Abstract
Aims
Since 2005, several glucagon‐like peptide‐1 receptor agonists (GLP‐1 RAs) have been approved to treat people with type 2 diabetes. These agents are considered for use at the same point in the treatment paradigm as basal insulins. A comprehensive comparison of these drug classes, therefore, can help inform treatment decisions. This systematic review and meta‐analysis assessed the clinical efficacy and safety of GLP‐1 RAs compared with basal insulins.
Materials and methods
MEDLINE, EMBASE, CENTRAL and PubMed databases were searched. Randomized clinical trials (RCTs) of ≥16 weeks’ duration comparing GLP‐1 RAs vs basal insulins in adults with type 2 diabetes inadequately controlled with oral antihyperglycemic drugs were included. Data on the change from baseline to 26 weeks (±10 weeks) of treatment in hemoglobin A1c (HbA1c) and weight, as well as the proportion of patients experiencing hypoglycaemia, were extracted. Fixed‐effect pairwise meta‐analyses were conducted where data were available from ≥2 studies.
Results
Fifteen RCTs were identified and 11 were meta‐analysed. The once‐weekly GLP‐1 RAs, exenatide long acting release (LAR) and dulaglutide, led to greater, statistically significant mean HbA1c reductions vs basal insulins (exenatide: −0.31% [95% confidence interval −0.42, −0.19], dulaglutide: −0.39% [−0.49, −0.29]) whilst once‐daily liraglutide and twice‐daily exenatide did not (liraglutide: 0.06% [−0.06, 0.18], exenatide: 0.01% [−0.11, 0.13]). Mean weight reduction was seen with all GLP‐1 RAs while mean weight gain was seen with basal insulins. Interpretation of the analysis of hypoglycaemia was limited by inconsistent definitions and reporting. Because of the limited number of available studies sensitivity analyses to explore heterogeneity could not be conducted.
Conclusions
Although weight reduction is seen with all GLP‐1 RA’s, only the once‐weekly agents, exenatide LAR and dulaglutide, demonstrate significant HbA1c reductions when compared to basal insulins.
Keywords: basal insulin, GLP‐1 RAs, glycaemic control, meta‐analysis, systematic review, type 2 diabetes
1. INTRODUCTION
Several drug classes provide options for physicians to improve patients’ control of type 2 diabetes. Glucagon‐like peptide‐1 receptor agonists (GLP‐1 RAs) are a novel class of injectable antihyperglycaemic treatments that, when compared to traditional treatment options such as insulin and sulfonylureas (SUs), offer the advantage of regulating insulin secretion in proportion to ambient glucose levels, thus reducing the risk of hypoglycaemia and, at the same time, facilitating weight loss.1 Various diabetes treatment algorithms include GLP‐1 RAs as a therapy option after initial treatment with metformin (MET).2, 3, 4 Since the introduction of exenatide twice daily (BID) for the treatment of type 2 diabetes in 2005, several GLP‐1 RAs have been developed and marketed. Increasingly, new GLP‐1 RAs are available as once‐weekly treatments rather than once‐ or twice‐daily options and in 2014, two new once‐weekly GLP‐1 RAs received marketing authorization: albiglutide and dulaglutide.5, 6, 7, 8
The clinical effectiveness and safety of GLP‐1 RAs compared to each other and to oral antihyperglycemic drugs (OAD) have been assessed in several meta‐analyses.9, 10, 11 However, the positioning of GLP‐1 RAs within the treatment paradigm is at the point when the use of basal insulin might also be considered; therefore, there is an increasing desire to understand the similarities and differences between GLP‐1 RAs and basal insulins. Although such comparisons have been published,11, 12, 13, 14, 15, 16 they all have limitations to consider. Wang et al. 15 do not include the two new agents (dulaglutide and albiglutide) and, although Karagiannis et al. 10 do include the new treatments, their analysis is limited to only once‐weekly GLP‐1 RAs. Liu et al. 13 on the other hand, pooled all GLP‐1 RAs together when comparing to insulin glargine. Such pooling assumes, a priori, that all GLP‐1 RAs are similar in efficacy and pharmacodynamic profile, which is not the case as demonstrated in head‐to‐head studies.17, 18 More recently, Zaccardi et al.16 conducted an analysis where GLP‐1 RAs were considered independently, but basal insulins were pooled, again making an a priori assumption that all basal insulins have the same efficacy and pharmaocodynamic profile. Pooling also discounts the heterogeneity between GLP‐1 RA trials regarding background therapy and drug dosage; as such, it is imperative that heterogeneity using appropriate measures is assessed prior to combining treatments for analytical purposes. To this end, to evaluate the clinical efficacy of GLP‐1 RAs, by type, vs basal insulins, we conducted a systematic review of the literature and a series of paired meta‐analyses to assess the differences in glycaemic control, weight change and the risk of hypoglycaemia in adults with type 2 diabetes.
