ILLUSTRATIVE CASE
A 4-year-old boy presented with papilledema and nystagmus. He had developed eye deviation, clumsiness, and episodes of dizziness starting 6 months prior to presentation, which progressed in frequency. Following an episode of emesis, he was brought to our attention. One week prior to presentation, the patient had one episode of emesis in the morning. His development had been notable for speech delay, with expressive language limited to speaking in short phrases without full sentence formation; he otherwise had normal growth and motor development.
Physical examination was significant for sluggish and dilated pupils, agitation, crying, and attention difficulty. Computer tomography (CT) scan of the brain revealed a large posterior fossa mass arising from the vermis with multiple calcifications and associated obstructive hydrocephalus. Magnetic resonance imaging (MRI) with and without contrast of the brain showed a mass with patchy enhancement and associated metastatic lesions located throughout the cerebellar hemispheres [Figure 1]. MRI of the spine demonstrated two focal enhancing nodules of spinal cord in the cervical and thoracic spine.
Figure 1.
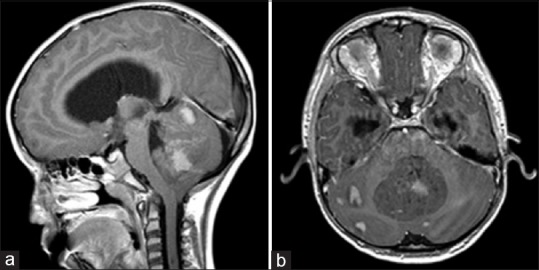
Preoperative MRI Brain, T1 post-gadolinium in sagittal (a) and axial (b) sections from patient in the example case. The large midline medulloblastoma arises from the cerebellar vermis and compresses the fourth ventricle, causing obstructive hydrocephalus. There is nodular leptomeningeal spread throughout the posterior fossa
Once in the operating room, a frontal external ventricular drain was placed prior to positioning the patient prone for a midline suboccipital craniotomy with transvermian approach splitting the inferior aspect of the vermis. The tumor was highly vascular and noted to be involving the floor of the fourth ventricle, bilateral foramina of Luschka, and left cerebellar peduncle, precluding a complete resection. Histopathology confirmed a medulloblastoma with subsequent molecular definition of a non-WNT(wingless)/non-SHH(sonic hedgehog) subgroup.
Postoperatively, the patient exhibited decreased responsiveness, mutism, fixed downward gaze, and inability to follow commands. Postoperative imaging did not demonstrate any hemorrhage [Figure 2]. He was dependent on cerebrospinal fluid (CSF) drainage, requiring a ventriculoperitoneal shunt on postoperative day 14. He had an extended postoperative course complicated by shunt infection but eventually was able to undergo adjuvant radiation and chemotherapy. Following craniospinal proton radiotherapy, he improved in his mental status and purposeful interaction and regained three-word speech. However, significant disease burden still persisted on imaging [Figure 3].
Figure 2.
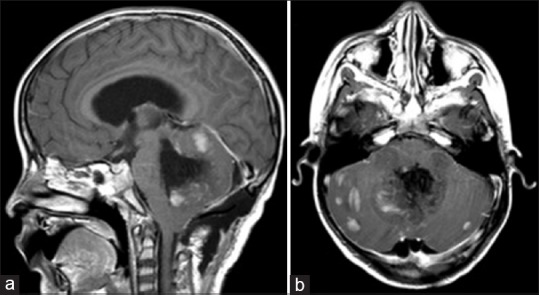
Postoperative MRI Brain, T1 post-gadolinium in sagittal (a) and axial (b) sections. The medial portion of the lesion has been largely resected, but the lesion remains in the bilateral cerebellar hemispheres, totaling >1.5 cm2
Figure 3.
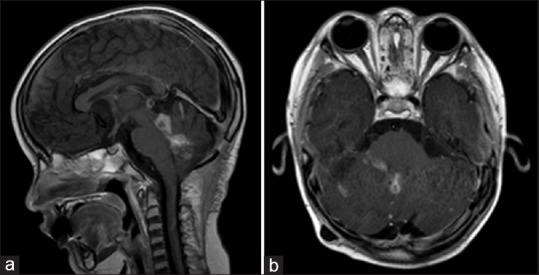
Post-radiotherapy MRI Brain, T1 post-gadolinium in sagittal (a) and axial (b) sections. There has been some interval improvement of leptomeningeal spread and nodular lesions. However, there has been recurrence of disease in the fourth ventricle
OVERVIEW OF MEDULLOBLASTOMA
Medulloblastoma (MB) is the most common posterior fossa tumor in children, presenting at a mean age of 9 years and, more commonly, in males (ratio 2:1).[2] It usually presents with obstructive hydrocephalus and resultant symptoms, which may also be accompanied by ataxia, cranial neuropathies, brainstem dysfunction, or nerve root/spinal cord compression from metastatic disease. Symptoms are often progressive over weeks to months, and it is not uncommon for patients to have an extended symptomatic period prior to initial diagnosis. Metastatic disease is commonly present at diagnosis (40%), and imaging of the entire craniospinal axis is an essential part of the initial diagnostic evaluation. Most cases of MB arise sporadically but are also linked to a number of syndromes, including Gorlin, Li-Fraumeni, and Turcot syndromes.
