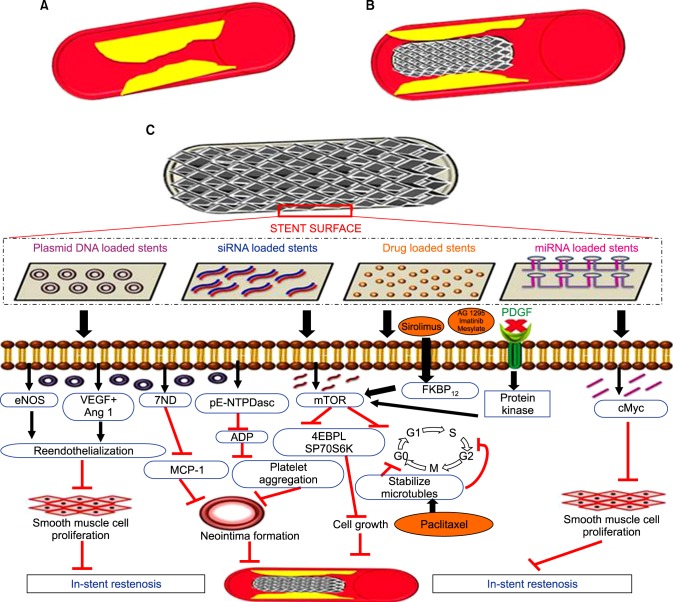
The anti-proliferative drug delivery using DES showed a significant reduction in restenosis but questions as to its long term efficacy remain unanswered.48 DES has shown poor long-term outcomes. It has resulted in incomplete re-endothelialization due to inappropriate inhibition of endothelial and SMC proliferation by the anti-proliferative drugs.49 This has turned out to be a risk factor for late-stent thrombosis, demanding a better therapeutic agent. And so, gene therapy has emerged as an efficient method for ISR therapy (Table 4 and Fig. 1).
TABLE 4. Summary on therapeutic gene delivered via stent.
GFP: green fluorescent protein, VEGF: vascular endothelial growth factor, miR: micro RNA.
FIG. 1. (A) Coronary artery with restenosis, (B) coronary artery with ISR after stent implantation, and (C) a scheme representing the mechanisms of gene- and drug-eluting for preventing ISR. This illustration was conceived and drawn by Kamali ML.
Cardiovascular gene therapy requires a therapeutic gene, a vector to encode the gene, and a carrier to deliver the vector. Carriers that are considered for vascular gene therapy are double-balloon catheters,51 porous and microporous catheters,52 hydrogel catheters,53 dispatch catheters,54 and infiltration catheters.55
Endovascular stents are an ideal platform for delivering genes to injured arteries because of their permanent scaffolding structures,3 although new studies should be conducted to help increase stent compatibility for gene delivery and overcome existing limitations.
For stent design, the coating on the stent surface is crucial as it acts as a reservoir for the therapeutic gene. The coating has to be biocompatible, and it should not cause any inflammatory or thrombogenic effects.3 After stent implantation, these coatings will be proximal to the circulatory system, and their usage as a reservoir is crucial for maintaining a prolonged local drug concentration. An ideal coating should possess certain characteristics such as excellent binding ability, targeted and sustained release, and sufficient vector capacity for significant transfection.56 Vectors for transfecting genes could be either viral vectors such as adenoviruses,57 retroviruses,58 or non-viral vectors such as liposomes and polymers.59 Naked plasmids60 are also transfected without vectors.
PLASMID DNA-ELUTING STENTS
Klugherz et al.61 first reported the successful delivery of green fluorescent protein (GFP) plasmid DNA into the coronary arteries of a pig using a DNA-eluting stent for cardiovascular disease treatment. They synthesized a coronary stent coated with a mixture of PLGA and DNA. They observed the sustained release of DNA and also obtained a structurally intact and functional DNA sample throughout their study. One of the major hindrances faced during this study was the biodistribution of DNA in downstream coronary sites and distal organs. They also studied the site-specific delivery of DNA by synthesizing a collagen-coated stent tethered with an antibody. Collagen-coated stents were conjugated with an adenoviral monoclonal antibody using covalent bonding, and a replication-deficient adenovirus-encoding GFP was conjugated to the antibody. The delivery of DNA to the smooth muscle cells in a pig artery was obtained with excellent efficiency and localization. They did not detect any vectors in either the downstream sites or distal organs during a biodistribution study. Although they observed occasional tearing in the collagen coating with deployment, no detachment of the collagen coating from the stent occurred, and no evidence of thrombogenesis secondary to hemocompatibility of the collagen was observed.62
However, the transfection efficiency was low, although it seemed to be promising. Therefore, in their consecutive study, they enhanced the transfection efficiency from 1% to approximately 10% by incorporating denatured collagen both in vitro and in vivo. It was based on a hypothesis involving adhesion molecule interaction through an integrin-related mechanism, as well as changes associated with the arterial smooth muscle cells through an actin-related mechanism.63
Jin et al.64 synthesized a coronary artery stent that delivered plasmid DNA in a site-specific manner. They first reported the synthesis of a coronary artery stent coated with anti-DNA antibodies to which the plasmid DNA was bound. The successful delivery of plasmid DNA with a high efficiency and with a neointimal transfection of about 7% was achieved, demonstrating that the system could be used as a cardiovascular gene therapy.
Kim et al.65 synthesized a gene-eluting stent that could deliver plasmid DNA with structural integrity. They designed an HA-coated stent, and plasmid DNA was loaded onto the surface using ionic interactions in a polyplex formation with polyethyleneimine (PEI) . This system has been demonstrated to have improved transfection efficiency and biocompatibility. These stents have dual functionality with HA and plasmid DNA, and have shown efficiency against restenosis.
