Abstract
The beneficial effects of oxygen are widely known, but the potentially harmful effects of high oxygenation concentrations in blood and tissues have been less widely discussed. Providing supplementary oxygen can increase oxygen delivery in hypoxaemic patients, thus supporting cell function and metabolism and limiting organ dysfunction, but, in patients who are not hypoxaemic, supplemental oxygen will increase oxygen concentrations into nonphysiological hyperoxaemic ranges and may be associated with harmful effects. Here, we discuss the potentially harmful effects of hyperoxaemia in various groups of critically ill patients, including postcardiac arrest, traumatic brain injury or stroke, and sepsis. In all these groups, there is evidence that hyperoxia can be harmful and that oxygen prescription should be individualized according to repeated assessment of ongoing oxygen requirements.
1. Introduction
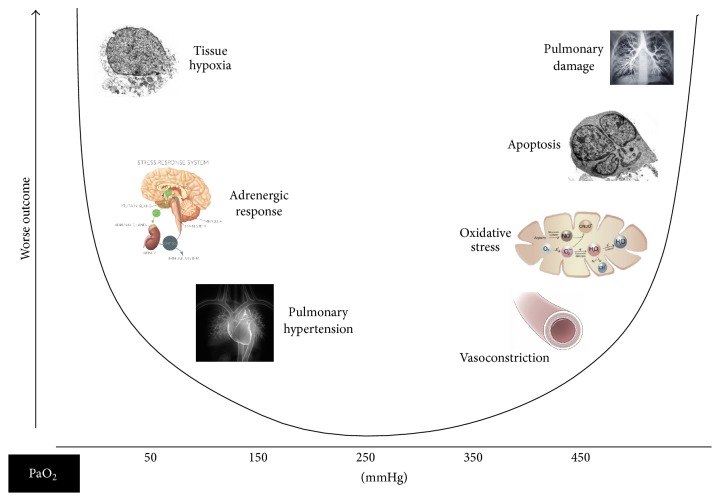
Oxygen is the third most abundant element in the universe and essential for life, but it was only officially “discovered” in the early 1770s separately by the British-born theologian, Joseph Priestly, and the Swedish apothecary, Carl Scheele [1, 2]. It took another few years for its role in respiration to be identified by the French chemist, Antoine-Laurent Lavoisier, who also gave it its name [1, 2]. Introduced into anaesthetic practice in the 1930s, oxygen is now one of the most widely used “drugs” in hospitalized patients. In a point-prevalence study conducted in 40 intensive care units (ICUs) in Australia and New Zealand in 2012, 59% of patients were receiving mechanical ventilation; among those not receiving mechanical ventilation, 86% were receiving oxygen via nasal cannulas, facial masks, or noninvasive ventilation [3]. However, although oxygen therapy clearly has important benefits in many patients, we have become increasingly aware of the potential harmful effects of high oxygenation concentrations in blood and tissues (Figure 1). In a retrospective study comparing mortality rates and PaO2 levels in mechanically ventilated ICU patients, de Jonge et al. reported a U-shaped relationship with increased mortality rates at low and high PaO2 [4]. The potential risks of hyperoxia, with a focus on recent clinical evidence in specific groups of critically ill patients (Table 1), will be the emphasis of this short narrative review.
Figure 1.
Schematic showing U-shaped association of PaO2 with outcome.
Table 1.
Some recent clinical studies on the risks of hyperoxia after cardiac arrest or myocardial infarction, in traumatic brain injury, stroke, sepsis, and mixed ICU patients.