2. MATERIALS AND METHODS
2.1. Data sources and searches
MEDLINE (including Epub ahead of print and In‐process citations), EMBASE and the Cochrane Central Register of Controlled Trials (CENTRAL) databases were searched from database inception to September 9, 2016. The searches were limited to peer‐reviewed studies in the English language. Separate search strategies were designed for each database (MEDLINE strategy is included in Appendix S1, Supporting Information). Each search strategy included free‐text, MeSH and EMTREE terms, where appropriate, for type 2 diabetes and GLP‐1 RAs, and a randomized controlled trial (RCT) study design filter in MEDLINE and EMBASE.
2.2. Study selection
Included RCTs were selected based on predefined eligibility criteria using the population, intervention, comparators, outcomes and study design (PICOS) framework. Eligibility criteria included: (1) adults with type 2 diabetes inadequately controlled by OADs; (2) treatment of the majority of subjects with US approved dosages of GLP‐1 RA (exenatide 10 µg twice daily, liraglutide 1.2 or 1.8 mg once daily, exenatide 2 mg once weekly (long acting release [LAR]), lixisenatide 20 µg once daily, albiglutide 30 or 50 mg once weekly, and dulaglutide 0.75 or 1.5 mg once weekly plus at least one OAD; (3) comparator arm of basal insulin (ie, insulin detemir, insulin glargine, insulin degludec, neutral protamine hagedorn [NPH] insulin) plus at least one OAD; and (4) RCT duration of at least 16 weeks. Trials performed in treatment‐naïve patients with type 2 diabetes, or samples that recruited only patients with comorbidities including renal failure or cardiovascular comorbidities, were excluded. Two reviewers independently determined whether the RCTs met eligibility criteria. Each reviewer first reviewed the titles and abstracts; full‐text articles were reviewed where eligibility could not be determined from the title and abstract review alone. Discrepancies between reviewers were resolved by consensus, or adjudicated by a third reviewer.
2.3. Data extraction and quality assessment
Data extraction was performed by a single experienced data extractor into a customized spreadsheet; key fields were validated by a second extractor. Discrepancies were resolved as described previously for study selection. The extraction form was designed to collect data reporting study design features, baseline patient demographics and clinical characteristics, treatment arm details, efficacy (glycated hemoglobin [HbA1c], weight) and safety outcomes (hypoglycemia, gastrointestinal adverse events).
Endpoints at week 26 (±10 weeks) were extracted and reported. If data at week 26 were unavailable, the data closest to week 26 between weeks 16 and 36 were included. For trials that presented endpoints in graph format only, values were derived by digitizing the graph, using the WebPlotDigitizer program available online (http://arohatgi.info/WebPlotDigitizer/app/). This was necessary for 4 studies.19, 20, 21, 22 A risk assessment of bias was performed for each included RCT using the Cochrane Collaboration's tool.23 Two reviewers independently assessed the quality of each included RCT.
2.4. Data synthesis and statistical analysis
Mean values and associated measures of variability (variance, standard deviation [SD], standard error [SE] or confidence interval [CI]) for continuous endpoints, and counts and proportions for categorical endpoints were extracted. For the purposes of the meta‐analyses, if SD was not reported, it was imputed from other available information (eg, SE or 95% CI), using the prognostic method described by Ma et al.24 Where sample sizes for HbA1c and weight outcomes were not reported, the intention‐to‐treat (ITT) population was assumed. Where sample sizes for hypoglycaemia endpoints were not reported, if available, the safety population was assumed; otherwise the ITT population was assumed.