Molecular subgrouping
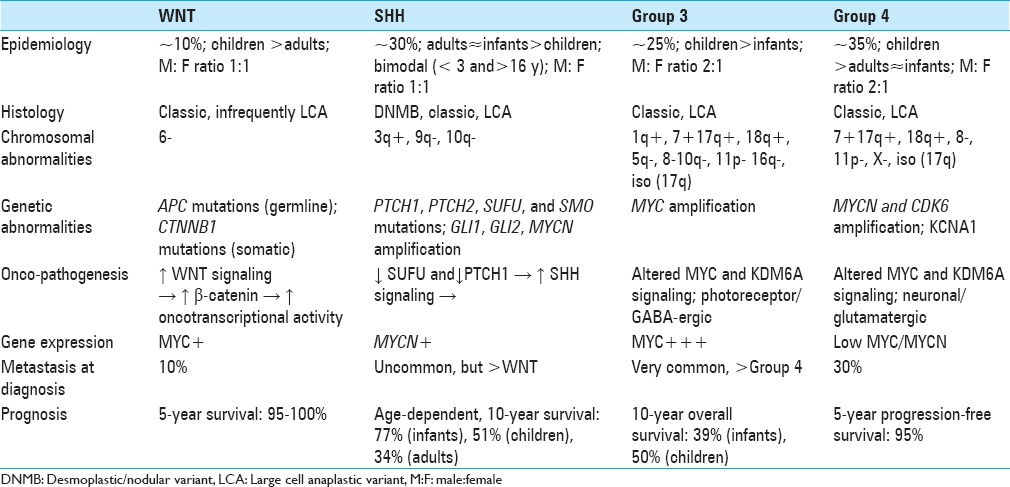
In addition to traditional histologic classification [classic, desmoplastic/nodular (DNMB), MB with extensive nodularity (MBEN), large cell/anaplastic (LCA)), MB has been classified into four distinct molecular subgroups according to transcriptional profiling studies.[41] The consensus four subgroups, which include WNT, SHH, Group 3, and Group 4, have been correlated to both clinical outcomes and histologic appearance.[2,41] These genetically defined molecular subgroups form a crucial aspect of the forthcoming World Health Organization 2016 guidelines, which divides MB into WNT, SHH-TP53 wild type, SHH-TP53 mutant, non-WNT/non-SHH, and MB-NOS.[31] Unfortunately, molecular studies, while available, remain isolated to large tertiary centers, even in developed countries.
Genetics
The WNT and SHH classifications identify the underlying oncopathogenic pathway, whereas Groups 3 and 4 retain generic designations pending elucidation of underlying cellular pathobiology. WNT tumors result from unregulated WNT signaling leading to increased beta-catenin-mediated increase in transcriptional activity and consequent tumorigenesis. They are associated with monosomy 6, germline APC mutations (Turcot syndrome), somatic CTNNB1 mutations, and nuclear positivity for β-catenin.[17,48] Oncogenesis of SHH MBs results from upregulation of sonic hedgehog signaling as a consequence of loss of function of the tumor suppressors suppressor of fused gene (SUFU) and patched-1 (PTCH1). Other events implicated in pathogenesis of SHH MBs include mutations of PTCH1, PTCH2, SUFU, and SMO, as well as amplification of GLI1 and GLI2.[7,8,26,27,28,37,39,40]
The precise oncopathogenic mechanisms for non-WNT/SHH tumors are still under investigation. Altered MYC and KDM6A signaling has been implicated in Groups 3 and 4 MBs. Whereas MYC is often overamplified in Group 3 MBs, Group 4 MBs are occasionally characterized by MYCN and CDK6 amplification. Isochromosome 17q is characteristic of Group 4 tumors but is also occasionally observed in Group 3 MBs, making it poorly specific. An alternative proposed marker for Group 4 lesions is KCNA1.[26]
Whereas molecular subgrouping of MB is based on differential gene transcriptional profiles, the different subgroups are recapitulated by subgroup-specific differential heterogeneity of cis-regulatory elements in the epigenome.[21] Based on these studies, it has been proposed that Group 4 MBs may arise from cells in the deep cerebellar nuclei of the cerebellar nuclear transitory zone or the upper rhombic lip.[21] Probing and further delineating epigenetic and gene regulatory differences may serve not only as better molecular subgrouping classification metrics but also the dual purpose of uncovering oncopathogenesis of different types of MB as well as of offering novel therapeutic targets.