| References | Study design | Hyperoxia measurements | Main finding |
|---|---|---|---|
| After cardiac arrest or myocardial infarction | |||
|
| |||
| Kilgannon et al. 2010 [11]. | Retrospective cohort study, 120 hospitals, 6326 patients (nontraumatic cardiac arrest) | First PaO2 in the first 24 hours. Hyperoxia: PaO2 ≥ 300 mmHg |
Hyperoxia was associated with an increased hospital mortality compared with either hypoxia or normoxia (OR 1.8 [1.5–2.2]) |
|
| |||
| Bellomo et al. 2011 [23] | Retrospective cohort study, 125 ICUs, 12108 patients (nontraumatic cardiac arrest) | Worst PaO2 in first 24 h. Hyperoxia: PaO2 ≥ 300 mmHg Normoxia: PaO2 60–300 mmHg |
Hyperoxia group had a higher hospital mortality than normoxia (OR 1.2 [1.1–1.6]) |
|
| |||
| Kilgannon et al. 2011 [24] | Retrospective cohort study, 120 hospitals, 4459 patients (nontraumatic cardiac arrest) | Highest PaO2 in the first 24 hours | A 100 mmHg increase in PaO2 was associated with a 24% increase in mortality risk (OR 1.24 [1.18 to 1.31]) |
|
| |||
| Ranchord et al. 2012 [25] | Pilot randomized controlled trial, single-centre, 136 patients with STEMI | Patients randomized to receive high-concentration (6 L/min) or titrated oxygen (to achieve oxygen saturation 93%–96%) for 6 hours after presentation | No differences in number of deaths in the two groups (relative risk 0.5, 95% CI 0.05–5.4, p = 0.56) |
|
| |||
| Janz et al. 2012 [26] | Retrospective analysis of a prospective cohort study, single-centre 170 patients (cardiac arrest treated with mild therapeutic hypothermia) | Highest PaO2 in first 24 h. | Increased hospital mortality for every 100 mmHg increase in PaO2 (OR 1.49 [1.03, 2.14]) |
|
| |||
| Lee et al. 2014 [27] | Retrospective cohort study, single-centre, 213 patients (cardiac arrest treated with therapeutic hypothermia) | Average PaO2 between ROSC and the end of rewarming. Hyperoxia: PaO2 > 157 mmHg Normoxia: PaO2 117–135 mmHg |
V-shaped association between PaO2 and poor neurologic outcome at hospital discharge (OR 6.47 [1.68, 24.91]) |
|
| |||
| Stub et al. 2015 [14] | Prospective, randomized, controlled trial, 9 hospitals, 441 patients with STEMI | Patients with an SpO2 > 94% were randomized to receive 8 L/min of oxygen or no supplemental oxygen from arrival of paramedics until transfer to the cardiac care unit | An increased rate of recurrent myocardial infarction, an increase in the frequency of cardiac arrhythmias, and an increase in myocardial infarct size at 6 months on magnetic resonance imaging in the supplement group |
|
| |||
| Elmer et al. 2015 [12] | Retrospective analysis of a high-resolution database, single-centre, 184 patients postcardiac arrest | Mean hourly exposure in first 24 h. Normoxia: PaO2 60–100 mmHg; Moderate hyperoxia: PaO2 101–299 mmHg; Severe hyperoxia: PaO2≥ 300 mmHg |
Severe hyperoxia was associated with decreased survival (OR 0.83 [0.69–0.99] per hour exposure); moderate hyperoxia was not associated with survival but with improved SOFA score 24 h (OR 0.92 [0.87–0.98]) |
|
| |||
| Eastwood et al. 2016 [13] | Retrospective before-after nested cohort study, single-centre, 50 patients postcardiac arrest | Conservative oxygenation: SpO2 88–92% | Conservative group had a shorter ICU length of stay; no difference in the proportion of survivors discharged from hospital with good neurological outcome compared to conventional group |
|
| |||
| In traumatic brain injury (TBI) and stroke | |||
|
| |||
| Davis et al. 2009 [18] | Retrospective cohort study, 5 trauma centres, 3420 moderate-to-severe patients | Extreme hyperoxia: first PaO2 > 487 mmHg | A PaO2 value of 110–487 mmHg was considered optimal. Extreme hyperoxia had an independent association with decreased survival (OR 0.50 [0.36, 0.71]) compared to optimal range |
|
| |||
| Brenner et al. 2012 [19] | Retrospective study, single-centre, 1547 severe TBI patients | Mean PaO2 in first 24 h hospital admission: Hyperoxia: PaO2 > 200 mmHg Normoxia: PaO2 100–200 mmHg |
Both low and high PaO2 had increased mortality. Patients with hyperoxia had higher hospital mortality (OR 1.50 [1.15–1.97]) and lower discharge GCS scores at discharge (OR 1.52 [1.18–1.96]) |
|
| |||
| Raj et al. 2013 [20] | Retrospective nested cohort analysis, 5 hospitals, 1116 ventilated moderate-to-severe TBI patients | Worst PaO2 in first 24 h ICU admission: Hyperoxia: PaO2 > 100 mmHg Normoxia: PaO2 75–100 mmHg |
