Abstract
Chimeric somatostatin/dopamine compounds such as BIM-23A760, an sst2/sst5/D2 receptors-agonist, have emerged as promising new approaches to treat pituitary adenomas. However, information on direct in vitro effects of BIM-23A760 in normal and tumoral pituitaries remains incomplete. The objective of this study was to analyze BIM-23A760 effects on functional parameters (Ca2+ signaling, hormone expression/secretion, cell viability and apoptosis) in pituitary adenomas (n = 74), and to compare with the responses of normal primate and human pituitaries (n = 3–5). Primate and human normal pituitaries exhibited similar sst2/sst5/D2 expression patterns, wherein BIM-23A760 inhibited the expression/secretion of several pituitary hormones (specially GH/PRL), which was accompanied by increased sst2/sst5/D2 expression in primates and decreased Ca2+ concentration in human cells. In tumoral pituitaries, BIM-23A760 also inhibited Ca2+ concentration, hormone secretion/expression and proliferation. However, BIM-23A760 elicited stimulatory effects in a subset of GHomas, ACTHomas and NFPAs in terms of Ca2+ signaling and/or hormone secretion, which was associated with the relative somatostatin/dopamine-receptors levels, especially sst5 and sst5TMD4. The chimeric sst2/sst5/D2 compound BIM-23A760 affects multiple, clinically relevant parameters on pituitary adenomas and may represent a valuable therapeutic tool. The relative ssts/D2 expression profile, particularly sst5 and/or sst5TMD4 levels, might represent useful molecular markers to predict the ultimate response of pituitary adenomas to BIM-23A760.
Pituitary adenomas represent one of the most common intracranial neoplasms. They are often accompanied by serious comorbidities, due to excessive hormonal secretion and/or compression of intracranial structures, such as amenorrhea, galactorrhea, growth abnormalities, hypopituitarism, cognitive and emotional disturbances and sexual dysfunctions1,2,3,4,5.
Somatostatin (SST) and dopamine (DA) are two well-known factors that regulate numerous, often overlapping, (patho)physiological functions6,7,8. Both, SST and DA bind to its own family of receptors (sst1–5 and D1–5, respectively), which exhibit a wide expression pattern in normal and tumoral tissues, including pituitary adenomas7,9. Activation of SST- and DA-receptors results in multiple, mostly inhibitory actions on endocrine and/or exocrine hormonal secretions and cellular proliferation7,8. Accordingly, these receptors serve as valuable targets for the pharmacological management of pituitary adenomas and other tumoral pathologies. Interestingly, pituitary adenomas often express, simultaneously, high levels of various ssts and Ds, showing expression profiles substantially altered compared with normal pituitaries or to cell types from which pituitary adenoma are originated10,11. Based on this and additional evidences, pharmaceutical companies have developed functional compounds selective for one or multiple sst-subtypes, with those selective for sst2 and sst5 being particularly useful (e.g., lanreotide, octreotide)8. Similarly, DA agonists selective for D2 (e.g., cabergoline), have been also generated and are efficiently used to treat some pituitary adenomas types, especially prolactin-secreting adenomas12.
Although ssts and Ds are highly present in pituitary adenomas, and the efficiency of the individual selective sst2/5 or D2 compounds have been proven in the treatment of pituitary adenomas, an appreciable subset of patients are poorly responsive or totally resistant to conventional therapy with SST- or DA-analogs8,12,13. Therefore, new approaches are already being tested or are currently under clinical investigation, such as the use of combined therapies (SST- plus DA-analogs), which have been shown to be more effective than individual compounds8.
Therefore, based on the well-known interaction between the SST- and DA-systems14 and on the ability of sst2 and sst5 to physically and functionally interact with D2 resulting in altered pharmacological or/and signaling properties14,15, an interesting new approach that is currently under basic and clinical investigation is the development and application of chimeric SST/DA compounds. As previously shown16, these drugs can retain the ability to interact with ssts and D2, and can display greater effects in reducing pituitary secretions than individual compounds. One of these promising chimeric SST/DA compounds is BIM-23A760, an agonist for sst2/sst5/D2 receptors used in clinical trials17,18,19. Specifically, the effect of BIM-23A760 has been tested in pituitary cell lines20 and in limited series of primary pituitary adenoma cell cultures16,21,22,23,24,25,26; however, the data collected to date on the in vitro effect of BIM-23A760 in pituitary adenomas is still incomplete, with some apparently contradictory results, which renders the available evidence somewhat inconclusive. Moreover, previous studies have not been focused to specifically analyze and compare the distinct effects of BIM-23A760 on several functional parameters in parallel in the same samples and/or in a wide range of cell types from pituitary adenomas, nor the responses of these pituitary adenoma cells have been compared with those observed in normal pituitary cells in vitro. Therefore, the aim of this study was to analyze a set of relevant functional parameters (Ca2+ signaling, hormonal expression and secretion, cell viability and apoptosis), in response to BIM-23A760 in the main types of pituitary adenomas, and in human/primate normal pituitary cells. Although BIM-23A760 has been withdrawn from clinical development after discovering a dopaminergic metabolite that accumulates and interferes with the activity of the parent compound in vivo19,27, it is still considered a good prototype molecule and therefore, the results generated using primary pituitary cell cultures from pituitary adenomas and normal pituitaries may be really useful in predicting the response to members of this class of compounds (i.e. new generation of chimeric agonist for sst2/sst5/D2 receptors) that may be used for clinical purposes in the future.
Results
Expression profile of sst2, sst5 and D2 in human and baboon normal pituitaries
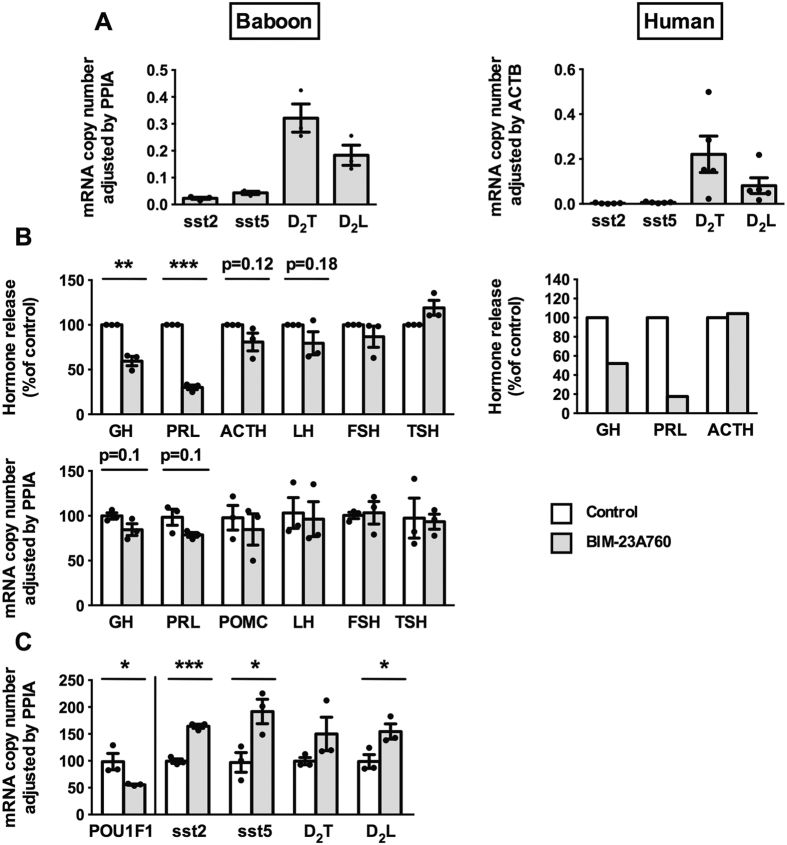
We found that sst5, sst2 and D2 were highly expressed in both baboon and human pituitary (Fig. 1A). Notably, the expression profile of sst2/sst5/D2, the target receptors for BIM-23A760, was virtually identical in both species (Fig. 1A). Specifically, pituitary cultures of baboons (n = 3) and humans (n = 5) cells expressed high levels of sst5, sst2 and D2, with relative order of D2T > D2L > sst5 > sst2. Notably, we found that cultures of baboon normal pituitaries maintain the same expression profile after dispersion and culture as whole normal pituitary tissues from baboons (Supplemental Table 1) which, together with the results presented in Fig. 1A, suggest that the baboon normal pituitary cultures might represent an appropriate model to study how BIM-23A760 modulate human pituitary cell function.
Figure 1. Expression profile and hormone release in the presence or absence of BIM-23A760 in normal pituitaries from baboons and humans.
(A) Expression profile of sst2, sst5, D2 (Total and Long) receptors in whole pituitary of female baboons (n = 3) and human pituitary (n = 5). (B) Effect of BIM-23A760 (100 nM) after 4-h treatment on the secretion and mRNA expression of all pituitary hormones in baboon primary pituitary cell cultures (n = 3; top and bottom panel on the left, respectively), and 24-h treatment on GH, PRL and ACTH release in human primary pituitary cell culture (n = 1; right-panel). (C) Effect of BIM-23A760 on POU1F1, sst2, sst5, D2 (Total and Long) after 4-h treatment in baboon primary pituitary cell cultures (n = 3). Values in figure B/C are expressed as percent of vehicle-treated controls, set at 100% within experiment. Hormonal release was determined by commercial ELISA kits. mRNA expression levels were measured by qPCR, and mRNA copy numbers were adjusted by Cyclophilin A (PPIA) and beta-actin (ACTB) mRNA copy number expression in baboons and humans, respectively. Values represent the mean ± SEM (3–4 wells/treatment/experiment). Asterisks show significant differences between BIM-23A760 and vehicle-treated controls in figure B and C (*p < 0.05, **p < 0.01; ***p < 0.001). In cases where only one experiment (n = 1) could be performed, no error bars are presented and no significance tests were performed.