Another compelling area of investigation in the genetics of MB is recurrent disease. Much of our understanding of the molecular and genetic profiles of MB is derived from experiments with treatment naïve and primary site disease. However, much like other malignancies, there is mounting evidence that suggests clonal selection and genetic divergence may play an important role in MB recurrence.[25] Although it has been demonstrated that molecular subgroups (WNT, SHH, Group 3, Group 4) are preserved in both metastatic and recurrent disease, for recurrence this may be insufficient to guide molecularly targeted therapies because significant genetic divergence, including increased mutational burden with both loss and gain of actionable molecular targets, has been demonstrated.[25,44]
Histologic correlation
There is an association between molecular subgroup and histologic type. For instance, 97% of WNT MBs are of the classic histologic variant.[19] However, this correlation is not absolute; in infants, children, and adults, 89%, 25%, and 100% of DNMBs were of the SHH molecular subgroup, respectively.[19] LCA tumors in infants are most commonly Group 3 lesions, however, in other ages they are evenly distributed across molecular subgroups.
Epidemiologic correlation
WNT, SHH, Group 3, and Group 4 MBs account for 10%, 30%, 25%, and 35% of MBs overall, respectively, with a 1:1 M:F ratio for WNT and SHH subgroups, and a 2:1 male predominance for non-SHH/WNT tumors. WNT tumors are typically seen in children and adults, whereas Group 3 tumors are more often seen in infants and children. SHH and Group 4 tumors are seen across all age groups, with the former exhibiting a bimodal age distribution, most typically occurring in patients <4 and >16 years of age.[10]
Surgical treatment
Extent of resection
The extent of resection indicated in MBs largely depends on the unique anatomy of the tumor and what can be done safely and without the incurrence of neurological deficit, as with all tumors in the eloquent areas of brain. Although no clinical trials have been designed to specifically evaluate the role of surgery for MB, there have been many studies supporting a relationship of extent of resection with event-free survival. The most influential is likely a retrospective analysis of 233 children involved in a randomized controlled trial of differing chemotherapy regimens by Albright et al. determining that a radiographically measured residual tumor less than 1.5 cm3 was associated with improvement in 5-year PFS of greater than 20% in patients with M0 disease and an 11% difference for all patients irrespective of age, M stage, or any other measured factors.[3] However, there have also been a number of studies that question a definitive association between the extent of resection and survival. Possibly, the most compelling of these studies was a recent retrospective analysis of 787 patients by Thompson et al. They demonstrated that the benefit of increased extent of resection is largely attenuated after taking into account molecular subtype and not significant when comparing STR (>1.5 cm3) versus NTR (<1.5 cm3) or gross total resection (GTR; no radiographic residual) versus NTR (<1.5 cm3).[43] In addition, aggressive resection of brainstem disease is not indicated owing to the high potential for morbidity incurred with this approach as well as the high sensitivity of the tumor to radiation and chemotherapy. For tumors involving the brainstem, investigators have found no difference in outcome between GTR and residual tumor <1.5 cc.[46]
Hydrocephalus
Following resection, between 10 and 40% of the patients have hydrocephalus requiring CSF diversion.[2,20,33] Riva-Cambrin et al. developed the Canadian Preoperative Prediction Rule for Hydrocephalus (CPPRH) for use in the preoperative prediction of shunt dependence, which can aid in surgical planning and patient counseling.[33] Our patient had a CPPRH score of 7/10 and, thus, a predicted risk of hydrocephalus of 79.9%. It is important to expedite shunt placement and not to delay adjuvant therapies, especially if leptomeningeal spread or metastases have occurred.
Adjuvant therapies
Risk stratification and radiotherapy
Traditionally, children older than 3 years of age are stratified into average and high-risk prognostic groups based on the presence of metastatic disease and post-resection residual less or greater than 1.5 cm3. “High-risk” MB is defined as having any one of the following characteristics: >1.5 cm3 postoperative residual, evidence of radiographic metastases, or presence of leptomeningeal disease/CSF seeding, with the remaining patients defined as “average-risk.”[2] Those less than 3 years of age constitute a unique group in which current standard of care is chemotherapy alone as first-line adjunctive therapy, with radiation therapy eschewed in order to avoid the very poor neurocognitive outcomes associated with typical doses of craniospinal irradiation (CSI) in such young patients.
