Abstract
Objective
Cerebral blood flow (CBF) plays a critical role in the maintenance of neuronal integrity, and CBF alterations have been linked to deleterious white matter changes. Although both CBF and white matter microstructural alterations have been observed within the context of traumatic brain injury (TBI), the degree to which these pathological changes relate to one another and whether this association is altered by time since injury have not been examined. The current study therefore sought to clarify associations between resting CBF and white matter microstructure post-TBI.
Methods
37 veterans with history of mild or moderate TBI (mmTBI) underwent neuroimaging and completed health and psychiatric symptom questionnaires. Resting CBF was measured with multiphase pseudocontinuous arterial spin labeling (MPPCASL), and white matter microstructural integrity was measured with diffusion tensor imaging (DTI). The cingulate cortex and cingulum bundle were selected as a priori regions of interest for the ASL and DTI data, respectively, given the known vulnerability of these regions to TBI.
Results
Regression analyses controlling for age, sex, and posttraumatic stress disorder (PTSD) symptoms revealed a significant time since injury × resting CBF interaction for the left cingulum (p < 0.005). Decreased CBF was significantly associated with reduced cingulum fractional anisotropy (FA) in the chronic phase; however, no such association was observed for participants with less remote TBI.
Conclusions
Our results showed that reduced CBF was associated with poorer white matter integrity in those who were further removed from their brain injury. Findings provide preliminary evidence of a possible dynamic association between CBF and white matter microstructure that warrants additional consideration within the context of the negative long-term clinical outcomes frequently observed in those with history of TBI. Additional cross-disciplinary studies integrating multiple imaging modalities (e.g., DTI, ASL) and refined neuropsychiatric assessment are needed to better understand the nature, temporal course, and dynamic association between brain changes and clinical outcomes post-injury.
Keywords: Perfusion, Arterial spin labeling, ASL, Cerebral blood flow, CBF, White matter microstructure, Diffusion tensor imaging, DTI, Traumatic brain injury, TBI, Time since injury, White matter
Highlights
-
•
Oftentimes unconsidered are (1) the dynamic relationship between brain variables of interest and (2) how time since injury may factor into brain changes.
-
•
Cerebral blood flow (CBF) plays a critical role in the maintenance of neuronal integrity, and CBF alterations have been linked to deleterious white matter (WM) changes.
-
•
Although both CBF and WM alterations have been observed within the context of TBI, the degree to which these pathological changes relate to one another has not been examined.
-
•
This is especially critical given CBF reductions could serve to exacerbate or contribute to any trauma-induced WM alterations well beyond the time of initial injury.
-
•
The current study explored the association between CBF and WM, and the potential influence of time since injury on this relationship in Veterans with history of TBI.
-
•
Results showed an interaction between time since injury and CBF of the left cingulate cortex on WM integrity of the left cingulum bundle.
-
•
These findings may assist in clarifying mechanisms underlying the poor long-term outcomes observed in those with history of TBI.
1. Introduction
Traumatic brain injury (TBI) has come to be known as the predominant injury of U.S. Veterans returning from the recent wars in Iraq and Afghanistan (Hoge et al., 2008). Of the nearly two million military service members that have been deployed since the beginning of these wars, estimates suggest that an astounding 15–25% of these individuals have sustained at least one TBI during deployment (Fortier et al., 2014, Hoge et al., 2008, Terrio et al., 2011, Warden, 2006). The vast majority of these injuries can be classified as either mild or moderate (Defense and Veterans Brain Injury Center, 2016), and are often the direct result of either blunt-force (i.e., direct blow to the head) or blast-related (i.e., pressure wave from an explosive device) trauma. While most Veterans who experience mild neurotrauma do not require immediate or emergency medical care at the time of injury, a host of troubling cognitive (e.g., executive dysfunction, attention and memory deficits) (Combs et al., 2015, Vanderploeg et al., 2005), post-concussive (e.g., headaches, dizziness, fatigue) (King et al., 2012, Lippa et al., 2010), and psychiatric symptoms (e.g, anxiety, depression) (Brenner, 2011, Yurgil et al., 2014) frequently emerge post-injury. Collectively, these enduring neurobehavioral symptoms contribute to considerable health care costs (Stroupe et al., 2013, Tanielian and Jaycox, 2008), and they play a fundamental role in frequently reported decreased quality of life (Schiehser et al., 2015), and increased rates of disability and unemployment observed in Veterans with history of head injury (Lippa et al., 2015). Importantly, although most individuals with mild TBI appear to fully recover within about one year post-injury, a subset of individuals—oftentimes referred to as the “miserable minority”—continue to experience long-term cognitive, psychiatric, and behavioral difficulties (Bigler, 2013a, Bigler, 2013b, Ruff et al., 1996, Vanderploeg et al., 2007). Unfortunately, the exact neuropathological mechanisms underlying the persistent sequelae of mild neurotrauma remain poorly understood since traditional neuroimaging techniques are generally insensitive to subtle neuropathological changes associated with mTBI, as conventional computed tomography (CT) and magnetic resonance imaging (MRI) scans have largely yielded normal results (Bigler, 2013a, Bigler, 2013b, Brenner, 2011, McAllister et al., 2001).
The advent of more sophisticated neuroimaging technology, coupled with experimental animal modeling of TBI, has provided insight into pathophysiological mechanisms thought to underlie the negative health outcomes of Veterans with history of TBI. Biomechanical and animal models of TBI have demonstrated that direct, or primary injury, to neurons, glia, and vessels occurs during neurotrauma (Bigler and Maxwell, 2012, Blennow et al., 2012, Chatelin et al., 2011, Kenney et al., 2015, LaPlaca et al., 2007, LaPlaca and Prado, 2010, Povlishock and Katz, 2005). Moreover, secondary pathophysiological cascades (i.e., neuroinflammation, edema, ischemia, Wallerian degeneration) exacerbate local injury sites and contribute to diffuse damage post-injury (Bigler and Maxwell, 2012, DeKosky et al., 2013, Farkas and Povlishock, 2007, Johnson et al., 2013, Magnuson et al., 2012). While more severe and heterogeneous pathology is observed in those with moderate or severe TBIs, the mechanisms underlying milder forms of neurotrauma remain less well understood, as precise modeling of mild TBI in experimental studies is challenging, and autopsy cases are exceedingly rare. Nevertheless, advanced MRI techniques (e.g., diffusion tensor imaging [DTI], arterial spin labeling [ASL]) have been utilized for in-vivo quantification of neurotrauma-related brain changes in Veterans (see Wilde et al., 2015 for review). However, while some studies find robust brain differences in Veterans with history of TBI relative to those with no history of head trauma (Mac Donald et al., 2011, Miller et al., 2016, Petrie et al., 2014, Ponto et al., 2016), others fail to detect any alterations (Jorge et al., 2012, Levin et al., 2010).
The inconsistent nature of neuroimaging findings following TBI may be partially explained by the heterogeneous nature of injury, or alternatively, differences in sample characteristics, scanning parameters, and analytic techniques utilized. However, oftentimes unconsidered are (1) the dynamic relationship between brain variables of interest and (2) how time since injury may factor into brain changes. With respect to the former, studies of normal and pathological aging have consistently demonstrated that cerebral blood flow (CBF) plays a pivotal role in the maintenance of white matter (WM) tissue integrity (Burzynska et al., 2015, Chen et al., 2013, O'Sullivan et al., 2002, Salat, 2014, Steketee et al., 2016). Reduced CBF has been demonstrated to not only precede, but also directly contribute to negative WM micro- and macro-structural changes in older adults (Bernbaum et al., 2015, Brickman et al., 2009, Promjunyakul et al., 2015, Promjunyakul et al., 2016, ten Dam et al., 2007). Importantly, while both CBF and WM changes have been independently examined within TBI (Delano-Wood et al., 2015, Ponto et al., 2016, Vas et al., 2016), few studies have explored relationships between CBF and WM within this population. This is especially critical given CBF reductions could serve to exacerbate or contribute to any trauma-induced WM alterations well beyond the time of initial injury.
