Abstract
Background
The erosion of the early mortality advantage of elective endovascular aneurysm repair (EVAR) compared with open repair of abdominal aortic aneurysm remains without a satisfactory explanation.
Methods
An individual‐patient data meta‐analysis of four multicentre randomized trials of EVAR versus open repair was conducted to a prespecified analysis plan, reporting on mortality, aneurysm‐related mortality and reintervention.
Results
The analysis included 2783 patients, with 14 245 person‐years of follow‐up (median 5·5 years). Early (0–6 months after randomization) mortality was lower in the EVAR groups (46 of 1393 versus 73 of 1390 deaths; pooled hazard ratio 0·61, 95 per cent c.i. 0·42 to 0·89; P = 0·010), primarily because 30‐day operative mortality was lower in the EVAR groups (16 deaths versus 40 for open repair; pooled odds ratio 0·40, 95 per cent c.i. 0·22 to 0·74). Later (within 3 years) the survival curves converged, remaining converged to 8 years. Beyond 3 years, aneurysm‐related mortality was significantly higher in the EVAR groups (19 deaths versus 3 for open repair; pooled hazard ratio 5·16, 1·49 to 17·89; P = 0·010). Patients with moderate renal dysfunction or previous coronary artery disease had no early survival advantage under EVAR. Those with peripheral artery disease had lower mortality under open repair (39 deaths versus 62 for EVAR; P = 0·022) in the period from 6 months to 4 years after randomization.
Conclusion
The early survival advantage in the EVAR group, and its subsequent erosion, were confirmed. Over 5 years, patients of marginal fitness had no early survival advantage from EVAR compared with open repair. Aneurysm‐related mortality and patients with low ankle : brachial pressure index contributed to the erosion of the early survival advantage for the EVAR group. Trial registration numbers: EVAR‐1, ISRCTN55703451; DREAM (Dutch Randomized Endovascular Aneurysm Management), NCT00421330; ACE (Anévrysme de l'aorte abdominale, Chirurgie versus Endoprothèse), NCT00224718; OVER (Open Versus Endovascular Repair Trial for Abdominal Aortic Aneurysms), NCT00094575.
Short abstract
Survival comparable
Introduction
Open repair of abdominal aortic aneurysm (AAA) was first introduced by Dubost in 19511. In the 1990s, the less invasive endovascular aneurysm repair (EVAR) was introduced; EVAR‐12, the first multicentre randomized trial of EVAR versus open repair, was started in 1999 in the UK. This was soon followed by the DREAM3 and ACE4 multicentre trials in Europe, and the OVER trial5 in the USA.
Each of the randomized trials of EVAR versus open repair recruited patients (suitable for either open or endovascular repair) with slightly different entry characteristics with respect to age, sex, aneurysm morphology and other demographics. The EVAR‐1, DREAM and OVER trials all showed an early survival benefit for EVAR, whereas ACE did not. This early survival benefit for EVAR was lost within 1–3 years in the EVAR‐1 and DREAM trials, but not until later in the OVER trial3, 5, 6. This ‘catch‐up’ in mortality has been noted in many other studies, including a Cochrane review7 and analyses of the Medicare database8, but no satisfactory explanation for this phenomenon has emerged. The Cochrane review7 was limited by not being able to report aneurysm‐related mortality or subgroup analyses. Each trial individually has been too small to investigate the reasons for the catch‐up mortality in the EVAR groups, or to answer the much discussed question of whether younger and fitter patients (or other subgroups) should be offered open repair, which is considered more durable than EVAR9, 10. This catch‐up in mortality needs to be avoided if EVAR is to outperform open repair in the longer term. To try to address some of these issues, the investigators of the four RCTs agreed to pool their data for an individual‐patient data meta‐analysis.
Methods
In July 2013, MEDLINE, Embase and clinical trial databases were searched for randomized trials comparing open and endovascular repair of AAAs. The search terms used were: abdominal aortic aneurysm, AAA, endovascular, stent, open repair and randomized trial. From 275 reports, four eligible trials reporting mid‐term follow‐up were identified (Appendix S1, supporting information).
The methods for the four multicentre trials included in this meta‐analysis have been published previously6, 11, 12, 13. The EVAR‐1 trial randomized 1252 patients (90·7 per cent men), with aneurysm diameter more than 5·5 cm, between September 1999 and August 2004 in the UK. The DREAM trial randomized 351 patients (91·7 per cent men), with aneurysm diameter at least 5 cm, between November 2000 and December 2003 in the Netherlands and Belgium. The OVER trial randomized 881 patients (99·4 per cent men), with aneurysm diameter 5·0 cm or larger, between October 2002 and April 2008 at Veterans Affairs hospitals in the USA. The ACE trial randomized 306 patients (99·0 per cent men), with aneurysm diameter exceeding 5·0 cm, between March 2003 and March 2008 in France; seven patients withdrew consent before discharge from hospital. All patients were considered fit for open surgery under general anaesthesia and all trials used approved devices for EVAR, predominantly within the manufacturers' instructions for use, and followed up patients for a minimum of 3 years.
The four data sets were merged based on fields available in the case record forms of the largest trial (EVAR‐1); range checks were conducted and queries resolved with the individual trial coordinating centres. The common baseline variables across the trials were: age, sex, history of smoking, diabetes, coronary artery disease (defined as previous stable or unstable angina or myocardial infarction), BMI, maximum aneurysm diameter, proximal aortic neck length and diameter, ankle : brachial pressure index (ABPI) and creatinine concentration, used for calculation of estimated glomerular filtration rate (eGFR)14 but without information on ethnicity. Each trial also contained some data on hypertension, which was included in a modified Wilkins cardiovascular survival risk score15 (Table S1, supporting information). The reporting of both drug use (including antiplatelet and lipid‐lowering agents) and reinterventions was very different in the four trials (particularly intestinal and wound‐related reinterventions following open repair). The postoperative surveillance protocol was identical for both randomized groups in all trials, except for DREAM where, after 2 years, surveillance was relaxed for the open repair group. However, for complications, only endoleaks after EVAR were reported similarly across the trials; laparotomy‐related complications were not.
Statistical analysis
The primary analyses considered the groups as randomized within each trial. Mortality after randomization was assessed at both 30 days, in hospital and then in three defined intervals: 0–6 months, 6 months to 4 years and more than 4 years after randomization. Aneurysm‐related mortality included: death from primary aneurysm rupture; within 30 days of aneurysm repair or any reintervention; and rupture after repair. Given the different times between randomization and aneurysm repair in the four trials, aneurysm‐related mortality also was assessed at 30 days, 31 days to 3 years and more than 3 years after aneurysm repair. Kaplan–Meier survival curves by randomized group were generated from the combined data from all four trials, and the restricted mean life‐years up to a certain time estimated by the area under the curve up to that time16. Logistic regression was used to compare operative (30‐day) and in‐hospital mortality among patients who underwent aneurysm repair, and Cox proportional hazards regression to compare total and aneurysm‐related mortality and time to reintervention. A two‐stage individual‐patient data meta‐analysis was performed. First analyses were conducted separately within each trial and then pooled time period‐specific estimates were calculated using random‐effects meta‐analysis, with between‐study heterogeneity estimated using the method of DerSimonian and Laird17. The proportion of between‐trial variability beyond that expected by chance was quantified using the I 2 statistic18. All analyses were then repeated, adjusting for the following baseline co‐variables: age, sex, maximum aneurysm diameter and log creatinine.
The subgroups age, sex, eGFR, coronary artery disease, ABPI, modified Wilkins cardiovascular survival risk score, maximum aneurysm diameter, proximal aneurysm neck diameter and neck length were assessed for differences in the effect of the EVAR and open strategies by including an interaction term between the subgroup and randomized group in a Cox regression model. All measures except for sex and coronary artery disease were entered as continuous variables to assess effect modification. Each interaction term was pooled across the trials using random‐effects meta‐analysis and its statistical significance assessed using a Wald test, taking the 5 per cent level as significant. For presentation purposes only (and not for assessing significance), hazard ratios are shown by dichotomizing continuous measures at chosen cut‐off points (age 72 years, eGFR 68·4 ml per min per 1·73 m2, ABPI 0·9, cardiovascular risk score 2 major risk factors, maximum AAA diameter 5·9 cm, neck diameter 2·3 cm, neck length 2·5 cm), estimating the hazard ratios within each subgroup and pooling these across studies.
The hazard of reintervention following aneurysm repair was analysed using a multiple failure time model (Appendix S1, supporting information). In addition, for patients who received EVAR, mortality hazard ratios were investigated in individuals with and without a detected/treated type II endoleak (Appendix S1, supporting information).
All analyses were performed using Stata® version 13 (StataCorp, College Station, Texas, USA).