The meta‐analyses were conducted using the “meta” statistical package in R version 3.1.3.25 To understand the difference between GLP‐1 RAs and basal insulins by drug, fixed‐effect pairwise meta‐analyses using the frequentist approach were conducted for the changes in HbA1c and weight, and the occurrence of hypoglycaemia outcomes. Random effects analysis were considered where appreciable statistical heterogeneity was observed. However, when a small number of studies are available for analysis, as was the case for this analysis, random effects analysis shows poor precision of between‐ study variance, and are therefore not appropriate.26 In keeping with the Cochrane handbook, meta‐analyses were conducted where data were available for at least 2 separate trials with the same treatments.27 If data for a treatment were identified in only 1 trial, meta‐analysis was not conducted; however, the trial results are shown. “Hypoglycaemia” outcomes included all attributions of hypoglycaemia in a publication (irrespective of blood glucose value), including severe hypoglycaemia. The “severe hypoglycaemia” category included publication attribution as severe or major hypoglycaemia or definition of “requiring third‐party assistance.” However, meta‐analysis for severe hypoglycaemia could not be conducted because of low event rates; as such, only the proportion of patients experiencing severe hypoglycaemia events in GLP‐1 RA groups vs basal insulins were extracted and presented. To calculate the weight contribution by individual trials to the overall effect estimate, the inverse variance method for continuous outcomes and the Mantel‐Haenszel for categorical outcomes were used.
Statistical tests of heterogeneity were conducted to understand the extent to which the results of trials included in the meta‐analysis were consistent. The main measure of heterogeneity used for evaluation was I2 value.27 Sub‐group sensitivity analysis was conducted by excluding one study to understand the impact of non‐standard dosing used in a minority (37.9%) of patients.20
Each model was coded, analysed and summarized using forest plots. The forest plots summarize effect estimates from all included trials to provide a comprehensive view of available evidence, as well as a pooled effect size from meta‐analysis. For change in HbA1c and weight, a mean difference <0 signifies the result favouring the GLP‐1 RA arm compared to the basal insulin arm, ie, a greater reduction in HbA1c or weight compared to basal insulin. For hypoglycaemia, an odds ratio <1 indicates lower odds in the GLP‐1 RA arm compared to basal insulin. Where meta‐analyses were conducted (ie, 2 or more trials were available for a treatment and comparator), I2 value was also reported. Codes designed for meta‐analyses and their outputs were validated by a second analyst.
3. RESULTS
The Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow diagram documenting the RCT selection process is provided in Figure 1. A total of 2694 articles were identified, of which 19 articles describing 15 RCTs were eligible for inclusion in the systematic literature review. Key study and baseline characteristics, including background therapy, are presented in Table 1. The eligible RCTs included comparisons of exenatide 10 µg or 2 mg, liraglutide 1.8 mg, albiglutide 30 mg (uptitrated to 50 mg as needed), dulaglutide 0.75 or 1.5 mg or lixisenatide 20 µg with insulin detemir, glargine or degludec. Eligible studies reporting NPH insulin were not identified. Study endpoints were reported at 16, 24, 26, 28, 52, 78, 156 and 168 weeks. Fifteen RCTs reported HbA1c and bodyweight change from baseline, 9 reported hypoglycemic episodes and 11 reported severe hypoglycemic episodes. As data for the dulaglutide 1.5 mg dose, albiglutide 30 and 50 mg doses and lixisentatide 20 µg were identified in only 1 study each, meta‐analysis could not be performed for these doses. The insulin titration algorithms and fasting plasma glucose (FPG) targets are shown in Table S1, Supporting Information. HbA1c, weight and FPG change from baseline are shown in Tables S2 to S4, Supporting Information respectively.
Figure 1.