Under the above scheme, “high-risk” patients undergo posterior fossa or surgical bed radiation (54–55.8 Gy) with high-dose CSI (36.0 Gy) followed by adjuvant chemotherapy, whereas “average-risk” patients undergo posterior fossa or surgical bed radiation (54–55.8 Gy) with reduced-dose CSI (23.4 Gy) followed by adjuvant chemotherapy.[2] However, molecular subgrouping is already being applied to clinical trials of varied adjuvant therapy schemes, and this classic scheme is likely to be modified or supplanted by a scheme reliant on molecular subgroups, as suggested in a recent consensus paper by Ramaswamy et al.[31]
Chemotherapy
Cytotoxic chemotherapy may be used in the initial treatment, maintenance therapy, or for recurrent disease. It may be radiation sparing, which would allow one to eschew the use of radiation in children <3 years of age and permit dose-reduction in older patients. Various regimens exist for initial treatment, with standard therapy being post-radiation cisplatin-based chemotherapy for 4–9 cycles.[42]
Many previous trials investigating chemotherapy and radiation regimens have compared outcomes based on the histologic type. However, the histologic types and molecular subgroups have not been completely congruent. Following the new stratification of MB by molecular subgroup, inroads have been made to tailor therapies to these pathways or predict response to traditional therapy.[47] In cases where tumor characteristics or metastases preclude adequate resection, biopsy followed by targeted chemotherapy may serve as a favorable alternative.[46]
Specifically targeted chemotherapies based on pathways believed to be involved in oncogenesis are a promising future application of molecular subgrouping. At present, molecularly targeted agents for each of the four molecular subgroups are being studied in either clinical and pre-clinical models—the most well-studied of these being smoothened (SMO) receptor antagonists, such as vismodigib, that have demonstrated some utility in both preclinical and clinical models.[9]
Alternative strategies include sensitization of MB tumor cells to chemotherapeutic treatment. For example, thiostrepton, an antagonist of FOXM1 (an oncogene shown to be upregulated in a variety of malignancies), was shown to sensitize MB cells to cisplatin in vitro.[22]
Options for recurrent disease are more limited. A regimen of ifosfamide, cisplatin, and etoposide has been investigated but may be limited by significant attendant toxicities, most notable for profound myelosuppression.[18] An alternative regimen combines bevacizumab and irinotecan with or without temozolomide, with an objective response rate of 55% at 6 months and may be better tolerated.[1] Further studies are required to identify an efficacious regimen with tolerable toxicity for recurrent medulloblastoma, perhaps targeted to molecular subgroup.
Prognosis and outcomes
The overall prognosis of MB is relatively good compared to other high-grade tumors, with a 5-year overall survival of approximately 70%.[38] Prognostic factors include age at diagnosis, post-resection residual, histologic type, presence of metastasis, and molecular subgroup. Positive prognostic markers include DNMB and MBEN histologic types, WNT subgroup tumors, and expression of beta-catenin in the nucleus[11,13] and TrkB.[16,35]
Histological phenotype has classically been very important in prognostication, especially in young children in whom it is highly predictive of outcome. Favorable results can be seen in DNMBs, which account for half of MB cases seen in children ≤3 years old.[46] In another study, DNMB and MBEN accounted for more than half of the cases in patients <3 years old and had a 5-year overall survival of ~53% compared to ~17% for the classic variant of MB.[23] DNMB and MBEN are also unique in that complete remission is a realistic and realizable goal with resection and postoperative chemotherapy.[14,15,34,36] In patients >3 years old, MB histology still retains prognostic significance, with significant differences in outcome for DNMB versus classic versus large cell/anaplastic types.
The WNT subgroup has a very favorable prognosis, with only a 10% probability of metastasis at diagnosis and a 5-year overall survival of 95–100%. SHH MBs carry a slightly greater risk of metastasis than WNT MBs but less than the Groups 3 and 4 subgroups. Prognosis of SHH tumors is inversely correlated with age, with 10-year overall survival of 77%, 51%, and 34% in infants, children, and adults, respectively.[32] The presence of TP53 mutations in SHH tumors, unlike its presence in the WNT subgroup MBs, carries an additional poor prognostic risk.