For the gene therapy method, the therapeutic efficiency and feasibility for the prevention of ISR was enhanced by coating the gene-eluting stents with dodecylated chitosan-plasmid DNA nanoparticles (DCDNPs).34 The DCDNPs were used for the local delivery of plasmid DNA into injured blood vessels over a longer time period. The sizes of the DCDNPs ranged from about 90 nm to 180 nm and were prepared by spray-coating dodecylated chitosan-plasmid DNA onto the stent. The target gene was released only in the arterial segment that was directly in contact with the stent, but not in an adjacent region or a distal organ, suggesting that coating the stents with DCDNPs is likely to be a reliable strategy for preventing ISR.34
VEGF GENE-ELUTING STENTS
The delivery of phosphorylcholic (PC) polymer stents coated with naked plasmid DNA that encoded human vascular endothelial growth factor (pHVEGF)-2 was studied.66 The results showed a reduction in the formation of neointima by accelerating re-endothelialization, instead of inhibition. This was considered an alternative for preventing restenosis. The VEGF gene was also used in combination with the angiopoietin-1 (Ang 1) gene to prevent ISR. The VEGF and Ang-1 genes were loaded into nanoparticles to deliver the genes to the target site. The stent was designed as a nanohybrid hydrogel-based endovascular stent in which the therapeutic genes were carried using a fibrin hydrogel, and it was conjugated to a carbon nanotube coated onto the stent. The hydrogel acts as a reservoir and delivers the genes to the target site, thereby enhancing re-endothelialization, whereas carbon nanotubes aid in tuning the bioactivity of the stent. The results clearly showed enhancement in re-endothelialization, attenuation of stenosis, and prevention of neointimal formation.67 Yang et al.68 developed a PLGA nanoparticle-coated stent containing VEGF and paclitaxel as a combined therapeutic agent for ISR. The stent comprised a bilayer of paclitaxel in the inner core and a PLGA nanoparticle containing VEGF in the outer core. After stent deployment, VEGF genes were initially released, followed by paclitaxel. Thus, at first, endothelial healing occurred, followed by the inhibition of smooth muscle cell proliferation. The occurrence of ISR was significantly reduced, and complete re-endothelialization occurred within 1 month of implantation.
7ND GENE-ELUTING STENTS
The onset of neointimal formation was inhibited by the delivery of the 7ND gene (cDNA) using a gene-eluting stent69 because the 7ND gene inhibits the action of monocyte chemoattractant protein-1 (MCP-1), which is vital for neointimal formation, thereby preventing it without any undesirable effects. Further, it was reported that the 7ND did not show any effect on the proliferation of human endothelial cells, suggesting that arterial wall healing would not be impaired.69
ENOS-ELUTING STENTS
The overexpression of eNOS is known to inhibit the proliferation of endothelial cells and accelerates re-endothelialization.70 Therefore, it could be utilized in the prevention of ISR. Sharif et al.71 first demonstrated that the delivery of eNOS using gene-eluting stents suppressed ISR and accelerated re-endothelialization. They used a PC-coated stent and delivered eNOS using an adenoviral vector. This may be considered an ideal strategy for managing the ISR problem. The non-viral delivery of eNOS has also been reported by Brito et al.72 The plasmid DNA encoding eNOS in a lipopolyplex formulation was immobilized on type B collagen-coated stainless steel stents; upon implantation, these stents were found to result in efficient transgene expression, suggesting that ISR could be inhibited and re-endothelialization could be achieved by delivering eNOS using non-viral vectors. In contrast, Sharif et al.73 reported that the non-viral delivery of eNOS did not reduce restenosis. They employed liposome-mediated gene delivery and observed that the lipo/eNOS delivered to the injured blood vessel accelerated re-endothelialization, but it did not inhibit neointimal formation, as compared with that of viral-mediated eNOS delivery.
ENTPDase-ELUTING STENTS
Degradation or blocking of adenosine diphosphate (ADP) could help in preventing ISR because ADP is known to be a major factor in platelet aggregation.74 This was demonstrated by the local delivery of human placental ectonucleoside triphosphate diphosphohydrolase (pENTPDase) into diseased coronary arteries through gene-eluting stents because pENTPDase is an enzyme that could hydrolyze ADP rapidly and inhibit platelet aggregation, suggesting that neointimal hyperplasia and ISR were suppressed efficiently using this strategy.74
INTERFERENCE RNA-ELUTING STENTS
Mitra and Agarwal reported that vascular, smooth muscle cells (VSMC) play a major role in ISR.75 Medial VSMCs underwent apoptosis because of injury caused by stent implantation,76 and the repair mechanism involved the coordination of thrombus deposition, leukocyte trafficking to the stent site, and VSMC mitogenic stimuli.77 ISR resulted because of dysregulation of the repair mechanism and increased VSMC proliferation.78
After endothelial injury, three microRNAs such as miR-21, miR-145, and miR-221 are involved in modification of vessel restenosis.79 Ji et al.80 reported that the intensity of neointimal lesion formation was lessened if the antisense of miR-21 was knocked down. In addition, they identified an enhancement in VSMC proliferation using miR-21 through Akt and Bcl-2 activation, along with the inhibition of phosphate and tension homology deleted from chromosome 10, which is a common target for silencing miR-21. The over-expression of miR-145 secondary to carotid balloon injury resulted in the promotion of VSMC marker expression with a reduction in neointimal formation.81 Platelet-derived growth factor (PDGF) β is one of the most potent VSMC mitogens that induces miR-221 upon stimulation; it also down-regulates cKit and inhibits p27kip1, which in turn decreases and increases, respectively, SMC proliferation, thereby contributing to neointimal formation82 and indicating that neointimal formation could be blocked by the over-expression of miR145 and knockdown of miR-21 and miR-221.
Park and his coworkers developed a system to deliver miR-145 using a polysorbitol-based osmotically active transporter (POAST) to treat restenosis.83 They designed a method to coat miR-145 over the stent surface, for which miR-145 was made into a complex with PSOAT to form PSOAT/miR-145 nanoparticles (PMN); then, it was immobilized on the heparin/dopamine-conjugated stent surface. From in vitro studies, the suppression of VSMC proliferation was evident after delivering miR-145 because of c-Myc downregulation, as c-Myc is the target gene for miR-145; heparin/dopamine-conjugated stents have demonstrated high transfection efficiency with less toxicity.