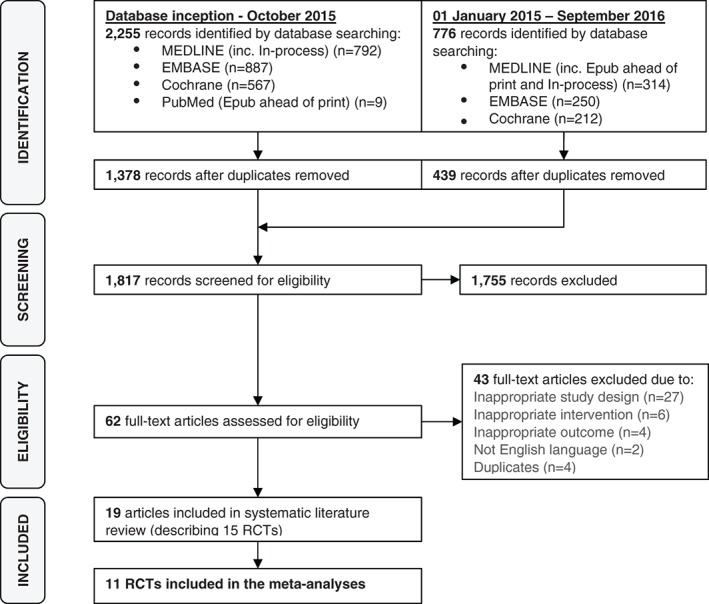
Study PRISMA diagram. Abbreviations: RCT, randomized clinical trial.
Table 1.
Study population baseline characteristics, background therapies, and presence of key three endpoints
| Trial name and author | Study duration (weeks) | Intervention | Mean age (SD), years | Female, % | White, % | Mean disease duration (SD), years | Mean HbA1c (SD), % | Mean HbA1c (mmol/mol) | Mean bodyweight (SD), kg | Mean BMI (SD), kg/m2 | Background therapy, % |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Exenatide 10 µg twice daily vs insulin glargine | |||||||||||
| Bunck 2009 | 52 | Exenatide1 | 58.4 (−) | 36.1 | – | 5.7 (−) | 7.6 (−) | 60 | 90.6 (−) | 30.9 (−) | MET (100%) |
| Glargine | 58.3 (−) | 33.3 | – | 4.0 (−) | 7.4 (−) | 57 | 92.4 (−) | 30.1 (−) | |||
| HEELA (Davies 2009) | 26 | Exenatide | 56.8 (10.2) | 29.7 | – | 9.0 (4.6) | 8.7 (0.7) | 72 | 101.4 (19.8) | 34.6 (5.7) | Double/triple therapy MET ± SU ± TZD |
| Glargine | 56.2 (7.9) | 33.6 | – | 8.4 (4.4) | 8.5 (0.7) | 69 | 97.6 (16.4) | 33.7 (4.9) | |||
| Heine 2005 | 26 | Exenatide | 59.8 (8.8) | 45.0 | 80 | 9.9 (6.0) | 8.2 (1.0) | 66 | 87.5 (16.9) | 31.4 (4.4) | MET + SU (100%) |
| Glargine | 58.0 (9.5) | 43.4 | 81 | 9.2 (5.7) | 8.3 (1.0) | 67 | 88.3 (17.9) | 31.3 (4.6) | |||
| Gurkan 2014 | 26 | Exenatide | 52.2 (7.3) | 70.0 | – | 6.9 (3.3) | 8.0 (0.8) | 64 | 94.3 (11.8) | 35.9 (3.7) | MET (100%) |
| Glargine | 53.1 (7.0) | 58.8 | – | 7.6 (4.3) | 8.1 (0.8) | 65 | 90.5 (14.3) | 33.2 (4.5) | |||
| Barnett 2007 | 32 | Exenatide | 54.5 (−) | 51.5 | – | 6.6 (−) | 8.9 (−) | 74 | 85.6 (−) | 31.3 (−) | MET (55%‐56%) or SU (44%‐46%) |
| Glargine | 55.3 (−) | 54.3 | – | 8.3 (−) | 9.0 (−) | 75 | 84.0 (−) | 30.9 (−) | |||
| Exenatide 2 mg once weekly vs insulin glargine | |||||||||||
| DURATION‐3 (Diamant 2010, 2014) | 156 | Exenatide | 58.0 (10.0) | 48.0 | 82 | 8.0 (6.0) | 8.3 (1.1) | 67 | 91.2 (18.6) | 32.3 (5.4) | MET (70%) |
| Glargine | 58.0 (9.0) | 45.0 | 85 | 7.8 (6.0) | 8.3 (1.0) | 67 | 90.6 (16.4) | 32.3 (4.8) | |||
| Inagaki 2012 | 62 | Exenatide | 57.1 (10.4) | 34.0 | 02 | 8.9 (6.1) | 8.5 (0.8) | 69 | 70.0 (13.3) | 26.1 (4.03) | MET (67%), BG + TZD (33%) |
| Glargine | 56.4 (11.2) | 30.2 | 02 | 9.2 (6.0) | 8.5 (0.8) | 69 | 71.0 (13.9) | 26.2 (3.8) | |||
| Exenatide 2 mg once weekly vs insulin detemir | |||||||||||
| Davies 2013 | 30 | Exenatide | 59.0 (10.0) | 36.0 | 94 | 8.0 (6.0) | 8.4 (0.9) | 68 | 96.7 (17.0) | 33.7 (4.7) | MET (100%) + SU (70%‐72%) |