Hyperoxia had no independent relationship with in-hospital mortality (OR 0.94 [0.65–1.36]) and 6-month mortality (OR 0.88 [0.63–1.22]) |
|
| |||
| Rincon et al. 2014 [28] | Retrospective cohort, 84 ICUs, 2894 stroke patients | PaO2 in the first 24 hours. Hyperoxia: PaO2 ≥ 300 mmHg Normoxia: PaO2 60–300 mmHg |
Hyperoxia was independently associated with in-hospital mortality (OR 1.22 [1.04–1.48]) |
|
| |||
| Rincon et al. 2014 [29] | Retrospective cohort study, 61 hospitals, 1212 ventilated TBI patients | Hyperoxia: PaO2 > 300 mmHg Normoxia: PaO2 60–300 mmHg |
Hyperoxia was associated with a higher in-hosptial case fatality (OR 1.5 [1.02–2.4]) |
|
| |||
| Jeon et al. 2014 [30] | Prospective, observational cohort database analysis, single-centre, 252 patients (subarachnoid haemorrhage) | PaO2 AUC by observation time until delayed cerebral ischemia (DCI). Hyperoxia: PaO2 ≥ 173 mmHg (upper quartile) | Hyperoxia group had a higher incidence of DCI (OR 3.16 [1.69 to 5.92]) and poor outcome (modified Rankin Scale 4–6 at 3 months after subarachnoid haemorrhage) (OR 2.30 [1.03 to 5.12]) |
|
| |||
| Quintard et al. 2015 [17] | Retrospective analysis of a database, single-centre, 36 severe TBI patients | Hyperoxia: PaO2 > 150 mmHg | Hyperoxia was associated with increased cerebral microdialysis glutamate, indicating cerebral excitotoxicity |
|
| |||
| Lang et al. 2016 [31] | Retrospective analysis using 2 databases, 432 ventilated patients (subarachnoid haemorrhage) | Time-weighted average PaO2 during the first 24 hours Low PaO2 < 97.5 mmHg; Intermediate PaO2 97.5–150 mmHg; High PaO2 >150 mmHg |
Patients with an unfavorable outcome had significantly higher PaO2, but high PaO2 has no effect on 3-month neurological outcomes (OR 1.09 [0.61–1.97]) or mortality (OR 0.73 [0.38–1.40]) |
|
| |||
| In sepsis | |||
|
| |||
| Stolmeijer et al. 2014 [32] | Prospective pilot study, 83 sepsis patients in emergency department, single-centre | PaO2 after 5 min of a VentiMask 40% with 10 L O2/min. Hyperoxia: PaO2 > 100 mmHg |
Of the hyperoxic patients, 8% died in hospital versus 6% with normoxia |
|
| |||
| In mixed ICU patients | |||
|
| |||
| de Jonge et al. 2008 [4] | Retrospective observational study, 50 ICUs, 36307 ventilated patients | Worst PaO2 in first 24 h. Hyperoxia: PaO2 ≥ 123 mmHg (upper quintile) compared with PaO2 between 67 and 80 mmHg |
In-hospital mortality was linearly related to FiO2 value and had a U-shaped relationship with PaO2. Hyperoxia had a higher mortality (OR 1.23 [1.13–1.34]) |
|
| |||
| Panwar et al. 2016 [33] | Pilot randomized controlled trial, 4 ICUs, 103 patients | Conservative oxygenation: SpO2 88–92% Liberal oxygenation: SpO2 ≥ 96% |
No significant differences in measures of new organ dysfunction, or ICU or 90-day mortality (OR 0.77 [0.40–1.50]) |
|
| |||
| Girardis et al. 2016 [34] | Open-label randomized trial, single-centre, 434 patients | Conservative oxygenation: PaO2 70–100 mmHg (SpO2 94–98%) Conventional oxygenation: PaO2 > 150 mmHg (SpO2 97–100%) |
Patients in the conservative group had lower ICU mortality (RR 0.57 [0.37–0.9]) and fewer episodes of shock, liver failure, and bacteraemia |
|
| |||
| Helmerhorst et al. 2017 [35] | Observational cohort study, 3 ICUs, 14441 ventilated patients | First PaO2 at ICU admission Mild hyperoxia: PaO2 120–200 mmHg Severe hyperoxia: PaO2 > 200 mmHg |
Severe hyperoxia was associated with higher mortality rates and fewer ventilator-free days in comparison to both mild hyperoxia and normoxia Time spent in hyperoxia had a linear and positive relationship with hospital mortality |
OR: odds ratio; SOFA: sequential organ failure assessment; ROSC: return of spontaneous circulation; DIC: delayed cerebral ischemia; AUC: area under the curve; STEMI: ST-segment elevated myocardial infarction.
2. Effects of Hyperoxia
Adequate cellular oxygenation is essential for normal cell function, and a low SaO2 is life-threatening, especially in acute conditions. Providing supplementary oxygen will increase oxygen delivery in hypoxaemic patients, thus supporting cell function and metabolism and limiting organ dysfunction. However, in patients who are not hypoxaemic, supplemental oxygen will increase oxygen concentrations into hyperoxaemic ranges. Although human beings may be exposed to hypoxia, for example, when at altitude or as a result of pulmonary disease, we are never exposed to hyperoxia, so that supplying extra oxygen to individuals who are not hypoxaemic is always a “nonphysiological” event.
