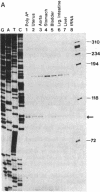
Abstract
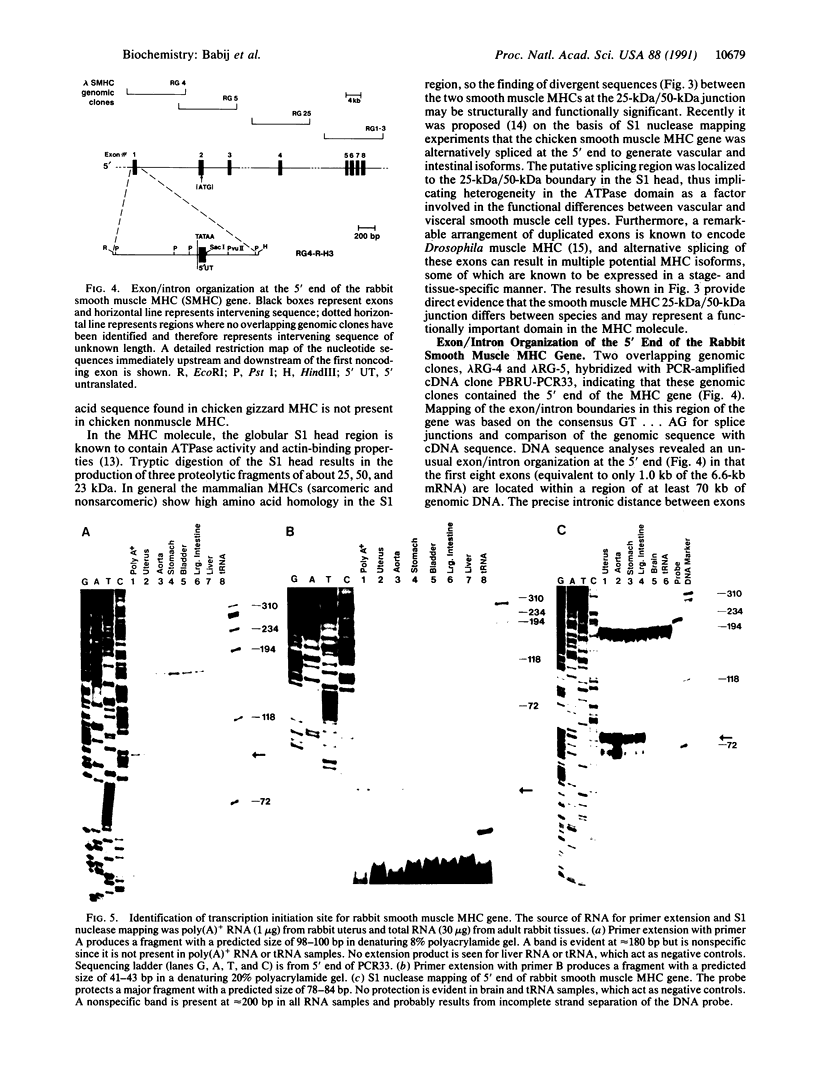
The purpose of this study was to characterize the complete cDNA sequence encoding the rabbit smooth muscle myosin heavy chain (MHC) and determine the exon/intron organization at the 5' end of the corresponding gene. The full-length cDNA sequence of 6644 base pairs encoding a protein of 1972 amino acids was generated from two cDNA clones: PBRUC1 (approximately 6.3 kilobases), isolated from a rabbit uterus cDNA library, and PBRU-PCR33 (420 base pairs), produced by primer extension and PCR amplification. Compared with the chicken smooth muscle MHC sequence [Yanagisawa, M., Hamada, Y., Katsuragawa, Y., Imamura, M., Mikawa, T. & Masaki, T. (1987) J. Mol. Biol. 198, 143-157] the rabbit MHC shares about 90% amino acid identity in the S1 globular head region but shows a striking sequence divergence at the junction between the 25-kDa and 50-kDa proteolytic fragments of the functionally important S1 head domain. Genomic cloning shows that the rabbit smooth muscle MHC gene is large and has an unusual exon/intron organization at the 5' end. The first eight contiguous exons are located within a region of at least 70 kilobases of genomic DNA. Some introns span several kilobases of DNA and others at the 5' end show a high degree of intron conservation in the Mg(2+)-ATPase domain when compared with more distantly related sarcomeric MHC genes. Primer extension and S1 nuclease mapping analysis demonstrate that transcription initiates from a single site in the rabbit smooth muscle MHC gene.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babij P., Periasamy M. Myosin heavy chain isoform diversity in smooth muscle is produced by differential RNA processing. J Mol Biol. 1989 Dec 5;210(3):673–679. doi: 10.1016/0022-2836(89)90142-3. [DOI] [PubMed] [Google Scholar]
- Burke J. F. High-sensitivity S1 mapping with single-stranded [32P]DNA probes synthesized from bacteriophage M13mp templates. Gene. 1984 Oct;30(1-3):63–68. doi: 10.1016/0378-1119(84)90105-7. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Emerson C. P., Jr, Bernstein S. I. Molecular genetics of myosin. Annu Rev Biochem. 1987;56:695–726. doi: 10.1146/annurev.bi.56.070187.003403. [DOI] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George E. L., Ober M. B., Emerson C. P., Jr Functional domains of the Drosophila melanogaster muscle myosin heavy-chain gene are encoded by alternatively spliced exons. Mol Cell Biol. 1989 Jul;9(7):2957–2974. doi: 10.1128/mcb.9.7.2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada Y., Yanagisawa M., Katsuragawa Y., Coleman J. R., Nagata S., Matsuda G., Masaki T. Distinct vascular and intestinal smooth muscle myosin heavy chain mRNAs are encoded by a single-copy gene in the chicken. Biochem Biophys Res Commun. 1990 Jul 16;170(1):53–58. doi: 10.1016/0006-291x(90)91239-o. [DOI] [PubMed] [Google Scholar]