Results
A total of 2783 patients, with 14 245 person‐years of follow‐up, were included in this meta‐analysis. Baseline characteristics are shown in Table 1; there were significant intertrial differences for all variables. Patients in the EVAR‐1 trial were older and had larger aneurysms than patients in the other trials. Nearly all patients in the OVER and EVAR‐1 trials had a history of smoking, compared with about half of those in the DREAM and ACE trials. Patients in the OVER trial had the highest BMI and this trial had the highest proportion of patients with diabetes. Postrandomization characteristics of the trials are also are summarized in Table 1. The median follow‐up was 6·0, 6·0, 5·4 and 3·1 years for EVAR‐1, DREAM, OVER and ACE respectively, and 5·5 years for the pooled data. Compliance with randomized allocation was at least 92 per cent.
Table 1.
Baseline and postrandomization characteristics of patients in the four trials
| EVAR‐1 (n =1252) | DREAM (n = 351) | OVER (n = 881) | ACE (n = 299) | |
|---|---|---|---|---|
| Baseline variables | ||||
| Age (years)* | 74(6) | 70(7) | 70(8) | 69(7) |
| Men | 1135 (90·7) | 322 (91·7) | 876 (99·4) | 296 (99·0) |
| BMI (kg/m2)* | 26·5(4·5) | 26·7(4·7) | 28·6(5·4) | 27·2(3·5) |
| Smoking status§ | ||||
| Current smoker | 270 (21·6) | 130 (37·0) | 363 (41·2) | 72 (24·1) |
| Ex‐smoker | 863 (68·9) | 78 (22·2) | 481 (54·6) | 75 (25·1) |
| Diabetes | 128 (10·2) | 35 (10·0) | 200 (22·7) | 49 (16·4) |
| Previous angina/MI | 492 (39·3) | 153 (43·6) | 268 (30·4) | 115 (38·5) |
| ABPI*, ¶ | 1·0(0·2) | 1·0(0·2) | 1·0(0·2) | n.a. |
| Creatinine (µmol/l)† | 102 (90–119) | 95 (84–109) | 97 (80–110) | 93 (82–110) |
| EQ‐5D™ score* | 0·82(0·12) | 0·84(0·11) | 0·85(0·09) | n.a. |
| AAA diameter (cm)* | 6·5(0·9) | 6·0(0·9) | 5·7(0·9) | 5·6(0·7) |
| AAA neck length (cm)* | 2·8(1·2) | 2·5(1·2) | 2·6(1·2) | 2·8(1·0) |
| AAA neck diameter (cm)* | 2·35(0·30) | 2·39(0·33) | 2·26(0·35) | 2·36(0·33) |
| Postrandomization parameters | ||||
| Time from randomization to repair (days)† # | 40 (1–576) | 39 (3–209) | 17 (0–290) | 27 (1–203) |
| Commenced repair in compliance with randomization | 1165 (93·1) | 339 (96·6) | 853 (96·8) | 277 (92·6) |
| Follow‐up for mortality (years)† | 6·0 (3·9–7·3) | 6·0 (5·0–6·8) | 5·4 (4·1–6·8) | 3·1 (2·1–3·4) |
| 30‐day operative mortality | ||||
| EVAR | 11 of 614 (1·8) | 2 of 170 (1·2) | 1 of 439 (0·2) | 2 of 150 (1·3) |
| Open repair | 26 of 602 (4·3) | 5 of 173 (2·9) | 8 of 429 (1·9) | 1 of 147 (0·7) |
| Reintervention rate‡ | ||||
| EVAR | 174 of 3381 (5·1) | 77 of 906 (8·5) | 155 of 2334 (6·6) | 32 of 419 (7·6) |
| Open repair | 64 of 3309 (1·9) | 41 of 932 (4·4) | 104 of 2276 (4·6) | 10 of 408 (2·5) |
Values in parentheses are percentages unless indicated otherwise;
values are mean(s.d.),
median (i.q.r.) and
rate per 100 person‐years.
Ex‐smokers in ACE and DREAM were defined as those smoking in the 10 years before randomization.
Mean and median ankle : brachial pressure index (ABPI) were almost identical.
For those who underwent aneurysm repair. EVAR, endovascular aneurysm repair; MI, myocardial infarction; n.a., not available; EQ, EuroQol (EuroQoLGroup, Rotterdam, The Netherlands); AAA, abdominal aortic aneurysm. Between‐trial differences were observed for all baseline characteristics (P < 0·001, Kruskal–Wallis test for continuous variables, Pearson's χ2 test for categorical variables). Drug therapy was recorded so differently for each trial that it is not reported here.
Total mortality
Over the follow‐up of all four trials there were 481 deaths in the EVAR groups and 482 in the open repair groups. Kaplan–Meier curves by randomized group for total mortality across all four trials are shown in Fig. 1. Overall, there was no difference in total mortality over the follow‐up period of the trials (hazard ratio 0·99, 95 per cent c.i. 0·87 to 1·13) (Table 2, Fig. 2). Between 0 and 6 months, mortality was lower for the EVAR groups, with 46 deaths versus 73 for open repair (hazard ratio 0·61, 0·42 to 0·89) and no evidence of heterogeneity between the trials. The early survival advantage of EVAR in the first 6 months was largely attributable to the lower 30‐day operative mortality for EVAR versus open repair groups (unadjusted pooled odds ratio 0·40, 95 per cent c.i. 0·22 to 0·74) (Fig. 3). After this, the early advantage for the EVAR group was lost and the hazard ratios moved (non‐significantly) in the direction of open repair. Adjusted hazard ratios were similar. By 5 years, the estimated survival rate was 73·6 (95 per cent c.i. 71·1 to 75·9) per cent in both the EVAR and open repair groups, with an expected 0·06 additional life‐years in the EVAR group, corresponding to 23 (95 per cent c.i. –16 to 61) days (P = 0·246). The causes of death by time period are shown in Table S2 (supporting information).
Figure 1.
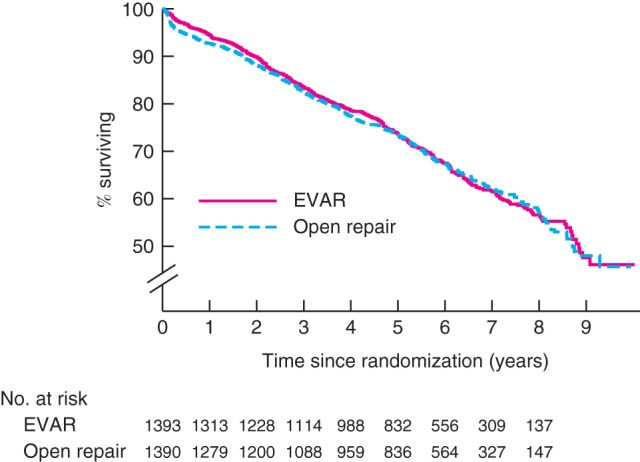
Kaplan–Meier survival curves for overall total mortality, by randomized group, for all 2783 patients in the four trials combined. EVAR, endovascular aneurysm repair
Table 2.
Unadjusted and adjusted hazard ratios for total mortality by time since randomization
| EVAR‐1 (n = 1252) | DREAM (n = 351) | OVER (n = 881) | ACE (n = 299) | Pooled (n = 2783) | |
|---|---|---|---|---|---|
| Proportion of patients who died* | |||||
| All patients | |||||
| EVAR | 260 of 626 (7·5) | 58 of 173 (6·2) | 146 of 444 (6·3) | 17 of 150 (4·1) | 481 of 1393 (6·7) |
| Open repair | 264 of 626 (7·7) | 60 of 178 (6·2) | 146 of 437 (6·4) | 12 of 149 (2·9) | 482 of 1390 (6·8) |
| Time since randomization | |||||
| 0–6 months | |||||
| EVAR | 26 of 626 (8·5) | 6 of 173 (7·1) | 11 of 444 (5·0) | 3 of 150 (4·6) | 46 of 1393 (6·7) |
| Open repair | 45 of 626 (15·0) | 10 of 178 (11·6) | 17 of 437 (8·0) | 1 of 149 (1·4) | 73 of 1390 (10·9) |
| 6 months to 4 years | |||||
| EVAR | 125 of 599 (6·7) | 33 of 167 (6·2) | 73 of 433 (5·2) | 13 of 146 (3·8) | 244 of 1345 (5·9) |
| Open repair | 116 of 581 (6·3) | 25 of 168 (4·6) | 78 of 420 (5·9) | 10 of 146 (3·0) | 229 of 1315 (5·7) |
| > 4 years | |||||
| EVAR | 109 of 472 (8·4) | 19 of 134 (6·0) | 62 of 348 (8·6) | 1 of 33 (17·7) | 191 of 987 (8·2) |
| Open repair | 103 of 461 (7·9) | 25 of 143 (7·4) | 51 of 331 (7·0) | 1 of 23 (17·5) | 180 of 958 (7·6) |
| Unadjusted hazard ratio† | |||||
| All patients | 0·98 | 1·00 | 0·97 | 1·52 | 0·99 |
| (0·82, 1·16) | (0·70, 1·44) | (0·77, 1·23) | (0·71, 3·25) | (0·87, 1·13) | |
| Time since randomization | |||||
| 0–6 months | 0·57 | 0·60 | 0·63 | 2·97 | 0·61 |
| (0·35, 0·92) | (0·22, 1·66) | (0·29, 1·34) | (0·31, 28·60) | (0·42, 0·89)¶ | |
| 6 months to 4 years | 1·06 | 1·36 | 0·89 | 1·25 | 1·04 |
| (0·82, 1·37) | (0·81, 2·28) | (0·65, 1·23) | (0·55, 2·86) | (0·87, 1·25) | |
| > 4 years | 1·06 | 0·81 | 1·22 | – | 1·07 |
| (0·81, 1·39) | (0·45, 1·47) | (0·85, 1·77) | (0·88, 1·32) | ||
| No. of patients in adjusted analysis | 1246 | 339 | 881 | 281 | 2747 |
| Adjusted hazard ratio† ‡ | |||||
| All patients | 1·00 | 0·88 | 1·04 | 1·43 | 1·01 |
| (0·84, 1·19) | (0·61, 1·27) | (0·82, 1·31) | (0·63, 3·22) | (0·89, 1·14) | |
| Time since randomization | |||||
| 0–6 months | 0·58 | 0·42 | 0·62 | –§ | 0·57 |
| (0·36, 0·95) | (0·14, 1·25) | (0·29, 1·33) | (0·39, 0·84)¶ | ||
| 6 months to 4 years | 1·08 | 1·15 | 0·94 | 1·14 | 1·05 |
| (0·84, 1·40) | (0·68, 1·95) | (0·69, 1·30) | (0·49, 2·68) | (0·87, 1·26) | |
| > 4 years | 1·11 | 0·79 | 1·30 | –§ | 1·12 |
| (0·85, 1·46) | (0·43, 1·44) | (0·89, 1·90) | (0·91, 1·38) | ||
Value in parentheses are *rate per 100 person‐years and
95 per cent confidence intervals.
