Abstract
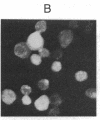
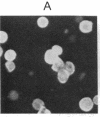
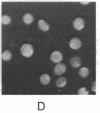
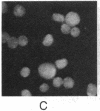
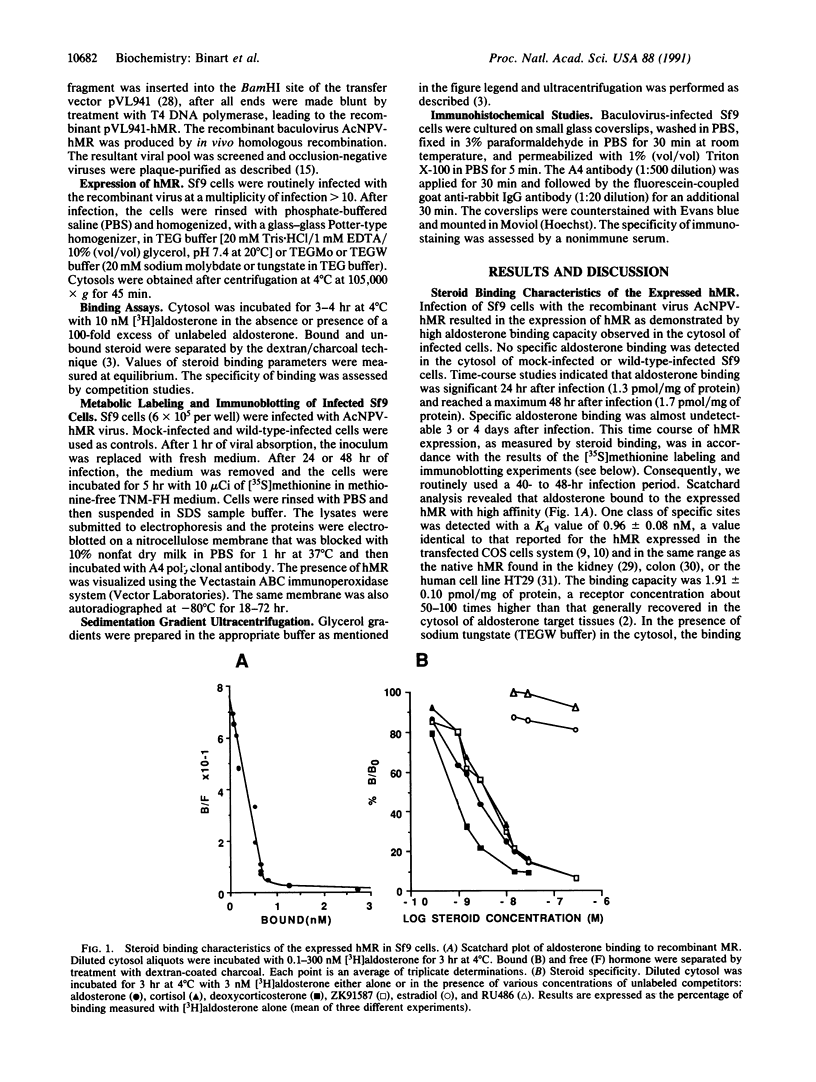
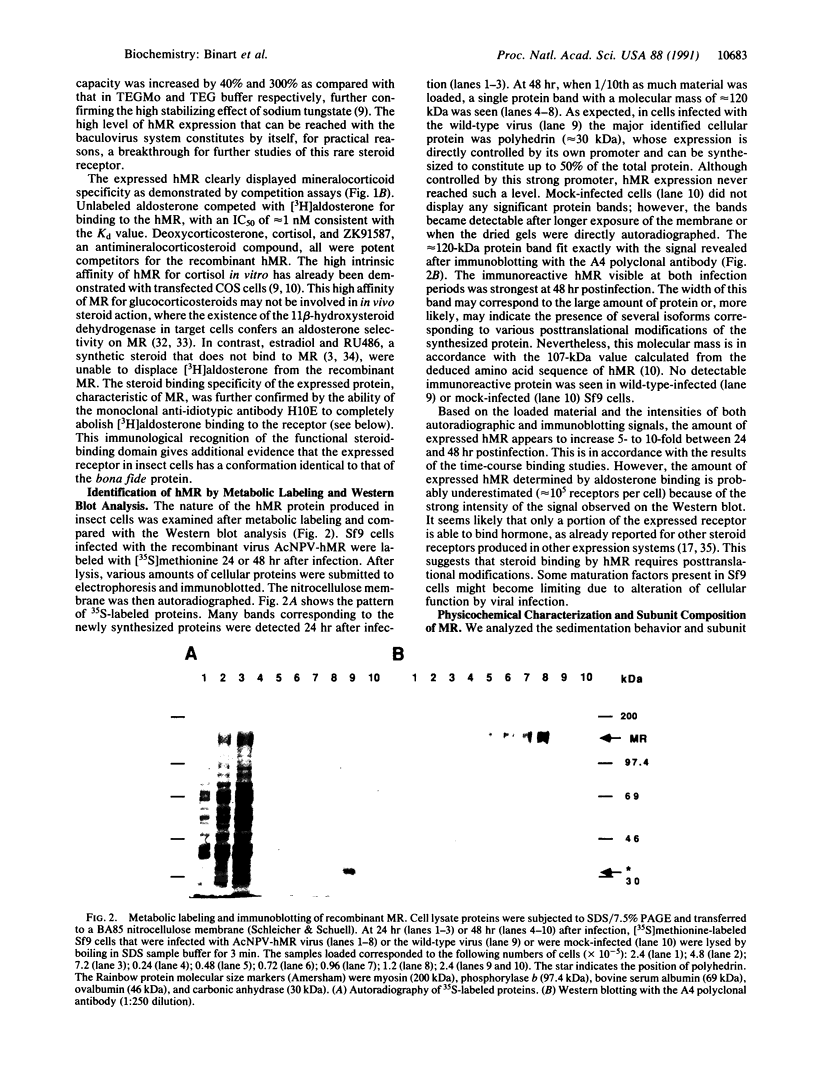
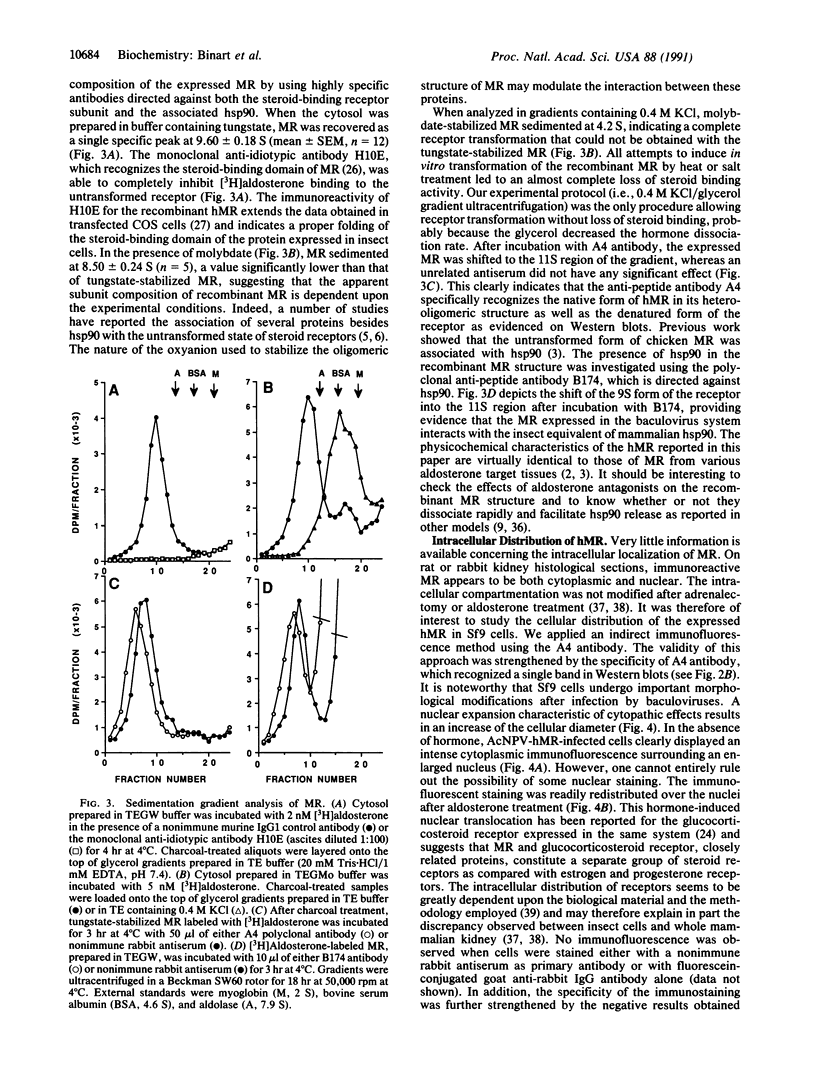
To investigate the structure and function of the mineralocorticosteroid receptor (MR), one has to circumvent the major difficulty related to its very low abundance. For this purpose, the full-length human MR (hMR) has been produced using the efficient baculovirus system. The recombinant protein is overexpressed in Sf9 insect cells at a concentration of approximately 2 pmol/mg of protein, which is 50-100 times more than the concentration in aldosterone target tissues. It binds aldosterone with high affinity (Kd approximately 1 nM) and clearly displays a mineralocorticosteroid specificity as evidenced by competition studies with steroid ligands and by the monoclonal anti-idiotypic antibody H10E interacting with the steroid-binding domain of MR. After [35S]methionine labeling, a single polypeptide band at approximately 120 kDa is detected and further identified as hMR by immunoblotting with A4, an anti-peptide antibody. Sedimentation analyses show that the native form of MR is recognized by A4 and B174, an antibody to the 90-kDa heat shock protein, since they both induce a shift of the receptor from 9S to 11S sedimentation coefficient. These results clearly demonstrate that MR is a heterooligomer containing the insect equivalent of the 90-kDa heat shock protein. This 9S receptor complex is converted to a 4S form during high-salt gradient ultracentrifugation, suggesting that MR can undergo a complete in vitro transformation. Immunofluorescence studies indicate that hMR, which is almost exclusively a cytoplasmic protein in Sf9 cells, is translocated to the nucleus after aldosterone exposure. Therefore, the recombinant hMR seems to behave as the native receptor. Given the possibility of large-scale protein production, the baculovirus system should prove useful in studies of the molecular basis of MR function as a transcription factor.