Unfortunately, the temporal course of the neuropathological consequences of TBI remains poorly understood (Greve and Zink, 2009, Povlishock and Katz, 2005). However, there is some evidence to suggest that both CBF and WM changes may differ depending upon phase of injury (Eierud et al., 2014, Niogi and Mukherjee, 2010). For example, though findings are mixed, fractional anisotropy (FA)—a marker of WM microstructural integrity derived from diffusion tensor imaging (DTI)—has been observed to be both elevated and decreased in various studies examining those with history of TBI in the acute phase of injury relative to those without history of head trauma (Croall et al., 2014, Ling et al., 2012, Mayer et al., 2012). On the other hand, decreased FA is more commonly reported in individuals with history of TBI in the chronic phase of injury (Miller et al., 2016, Wada et al., 2012). Similarly, while studies vary in reporting either elevated or decreased CBF in the acute phase of injury (Doshi et al., 2015, Meier et al., 2015), decreased CBF is most commonly observed in those with history of TBI who are further removed for their initial injury when compared to controls (Fridley et al., 2015, Ge et al., 2009). While there is no general consensus as to what constitutes acute versus chronic phases of injury, most Veterans are many months to years removed from their initial injury (i.e., in the chronic phase) during assessment; although, there is considerable inter-subject variability in the time between injury and assessment within and across previous Veteran TBI studies (Delano-Wood et al., 2015, Jorge et al., 2012, Mac Donald et al., 2011, Miller et al., 2016). It is especially critical to take into account time since injury when relating CBF and WM integrity given there is some evidence—at least in the aging literature—to suggest that CBF changes may persist for some time before negative WM alterations are subsequently observed (Brickman et al., 2009, Promjunyakul et al., 2015, ten Dam et al., 2007).
Therefore, there is a critical need to not only consider how CBF and WM relate to one another, but also how this relationship may depend on time since a TBI event. The current study sought to examine the link between resting CBF of the cingulate cortex and WM integrity of the cingulum bundle—two largely overlapping neuroanatomical regions that are known to be especially vulnerable to TBI effects (Bigler, 2007, Wu et al., 2010). Clarification of such relationships may assist in providing insight into factors influencing disparate brain findings in the TBI literature and elucidate findings that show WM degeneration may evolve over time during the chronic phase of injury (Bendlin et al., 2008, Yeh et al., 2017). We hypothesize that (1) decreased CBF of the cingulate cortex will be associated with reduced WM integrity of the cingulum bundle and (2), that this association will become more pronounced the further removed individuals are from their injuries. Importantly, findings may assist in clarifying mechanisms underlying the poor long-term outcomes and increased risk for stroke and dementia observed in those with history of TBI (Barnes et al., 2014, Burke et al., 2013, Chen et al., 2011, Lee et al., 2013).
2. Methods
Study participants were 37 Operation Enduring Freedom, Operation Iraqi Freedom, and Operation New Dawn (OEF/OIF/OND) Veterans with history of mild or moderate TBI (mmTBI) recruited from outpatient clinics and posted recruitment flyers at the VA San Diego Hospital (VASDH) in La Jolla, California. The institutional review boards (IRBs) at the VA San Diego Healthcare System (VASDHS) and University of California, San Diego (UCSD) approved the study, and all study participants provided written and informed consent. Neuropsychological testing, TBI history interviews, and completion of questionnaires occurred at the Veterans Medical Research Foundation building located on the VASDHS campus. All MRI scanning took place at the UCSD Center for Functional MRI.
2.1. TBI diagnostic procedure
The Department of Defense (DoD)/VA TBI Task Force criteria (2009) was used for diagnosis of mild or moderate TBI. The criteria for mild TBI include loss of consciousness (LOC) < 30 min, or alteration of consciousness (AOC) or post traumatic amnesia (PTA) < 24 h, while the criteria for moderate TBI were LOC > 30 min but < 24 h, or AOC > 24 h or PTA > 1 day but < 7 days. Per Clark et al. (2016) trained graduate level and post-baccalaureate research assistants completed TBI history interviews. Each study participant was assessed for both military (i.e., during enlistment in the US armed services) and non-military (i.e., prior to or after discharge from the military) related head injuries. All reported military-related injuries also include assessment of whether the mechanism of injury was blunt or blast-related. For any injury that met diagnostic criteria for mild or moderate TBI, the date of occurrence was recorded and time since the most recent TBI and date of evaluation was calculated for use in subsequent analyses.
The following exclusionary criteria were applied to the study sample overall: (1) severe TBI (loss of consciousness [LOC] ≥ 24 h, alteration of consciousness [AOC] > 24 h, or posttraumatic amnesia [PTA] ≥ 7 days); (2) prior history of major medical illnesses (e.g., myocardial infarction) or neurological conditions (e.g., multiple sclerosis, stroke); (3) current active suicidal and/or homicidal ideation, intent, or plan requiring crisis intervention; (4) current or past history of DSM-IV diagnosis of bipolar disorder, schizophrenia, other psychotic disorder, or cognitive disorder due to a general medical condition other than TBI; (5) DSM-IV diagnosis of current substance/alcohol dependence or abuse; (6) a positive toxicology screen as measured by the Rapid Response 10-drug Test Panel; and (7) any contraindications that prevented MRI scanning. Participants were included in the study if they were OEF/OIF/OND Veterans between the ages of 18–65, completed neuropsychological testing, and received both DTI and MPPCASL sequences.
2.2. Health status, combat exposure, & symptom rating scales
All study participants completed a background health questionnaire and height, weight, and blood pressure was collected at the time of their study visit. Exposure to wartime stressors and combat situations while on deployment was assessed using the Combat Exposure Scale (CES; Keane et al., 1989). Symptom rating scales that quantified current levels of posttraumatic stress (PTSD Checklist [PCL-M]; (Weathers et al., 1993), depression (Beck-Depression Inventory-II [BDI-II]; (Beck et al., 1996), and neurological symptoms (Neurobehavioral Symptom Inventory [NSI]; King et al., 2012) were also completed.
2.3. Neuroimaging data acquisition
All participants were scanned on a 3-Tesla General Electric MR750 whole-body MRI system with an eight-channel head coil. T1-weighted Anatomical Scan: A sagitally acquired high-resolution T1-weighted anatomical scan was collected using a 3D FSPGR sequence with the following parameters: FOV = 24 cm, 256 × 192 matrix, TR = 8.1 ms, TE = 3.192 ms, flip angle = 12°, TI = 550 ms, bandwidth = 31.25 kHz, and 172 1.2 mm slices.
DTI: DTI images were collected via dual spin echo EPI acquisition (Reese, Heid, Weisskoff, & Wedeen, 2003) with the following parameters: FOV = 24 cm, slice thickness = 3 mm, matrix size 128 × 128, in-plane resolution = 1.875 × 1.875 mm, TR = 8000 ms, TE = 88 ms, scan time: 12 min. Forty-three slices were acquired with 61 diffusion directions distributed on the surface of a sphere in conjunction with the electrostatic repulsion model (Jones, Horsfield, & Simmons, 1999) and a b value of 1500 s/mm2. Collection also included one T2 weighted image with no diffusion (b = 0). Distortions due to a lack of magnetic field homogeneity were reduced via field map corrections.
Resting CBF: Time-of-flight angiogram was collected with a three-dimensional spoiled gradient echo sequence (FOV = 22 × 16.5 cm, slice thickness = 1 mm, 0.57 × 0.74 × 1 mm3 resolution, TE = 2.7 ms, TR = 20 ms, flip angle 15°) in order to define the location for PCASL labeling. The imaging volume was prescribed to visualize arteries above the vertebral crossing, but below the basilar artery. Axial images were used to select the slice most perpendicular to bilateral vertebral and carotid arteries and this location was then set as the labeling plane in an effort to achieve optimal tagging efficiency for the whole brain PCASL scan.