| Detemir | 58.0 (10.0) | 31.0 | 97 | 7.0 (5.0) | 8.4 (0.9) | 68 | 97.9 (15.8) | 33.7 (4.7) | |||
| Liraglutide 1.8 mg once daily vs insulin glargine | |||||||||||
| EAGLE (D'Alessio 2015) | 24 | Liraglutide | 57.4 (8.9) | 44.0 | – | – | 9.1 (1.1) | 76 | 90.1 (16.7) | 31.8 (4.1) | MET + SU3 |
| Glargine | 57.1 (8.8) | 47.3 | – | – | 9.0 (1.0) | 75 | 90.8 (16.6) | 32.0 (4.2) | |||
| LEAD‐5 (Russell‐Jones 2009) | 26 | Liraglutide | 57.6 (9.5) | 43.0 | – | 9.2 (5.8) | 8.3 (0.9) | 67 | 85.5 (19.4) | 30.4 (5.3) | MET + SU (94%‐95%) |
| Glargine | 57.5 (10.5) | 40.0 | – | 9.7 (6.4) | 8.2 (0.9) | 66 | 85.0 (17.9) | 30.3 (5.3) | |||
| Liraglutide 1.8 mg once daily vs insulin degludec | |||||||||||
| DUAL‐I (Gough 2014, 2015) | 52 | Liraglutide | 55.0 (10.2) | 50.0 | 62 | 7.2 (6.1) | 8.3 (0.9) | 67 | 87.4 (18.0) | 31.3 (4.8) | MET (82%‐83%), MET + TZD (17%‐18%) |
| Degludec | 54.9 (9.7) | 52.0 | 62 | 7.0 (5.3) | 8.3 (1.0) | 67 | 87.4 (19.2) | 31.2 (5.3) | |||
| Albiglutide 30 mg once weekly vs insulin glargine | |||||||||||
| HARMONY4 (Weissman 2014) | 156 | Albiglutide | 55.8 (9.3) | 43.3 | 69 | 8.9 (6.5) | 8.3 (0.9) | 67 | 95.1 (19.7) | 33.2 (5.6) | MET + SU (82%) |
| Glargine | 54.7 (9.8) | 45.2 | 66 | 8.4 (5.7) | 8.4 (1.0) | 68 | 94.6 (19.1) | 33.0 (5.4) | |||
| Dulaglutide once weekly vs insulin glargine | |||||||||||
| AWARD‐2 (Giorgino 2015) | 78 | Dulaglutide 0.75 mg | 57.0 (9.0) | 50.0 | 71 | 9.0 (6.0) | 8.1 (1.0) | 65 | 86.0 (18.0) | 32.0 (5.0) | MET + SU (65%‐68%) |
| Dulaglutide 1.5 mg | 56.0 (10.0) | 47.0 | 71 | 9.0 (6.0) | 8.2 (1.0) | 66 | 85.0 (18.0) | 31.0 (5.0) | |||
| Glargine | 57.0 (9.0) | 49.0 | 70 | 9.0 (6.0) | 8.1 (1.0) | 65 | 88.0 (20.0) | 32.0 (6.0) | |||
| Araki 2015 | 34 | Dulaglutide 0.75 mg | 57.5 (10.5) | 31.0 | 02 | 8.9 (6.7) | 8.1 (0.8) | 65 | 70.9 (13.7) | 26.1 (3.6) | MET ± SU4 |
| Glargine | 56.1 (11.3) | 26.0 | 02 | 8.8 (6.1) | 8.0 (0.9) | 64 | 71.1 (13.8) | 25.9 (3.9) | |||
| Lixisenatide versus insulin glargine | |||||||||||
| LixiLan‐O (Rosenstock 2016) | Lixisenatide 20 µg | 58.7 (8.7) | 43.2 | 92.3 | 8.9 (6.3) | 8.1 (0.7) | 65 | 90.8 (16.3) | 32.0 (4.4) | MET | |
| 30 | Glargine | 58.3 (9.4) | 49.3 | 90.1 | 8.7 (5.6) | 8.1 (0.7) | 65 | 89.8 (16.3) | 31.7 (4.5) | ||
Abbreviations: BG, biguanine; BMI, body mass index; HbA1c, glycated hemoglobin; MET, metformin; SD, standard deviation; SU, sulfonylurea; TZD, thiazolidinedione.