Non-WNT/SHH (Groups 3 and 4) MBs are more likely to be metastatic than WNT and SHH subgroups, with approximately 30% of the patients having metastasis at diagnosis. Group 4 tumors carry an intermediate prognosis with 5-year progression-free survival of 95%, with negative risk modifiers including MYCN amplification and presence of metastasis.[19,32] A categorically poor prognosis is carried by Group 3 MBs, with a 10-year overall survival of 39% and 50% in infants and children, respectively; these tumors are associated with large cell/anaplastic histology and MYC amplification.[29] These and other distinguishing features are summarized in Table 1.
Table 1.
Summary of characteristics and prognosis of the four recognized subgroups of medulloblastoma
A meta-analysis examining event-free survival/disease-free survival favored the inclusion of chemotherapy in treatment of pediatric MB when omitting, but not when including, disease progression as an event.[24] The authors could not discount a benefit for chemotherapy in the treatment of MB, but also could not make firm positive recommendations for the same. The decision regarding whether or not to include chemotherapy in the treatment of a specific patient and what intensity to use should be individualized based on patient risk factors and tumor characteristics (i.e., molecular subgroup, histology, presence of metastasis).
Posterior fossa syndrome
The posterior fossa syndrome (PFS), also known as cerebellar mutism syndrome, is a complication that occurs in 8–24% of infratentorial brain tumor resections.[12] PFS usually presents between 1 and 2 days postoperatively and is characterized as a triad of cerebellar mutism, ataxia or axial hypotonia, and affective symptoms such as irritability and emotional lability. These children are commonly inconsolable, apathetic, and/or hypokinetic. While the pathophysiology is poorly understood, there are some purported mechanisms, including disruption of the dentate-thalamo-cortical (DTC) pathway, which has been identified on diffusion tensor imaging of patients affected with PFS.[5] Specifically, Avula et al. have shown, across several imaging analyses, possible involvement of the proximal efferent cerebellar pathway (pECP), which interconnects the dentate nucleus, superior cerebellar peduncle, and midbrain tegmentum.[4] The DTC and pECP constitute a sufficiently large area that may account for the anatomic heterogeneity of lesions associated with PFS.
While some studies have suggested vermian injury as the cause of PFS,[30] equivocal data exist for this hypothesis.[45] While potentially associated with a variety of posterior fossa tumors and even hemorrhagic AVMs,[6] MB appears to be associated with the highest risk for development of PFS. While some recent progress has been made regarding the anatomical pathways associated with PFS, a wide variety of partially supported hypotheses still exist regarding the pathophysiology, including postoperative vasospasm, axonal injury, neuronal dysfunction, thermal injury, and postoperative edema.[5]
CONCLUSION
MB is one of the most well-studied and frequently encountered CNS malignancies with a relatively good response to current treatments. However, there remains a subset of patients with poor outcomes despite numerous studies trying to optimize chemotherapy and radiation regimens. The current understanding of MB biology has vastly outpaced breakthroughs in treatment over the past 5–10 years, and application of this knowledge holds promise for continued improvements in outcomes for the future.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Contributor Information
Visish M. Srinivasan, Email: vsriniva@bcm.edu.
Michael G. Z. Ghali, Email: mgzghali@gmail.com.
Robert Y. North, Email: north@bcm.edu.
Zain Boghani, Email: zboghani@houstonmethodist.org.
Daniel Hansen, Email: dxhanse1@texaschildrens.org.
Sandi Lam, Email: sklam@texaschildrens.org.