Whole-brain ASL data was acquired during a resting state using an MPPCASL sequence. Importantly, MPPCASL mitigates the adverse effects of off-resonance fields and gradient imperfections on the inversion efficiency in traditional PCASL techniques (Jung, Wong, & Liu, 2010). In MPPCASL, the blood magnetization is modulated with multiple RF phase offsets, and the resulting signal is then fit to a model function to generate a CBF estimate. Parameters included 20 5 mm thick axial slices (1 mm gap), FOV = 24 cm, matrix 64 × 64, PCASL labeling duration = 2000 ms, post-labeling delay = 1600 ms, TR = 4200 ms, TE = minimum, volumes = 60, scan time = 5 min. To achieve CBF quantification in physiological units (mL/100 g-min), a 36-s cerebrospinal fluid (CSF) reference scan was obtained to estimate of the magnetization of CSF (TR = 4000 ms, TE = 3.3 ms, NEX = 9 90°excitation pulse which is turned off for first 8 repetitions to create PDW image contrast; Chalela et al., 2000). A 32-s minimum contrast scan was also acquired to adjust for coil inhomogeneities (TR = 2000 ms, TE = 11 ms, NEX = 2) during the CBF quantification step. Finally, a field map was acquired using a spoiled gradient echo sequence to correct for field inhomogeneities (TR = 500 ms, TE1 = 6.5 ms, TE2 = 8.5 ms, flip angle 45°, scan time = 1:10 min).
2.4. Neuroimaging data processing
2.4.1. T1-weighted anatomical image processing
T1 anatomical images were reconstructed and parcellated into regions of interest using FreeSurfer software (Dale, Fischl, & Sereno, 1999). Manual edits were performed to ensure proper region of interest (ROI) segmentation and gray and white matter differentiation.
2.4.2. DTI processing
DTI preprocessing utilized the Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library (FSL) (Smith et al., 2004). Two field maps were utilized to unwarp EPI acquisitions, and all images were motion corrected and visually inspected occurred for quality control purposes. The FSL program dtifit was used for voxel-by-voxel calculation of the diffusion eigenvalues and to provide fractional anisotropy (FA), a directional measure of diffusion ranging from 0 (isotropic diffusion) and 1 (perfectly anisotropic diffusion) that is reflective of fiber integrity.
2.4.3. Tractography
TrackVis (Wang et al., 2007), using the fiber assignment by continuous tracking (FACT) algorithm, was used to generate the left and right cingulum bundle for each participant. First, a color-coded map, seen by loading the principle eigenvector image in FSL, was generated to display each voxel's main orientation of diffusion. This information, in conjunction with a non-diffusion weighted map, allowed the rater to place seed points for fiber tracking. An initial seed was placed inferior to the cingulum gyrus and superior to the corpus callosum in the coronal plane. Next, three additional seeds in the anterior portion, the middle, and the posterior portion were placed following the description of Concha, Gross, & Beaulieu (2005) to generate the entire cingulum bundle for each hemisphere. Finally, mean FA was extracted from the length of each generated tract for use in statistical analyses. See Fig. 1 for depiction of left cingulum bundle ROI used in analyses.
Fig. 1.
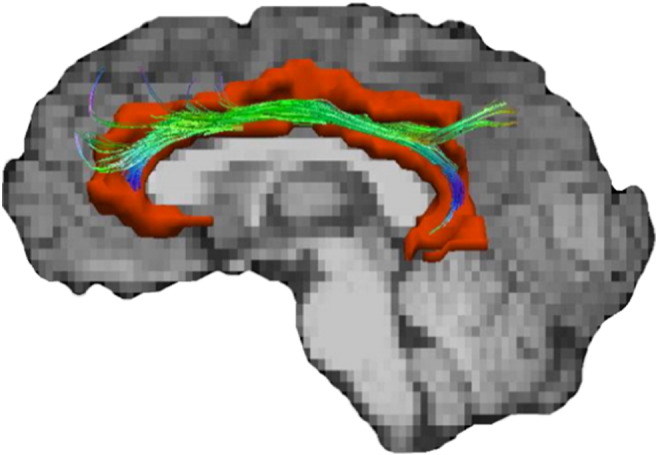
Lateralized depiction of the regions of interested utilized in the current study. The left cingulum bundle fiber track is superimposed on the left cingulate cortex (red).
2.4.4. Resting CBF
Each subject's raw ASL data, field map, and anatomical data were uploaded for processing to the Cerebral Blood Flow Biomedical Informatics Research Network (CBFBIRN; cbfbirn.ucsd.edu;Shin, Ozyurt, & Liu, 2013) established at the UCSD Center for Functional Imaging. Field map and motion correction, skull-stripping, tissue segmentation, and conversion to absolute physiological units of CBF (mL/100 g tissue/min) were completed through CBFBIRN. Quantified CBF maps for each participant were downloaded to a local server where they were blurred to 4 mm full-width at half maximum. Next, T1 images and partial volume segmentations were registered to ASL space and down-sampled to the resolution of the ASL images using the Analysis of Functional NeuroImages (AFNI) package (Cox, 1996). A threshold was applied that removed values outside of the expected physiological range of CBF (< 10 or > 150; (Bangen et al., 2014), then whole brain gray matter CBF and regional gray matter CBF values from the Desikan et al. (2006) atlas were extracted. Mean perfusion of the cingulate cortex was calculated as the average of the following gray matter ROIs for each hemisphere with each region's contribution to the average weighted by the volume of the region: rostral and caudal anterior cingulate, posterior cingulate and isthmus of the cingulate. See Fig. 1 for a lateralized depiction of the left cingulate cortex utilized in this study.
2.5. Statistical analyses
Multiple linear regressions were performed to determine (1) whether there was an association between CBF of the cingulate cortex and WM microstructural integrity of the cingulum bundle and (2) whether this association was modified by time since injury. A median split for time since injury was conducted to dichotomize TBI participants into two groups. Chi-squared analyses were utilized to compare the groups in terms of categorical variables and analysis of variance (ANOVA) was used for continuous variables. All statistical analyses were conducted using the Statistical Package for the Social Sciences (SPSS) version 21 (SPSS IBM, New York, USA).
3. Results
Participant demographics, TBI characteristics, and symptom rating scales for the sample are presented in Table 1. Participants were predominantly young (Mean age = 33.38 years) male (89%) Veterans who were blast-exposed (49%) and experienced moderate levels of combat exposure (Mean total score of 16.23 on Combat Exposure Scale) while on deployment. With respect to TBI injury characteristics, a greater proportion of Veterans experienced loss (65%) versus alteration of consciousness during their most significant TBI, these injuries were predominantly mild in severity (81%), and on average many months had passed since their most recent head injury (Mean time = 69.05). Symptom rating scales revealed participants endorsed sub-threshold levels of posttraumatic stress symptoms (Mean PCL-M total score = 47.57) and depressive symptoms were moderate in severity (Mean BDI-II total score = 21.14).
Table 1.
Participant characteristics.
| Mean (SD) | |
|---|---|
| Age | 33.38 (6.05) |
| Education | 14.54 (1.56) |
| WRAT-4 reading standard score | 102.63 (12.45) |
| Gender (% Male) | 89.2% |
| Ethnicity | |
| Caucasian | 35.1% |
| African-American | 8.1% |
| Hispanic | 37.8% |
| Asian | 16.2% |
| Native American | 2.7% |
| Combat exposure scale | 16.23 (12.06) |
| PCL-M total score | 47.57 (18.36) |
| BDI-II total score | 21.14 (12.52) |
| NSI total score | 37.11 (18.96) |
| Months since injury (months) | 69.05 (38.09), median = 62.00, range = 148 |
| Total # of TBIs | 2.84 (1.48) |
| TBI severity (% mild) | 81% |
| % most significant TBI with LOC | 65% |
| Blast-exposed (% yes) | 49% |
| Pulse pressure (n = 36) | 45.65 (8.28) |
| Height (in.) | 67.93 (3.30) |
| Weight (lbs) | 193.03 (45.58) |
| Current smoker (% yes) | 16% |
| APOE-ε4 genotype (n = 35; % yes) | 31% |
WRAT-4 = wide range achievement test-4th edition; PCL-M = posttraumatic stress disorder checklist; BDI-II = Beck depression inventory 2nd edition; NSI = neurobehavioral symptom inventory; APOE-ε4 = apolipoprotein-ε4 carrier.