Hyperoxia is associated with multiple effects in different organ systems. It can directly damage tissues via the production of reactive oxygen species (ROS) in excess of physiological antioxidant defence capabilities [5], leading to increased cell death by apoptosis and increased release of endogenous damage-associated molecular pattern molecules (DAMPs) that stimulate an inflammatory response, notably in the lungs [6] and vasoconstriction, likely as a result of reduced nitric oxide levels [7]. Orbegozo Cortés et al. recently reported that normobaric hyperoxia in healthy volunteers was associated with reduced capillary perfusion as assessed using sublingual side-stream dark field (SDF) video-microscopy [8]. It has been suggested that these vasoconstrictive effects may provide a means of protecting cells from the harmful effects of high PaO2 [9].
2.1. After Cardiac Arrest or Myocardial Infarction
Given the associated vasoconstriction and increased ROS release, hyperoxia may be particularly harmful after cardiac arrest [10]. Experimental and observational data have given conflicting results regarding the effects of hyperoxia in this setting [10]. In a retrospective analysis of data from 6326 postcardiac arrest patients admitted to ICUs in 120 US hospitals between 2001 and 2005, patients with hyperoxia (defined as PaO2 of ≥300 mmHg) on arrival in the ICU had higher mortality rates than those with normoxia or hypoxia; in multivariable analysis, hyperoxia exposure was an independent predictor of in-hospital death (odds ratio 1.8 [95% CI 1.5–2.2], p < 0.001) [11]. In an analysis of a registry database, severe hyperoxia (as identified by a PaO2 > 300 mmHg) was associated with increased mortality in postcardiac arrest patients, whereas moderate or “probable” hyperoxia (PaO2 101–299 mmHg) was not [12]. In a retrospective cohort study, patients managed according to a conservative oxygen approach after cardiac arrest, targeting an pulse oximetry oxygen saturation (SpO2) of 88–92% had shorter lengths of ICU stay, although there were no differences in neurological outcomes [13]. In a multicentre trial conducted in 441 patients with ST-elevation myocardial infarction, patients with an SpO2 > 94% were randomized to receive 8 L/min of oxygen or no supplemental oxygen from arrival of paramedics until transfer to the cardiac care unit. Patients treated with oxygen had an increased rate of recurrent myocardial infarction, an increase in the frequency of cardiac arrhythmias, and an increase in myocardial infarct size at 6 months on magnetic resonance imaging [14]. These results do not support the use of routine supplemental oxygen after cardiac arrest or myocardial infarction. A randomized multicentre study is ongoing in Sweden aiming to randomize 6,600 patients with suspected acute myocardial infarction and SpO2 ≥ 90% to either 6 L/min of supplemental oxygen for 6 to 12 hours or room air [15].
2.2. In Traumatic Brain Injury and Stroke
Reduced cerebral oxygenation after brain injury is associated with impaired mitochondrial function and reduced metabolic rate and may be associated with an increased risk of secondary brain damage [16, 17]. Treating such patients with hyperoxia may, therefore, be expected to have beneficial effects on outcomes. However, clinical studies have given conflicting results. In a retrospective study of more than 3000 patients with traumatic brain injury (TBI), hypoxaemia, and extreme hyperoxaemia (PaO2 > 487 mmHg) on admission were both associated with worse outcomes; a PaO2 value of 110–487 mmHg was considered optimal in this study [18]. Similar findings were reported by a more recent retrospective study in 1547 patients with TBI, with both low and high admission PaO2 levels independently associated with worse outcomes [19]. In a long-term outcomes study after TBI, although there was a significant association between hyperoxaemia and a decreased risk of 6-month mortality in univariate analysis, in multivariable analysis, hyperoxaemia was not independently associated with outcome [20]. In a small randomized trial, 68 patients with severe TBI received either 80% or 50% oxygen via mechanical ventilation in the first 6 hours after the TBI. Patients in the hyperoxia group had better outcomes at 6 months as assessed using the Glasgow Outcome Scale than patients in the normoxia group [21]. A planned larger study to compare treatment with an FiO2 of 0.4 or 0.7 in patients with TBI was terminated because of slow recruitment (NCT01201291). Interestingly, in a prospective study of 30 patients monitored with a brain tissue oxygen sensor, Vilalta et al. reported that a hyperoxia challenge was associated with improved cerebral metabolism only in patients with reduced metabolism at baseline [22].