Table 1. Demographic data of all the patients included in this study.
| n | Age, years (min-max) | Gender (male/female) | |
|---|---|---|---|
| Normal pituitary | 5 | 43 (21–69) | 0/5 |
| Somatotropinoma | 22 | 43 (13–64) | 8/14 |
| Mixed GH-PRL adenomas | 5 | 39 (32–60) | 2/3 |
| Prolactinoma | 6 | 32 (18–47) | 3/3 |
| Corticotropinoma | 11 | 38 (23–68) | 1/10 |
| Non functioning pituitary adenoma | 26 | 58 (22–74) | 14/12 |
| Gonadotropinoma | 1 | 35 | 0/1 |
| Thyrotropinoma | 3 | 58 (47–71) | 1/2 |
Incubation of cultured baboon pituitary cells with BIM-23A760 revealed clear inhibitory effects on GH and PRL release and a tendency to inhibit their expression levels (Fig. 1B, left-panels). Conversely, expression and/or release of proopiomelanocortin (ACTH and POMC, the ACTH-precursor, respectively), LH, FSH or TSH were not significantly altered in response to BIM-23A760 (Fig. 1B, left-panels). Because of the limited available amount of human normal pituitary samples, we could not study in depth the effects of BIM-23A760 on human normal pituitaries; however, we obtained a limited number of cells derived from a dispersed human normal pituitary preparation to study the effect of BIM-23A760 on the secretion of some, selected, hormones. Of note, as observed in baboons, we found that BIM-23A760 treatment seemed to inhibit GH and PRL, but not ACTH release in this culture of human normal pituitary cells (Fig. 1B, right-panel). Interestingly, we also observed that BIM-23A760 treatment evoked an up-regulation of sst2, sst5 and D2L expression, and a down-regulation of POU1F1 mRNA levels in baboons normal pituitary cell cultures (Fig. 1C).
Direct effects of BIM-23A760 on [Ca2+]i levels in human pituitary adenomas and normal pituitaries
It should be mentioned that free cytosolic calcium ([Ca2+]i) acts as a second messenger well-known for being directly involved and required in hormone secretory vesicle release28. The measurement of [Ca2+]i provides useful information about several parameters including: (1) the percentage of maximum response (PMR), which indicates the maximal response (positive or negative) achieved in [Ca2+]i in response to a specific treatment; (2) the proportion of responsive cells (PRC), which indicates the percentage of cells that elicit a positive or negative response in terms of [Ca2+]i levels in response to an specific treatment; and (3) the time to maximal response of sensitive cells to the specific compound, which indicates the time when the maximal response (positive or negative) is achieved in terms of [Ca2+]i levels in response to the specific treatment29,30. BIM-23A760 treatment inhibited [Ca2+]i levels (Table 2) in cell cultures of all normal pituitaries tested (n = 4; PMR: 77%, PRC: 42%), in 65% of GHomas (n = 13/20; PMR: 72%, PRC: 64%), in 80% of mixed GH/PRLomas (n = 4/5; PMR: 79%, PRC: 42%), in 67% of PRLomas (n = 4/6; PMR: 69%, PRC: 30%), in 30% of ACTHomas (n = 3/10; PMR: 73%, PRC: 24%), in 25% of NFPA (n = 4/16; PMR: 81%, PRC: 44%), in the available FSHoma (PMR: 65%, PRC: 66%), and in one of the two TSHomas included in the study (PMR: 53%, PRC: 65%). BIM-23A760 did not alter [Ca2+]i levels in 5%, 20%, 33%, 40%, 63% and 50% of the GHomas, mixed GH/PRLomas, PRLomas, ACTHomas, NFPAs and TSHomas analyzed, respectively (Table 2). Interestingly, we also found that BIM-23A760 increased [Ca2+]i levels in 30% of GHomas and ACTHomas (PMR: 212 and 223%; PRC: 69 and 35%, respectively), and in 13% of the NFPAs analyzed (PMR: 170%, PRC: 19%). Representative profiles depicting changes in [Ca2+]i levels in normal and tumoral pituitary cell cultures in response to BIM-23A760 are presented in Supplemental Figure 1.
Table 2. Effect of BIM-23A760 on free cytosolic calcium kinetics.
| PRC (%) | PMR (%) ± SEM | Time ± SEM | |
|---|---|---|---|
| Normal pituitary-1 | 60.0 | 71.2 ± 2.4 | 109.4 ± 0.5 |
| Normal pituitary-2 | 52.0 | 79.1 ± 2.2 | 74.2 ± 0.7 |
| Normal pituitary-3 | 16.3 | 76.0 ± 3.0 | 59.1 ± 3.4 |
| Normal pituitary-4 | 37.5 | 83.2 ± 1.1 | 69.7 ± 0.3 |
| GHoma-1 | 52.5 | 65.0 ± 2.2 | 38.3 ± 2.4 |
| GHoma-2 | 83.5 | 71.9 ± 6.0 | 42.11 ± 7.2 |
| GHoma-3 | 97.5 | 81.3 ± 1.05 | 30.13 ± 0.97 |
| GHoma-4 | 90.0 | 74.2 ± 2.8 | 30.0 ± 0.6 |
| GHoma-5 | 80.0 | 68.8 ± 2.0 | 42.0 ± 1.7 |
| GHoma-6 | 76.6 | 68.3 ± 8.7 | 47.8 ± 2.4 |
| GHoma-7 | 77.3 | 72.5 ± 2.3 | 25.6 ± 2.0 |
| GHoma-8 | 87.5 | 61.1 ± 2.2 | 54.3 ± 2.0 |
| GHoma-9 | 65.0 | 74.8 ± 0.8 | 104.8 ± 0.2 |
| GHoma-10 | 58.2 | 80.6 ± 3.2 | 38.6 ± 0.3 |
| GHoma-11 | 20.0 | 64.8 ± 3.2 | 36 ± 3.5 |
| GHoma-12 | 12.5 | 72.6 ± 4.3 | 61.0 ± 5.7 |
| GHoma-13 | 27.5 | 77.4 ± 1.3 | 71.4 ± 4.5 |
| GHoma-14* | 90.0 | 243.8 ± 9.1 | 27.8 ± 1.6 |
| GHoma-15* | 89.5 | 228.4 ± 3.3 | 18.0 ± 7.1 |
| GHoma-16* | 86.3 | 254.7 ± 15.2 | 37.0 ± 4.4 |
| GHoma-17* | 11.3 | 123.3 ± 6.7 | 31.5 ± 3.5 |
| GHoma-18* | 97.5 | 199.5 ± 6.8 | 26.2 ± 0.7 |
| GHoma-19* | 37.5 | 222.7 ± 13.6 | 56.3 ± 3.7 |
| GHoma-20† | 0.0 | ||
| Mixed GH/PRLoma-1 | 30.0 | 76.9 ± 2.0 | 43.8 ± 2.0 |
| Mixed GH/PRLoma-2 | 63.9 | 63.1 ± 2.4 | 53.0 ± 2.0 |
| Mixed GH/PRLoma-3 | 32.5 | 77.0 ± 1.7 | 77.7 ± 4.5 |
| Mixed GH/PRLoma-4 | 41.2 | 81.5 ± 2.4 | 26.0 ± 3.0 |
| Mixed GH/PRLoma-5† | 0.0 | ||
| PRLoma-1 | 41.0 | 63.0 ± 3.0 | 49.0 ± 2.4 |
| PRLoma-2 | 25.0 | 59.9 ± 2.8 | 92.0 ± 4.5 |
| PRLoma-3 | 42.9 | 63.0 ± 5.5 | 48.3 ± 4.9 |
| PRLoma-4 | 9.5 | 88.0 ± 1.3 | 35 ± 0 |
| PRLoma-5† | 0.0 | ||
| PRLoma-6† | 0.0 | ||
| ACTHoma-1 | 100.0 | 58.5 ± 3.8 | 82.5 ± 8.8 |
| ACTHoma-2 | 32.5 | 58.5 ± 4.3 | 53.1 ± 3.2 |
| ACTHoma-3 | 15.0 | 86.5 ± 3.7 | 48.3 ± 4.9 |
| ACTHoma-4* | 2.5 | 232.7 ± 0.0 | 20.0 ± 0.0 |
| ACTHoma-5* | 15.0 | 172.0 ± 17.0 | 28.0 ± 4.5 |
| ACTHoma-6* | 55.0 | 247.7 ± 13.0 | 67.5 ± 2.4 |
| ACTHoma-7† | 0.0 | ||
| ACTHoma-8† | 0.0 | ||
| ACTHoma-9† | 0.0 | ||
| ACTHoma-10† | 0.0 | ||
| NFPA-1 | 70.7 | 82.4 ± 0.3 | 52.9 ± 24.6 |
| NFPA-2 | 20.5 | 78.1 ± 2.3 | 58.4 ± 4.6 |
| NFPA-3 | 42.5 | 81.3 ± 8.8 | 47.8 ± 4.8 |
| NFPA-4 | 43.5 | 83.3 ± 0.6 | 65.0 ± 2.0 |
| NFPA-5* | 17.6 | 163.0 ± 17.0 | 51.0 ± 2.7 |
| NFPA-6* | 20.0 | 177.1 ± 18.8 | 30.0 ± 5.0 |
| NFPA-7† | 0.0 | ||
| NFPA-8† | 0.0 | ||
| NFPA-9† | 0.0 | ||
| NFPA-10† | 0.0 | ||
| NFPA-11† | 0.0 | ||
| NFPA-12† | 0.0 | ||
| NFPA-13† | 0.0 | ||
| NFPA-14† | 0.0 | ||
| NFPA-15† | 0.0 | ||
| NFPA-16† | 0.0 | ||
| FSHoma-1 | 66.2 | 64.5 ± 0.6 | 52.9 ± 5.7 |
| TSHoma-1 | 64.7 | 52.9 ± 3.0 | 14.0 ± 0.8 |
| TSHoma-2† | 0.0 |
The proportion of responsive cells (PRC) showing changes in [Ca2+]i levels in response to BIM-23A760 is indicated for all the pituitary cell culture preparations. Percentage of maximum response (PMR) and time of response to BIM-23A760 administration are also indicated. Pituitary adenomas in which an stimulatory response to BIM-23A760 was observed are indicated by an asterisk (*) while adenomas that did not respond to BIM-23A760 are indicated by a cross (†). The percentage of response (in %) and the time (in seconds) is indicated as mean ± SEM of 20–120 individual cells (depending on the experiment).