Adjusted for age, sex, maximum aneurysm diameter and log creatinine.
Too few events to estimate a hazard ratio. EVAR, endovascular aneurysm repair.
P < 0·050.
Figure 2.
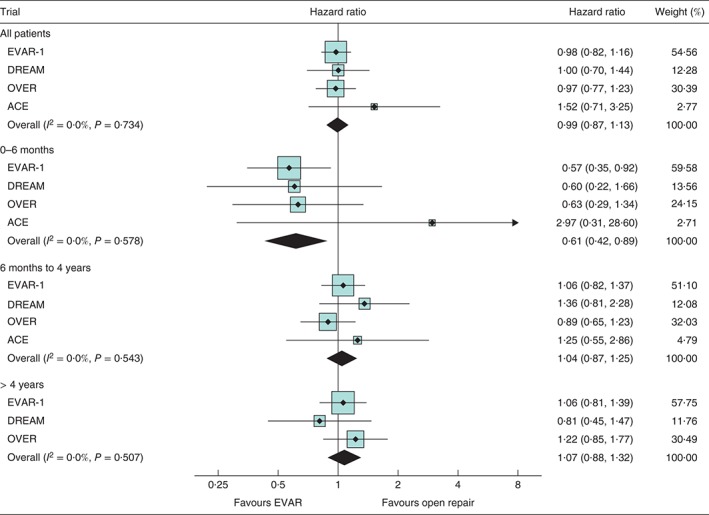
Unadjusted hazard ratios, with 95 per cent confidence intervals, for total mortality overall and at 0–6 months, 6 months to 4 years and more than 4 years since randomization. EVAR, endovascular aneurysm repair
Figure 3.
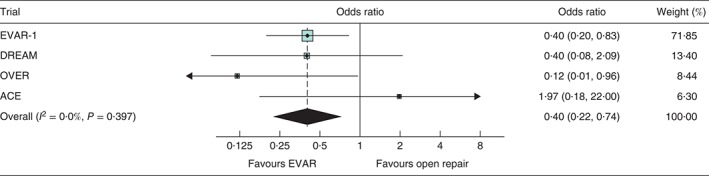
Odds ratios, with 95 per cent confidence intervals, for mortality within 30 days of operation. EVAR, endovascular aneurysm repair
Aneurysm‐related mortality
The findings for aneurysm‐related mortality were similar in direction, with relative benefit for the EVAR groups 0–6 months after randomization (25 in EVAR groups versus 55 in open repair groups; pooled unadjusted hazard ratio 0·44, 95 per cent c.i. 0·26 to 0·76). In later time periods, the results moved in the direction of open repair: the pooled hazard ratio was 1·43 (95 per cent c.i. 0·61 to 3·34) for 6 months to 4 years and 2·29 (0·49 to 10·85) for over 4 years (Fig. S1, supporting information). For those who received aneurysm repair, analysis by time from repair showed a strong relative advantage for the EVAR group in the first 30 days; between 30 days and 3 years there was no difference between the groups, but after 3 years there was a significant relative advantage for the open repair group, with three aneurysm‐related deaths versus 19 in the EVAR groups (hazard ratio 5·16, 1·49 to 17·89; P = 0·010) (Table 3).
Table 3.
Unadjusted and adjusted hazard ratios for aneurysm‐related mortality by time since operation for those who underwent surgery
| EVAR‐1 (n = 1216) | DREAM (n = 343) | OVER (n = 868) | ACE (n = 297) | Pooled (n = 2724) | |
|---|---|---|---|---|---|
| Proportion of patients who died* | |||||
| All patients | |||||
| EVAR | 31 of 614 (0·9) | 6 of 170 (0·7) | 9 of 439 (0·4) | 7 of 150 (1·7) | 53 of 1373 (0·8) |
| Open repair | 32 of 602 (1·0) | 10 of 173 (1·1) | 13 of 429 (0·6) | 1 of 147 (0·3) | 56 of 1351 (0·8) |
| Time since operation | |||||
| 0–30 days | |||||
| EVAR | 11 of 614 (22·0) | 2 of 170 (14·3) | 1 of 439 (2·8) | 2 of 150 (16·4) | 16 of 1373 (14·2) |
| Open repair | 26 of 602 (53·7) | 5 of 173 (35·5) | 8 of 429 (22·9) | 1 of 147 (8·3) | 40 of 1351 (36·5) |
| 31 days to 3 years | |||||
| EVAR | 7 of 603 (0·4) | 2 of 168 (0·4) | 5 of 438 (0·4) | 4 of 148 (1·1) | 18 of 1357 (0·5) |
| Open repair | 4 of 576 (0·3) | 5 of 168 (1·1) | 4 of 421 (0·3) | 0 of 146 (0) | 13 of 1311 (0·4) |
| > 3 years | |||||
| EVAR | 13 of 498 (0·8) | 2 of 140 (0·5) | 3 of 380 (0·3) | 1 of 78 (2·3) | 19 of 1096 (0·6) |
| Open repair | 2 of 484 (0·1) | 0 of 146 (0) | 1 of 352 (0·1) | 0 of 72 (0) | 3 of 1054 (0·1) |
| Unadjusted hazard ratio* | |||||
| All patients | 0·94 | 0·61 | 0·68 | 6·86 | 0·89 |
| (0·57, 1·54) | (0·22, 1·68) | (0·29, 1·59) | (0·84, 55·78) | (0·51, 1·56) | |
| Time since operation | |||||
| 0–30 days | 0·41 | 0·40 | 0·12 | 1·97 | 0·41 |
| (0·20, 0·83) | (0·08, 2·08) | (0·02, 0·97) | (0·18, 21·76) | (0·22, 0·74)¶ | |
| 31 days to 3 years | 1·69 | 0·40 | 1·20 | –§ | 1·07 |
| (0·50, 5·77) | (0·08, 2·08) | (0·32, 4·47) | (0·49, 2·36) | ||
| > 3 years | 6·35 | – | 3·18 | –§ | 5·16 |
| (1·43, 28·15) | (0·33, 30·64) | (1·49, 17·89)¶ | |||
| No. of patients in adjusted analysis | 1211 | 331 | 868 | 280 | 2690 |
| Adjusted hazard ratio† ‡ | |||||
| All patients | 0·97 | 0·44 | 0·71 | –§ | 0·81 |
| (0·59, 1·59) | (0·15, 1·30) | (0·30, 1·67) | (0·55, 1·21) | ||
| Time since operation | |||||
| 0–30 days | 0·42 | 0·21 | 0·13 | –§ | 0·36 |
| (0·21, 0·86) | (0·02, 1·85) | (0·02, 1·05) | (0·19, 0·67)¶ | ||
| 31 days to 3 years | 1·82 | 0·32 | 1·13 | –§ | 0·98 |
| (0·52, 6·36) | (0·06, 1·65) | (0·29, 4·39) | (0·38, 2·55) | ||
| > 3 years | 6·58 | – | 3·19 | –§ | 5·30 |
| (1·48, 29·21) | (0·33, 31·26) | (1·52, 18·46)¶ | |||
Value in parentheses are
rate per 100 person‐years and
95 per cent confidence intervals.
Adjusted for age, sex, maximum aneurysm diameter and log creatinine.