Similar to microRNAs, short interfering RNA (siRNA) could also induce RNA silencing and could be used for the treatment of restenosis. It was also demonstrated to suppress restenosis using an Akt1 siRNA-embedded coronary stent, suggesting that the inhibition of Akt1 protein, which is responsible for cell proliferation, could reduce cell growth. Furthermore, a coronary stent, coated with dopamine-conjugated HA and Akt1 siRNA, was loaded on the HA surface using an electrostatic interaction.84 It was found that the delivery of Akt1 siRNA suppressed VSMC proliferation, and downstream Akt1 signaling proteins such as mTOR, 4E-BPI, and p70S6K were also downregulated. The therapeutic efficiency of an Akt1 siRNA-loaded HA-coated stent (ASN/HA) stent was investigated in a rabbit restenosis model with BMS as a control.85 Micro-computed tomography (micro-CT) imaging confirmed the suppression of ISR at the stent-implanted region. Vascular growth around the stent was thin with an ASN/HA stent and an HA-coated stent that were implanted in an animal model; however, in a BMS-implant model, thick vascular growth was observed.
In addition, the ISR area, SMC deposition, and ISR rate were significantly reduced with the ASN/HA stent, as compared with the BMS stent. Histopathological analysis revealed that neointima hyperplasia was completely absent in the ASN/HA stent implant model, but BMS demonstrated neointima formation to a large extent. The neointima suppression by the BMS HA coated stent and the ASN/HA stent were evaluated after 2 and 4 weeks post implantation. The thickness and pathology of the stenotic region were significantly reduced in arteries implanted with an ASN/HA stent, as compared with BMS- and HA-coated stents.
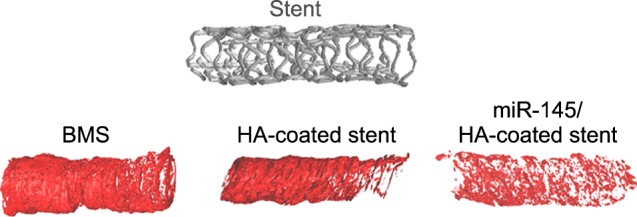
Verhoeff-Van Gieson staining of the arteries confirmed the suppression of neointima formation with the ASN/HA stent, and with a BMS-treated artery, neointima formation was larger. Similarly, a microRNA 145 nanoparticle-immobilized HA-coated coronary stent (Fig. 2) was also developed by them for targeting C-Myc protein.86 In this system, disulfide–cross-linked PEI was used as a carrier to transfect miR145. The miR145 was labeled with YOYO-1, a fluorescent dye, which enabled the imaging of miR145 after transfection. The stents were then implanted into the coronary arteries of a rabbit model. CT imaging and histological analyses revealed that SMC proliferation was inhibited and resulted in the prevention of ISR secondary to the release of miR145. They have observed suppression of neointimal formation, indicating that the RNA interference strategy is promising for treating ISR.
FIG. 2. Micro-CT image of a BMS and HA-coated and miR145-loaded stents.86.
BIOSENSOR INTEGRATED CARDIOVASCULAR STENTS
DES was created for treating restenosis and could also be used as biosensors to monitor blood flow and arterial pressure. Implantation of cardiovascular stents is known to cause inflammation and neointimal formation that can hinder blood flow. Therefore, when DES is implanted in together with a biosensor to monitor pulmonary artery pressure, the treatment efficacy will be improved. Arterial pressure load is a major problem in other cardiac ailments. Implanting sensors in the heart and monitoring blood flow could help in the diagnosis of a variety of cardiac ailments. Chow et al.87 developed an implantable cardiac monitoring system that was integrated with a cardiovascular stent. The system consists of an FDA-approved stent tailored with a miniature cardiac pressure sensor and wireless transmitter. In this design, the stent serves as an antenna, which transmits a quantified absorption to the surrounding region using a transmitter with wireless telemetry. One of the major drawbacks reported in this study was that the monitoring system was affected by electromagnetic interference secondary to Bluetooth and Wi-Fi devices.
In their subsequent study, Chow et al.88 improved their system by designing an application-specific integrated circuit (ASIC) made for wireless telemetry consisting of a voltage regulator and radio frequency power component. As a capacitive sensor, microelectromechanical systems were packed with ASIC for processing data, and the stent that was integrated with the system acted as an antenna and provided structural support. The final device included a radio frequency-powering component as an optimized, power transfer feature was implanted on the chip. The ASIC provided precise and accurate measurements with a sensitivity of 0.5 mmHg.
Recently, Son et al.89 designed a novel bioresorbable electronic stent, integrated with a sensor system and memory storage devices. The stent was fitted with core shell nanospheres loaded with drug and anti-inflammatory nanoparticles; the nanoparticle release was controlled by an external optical stimulus. The flow sensor measures blood flow and stores the flow pattern in its memory that would be helpful for follow-up treatment. Anti-inflammatory drug nanoparticles were released using a reactive oxygen species (ROS) scavenging effect where the stent was deployed, reducing inflammatory responses and inhibiting ISR occurrence. A temperature sensor regulated hyperthermia and controlled drug release as a photothermal therapy, suggesting that future designs of multifunctional stents could incorporate biosensors and deliver drugs based on physiological conditions.
FUTURE SCOPE
Coronary artery stents have been widely used to treat restenosis. However, implantation of coronary stents could result in ISR (i.e., development of plaque that is secondary to stent deployment). Using stents, this issue is resolved by delivering drugs to prevent ISR. A DES was coated with various non-erodible and biodegradable polymers to achieve sustained drug release; the polymer coating also serves as a reservoir for drugs. Drugs such as tyrphostin AG1295, Imatinib mesylate, paclitaxel, pitavastatin, sirolimus, and S-nitrosoglutathione that could inhibit the proliferation of smooth muscle cells and thrombosis at the injured site were loaded onto polymeric stents to prevent ISR. However, because of a lack of understanding of the mechanism of DES and other significant technical hurdles, the use of DES in clinical practice has been hampered. Over the last 30 years, many studies have reported on the possible mechanism of action of stents, thus providing a biological rationale for their use. This issue pertains to the use of coronary stents as scaffolding for the targeted and sustained delivery of therapeutic genes. Gene-eluting stents have been under development for a while and are inherently capable of providing a permanent solution for ISR. Thus, gene therapy became an appealing strategy to prevent restenosis and ISR through the delivery of therapeutic genes into the coronary artery. Many studies were conducted on the delivery of therapeutic genes using balloon catheters and intravenous injections, although targeted delivery remains an obstacle for cardiovascular gene therapy. Therapeutic genes that encode VEGF, MCP-1, eNOS, and pENTPDase were delivered using stents and were reported as a promising strategy to prevent ISR, although significant technical limitations such as vector development, percutaneous deployment, and gene incorporation still necessitate resolution. Vector development has also led to the improvement of gene-eluting stents.