3.1. Resting CBF and WM associations
A set of multiple linear regressions were performed for each hemisphere in an effort to determine if there was an association between resting CBF of the cingulate cortex and white matter microstructural integrity of the cingulum bundle. In each model age, sex, PCL-M total score, and resting CBF of the cingulate cortex were entered as predictors. Results revealed that neither the left (β = 0.08, p = 0.67) or right (β = 0.03, p = 0.88) cingulate cortex CBF predicted left or right cingulum bundle FA, respectively.
3.2. Resting CBF, time since injury, and WM integrity
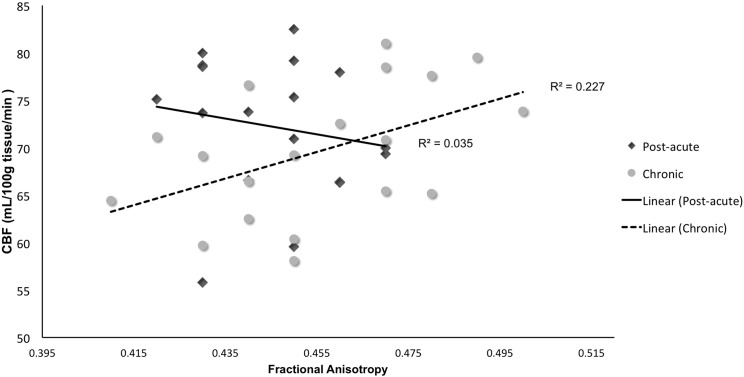
A second set of multiple linear regressions were performed for each hemisphere to determine whether time since injury moderated the association between resting CBF of the cingulate cortex and cingulum bundle FA. In the first model, FA of the left cingulum bundle was entered as the dependent variable; age, sex, PCL-M total score, resting CBF of the left cingulate cortex, time since injury, and an interaction term (resting CBF of left cingulate cortex X time since injury) were entered as independent variables. As shown in Table 2, results revealed there was a significant resting CBF of the left cingulate cortex × time since injury interaction on FA of the left cingulum bundle (β = 7.05, t = 3.99, p < 0.001). A median split for time since injury was conducted for further inspection of this relationship (see Fig. 2). Examination of simple main effects revealed that there was a significant positive correlation between resting CBF of left cingulate cortex and left cingulum bundle FA (r = 0.48, p = 0.04, n = 19) in Veterans furthest removed from their time since injury (≥ 62 months). However, for Veterans whose injuries were more recent (< 62 months), there was no significant association between resting CBF of the left cingulate cortex and left cingulum bundle FA (r = − 0.19, p = 0.46, n = 18). Results did not differ when total number of TBIs was included as a covariate in a secondary set of analyses and total number of TBIs (β = 0.13, t = 0.67, p = 0.51) was not a significant predictor of FA of the left cingulum bundle in the model. When this set of analyses was performed for the right hemisphere, there was no significant resting CBF of right cingulate cortex X time since injury interaction on FA of the right cingulum bundle (β = − 0.99, t = 0.44, p = 0.66).
Table 2.
Multiple linear regression models for left cingulum bundle FA.
| R2 | F | Standardized β | t | p | |
|---|---|---|---|---|---|
| 0.445 | 4.01 | 0.005 | |||
| Age | − 0.304 | − 1.90 | 0.07 | ||
| Gender | − 0.359 | − 2.55 | 0.02 | ||
| PCL-M total score | − 0.007 | − 0.052 | 0.96 | ||
| Time since injury | − 7.02 | − 3.97 | 0.000 | ||
| CBF of left cingulate cortex | − 1.19 | − 3.37 | 0.002 | ||
| CBF × time since injury | 7.05 | 3.99 | 0.000 |
Fig. 2.
Cerebral blood flow (CBF) of left cingulate cortex × time since injury for left cingulum bundle fractional anisotropy.
3.3. Group comparisons for phase of injury
In an effort to further understand the significant association between resting CBF of the left cingulate cortex and FA of the left cingulum bundle, participant demographics, TBI injury characteristics, and symptom rating scales were compared for participants who were closer versus further removed from their time since injury (see Table 3). Results revealed the groups were comparable on all comparisons except for time since injury. Although not statistically significant, there were a greater proportion of individuals in those further removed from their TBI whose injuries were moderate (rather than mild) in severity. However, when a secondary set of analyses where TBI injury severity was included in the original regression model results remained the same and TBI injury severity was not a significant predictor of FA of the left cingulum bundle (β = 0.04, t = 0.25, p = 0.80). Moreover, sensitivity analyses revealed that when those with moderate TBIs (n = 7) were excluded from this analysis entirely, the significant interaction of resting CBF x time since injury on FA of the left cingulum bundle remained (β = 6.84, t = 3.58, p = 0.002).
Table 3.
Group comparisons for phase of injury.
| Post-acute group mean (sd) n = 18 |
Chronic group mean (sd) n = 19 |
F or χ2 | p | |
|---|---|---|---|---|
| Age | 33.11 (1.71) | 33.63 (4.79) | 0.07 | 0.80 |
| Gender (% male)^ | 83.3% | 94.7% | 1.29 | 0.26 |
| Ethnicity^ | 2.74 | 0.34 | ||
| Caucasian | 33.8% | 36.8% | ||
| African-American | 5.6% | 10.5% | ||
| Hispanic | 33.3% | 42.1% | ||
| Asian | 22.2% | 10.5% | ||
| Native American | 5.6% | 0% | ||
| Combat exposure scale | 13.96 (11.97) | 18.38 (12.09) | 1.25 | 0.27 |
| PCL-M total score | 51.28 (16.92) | 44.07 (19.41) | 1.44 | 0.24 |
| BDI-II total score | 24.44 (12.11) | 18.01 (12.39) | 2.55 | 0.12 |
| NSI total score | 42.30 (18.47) | 32.11 (18.51) | 2.86 | 0.10 |
| Months since injury (months) | 39.28 (15.97) | 97.26 (30.57) | 51.39 | < 0.001 |
| Total # of TBIs | 2.89 (1.56) | 2.79 (1.43) | 0.04 | 0.84 |
| TBI Severity (% mild)^ | 88.9% | 73.7% | 1.44 | 0.23 |
| % most significant TBI with LOC | 55.6% | 73.7% | 1.34 | 0.25 |
| Blast-exposed (% yes) | 44.4% | 52.6% | 0.25 | 0.62 |
| Pulse pressure (n = 18, n = 18) | 46.22 (7.34) | 45.08 (9.31) | 0.17 | 0.69 |
| Height | 67.75 (3.93) | 68.11 (2.70) | 0.75 | 0.75 |
| Weight | 191.78 (48.92) | 194.28 (43.37) | 0.87 | 0.87 |
| Current smoker (% yes)^ | 17% | 16% | 0.23 | 0.24 |
| APOE-ε4 genotype (% yes) | 35% | 28% | 0.63 | 0.63 |
^Likelihood ratio; PCL-M = posttraumatic stress disorder checklist; BDI-II = Beck depression inventory 2nd edition; NSI = neurobehavioral symptom inventory; APOE-ε4 = apolipoprotein-ε4 carrier.
4. Discussion
The current study explored (1) the association between neuroimaging biomarkers of CBF and WM, and (2) the potential influence of time since injury on this relationship in Veterans with history of mmTBI. Results showed an interaction between time since injury and CBF of the left cingulate cortex on WM integrity of the left cingulum bundle. Specifically, in Veterans who were furthest removed from their time since injury, decreased CBF was significantly associated with reduced FA of the cingulum region. These findings provide preliminary evidence for a dynamic association between CBF and WM that may also play a pivotal role in increased risk for negative health outcomes (e.g. stroke, dementia) commonly observed in individuals with history of TBI. It is possible that this dynamic association observed between CBF and WM may partially explain the mixed findings in the neuroimaging literature, particularly since the vast majority of existing studies have focused on a single neuroimaging modality.