Evidence from studies in patients with stroke is also conflicting. Lang et al. reported no effect on 3-month neurological outcomes or mortality of moderate hyperoxaemia during the first 24 hours after ICU admission in patients after subarachnoid haemorrhage [31], and Young et al. similarly reported no association between worst PaO2 in the first 24 hours after ICU admission and mortality in patients with acute ischaemic stroke [36]. However, other observational studies have reported detrimental effects on short and longer term outcomes in different groups of stroke patients [28, 30]. A study randomizing patients to room air or supplemental oxygen administered at 30–45 L/min for 8 hours was terminated early because of more deaths in the oxygen group (NCT00414726). In a pilot study comparing oxygen supplementation for 72 h via nasal cannulae with room air in 289 patients with acute stroke, there was a small improvement in neurological recovery at one week [37], but there were no significant differences between the groups at 6 months [38], findings supported by the larger Stroke Oxygen Study in more than 8000 patients [39].
2.3. In Sepsis and Septic Shock
The use of hyperoxia in patients with sepsis is also controversial [40]. Sepsis is already associated with increased formation of ROS, believed to play a role in the tissue damage and organ dysfunction seen during sepsis. Hyperoxaemia is known to stimulate release of ROS and could therefore further worsen organ function in these patients. In a rat caecal ligation and puncture model, hyperoxia was associated with increased inflammatory cytokine release and organ dysfunction compared to normoxia [41]. However, in other animal models of sepsis, hyperoxia has been associated with improved haemodynamics and anti-inflammatory effects [42, 43]. And in a sheep model of sepsis, we showed that hyperoxia was associated with better haemodynamics and organ function compared to normoxia (unpublished data). In experimental human endotoxaemia, hyperoxia had no effect on levels of inflammatory mediators [44], and in a small observational study of patients with suspected sepsis in the emergency department, there were no significant differences in mortality rates between hyperoxic and normoxic patients [32]. A clinical trial in patients with sepsis randomized to hyperoxia or normoxia and hypertonic or isotonic saline in a 2 × 2 factorial design was stopped prematurely because of increased mortality rates in the hyperoxia and hypertonic saline arms (NCT01722422). Two randomized studies, one comparing supplemental oxygen titrated to different PaO2 targets (105–135 mmHg versus 60–90 mmHg, O2-ICU study, NCT02321072) and one comparing supplemental oxygen at 15 L/min to no supplemental oxygen (NCT02378545), are currently ongoing and should provide final answers as to whether or not patients with sepsis may benefit from hyperoxia.
2.4. In Mixed ICU Patients
Use of liberal oxygen therapy is frequent in critically ill patients [45] and severe hyperoxaemia (PaO2 > 200 mmHg) is associated with higher mortality rates [35]. Interestingly, in three ICUs in the Netherlands, more than 70% of ICU patients had PaO2 levels that were higher than the upper limits identified by the ICU clinicians treating them [46]. Several studies have now compared so-called conservative oxygen strategies targeting lower PaO2 or SpO2 values with conventional oxygen administration. Panwar et al. compared target SpO2 values of 88–92% and ≥96% in 103 ICU patients and reported no significant differences between the groups in terms of organ function or ICU and 90-day mortality [33]. In the Oxygen-ICU study, which was terminated early, 434 patients were randomized to receive supplemental oxygen to maintain PaO2 at 70–100 mmHg (SpO2 94–98%) or to be managed conventionally allowing PaO2 to reach 150 mmHg (SpO2 97–100%) [34]. Patients in the conservative group had lower ICU mortality than those in the conventional group (relative risk 0.57 [95% CI 0.37–0.9]; p = 0.01).
3. Conclusions
For many years, the known risks of hypoxia and less known adverse effects of hyperoxia have led to many patients receiving liberal oxygenation to avoid hypoxaemia at all costs. Although good quality data remain limited, results from the latest clinical studies seem to suggest that hyperoxaemia may be associated with worse outcomes in some critically ill patients (Table 1). The trend is therefore moving towards a more conservative approach to oxygenation aimed at maintaining SpO2 targets at 95–97%, although the optimal PaO2 level has not yet been defined and will likely change during the course of a patient's illness. Indeed, there may be a time window during which patients may benefit from higher oxygen levels [47]. Further well-designed randomized controlled trials in carefully selected groups of patients may help provide some definitive answers to these questions. As with other areas of intensive care management, oxygen therapy should be individualized. Patients who are hypoxaemic clearly need to receive supplemental oxygen, but ongoing requirements need to be reassessed on a regular basis to limit any risks associated with hyperoxia.