RNA interference technology could also be utilized for treating restenosis, which is currently not well exploited. The utilization of RNA interference to treat cardiovascular diseases is a significant discovery and will be employed in clinical practice in the near future. Future studies may incorporate novel strategies including miRNA- and si-RNA-eluting stents to treat ISR with the evolution of DNA technology. Furthermore, multifunctional electronic stents could be used as biosensors and to deliver drugs or genes based on physiological conditions, and intravascular MRI combined with gene-eluting stents would be a promising treatment to prevent ISR.
CONCLUSION
The development in coronary stents has increasingly gained researchers' interest over the past decade because coronary stent implantation is currently considered a safe and widely accepted strategy to treat CAD, and it could treat restenosis resulting from coronary balloon or catheter implantation. However, a limitation of coronary stents is ISR that leads to the development of DES. To overcome ISR, many researchers have studied the delivery of drugs using a DES to inhibit the proliferation of SMCs, however, the current therapeutic approaches are still insufficient; therefore, a new strategy is required to prevent ISR. The development of gene-eluting stents was a breakthrough stent technology. The first gene-eluting stent was studied in the year 2000 for plasmid DNA delivery. An ideal gene-eluting stent should be non-inflammatory, non-thrombotic, and biocompatible, and it should also facilitate vascular healing. The development of gene-eluting stents requires a clear understanding of the molecular mechanisms, cytokines, and growth factors involved in neointimal formation. Gene-eluting stents are a promising strategy that will be available for the treatment of restenosis within a few years. The disadvantages of DES could be overcome by employing gene-eluting stents. Gene-eluting stents use viral and non-viral vectors to carry genes to the target site. Plasmids encode genes with a specific protein that could interfere or completely inhibit the formation of ISR and have been studied by many researchers. In this review, we briefly discussed the current progress in the development of gene-eluting stents. Genes encoding growth factors or cytokines that are involved in the proliferation of smooth muscle cells were inserted into a plasmid and directed toward the diseased area using stents, although significant technical hurdles have to be overcome for use in clinical practice. Restenosis could also be prevented at the molecular level by administering either microRNA or siRNA that could directly inhibit the mRNA involved in the proliferation of vascular smooth muscle cells, although future studies should focus on developing microRNA- or siRNA-eluting stents that could inhibit ISR via more than one pathway. Furthermore, the development of new multifunctional electronic stents that could be used as biosensors and deliver drugs or genes based on physiological conditions would be helpful in the future, and intravascular MRI should be combined with gene-eluting stents.
ACKNOWLEDGEMENTS
This work was financially supported by the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (HI12C0810 & HI14C0187); the Leading Foreign Research Institute Recruitment Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology (MEST) (2011-0030034 & NRF-2013R1A2A2A01004668); and the Pioneer Research Center Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT & Future Planning (2014M3C1A3053035). IKP acknowledges support from a grant (CRI14073-3) by the Chonnam National University Hospital Research Institute of Clinical Medicine. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Footnotes
CONFLICT OF INTEREST STATEMENT: None declared.
References
- 1.Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet. 1997;349:1269–1276. doi: 10.1016/S0140-6736(96)07493-4. [DOI] [PubMed] [Google Scholar]
- 2.WHO. World Health Statistics 2008. [Internet] Geneva: World Health Organization; c2008. [cited 2015 Jun 26]. Available from: http://www.who.int/whosis/whostat/2008/en/ [Google Scholar]
- 3.Sharif F, Daly K, Crowley J, O’Brien T. Current status of catheter- and stent-based gene therapy. Cardiovasc Res. 2004;64:208–216. doi: 10.1016/j.cardiores.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Dotter CT, Judkins MP. Transluminal treatment of arteriosclerotic obstruction. Description of a new technic and a preliminary report of its application. Circulation. 1964;30:654–670. doi: 10.1161/01.cir.30.5.654. [DOI] [PubMed] [Google Scholar]
- 5.Serruys PW, de Jaegere P, Kiemeneij F, Macaya C, Rutsch W, Heyndrickx G, et al. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. Benestent Study Group. N Engl J Med. 1994;331:489–495. doi: 10.1056/NEJM199408253310801. [DOI] [PubMed] [Google Scholar]
- 6.Sigwart U, Puel J, Mirkovitch V, Joffre F, Kappenberger L. Intravascular stents to prevent occlusion and restenosis after transluminal angioplasty. N Engl J Med. 1987;316:701–706. doi: 10.1056/NEJM198703193161201. [DOI] [PubMed] [Google Scholar]