The majority of participants received exenatide 10 µg twice daily.
Trial conducted in a Japanese population.
Insulin glargine arm: MET = 99.6%, SU = 67.5%, SUs taken by 60% of participants at baseline and reduced to 49% at 24 weeks, liraglutide arm: MET = 99.8%, SU = 68.3%, SUs taken by 63% of participants at baseline and reduced to 48% at 24 weeks. SUs were reduced or discontinued at investigators discretion.
Dulaglutide arm: SU monotherapy (19%), MET monotherapy (35%), SU + MET (46%). Insulin glargine arm: SU monotherapy (18%), MET monotherapy (37%), SU + MET (45%).
Mean patient age in the included studies ranged from 52.2 to 59.8 years and mean disease duration ranged from 4.0 to 9.9 years. Slightly more males were represented in the study populations. Mean baseline HbA1c levels ranged from 7.4% to 9.1% (57‐76 mmol/mol); the median across trials was 8.2%. Mean bodyweight ranged from 69.95 to 101.40 kg and mean body mass index (BMI) ranged from 25.90 to 35.89 kg/m2. Mean fasting glucose values at baseline varied from 8.6 to 12.2 mmol/L (Table S4, Supporting Information). Two trials were conducted in Asian populations28, 29 and the other trials that reported ethnicity were conducted in mostly white populations.21, 22, 30, 31, 32, 33, 34, 35
3.1. Risk of bias
The risk of bias for all trials included is presented in Figure S1, Supporting Information. Selection bias was assessed as unclear in 7 trials20, 22, 31, 36, 37, 38, 39 for random sequence generation and in 4 trials20, 36, 37, 38 for allocation concealment. Absence of reporting was the main reason for the decision to assign an RCT as presenting unclear risk of bias. All trials were open label. Attrition bias (see incomplete outcome data) was assessed to be low in 12 trials19, 20, 21, 22, 28, 29, 30, 31, 33, 34, 35, 38 (percentage of patients lost to follow‐up reported as 0%‐3% in all arms) and was unclear in 3.36, 37, 39 Two trials were deemed to have an unclear risk with regard to reporting.36, 37
3.2. Efficacy outcomes
3.2.1. Glycemic control
Of the 15 RCTs identified by the systematic review, 11 studies reported change in HbA1c at 26 weeks (±10 weeks) and had comparable data from at least 2 studies to allow for a pairwise meta‐analysis. These included 5 trials of exenatide 10 µg vs insulin glargine19, 20, 34, 37, 38; 2 trials of exenatide 2 mg LAR vs insulin glargine29, 33; 2 trials of liraglutide 1.8 mg vs insulin glargine36, 39; and 2 trials of dulaglutide 0.75 mg vs insulin glargine.21, 28 Four studies could not be included in the pair‐wise meta‐analysis as the requirement of comparable data for the same dose or comparator insulin from 2 studies was not met. These studies compared exenatide 2 mg LAR vs insulin detemir,30 liraglutide 1.8 mg vs insulin degludec,31 albiglutide 30 mg titrated to 50 mg (as required) vs insulin glargine22 and lixisentatide 20 µg vs insulin glargine.35 In addition, although 2 studies were available for a pair‐wise meta‐analysis of the 0.75 mg dose of dulaglutide21, 28 to be performed, only 1 study reported both doses of dulaglutide (0.75 and 1.5 mg).21 In the absence of another study with the 1.5 mg dose, a pair‐wise analysis for this dose could not be performed. A summary of all evidence identified by the systematic review and the results of the pairwise meta‐analyses are presented in Figure 2A. Mean HbA1c changes from baseline as well as the percentage of patients achieving HbA1c < 7% (53 mmol/L) are presented in Table S2, Supporting Information.