Too few events to estimate a hazard ratio. EVAR, endovascular aneurysm repair.
P < 0·050.
Total mortality by subgroups
There was no significant effect of age or sex on the relative effectiveness of EVAR in preventing deaths in any time period, including the first 6 months after randomization (Fig. 4). There were two subgroups of patients who appeared to have no early benefit (to 6 months) under EVAR versus open repair: patients with moderate renal dysfunction and those with coronary artery disease. For those with above‐median eGFR, the pooled hazard ratio of 0·42 (95 per cent c.i. 0·21 to 0·84) was significantly in favour of EVAR, compared with the less favourable and non‐significant pooled hazard ratio of 0·68 (0·43 to 1·08) for those with worse renal function; therefore, the interaction between eGFR measure and treatment group was significant (interaction P = 0·024) (Fig. 4). Similarly, patients with coronary artery disease gained no early advantage of being in the EVAR group, in comparison with patients without previous coronary artery disease (interaction P = 0·047) (Fig. 5). None of the morphological aneurysm characteristics, smoking, diabetes or BMI was associated with mortality (Figs S2 and S3, supporting information).
Figure 4.
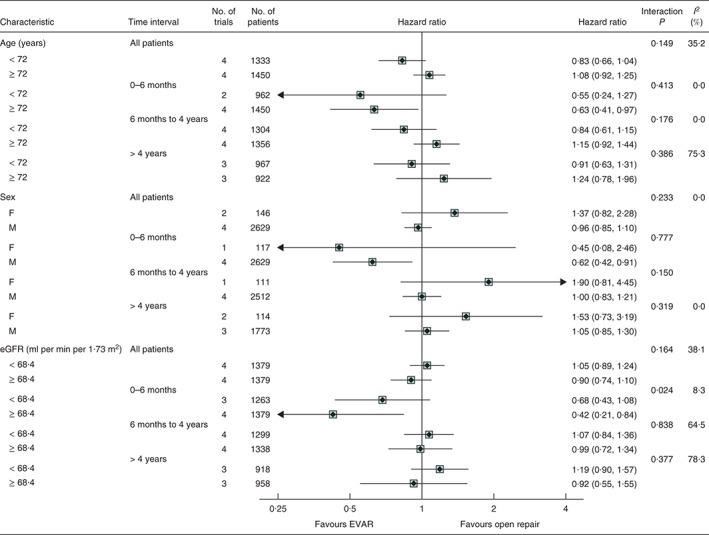
Unadjusted hazard ratios, with 95 per cent confidence intervals, for total mortality by subgroups of age, sex and estimated glomerular filtration rate (eGFR), overall and at 0–6 months, 6 months to 4 years and more than 4 years since randomization. Interaction P values for age and eGFR were calculated using continuous measures (median eGFR 68·4 ml per min per 1·73 m2). Not all trials contributed to the subgroup analyses or every time point. Hazard ratios for sex could not be estimated in the OVER and ACE trials owing to small numbers of women. EVAR, endovascular aneurysm repair
Figure 5.
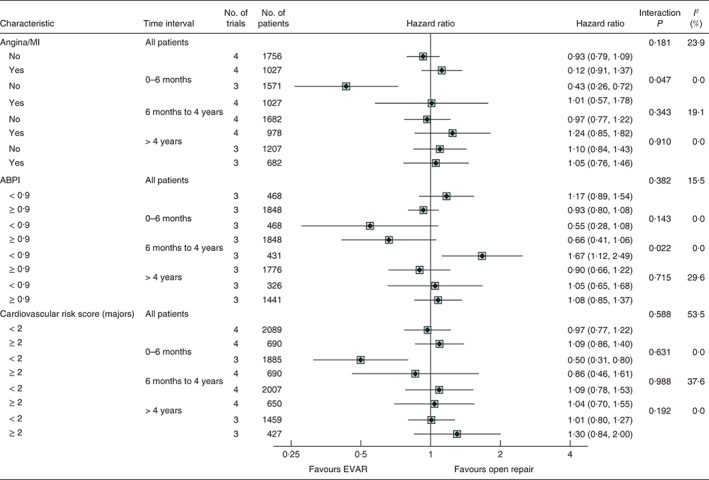
Unadjusted hazard ratios, with 95 per cent confidence intervals, for total mortality by subgroups of history of angina or myocardial infarction (MI), ankle : brachial pressure index (ABPI) and cardiovascular risk score, overall and at 0–6 months, 6 months to 4 years and more than 4 years since randomization. Interaction P values for ABPI and cardiovascular risk score were calculated using continuous measures. Not all trials contributed to the subgroup analyses or every time point. The ACE trial did not report ABPI so is not included in these results. EVAR, endovascular aneurysm repair
Baseline ABPI was not available for the ACE trial. In the other trials, patients with peripheral artery disease (ABPI below 0·9) had a similar early survival advantage from being in the EVAR group as those with a higher ABPI (0·9 or above). However, in the 6‐month to 4‐year time interval, among those with an ABPI lower than 0·9, the open repair group had a survival advantage (39 deaths versus 62 in EVAR group; hazard ratio 1·67, 1·12 to 2·49), in comparison with patients with an ABPI of at least 0·9 (interaction P = 0·022). During this interval, in those with an ABPI lower than 0·9, total mortality rates were 9·6 and 5·7 per 100 person‐years in the EVAR and open repair groups respectively, compared with 5·1 and 5·8 per 100 person‐years respectively in the group with a higher ABPI. The cause of death in the two ABPI groups by time period is shown in Table S3 (supporting information). Analysis of the operative mortality by subgroup showed that patients with a low ABPI had the highest mortality rate (Table S4, supporting information), and this group had higher aneurysm‐related mortality throughout. Finally, a cardiovascular risk score was not discriminatory in any time period.
Complications and reinterventions
Complications (apart from endoleaks after EVAR) and reinterventions were reported heterogeneously across the four trials. The overall rates of reintervention reported were higher in the EVAR group than in the open repair group for all trials (Table 1). The risk of reintervention by time period following aneurysm repair is shown in Fig. S4 (supporting information); there was substantial heterogeneity between trials for reinterventions recorded between 31 days and 3 years.
There was no indication that complications following EVAR decreased with the year in which the trial commenced: these rates, together with types and numbers of complications, are reported in Table S5 (supporting information). The commonest reported complication after EVAR was type II endoleak, which overall was reported 435 times in 325 of 2783 patients (11·7 per cent). Corrective reintervention was performed in 99 of 435 detected type II endoleaks (22·8 per cent). There was no evidence that a detected type II endoleak (either treated or untreated) was associated with worse overall survival (Table S6, supporting information). The second most common type of complication was type I endoleak, for which 79 of 120 (65·8 per cent) received an early reintervention. Similarly, early correction of other serious EVAR‐related complications was attempted in less than two‐thirds of patients. Secondary sac rupture was reported in 37 patients: 33 in the EVAR randomized groups (2·4 per cent of patients) and four in the open groups (0·3 per cent), although all four of the latter patients were treated with EVAR. For those with secondary rupture following treatment with EVAR, the median time to rupture was 3·5 years (Fig. S5, supporting information). Of these patients, 11 had a type I endoleak, of which seven were treated, seven had a type II endoleak, of which three were treated, two had a type III endoleak, of which one was treated, and nine had known graft migration. Nineteen patients had no endoleaks detected before secondary sac rupture and one further patient had a thoracic endograft for proximal aortic dissection 3 days earlier. The mean time between detection of the first endoleak and rupture was 1·8 years. The 30‐day mortality rate following rupture was 62 per cent (23 of 37).
Discussion
These four randomized trials, in Europe and the USA, provide the best evidence for the early survival advantage offered by EVAR rather than open repair. Patients prefer EVAR, the less invasive method of AAA repair, which has been adopted widely, and the majority of elective repairs are now performed using EVAR8, 19, 20. This meta‐analysis, over a 5‐year time horizon, confirms that, overall, there is an early survival advantage for EVAR, which is lost within 3 years of randomization, so that the life‐years saved from EVAR over 5 years are minimal. Between 0 and 6 months after randomization, total and aneurysm‐related mortality rates were lower for the EVAR group, mainly because of the 2·5‐fold lower 30‐day operative mortality in this group. However, after this interval, the early EVAR group advantage was eroded progressively. By 3 years after aneurysm repair, aneurysm‐related mortality was five times higher in the EVAR group (mainly due to secondary rupture or reinterventions) and this is likely to have contributed to the ‘catch‐up’ in mortality.
Further investigations focused on whether the early survival advantage was either maintained or lost in subgroups of patients categorized by preoperative characteristics. Over a 5‐year time horizon, there was no convincing evidence that being randomized to EVAR or open repair resulted in differential survival between any subgroups of the population. This does not support the suggestion that younger and fitter patients with aortic morphology suitable for EVAR are likely to benefit from open repair over 5 years9. However, differential effect modification was suggested in some subgroups (renal dysfunction, coronary artery disease) in the first 6 months and for those with peripheral artery disease in the later 6‐month to 4‐year time interval, possibly owing to frailty effects.