- 7.Garg S, Serruys PW. Coronary stents: current status. J Am Coll Cardiol. 2010;56(10 Suppl):S1–S42. doi: 10.1016/j.jacc.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 8.Tamai H, Igaki K, Kyo E, Kosuga K, Kawashima A, Matsui S, et al. Initial and 6-month results of biodegradable poly-l-lactic acid coronary stents in humans. Circulation. 2000;102:399–404. doi: 10.1161/01.cir.102.4.399. [DOI] [PubMed] [Google Scholar]
- 9.Regar E, Sianos G, Serruys PW. Stent development and local drug delivery. Br Med Bull. 2001;59:227–248. doi: 10.1093/bmb/59.1.227. [DOI] [PubMed] [Google Scholar]
- 10.Venkatraman S, Boey F. Release profiles in drug-eluting stents: issues and uncertainties. J Control Release. 2007;120:149–160. doi: 10.1016/j.jconrel.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 11.Simons M, Edelman ER, DeKeyser JL, Langer R, Rosenberg RD. Antisense c-myb oligonucleotides inhibit intimal arterial smooth muscle cell accumulation in vivo. Nature. 1992;359:67–70. doi: 10.1038/359067a0. [DOI] [PubMed] [Google Scholar]
- 12.Mach F. Toward new therapeutic strategies against neointimal formation in restenosis. Arterioscler Thromb Vasc Biol. 2000;20:1699–1700. doi: 10.1161/01.atv.20.7.1699. [DOI] [PubMed] [Google Scholar]
- 13.Kibos A, Campeanu A, Tintoiu I. Pathophysiology of coronary artery in-stent restenosis. Acute Card Care. 2007;9:111–119. doi: 10.1080/17482940701263285. [DOI] [PubMed] [Google Scholar]
- 14.Andersen HR, Maeng M, Thorwest M, Falk E. Remodeling rather than neointimal formation explains luminal narrowing after deep vessel wall injury: insights from a porcine coronary (re)stenosis model. Circulation. 1996;93:1716–1724. doi: 10.1161/01.cir.93.9.1716. [DOI] [PubMed] [Google Scholar]
- 15.Moreno PR, Palacios IF, Leon MN, Rhodes J, Fuster V, Fallon JT. Histopathologic comparison of human coronary in-stent and post-balloon angioplasty restenotic tissue. Am J Cardiol. 1999;84:462–466. A9. doi: 10.1016/s0002-9149(99)00334-3. [DOI] [PubMed] [Google Scholar]
- 16.Mintz GS, Popma JJ, Pichard AD, Kent KM, Satler LF, Wong C, et al. Arterial remodeling after coronary angioplasty: a serial intravascular ultrasound study. Circulation. 1996;94:35–43. doi: 10.1161/01.cir.94.1.35. [DOI] [PubMed] [Google Scholar]
- 17.Brewster LP, Brey EM, Greisler HP. Cardiovascular gene delivery: the good road is awaiting. Adv Drug Deliv Rev. 2006;58:604–629. doi: 10.1016/j.addr.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation. 2005;111:3481–3488. doi: 10.1161/CIRCULATIONAHA.105.537878. [DOI] [PubMed] [Google Scholar]
- 19.Schainfeld RM. Potential emerging therapeutic strategies to prevent restenosis in the peripheral vasculature. Catheter Cardiovasc Interv. 2002;56:421–431. doi: 10.1002/ccd.10211. [DOI] [PubMed] [Google Scholar]
- 20.Tsigkas GG, Karantalis V, Hahalis G, Alexopoulos D. Stent restenosis, pathophysiology and treatment options: a 2010 update. Hellenic J Cardiol. 2011;52:149–157. [PubMed] [Google Scholar]
- 21.Byrne RA, Kastrati A, Kufner S, Massberg S, Birkmeier KA, Laugwitz KL, et al. Randomized, non-inferiority trial of three limus agent-eluting stents with different polymer coatings: the Intracoronary Stenting and Angiographic Results: Test Efficacy of 3 Limus-Eluting Stents (ISAR-TEST-4) Trial. Eur Heart J. 2009;30:2441–2449. doi: 10.1093/eurheartj/ehp352. [DOI] [PubMed] [Google Scholar]
- 22.Lowe HC, Oesterle SN, Khachigian LM. Coronary in-stent restenosis: current status and future strategies. J Am Coll Cardiol. 2002;39:183–193. doi: 10.1016/s0735-1097(01)01742-9. [DOI] [PubMed] [Google Scholar]
- 23.Abizaid A, Kornowski R, Mintz GS, Hong MK, Abizaid AS, Mehran R, et al. The influence of diabetes mellitus on acute and late clinical outcomes following coronary stent implantation. J Am Coll Cardiol. 1998;32:584–589. doi: 10.1016/s0735-1097(98)00286-1. [DOI] [PubMed] [Google Scholar]
- 24.Dangas G, Mehran R, Lansky AJ, Waksman R, Satler LF, Pichard AD, et al. Acute and long-term results of treatment of diffuse in-stent restenosis in aortocoronary saphenous vein grafts. Am J Cardiol. 2000;86:777–779. A6. doi: 10.1016/s0002-9149(00)01080-8. [DOI] [PubMed] [Google Scholar]
- 25.Welt FG, Rogers C. Inflammation and restenosis in the stent era. Arterioscler Thromb Vasc Biol. 2002;22:1769–1776. doi: 10.1161/01.atv.0000037100.44766.5b. [DOI] [PubMed] [Google Scholar]
- 26.Scott NA. Restenosis following implantation of bare metal coronary stents: pathophysiology and pathways involved in the vascular response to injury. Adv Drug Deliv Rev. 2006;58:358–376. doi: 10.1016/j.addr.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 27.Costa MA, Simon DI. Molecular basis of restenosis and drug-eluting stents. Circulation. 2005;111:2257–2273. doi: 10.1161/01.CIR.0000163587.36485.A7. [DOI] [PubMed] [Google Scholar]
- 28.Zohlnhöfer D, Klein CA, Richter T, Brandl R, Murr A, Nührenberg T, et al. Gene expression profiling of human stent-induced neointima by cDNA array analysis of microscopic specimens retrieved by helix cutter atherectomy: detection of FK506-binding protein 12 upregulation. Circulation. 2001;103:1396–1402. doi: 10.1161/01.cir.103.10.1396. [DOI] [PubMed] [Google Scholar]
- 29.Sousa JE, Costa MA, Abizaid A, Abizaid AS, Feres F, Pinto IM, et al. Lack of neointimal proliferation after implantation of sirolimus-coated stents in human coronary arteries: a quantitative coronary angiography and three-dimensional intravascular ultrasound study. Circulation. 2001;103:192–195. doi: 10.1161/01.cir.103.2.192. [DOI] [PubMed] [Google Scholar]
