Abstract
BACKGROUND: Cancer stem cells (CSCs) are considered a pivotal target for the eradication of hepatocellular carcinoma (HCC). Recently, we reported that the CSC markers epithelial cell adhesion molecule (EpCAM) and CD90 are expressed independently in primary HCCs and cell lines, and CD90+ cells share features of metastatic vascular endothelial cells and express the vascular endothelial marker CD105, a co-receptor of transforming growth factor-beta. METHODS: The EpCAM+ cell lines HuH1 and HuH7 were treated with 5-fluorouracil (5-FU) or epirubicin in vitro. Gene and protein expression levels were evaluated by quantitative reverse transcription-polymerase chain reaction (qRT-PCR) and fluorescence-activated cell sorting, respectively. The expression of CD105 in primary HCC was evaluated by immunohistochemistry. The relationship of CD105 expression status and HCC prognosis was evaluated using 85 surgically resected HCC tissues by Kaplan–Meier survival analysis. RESULTS: 5-FU or epirubicin treatment resulted in the generation of CD90+ and CD105+ cells in vitro in HuH1 and HuH7 cells, which originally contain no CD90+ or CD105+ cells. This phenomenon was validated by qRT-PCR analysis with activation of the epithelial-mesenchymal transition (EMT) program regulators Snail family zinc finger 1 (SNAI1) and SNAI2. Immunohistochemical analysis indicated that CD105+ cells were morphologically identical to vascular endothelial cells in untreated primary HCCs. However, surgically resected specimens after transcatheter arterial chemoembolization clearly indicated that CD105+ cancer cells survived at the peripheral edge of the tumor. Kaplan–Meier survival analysis indicated that HCCs expressing CD105 showed poor prognosis after surgery with statistical significance. CONCLUSIONS: Taken together, our data highlight the role of CD105+ HCC cells with activation of the EMT program generated de novo after cytotoxic therapy on the prognosis of HCC patients.
Introduction
Hepatocellular carcinoma (HCC) is one of the most common causes of cancer death worldwide [1], partially due to the lack of effective chemotherapeutic options for patients with advanced-stage disease [2]. Various molecular profiling approaches have been applied to identify therapeutic targets specifically activated in HCC [3]. Cancer stem cells (CSCs) are considered a pivotal target for the eradication of HCC [4]. Some studies have suggested the importance of evaluating stemness in HCC because it reflects the malignant nature of the tumor closely and is related to poor prognosis after surgery [5], [6], [7], [8]. In HCC, several stem cell markers including CD133, CD90, CD13, epithelial cell adhesion molecule (EpCAM), CD24, and side populations are reportedly enriched in CSC populations [9].
Recently, we reported that the CSC markers EpCAM and CD90 are expressed independently in primary HCCs and cell lines [10], and CD90+ cells share features of metastatic vascular endothelial cells and express the vascular endothelial marker CD105, a co-receptor of transforming growth factor (TGF)-β [11]. Our previous data suggested that CD105 is not only a vascular endothelial cell marker but also a marker of CSCs with mesenchymal cell features, but the significance of CD105 expression on HCC phenotypes remains to be elucidated. In this study, we evaluated the expression of CD105 in human HCC and found that CD105+ HCC cells could be generated from CD105− HCC cells de novo after treatment with cytotoxic reagents with activation of the expression of the epithelial-mesenchymal transition (EMT) inducers Snail family zinc finger 1 (SNAI1) and SNAI2.
Materials and Methods
Patients
HCC samples were obtained with informed consent from patients who had undergone radical resection at the Department of Gastroenterologic Surgery in Kanazawa University Hospital, Kanazawa, Japan, and tissue acquisition procedures were approved by the Ethics Committee of Kanazawa University. A total of 85 formalin-fixed and paraffin-embedded HCC samples obtained from 2002 to 2008 were used for immunohistochemical analyses.