REFERENCES
- 1.Aguilera D, Mazewski C, Fangusaro J, MacDonald TJ, McNall-Knapp RY, Hayes LL, et al. Response to bevacizumab, irinotecan, and temozolomide in children with relapsed medulloblastoma: A multi-institutional experience. Childs Nerv Syst. 2013;29:589–96. doi: 10.1007/s00381-012-2013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albright AL, Pollack IF, Adelson PD. Principles and practice of pediatric neurosurgery. 3rd ed. New York: Thieme; 2014. [Google Scholar]
- 3.Albright AL, Wisoff JH, Zeltzer PM, Boyett JM, Rorke LB, Stanley P. Effects of medulloblastoma resections on outcome in children: A report from the Children's Cancer Group. Neurosurgery. 1996;38:265–71. doi: 10.1097/00006123-199602000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Avula S, Kumar R, Pizer B, Pettorini B, Abernethy L, Garlick D, et al. Diffusion abnormalities on intraoperative magnetic resonance imaging as an early predictor for the risk of posterior fossa syndrome. Neuro Oncol. 2015;17:614–22. doi: 10.1093/neuonc/nou299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avula S, Mallucci C, Kumar R, Pizer B. Posterior fossa syndrome following brain tumour resection: Review of pathophysiology and a new hypothesis on its pathogenesis. Childs Nerv Syst. 2015;31:1859–67. doi: 10.1007/s00381-015-2797-0. [DOI] [PubMed] [Google Scholar]
- 6.Baillieux H, Weyns F, Paquier P, De Deyn PP, Marien P. Posterior fossa syndrome after a vermian stroke: A new case and review of the literature. Pediatr Neurosurg. 2007;43:386–95. doi: 10.1159/000106388. [DOI] [PubMed] [Google Scholar]
- 7.Brechbiel J, Miller-Moslin K, Adjei AA. Crosstalk between hedgehog and other signaling pathways as a basis for combination therapies in cancer. Cancer Treat Rev. 2014;40:750–9. doi: 10.1016/j.ctrv.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Brugieres L, Pierron G, Chompret A, Paillerets BB, Di Rocco F, Varlet P, et al. Incomplete penetrance of the predisposition to medulloblastoma associated with germ-line SUFU mutations. J Med Genet. 2010;47:142–4. doi: 10.1136/jmg.2009.067751. [DOI] [PubMed] [Google Scholar]
- 9.Coluccia D, Figuereido C, Isik S, Smith C, Rutka JT. Medulloblastoma: Tumor Biology and Relevance to Treatment and Prognosis Paradigm. Curr Neurol Neurosci Rep. 2016;16:43. doi: 10.1007/s11910-016-0644-7. [DOI] [PubMed] [Google Scholar]
- 10.DeSouza RM, Jones BR, Lowis SP, Kurian KM. Pediatric medulloblastoma-Update on molecular classification driving targeted therapies. Front Oncol. 2014;4:176. doi: 10.3389/fonc.2014.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellison DW, Onilude OE, Lindsey JC, Lusher ME, Weston CL, Taylor RE, et al. beta-Catenin status predicts a favorable outcome in childhood medulloblastoma: The United Kingdom Children's Cancer Study Group Brain Tumour Committee. J Clin Oncol. 2005;23:7951–7. doi: 10.1200/JCO.2005.01.5479. [DOI] [PubMed] [Google Scholar]
- 12.Gadgil N, Hansen D, Barry J, Chang R, Lam S. Posterior fossa syndrome in children following tumor resection: Knowledge update. Surg Neurol Int. 2016;7(Suppl 6):S179–83. doi: 10.4103/2152-7806.178572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gajjar A, Chintagumpala M, Ashley D, Kellie S, Kun LE, Merchant TE, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): Long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7:813–20. doi: 10.1016/S1470-2045(06)70867-1. [DOI] [PubMed] [Google Scholar]
- 14.Giangaspero F, Perilongo G, Fondelli MP, Brisigotti M, Carollo C, Burnelli R, et al. Medulloblastoma with extensive nodularity: A variant with favorable prognosis. J Neurosurg. 1999;91:971–7. doi: 10.3171/jns.1999.91.6.0971. [DOI] [PubMed] [Google Scholar]
- 15.Grill J, Sainte-Rose C, Jouvet A, Gentet JC, Lejars O, Frappaz D, et al. Treatment of medulloblastoma with postoperative chemotherapy alone: An SFOP prospective trial in young children. Lancet Oncol. 2005;6:573–80. doi: 10.1016/S1470-2045(05)70252-7. [DOI] [PubMed] [Google Scholar]
- 16.Grotzer MA, Janss AJ, Fung K, Biegel JA, Sutton LN, Rorke LB, et al. TrkC expression predicts good clinical outcome in primitive neuroectodermal brain tumors. J Clin Oncol. 2000;18:1027–35. doi: 10.1200/JCO.2000.18.5.1027. [DOI] [PubMed] [Google Scholar]