Hormonal expression/release
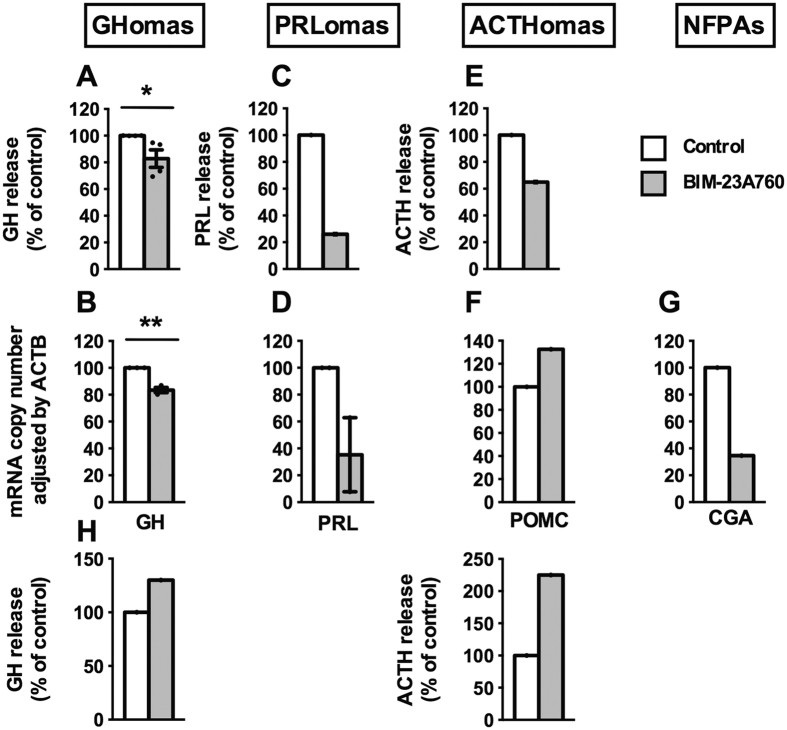
Incubation with BIM-23A760 (24 h) inhibited GH release (Fig. 2A) and expression (Fig. 2B) in GHomas. It also seemed to decrease PRL release/expression in PRLomas (Fig. 2C–D) and ACTH release in one ACTHoma (Fig. 2E) but not POMC expression (Fig. 2F). BIM-23A760 also seemed to reduce CGA expression in one NFPA (Fig. 2G); whereas it increased hormonal levels in one GHoma and one ACTHoma (Fig. 2H).
Figure 2.
Effect of BIM-23A760 (100 nM; 24 h) on pituitary hormone release and/or expression in primary human pituitary cell cultures from GHomas (A and B; n = 3–4), PRLomas (C and D; n = 1–2), ACTHomas (E and F; n = 1) and NFPAs (G; alpha-subunit (CGA); n = 1). (H) Stimulatory effect of BIM-23A760 on GH and ACTH release in GHomas and ACTHomas (n = 1). Values are expressed as percent of vehicle-treated controls, set at 100% within experiment. Hormonal release was determined by commercial ELISA kits, and mRNA expression levels were measured by qPCR. mRNA copy numbers were adjusted by ACTB mRNA copy number. Values represent the mean ± SEM. Asterisks show significant differences between BIM-23A760 and vehicle-treated controls (*p < 0.05, **p < 0.01). In cases where only one experiment (n = 1) could be performed, no error bars are presented and no significance tests were performed.
Cell viability and apoptotic rate
Direct effects of BIM-23A760 on cell viability and apoptotic rate were also tested in human normal pituitaries and pituitary adenomas cultures. BIM-23A760 seemed to moderately inhibit cell viability at different incubation-times in one cultured normal pituitary (Fig. 3A). Similarly, BIM-23A760 also inhibited cell viability in GHomas (Fig. 3B), one mixed GH/PRLoma (Fig. 3C) and NFPAs (Fig. 3E). Additionally, BIM-23A760 down-regulated cell viability in PRLomas at 72 h (Fig. 3D), but had no effect in the TSHoma analyzed (Fig. 3F). Moreover, BIM-23A760 increased apoptotic rate in GHomas, as observed using Annexin-V-FITC/propidium iodide staining assay (Fig. 3G).
Figure 3.
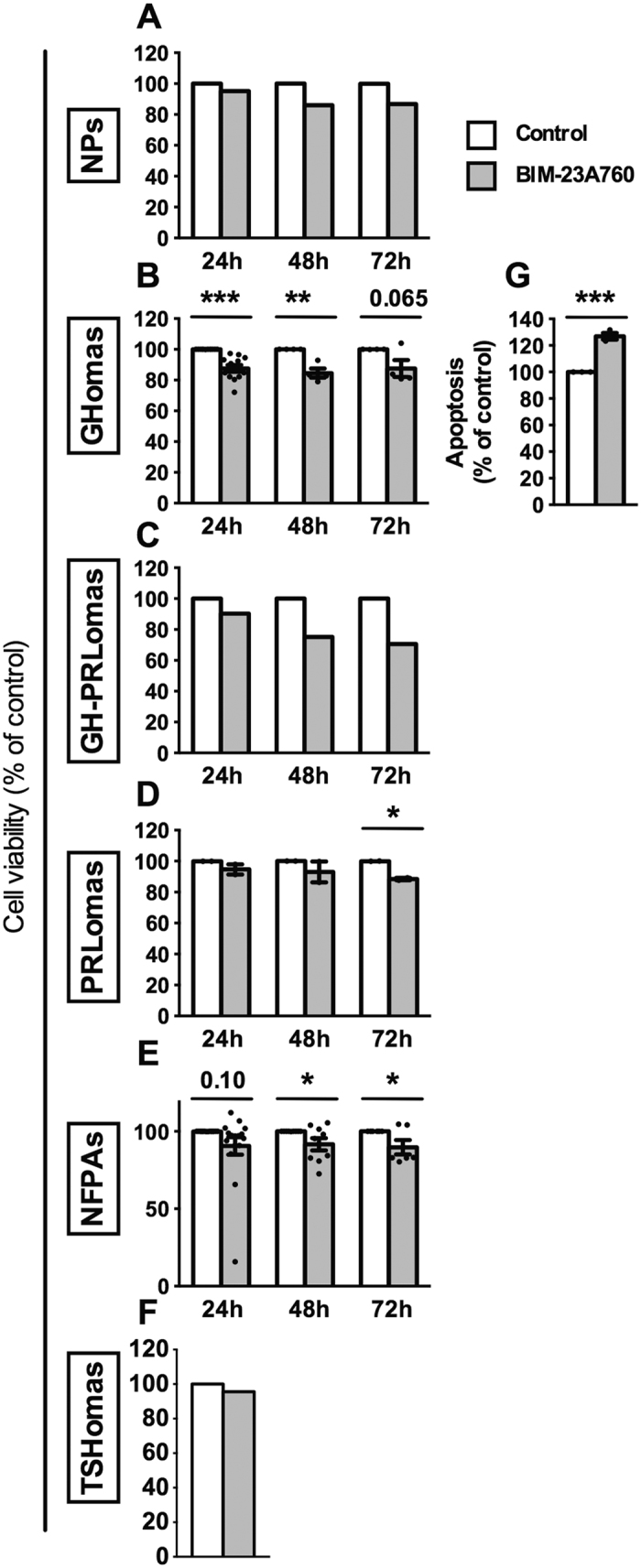
(A) Effect of BIM-23A760 (100 nM) on cell viability (24–72-h treatment) in human normal pituitaries (NP; n = 1), GHomas (n = 11), mixed GH/PRLomas (n = 1), PRLomas (n = 2), NFPAs (n = 18) and TSHomas (n = 1). Effect of BIM-23A760 on apoptosis in GHomas (n = 3). Values represent the mean ± SEM. Asterisks show significant differences between BIM-23A760 and vehicle-treated controls (*p < 0.05, ***p < 0.001). In cases where only one experiment (n = 1) could be performed, no error bars are presented and no significance tests were performed.