Cell Culture and Reagents
The human liver cancer cell lines HuH1 and HuH7 were obtained from the Japanese Collection of Research Bioresources (Osaka, Japan) and routinely cultured with Dulbecco's modified Eagle's medium supplemented with 10% FBS. Epirubicin and 5-fluorouracil (5-FU) were obtained from Wako Pure Chemical Industries, Ltd. (Tokyo, Japan) and Kyowa Kirin (Tokyo, Japan), respectively. HuH1 and HuH7 cell lines were seeded at 10,000 cells per well in a 6-well plate treated with epirubicin (0.5 μg/ml for HuH1 and 0.1 μg/ml for HuH7) or 5-FU (2.0 μg/ml for HuH1 and 2.5 μg/ml for HuH7) for 5 days based on IC50 data obtained from a cell proliferation assay. These cells were then used for quantitative reverse transcription-polymerase chain reaction (qRT-PCR), fluorescence-activated cell sorting (FACS), and immunofluorescence analyses, as described previously [12].
qRT-PCR
Total RNA was extracted using TRIzol (Thermo Fisher Scientific K.K., Yokohama, Japan) according to the manufacturer's instructions. The expression of selected genes was determined in triplicate using the 7900 Sequence Detection System (Applied Biosystems, Foster City, CA). Each sample was normalized relative to 18S rRNA expression. The following probes were used: ENG, Hs00923996_m1; KRT19, Hs00761767_s1; THY1, Hs00264235_21; and 18S rRNA, Hs99999901_s1 (Applied Biosystems).
FACS
Cultured cells were trypsinized, washed, and resuspended in Hank's balanced salt solution (Lonza, Basel, Switzerland) supplemented with 1% HEPES and 2% FBS. The cells were then incubated with antibodies on ice for 30 minutes. Labeled cells were analyzed by FACS using a FACSCalibur (BD Biosciences, San Jose, CA). The following antibodies were used: FITC-conjugated anti-EpCAM monoclonal antibody Clone Ber-EP4 (DAKO, Carpinteria, CA), APC-conjugated anti-CD326 (EpCAM) antibody (Miltenyi Biotec K.K., Tokyo, Japan), FITC-conjugated anti-CD90 monoclonal antibody Clone 5E10 (STEMCELL Technologies, Seattle, WA), APC-conjugated anti-CD133/2 antibody Clone 293C3 (Miltenyi Biotec K.K.), and APC-conjugated anti-CD105 mouse monoclonal antibody (BD Biosciences).
Immunohistochemistry and Immunofluorescence
Immunohistochemistry was performed using Envision+ kits (DAKO, Carpinteria, CA) according to the manufacturer's instructions. Anti-CD105 rabbit monoclonal antibody EPR10145 (Abcam, Cambridge, UK) was used for detecting CD105. CD105 expression was measured and categorized into two groups, CD105+ and CD105−, according to the expression status not in vascular endothelial cells but in cancer cells. Immunofluorescence was performed using anti-CD105 mouse monoclonal antibody clone SN6h (DAKO) and Alexa 488 FITC-conjugated anti-mouse IgG as primary and secondary antibodies, respectively. Fluorescence microscopic analysis was performed as described previously [6].
Statistical Analysis
Student's t test, chi-square test, and unpaired t test were performed with GraphPad Prism software 5.0 (GraphPad Software, San Diego, CA) to compare various test groups. Kaplan–Meier survival analysis was also performed with GraphPad Prism software 5.0 (GraphPad Software).