- 30.Yin RX, Yang DZ, Wu JZ. Nanoparticle drug- and gene-eluting stents for the prevention and treatment of coronary restenosis. Theranostics. 2014;4:175–200. doi: 10.7150/thno.7210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Remuzzi A, Mantero S, Colombo M, Morigi M, Binda E, Camozzi D, et al. Vascular smooth muscle cells on hyaluronic acid: culture and mechanical characterization of an engineered vascular construct. Tissue Eng. 2004;10:699–710. doi: 10.1089/1076327041348347. [DOI] [PubMed] [Google Scholar]
- 32.Jiang HL, Hong SH, Kim YK, Islam MA, Kim HJ, Choi YJ, et al. Aerosol delivery of spermine-based poly(amino ester)/Akt1 shRNA complexes for lung cancer gene therapy. Int J Pharm. 2011;420:256–265. doi: 10.1016/j.ijpharm.2011.08.045. [DOI] [PubMed] [Google Scholar]
- 33.Jensen DK, Jensen LB, Koocheki S, Bengtson L, Cun D, Nielsen HM, et al. Design of an inhalable dry powder formulation of DOTAP-modified PLGA nanoparticles loaded with siRNA. J Control Release. 2012;157:141–148. doi: 10.1016/j.jconrel.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 34.Zhu D, Jin X, Leng X, Wang H, Bao J, Liu W, et al. Local gene delivery via endovascular stents coated with dodecylated chitosan-plasmid DNA nanoparticles. Int J Nanomedicine. 2010;5:1095–1102. doi: 10.2147/IJN.S14358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Radeleff B, Thierjung H, Stampfl U, Stampfl S, Lopez-Benitez R, Sommer C, et al. Restenosis of the CYPHER-Select, TAXUS-Express, and Polyzene-F nanocoated cobalt-chromium stents in the minipig coronary artery model. Cardiovasc Intervent Radiol. 2008;31:971–980. doi: 10.1007/s00270-007-9243-y. [DOI] [PubMed] [Google Scholar]
- 36.La Manna A, Capodanno D, Cera M, Di Salvo ME, Sacchetta G, Corcos T, et al. Optical coherence tomographic results at six-month follow-up evaluation of the CATANIA coronary stent system with nanothin Polyzene-F surface modification (from the Assessment of The LAtest Non-Thrombogenic Angioplasty Stent [ATLANTA] trial) Am J Cardiol. 2009;103:1551–1555. doi: 10.1016/j.amjcard.2009.01.378. [DOI] [PubMed] [Google Scholar]
- 37.Banai S, Wolf Y, Golomb G, Pearle A, Waltenberger J, Fishbein I, et al. PDGF-receptor tyrosine kinase blocker AG1295 selectively attenuates smooth muscle cell growth in vitro and reduces neointimal formation after balloon angioplasty in swine. Circulation. 1998;97:1960–1969. doi: 10.1161/01.cir.97.19.1960. [DOI] [PubMed] [Google Scholar]
- 38.Masuda S, Nakano K, Funakoshi K, Zhao G, Meng W, Kimura S, et al. Imatinib mesylate-incorporated nanoparticle-eluting stent attenuates in-stent neointimal formation in porcine coronary arteries. J Atheroscler Thromb. 2011;18:1043–1053. doi: 10.5551/jat.8730. [DOI] [PubMed] [Google Scholar]
- 39.Gingras AC, Raught B, Sonenberg N. mTOR signaling to translation. Curr Top Microbiol Immunol. 2004;279:169–197. doi: 10.1007/978-3-642-18930-2_11. [DOI] [PubMed] [Google Scholar]
- 40.Luderer F, Löbler M, Rohm HW, Gocke C, Kunna K, Köck K, et al. Biodegradable sirolimus-loaded poly(lactide) nanoparticles as drug delivery system for the prevention of in-stent restenosis in coronary stent application. J Biomater Appl. 2011;25:851–875. doi: 10.1177/0885328209360696. [DOI] [PubMed] [Google Scholar]
- 41.Räthel T, Mannell H, Pircher J, Gleich B, Pohl U, Krötz F. Magnetic stents retain nanoparticle-bound antirestenotic drugs transported by lipid microbubbles. Pharm Res. 2012;29:1295–1307. doi: 10.1007/s11095-011-0643-y. [DOI] [PubMed] [Google Scholar]
- 42.Giannakakou P, Robey R, Fojo T, Blagosklonny MV. Low concentrations of paclitaxel induce cell type-dependent p53, p21 and G1/G2 arrest instead of mitotic arrest: molecular determinants of paclitaxel-induced cytotoxicity. Oncogene. 2001;20:3806–3813. doi: 10.1038/sj.onc.1204487. [DOI] [PubMed] [Google Scholar]
- 43.Bhargava B, Reddy NK, Karthikeyan G, Raju R, Mishra S, Singh S, et al. A novel paclitaxel-eluting porous carbon-carbon nanoparticle coated, nonpolymeric cobalt-chromium stent: evaluation in a porcine model. Catheter Cardiovasc Interv. 2006;67:698–702. doi: 10.1002/ccd.20698. [DOI] [PubMed] [Google Scholar]
- 44.Chorny M, Fishbein I, Yellen BB, Alferiev IS, Bakay M, Ganta S, et al. Targeting stents with local delivery of paclitaxel-loaded magnetic nanoparticles using uniform fields. Proc Natl Acad Sci U S A. 2010;107:8346–8351. doi: 10.1073/pnas.0909506107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsukie N, Nakano K, Matoba T, Masuda S, Iwata E, Miyagawa M, et al. Pitavastatin-incorporated nanoparticle-eluting stents attenuate in-stent stenosis without delayed endothelial healing effects in a porcine coronary artery model. J Atheroscler Thromb. 2013;20:32–45. doi: 10.5551/jat.13862. [DOI] [PubMed] [Google Scholar]
- 46.Yoo JW, Lee JS, Lee CH. Characterization of nitric oxide-releasing microparticles for the mucosal delivery. J Biomed Mater Res A. 2010;92:1233–1243. doi: 10.1002/jbm.a.32434. [DOI] [PubMed] [Google Scholar]
- 47.Acharya G, Lee CH, Lee Y. Optimization of cardiovascular stent against restenosis: factorial design-based statistical analysis of polymer coating conditions. PLoS One. 2012;7:e43100. doi: 10.1371/journal.pone.0043100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shuchman M. Debating the risks of drug-eluting stents. N Engl J Med. 2007;356:325–328. doi: 10.1056/NEJMp068300. [DOI] [PubMed] [Google Scholar]