Competing Interests
The authors declare that there are no competing interests regarding the publication of this paper.
References
- 1.Heffner J. E. The story of oxygen. Respiratory Care. 2013;58(1):18–31. doi: 10.4187/respcare.01831. [DOI] [PubMed] [Google Scholar]
- 2.Severinghaus J. W. Priestley, the furious free thinker of the enlightenment, and Scheele, the taciturn apothecary of Uppsala. Acta Anaesthesiologica Scandinavica. 2002;46(1):2–9. doi: 10.1046/j.0001-5172.2001.00351.x. [DOI] [PubMed] [Google Scholar]
- 3.Parke R. L., Eastwood G. M., McGuinness S. P. Oxygen therapy in non-intubated adult intensive care patients: a point prevalence study. Critical Care and Resuscitation. 2013;15(4):287–293. [PubMed] [Google Scholar]
- 4.de Jonge E., Peelen L., Keijzers P. J., et al. Association between administered oxygen, arterial partial oxygen pressure and mortality in mechanically ventilated intensive care unit patients. Critical Care. 2008;12(6, article R156) doi: 10.1186/cc7150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brueckl C., Kaestle S., Kerem A., et al. Hyperoxia-induced reactive oxygen species formation in pulmonary capillary endothelial cells in situ. American Journal of Respiratory Cell and Molecular Biology. 2006;34(4):453–463. doi: 10.1165/rcmb.2005-0223OC. [DOI] [PubMed] [Google Scholar]
- 6.Zaher T. E., Miller E. J., Morrow D. M. P., Javdan M., Mantell L. L. Hyperoxia-induced signal transduction pathways in pulmonary epithelial cells. Free Radical Biology and Medicine. 2007;42(7):897–908. doi: 10.1016/j.freeradbiomed.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sjöberg F., Singer M. The medical use of oxygen: a time for critical reappraisal. Journal of Internal Medicine. 2013;274(6):505–528. doi: 10.1111/joim.12139. [DOI] [PubMed] [Google Scholar]
- 8.Orbegozo Cortés D., Puflea F., Donadello K., et al. Normobaric hyperoxia alters the microcirculation in healthy volunteers. Microvascular Research. 2015;98:23–28. doi: 10.1016/j.mvr.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Calzia E., Asfar P., Hauser B., et al. Hyperoxia may be beneficial. Critical Care Medicine. 2010;38(10):S559–S568. doi: 10.1097/CCM.0b013e3181f1fe70. [DOI] [PubMed] [Google Scholar]
- 10.Dell'Anna A. M., Lamanna I., Vincent J.-L., Taccone F. S. How much oxygen in adult cardiac arrest? Critical Care. 2014;18(5, article 555) doi: 10.1186/s13054-014-0555-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kilgannon J. H., Jones A. E., Shapiro N. I., et al. Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. JAMA—Journal of the American Medical Association. 2010;303(21):2165–2171. doi: 10.1001/jama.2010.707. [DOI] [PubMed] [Google Scholar]
- 12.Elmer J., Scutella M., Pullalarevu R., et al. The association between hyperoxia and patient outcomes after cardiac arrest: analysis of a high-resolution database. Intensive Care Medicine. 2015;41(1):49–57. doi: 10.1007/s00134-014-3555-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eastwood G. M., Tanaka A., Espinoza E. D. V., et al. Conservative oxygen therapy in mechanically ventilated patients following cardiac arrest: a retrospective nested cohort study. Resuscitation. 2016;101:108–114. doi: 10.1016/j.resuscitation.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 14.Stub D., Smith K., Bernard S., et al. Air versus oxygen in ST-segment-elevation myocardial infarction. Circulation. 2015;131(24):2143–2150. doi: 10.1161/CIRCULATIONAHA.114.014494. [DOI] [PubMed] [Google Scholar]
- 15.Hofmann R., James S. K., Svensson L., et al. DETermination of the role of OXygen in suspected Acute Myocardial Infarction trial. American Heart Journal. 2014;167(3):322–328. doi: 10.1016/j.ahj.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 16.Xu F., Liu P., Pascual J. M., Xiao G., Lu H. Effect of hypoxia and hyperoxia on cerebral blood flow, blood oxygenation, and oxidative metabolism. Journal of Cerebral Blood Flow and Metabolism. 2012;32(10):1909–1918. doi: 10.1038/jcbfm.2012.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quintard H., Patet C., Suys T., Marques-Vidal P., Oddo M. Normobaric hyperoxia is associated with increased cerebral excitotoxicity after severe traumatic brain injury. Neurocritical Care. 2015;22(2):243–250. doi: 10.1007/s12028-014-0062-0. [DOI] [PubMed] [Google Scholar]