Molecular mechanisms underlying the differential, inhibitory/stimulatory, response to BIM-23A760 in human GHomas and ACTHomas
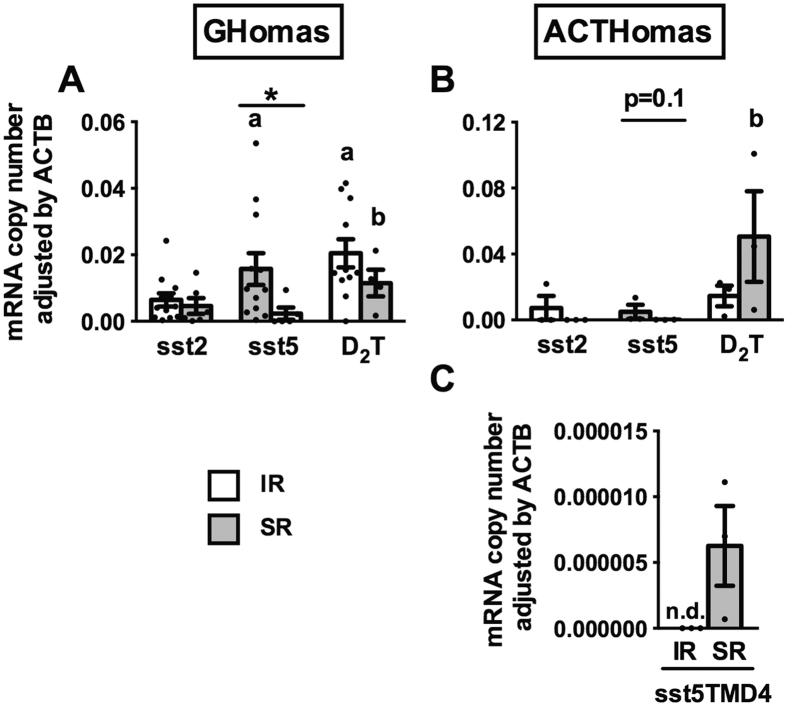
The results previously presented suggest that two populations may exist in pituitary adenomas (at least within GHomas and ACTHomas) that respond differentially, even oppositely, to BIM-23A760 (hence forth referred to as “inhibited pituitary adenoma” and “stimulated pituitary adenoma”). Analysis and comparison of the expression levels profile of D2 and sst2/sst5-subtypes in human GHomas and ACTHomas revealed that D2 and sst5 expression levels were significantly higher than sst2 in inhibited GHomas (Fig. 4A); whereas, sst5 levels were lower than those of D2 in stimulated GHomas. When comparing the expression levels across GHomas, the only difference found was a significantly lower sst5 expression in stimulated GHomas vs. inhibited GHomas (Fig. 4A). In ACTHomas, there were no differences between the average expression levels of sst2, sst5 and D2 in inhibited ACTHomas (Fig. 4B). In contrast, D2 expression levels were significantly higher than those of sst2 and sst5 in stimulated ACTHomas. Interestingly, when expression levels were compared across ACTHomas, we found a similar situation to that previously observed in GHomas, in that sst5 levels were lower in stimulated vs. inhibited ACTHomas (Fig. 4B).
Figure 4.
Differential expression profile of sst2, sst5 and D2T according to the inhibitory and stimulatory responses observed in terms of [Ca2+]i kinetics (Table 2) in GHomas (A; n = 12, and n = 6, inhibited- and stimulated- adenomas, respectively) and ACTHomas (B); n = 3 and n = 3, inhibited- and stimulated- adenomas populations. Inhibitory and stimulatory responders were indicated as IR (white bars) and SR (black bars), respectively. (C) Expression profile of sst5TMD4 in the inhibited population (n = 3) and stimulated population (n = 3) of ACTHomas. Values represent the mean ± SEM. Asterisks show significant differences between the same receptor-subtype in inhibited- and stimulated- adenoma populations of GHomas and ACTHomas (*p < 0.05). “a” indicates a statistical difference in the expression levels of sst5 or D2 as compared with sst2 expression in the inhibited population. “b” indicates a statistical difference in the expression levels between of sst5 compared with D2 expression in the stimulated population.
Finally, truncated sst5TMD4 expression levels did not change between both GHomas populations (mean of 187 copies/0.05 μg of total RNA); however, the stimulated population of ACTHomas expressed higher mRNA levels of sst5TMD4 (mean of 96 copies/0.05 μg of total RNA), while no expression was detectable in the inhibited population of ACTHomas (0 copies/0.05 μg of total RNA; Fig. 4C).
Discussion
The results of this study indicated that, consistent with previous reports11,31, sst5, sst2 and D2 were highly expressed in both baboon and human normal pituitaries, showing a similar expression pattern (i.e. D2T > D2L > sst5 > sst2). These results, coupled with our data showing that baboon pituitary cultures maintain the same expression profile after dispersion and culture as whole-pituitaries, and with previous reports aimed to analyze pituitary physiology32,33, strongly suggest that baboon normal pituitaries might be an appropriate model to study human pituitary function. Thus, in vitro treatment of cultured baboon pituitary cells with BIM-23A760 demonstrated a decrease of GH and PRL expression/release, which was comparable to that shown in human pituitary. To the best of our knowledge, this is the first study showing, the direct effect of in vitro BIM-23A760 on the expression and/or secretion of all major pituitary hormones in primates and/or humans normal pituitaries. As such, our results extend and reinforce previous data, which indicated that s.c. administration of BIM-23A760 suppressed circulating PRL secretion in healthy male volunteers17, and GH, IGF1 and PRL secretion in normal cynomolgus monkeys in vivo34. Furthermore, the present work demonstrates that BIM-23A760 effects on normal pituitary are not just confined to regulate the release or expression of pituitary hormones, but also include an up-regulation of sst2, sst5 and D2L expression. These findings suggest that, even though mRNA may not always necessarily reflect the precise, final protein levels of functional receptors available at the cell membrane (a technically challenging assay that we could not perform given the limited tissue availability), the changes observed herein likely represent a relevant up-regulation in the expression of these receptors, which would reflect an additional regulatory mechanism, that is typically observed in GPCRs33, on pituitary cells35, and enables to finely tune the response of pituitary cells to their ligands. Moreover, our data invite to speculate that the effects of BIM-23A760 on somatotropes/lactotropes might be mediated by the inhibition of POU1F1 mRNA production. Altogether, these novel results support the notion that the use of chimeric molecules as BIM-23A760 could provide a new, valuable therapeutic tool for medical treatment of different pituitary adenoma types, and thereby invite further exploration of the underlying molecular mechanisms of its effects.
The central aim of this study was to systematically analyze the effect of BIM-23A760 on essential functional parameters in all major human pituitary adenoma cell types. Because of the limited cells available after surgical resection and dispersion of pituitary adenomas, we decided to test the effect of BIM-23A760 on [Ca2+]i kinetics assay, a sensitive assay that requires a small number of cells compared to other assays and provides useful information about several parameters (PRC, PMR,…). Our data indicate that BIM-23A760 inhibited [Ca2+]i levels in normal and tumoral pituitary cells; but increased [Ca2+]i levels in a population of GHomas, ACTHomas and NFPAs, demonstrating that BIM-23A760 directly acts on human pituitary adenomas and normal pituitary cells by activating [Ca2+]i levels. In this regard, the only previous study exploring activation of signaling pathways in response to BIM-23A760 indicated that the antiproliferative effect of BIM-23A760 involved phosphorylation of ERK1/2 and p38 MAPK pathways, and that this antiproliferative effect was mainly exerted through the activation of D2 in NFPAs23. However, our study is the first to characterize, in a wide range of pituitary adenomas and in normal pituitaries, a key element of the signaling pathway activated by BIM-23A760 directly linked to hormone release, i.e. [Ca2+]i28. When remaining cells were available from normal pituitaries and pituitary adenomas cultures, we decided to additionally measured hormonal secretion and/or expression, cell viability and apoptosis in response to BIM-23A760 but, unfortunately, the limited amount of cells available precluded the desired in-depth study on the possible mechanisms associated with these changes in [Ca2+]i on human pituitary adenoma and normal pituitary cells in response to BIM-23A760. Nevertheless, the question still arises, why some pituitary adenomas exhibit a differential, even opposite, response to BIM-23A760? As will be discussed further below, a different receptor expression profile, and the possible interactions between receptors in the various pituitary adenoma types, might provide at least a partial explanation for the effects of BIM-23A760.
We found that treatment with BIM-23A760 inhibited hormone secretion and/or expression in GHomas; and in individual observations in PRLomas, ACTHomas and NFPAs, which is in line with previous reports showing that BIM-23A760 potently decreased hormone release in cultures from GHomas and the GH3 cell line16,20,26,36, PRLomas and the MMQ cell line20,22, mixed GH/PRLomas16,26 and ACTHomas37, and it also agrees with a Phase-II clinical study in acromegalic patients showing that BIM-23A760 inhibits basal circulating GH levels18,19. In favor of the idea of using chimeric SST/DA compounds to reduce hormonal expression/secretion from various pituitary adenoma types is the report of Saveanu and coauthors demonstrating that the potency of another chimeric compound (BIM-23A387) in suppressing GH secretion was 100-times higher than individual sst2 or D2 analogs38. However, it should be noted that, in line with both the stimulatory and inhibitory effects of BIM-23A760 observed on [Ca2+]i levels, we also observed that BIM-23A760 increased GH and ACTH release in individual cases of GHomas and ACTHomas, which suggest that there are different subpopulations of adenomas. We could not corroborate whether NFPAs also have a differential response to BIM-23A760 as these tumors respond poorly in terms of hormone secretion.