While our understanding of the pathophysiological consequences of TBI has improved, the time course of brain changes post-injury remains less well understood. Recent evidence suggests that TBI-related brain changes are not static, but may continue to evolve many months to years following the initial insult. For example, Venkatesan et al. (2015) utilized resting state functional MRI (rs-fMRI) to explore the trajectory of connectivity patterns between the acute and chronic phase of injury in individuals with history of moderate-to-severe TBI. Results revealed that, relative to controls, the TBI group not only demonstrated altered connectivity patterns, but that these differences intensified from the acute to chronic phase of injury. In the present study, CBF and WM associations were only evident in those furthest removed from injury. It may be that this association is a manifestation of pathological processes that are characteristic of more chronic injury phases. Alternatively, as the aging literature has shown, CBF reductions may need to persist for some time before WM alterations arise (ten Dam et al., 2007, Brickman et al., 2009, Promjunyakul et al., 2015). Importantly, we cannot ascribe our results to exact causal or directional etiologies given the cross-sectional nature of this study and future studies are needed to further elucidate the time course of these dynamic relationships. Moreover, given this sample reflects mild TBI, there is also a critical need to clarify to what extent the observed findings may apply to samples comprising primarily moderate or severe injuries.
Our finding of an association between CBF and WM in those most remote from their injury aligns well with existing literature demonstrating a pronounced co-variation between WM integrity and vascular function in both healthy and pathological aging samples (Burzynska et al., 2015, Chen et al., 2013, O'Sullivan et al., 2002, Steketee et al., 2016). Within the context of TBI, CBF may play an important role in identifying those at risk for secondary WM changes following injury. For example, in an emergency room sample with mild TBI, decreased CBF at baseline assessment (within hours of injury) was tightly linked with reduced WM integrity at follow-up (on average 5 months post-injury; Metting, Cerliani, Rodiger, & van der Naalt, 2013). The establishment of a relationship between CBF and WM within the context of head injury is critical, as maintenance of vascular health may be a critical point of intervention in the prevention of additional brain damage in those with history of TBI. Indeed, population-based studies have demonstrated that history of TBI is associated with increased risk for stroke (Burke et al., 2013, Chen et al., 2011), which reportedly persists for many years following the initial trauma (Chen et al., 2011).
Capturing brain changes in mild TBI is difficult, and it is possible that other neuroimaging metrics not directly examined here (e.g., cortical thickness) may also influence the CBF-WM associations observed in the current study. For example, a study by Duering et al. (2012) used longitudinal MRI methods to study how subcortical infarcts influence cortical morphology post-stroke. They found that damage to subcortical white matter initiated a secondary neurodegenerative process within cortical gray matter. Moreover, structural changes in the form of cerebral atrophy have also been linked to CBF reductions in other clinical populations (Appelman et al., 2008, Wirth et al., 2016). Unfortunately, work teasing apart primary and secondary injury processes within the context of TBI is still in its infancy, and prospective and longitudinal study designs with well-characterized samples are needed to tease apart how brain variables may interact with one another, especially over time, and ultimately influence behavioral outcomes.
Our secondary analyses revealed that the observed CBF and WM relationship in those furthest removed from their injury was not driven by fundamental differences in psychological, post-concussive, health, or injury characteristics relative to those whom were closer in time to their injury. Interestingly, both CBF and WM alterations have also been observed in those with elevated vascular risk in mid-to-late life (Beason-Held et al., 2012, Bangen et al., 2014, Maillard et al., 2015, Wang et al., 2007); however, it is unclear to what extent TBI may increase the prevalence of vascular risk factors and whether individuals with elevated vascular risk are uniquely vulnerable to negative brain changes post-TBI. Future studies that include more comprehensive assessment of vascular risk are needed to understand how history of TBI and vascular risk factors may interact to affect the brain, cognition, and functional outcomes post-injury.
To our knowledge, this is the first study to investigate both ASL and DTI in the context of military TBI. However, there are several limitations that warrant discussion. Given the cross-sectional nature of this study, we cannot determine causal relationships between reduced CBF and reduced FA. Secondly, we were unable to explore whether these associations differ across mechanism of injury (i.e., blast versus blunt) or with blast-exposure given sample size restrictions. As is common with military studies of TBI, diagnosis of mild or moderate TBI was based entirely upon retrospective self-report of injuries and may therefore be subject to recall bias. We chose to examine CBF and WM of two closely linked neuroanatomical regions that are known to be vulnerable to the effects of neurotrauma; however, future studies will need to examine these effects across the brain and with other DTI metrics (i.e., axial and radial diffusivity) to further elucidate CBF and WM relationships. Replication with larger sample sizes and longitudinal designs are also needed to provide more insight into the complex associations between WM integrity and CBF at different stages post-TBI.
5. Conclusion
Taken together, results indicate that, even after adjusting for psychiatric symptomatology, an association between CBF and WM exists in those with history of mmTBI. Although the exact nature and timeline of brain changes post-TBI is unclear, CBF and WM alterations may play a pivotal role in the increased risk for negative health outcomes (e.g. stroke, dementia) that are observed in individuals with history of TBI. Currently, there is an ever-pressing need to consider how brain changes may differ with time and what might mediate or moderate these changes following injury. These findings contribute to our understanding of the possible dynamic relationship between CBF and white matter integrity, and they enhance our understanding of potential pathophysiological mechanisms that exist in the post-acute phase of injury.
Compliance with ethical standards & disclosures
All procedures involved in this study were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975. Informed consent was obtained from all patients included in the study. Alexandra Clark, Katherine Bangen, Lisa Delano-Wood, Scott Sorg, Dawn Schiehser, Nicole Evangelista, and Thomas Liu declare no conflicts of interest.
Sources of funding
This work was supported by grants awarded by the Veterans Affairs: a Career Development Award to D.S. (2-065-10S) and Merit Award to L.D.-W (829-MR-NB-25860). This work was further supported by a Department of Defense Investigator-Initiated Research Grant to L.D.-W. (W81XWH-10-2-0169).
Acknowledgements
The authors would like to thank all OEF/OIF/OND Veterans for their service and are extremely appreciative of those who volunteered to participate in this study. In addition, a special thanks is owed to the Veterans Affairs CESAMH at the Veterans Affairs San Diego Healthcare System for their organizational assistance and to the wonderful research assistants (RK, EL, NL) who dedicate their time to our research lab.