- 18.Davis D. P., Meade W., Jr., Sise M. J., et al. Both hypoxemia and extreme hyperoxemia may be detrimental in patients with severe traumatic brain injury. Journal of Neurotrauma. 2009;26(12):2217–2223. doi: 10.1089/neu.2009.0940. [DOI] [PubMed] [Google Scholar]
- 19.Brenner M., Stein D., Hu P., Kufera J., Wooford M., Scalea T. Association between early hyperoxia and worse outcomes after traumatic brain injury. Archives of Surgery. 2012;147(11):1042–1046. doi: 10.1001/archsurg.2012.1560. [DOI] [PubMed] [Google Scholar]
- 20.Raj R., Bendel S., Reinikainen M., et al. Hyperoxemia and long-term outcome after traumatic brain injury. Critical Care. 2013;17(4, article no. R177) doi: 10.1186/cc12856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taher A., Pilehvari Z., Poorolajal J., Aghajanloo M. Effects of normobaric hyperoxia in traumatic brain injury: a randomized controlled clinical trial. Trauma Monthly. 2016;21(1) doi: 10.5812/traumamon.26772.e26772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vilalta A., Sahuquillo J., Merino M.-A., et al. Normobaric hyperoxia in traumatic brain injury: does brain metabolic state influence the response to hyperoxic challenge? Journal of Neurotrauma. 2011;28(7):1139–1148. doi: 10.1089/neu.2010.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bellomo R., Bailey M., Eastwood G. M., et al. Arterial hyperoxia and in-hospital mortality after resuscitation from cardiac arrest. Critical Care. 2011;15(2, article no. R90) doi: 10.1186/cc10090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kilgannon J. H., Jones A. E., Parrillo J. E., et al. Relationship between supranormal oxygen tension and outcome after resuscitation from cardiac arrest. Circulation. 2011;123(23):2717–2722. doi: 10.1161/CIRCULATIONAHA.110.001016. [DOI] [PubMed] [Google Scholar]
- 25.Ranchord A. M., Argyle R., Beynon R., et al. High-concentration versus titrated oxygen therapy in ST-elevation myocardial infarction: a pilot randomized controlled trial. American Heart Journal. 2012;163(2):168–175. doi: 10.1016/j.ahj.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 26.Janz D. R., Hollenbeck R. D., Pollock J. S., McPherson J. A., Rice T. W. Hyperoxia is associated with increased mortality in patients treated with mild therapeutic hypothermia after sudden cardiac arrest. Critical Care Medicine. 2012;40(12):3135–3139. doi: 10.1097/CCM.0b013e3182656976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee B. K., Jeung K. W., Lee H. Y., et al. Association between mean arterial blood gas tension and outcome in cardiac arrest patients treated with therapeutic hypothermia. American Journal of Emergency Medicine. 2014;32(1):55–60. doi: 10.1016/j.ajem.2013.09.044. [DOI] [PubMed] [Google Scholar]
- 28.Rincon F., Kang J., Maltenfort M., et al. Association between hyperoxia and mortality after stroke: a multicenter cohort study. Critical Care Medicine. 2014;42(2):387–396. doi: 10.1097/ccm.0b013e3182a27732. [DOI] [PubMed] [Google Scholar]
- 29.Rincon F., Kang J., Vibbert M., Urtecho J., Athar M. K., Jallo J. Significance of arterial hyperoxia and relationship with case fatality in traumatic brain injury: a multicentre cohort study. Journal of Neurology, Neurosurgery and Psychiatry. 2014;85(7):799–805. doi: 10.1136/jnnp-2013-305505. [DOI] [PubMed] [Google Scholar]
- 30.Jeon S.-B., Choi H. A., Badjatia N., et al. Hyperoxia may be related to delayed cerebral ischemia and poor outcome after subarachnoid haemorrhage. Journal of Neurology, Neurosurgery and Psychiatry. 2014;85:1301–1307. doi: 10.1136/jnnp-2013-307314. [DOI] [PubMed] [Google Scholar]
- 31.Lang M., Raj R., Skrifvars M. B., et al. Early moderate hyperoxemia does not predict outcome after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2016;78(4):540–545. doi: 10.1227/NEU.0000000000001111. [DOI] [PubMed] [Google Scholar]
- 32.Stolmeijer R., Ter Maaten J. C., Zijlstra J. G., Ligtenberg J. J. M. Oxygen therapy for sepsis patients in the emergency department: a little less? European Journal of Emergency Medicine. 2014;21(3):233–235. doi: 10.1097/mej.0b013e328361c6c7. [DOI] [PubMed] [Google Scholar]