Hence, and despite exercising due caution, our results reinforce the notion that at least two populations may exist in GHomas and ACTHomas that respond differentially, even oppositely, to BIM-23A760. Nevertheless, this stimulatory effect of BIM-23A760 should not necessarily be surprising, based on previous studies which have revealed that SST, DA and/or their analogs can directly stimulate pituitary hormone secretion in primates and other species31,39,40, and in a select population of human pituitary adenomas40,41,42. Furthermore, it is well-known that different SST-agonists can elicit different effects in the same pituitary adenoma cell-type, known as “biased agonism”43, which might be directly depending on the agonist-receptor and receptor-receptor interactions, on the active receptor-conformations, etc. Therefore, and although we could not perform a systematical analysis of the relative contribution of SST and DA receptors to this differential response due to a limitation in the availability of cells from the same pituitary adenoma, comparison of the receptor expression profiles between the pituitary adenoma inhibited-population and stimulated-population in response to BIM-23A760 might be important for determining the molecular mechanism involved in the opposite responses to this and other compounds (see below).
We also observed that BIM-23A760 treatment decreased cell viability in GHomas, NFPAs and PRLomas at different incubation-times and stimulates apoptosis in GHomas. These results are, in part, consistent with previous data showing that BIM-23A760 decreased cell viability/proliferation in NFPAs21,23,44 and TSHomas25, and increased apoptosis in NFPAs44. These results, therefore, suggest that in addition to suppressing [Ca2+]i levels and hormonal hypersecretion, BIM-23A760 may be able to decrease cell viability and increase apoptotic rate in pituitary adenomas. Taken together, our results provide relevant information from a mechanistic/therapeutic perspective, since demonstrate that BIM-23A760 can alter (decrease) key cellular parameters and also influences clinically relevant endpoints in a significant subset of pituitary adenomas. Once again, these findings invite further exploration of the potential value of SST-DA chimeric compounds as therapeutic approaches for patients with pituitary adenomas.
In order to identify molecular determinants that could explain the differential, inhibitory/stimulatory, responses to BIM-23A760, expression levels of sst2/sst5/D2 were measured in GHomas and ACTHomas and the results were compared between “inhibited pituitary adenomas” and “stimulated pituitary adenomas”, based on the data of [Ca2+]i kinetics. Interestingly, when results from both GHomas and ACTHomas are viewed together, we observed two common, distinctive molecular signatures, namely, a lower sst5 expression in stimulated vs. inhibited pituitary adenomas, and a higher D2 expression level as compared with sst5 expression in stimulated pituitary adenomas. Therefore, these findings might suggest that the relative sst5 expression levels (i.e. lower sst5 levels in stimulated vs. inhibited pituitary adenomas as well as, lower sst5 as compared with D2 levels in the stimulated pituitary adenomas) might represent a potential molecular signature contributing to the differential response to BIM-23A760.
These results, coupled to our previous demonstration that the truncated sst5TMD4 receptor is overexpressed in pituitary adenomas and plays relevant roles in GHomas29,45,46 and in other endocrine-related tumors47,48, prompted us to analyze its expression in the two pituitary adenoma populations. Notably, while sst5TMD4 expression levels did not change between both GHomas populations, the stimulated-population of ACTHomas expressed appreciable levels of sst5TMD4, which were not detectable in the inhibited-population of ACTHomas. Although full-length sst5 mRNA is the dominant sst5 gene transcript in the stimulated population of ACTHomas, with absolute levels much greater than those of sst5TMD4 (27-fold), our results unveil that sst5TMD4 is selectively expressed in the stimulated population of ACTHomas, thus suggesting a potential involvement of sst5TMD4 in the unique stimulatory response of some ACTHomas to BIM-23A760.
Nevertheless, when viewed together, our results strongly suggest that sst5 expression in GHomas and ACTHomas, and sst5TMD4 expression in ACTHomas might represent useful molecular markers to predict the ultimate response of these pituitary adenoma types to BIM-23A760. This idea is supported by a study showing that presence of sst5TMD4 is linked to a reduced in vivo hormonal response to SST-analogue treatment in acromegalic patients45. Further support that the low sst5 expression level in the stimulated-population of GHomas and ACTHomas may serve as a potential signature to distinguish between the differential response of pituitary adenomas is provided by a previous study in patients with TSHomas, which showed that tumors displaying a stimulatory response to octreotide expressed relatively low levels of sst5 in comparison with sst2, while tumors with higher sst2 than sst5 expression obtained a beneficial response to octreotide25. Nevertheless, it should not be discounted that other factors could also contribute to the differential, inhibitory/stimulatory response of GHomas and ACTHomas to BIM-23A760. One of these factors could be the precise number of receptors and/or their specific proportion available on the tumor cells. In this scenario, it seems conceivable that a specific expression profile (such as high D2 vs. sst5 expression levels in stimulated-pituitary adenomas compared to similar D2/sst5 expression levels in inhibited-pituitary adenomas) could be a key molecular determinant for the response to BIM-23A760, as it could dictate the possible interactions between receptor subtypes (homo and/or heterodimerization). Obviously, further, specifically directed, studies will be required to formally prove this notion with regard to the effect of BIM-23A760 in pituitary adenoma cells. However, this concept is supported by several studies demonstrating that the therapeutic response of pituitary adenomas to different drugs is directly dependent on the relative expression pattern of both SST and DA receptors10,49. The existence of heterodimers between SST/DA receptors (e.g. sst5 and D2) that result in changes in the functional, pharmacological and signaling properties of the receptors is well established14. In particular, this concept is supported by results demonstrating that only the amount of dimers between sst5 and D2, and not between sst2 with D2, was directly and positively correlated with an enhanced antiproliferative effect of BIM-23A760 in prostate and lung cancer cell lines50.
Altogether, the results of the present study provide convincing evidence that chimeric compounds for the sst2/sst5/D2 system might represent valuable tools for the design and development of new therapeutic drugs for the management of certain pituitary adenomas and its associated comorbidities in the near future.
Materials and Methods
Reagents
All reagents used in this study were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise specified. BIM-23A760 was kindly provided by IPSEN Bioscience (Cambridge, MA, USA) and prepared as previously described26; specifically, dry powder was dissolved in 0.01 N acetic acid containing 0.1% bovine serum albumin, aliquoted and stored at −20 °C. Fresh working solutions were prepared for each experiment from a new aliquot.
Patients and animals tissue collection
Human pituitary specimens were obtained during transsphenoidal surgery (from 2008 to 2014) from a total of 74 patients [22 somatotropinomas (GHomas), 5 mixed GH/PRLomas, 26 nonfunctioning pituitary adenoma (NFPAs), 6 PRL-secreting adenomas (PRLomas), 11 corticotropinomas (ACTHomas), 1 FSH-secreting gonadotropinoma (FSHoma) and 3 thyrotropinomas]. Moreover, 5 samples corresponding to normal pituitary tissue were used, which were obtained from patients who underwent surgical removal of a pituitary adenoma, and the tissue piece obtained by our laboratory was confirmed as normal pituitary tissue. Specifically, both human normal and tumoral pituitary tissue pieces were confirmed by 3 separate methods: examination by an expert anatomopathologist, molecular screening by quantitative real time PCR of the main pituitary hormonal products and membrane receptors, and analysis of the hormonal phenotype using single-cell secretion by a cell-blotting assay, as previously described30. Before surgery, all patients with a GHoma or a PRLoma were treated with somatostatin and dopamine analogs, respectively. Available patients’ demographic data are summarized in Table 1. Due to the extensive time-range required for collection of samples, histological and genetic information of the patients and samples obtained were limited, and therefore additional information about immunohistochemistry, granulation pattern of the tumors, presence of AIP or GNAS mutations, or other novel factors that have been demonstrated to affect the response to pharmacological treatment could not be provided8. Informed consent from each patient was obtained. Primate (Olive Baboon, Papio anubis; n = 3, 9–10 yr of age) pituitaries were obtained from randomly cyclic control females within 15 min after sodium pentobarbital overdose as previously reported51. It should be mentioned that the baboons used represent control animals from a breeding colony, all under Institutional Animal Care and Use Committee approved studies conducted by other University of Illinois at Chicago investigators. All the methods were carried out in accordance with the approved guidelines of the University of Córdoba/IMIBIC and Hospital Ethics Committees were obtained. All experimental protocols were approved by University of Córdoba, IMIBIC and University of Illinois at Chicago institutional committees.
Primary pituitary cell culture
All human pieces (normal and tumoral pituitaries) were immediately collected after surgery, placed in sterile cold (4 °C) medium (S-MEM, Gibco, Madrid, Spain) supplemented with 0.1% BSA, 0.01% L-glutamine, 1% antibiotic-antimycotic solution, and 2.5% HEPES; then were rapidly moved to our laboratory on ice within 1–3 hours, where they were dispersed into single cells for culture by enzymatic and mechanical disruption following the methods and reagents previously reported30,52,53. Primate anterior pituitaries were cut into small pieces, and one-two fragments were rapidly frozen in liquid nitrogen and stored at −80 °C until extraction for total RNA, while the remaining pieces were placed in sterile cold α-Minimum essential medium (α-MEM) (Invitrogen, Grand Island, NY, USA) supplemented with 0.15% BSA, 6 mM HEPES, and 10 IU/ml penicillin and 10 μg/ml streptomycin (Invitrogen); and dispersed into single cells for culture following the methods/reagents previously reported33,51,54,55. Both human and primate pituitary samples were minced into 1–2 mm3 pieces under sterile conditions. Some pieces were stored for posterior RNA isolation at −80 °C and the remaining tissue was washed and incubated in 30 ml S-MEM medium complemented with 0.3% trypsin (Beckson, Dickinson and Company, Sparks, MD, USA) in a spinner flask (Bellco Glass, Vineland, NJ, USA) for 2 h at 37 °C under gentle shaking and then, incubation was continued for 5 min in presence of 1 mg of DNAse I (Roche, Mannheim, Germany). Dispersed cells were decanted by centrifugation and then, by repeated aspiration into a smooth tipped glass Pasteur pipette. Finally, human cell suspension was washed in 4.5 g/L glucose containing DMEM medium (Gibco, Madrid, Spain) complemented with 0.1% BSA, 0.01% L-glutamine, 1% antibiotic-antimycotic solution, and 2.5% HEPES, and baboon cell suspension was washed in supplemented α-MEM as above described but containing 10% horse serum (Invitrogen). To avoid fibroblast contamination, suspensions of dispersed human and baboon pituitary cells were filtered through a nylon gauze of 130 μm-mesh, and in both DMEM and α-MEM, D-Valine-was replaced for L-valine to selectively inhibit fibroblast proliferation/overgrowth as previously reported56. In addition, visual inspection of primary cell cultures at the time of experimental assays showed no sign of cells displaying the typical fibroblast-like morphology. All the individual pituitary cultures showed cell viability higher than 95%, as determined by the trypan blue dye exclusion method (American Type Culture Collection, Manassas, VA).