- 49.Iakovou I, Schmidt T, Bonizzoni E, Ge L, Sangiorgi GM, Stankovic G, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005;293:2126–2130. doi: 10.1001/jama.293.17.2126. [DOI] [PubMed] [Google Scholar]
- 50.Ernst A, Bulum J. New generations of drug-eluting stents - A brief review. EMJ Int Cardiol. 2014:100–106. [Google Scholar]
- 51.Goldman B, Blanke H, Wolinsky H. Influence of pressure on permeability of normal and diseased muscular arteries to horseradish peroxidase. A new catheter approach. Atherosclerosis. 1987;65:215–225. doi: 10.1016/0021-9150(87)90037-2. [DOI] [PubMed] [Google Scholar]
- 52.Wolinsky H, Thung SN. Use of a perforated balloon catheter to deliver concentrated heparin into the wall of the normal canine artery. J Am Coll Cardiol. 1990;15:475–481. doi: 10.1016/s0735-1097(10)80079-8. [DOI] [PubMed] [Google Scholar]
- 53.Landau C, Pirwitz MJ, Willard MA, Gerard RD, Meidell RS, Willard SE. Adenoviral mediated gene transfer to atherosclerotic arteries after balloon angioplasty. Am Heart J. 1995;129:1051–1057. doi: 10.1016/0002-8703(95)90383-6. [DOI] [PubMed] [Google Scholar]
- 54.Numaguchi Y, Okumura K, Harada M, Naruse K, Yamada M, Osanai H, et al. Catheter-based prostacyclin synthase gene transfer prevents in-stent restenosis in rabbit atheromatous arteries. Cardiovasc Res. 2004;61:177–185. doi: 10.1016/j.cardiores.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 55.Wang K, Kessler PD, Zhou Z, Penn MS, Forudi F, Zhou X, et al. Local adenoviral-mediated inducible nitric oxide synthase gene transfer inhibits neointimal formation in the porcine coronary stented model. Mol Ther. 2003;7:597–603. doi: 10.1016/s1525-0016(03)00061-3. [DOI] [PubMed] [Google Scholar]
- 56.Laçin NT, Utkan GG. Role of biomaterials in prevention of in-stent restenosis. J Biomed Mater Res B Appl Biomater. 2014;102:1113–1120. doi: 10.1002/jbm.b.33083. [DOI] [PubMed] [Google Scholar]
- 57.Guzman RJ, Lemarchand P, Crystal RG, Epstein SE, Finkel T. Efficient and selective adenovirus-mediated gene transfer into vascular neointima. Circulation. 1993;88:2838–2848. doi: 10.1161/01.cir.88.6.2838. [DOI] [PubMed] [Google Scholar]
- 58.Nabel EG, Plautz G, Nabel GJ. Site-specific gene expression in vivo by direct gene transfer into the arterial wall. Science. 1990;249:1285–1288. doi: 10.1126/science.2119055. [DOI] [PubMed] [Google Scholar]
- 59.Rolling F, Nong Z, Pisvin S, Collen D. Adeno-associated virus-mediated gene transfer into rat carotid arteries. Gene Ther. 1997;4:757–761. doi: 10.1038/sj.gt.3300465. [DOI] [PubMed] [Google Scholar]
- 60.Flugelman MY, Jaklitsch MT, Newman KD, Casscells W, Bratthauer GL, Dichek DA. Low level in vivo gene transfer into the arterial wall through a perforated balloon catheter. Circulation. 1992;85:1110–1117. doi: 10.1161/01.cir.85.3.1110. [DOI] [PubMed] [Google Scholar]
- 61.Klugherz BD, Jones PL, Cui X, Chen W, Meneveau NF, DeFelice S, et al. Gene delivery from a DNA controlled-release stent in porcine coronary arteries. Nat Biotechnol. 2000;18:1181–1184. doi: 10.1038/81176. [DOI] [PubMed] [Google Scholar]
- 62.Klugherz BD, Song C, DeFelice S, Cui X, Lu Z, Connolly J, et al. Gene delivery to pig coronary arteries from stents carrying antibody-tethered adenovirus. Hum Gene Ther. 2002;13:443–454. doi: 10.1089/10430340252792576. [DOI] [PubMed] [Google Scholar]
- 63.Perlstein I, Connolly JM, Cui X, Song C, Li Q, Jones PL, et al. DNA delivery from an intravascular stent with a denatured collagen-polylactic-polyglycolic acid-controlled release coating: mechanisms of enhanced transfection. Gene Ther. 2003;10:1420–1428. doi: 10.1038/sj.gt.3302043. [DOI] [PubMed] [Google Scholar]
- 64.Jin X, Mei L, Song C, Liu L, Leng X, Sun H, et al. Immobilization of plasmid DNA on an anti-DNA antibody modified coronary stent for intravascular site-specific gene therapy. J Gene Med. 2008;10:421–429. doi: 10.1002/jgm.1165. [DOI] [PubMed] [Google Scholar]
- 65.Kim TG, Lee Y, Park TG. Controlled gene-eluting metal stent fabricated by bio-inspired surface modification with hyaluronic acid and deposition of DNA/PEI polyplexes. Int J Pharm. 2010;384:181–188. doi: 10.1016/j.ijpharm.2009.09.042. [DOI] [PubMed] [Google Scholar]
- 66.Walter DH, Cejna M, Diaz-Sandoval L, Willis S, Kirkwood L, Stratford PW, et al. Local gene transfer of phVEGF-2 plasmid by gene-eluting stents: an alternative strategy for inhibition of restenosis. Circulation. 2004;110:36–45. doi: 10.1161/01.CIR.0000133324.38115.0A. [DOI] [PubMed] [Google Scholar]
- 67.Paul A, Shao W, Shum-Tim D, Prakash S. The attenuation of restenosis following arterial gene transfer using carbon nanotube coated stent incorporating TAT/DNA(Ang1+Vegf) nanoparticles. Biomaterials. 2012;33:7655–7664. doi: 10.1016/j.biomaterials.2012.06.096. [DOI] [PubMed] [Google Scholar]
- 68.Yang J, Zeng Y, Zhang C, Chen YX, Yang Z, Li Y, et al. The prevention of restenosis in vivo with a VEGF gene and paclitaxel co-eluting stent. Biomaterials. 2013;34:1635–1643. doi: 10.1016/j.biomaterials.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 69.Egashira K, Nakano K, Ohtani K, Funakoshi K, Zhao G, Ihara Y, et al. Local delivery of anti-monocyte chemoattractant protein-1 by gene-eluting stents attenuates in-stent stenosis in rabbits and monkeys. Arterioscler Thromb Vasc Biol. 2007;27:2563–2568. doi: 10.1161/ATVBAHA.107.154609. [DOI] [PubMed] [Google Scholar]