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alnemri E. S., Maksymowych A. B., Robertson N. M., Litwack G. Characterization and purification of a functional rat glucocorticoid receptor overexpressed in a baculovirus system. J Biol Chem. 1991 Feb 25;266(6):3925–3936. [PubMed] [Google Scholar]
- Arriza J. L., Simerly R. B., Swanson L. W., Evans R. M. The neuronal mineralocorticoid receptor as a mediator of glucocorticoid response. Neuron. 1988 Nov;1(9):887–900. doi: 10.1016/0896-6273(88)90136-5. [DOI] [PubMed] [Google Scholar]
- Arriza J. L., Weinberger C., Cerelli G., Glaser T. M., Handelin B. L., Housman D. E., Evans R. M. Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science. 1987 Jul 17;237(4812):268–275. doi: 10.1126/science.3037703. [DOI] [PubMed] [Google Scholar]
- Barkhem T., Carlsson B., Simons J., Möller B., Berkenstam A., Gustafsson J. A., Nilsson S. High level expression of functional full length human thyroid hormone receptor beta 1 in insect cells using a recombinant baculovirus. J Steroid Biochem Mol Biol. 1991 Jun;38(6):667–675. doi: 10.1016/0960-0760(91)90077-i. [DOI] [PubMed] [Google Scholar]
- Cato A. C., Weinmann J. Mineralocorticoid regulation of transcription of transfected mouse mammary tumor virus DNA in cultured kidney cells. J Cell Biol. 1988 Jun;106(6):2119–2125. doi: 10.1083/jcb.106.6.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards C. R., Stewart P. M., Burt D., Brett L., McIntyre M. A., Sutanto W. S., de Kloet E. R., Monder C. Localisation of 11 beta-hydroxysteroid dehydrogenase--tissue specific protector of the mineralocorticoid receptor. Lancet. 1988 Oct 29;2(8618):986–989. doi: 10.1016/s0140-6736(88)90742-8. [DOI] [PubMed] [Google Scholar]
- Eisen L. P., Harmon J. M. Activation of the rat kidney mineralocorticoid receptor. Endocrinology. 1986 Oct;119(4):1419–1426. doi: 10.1210/endo-119-4-1419. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller P. J., Funder J. W. Mineralocorticoid and glucocorticoid receptors in human kidney. Kidney Int. 1976 Aug;10(2):154–157. doi: 10.1038/ki.1976.89. [DOI] [PubMed] [Google Scholar]