References
- Appelman A.P., Van der Graaf Y., Vincken K.L., Tiehuis A.M., Witkamp T.D., Mali W.P.…SMART Study Group Total cerebral blood flow, white matter lesions and brain atrophy: the SMART-MR study. J. Cereb. Blood Flow Metab. 2008;28(3):633–639. doi: 10.1038/sj.jcbfm.9600563. [DOI] [PubMed] [Google Scholar]
- Bangen K.J., Nation D.A., Clark L.R., Harmell A.L., Wierenga C.E., Dev S.I.…Bondi M.W. Interactive effects of vascular risk burden and advanced age on cerebral blood flow. Front. Aging Neurosci. 2014;6:159. doi: 10.3389/fnagi.2014.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes D.E., Kaup A., Kirby K.A., Byers A.L., Diaz-Arrastia R., Yaffe K. Traumatic brain injury and risk of dementia in older veterans. Neurology. 2014;83(4):312–319. doi: 10.1212/WNL.0000000000000616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beason-Held L.L., Thambisetty M., Deib G., Sojkova J., Landman B.A., Zonderman A.B. Baseline cardiovascular risk predicts subsequent changes in resting brain function. Stroke. 2012;43(6):1542–1547. doi: 10.1161/STROKEAHA.111.638437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A.T., Steer R.A., Brown G.K. Psychology Corporation; San Antonio, TX: 1996. Manual for the Beck Depression Inventory-II. [Google Scholar]
- Bendlin B.B., Ries M.L., Lazar M., Alexander A.L., Dempsey R.J., Rowley H.A.…Johnson S.C. Longitudinal changes in patients with traumatic brain injury assessed with diffusion-tensor and volumetric imaging. NeuroImage. 2008;42(2):503–514. doi: 10.1016/j.neuroimage.2008.04.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernbaum M., Menon B.K., Fick G., Smith E.E., Goyal M., Frayne R., Coutts S.B. Reduced blood flow in normal white matter predicts development of leukoaraiosis. J. Cereb. Blood Flow Metab. 2015;35(10):1610–1615. doi: 10.1038/jcbfm.2015.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigler E.D. Anterior and middle cranial fossa in traumatic brain injury: relevant neuroanatomy and neuropathology in the study of neuropsychological outcome. Neuropsychology. 2007;21(5):515–531. doi: 10.1037/0894-4105.21.5.515. [DOI] [PubMed] [Google Scholar]
- Bigler E.D., Maxwell W.L. Neuropathology of mild traumatic brain injury: relationship to neuroimaging findings. Brain Imaging Behav. 2012;6(2):108–136. doi: 10.1007/s11682-011-9145-0. [DOI] [PubMed] [Google Scholar]
- Bigler E.D. Neuroimaging biomarkers in mild traumatic brain injury (mTBI) Neuropsychol. Rev. 2013;23(3):169–209. doi: 10.1007/s11065-013-9237-2. [DOI] [PubMed] [Google Scholar]
- Bigler E.D. Traumatic brain injury, neuroimaging, and neurodegeneration. Front. Hum. Neurosci. 2013;7:395. doi: 10.3389/fnhum.2013.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennow K., Hardy J., Zetterberg H. The neuropathology and neurobiology of traumatic brain injury. Neuron. 2012;76(5):886–899. doi: 10.1016/j.neuron.2012.11.021. [DOI] [PubMed] [Google Scholar]
- Brenner L.A. Neuropsychological and neuroimaging findings in traumatic brain injury and post-traumatic stress disorder. Dialogues in clinical neuroscience. 2011;13(3):311–323. doi: 10.31887/DCNS.2011.13.3/lbrenner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman A.M., Zahra A., Muraskin J., Steffener J., Holland C.M., Habeck C.…Stern Y. Reduction in cerebral blood flow in areas appearing as white matter hyperintensities on magnetic resonance imaging. Psychiatry Res. 2009;172(2):117–120. doi: 10.1016/j.pscychresns.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke J.F., Stulc J.L., Skolarus L.E., Sears E.D., Zahuranec D.B., Morgenstern L.B. Traumatic brain injury may be an independent risk factor for stroke. Neurology. 2013;81(1):33–39. doi: 10.1212/WNL.0b013e318297eecf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzynska A.Z., Wong C.N., Voss M.W., Cooke G.E., McAuley E., Kramer A.F. White matter integrity supports BOLD signal variability and cognitive performance in the aging human brain. PLoS One. 2015;10(4) doi: 10.1371/journal.pone.0120315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A.L., Delano-Wood L., Sorg S.F., Werhane M.L., Hanson K.L., Schiehser D.M. Cognitive fatigue is associated with reduced anterior internal capsule integrity in veterans with history of mild to moderate traumatic brain injury. Brain Imaging Behav. 2016:1–7. doi: 10.1007/s11682-016-9594-6. [ePub ahead of print] [DOI] [PubMed] [Google Scholar]
- Chalela J.A., Alsop D.C., Gonzalez-Atavales J.B., Maldjian J.A., Kasner S.E., Detre J.A. Magnetic resonance perfusion imaging in acute ischemic stroke using continuous arterial spin labeling. Stroke. 2000;31(3):680–687. doi: 10.1161/01.str.31.3.680. [DOI] [PubMed] [Google Scholar]
- Chatelin S., Deck C., Renard F., Kremer S., Heinrich C., Armspach J.P., Willinger R. Computation of axonal elongation in head trauma finite element simulation. J. Mech. Behav. Biomed. Mater. 2011;4(8):1905–1919. doi: 10.1016/j.jmbbm.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Chen J.J., Rosas H.D., Salat D.H. The relationship between cortical blood flow and sub-cortical white-matter health across the adult age span. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0056733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.H., Kang J.H., Lin H.C. Patients with traumatic brain injury: population-based study suggests increased risk of stroke. Stroke. 2011;42(10):2733–2739. doi: 10.1161/STROKEAHA.111.620112. [DOI] [PubMed] [Google Scholar]
- Combs H.L., Berry D.T., Pape T., Babcock-Parziale J., Smith B., Schleenbaker R.…High W.M., Jr. The effects of mild traumatic brain injury, post-traumatic stress disorder, and combined mild traumatic brain injury/post-traumatic stress disorder on returning veterans. J. Neurotrauma. 2015;32(13):956–966. doi: 10.1089/neu.2014.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concha L., Gross D.W., Beaulieu C. Diffusion tensor tractography of the limbic system. AJNR Am. J. Neuroradiol. 2005;26(9):2267–2274. [PMC free article] [PubMed] [Google Scholar]
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Croall I.D., Cowie C.J., He J., Peel A., Wood J., Aribisala B.S.…Blamire A.M. White matter correlates of cognitive dysfunction after mild traumatic brain injury. Neurology. 2014;83(6):494–501. doi: 10.1212/WNL.0000000000000666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- DeKosky S.T., Blennow K., Ikonomovic M.D., Gandy S. Acute and chronic traumatic encephalopathies: pathogenesis and biomarkers. Nat. Rev. Neurol. 2013;9(4):192–200. doi: 10.1038/nrneurol.2013.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delano-Wood L., Bangen K.J., Sorg S.F., Clark A.L., Schiehser D.M., Luc N.…Bigler E.D. Brainstem white matter integrity is related to loss of consciousness and postconcussive symptomatology in veterans with chronic mild to moderate traumatic brain injury. Brain Imaging Behav. 2015;9(3):500–512. doi: 10.1007/s11682-015-9432-2. [DOI] [PubMed] [Google Scholar]
- Defense and Veterans Brain Injury Center Department of Defense numbers for TBI–worldwide totals. 2016. http://dvbic.dcoe.mil/files/tbi-numbers/DoD-TBI-Worldwide-Totals_2016_Q1_May-16-2016_v1.0_2016-06-24_1.pdf Retrieved from:
- Department of Veterans Affairs and Department of Defense VA/DOD clinical practice guideline for the management of concussion/mild traumatic brain injury. 2009. http://www.healthquality.va.gov/guidelines/Rehab/mtbi/concussion_mtbi_full_1_0.pdf Retrieved from.