- 33.Panwar R., Hardie M., Bellomo R., et al. Conservative versus liberal oxygenation targets for mechanically ventilated patients. A pilot multicenter randomized controlled trial. American Journal of Respiratory and Critical Care Medicine. 2016;193(1):43–51. doi: 10.1164/rccm.201505-1019oc. [DOI] [PubMed] [Google Scholar]
- 34.Girardis M., Busani S., Damiani E., et al. Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit: the Oxygen-ICU randomized clinical trial. The Journal of the American Medical Association. 2016;316(15):1583–1589. doi: 10.1001/jama.2016.11993. [DOI] [PubMed] [Google Scholar]
- 35.Helmerhorst H. J., Arts D. L., Schultz M. J., et al. Metrics of arterial hyperoxia and associated outcomes in critical care. Critical Care Medicine. 2017;45(2):187–195. doi: 10.1097/ccm.0000000000002084. [DOI] [PubMed] [Google Scholar]
- 36.Young P., Beasley R., Bailey M., et al. The association between early arterial oxygenation and mortality in ventilated patients with acute ischaemic stroke. Critical Care and Resuscitation. 2012;14(1):14–19. [PubMed] [Google Scholar]
- 37.Roffe C., Ali K., Warusevitane A., et al. The SOS pilot study: a RCT of routine oxygen supplementation early after acute stroke—effect on recovery of neurological function at one week. PLoS ONE. 2011;6(5) doi: 10.1371/journal.pone.0019113.e19113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ali K., Warusevitane A., Lally F., et al. The stroke oxygen pilot study: a randomized controlled trial of the effects of routine oxygen supplementation early after acute stroke—effect on key outcomes at six months. PLoS ONE. 2013;8(6) doi: 10.1371/journal.pone.0059274.e59274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hafner S., Beloncle F., Koch A., Radermacher P., Asfar P. Hyperoxia in intensive care, emergency, and peri-operative medicine: Dr. Jekyll or Mr. Hyde? A 2015 update. Annals of Intensive Care. 2015;5(1, article 42):1–14. doi: 10.1186/s13613-015-0084-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Asfar P., Calzia E., Huber-Lang M., Ignatius A., Radermacher P. Hyperoxia during septic Shock—Dr. Jekyll or Mr. Hyde? Shock. 2012;37(1):122–123. doi: 10.1097/shk.0b013e318238c991. [DOI] [PubMed] [Google Scholar]
- 41.Rodríguez-González R., Martín-Barrasa J. L., Ramos-Nuez Á., et al. Multiple system organ response induced by Hyperoxia in a clinically relevant animal model of sepsis. Shock. 2014;42(2):148–153. doi: 10.1097/SHK.0000000000000189. [DOI] [PubMed] [Google Scholar]
- 42.Hauser B., Barth E., Bassi G., et al. Hemodynamic, metabolic, and organ function effects of pure oxygen ventilation during established fecal peritonitis-induced septic shock. Critical Care Medicine. 2009;37(8):2465–2469. doi: 10.1097/ccm.0b013e3181aee8ad. [DOI] [PubMed] [Google Scholar]
- 43.Waisman D., Brod V., Rahat M. A., et al. Dose-related effects of hyperoxia on the lung inflammatory response in septic rats. Shock. 2012;37(1):95–102. doi: 10.1097/SHK.0b013e3182356fc3. [DOI] [PubMed] [Google Scholar]
- 44.Kiers D., Gerretsen J., Janssen E., et al. Short-term hyperoxia does not exert immunologic effects during experimental murine and human endotoxemia. Scientific Reports. 2015;5, article 17441 doi: 10.1038/srep17441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suzuki S., Eastwood G. M., Peck L., Glassford N. J., Bellomo R. Current oxygen management in mechanically ventilated patients: a prospective observational cohort study. Journal of Critical Care. 2013;28(5):647–654. doi: 10.1016/j.jcrc.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 46.Helmerhorst H. J., Schultz M. J., van der Voort P. H., et al. Self-reported attitudes versus actual practice of oxygen therapy by ICU physicians and nurses. Annals of Intensive Care. 2014;4, article 23 doi: 10.1186/s13613-014-0023-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ridler N., Plumb J., Grocott M. Oxygen therapy in critical illness: friend or foe? A review of oxygen therapy in selected acute illnesses. Journal of the Intensive Care Society. 2014;15(3):190–198. doi: 10.1177/175114371401500303. [DOI] [Google Scholar]