- 17.Hamilton SR, Liu B, Parsons RE, Papadopoulos N, Jen J, Powell SM, et al. The molecular basis of Turcot's syndrome. N Engl J Med. 1995;332:839–47. doi: 10.1056/NEJM199503303321302. [DOI] [PubMed] [Google Scholar]
- 18.Kanamori M, Kumabe T, Saito R, Yamashita Y, Sonoda Y, Tominaga T. The safety of combination chemotherapy with ifosfamide, cisplatin, and etoposide (ICE): Single-institution retrospective review of 108 cases. No Shinkei Geka. 2010;38:997–1005. [PubMed] [Google Scholar]
- 19.Kool M, Korshunov A, Remke M, Jones DT, Schlanstein M, Northcott PA, et al. Molecular subgroups of medulloblastoma: An international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol. 2012;123:473–84. doi: 10.1007/s00401-012-0958-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lam S, Reddy GD, Lin Y, Jea A. Management of hydrocephalus in children with posterior fossa tumors. Surg Neurol Int. 2015;6(Suppl 11):S346–8. doi: 10.4103/2152-7806.161413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin CY, Erkek S, Tong Y, Yin L, Federation AJ, Zapatka M, et al. Active medulloblastoma enhancers reveal subgroup-specific cellular origins. Nature. 2016;530:57–62. doi: 10.1038/nature16546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin J, Zheng Y, Chen K, Huang Z, Wu X, Zhang N. Inhibition of FOXM1 by thiostrepton sensitizes medulloblastoma to the effects of chemotherapy. Oncol Rep. 2013;30:1739–44. doi: 10.3892/or.2013.2654. [DOI] [PubMed] [Google Scholar]
- 23.McManamy CS, Pears J, Weston CL, Hanzely Z, Ironside JW, Taylor RE, et al. Nodule formation and desmoplasia in medulloblastomas-defining the nodular/desmoplastic variant and its biological behavior. Brain Pathol. 2007;17:151–64. doi: 10.1111/j.1750-3639.2007.00058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michiels EM, Schouten-Van Meeteren AY, Doz F, Janssens GO, van Dalen EC. Chemotherapy for children with medulloblastoma. Cochrane Database Syst Rev. 2015;1:CD006678. doi: 10.1002/14651858.CD006678.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morrissy AS, Garzia L, Shih DJ, Zuyderduyn S, Huang X, Skowron P, et al. Divergent clonal selection dominates medulloblastoma at recurrence. Nature. 2016;529:351–7. doi: 10.1038/nature16478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Northcott PA, Hielscher T, Dubuc A, Mack S, Shih D, Remke M, et al. Pediatric and adult sonic hedgehog medulloblastomas are clinically and molecularly distinct. Acta Neuropathol. 2011;122:231–40. doi: 10.1007/s00401-011-0846-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Northcott PA, Nakahara Y, Wu X, Feuk L, Ellison DW, Croul S, et al. Multiple recurrent genetic events converge on control of histone lysine methylation in medulloblastoma. Nat Genet. 2009;41:465–72. doi: 10.1038/ng.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pastorino L, Ghiorzo P, Nasti S, Battistuzzi L, Cusano R, Marzocchi C, et al. Identification of a SUFU germline mutation in a family with Gorlin syndrome. Am J Med Genet A. 2009;149A:1539–43. doi: 10.1002/ajmg.a.32944. [DOI] [PubMed] [Google Scholar]
- 29.Pfister S, Remke M, Benner A, Mendrzyk F, Toedt G, Felsberg J, et al. Outcome prediction in pediatric medulloblastoma based on DNA copy-number aberrations of chromosomes 6q and 17q and the MYC and MYCN loci. J Clin Oncol. 2009;27:1627–36. doi: 10.1200/JCO.2008.17.9432. [DOI] [PubMed] [Google Scholar]
- 30.Puget S, Boddaert N, Viguier D, Kieffer V, Bulteau C, Garnett M, et al. Injuries to inferior vermis and dentate nuclei predict poor neurological and neuropsychological outcome in children with malignant posterior fossa tumors. Cancer. 2009;115:1338–47. doi: 10.1002/cncr.24150. [DOI] [PubMed] [Google Scholar]
- 31.Ramaswamy V, Remke M, Bouffet E, Bailey S, Clifford SC, Doz F, et al. Risk stratification of childhood medulloblastoma in the molecular era: The current consensus. Acta Neuropathol. 2016;131:821–31. doi: 10.1007/s00401-016-1569-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramaswamy V, Remke M, Bouffet E, Faria CC, Perreault S, Cho YJ, et al. Recurrence patterns across medulloblastoma subgroups: An integrated clinical and molecular analysis. Lancet Oncol. 2013;14:1200–7. doi: 10.1016/S1470-2045(13)70449-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riva-Cambrin J, Detsky AS, Lamberti-Pasculli M, Sargent MA, Armstrong D, Moineddin R, et al. Predicting postresection hydrocephalus in pediatric patients with posterior fossa tumors. J Neurosurg Pediatr. 2009;3:378–85. doi: 10.3171/2009.1.PEDS08298. [DOI] [PubMed] [Google Scholar]