- Desikan R.S., Segonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D.…Killiany R.J. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Doshi H., Wiseman N., Liu J., Wang W., Welch R.D., O'Neil B.J.…Kou Z. Cerebral hemodynamic changes of mild traumatic brain injury at the acute stage. PLoS One. 2015;10(2) doi: 10.1371/journal.pone.0118061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duering M., Righart R., Csanadi E., Jouvent E., Hervé D., Chabriat H., Dichgans M. Incident subcortical infarcts induce focal thinning in connected cortical regions. Neurology. 2012;79(20):2025–2028. doi: 10.1212/WNL.0b013e3182749f39. [DOI] [PubMed] [Google Scholar]
- Eierud C., Craddock R.C., Fletcher S., Aulakh M., King-Casas B., Kuehl D., LaConte S.M. Neuroimaging after mild traumatic brain injury: review and meta-analysis. NeuroImage. 2014;4:283–294. doi: 10.1016/j.nicl.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas O., Povlishock J.T. Cellular and subcellular change evoked by diffuse traumatic brain injury: a complex web of change extending far beyond focal damage. Prog. Brain Res. 2007;161:43–59. doi: 10.1016/S0079-6123(06)61004-2. [DOI] [PubMed] [Google Scholar]
- Fortier C.B., Amick M.M., Grande L., McGlynn S., Kenna A., Morra L.…McGlinchey R.E. The Boston Assessment of Traumatic Brain Injury-Lifetime (BAT-L) semistructured interview: evidence of research utility and validity. J. Head Trauma Rehabil. 2014;29(1):89–98. doi: 10.1097/HTR.0b013e3182865859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridley J., Robertson C., Gopinath S. Quantitative lobar cerebral blood flow for outcome prediction after traumatic brain injury. J. Neurotrauma. 2015;32(2):75–82. doi: 10.1089/neu.2014.3350. [DOI] [PubMed] [Google Scholar]
- Ge Y., Patel M.B., Chen Q., Grossman E.J., Zhang K., Miles L.…Grossman R.I. Assessment of thalamic perfusion in patients with mild traumatic brain injury by true FISP arterial spin labelling MR imaging at 3T. Brain Inj. 2009;23(7):666–674. doi: 10.1080/02699050903014899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve M.W., Zink B.J. Pathophysiology of traumatic brain injury. Mt Sinai J. Med. 2009;76(2):97–104. doi: 10.1002/msj.20104. [DOI] [PubMed] [Google Scholar]
- Hoge C.W., McGurk D., Thomas J.L., Cox A.L., Engel C.C., Castro C.A. Mild traumatic brain injury in U.S. soldiers returning from Iraq. N. Engl. J. Med. 2008;358(5):453–463. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- Johnson V.E., Stewart J.E., Begbie F.D., Trojanowski J.Q., Smith D.H., Stewart W. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain. 2013;136(Pt 1):28–42. doi: 10.1093/brain/aws322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D.K., Horsfield M.A., Simmons A. Optimal strategies for measuring diffusion in anisotropic systems by magnetic resonance imaging. Magnetic Resonance in Medicine. 1999;42(3):515–525. [PubMed] [Google Scholar]
- Jorge R.E., Acion L., White T., Tordesillas-Gutierrez D., Pierson R., Crespo-Facorro B., Magnotta V.A. White matter abnormalities in veterans with mild traumatic brain injury. Am. J. Psychiatry. 2012;169(12):1284–1291. doi: 10.1176/appi.ajp.2012.12050600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y., Wong E.C., Liu T.T. Multiphase pseudocontinuous arterial spin labeling (MP-PCASL) for robust quantification of cerebral blood flow. Magn. Reson. Med. 2010;64(3):799–810. doi: 10.1002/mrm.22465. [DOI] [PubMed] [Google Scholar]
- Keane T.M., Fairbank J.A., Caddell J.M., Zimering R.T., Taylor K.L., Mora C.A. Clinical evaluation of a measure to assess combat exposure. Psychol. Assess. 1989;1(1):53. [Google Scholar]
- Kenney K., Amyot F., Haber M., Pronger A., Bogoslovsky T., Moore C., Diaz-Arrastia R. Cerebral vascular injury in traumatic brain injury. Exp. Neurol. 2015 doi: 10.1016/j.expneurol.2015.05.019. [DOI] [PubMed] [Google Scholar]
- King P.R., Donnelly K.T., Donnelly J.P., Dunnam M., Warner G., Kittleson C.J.…Meier S.T. Psychometric study of the neurobehavioral symptom inventory. J. Rehabil. Res. Dev. 2012;49(6):879–888. doi: 10.1682/jrrd.2011.03.0051. [DOI] [PubMed] [Google Scholar]
- LaPlaca M.C., Simon C.M., Prado G.R., Cullen D.K. CNS injury biomechanics and experimental models. Prog. Brain Res. 2007;161:13–26. doi: 10.1016/S0079-6123(06)61002-9. [DOI] [PubMed] [Google Scholar]
- LaPlaca M.C., Prado G.R. Neural mechanobiology and neuronal vulnerability to traumatic loading. J. Biomech. 2010;43(1):71–78. doi: 10.1016/j.jbiomech.2009.09.011. [DOI] [PubMed] [Google Scholar]
- Lee Y.K., Hou S.W., Lee C.C., Hsu C.Y., Huang Y.S., Su Y.C. Increased risk of dementia in patients with mild traumatic brain injury: a nationwide cohort study. PLoS One. 2013;8(5) doi: 10.1371/journal.pone.0062422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin H.S., Wilde E., Troyanskaya M., Petersen N.J., Scheibel R., Newsome M.…Li X. Diffusion tensor imaging of mild to moderate blast-related traumatic brain injury and its sequelae. J. Neurotrauma. 2010;27(4):683–694. doi: 10.1089/neu.2009.1073. [DOI] [PubMed] [Google Scholar]
- Ling J.M., Pena A., Yeo R.A., Merideth F.L., Klimaj S., Gasparovic C., Mayer A.R. Biomarkers of increased diffusion anisotropy in semi-acute mild traumatic brain injury: a longitudinal perspective. Brain. 2012;135(Pt 4):1281–1292. doi: 10.1093/brain/aws073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippa S.M., Pastorek N.J., Benge J.F., Thornton G.M. Postconcussive symptoms after blast and nonblast-related mild traumatic brain injuries in Afghanistan and Iraq war veterans. J. Int. Neuropyschol. Soc. 2010;16(05):856–866. doi: 10.1017/S1355617710000743. [DOI] [PubMed] [Google Scholar]
- Lippa S.M., Fonda J.R., Fortier C.B., Amick M.A., Kenna A., Milberg W.P., McGlinchey R.E. Deployment-related psychiatric and behavioral conditions and their association with functional disability in OEF/OIF/OND veterans. J. Trauma. Stress. 2015;28(1):25–33. doi: 10.1002/jts.21979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillard P., Carmichael O.T., Reed B., Mungas D., DeCarli C. Cooccurrence of vascular risk factors and late-life white-matter integrity changes. Neurobiol. Aging. 2015;36(4):1670–1677. doi: 10.1016/j.neurobiolaging.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Donald C.L., Johnson A.M., Cooper D., Nelson E.C., Werner N.J., Shimony J.S.…Brody D.L. Detection of blast-related traumatic brain injury in U.S. military personnel. N. Engl. J. Med. 2011;364(22):2091–2100. doi: 10.1056/NEJMoa1008069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnuson J., Leonessa F., Ling G.S. Neuropathology of explosive blast traumatic brain injury. Curr. Neurol. Neurosci. Rep. 2012;12(5):570–579. doi: 10.1007/s11910-012-0303-6. [DOI] [PubMed] [Google Scholar]
- Mayer A.R., Ling J.M., Yang Z., Pena A., Yeo R.A., Klimaj S. Diffusion abnormalities in pediatric mild traumatic brain injury. J. Neurosci. 2012;32(50):17961–17969. doi: 10.1523/JNEUROSCI.3379-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister T.W., Sparling M.B., Flashman L.A., Saykin A.J. Neuroimaging findings in mild traumatic brain injury. J. Clin. Exp. Neuropyschol. 2001;23(6):775–791. doi: 10.1076/jcen.23.6.775.1026. [DOI] [PubMed] [Google Scholar]
- Meier T.B., Bellgowan P.S., Singh R., Kuplicki R., Polanski D.W., Mayer A.R. Recovery of cerebral blood flow following sports-related concussion. JAMA Neurol. 2015;72(5):530–538. doi: 10.1001/jamaneurol.2014.4778. [DOI] [PubMed] [Google Scholar]
- Metting Z., Cerliani L., Rodiger L.A., van der Naalt J. Pathophysiological concepts in mild traumatic brain injury: diffusion tensor imaging related to acute perfusion CT imaging. PLoS One. 2013;8(5) doi: 10.1371/journal.pone.0064461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D.R., Hayes J.P., Lafleche G., Salat D.H., Verfaellie M. White matter abnormalities are associated with chronic postconcussion symptoms in blast-related mild traumatic brain injury. Hum. Brain Mapp. 2016;37(1):220–229. doi: 10.1002/hbm.23022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niogi S.N., Mukherjee P. Diffusion tensor imaging of mild traumatic brain injury. J. Head Trauma Rehabil. 2010;25(4):241–255. doi: 10.1097/HTR.0b013e3181e52c2a. [DOI] [PubMed] [Google Scholar]