Results
De Novo Emergence of CD105+ HCC Cells after Treatment with Cytotoxic Reagents
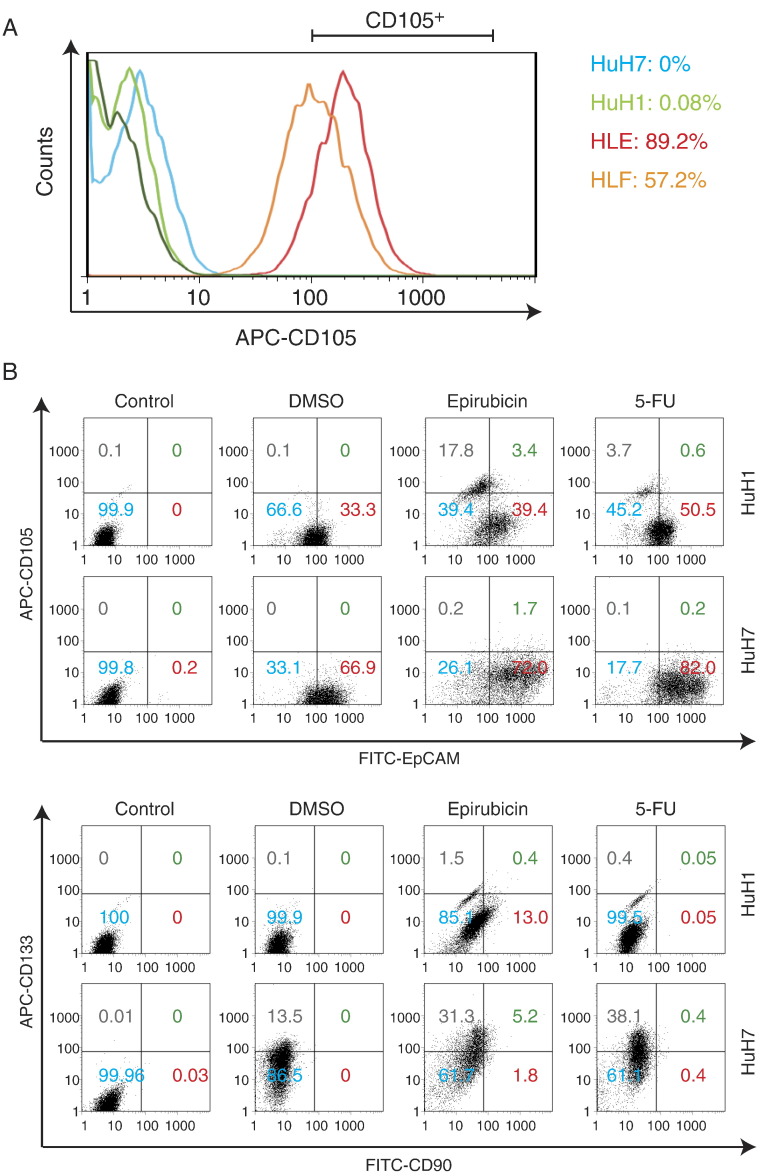
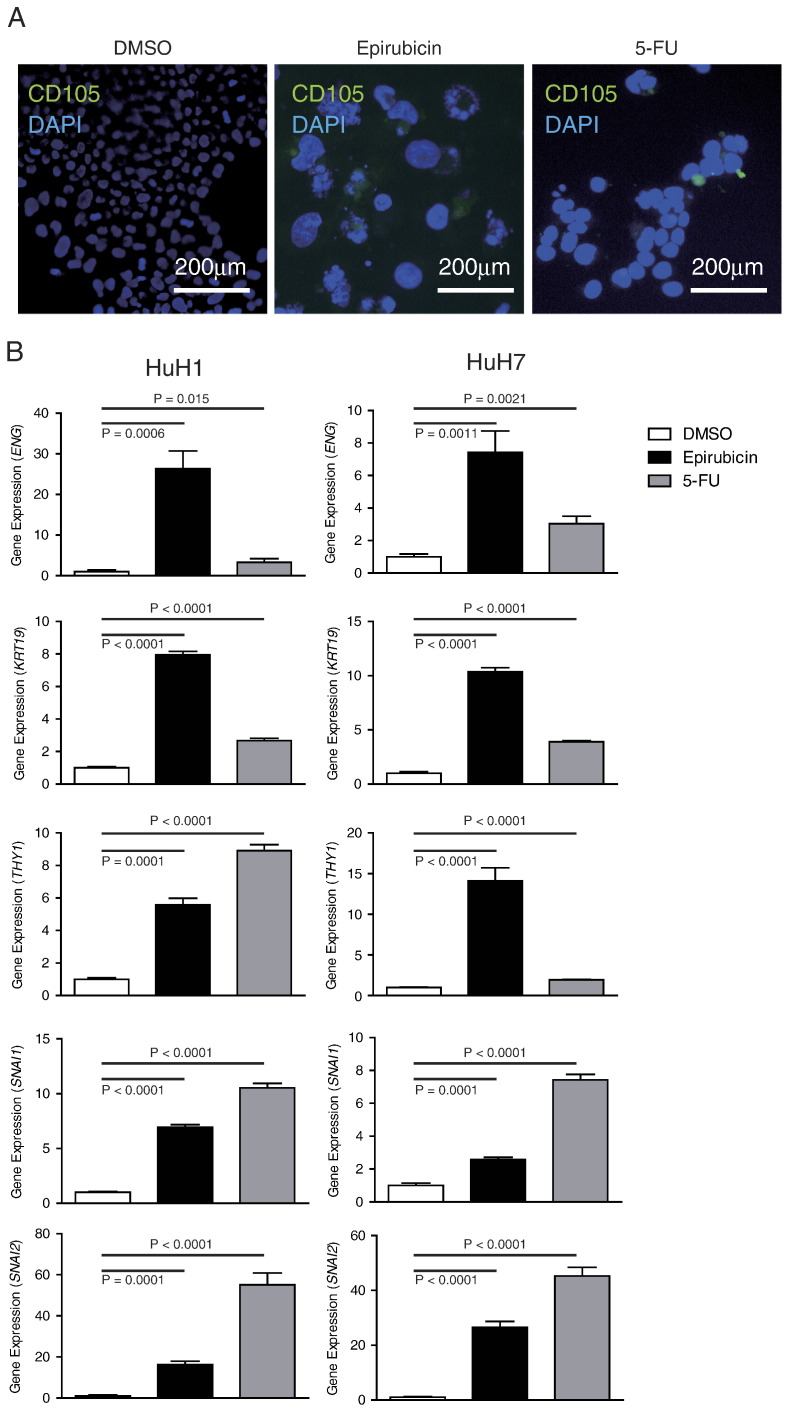
Previously, we evaluated the expression of the CSC markers EpCAM and CD90 and their tumorigenicity in representative HCC cell lines. We found that the EpCAM+ cell lines HuH1 and HuH7 do not express CD90 and show an epithelial cell shape with high tumorigenic capacity, whereas the CD90+ cell lines HLE and HLF also do not express EpCAM but show a mesenchymal cell shape with high metastatic capacity. Interestingly, when we explored the expression of CD105 in these cell lines, we identified the abundant expression of CD105 in the CD90+ cell lines (89.2% in HLE and 57.2% in HLF) but not in the EpCAM+ cell lines (0% in HuH7 and 0.08% in HuH1) (Figure 1A), suggesting that CD105 may be a marker of mesenchymal liver CSCs. Next, we evaluated the expression of CD105 in the EpCAM+ cell lines HuH1 and HuH7 treated with the cytotoxic reagents epirubicin and 5-FU. Originally, HuH1 and HuH7 cells contained a population of EpCAM+ cells (33.3% in HuH1 and 66.9% in HuH7) but no CD90+ and CD105+ cells as evaluated by FACS analysis (Figure 1B). Furthermore, we identified the enrichment of the EpCAM+ cell population after treatment with epirubicin and 5-FU for 72 hours in