- Funder J. W., Pearce P. T., Smith R., Smith A. I. Mineralocorticoid action: target tissue specificity is enzyme, not receptor, mediated. Science. 1988 Oct 28;242(4878):583–585. doi: 10.1126/science.2845584. [DOI] [PubMed] [Google Scholar]
- Gasc J. M., Delahaye F., Baulieu E. E. Compared intracellular localization of the glucocorticosteroid and progesterone receptors: an immunocytochemical study. Exp Cell Res. 1989 Apr;181(2):492–504. doi: 10.1016/0014-4827(89)90106-7. [DOI] [PubMed] [Google Scholar]
- Greenfield C., Patel G., Clark S., Jones N., Waterfield M. D. Expression of the human EGF receptor with ligand-stimulatable kinase activity in insect cells using a baculovirus vector. EMBO J. 1988 Jan;7(1):139–146. doi: 10.1002/j.1460-2075.1988.tb02793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera R., Lebwohl D., Garcia de Herreros A., Kallen R. G., Rosen O. M. Synthesis, purification, and characterization of the cytoplasmic domain of the human insulin receptor using a baculovirus expression system. J Biol Chem. 1988 Apr 25;263(12):5560–5568. [PubMed] [Google Scholar]
- Ikeda U., Hyman R., Smith T. W., Medford R. M. Aldosterone-mediated regulation of Na+, K(+)-ATPase gene expression in adult and neonatal rat cardiocytes. J Biol Chem. 1991 Jun 25;266(18):12058–12066. [PubMed] [Google Scholar]
- Krozowski Z. S., Rundle S. E., Wallace C., Castell M. J., Shen J. H., Dowling J., Funder J. W., Smith A. I. Immunolocalization of renal mineralocorticoid receptors with an antiserum against a peptide deduced from the complementary deoxyribonucleic acid sequence. Endocrinology. 1989 Jul;125(1):192–198. doi: 10.1210/endo-125-1-192. [DOI] [PubMed] [Google Scholar]
- Lombes M., Claire M., Lustenberger P., Michaud A., Rafestin-Oblin M. E. A new affinity matrix for mineralocorticoid receptors. J Biol Chem. 1987 Jun 15;262(17):8121–8127. [PubMed] [Google Scholar]
- Lombes M., Claire M., Pinto M., Michaud A., Rafestin-Oblin M. E. Aldosterone binding in the human colon carcinoma cell line HT29: correlation with cell differentiation. J Steroid Biochem. 1984 Jan;20(1):329–333. doi: 10.1016/0022-4731(84)90227-9. [DOI] [PubMed] [Google Scholar]