- O'Sullivan M., Lythgoe D.J., Pereira A.C., Summers P.E., Jarosz J.M., Williams S.C., Markus H.S. Patterns of cerebral blood flow reduction in patients with ischemic leukoaraiosis. Neurology. 2002;59(3):321–326. doi: 10.1212/wnl.59.3.321. [DOI] [PubMed] [Google Scholar]
- Petrie E.C., Cross D.J., Yarnykh V.L., Richards T., Martin N.M., Pagulayan K.…Peskind E.R. Neuroimaging, behavioral, and psychological sequelae of repetitive combined blast/impact mild traumatic brain injury in Iraq and Afghanistan war veterans. J. Neurotrauma. 2014;31(5):425–436. doi: 10.1089/neu.2013.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponto L.L., Brashers-Krug T.M., Pierson R.K., Menda Y., Acion L., Watkins G.L.…Jorge R.E. Preliminary investigation of cerebral blood flow and amyloid burden in veterans with and without combat-related traumatic brain injury. J. Neuropsychiatry Clin. Neurosci. 2016;28(2):89–96. doi: 10.1176/appi.neuropsych.15050106. [DOI] [PubMed] [Google Scholar]
- Povlishock J.T., Katz D.I. Update of neuropathology and neurological recovery after traumatic brain injury. J. Head Trauma Rehabil. 2005;20(1):76–94. doi: 10.1097/00001199-200501000-00008. [DOI] [PubMed] [Google Scholar]
- Promjunyakul N., Lahna D., Kaye J.A., Dodge H.H., Erten-Lyons D., Rooney W.D., Silbert L.C. Characterizing the white matter hyperintensity penumbra with cerebral blood flow measures. Neurol. Clin. 2015;8:224–229. doi: 10.1016/j.nicl.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Promjunyakul N.O., Lahna D.L., Kaye J.A., Dodge H.H., Erten-Lyons D., Rooney W.D., Silbert L.C. Comparison of cerebral blood flow and structural penumbras in relation to white matter hyperintensities: a multi-modal magnetic resonance imaging study. J. Cereb. Blood Flow Metab. 2016 doi: 10.1177/0271678X16651268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese T.G., Heid O., Weisskoff R.M., Wedeen V.J. Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Magn. Reson. Med. 2003;49(1):177–182. doi: 10.1002/mrm.10308. [DOI] [PubMed] [Google Scholar]
- Ruff R.M., Camenzuli L., Mueller J. Miserable minority: Emotional risk factors that influence the outcome of a mild traumatic brain injury. Brain Inj. 1996;10(8):551–566. doi: 10.1080/026990596124124. [DOI] [PubMed] [Google Scholar]
- Salat D.H. Imaging small vessel-associated white matter changes in aging. Neuroscience. 2014;276:174–186. doi: 10.1016/j.neuroscience.2013.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiehser D.M., Twamley E.W., Liu L., Matevosyan A., Filoteo J.V., Jak A.J.…Delano-Wood L. The relationship between postconcussive symptoms and quality of life in veterans with mild to moderate traumatic brain injury. J. Head Trauma Rehabil. 2015;30(4):E21–E28. doi: 10.1097/HTR.0000000000000065. [DOI] [PubMed] [Google Scholar]
- Shin D.D., Ozyurt I.B., Liu T.T. The cerebral blood flow biomedical informatics research network (CBFBIRN) database and analysis pipeline for arterial spin labeling MRI data. Front. Neuroinform. 2013;7:21. doi: 10.3389/fninf.2013.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E., Johansen-Berg H.…Matthews P.M. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl. 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Steketee R.M., Meijboom R., de Groot M., Bron E.E., Niessen W.J., van der Lugt A.…Smits M. Concurrent white and gray matter degeneration of disease-specific networks in early-stage Alzheimer's disease and behavioral variant frontotemporal dementia. Neurobiol. Aging. 2016;43:119–128. doi: 10.1016/j.neurobiolaging.2016.03.031. [DOI] [PubMed] [Google Scholar]
- Stroupe K.T., Smith B.M., Hogan T.P. Healthcare utilization and costs of veterans screened and assessed for traumatic brain injury. J. Rehabil. Res. Dev. 2013;50:1047–1068. doi: 10.1682/JRRD.2012.06.0107. [DOI] [PubMed] [Google Scholar]
- Tanielian T., Jaycox L.H. 2008. Invisible wounds of war: Psychological and cognitive injuries, their consequences, and services to assist recovery. Santa Monica CA. [Google Scholar]
- ten Dam V.H., van den Heuvel D.M., de Craen A.J., Bollen E.L., Murray H.M., Westendorp R.G.…van Buchem M.A. Decline in total cerebral blood flow is linked with increase in periventricular but not deep white matter hyperintensities. Radiology. 2007;243(1):198–203. doi: 10.1148/radiol.2431052111. [DOI] [PubMed] [Google Scholar]
- Terrio H.P., Nelson L.A., Betthauser L.M., Harwood J.E., Brenner L.A. Postdeployment traumatic brain injury screening questions: sensitivity, specificity, and predictive values in returning soldiers. Rehabil. Psychol. 2011;56(1):26–31. doi: 10.1037/a0022685. [DOI] [PubMed] [Google Scholar]
- Vanderploeg R.D., Curtiss G., Belanger H.G. Long-term neuropsychological outcomes following mild traumatic brain injury. J. Int. Neuropsychol. Soc. 2005;11(3):228–236. doi: 10.1017/S1355617705050289. [DOI] [PubMed] [Google Scholar]
- Vanderploeg R.D., Curtiss G., Luis C.A., Salazar A.M. Long-term morbidities following self-reported mild traumatic brain injury. J. Clin. Exp. Neuropsychol. 2007;29(6):585–598. doi: 10.1080/13803390600826587. [DOI] [PubMed] [Google Scholar]
- Vas A., Chapman S., Aslan S., Spence J., Keebler M., Rodriguez-Larrain G.…Krawczyk D. Reasoning training in veteran and civilian traumatic brain injury with persistent mild impairment. Neuropsychol. Rehabil. 2016;26(4):502–531. doi: 10.1080/09602011.2015.1044013. [DOI] [PubMed] [Google Scholar]
- Venkatesan U.M., Dennis N.A., Hillary F.G. Chronology and chronicity of altered resting-state functional connectivity after traumatic brain injury. J. Neurotrauma. 2015;32(4):252–264. doi: 10.1089/neu.2013.3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada T., Asano Y., Shinoda J. Decreased fractional anisotropy evaluated using tract-based spatial statistics and correlated with cognitive dysfunction in patients with mild traumatic brain injury in the chronic stage. AJNR Am. J. Neuroradiol. 2012;33(11):2117–2122. doi: 10.3174/ajnr.A3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., Benner T., Soensen A.G., Wedeen V.J. Diffusion toolkit: a software package for diffusion imaging data processing and tractography. Proc. Int. Soc. Magn. Reson. Med. 2007;15:3720. [Google Scholar]
- Warden D. Military TBI during the Iraq and Afghanistan wars. J. Head Trauma Rehabil. 2006;21(5):398–402. doi: 10.1097/00001199-200609000-00004. [DOI] [PubMed] [Google Scholar]
- Weathers F.W., Litz B.T., Herman D.S., Huska J.A., Keane T.M. Paper Presented at the the Annual Meeting of the International Society for Traumatic Stress Studies, San Antonio, TX, USA. 1993. The PTSD checklist (PCL): reliability, validity, and diagnostic utility. [Google Scholar]
- Wilde E.A., Bouix S., Tate D.F., Lin A.P., Newsome M.R., Taylor B.A.…York G. Advanced neuroimaging applied to veterans and service personnel with traumatic brain injury: state of the art and potential benefits. Brain Imaging Behav. 2015;9(3):367–402. doi: 10.1007/s11682-015-9444-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth M., Binette A.P., Brunecker P., Köbe T., Witte A.V., Flöel A. Divergent regional patterns of cerebral hypoperfusion and gray matter atrophy in mild cognitive impairment patients. J. Cereb. Blood Flow Metab. 2016 doi: 10.1177/0271678X16641128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T.C., Wilde E.A., Bigler E.D., Yallampalli R., McCauley S.R., Troyanskaya M.…Levin H.S. Evaluating the relationship between memory functioning and cingulum bundles in acute mild traumatic brain injury using diffusion tensor imaging. J. Neurotrauma. 2010;27(2):303–307. doi: 10.1089/neu.2009.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh P.H., Guan Koay C., Wang B., Morissette J., Sham E., Senseney J.…Liu W. Compromised Neurocircuitry in Chronic Blast‐Related Mild Traumatic Brain Injury. Hum. Brain Mapp. 2017;38(1):352–369. doi: 10.1002/hbm.23365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurgil K.A., Barkauskas D.A., Vasterling J.J., Nievergelt C.M., Larson G.E., Schork N.J.…Baker D.G. Association between traumatic brain injury and risk of posttraumatic stress disorder in active-duty marines. JAMA Psychiat. 2014;71(2):149–157. doi: 10.1001/jamapsychiatry.2013.3080. [DOI] [PubMed] [Google Scholar]