HuH1 (39.4% by epirubicin and 50.5% by 5-FU) and HuH7 cells (72% by epirubicin and 82% by 5-FU), consistent with the highly chemoresistant capacity of EpCAM+ CSCs. However, surprisingly, we also identified a small population of CD90+ and CD105+ cells generated de novo in HuH1 and HuH7 cells after treatment with these cytotoxic reagents. When we evaluated the nuclear size of HuH1 cells, we identified strong and modest increases of nuclear size following treatment with epirubicin and 5-FU compared with control, respectively (Figure 2A). We confirmed the de novo expression of CD105 in HuH1 cells by immunofluorescence (Figure 2A). The induction of genes encoding CD105 (ENG), CK19 (KRT19), and CD90 (THY1) was further confirmed by qRT-PCR analysis in HuH1 and HuH7 cells (Figure 2B). The de novo emergence of CD105+ cells in HuH1 and HuH7 cells was accompanied by the upregulation of genes encoding the transcription factors SNAI1 and SNAI2, master regulators of genes regulating EMT. These data suggest that CD105, previously recognized as a vascular endothelial marker, was induced de novo in EpCAM+ HCC cell lines with activation of genes regulating EMT.
Figure 1.
FACS analysis of representative CSC markers.
(A) Expression of CD105 in CD90+ (89.2% in HLE and 57.2% in HLF) and EpCAM+ cell lines (0% in HuH7 and 0.08% in HuH1).