- 34.Rutkowski S, Bode U, Deinlein F, Ottensmeier H, Warmuth-Metz M, Soerensen N, et al. Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. N Engl J Med. 2005;352:978–86. doi: 10.1056/NEJMoa042176. [DOI] [PubMed] [Google Scholar]
- 35.Rutkowski S, von Bueren A, von Hoff K, Hartmann W, Shalaby T, Deinlein F, et al. Prognostic relevance of clinical and biological risk factors in childhood medulloblastoma: Results of patients treated in the prospective multicenter trial HIT’91. Clin Cancer Res. 2007;13:2651–7. doi: 10.1158/1078-0432.CCR-06-1779. [DOI] [PubMed] [Google Scholar]
- 36.Saito R, Kumabe T, Sonoda Y, Kanamori M, Yamashita Y, Watanabe M, et al. Combination chemotherapy with ifosfamide, cisplatin, and etoposide for medulloblastoma: Single-institute experience and differences in efficacy for subgroups of medulloblastoma. Childs Nerv Syst. 2011;27:1399–406. doi: 10.1007/s00381-011-1485-y. [DOI] [PubMed] [Google Scholar]
- 37.Slade I, Murray A, Hanks S, Kumar A, Walker L, Hargrave D, et al. Heterogeneity of familial medulloblastoma and contribution of germline PTCH1 and SUFU mutations to sporadic medulloblastoma. Fam Cancer. 2011;10:337–42. doi: 10.1007/s10689-010-9411-0. [DOI] [PubMed] [Google Scholar]
- 38.Smoll NR. Relative survival of childhood and adult medulloblastomas and primitive neuroectodermal tumors (PNETs) Cancer. 2012;118:1313–22. doi: 10.1002/cncr.26387. [DOI] [PubMed] [Google Scholar]
- 39.Taylor MD, Liu L, Raffel C, Hui CC, Mainprize TG, Zhang X, et al. Mutations in SUFU predispose to medulloblastoma. Nat Genet. 2002;31:306–10. doi: 10.1038/ng916. [DOI] [PubMed] [Google Scholar]
- 40.Taylor MD, Mainprize TG, Rutka JT. Molecular insight into medulloblastoma and central nervous system primitive neuroectodermal tumor biology from hereditary syndromes: A review. Neurosurgery. 2000;47:888–901. doi: 10.1097/00006123-200010000-00020. [DOI] [PubMed] [Google Scholar]
- 41.Taylor MD, Northcott PA, Korshunov A, Remke M, Cho YJ, Clifford SC, et al. Molecular subgroups of medulloblastoma: The current consensus. Acta Neuropathol. 2012;123:465–72. doi: 10.1007/s00401-011-0922-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor RE, Bailey CC, Robinson K, Weston CL, Ellison D, Ironside J, et al. Results of a randomized study of preradiation chemotherapy versus radiotherapy alone for nonmetastatic medulloblastoma: The International Society of Paediatric Oncology/United Kingdom Children's Cancer Study Group PNET-3 Study. J Clin Oncol. 2003;21:1581–91. doi: 10.1200/JCO.2003.05.116. [DOI] [PubMed] [Google Scholar]
- 43.Thompson EM, Hielscher T, Bouffet E, Remke M, Luu B, Gururangan S, et al. Prognostic value of medulloblastoma extent of resection after accounting for molecular subgroup: A retrospective integrated clinical and molecular analysis. Lancet Oncol. 2016;17:484–95. doi: 10.1016/S1470-2045(15)00581-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang X, Dubuc AM, Ramaswamy V, Mack S, Gendoo DM, Remke M, et al. Medulloblastoma subgroups remain stable across primary and metastatic compartments. Acta Neuropathol. 2015;129:449–57. doi: 10.1007/s00401-015-1389-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wells EM, Khademian ZP, Walsh KS, Vezina G, Sposto R, Keating RF, et al. Postoperative cerebellar mutism syndrome following treatment of medulloblastoma: Neuroradiographic features and origin. J Neurosurg Pediatr. 2010;5:329–34. doi: 10.3171/2009.11.PEDS09131. [DOI] [PubMed] [Google Scholar]
- 46.Wong TT, Liu YL, Ho DM, Chang KP, Liang ML, Chen HH, et al. Factors affecting survival of medulloblastoma in children: The changing concept of management. Childs Nerv Syst. 2015;31:1687–98. doi: 10.1007/s00381-015-2884-2. [DOI] [PubMed] [Google Scholar]
- 47.Zhang ZY, Xu J, Ren Y, Li KK, Ng HK, Mao Y, et al. Medulloblastoma in China: Clinicopathologic analyses of SHH, WNT, and non-SHH/WNT molecular subgroups reveal different therapeutic responses to adjuvant chemotherapy. PLoS One. 2014;9:e99490. doi: 10.1371/journal.pone.0099490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zurawel RH, Chiappa SA, Allen C, Raffel C. Sporadic medulloblastomas contain oncogenic beta-catenin mutations. Cancer Res. 1998;58:896–9. [PubMed] [Google Scholar]