- Ketchum A. S., Stewart C. T., Stewart M., Kiehart D. P. Complete sequence of the Drosophila nonmuscle myosin heavy-chain transcript: conserved sequences in the myosin tail and differential splicing in the 5' untranslated sequence. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6316–6320. doi: 10.1073/pnas.87.16.6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug M. S., Berger S. L. First-strand cDNA synthesis primed with oligo(dT). Methods Enzymol. 1987;152:316–325. doi: 10.1016/0076-6879(87)52036-5. [DOI] [PubMed] [Google Scholar]
- Lytton J., Zarain-Herzberg A., Periasamy M., MacLennan D. H. Molecular cloning of the mammalian smooth muscle sarco(endo)plasmic reticulum Ca2+-ATPase. J Biol Chem. 1989 Apr 25;264(12):7059–7065. [PubMed] [Google Scholar]
- Mahdavi V., Chambers A. P., Nadal-Ginard B. Cardiac alpha- and beta-myosin heavy chain genes are organized in tandem. Proc Natl Acad Sci U S A. 1984 May;81(9):2626–2630. doi: 10.1073/pnas.81.9.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina M. I., Kropp K. E., Gulick J., Robbins J. The sequence of an embryonic myosin heavy chain gene and isolation of its corresponding cDNA. J Biol Chem. 1987 May 15;262(14):6478–6488. [PubMed] [Google Scholar]
- Nagai R., Kuro-o M., Babij P., Periasamy M. Identification of two types of smooth muscle myosin heavy chain isoforms by cDNA cloning and immunoblot analysis. J Biol Chem. 1989 Jun 15;264(17):9734–9737. [PubMed] [Google Scholar]
- Nagai R., Larson D. M., Periasamy M. Characterization of a mammalian smooth muscle myosin heavy chain cDNA clone and its expression in various smooth muscle types. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1047–1051. doi: 10.1073/pnas.85.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovner A. S., Thompson M. M., Murphy R. A. Two different heavy chains are found in smooth muscle myosin. Am J Physiol. 1986 Jun;250(6 Pt 1):C861–C870. doi: 10.1152/ajpcell.1986.250.6.C861. [DOI] [PubMed] [Google Scholar]
- Shohet R. V., Conti M. A., Kawamoto S., Preston Y. A., Brill D. A., Adelstein R. S. Cloning of the cDNA encoding the myosin heavy chain of a vertebrate cellular myosin. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7726–7730. doi: 10.1073/pnas.86.20.7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strehler E. E., Mahdavi V., Periasamy M., Nadal-Ginard B. Intron positions are conserved in the 5' end region of myosin heavy-chain genes. J Biol Chem. 1985 Jan 10;260(1):468–471. [PubMed] [Google Scholar]
- Strehler E. E., Strehler-Page M. A., Perriard J. C., Periasamy M., Nadal-Ginard B. Complete nucleotide and encoded amino acid sequence of a mammalian myosin heavy chain gene. Evidence against intron-dependent evolution of the rod. J Mol Biol. 1986 Aug 5;190(3):291–317. doi: 10.1016/0022-2836(86)90003-3. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Hamada Y., Katsuragawa Y., Imamura M., Mikawa T., Masaki T. Complete primary structure of vertebrate smooth muscle myosin heavy chain deduced from its complementary DNA sequence. Implications on topography and function of myosin. J Mol Biol. 1987 Nov 20;198(2):143–157. doi: 10.1016/0022-2836(87)90302-0. [DOI] [PubMed] [Google Scholar]
- Yutzey K. E., Kline R. L., Konieczny S. F. An internal regulatory element controls troponin I gene expression. Mol Cell Biol. 1989 Apr;9(4):1397–1405. doi: 10.1128/mcb.9.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]