Measure of pituitary hormones
Pituitary cell cultures (100,000–200,000 cell/well, n = 3–4 wells/treatment) were incubated with media alone (controls) or media containing BIM-23A760 (100 nM) for 4 h (primate normal pituitary cells) or 24 h (human normal pituitary and pituitary adenoma cells). At the end of the incubation with the corresponding treatment, media were recovered for hormone analysis and, whenever possible, cells were recovered for RNA analysis (see below). As previously reported elsewhere51, hormone concentrations were measured in the culture media derived from human samples according to the pituitary adenoma type and, when possible, all pituitary hormones concentrations were measured in the human and baboon normal pituitary samples using human/primate commercial ELISA kits [GH, LH, FSH, PRL, ACTH and TSH (reference numbers: EIA-3552, EIA-1289, EIA-1288, EIA-1291, EIA-3647 and EIA-1790, respectively; DRG, Mountainside, NJ, USA)] following the manufacturer’s instructions. All the information regarding specificity, detectability, and reproducibility for each of the assays can be accessed at the website of the company.
RNA isolation, reverse transcription, and analysis of gene expression by quantitative real-time PCR (qPCR)
Details of RNA extraction, quantification, reverse-transcription (RT), application of qPCR and primer sequences used to measure the expression levels of human and baboon transcripts included in this study (pituitary hormones, sst2, sst5 and D2 [long (D2L) and total (D2T) isoforms] and POU1F1) have been previously reported elsewhere by our group10,11,31. Briefly, human and baboon frozen tissues were processed for recovery of total RNA with two commercial kits following the manufacturer’s protocols: Absolutely RNA RT-PCR Miniprep Kit (Agilent, La Jolla, CA, USA) with deoxyribonuclease treatment and AllPrep DNA/RNA/Protein Mini Kit followed by deoxyribonuclease treatment using RNase-Free DNase Set (Qiagen, Limburg, Netherlands). Total RNA from pituitary cell cultures was isolated using TRIzol Reagent (Life Technologies, Barcelona, Spain) following the manufacturer’s protocol and subsequently treated with DNase (Promega, Barcelona, Spain). Total RNA amount was quantified using the Ribogreen RNA Quantification Kit (Molecular Probes, Eugene, OR). Total RNA (1 μg for whole human and baboon tissues; ∼0.15 μg for pituitary cell cultures treated with vehicle or BIM-23A760) was reversed transcribed using random hexamers and the cDNA First Strand Synthesis kit (MRI Fermentas, Hanover, MD, USA); and the cDNAs were amplified by qPCR using a Stratagene Mx3000p real-time PCR machine and the brilliant SYBR Green QPCR Master Mix (Stratagene, La Jolla, CA, USA). Samples were run against synthetic standards (1, 101, 102, 103, 104, 105, and 106 copies) for each transcript of interest to estimate mRNA copy number, and a No-RT sample was used as a negative control. Thermal profile: one step at 95 °C for 10 minutes; 40 cycles of denaturation (95 °C for 30 seconds), annealing (61 °C for 1 minute), and extension (72 °C for 30 seconds); and one dissociation cycle to verify the reaction. Since it is not possible to design a specific set of primers for qPCR that only amplified the short isoform of D211, a set of primers that amplify both, the long and short, isoforms (D2T) and a set of primers that only amplify the long isoform (D2L) were used in this study. As previously reported, to control for variations in the amount of RNA used in the RT reaction and the efficiency of the RT reaction, the expression level (copy-number) of each transcript was adjusted by ACTB (human samples30) or by PPIA (baboon samples51) expression, used as control genes based on their stability among the different samples (no significant changes were observed in the expression of these genes among experimental groups).
Measurements of free cytosolic calcium concentration ([Ca2+]i)
As we have previously described in detail elsewhere29,30,53, changes in [Ca2+]i in response to treatment with BIM-23A760 were measured in single cultured human pituitary cells (50,000 cell/well) by using fura-2AM probe (Molecular Probes, Eugene, OR, USA) and MetaFluor Software (Imaging Corp, West Chester, PA, USA). Particularly, human primary pituitary cells were plated for 36–48 h onto glass coverslips (35-mm plates); then they were incubated at 37 °C for 30 min with fura-2AM in phenol red-free DMEM containing 20 mM NaHCO3 (pH 7.4). Coverslips were washed with phenol red-free DMEM and placed on a Sykes-Moore chamber (Bellco Glass, Madrid, Spain) and set on an inverted microscope Eclipse TE2000-E (Nikon, Tokyo, Japan) attached to a digital camera ORCA II BT (Hamamatsu Photonics, Hamamatsu, Japan). Briefly, cells were examined for changes in [Ca2+]i after BIM-23A760 treatment while exposed to alternating 340–380 nm light beams, and the intensity of light emission at 505 nm was measured every 5 seconds using a 40x objective with Immersion Oil Type NF (Nikon, Tokyo, Japan). Data was collected and processed using MetaFluor Software (Imaging Corp., West Chester, PA, USA). Phenol red-free DMEM and ionomycin (Sigma-Aldrich, Madrid, Spain) were used, respectively, as negative and positive controls.
Measurements of cell viability
Cell viability was evaluated in primary cell cultures (10,000 cell/well; n = 4–5 well/treatment) treated with BIM-23A760 as compared with vehicle-treated controls using the alamar-Blue reagent (Biosource International; Camarillo, CA, USA). Specifically, cells were serum-starved for 12–16 h and then, treated with serum-free medium containing 10% alamar-Blue for 3 h, and subsequently treated for 24-, 48-, and 72-h with BIM-23A760 or vehicle-controls. Reduction of alamar-Blue was quantified exciting at 560 nm and reading at 590 nm in the FlexStation III system (Molecular Devices, Sunnyvale, CA, USA) following the manufacturer’s instructions, as previously reported29,30,53.
Measurements of apoptosis
To evaluate the apoptotic rate in GHomas, 150,000 cells/well were plated and cultured for 36 h. Cell cultures were incubated for 12 h with BIM-23A760 as compared with vehicle-treated controls; after that: media were collected, centrifuged 5 min at 1,200 rpm, and the supernatant was discarded and the pellet was maintained; then cells were washed with PBS, detached with a cell scrapper and collected together with the previous pellet. Then, centrifuged for 5 min at 1,200 rpm and the supernatant was discarded while the pellet was processed following manufacturer’s instructions of Annexin-V-FITC/propidium iodide staining assay (Bender Medsystems, Barcelona, Spain) and measurement of apoptotic rate were carried out by flow cytometry (Beckman Coulter, Coulter Epics XL, Madrid, Spain).
Statistical analysis
Statistical differences were assessed by unpaired parametric t-test (using Welch correction in the case of unequal variances) or nonparametric Mann-Whitney tests according to normality, assessed by Kolmogorov-Smirnov test. No corrections for multiple comparisons were performed. As previously reported30,51,53 to normalize values within each treatment and minimize intragroup variations in the different in vitro experiments (i.e. different age of the tissue donor, different stage of the estrus cycle or metabolic environment), the values obtained were compared with vehicle-treated controls (set at 100%). Specifically, to generate these values, individual values (adjusted by the corresponding level of control gene in the case of qPCR), within each individual experiment were divided by the mean value of the control group and multiplied times 100, and the means of these adjusted values are presented with their associated standard error. It should be emphasized that this style of data presentation does not alter the relative differences between BIM-23A760-treated and vehicle-treated groups. All data are expressed as mean ± SEM. In the cases wherein only one (n = 1) experiments could be performed due to a limitation in the availability of pituitary adenoma samples/cells, mean from tri-quadruplicate measures without error bars are depicted. p < 0.05 was considered significant; when p-values ranged between <0.1 and >0.05, a trend for significance was indicated where appropriate. All statistical analyses were performed using GraphPad Prism 6 (GraphPad Software; La Jolla, CA, USA).