Low ABPI was introduced as a measure of peripheral atherosclerosis21 and is a marker of generalized atherosclerosis22. This subgroup had the highest pooled operative mortality, although the relative early advantage of EVAR was maintained. However, between 6 months and 4 years, fortunes reversed in favour of a survival advantage for the open repair group, a pattern that indicates those with a low ABPI are another contributor to the ‘catch‐up’ in mortality.
All trials enrolled patients with evidence of moderate renal dysfunction, with 35 per cent of the overall enrolment having an eGFR below 60 ml per min per 1·73 m2 (chronic kidney disease stage 3 or above). For these patients, there was no evidence of a benefit from being randomized to EVAR (versus open repair), even in the first 6 months. These data are in agreement with an earlier observational study23 showing a high early postoperative event rate for patients with low eGFR. Whether renal function deteriorates more rapidly after either elective EVAR or open repair of an AAA has been debated fiercely, and less attention has been focused on improving perioperative care24. Similarly, patients with known coronary artery disease had no evidence of an early survival advantage from being randomized to EVAR. Given that EVAR is less invasive than open repair, these findings for patients with moderate renal dysfunction and coronary artery disease are surprising. Perhaps the stress of EVAR in these subgroups has been underestimated. It also is possible that those randomized to EVAR received less stringent preoperative evaluations, resulting in better perioperative care for the open repair group with these co‐morbidities.
This study has several limitations that restrict its scope. First, endografts were implanted between 1999 and 2008 using general anaesthesia and today newer endografts are being used, often with local anaesthesia. Second, there were different reporting standards across the trials for baseline characteristics such as smoking and drugs. Third, the reporting of complications and reinterventions (aneurysm‐related and other cardiovascular) was very heterogeneous across the trials. Fourth, today it is recognized that type II endoleaks with sac enlargement can be dangerous25 and that a type II endoleak might even hide a type I or type III endoleak. Fifth, at the time these trials recruited (1999–2008), no trial had a clear policy for reintervention in the presence of endoleaks after EVAR; even serious complications such as type I endoleak were not always corrected quickly, which may have contributed to the increasing aneurysm‐related mortality rate in the EVAR group at 3 or more years after aneurysm repair.
The reintervention rate was consistently higher in the EVAR groups (Table 1), although the data are heterogeneous and the largest trial did not report incision‐related complications after open repair. It would be reassuring to learn that by using more recent EVAR devices, according to the instructions for use, coupled with more rigorous surveillance, the continuing aneurysm‐related mortality in the EVAR group could be attenuated to minimize the ‘catch‐up’ in mortality. To rely entirely on the introduction of new devices to prevent aneurysm‐related mortality in the EVAR group, especially without adequate surveillance, may be unwise. Recent analyses8 of the Medicare database support this caution. Correction of the commonest reported complication of EVAR, type II endoleak, had no effect on survival.
This meta‐analysis confirms the advantage of lower mortality in the EVAR group in the first 6 months and provides some new insight of how this early advantage of EVAR is eroded (aneurysm‐related mortality and inclusion of those with peripheral artery disease). Additionally, two subgroups of patients were identified who do not have lower mortality with EVAR at any time, suggesting that patients with moderate renal dysfunction and those with established coronary artery disease might benefit from improved perioperative care, especially for EVAR. Surveillance must focus on reducing aneurysm‐related deaths in the mid and longer term, particularly deaths resulting from reinterventions and secondary ruptures after EVAR.
Collaborators
EVAR‐1 trial
Trial Management Committee: R. M. Greenhalgh (Chief Investigator), J. D. Beard, M. J. Buxton, L. C. Brown, P. L. Harris, J. T. Powell, J. D. G. Rose, I. T. Russell, M. J. Sculpher, S. G. Thompson; Trial Steering Committee: R. J. Lilford (Chair), P. R. F. Bell, R. M. Greenhalgh, S. C. Whitaker; Data Monitoring and Ethics Committee: the late P. A. Poole‐Wilson (Chair), C. V. Ruckley, W. B. Campbell, M. R. E. Dean, M. S. T. Ruttley, E. C. Coles; End‐Points Committee: J. T. Powell (Chair), A. Halliday, S. J. Gibbs; Statistical and costs analyses: L. C. Brown, D. Epstein, M. J. Sculpher, S. G. Thompson.
Regional Trial Investigators (represented by 1 surgeon, radiologist and coordinator per centre; numbers indicate the number of patients entered into both the EVAR‐1 and EVAR‐2 trials): K. Varty, C. Cousins (Addenbrookes Hospital, Cambridge, 10); R. J. Hannon, L. Johnston (Belfast City Hospital, Belfast, 53); A. W. Bradbury, M. J. Henderson (Birmingham Heartlands Hospital, Birmingham, 8); S. D. Parvin, D. F. C. Shepherd (Bournemouth General Hospital, Bournemouth, 68); R. M. Greenhalgh, A. W. Mitchell (Charing Cross Hospital, London, 27); P. R. Edwards, G. T. Abbott (Countess of Chester Hospital, Chester, 15); D. J. Higman, A. Vohra (Coventry and Walsgrave Hospital, Coventry, 8); S. Ashley, C. Robottom (Derriford Hospital, Plymouth, 2); M. G. Wyatt, J. D. G. Rose (Freeman Hospital, Newcastle upon Tyne, 121); D. Byrne, R. Edwards, (Gartnavel General Hospital, Glasgow, 12); D. P. Leiberman, D. H. McCarter (Glasgow Royal Infirmary, Glasgow, 19); P. R. Taylor, J. F. Reidy (Guy's and St Thomas' Hospital, London, 124); A. R. Wilkinson, D. F. Ettles (Hull Royal Infirmary, Hull, 29); A. E. Clason, G. L. S. Leen (James Cook University Hospital, Middlesborough, 19); N. V. Wilson, M. Downes (Kent and Canterbury Hospital, Canterbury, 1); S. R. Walker, J. M. Lavelle (Lancaster General Infirmary, Lancaster, 12); M. J. Gough, S. McPherson (Leeds General Infirmary, Leeds, 38); D. J. A. Scott, D. O. Kessell (Leeds St James's Hospital, Leeds, 11); R. Naylor, R. Sayers, N. G. Fishwick (Leicester Royal Infirmary, Leicester, 148); P. L. Harris, D. A. Gould (Liverpool Royal Hospital, Liverpool, 143); M. G. Walker, N. C. Chalmers (Manchester Royal Infirmary, Manchester, 96); A. Garnham, M. A. Collins (New Cross Hospital, Wolverhampton, 1); J. D. Beard, P. A. Gaines (Northern General Hospital, Sheffield, 77); M. Y. Ashour, R. Uberoi (Queen Elizabeth Hospital, Gateshead, 18); B. Braithwaite, S. C. Whitaker, Queen's Medical Centre, Nottingham, 116); J. N. Davies, S. Travis (Royal Cornwall Hospital, Truro, 26); G. Hamilton, A. Platts (Royal Free Hospital, London, 42); A. Shandall, B. A. Sullivan (Royal Gwent Hospital, Newport, 1); M. Sobeh, M. Matson (Royal London Hospital, London, 7); A. D. Fox, R. Orme (Royal Shrewsbury Hospital, Shrewsbury, 7); W. Yusef, T. Doyle (Royal Sussex County Hospital, Brighton, 6); M. Horrocks, J. Hardman (Royal United Hospital, Bath, 34); P. H. B. Blair, P. K. Ellis (Royal Victoria Hospital, Belfast, 46); G. Morris, A. Odurny (Southampton General Hospital, Southampton, 39); R. Vohra, M. Duddy (Selly Oak Hospital, Birmingham, 22); M. Thompson, T. M. L. Loosemore, A. M. Belli, R. Morgan (St George's Hospital, London, 54); M. Adiseshiah, J. A. S. Brookes (University College Hospital, London, 69); C. N. McCollum, R. Ashleigh (University Hospital of South Manchester, Manchester, 127).
Trial Coordinators: M. Aukett, S. Baker, E. Barbe, N. Batson, J. Bell, J. Blundell, D. Boardley, S. Boyes, O. Brown, J. Bryce, M. Carmichael, T. Chance, J. Coleman, C. Cosgrove, G. Curran, T. Dennison, C. Devine, N. Dewhirst, B. Errington, H. Farrell, C. Fisher, P. Fulford, M. Gough, C. Graham, R. Hooper, G. Horne, L. Horrocks, B. Hughes, T. Hutchings, M. Ireland, C. Judge, L. Kelly, J. Kemp, A. Kite, M. Kivela, M. Lapworth, C. Lee, L. Linekar, A. Mahmood, L. March, J. Martin, N. Matharu, K. McGuigen, P. Morris‐Vincent, S. Murray, A. Murtagh, G. Owen, V. Ramoutar, C. Rippin, J. Rowley, J. Sinclair, S. Spencer, V. Taylor, C. Tomlinson, S. Ward, V. Wealleans, J. West, K. White, J. Williams, L. Wilson.