(B) Expression of EpCAM, CD105, CD90, and CD133 in HuH1 and HuH7 cells treated with DMSO, epirubicin, and 5-FU.
Figure 2.
De novo expression of CD105 in EpCAM+ CD90− CD105− HCC cell lines.
(A) Immunofluorescence analysis of CD105 expression in HuH1 cells treated with DMSO, epirubicin, and 5-FU.
(B) qRT-PCR analysis of ENG (encoding CD105), KRT19, THY1, SNAI1, and SNAI2 in HuH1 and HuH7 cells treated with DMSO (white bar), epirubicin (black bar), and 5-FU (gray bar).
CD105+ HCCs Correlate with Microvascular Invasion and Poor Prognosis
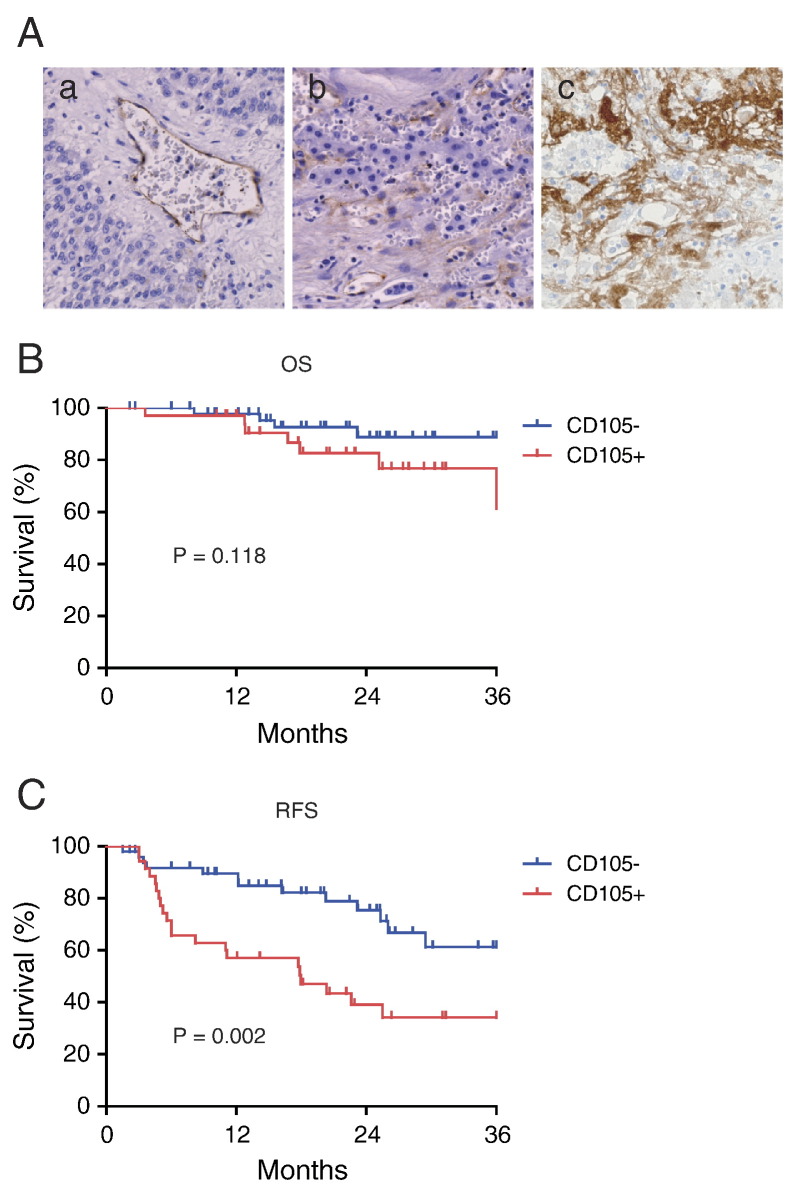
To elucidate the expression of CD105 in primary HCC tissues, we immunohistochemically evaluated the expression of CD105 in a total of 85 surgically resected HCC tissue samples. In most cases, CD105 staining was detected in vascular endothelial cells (Figure 3A, panel a); however, we also detected CD105 staining in HCC cells with a mesenchymal cell shape (Figure 3A, panel b). Most strikingly, CD105+ cancer cells were detected in surgically resected HCC patients' tissues who received transcatheter arterial chemoembolization (TACE) with epirubicin prior to surgery (Figure 3A, panel c).