- 70.Cooney R, Hynes SO, Sharif F, Howard L, O'Brien T. Effect of gene delivery of NOS isoforms on intimal hyperplasia and endothelial regeneration after balloon injury. Gene Ther. 2007;14:396–404. doi: 10.1038/sj.gt.3302882. [DOI] [PubMed] [Google Scholar]
- 71.Sharif F, Hynes SO, Cooney R, Howard L, McMahon J, Daly K, et al. Gene-eluting stents: adenovirus-mediated delivery of eNOS to the blood vessel wall accelerates re-endothelialization and inhibits restenosis. Mol Ther. 2008;16:1674–1680. doi: 10.1038/mt.2008.165. [DOI] [PubMed] [Google Scholar]
- 72.Brito LA, Chandrasekhar S, Little SR, Amiji MM. Non-viral eNOS gene delivery and transfection with stents for the treatment of restenosis. Biomed Eng Online. 2010;9:56. doi: 10.1186/1475-925X-9-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sharif F, Hynes SO, McCullagh KJ, Ganley S, Greiser U, McHugh P, et al. Gene-eluting stents: non-viral, liposome-based gene delivery of eNOS to the blood vessel wall in vivo results in enhanced endothelialization but does not reduce restenosis in a hypercholesterolemic model. Gene Ther. 2012;19:321–328. doi: 10.1038/gt.2011.92. [DOI] [PubMed] [Google Scholar]
- 74.Takemoto Y, Kawata H, Soeda T, Imagawa K, Somekawa S, Takeda Y, et al. Human placental ectonucleoside triphosphate diphosphohydrolase gene transfer via gelatin-coated stents prevents in-stent thrombosis. Arterioscler Thromb Vasc Biol. 2009;29:857–862. doi: 10.1161/ATVBAHA.109.186429. [DOI] [PubMed] [Google Scholar]
- 75.Mitra AK, Agrawal DK. In stent restenosis: bane of the stent era. J Clin Pathol. 2006;59:232–239. doi: 10.1136/jcp.2005.025742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Isner JM, Kearney M, Bortman S, Passeri J. Apoptosis in human atherosclerosis and restenosis. Circulation. 1995;91:2703–2711. doi: 10.1161/01.cir.91.11.2703. [DOI] [PubMed] [Google Scholar]
- 77.Raines EW. PDGF and cardiovascular disease. Cytokine Growth Factor Rev. 2004;15:237–254. doi: 10.1016/j.cytogfr.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 78.Komatsu R, Ueda M, Naruko T, Kojima A, Becker AE. Neointimal tissue response at sites of coronary stenting in humans: macroscopic, histological, and immunohistochemical analyses. Circulation. 1998;98:224–233. doi: 10.1161/01.cir.98.3.224. [DOI] [PubMed] [Google Scholar]
- 79.Schaer GL, Zhang C. Implementation of miRNAs to reduce in-stent restenosis in the future. J Am Coll Cardiol. 2015;65:2328–2330. doi: 10.1016/j.jacc.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 80.Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen H, et al. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ Res. 2007;100:1579–1588. doi: 10.1161/CIRCRESAHA.106.141986. [DOI] [PubMed] [Google Scholar]
- 81.Cheng Y, Liu X, Yang J, Lin Y, Xu DZ, Lu Q, et al. MicroRNA-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation. Circ Res. 2009;105:158–166. doi: 10.1161/CIRCRESAHA.109.197517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Davis BN, Hilyard AC, Nguyen PH, Lagna G, Hata A. Induction of microRNA-221 by platelet-derived growth factor signaling is critical for modulation of vascular smooth muscle phenotype. J Biol Chem. 2009;284:3728–3738. doi: 10.1074/jbc.M808788200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Muthiah M, Islam MA, Cho CS, Hwang JE, Chung IJ, Park IK. Substrate-mediated delivery of microRNA-145 through a polysorbitol-based osmotically active transporter suppresses smooth muscle cell proliferation: implications for restenosis treatment. J Biomed Nanotechnol. 2014;10:571–579. doi: 10.1166/jbn.2014.1737. [DOI] [PubMed] [Google Scholar]
- 84.Che HL, Bae IH, Lim KS, Song IT, Lee H, Muthiah M, et al. Suppression of post-angioplasty restenosis with an Akt1 siRNA-embedded coronary stent in a rabbit model. Biomaterials. 2012;33:8548–8556. doi: 10.1016/j.biomaterials.2012.07.045. [DOI] [PubMed] [Google Scholar]
- 85.Che HL, Bae IH, Lim KS, Song IT, Lee H, Lee D, et al. Therapeutic Effect of Akt1 siRNA nanoparticle eluting coronary stent on suppression of post-angioplasty restenosis. J Biomed Nanotechnol. 2016;12:1211–1222. doi: 10.1166/jbn.2016.2255. [DOI] [PubMed] [Google Scholar]
- 86.Che HL, Bae IH, Lim KS, Uthaman S, Song IT, Lee H, et al. Novel fabrication of microRNA nanoparticle-coated coronary stent for prevention of post-angioplasty restenosis. Korean Circ J. 2016;46:23–32. doi: 10.4070/kcj.2016.46.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chow EY, Beier BL, Francino A, Chappell WJ, Irazoqui PP. Toward an implantable wireless cardiac monitoring platform integrated with an FDA-approved cardiovascular stent. J Interv Cardiol. 2009;22:479–487. doi: 10.1111/j.1540-8183.2009.00483.x. [DOI] [PubMed] [Google Scholar]
- 88.Chow EY, Chlebowski AL, Chakraborty S, Chappell WJ, Irazoqui PP. Fully wireless implantable cardiovascular pressure monitor integrated with a medical stent. IEEE Trans Biomed Eng. 2010;57:1487–1496. doi: 10.1109/TBME.2010.2041058. [DOI] [PubMed] [Google Scholar]
- 89.Son D, Lee J, Lee DJ, Ghaffari R, Yun S, Kim SJ, et al. Bioresorbable electronic stent integrated with therapeutic nanoparticles for endovascular diseases. ACS Nano. 2015;9:5937–5946. doi: 10.1021/acsnano.5b00651. [DOI] [PubMed] [Google Scholar]