Additional Information
How to cite this article: Ibáñez-Costa, A. et al. BIM-23A760 influences key functional endpoints in pituitary adenomas and normal pituitaries: molecular mechanisms underlying the differential response in adenomas. Sci. Rep. 7, 42002; doi: 10.1038/srep42002 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
We thank Anabel Pozo-Salas and Fernando L-López (University of Córdoba/ Instituto Maimónides de Investigación Biomédica de Córdoba), and Ainara Madrazo-Atutxa (Instituto de Biomedicina de Sevilla) for their invaluable help. This work has been funded by the following grants: Junta de Andalucía (BIO-0139, CTS-1406, PI-639-2012); Ministerio de Economía y Competitividad, Gobierno de España (BFU2013-43282) ; Proyectos de Investigación en Salud FIS, funded by Instituto de Salud Carlos III (PI13-00651, co-funded by ERDF/ESF, “Investing in your future”); Instituto de Salud Carlos III, Ministerio de Sanidad, Servicios Sociales e Igualdad ‘CIBERobn’; and Ayuda Merck Serono 2013 (to RML and JPC). Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development Merit Award and the National Institutes of Health: R21AG031465 and R01DK088133 (to RDK).
Footnotes
M.D. Culler is an employee of IPSEN. E. Venegas-Moreno, A. Soto-Moreno, Susan M. Webb, J.P. Castaño and R.M. Luque have received lecture fees and/or research grant support from Ipsen and Novartis. The rest of the authors have nothing to disclose.
Author Contributions Conception of the work: A.I.-C., M.D.C., J.P.C., R.M.L.; Design of research: A.I.-C., R.D.K., J.P.C., R.M.L.; Performed experiments: A.I.-C., L.M.L.-S., M.D.G., E.R.-C., M.C.V.-B., R.M.L.; Analyzed data: AI-C, JPC, RML; Interpreted results of experiments: A.I.-C., M.D.G., M.D.C., J.P.C., R.M.L.; Prepared figures: A.I.-C., M.D.G., R.M.L.; Acquisition of clinical/pathological data and samples: M.A.G., A.d.l.R., EV-M, LJR, A.M.-C., F.J.T., S.M.-S., M.A.J., J.A.G.-A., A.S.-M., S.M.W., R.D.K.; Draft the work: A.I.-C., L.M.L.-S., M.D.G., E.R.-C., M.C.V.-B., M.A.G., A.d.l.R., E.V.-M., L.J.R., A.M.-C., F.J.T., S.M.-S., M.A.J., J.A.G.-A., A.S.-M., S.M.W., R.D.K., M.D.C., J.P.C., R.M.L.; Wrote the manuscript: A.I.-C., J.P.C., R.M.L.; Critically revised the manuscript for important intellectual content and approved final version of manuscript, A.I.-C., L.M.L.-S., M.D.G., E.R.-C., M.C.V.-B., M.A.G., A.d.l.R., E.V.-M., L.J.R., A.M.-C., F.J.T., S.M.-S., M.A.J., J.A.G.-A., A.S.-M., S.M.W., R.D.K., M.D.C., J.P.C., R.M.L.
References
- Asa S. L. & Ezzat S. The pathogenesis of pituitary tumours. Nat Rev Cancer 2, 836–849, doi: 10.1038/nrc926 (2002). [DOI] [PubMed] [Google Scholar]
- Leon-Carrion J. et al. Evidence of cognitive and neurophysiological impairment in patients with untreated naive acromegaly. J Clin Endocrinol Metab 95, 4367–4379, doi: 10.1210/jc.2010-0394 (2010). [DOI] [PubMed] [Google Scholar]
- Martin-Rodriguez J. F. et al. Neurocognitive function in acromegaly after surgical resection of GH-secreting adenoma versus naive acromegaly. PLoS One 8, e60041, doi: 10.1371/journal.pone.0060041] (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forget H., Lacroix A. & Cohen H. Persistent cognitive impairment following surgical treatment of Cushing’s syndrome. Psychoneuroendocrinology 27, 367–383, doi: S0306453001000592 (2002). [DOI] [PubMed] [Google Scholar]
- Valassi E., Crespo I., Santos A. & Webb S. M. Clinical consequences of Cushing’s syndrome. Pituitary 15, 319–329, doi: 10.1007/s11102-012-0394-8 (2012). [DOI] [PubMed] [Google Scholar]
- Brazeau P. et al. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science 179, 77–79 (1973). [DOI] [PubMed] [Google Scholar]
- Missale C., Nash S. R., Robinson S. W., Jaber M. & Caron M. G. Dopamine receptors: from structure to function. Physiol Rev 78, 189–225 (1998). [DOI] [PubMed] [Google Scholar]
- Theodoropoulou M. & Stalla G. K. Somatostatin receptors: from signaling to clinical practice. Front Neuroendocrinol 34, 228–252, doi: 10.1016/j.yfrne.2013.07.005 (2013). [DOI] [PubMed] [Google Scholar]
- Gahete M. D. et al. Somatostatin and its receptors from fish to mammals. Annals of the New York Academy of Sciences 1200, 43–52, doi: 10.1111/j.1749-6632.2010.05511.x (2010). [DOI] [PubMed] [Google Scholar]
- Taboada G. F. et al. Quantitative analysis of somatostatin receptor subtype (SSTR1–5) gene expression levels in somatotropinomas and non-functioning pituitary adenomas. Eur J Endocrinol 156, 65–74, doi: 10.1530/eje.1.02313 (2007). [DOI] [PubMed] [Google Scholar]
- Neto L. V. et al. Expression analysis of dopamine receptor subtypes in normal human pituitaries, nonfunctioning pituitary adenomas and somatotropinomas, and the association between dopamine and somatostatin receptors with clinical response to octreotide-LAR in acromegaly. J Clin Endocrinol Metab 94, 1931–1937, doi: 10.1210/jc.2008-1826 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colao A. & Savastano S. Medical treatment of prolactinomas. Nat Rev Endocrinol 7, 267–278, doi: 10.1038/nrendo.2011.37 (2011). [DOI] [PubMed] [Google Scholar]
- Feelders R. A. et al. Pasireotide alone or with cabergoline and ketoconazole in Cushing’s disease. N Engl J Med 362, 1846–1848, doi: 10.1056/NEJMc1000094 (2010). [DOI] [PubMed] [Google Scholar]
- Rocheville M. et al. Receptors for dopamine and somatostatin: formation of hetero-oligomers with enhanced functional activity. Science 288, 154–157 (2000). [DOI] [PubMed] [Google Scholar]
- Baragli A., Alturaihi H., Watt H. L., Abdallah A. & Kumar U. Heterooligomerization of human dopamine receptor 2 and somatostatin receptor 2 Co-immunoprecipitation and fluorescence resonance energy transfer analysis. Cell Signal 19, 2304–2316, doi: S0898-6568(07)00207-0 (2007). [DOI] [PubMed] [Google Scholar]
- Jaquet P. et al. Efficacy of chimeric molecules directed towards multiple somatostatin and dopamine receptors on inhibition of GH and prolactin secretion from GH-secreting pituitary adenomas classified as partially responsive to somatostatin analog therapy. Eur J Endocrinol 153, 135–141, doi: 10.1530/eje.1.01950 (2005). [DOI] [PubMed] [Google Scholar]
- Froehlich J., Ramis J., Lesage C. & Obach R. Safety, Pharmacokinetics (PK) and Pharmacodynamics (PD) after Subcutaneous (s. c.) Administration (adm) of BIM23A760, a Chimeric Compound Combining Dopaminergic Agonist and Somatostatin Analogue, in Healthy Male Volunteers. 91st Annual Meeting of the Endocrine Society, Washington DC; Abstract P3–685 (2009). [Google Scholar]
- Lesage C. et al. A Phase II Exploratory Study of BIM23A760 in Acromegalic Patients: Preliminary Results of Safety and Efficacy after a Single-Dose Administration. 91st Annual Meeting of the Endocrine Society, Washington DC; Abstract P3–673 (2009). [Google Scholar]
- Culler M. D. Somatostatin-dopamine chimeras: a novel approach to treatment of neuroendocrine tumors. Horm Metab Res 43, 854–857, doi: 10.1055/s-0031-1287769 (2011). [DOI] [PubMed] [Google Scholar]
- Gruszka A., Ren S. G., Dong J., Culler M. D. & Melmed S. Regulation of growth hormone and prolactin gene expression and secretion by chimeric somatostatin-dopamine molecules. Endocrinology 148, 6107–6114, doi: 10.1210/en.2007-0378 (2007). [DOI] [PubMed] [Google Scholar]
- Florio T. et al. Efficacy of a dopamine-somatostatin chimeric molecule, BIM-23A760, in the control of cell growth from primary cultures of human non-functioning pituitary adenomas: a multi-center study. Endocr Relat Cancer 15, 583–596, doi: 10.1677/ERC-07-0271 (2008). [DOI] [PubMed] [Google Scholar]
- Fusco A. et al. Somatostatinergic ligands in dopamine-sensitive and -resistant prolactinomas. Eur J Endocrinol 158, 595–603, doi: 10.1530/EJE-07-0806 (2008). [DOI] [PubMed] [Google Scholar]
- Peverelli E. et al. The dopamine-somatostatin chimeric compound BIM-23A760 exerts antiproliferative and cytotoxic effects in human non-functioning pituitary tumors by activating ERK1/2 and p38 pathways. Cancer Lett 288, 170–176, doi: 10.1016/j.canlet.2009.06.034 (2010). [DOI] [PubMed] [Google Scholar]
- Cuny T. et al. Somatostatin receptor sst2 gene transfer in human prolactinomas in vitro: impact on sensitivity to dopamine, somatostatin and dopastatin, in the control of prolactin secretion. Mol Cell Endocrinol 355, 106–113, doi: 10.1016/j.mce.2012.01.026 (2012). [DOI] [PubMed] [Google Scholar]
- Gatto F. et al. Balance between somatostatin and D2 receptor expression drives TSH-secreting adenoma response to somatostatin analogues and dopastatins. Clin Endocrinol (Oxf) 76, 407–414, doi: 10.1111/j.1365-2265.2011.04200.x (2012). [DOI] [PubMed] [Google Scholar]