Figure 3.
CD105 expression and prognosis in HCC.
(A) Panel (a) shows CD105 staining in vascular endothelial cells in HCC. Panel (b) shows CD105 staining in HCC cells with a mesenchymal cell shape. Panel (c) shows the enrichment of CD105+ cells in HCC tissues surgically resected after treatment with epirubicin TACE.
(B). Kaplan–Meier survival analysis of CD105+ and CD105− HCCs (overall survival [OS]).
(C) Kaplan–Meier survival analysis of CD105+ and CD105− HCCs (recurrence-free survival [RFS]). CD105+ HCCs showed poor RFS compared with CD105− HCCs with statistical significance (P = .002).
We classified the HCC cases into CD105+ and CD105− according to the expression of CD105 in cancer cells, not in vascular endothelial cells. We defined HCC as CD105+ even if we could detect only a small subset of cancer cells (<1%) stained by the CD105 antibody, unless the staining was restricted to vascular endothelial cells. Accordingly, 35 of 85 HCCs were defined as CD105+. When we evaluated the clinicopathological characteristics of the CD105+ and CD105− HCCs, we could not detect any differences in terms of age; sex; virus infection status; presence of liver cirrhosis; tumor size; serum alpha-fetoprotein values; histological grades; distant organ metastasis; and tumor, node, and metastasis stages (See Table 1). However, we found that CD105+ HCC could be characterized by a high frequency of microvascular invasion of the tumor. Consistently, although we could not detect differences in overall survival between CD105+ and CD105− HCC cases, we observed the poor recurrence-free survival of CD105+ HCCs compared with CD105− HCCs with statistical significance (P = .002). These data suggest that the expression of CD105 in HCC tissues may not only correlate with vascular density, as reported previously [13], but also correlate with the abundance of cancer cells with a mesenchymal cell shape expressing CD105, potentially induced by cytotoxic reagents or activated by the EMT program.
Table 1.
Clinicopathological Characteristics of CD105+ and CD105− HCC
| Parameter | CD105+ (n = 35) | CD105− (n = 50) | P Value |
|---|---|---|---|
| Age | 62.2 ± 11.6 | 64.8 ± 9.1 | .25 |
| Sex (M/F) | 26/9 | 36/14 | 1 |
| Virus (HBV/HCV/HBV+HCV/NBNC) | 14/21/0/0 | 12/33/2/3 | |
| LC (yes/no) | 22/13 | 29/21 | .82 |
| Tumor size (>3 cm, ≦3 cm) | 16/19 | 20/30 | .66 |
| AFP (median, [25%-75%]) | 12.1 (10-418) | 16 (10-92.5) | .14 |
| Microvascular invasion | 16/19 | 8/42 | .0036 |
| Histological grade (well/moderate/poor)⁎ | 3/25/7 | 8/38/4 | .2 |
| Distant organ metastasis (yes/no) | 6/29 | 6/44 | .54 |
| TNM stages (I-II/III-IV) | 25/10 | 38/12 | .8 |
Edmondson-Steiner.
Discussion
Although considered monoclonal in origin, cancer cells are heterogeneous in terms of morphology, proliferation, invasion capacity, and drug resistance. This heterogeneity is currently explained by two models: the clonal evolution model and the CSC model [4]. Previously, we provided evidence that at least some HCCs follow the CSC model [7]. We further explored the CSC hypothesis using several CSC markers and found that liver CSCs can be divided into at least two distinct entities: tumorigenic epithelial CSCs expressing EpCAM and metastatic mesenchymal CSCs expressing CD90 [10]. Besides, morphologically, CD90+ CSCs show vascular endothelial cell features, which are closely correlated with the phenotype termed vasculogenic mimicry [10]. Here, we provided evidence that CD90+ CSCs also express the vascular endothelial marker CD105. CD105+ HCC is characterized by a high frequency of microvascular invasion and poor prognosis after surgery. CD105+ cancer cells were evident in HCC tissues previously treated by TACE, suggesting that CD105+ cancer cells may be resistant to chemotherapy and hypoxia. These findings suggest that CD105 may be a good candidate molecule to target HCC cells with a capacity for vascular invasion and resistance to cytotoxic reagents.