- Lombes M., Edelman I. S., Erlanger B. F. Internal image properties of a monoclonal auto-anti-idiotypic antibody and its binding to aldosterone receptors. J Biol Chem. 1989 Feb 15;264(5):2528–2536. [PubMed] [Google Scholar]
- Lombès M., Farman N., Oblin M. E., Baulieu E. E., Bonvalet J. P., Erlanger B. F., Gasc J. M. Immunohistochemical localization of renal mineralocorticoid receptor by using an anti-idiotypic antibody that is an internal image of aldosterone. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1086–1088. doi: 10.1073/pnas.87.3.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckow V. A., Summers M. D. High level expression of nonfused foreign genes with Autographa californica nuclear polyhedrosis virus expression vectors. Virology. 1989 May;170(1):31–39. doi: 10.1016/0042-6822(89)90348-6. [DOI] [PubMed] [Google Scholar]
- Metzger D., White J. H., Chambon P. The human oestrogen receptor functions in yeast. Nature. 1988 Jul 7;334(6177):31–36. doi: 10.1038/334031a0. [DOI] [PubMed] [Google Scholar]
- Moguilewsky M., Philibert D. RU 38486: potent antiglucocorticoid activity correlated with strong binding to the cytosolic glucocorticoid receptor followed by an impaired activation. J Steroid Biochem. 1984 Jan;20(1):271–276. doi: 10.1016/0022-4731(84)90216-4. [DOI] [PubMed] [Google Scholar]
- Power R. F., Conneely O. M., McDonnell D. P., Clark J. H., Butt T. R., Schrader W. T., O'Malley B. W. High level expression of a truncated chicken progesterone receptor in Escherichia coli. J Biol Chem. 1990 Jan 25;265(3):1419–1424. [PubMed] [Google Scholar]
- Rafestin-Oblin M. E., Couette B., Radanyi C., Lombes M., Baulieu E. E. Mineralocorticosteroid receptor of the chick intestine. Oligomeric structure and transformation. J Biol Chem. 1989 Jun 5;264(16):9304–9309. [PubMed] [Google Scholar]
- Rafestin-Oblin M. E., Lombes M., Michiel J. B., Michaud A., Claire M. Mineralocorticoid receptors in the epithelial cells of human colon and ileum. J Steroid Biochem. 1984 Jan;20(1):311–315. doi: 10.1016/0022-4731(84)90223-1. [DOI] [PubMed] [Google Scholar]