- Gruszka A., Culler M. D. & Melmed S. Somatostatin analogs and chimeric somatostatin-dopamine molecules differentially regulate human growth hormone and prolactin gene expression and secretion in vitro. Mol Cell Endocrinol 362, 104–109, doi: 10.1016/j.mce.2012.05.020 (2012). [DOI] [PubMed] [Google Scholar]
- Lesage C. & IPSEN. Phase II, Open, Adaptive, Dose Escalating, Multicentre Titration Study to Assess the Efficacy and Safety of Repeated Subcutaneous Administration of Different Doses of BIM 23A760 in Patients With Carcinoid Syndrome, https://clinicaltrials.gov/ct2/show/NCT01018953 (2011). [Google Scholar]
- Stojilkovic S. S., Izumi S. & Catt K. J. Participation of voltage-sensitive calcium channels in pituitary hormone release. J Biol Chem 263, 13054–13061 (1988). [PubMed] [Google Scholar]
- Durán-Prado M. et al. Identification and characterization of two novel truncated but functional isoforms of the somatostatin receptor subtype 5 differentially present in pituitary tumors. J Clin Endocrinol Metab 94, 2634–2643, doi: 10.1210/jc.2008-2564 (2009). [DOI] [PubMed] [Google Scholar]
- Luque R. M. et al. A cellular and molecular basis for the selective desmopressin-induced ACTH release in Cushing disease patients: key role of AVPR1b receptor and potential therapeutic implications. J Clin Endocrinol Metab 98, 4160–4169, doi: 10.1210/jc.2013-1992 (2013). [DOI] [PubMed] [Google Scholar]
- Cordoba-Chacon J. et al. Somatostatin dramatically stimulates growth hormone release from primate somatotrophs acting at low doses via somatostatin receptor 5 and cyclic AMP. J Neuroendocrinol 24, 453–463, doi: 10.1111/j.1365-2826.2011.02261.x (2012). [DOI] [PubMed] [Google Scholar]
- Luque R. M., Gahete M. D., Valentine R. J. & Kineman R. D. Examination of the direct effects of metabolic factors on somatotrope function in a non-human primate model, Papio anubis. J Mol Endocrinol 37, 25–38, doi: 37/1/25 (2006). [DOI] [PubMed] [Google Scholar]
- Kineman R. D., Gahete M. D. & Luque R. M. Identification of a mouse ghrelin gene transcript that contains intron 2 and is regulated in the pituitary and hypothalamus in response to metabolic stress. J Mol Endocrinol 38, 511–521 (2007). [DOI] [PubMed] [Google Scholar]
- Culler M. D. et al. The somatostatin-dopamine chimeric molecule, BIM-23A760, is highly efficacious in suppressing GH in normal, cynomolgus monkeys (Macaca fascicularis). Proceedings of the 88th Annual Meeting of the Endocrine Society, Philadelphia, PA, USA; Abstract O9-6 (2006). [Google Scholar]
- Mangalam H. J. et al. A pituitary POU domain protein, Pit-1, activates both growth hormone and prolactin promoters transcriptionally. Genes Dev 3, 946–958 (1989). [DOI] [PubMed] [Google Scholar]
- Jaquet P. et al. BIM-23A760, a chimeric molecule directed towards somatostatin and dopamine receptors, vs universal somatostatin receptors ligands in GH-secreting pituitary adenomas partial responders to octreotide. J Endocrinol Invest 28, 21–27 (2005). [PubMed] [Google Scholar]
- Hofland L. et al. A muliticenter in vitro study on the effects of the chimeric somatostatin/dopamine compound, BIM-23A760, on ACTH secretion by primary cultures of human corticotroph adenomas. 14 th Congress of the European NeuroEndocrine Association. Liege, Belgium. Abstract OC-4.7 (2010). [Google Scholar]
- Saveanu A. et al. Demonstration of enhanced potency of a chimeric somatostatin-dopamine molecule, BIM-23A387, in suppressing growth hormone and prolactin secretion from human pituitary somatotroph adenoma cells. J Clin Endocrinol Metab 87, 5545–5552, doi: 10.1210/jc.2002-020934 (2002). [DOI] [PubMed] [Google Scholar]
- Luque R. M. et al. Identification of the somatostatin receptor subtypes (sst) mediating the divergent, stimulatory/inhibitory actions of somatostatin on growth hormone secretion. Endocrinology 147, 2902–2908, doi: en.2005-1559 (2006). [DOI] [PubMed] [Google Scholar]
- Murray R. D. et al. The novel somatostatin ligand (SOM230) regulates human and rat anterior pituitary hormone secretion. J Clin Endocrinol Metab 89, 3027–3032, doi: 10.1210/jc.2003-031319 (2004). [DOI] [PubMed] [Google Scholar]
- Florio T. et al. Characterization of the intracellular mechanisms mediating somatostatin and lanreotide inhibition of DNA synthesis and growth hormone release from dispersed human GH-secreting pituitary adenoma cells in vitro. Clin Endocrinol (Oxf) 59, 115–128, doi: 10.1046/j.1365-2265.2003.01811.x (2003). [DOI] [PubMed] [Google Scholar]
- Spada A., Sartorio A., Bassetti M., Pezzo G. & Giannattasio G. In vitro effect of dopamine on growth hormone (GH) release from human GH-secreting pituitary adenomas. J Clin Endocrinol Metab 55, 734–740, doi: 10.1210/jcem-55-4-734 (1982). [DOI] [PubMed] [Google Scholar]
- Schonbrunn A. Selective agonism in somatostatin receptor signaling and regulation. Mol Cell Endocrinol 286, 35–39, doi: S0303-7207(07)00360-7 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruszka A. et al. The effect of selective sst1, sst2, sst5 somatostatin receptors agonists, a somatostatin/dopamine (SST/DA) chimera and bromocriptine on the “clinically non-functioning” pituitary adenomas in vitro. Life Sci 78, 689–693, doi: 10.1016/j.lfs.2005.05.061 (2006). [DOI] [PubMed] [Google Scholar]
- Durán-Prado M. et al. A potential inhibitory role for the new truncated variant of somatostatin receptor 5, sst5TMD4, in pituitary adenomas poorly responsive to somatostatin analogs. J Clin Endocrinol Metab 95, 2497–2502, doi: 10.1210/jc.2009-2247 (2010). [DOI] [PubMed] [Google Scholar]
- Luque R. M. et al. Truncated somatostatin receptor variant sst5TMD4 confers aggressive features (proliferation, invasion and reduced octreotide response) to somatotropinomas. Cancer Lett 359, 299–306, doi: 10.1016/j.canlet.2015.01.037 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durán-Prado M. et al. The new truncated somatostatin receptor variant sst5TMD4 is associated to poor prognosis in breast cancer and increases malignancy in MCF-7 cells. Oncogene 31, 2049–2061, doi: 10.1038/onc.2011.389 (2012). [DOI] [PubMed] [Google Scholar]
- Puig-Domingo M. et al. The Truncated Isoform of Somatostatin Receptor5 (sst5TMD4) Is Associated with Poorly Differentiated Thyroid Cancer. PLoS One 9, e85527, doi: 10.1371/journal.pone.0085527 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colao A., Auriemma R. S., Lombardi G. & Pivonello R. Resistance to somatostatin analogs in acromegaly. Endocr Rev 32, 247–271, doi: 10.1210/er.2010-0002 (2011). [DOI] [PubMed] [Google Scholar]
- Arvigo M. et al. Somatostatin and dopamine receptor interaction in prostate and lung cancer cell lines. J Endocrinol 207, 309–317, doi: 10.1677/JOE-10-0342 (2010). [DOI] [PubMed] [Google Scholar]
- Luque R. M. et al. Obestatin plays an opposite role in the regulation of pituitary somatotrope and corticotrope function in female primates and male/female mice. Endocrinology 155, 1407–1417, doi: 10.1210/en.2013-1728 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Fuentes A. J. et al. Ghrelin is produced by and directly activates corticotrope cells from adrenocorticotropin-secreting adenomas. J Clin Endocrinol Metab 91, 2225–2231 (2006). [DOI] [PubMed] [Google Scholar]
- Ibáñez-Costa A. et al. In1-ghrelin splicing variant is overexpressed in pituitary adenomas and increases their aggressive features. Sci Rep 5, 8714, doi: 10.1038/srep08714 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordoba-Chacon J., Gahete M. D., Castano J. P., Kineman R. D. & Luque R. M. Somatostatin and its receptors contribute in a tissue-specific manner to the sex-dependent metabolic (fed/fasting) control of growth hormone axis in mice. Am J Physiol Endocrinol Metab 300, E46–54, doi: 10.1152/ajpendo.00514.2010 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibáñez-Costa A. et al. Melatonin regulates somatotrope and lactotrope function through common and distinct signaling pathways in cultured primary pituitary cells from female primates. Endocrinology 156, 1100–1110, doi: 10.1210/en.2014-1819 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordoba-Chacon J., Gahete M. D., Castano J. P., Kineman R. D. & Luque R. M. Homologous and heterologous in vitro regulation of pituitary receptors for somatostatin, growth hormone (GH)-releasing hormone, and ghrelin in a nonhuman primate (Papio anubis). Endocrinology 153, 264–272, doi: 10.1210/en.2011-1677 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.