CD105 is a type I integral transmembrane glycoprotein that has predominantly been investigated in the vasculature where it is upregulated during angiogenesis [14]. In endothelial cells, CD105 is an accessory co-receptor for TGF-β and associates at the cell surface with type I and type II TGF-β receptors [14]. Therefore, CD105 has a key role in modulating downstream signaling molecules such as SMAD family proteins. Because of its role in neovascularization, CD105 is generally considered as one of the vascular endothelial cell markers, such as CD31 and VEGFRs, and reflects the vascular density of a tumor, which may correlate with tumor progression, metastasis, and poor prognosis [13]. Indeed, the increased expression of CD105 in vascular endothelial cells has been reported as a marker of invasiveness and metastatic potential in cancer, including HCC [11].
Although CD105 is expressed in vascular endothelial cells and strongly correlates with angiogenesis, recent studies suggested that CD105 is also expressed in tumor epithelial cells of renal cell carcinoma [15] and ovarian cancer [16]. Here, we reported that CD105 is also expressed in liver CSCs expressing CD90 with metastatic mesenchymal cell features. Furthermore, we provided evidence that mesenchymal CD105+ cells could originate from epithelial CD105− cells de novo. It may be possible that CD105 could be a direct target against HCC with chemoresistance and metastatic features. As CD105 is a co-receptor of TGF-β receptors, it is plausible that TGF-β signaling may be activated in CD105+ HCC cells. Indeed, we demonstrated the activation of SNAI1 and SNAI2 in CD105+ HCC cells, potentially resulting from the activation of TGF-β signaling. Therefore, it could be possible to suppress cancer cell growth by TGF-β receptor signaling inhibitors such as galunisertib (LY2157299) [17]. Furthermore, a recent phase II study evaluating the effects of TRC105, a chimeric IgG1 anti-CD105 monoclonal antibody, in human HCC patients showed that it was tolerated well [18]. Future studies are required to evaluate the role of CD105+ cells and the therapeutic effects of galunisertib or TRC105 in human HCC.
Acknowledgements
We would like to thank Ms. Masayo Baba for excellent technical assistance. This study was supported by a grant from the Japanese Ministry of Education, Culture, Sports, Science, and Technology (23590967).
Footnotes
This study was supported by a grant from the Japanese Ministry of Education, Culture, Sports, Science, and Technology (23590967).
Conflict of Interest: All authors declare that they have no conflicts of interest.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Villanueva A, Hoshida Y, Toffanin S, Lachenmayer A, Alsinet C, Savic R, Cornella H, Llovet JM. New strategies in hepatocellular carcinoma: genomic prognostic markers. Clin Cancer Res. 2010;16:4688–4694. doi: 10.1158/1078-0432.CCR-09-1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zucman-Rossi J, Villanueva A, Nault JC, Llovet JM. Genetic landscape and biomarkers of hepatocellular carcinoma. Gastroenterology. 2015;149:1226–1239. doi: 10.1053/j.gastro.2015.05.061. [e1224] [DOI] [PubMed] [Google Scholar]
- 4.Yamashita T, Wang XW. Cancer stem cells in the development of liver cancer. J Clin Invest. 2013;123:1911–1918. doi: 10.1172/JCI66024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma S, Chan KW, Hu L, Lee TK, Wo JY, Ng IO, Zheng BJ, Guan XY. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology. 2007;132:2542–2556. doi: 10.1053/j.gastro.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 6.Nio K, Yamashita T, Okada H, Kondo M, Hayashi T, Hara Y, Nomura Y, Zeng SS, Yoshida M, Sunagozaka H. Defeating EpCAM liver cancer stem cells by targeting chromatin remodeling enzyme CHD4 in human hepatocellular carcinoma. J Hepatol. 