DREAM trial
Members of the DREAM trial study group are as follows. Steering Committee: D. E. Grobbee, J. D. Blankensteijn, A. A. A. Bak, J. Buth, P. M. Pattynama, E. L. G. Verhoeven, A. E. van Voorthuisen; Executive and Writing Committee: J. D. Blankensteijn, R. Balm, J. Buth, P. W. M. Cuypers, D. E. Grobbee, M. Prinssen, M. R. H. M. van Sambeek, E. L. G. Verhoeven, A. F. Baas; Data Monitoring and Ethics Committee: M. G. Hunink, J. M. van Engelshoven, M. J. H. M. Jacobs, B. A. J. M. de Mol; Site and Device Selection Committee: J. H. van Bockel, R. Balm, J. Reekers, X. Tielbeek, E. L. G. Verhoeven, W. Wisselink; Data management: N. Boekema, L. M. Heuveling, I. Sikking; Outcome Adjudication Committee: M. Prinssen, R. Balm, J. D. Blankensteijn, J. Buth, P. W. M. Cuypers, M. R. H. M. van Sambeek, E. L. G. Verhoeven; Data analysis: J. L. de Bruin, A. F. Baas, J. D. Blankensteijn, M. Prinssen.
Clinical centres that participated in the study (with the number of randomized patients). The Netherlands: J. Buth, A. V. Tielbeek (Catharina Hospital, Eindhoven, 94); J. D. Blankensteijn (University Medical Centre, Utrecht, 35); R. Balm, J. A. Reekers (Academic Medical Centre, Amsterdam, 32); M. R. H. M. van Sambeek, P. Pattynama (Erasmus Medical Centre, Rotterdam, 30); E. L. G. Verhoeven, T. Prins (University Hospital, Groningen, 27); A. C. van der Ham, J. J. I. M. van der Velden (St Franciscus Gasthuis, Rotterdam, 27); S. M. M. van Sterkenburg, G. B. ten Haken (Rijnstate Hospital, Arnhem, 14); C. M. A. Bruijninckx, H. van Overhagen (Leyenburg Hospital, ′s‐Gravenhage, 9); R. P. Tutein Nolthenius, T. R. Hendriksz (Albert Schweitzer Hospital, Dordrecht, 8); J. A. W. Teijink, H. F. Odink (Atrium Medical Centre, Heerlen, 8); A. A. E. A. de Smet, D. Vroegindeweij (Medical Centre Rijnmond Zuid, Rotterdam, 7); R. M. M. van Loenhout, M. J. Rutten (Jeroen Bosch Hospital, den Bosch, 7); J. F. Hamming, L. E. H. Lampmann (St Elisabeth Hospital, Tilburg, 5); M. H. M. Bender, H. Pasmans (Maxima Medical Centre, Veldhoven, 5); A. C. Vahl, C. de Vries (Onze Lieve Vrouwe Gasthuis, Amsterdam, 5); A. J. C. Mackaay (Meander Medical Centre, Amersfoort, 4); L. M. C. van Dortmont (Vlietland Hospital, Schiedam, 4); A. J. van der Vliet, L. J. Schultze Kool (University Medical Centre, Nijmegen, 4); J. H. B. Boomsma, H. R. van Dop (Martini Hospital, Groningen, 3); J. C. A. de Mol van Otterloo, T. P. W. de Rooij (Medical Centre Haaglanden, `s‐Gravenhage, 3); T. M. Smits (Hospital Bernhoven, Oss, 3); E. N. Yilmaz (Oosterschelde Hospital, Goes, 3); W. Wisselink, F. G. van den Berg (Vrije Universiteit Medical Centre, Amsterdam, 2); M. J. T. Visser, E. van der Linden (Leiden University Medical Centre, Leiden, 1); G. W. H. Schurink, M. de Haan (University Medical Centre, Maastricht, 1); H. J. Smeets (Bronovo Hospital, `s‐Gravenhage, 1). Belgium: P. Stabel (St Jozef Hospital, Turnhout, 4); F. van Elst (St Trudo Hospital, St Truiden, 3); J. Poniewierski (University Hospital, Antwerp, 1); F. E. G. Vermassen (University Medical Centre, Ghent, 1).
OVER trial
Executive Committee: F. A. Lederle (Co‐Chairperson), J. A. Freischlag (Co‐Chairperson), T. R. Kohler, E. Latts, J. Matsumura, F. T. Padberg Jr, T. C. Kyriakides, K. M. Swanson; Cooperative Studies Program Coordinating Center VA Connecticut Healthcare System, West Haven, Connecticut: P. Guarino (Acting Director), P. Peduzzi, M. Antonelli (Assistant Director, Operations), C. Cushing (Programmer), E. Davis (Research Coordinator), L. Durant (Quality Assurance Officer), S. Joyner (Research Coordinator), the late A. Kossack (Research Coordinator), T. C. Kyriakides (Biostatistician), M. LeGwin (Project Manager), V. McBride (Research Coordinator), T. O'Connor (Biostatistician), J. Poulton (Research Coordinator), the late S. Stratton (Project Manager), S. Zellner (Research Coordinator); Regulatory Affairs and Clinical Compliance Section, Cooperative Studies Program Clinical Research Pharmacy Coordinating Center: A. J. Snodgrass, J. Thornton, K. M. Swanson; VA Research Site Management and Review Team (SMART): C. M. Haakenson (Pharmacist); Center for Management of Complex Care, Veterans Affairs Medical Center (VAMC), Albuquerque, New Mexico; K. T. Stroupe (Health Economist); Center for Chronic Disease Outcomes Research, VAMC, Minneapolis, Minnesota: Y. Jonk (Health Economist); Data and Safety Monitoring Board: J. W. Hallett, N. Hertzer, J. Towne, D. A. Katz, T. Karrison (Biostatistician), J. P. Matts (Biostatistician); Human Rights Committee, West Haven, Connecticut: R. Marottoli, S. Kasl, R. Mehta, R. Feldman, W. Farrell, H. Allore, E. Perry, J. Niederman, F. Randall, M. Zeman, the late D. Beckwith; Central Administration, Cooperative Studies Program (VA Central Office): T. J. O'Leary (Director, Clinical Science Research and Development Service), G. D. Huang (Deputy Director, Cooperative Studies Program); National Project Coordinators, Minneapolis VAMC: E. Latts, M. Bader.