- Renoir J. M., Radanyi C., Faber L. E., Baulieu E. E. The non-DNA-binding heterooligomeric form of mammalian steroid hormone receptors contains a hsp90-bound 59-kilodalton protein. J Biol Chem. 1990 Jun 25;265(18):10740–10745. [PubMed] [Google Scholar]
- Sanchez E. R., Faber L. E., Henzel W. J., Pratt W. B. The 56-59-kilodalton protein identified in untransformed steroid receptor complexes is a unique protein that exists in cytosol in a complex with both the 70- and 90-kilodalton heat shock proteins. Biochemistry. 1990 May 29;29(21):5145–5152. doi: 10.1021/bi00473a021. [DOI] [PubMed] [Google Scholar]
- Schulman G., Miller-Diener A., Litwack G., Bastl C. P. Characterization of the rat colonic aldosterone receptor and its activation process. J Biol Chem. 1986 Sep 15;261(26):12102–12108. [PubMed] [Google Scholar]
- Smith D. F., Faber L. E., Toft D. O. Purification of unactivated progesterone receptor and identification of novel receptor-associated proteins. J Biol Chem. 1990 Mar 5;265(7):3996–4003. [PubMed] [Google Scholar]
- Smith G. E., Summers M. D., Fraser M. J. Production of human beta interferon in insect cells infected with a baculovirus expression vector. Mol Cell Biol. 1983 Dec;3(12):2156–2165. doi: 10.1128/mcb.3.12.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan G., Thompson E. B. Overexpression of full-length human glucocorticoid receptor in Spodoptera frugiperda cells using the baculovirus expression vector system. Mol Endocrinol. 1990 Feb;4(2):209–216. doi: 10.1210/mend-4-2-209. [DOI] [PubMed] [Google Scholar]
- Tsai S. Y., Srinivasan G., Allan G. F., Thompson E. B., O'Malley B. W., Tsai M. J. Recombinant human glucocorticoid receptor induces transcription of hormone response genes in vitro. J Biol Chem. 1990 Oct 5;265(28):17055–17061. [PubMed] [Google Scholar]
- Wright A. P., Carlstedt-Duke J., Gustafsson J. A. Ligand-specific transactivation of gene expression by a derivative of the human glucocorticoid receptor expressed in yeast. J Biol Chem. 1990 Sep 5;265(25):14763–14769. [PubMed] [Google Scholar]