2015 doi: 10.1016/j.jhep.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Yamashita T, Ji J, Budhu A, Forgues M, Yang W, Wang HY, Jia H, Ye Q, Qin LX, Wauthier E. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology. 2009;136:1012–1024. doi: 10.1053/j.gastro.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeng SS, Yamashita T, Kondo M, Nio K, Hayashi T, Hara Y, Nomura Y, Yoshida M, Hayashi T, Oishi N. The transcription factor SALL4 regulates stemness of EpCAM-positive hepatocellular carcinoma. J Hepatol. 2014;60:127–134. doi: 10.1016/j.jhep.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 9.Oishi N, Yamashita T, Kaneko S. Molecular biology of liver cancer stem cells. Liver Cancer. 2014;3:71–84. doi: 10.1159/000343863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamashita T, Honda M, Nakamoto Y, Baba M, Nio K, Hara Y, Zeng SS, Hayashi T, Kondo M, Takatori H. Discrete nature of EpCAM(+) and CD90(+) cancer stem cells in human hepatocellular carcinoma. Hepatology. 2013;57:1484–1497. doi: 10.1002/hep.26168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benetti A, Berenzi A, Gambarotti M, Garrafa E, Gelati M, Dessy E, Portolani N, Piardi T, Giulini SM, Caruso A. Transforming growth factor-beta1 and CD105 promote the migration of hepatocellular carcinoma–derived endothelium. Cancer Res. 2008;68:8626–8634. doi: 10.1158/0008-5472.CAN-08-1218. [DOI] [PubMed] [Google Scholar]
- 12.Yamashita T, Honda M, Nio K, Nakamoto Y, Takamura H, Tani T, Zen Y, Kaneko S. Oncostatin m renders epithelial cell adhesion molecule-positive liver cancer stem cells sensitive to 5-fluorouracil by inducing hepatocytic differentiation. Cancer Res. 2010;70:4687–4697. doi: 10.1158/0008-5472.CAN-09-4210. [DOI] [PubMed] [Google Scholar]
- 13.Xiong YQ, Sun HC, Zhang W, Zhu XD, Zhuang PY, Zhang JB, Wang L, Wu WZ, Qin LX, Tang ZY. Human hepatocellular carcinoma tumor-derived endothelial cells manifest increased angiogenesis capability and drug resistance compared with normal endothelial cells. Clin Cancer Res. 2009;15:4838–4846. doi: 10.1158/1078-0432.CCR-08-2780. [DOI] [PubMed] [Google Scholar]
- 14.Nassiri F, Cusimano MD, Scheithauer BW, Rotondo F, Fazio A, Yousef GM, Syro LV, Kovacs K, Lloyd RV. Endoglin (CD105): a review of its role in angiogenesis and tumor diagnosis, progression and therapy. Anticancer Res. 2011;31:2283–2290. [PubMed] [Google Scholar]
- 15.Cheng B, Yang G, Jiang R, Cheng Y, Yang H, Pei L, Qiu X. Cancer stem cell markers predict a poor prognosis in renal cell carcinoma: a meta-analysis. Oncotarget. 2016 doi: 10.18632/oncotarget.11672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ziebarth AJ, Nowsheen S, Steg AD, Shah MM, Katre AA, Dobbin ZC, Han HD, Lopez-Berestein G, Sood AK, Conner M. Endoglin (CD105) contributes to platinum resistance and is a target for tumor-specific therapy in epithelial ovarian cancer. Clin Cancer Res. 2013;19:170–182. doi: 10.1158/1078-0432.CCR-12-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serova M, Tijeras-Raballand A, Dos Santos C, Albuquerque M, Paradis V, Neuzillet C, Benhadji KA, Raymond E, Faivre S, de Gramont A. Effects of TGF-beta signalling inhibition with galunisertib (LY2157299) in hepatocellular carcinoma models and in ex vivo whole tumor tissue samples from patients. Oncotarget. 2015;6:21614–21627. doi: 10.18632/oncotarget.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duffy AG, Ulahannan SV, Cao L, Rahma OE, Makarova-Rusher OV, Kleiner DE, Fioravanti S, Walker M, Carey S, Yu Y. A phase II study of TRC105 in patients with hepatocellular carcinoma who have progressed on sorafenib. United European Gastroenterol J. 2015;3:453–461. doi: 10.1177/2050640615583587. [DOI] [PMC free article] [PubMed] [Google Scholar]