Investigators and site coordinators at participating VAMCs: E. R. Ketteler, D. D. Kingsley, J. M. Marek, R. J. Massen, B. D. Matteson, J. D. Pitcher, M. Langsfeld, J. D. Corson, J. M. Goff Jr, K. Kasirajan, C. Paap, D. C. Robertson (Albuquerque, New Mexico); A. Salam, R. Veeraswamy, R. Milner, K. Kasirajan, J. Guidot, (Atlanta, Georgia); B. K. Lal, S. J. Busuttil, M. P. Lilly, M. Braganza, K. Ellis, (Baltimore, Maryland); M. A. Patterson, W. D. Jordan, D. Whitley, S. Taylor, M. Passman, D. Kerns, C. Inman, J. Poirier (Birmingham, Alabama); J. Ebaugh, J. Raffetto, D. Chew, S. Lathi, C. Owens, K. Hickson (Boston, Massachusetts); H. H. Dosluoglu, K. Eschberger (Buffalo, New York); M. R. Kibbe, H. M. Baraniewski, J. Matsumura, M. Endo, A. Busman, W. Meadows, M. Evans (Chicago, Illinois); J. S. Giglia, H. El Sayed, A. B. Reed, M. Ruf, S. Ross, (Cincinnati, Ohio); J. M. Jean‐Claude, G. Pinault, P. Kang, N. White, M. Eiseman, the late R. Jones (Cleveland, Ohio); C. H. Timaran, J. G. Modrall, M. B. Welborn III, J. Lopez, T. Nguyen (Dallas, Texas); J. K. Y. Chacko, K. Granke, A. G. Vouyouka, E. Olgren, P. Chand, B. Allende, M. Ranella, C. Yales (Detroit, Michigan); T. A. Whitehill, the late W. C. Krupski, M. R. Nehler, S. P. Johnson, D. N. Jones, P. Strecker, M. A. Bhola (Denver, Colorado); C. K. Shortell, J. L. Gray, J. H. Lawson, R. McCann, M. W. Sebastian, J. Kistler Tetterton, C. Blackwell, P. A. Prinzo, N. Lee (Durham, North Carolina); F. T. Padberg Jr, J. J. Cerveira, B. K. Lal, R. W. Zickler, K. A. Hauck, (East Orange, New Jersey); S. A. Berceli, W. A. Lee, C. K. Ozaki, P. R. Nelson, A. S. Irwin, R. Baum (Gainesville, Florida); B. Aulivola, H. Rodriguez, F. N. Littooy, H. Greisler, M. T. O'Sullivan (Hines, Illinois); P. Kougias, P. H. Lin, R. L. Bush, G. Guinn, C. Bechara, C. Cagiannos, G. Pisimisis, N. Barshes, S. Pillack, B. Guillory (Houston, Texas); D. Cikrit, S. G. Lalka, G. Lemmon, R. Nachreiner, M. Rusomaroff, E. O'Brien (Indianapolis, Indiana); J. J. Cullen, J. Hoballah, W. J. Sharp, J. L. McCandless, V. Beach (Iowa City, Iowa); D. Minion, T. H. Schwarcz, J. Kimbrough, L. Ashe, A. Rockich, J. Warner‐Carpenter (Lexington, Kentucky); M. Moursi, J. F. Eidt, S. Brock, (Little Rock, Arkansas); C. Bianchi, V. Bishop (Loma Linda, California); I. L. Gordon, R. Fujitani, S. M. Kubaska III, M. Behdad, R. Azadegan, C. Ma Agas, K. Zalecki (Long Beach, California); J. R. Hoch, S. C. Carr, C. Acher, M. Schwarze, G. Tefera, M. Mell, B. Dunlap, J. Rieder (Madison, Wisconsin); J. M. Stuart, D. S. Weiman, O. Abul‐Khoudoud, H. E. Garrett, S. M. Walsh, K. L. Wilson (Memphis, Tennessee); G. R. Seabrook, R. A. Cambria, K. R. Brown, B. D. Lewis, S. Framberg, C. Kallio (Milwaukee, Wisconsin); R. A. Barke, S. M. Santilli, A. C. d'Audiffret, N. Oberle, C. Proebstle, L. L. Johnson (Minneapolis, Minnesota); G. R. Jacobowitz, N. Cayne, C. Rockman, M. Adelman, P. Gagne, M. Nalbandian, L. J. Caropolo (New York, New York); I. I. Pipinos, J. Johanning, T. Lynch, H. DeSpiegelaere, G. Purviance (Omaha, Nebraska); W. Zhou, R. Dalman, J. T. Lee, B. Safadi, S. M. Coogan, S. M. Wren, D. D. Bahmani, D. Maples, S. Thunen (Palo Alto, California); M. A. Golden, M. E. Mitchell, R. Fairman, S. Reinhardt (Philadelphia, Pennsylvania); M. A. Wilson, E. Tzeng, S. Muluk, N. M. Peterson, M. Foster (Pittsburgh, Pennsylvania); J. Edwards, G. L. Moneta, G. Landry, L. Taylor, R. Yeager, E. Cannady (Portland, Oregon); G.Treiman, S. Hatton‐Ward, the late B. Salabsky (Salt Lake City, Utah); N. Kansal, E. Owens, M. Estes, B. A. Forbes, C. Sobotta (San Diego, California); J. H. Rapp, L. M. Reilly, S. L. Perez, K. Yan, R. Sarkar, S. S. Dwyer, S. Perez, K. Chong (San Francisco, California); T. R. Kohler, T. S. Hatsukami, D. G. Glickerman, M. Sobel, T. S. Burdick, K. Pedersen, P. Cleary (Seattle, Washington); M. Back, D. Bandyk, B. Johnson, M. Shames, R. L. Reinhard, S. C. Thomas (Tampa, Florida); G. C. Hunter, L. R. Leon Jr, A. Westerband, R. J. Guerra, M. Riveros, J. L. Mills Sr, J. D. Hughes, A. M. Escalante, S. B. Psalms, N. N. Day (Tucson, Arizona); R. Macsata, A. Sidawy, J. Weiswasser, S. Arora, B. J. Jasper (Washington, DC); A. Dardik, V. Gahtan, B. E. Muhs, B. E. Sumpio, R. J. Gusberg, M. Spector, J. Pollak, J. Aruny, E. L. Kelly, J. Wong, P. Vasilas, C. Joncas (West Haven, Connecticut); H. A. Gelabert, C. DeVirgillio, D. A. Rigberg, L. Cole (West Los Angeles, California).
ACE trial
ACE study committees and organization. Writing Committee: J.‐P. Becquemin, J. Marzelle; Chief investigators: J.‐P. Becquemin, M. Sapoval; Scientific Committee: J.‐P. Becquemin, J.‐P. Favre, J. Watelet, P. Lermusiaux, M. Sapoval; Statistics: E. Lepage, F. Hemery, G. Dolbeau, N. Hawajry, P. Cunin; Surveillance Committee: P. Harris, L. Stockx, G. Chatellier; Safety Issues Committee: C. Mialhe, J.‐N. Fiessinger, L. Pagny, H. Kobeiter; Endpoints and Adverse Events Validation Committee: C. Boissier, P. Lacroix, F. Ledru, J.‐J. Pinot, J.‐F. Deux, B. Tzvetkov, P. Duvaldestin, J. Watelet; Data management: C. Jourdain, V. David, D. Enouf, N. Ady, A. Krimi, N. Boudjema.
ACE trial participating centres and physicians with number of patients: Y. Jousset, B. Enon, V. Blin, J. Picquet, P. L'Hoste, F. Thouveny (Centre Hospitalier Universitaire (CHU) Angers–Hôtel Dieu, Angers, 3); H. Borie, S. Kowarski, J.‐M. Pernes, M. Auguste (Hôpital privé d'Antony, Antony, 5); J.‐P. Becquemin, P. Desgranges, E. Allaire, J. Marzelle, H. Kobeiter (Hôpital Henri Mondor, Créteil, 102); P.‐Y. Meaulle, D. Chaix (Clinique mutualiste des Eaux Claires, Grenoble, 1); P. Juliae, J. N. Fabiani, P. Chevalier, M. Combes, A. Seguin, D. Belhomme, M. Sapoval, J. Baque, O. Pellerin (Hôpital Européen Georges Pompidou, Paris, 3); J. P. Favre, X. Barral, C. Veyret (CHU Nord, Saint Étienne, 14); J. Watelet, C. Peillon, D. Plissonier, P. Thomas, E. Clavier (Hôpital Charles Nicolle, Rouen, 9); P. Lermusiaux, R. Martinez, F. Bleuet, C. Dupreix (Hôpital Trousseau, Tours, 17); J. P. Verhoye, T. Langanay, J. F. Heautot (CHU de Pontchaillou, Rennes, 2); M. Koussa, S. Haulon, P. Halna, L. Destrieux, C. Lions, S. Wiloteaux, J. P. Beregi (Hôpital Cardiologique, Lille, 5); P. Bergeron, J.‐J. Pinot (Hôpital Saint Joseph, Marseille, 11); P. Patra, A. Costargent, P. Chaillou, A. D'Alicourt, Y. Goueffic (Hôpital G et R Laennec, Nantes, 20); E. Cheysson, A. Parrot, P. Garance (Icham Abada Centre Hospitalier René Dubos, Pontoise, 1); A. Demon, A. Tyazi (Centre Hospitalier de Valenciennes, Valenciennes, 4); J.‐C. Pillet (Nouvelles Cliniques Nantaises, Nantes, 33); F. Lescalie, G. Tilly (Clinique Saint‐Augustin, Nantes, 25); E. Steinmetz, C. Favier, R. Brenot, D. Krause, J. P. Cercueil (Hôpital du Bocage, Dijon, 15); O. Vahdat, M. Sauer, P. Soula, A. Querian, O. Garcia, M. Levade, D. Colombier (Clinique Pasteur, Toulouse, 2); J.‐M. Cardon, A. Joyeux, P. Borrelly, G. Dogas (Clinique des Franciscaines, Nimes, 10); P.‐É. Magnan, A. Branchereau, J.‐M. Bartoli (Hôpital d'Adultes de la Timone, Marseille, 1); R. Hassen‐Khodja, M. Batt, P.‐F. Planchard, P.‐J. Bouillanne, P. Haudebourg, J. Bayne (Hôpital Saint Roch, Nice, 10); P. Gouny, A. Badra, J. Braesco, M. Nonent (Hôpital La Cavale Blanche, Brest, 2); A. Lucas, A. Cardon, Y. Kerdiles, Y. Rolland (Hôpital Sud, Rennes, 3); M. Kassab, C. Brillu, F. Goubault, L. Tailboux (Polyclinique de Poitiers, Poitiers, 2); H. Darrieux, O. Briand, J.‐C. Maillard (Clinique Marie Immaculée, Bourges, 6).
Supporting information.
Additional supporting information may be found in the online version of this article:
Appendix S1 Supplementary information on search and statistical methods (Word document)
Fig. S1 Aneurysm‐related mortality, overall and at 0–6 months, 6 months to 4 years and more than 4 years since randomization (Word document)
Fig. S2 Total mortality by aneurysm morphology subgroups (Word document)
Fig. S3 Total mortality by diabetes, BMI and smoking subgroups (Word document)
Fig. S4 Reinterventions by trial and time from aneurysm repair (Word document)
Fig. S5 Time from randomization to secondary sac rupture after endovascular aneurysm repair (Word document)
Table S1 Cardiovascular risk score (Word document)
Table S2 Causes of death by randomized group, trial and time since randomization (Word document)
Table S3 Causes of death by categorization of baseline ankle : brachial pressure index and time since randomization (Word document)
Table S4 Operative mortality by subgroup, for individuals who underwent an operation (Word document)
Table S5 Complications by trial, focusing on endovascular repair‐related complications (Word document)
Table S6 Effects of treated and untreated type II endoleak on survival (Word document)
Supporting information
Appendix S1 Supplementary information on search and statistical methods
Fig. S1 Aneurysm‐related mortality, overall and at 0–6 months, 6 months to 4 years and more than 4 years since randomization (Word document)
Fig. S2 Total mortality by aneurysm morphology subgroups (Word document)
Fig. S3 Total mortality by diabetes, BMI and smoking subgroups (Word document)
Fig. S4 Reinterventions by trial and time from aneurysm repair (Word document)
Fig. S5 Time from randomization to secondary sac rupture after endovascular aneurysm repair (Word document)
Table S1 Cardiovascular risk score (Word document)
Table S2 Causes of death by randomized group, trial and time since randomization (Word document)
Table S3 Causes of death by categorization of baseline ankle : brachial pressure index and time since randomization (Word document)
Table S4 Operative mortality by subgroup, for individuals who underwent an operation (Word document)
Table S5 Complications by trial, focusing on endovascular repair‐related complications (Word document)
Table S6 Effects of treated and untreated type II endoleak on survival (Word document)
Acknowledgements
The individual‐patient data meta‐analysis was funded by the National Institute for Health Research (NIHR), Health Technology Assessment (HTA) project 11/36/46 as a contract variation (Chief Investigator R. Greenhalgh). There was some support from the Camelia Botnar Research Foundation. They had no role in the study design, in the collection, merging, analysis or reporting of data, in the writing of the report, or in the decision to submit the paper for publication. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the HTA Programme, NIHR, National Health Service or the Department of Health.
Disclosure: The authors declare no conflict of interest.
Presented to the Charing Cross International Symposium, London, UK, April 2016
References
- 1. Dubost C, Allary M, Oeconomos N. [Treatment of aortic aneurysms; removal of the aneurysm; re‐establishment of continuity by grafts of preserved human aorta.] Mem Acad Chir (Paris) 1951; 77: 381–383. [PubMed] [Google Scholar]
- 2. Greenhalgh RM, Brown LC, Kwong GP, Powell JT, Thompson SG; EVAR trial participants. Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30‐day operative mortality results: randomised controlled trial. Lancet 2004; 364: 843–848. [DOI] [PubMed] [Google Scholar]
- 3. Blankensteijn JD, de Jong SE, Prinssen M, van der Ham AC, Buth J, van Sterkenburg SM et al; Dutch Randomized Endovascular Aneurysm Management (DREAM) Trial Group. Two‐year outcomes after conventional or endovascular repair of abdominal aortic aneurysms. N Engl J Med 2005; 352: 2398–2405. [DOI] [PubMed] [Google Scholar]
- 4. Becquemin JP, Pillet JC, Lescalie F, Sapoval M, Goueffic Y, Lermusiaux P et al; ACE trialists. A randomized controlled trial of endovascular aneurysm repair versus open surgery for abdominal aortic aneurysms in low‐to‐moderate‐risk patients. J Vasc Surg 2011; 53: 1167–1173. [DOI] [PubMed] [Google Scholar]
- 5. Lederle FA, Freischlag JA, Kyriakides TC, Matsumura JS, Padberg FT Jr, Kohler TR et al; OVER Veterans Affairs Cooperative Study Group. Long‐term comparison of endovascular and open repair of abdominal aortic aneurysm. N Engl J Med 2012; 367: 1988–1997. [DOI] [PubMed] [Google Scholar]
- 6. The UK EVAR Trial Investigators , Greenhalgh RM, Brown LC, Powell JT, Thompson SG, Epstein D, Sculpher MJ. Endovascular versus open repair of abdominal aortic aneurysm. N Engl J Med 2010; 362: 1863–1871.20382983 [Google Scholar]
- 7. Paravastu SC, Jayarajasingam R, Cottam R, Palfreyman SJ, Michaels JA, Thomas SM. Endovascular repair of abdominal aortic aneurysm. Cochrane Database Syst Rev 2014; (1)CD004178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schermerhorn ML, Buck DB, O'Malley AJ, Curran T, McCallum JC, Darling J et al Long‐term outcomes of abdominal aortic aneurysm in the Medicare population. N Engl J Med 2015; 373: 328–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cronenwett JL. Endovascular aneurysm repair: important mid‐term results. Lancet 2005; 365: 2156–2158. [DOI] [PubMed] [Google Scholar]
- 10. Kontopodis N, Antoniou SA, Georgakarakos E, Ioannou CV. Endovascular vs open aneurysm repair in the young: systematic review and meta‐analysis. J Endovasc Ther 2015; 22: 897–904. [DOI] [PubMed] [Google Scholar]
- 11. Brown LC, Epstein D, Manca A, Beard JD, Powell JT, Greenhalgh RM. The UK Endovascular Aneurysm Repair (EVAR) trials: design, methodology and progress. Eur J Vasc Endovasc Surg 2004; 27; 372–381. [DOI] [PubMed] [Google Scholar]
- 12. Prinssen M, Buskens E, Blankensteijn JD. The Dutch Randomised Endovascular Aneurysm Management (DREAM) trial. Background, design and methods. J Cardiovasc Surg (Torino) 2002; 43: 379–384. [PubMed] [Google Scholar]
- 13. ClinicalTrials.gov . Standard Open Surgery Versus Endovascular Repair of Abdominal Aortic Aneurysm (AAA) (OVER) . https://clinicaltrials.gov/ct2/show/NCT00094575 [accessed 2 March 2016].
- 14. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130: 461–470. [DOI] [PubMed] [Google Scholar]
- 15. Wilkins JT, Ning H, Berry J, Zhao L, Dyer AR, Lloyd‐Jones DM. Lifetime risk and years lived free of total cardiovascular disease. JAMA 2012; 308: 1795–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Parner ET, Andersen PK. Regression analysis of censored data using pseudo‐observations. Stata J 2010; 10: 408–422. [Google Scholar]
- 17. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 18. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reise JA, Sheldon H, Earnshaw J, Naylor AR, Dick F, Powell JT et al Patient preference for surgical method of abdominal aortic aneurysm repair: postal survey. Eur J Vasc Endovasc Surg 2010; 39: 55–61. [DOI] [PubMed] [Google Scholar]
- 20. Mani K, Bjorck M, Wanhainen A. Changes in the management of infrarenal abdominal aortic aneurysm disease in Sweden. Br J Surg 2013; 100: 638–444. [DOI] [PubMed] [Google Scholar]
- 21. Sumner DS, Strandness DE. The relationship between calf blood flow and ankle pressure in patients with intermittent claudication. Surgery 1969; 65: 763–771. [PubMed] [Google Scholar]
- 22. Heald CL, Fowkes FG, Murray GD, Price JF; Ankle Brachial Index Collaboration. Risk of mortality and cardiovascular disease associated with the ankle/brachial index: systematic review. Atherosclerosis 2006; 189: 61–69. [DOI] [PubMed] [Google Scholar]
- 23. Saratzis A, Sarafidis P, Melas N, Saratzis N, Kitas G. Impaired renal function is associated with mortality and morbidity after endovascular abdominal aortic aneurysm repair. J Vasc Surg 2013; 58: 879–885. [DOI] [PubMed] [Google Scholar]
- 24. Chaikof EL, Brewster DC, Dalman RL, Makaroun MS, Illig KA, Sicard GA et al SVS practice guidelines for the care of patients with an abdominal aortic aneurysm: executive summary. J Vasc Surg 2009; 50: 880–896. [DOI] [PubMed] [Google Scholar]
- 25. Wyss TR, Brown LC, Powell JT, Greenhalgh RM. Rate and predictability of graft rupture after endovascular and open abdominal aortic aneurysm repair: data from the EVAR trials. Ann Surg 2010; 252: 805–812. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supplementary information on search and statistical methods
Fig. S1 Aneurysm‐related mortality, overall and at 0–6 months, 6 months to 4 years and more than 4 years since randomization (Word document)
Fig. S2 Total mortality by aneurysm morphology subgroups (Word document)
Fig. S3 Total mortality by diabetes, BMI and smoking subgroups (Word document)
Fig. S4 Reinterventions by trial and time from aneurysm repair (Word document)
Fig. S5 Time from randomization to secondary sac rupture after endovascular aneurysm repair (Word document)
Table S1 Cardiovascular risk score (Word document)
Table S2 Causes of death by randomized group, trial and time since randomization (Word document)
Table S3 Causes of death by categorization of baseline ankle : brachial pressure index and time since randomization (Word document)
Table S4 Operative mortality by subgroup, for individuals who underwent an operation (Word document)
Table S5 Complications by trial, focusing on endovascular repair‐related complications (Word document)
Table S6 Effects of treated and untreated type II endoleak on survival (Word document)