Figure 2.
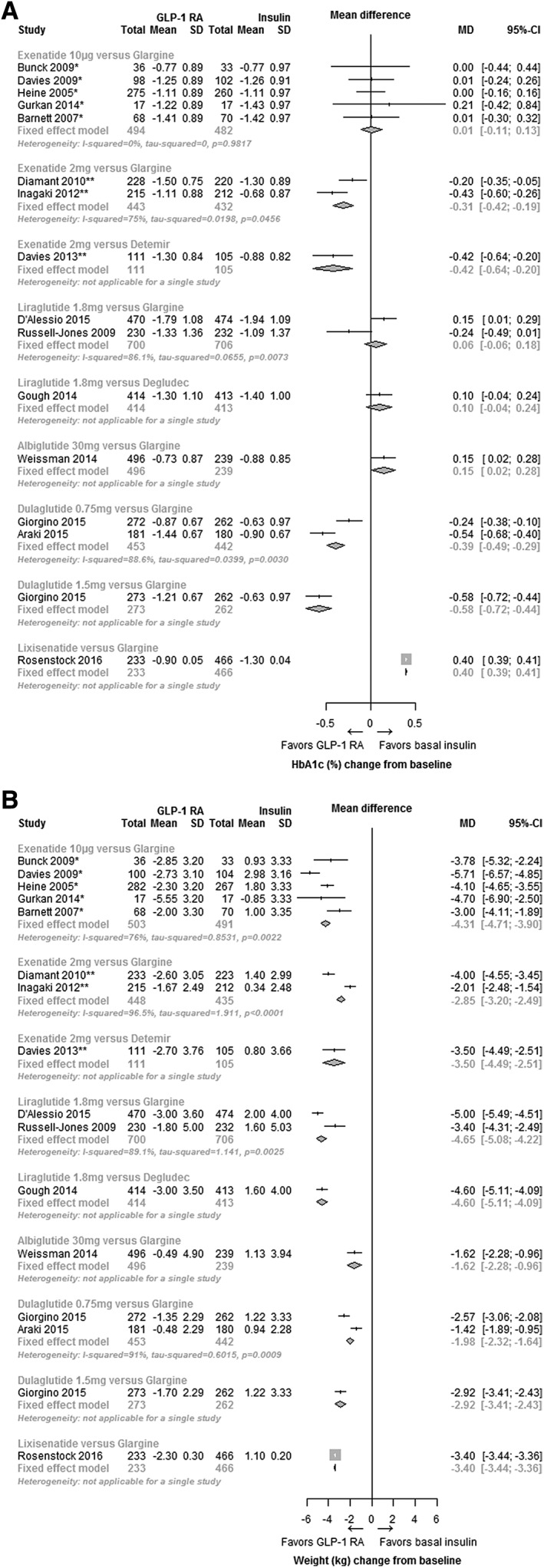
Effect of GLP‐1 RA compared to basal insulin at week 26 (±10 weeks). Change in HbA1c (%) (A), and change in bodyweight (kg) (B). Abbreviations: CI, confidence interval; GLP‐1 RA, glucagon‐like peptide‐1 receptor agonist; MD, mean difference; SD, standard deviation. *Twice daily; **once weekly.
Compared with insulin glargine, the mean difference in HbA1c change was +0.01% (95% CI, −0.11, 0.13; I2 = 0%) (+0.01 mmol/L [95% CI, −1.2, 1.4]) with exenatide 10 µg, −0.31% (95% CI, −0.42, −0.19; I2 = 75%) (−3.4 mmol/L [95% CI, −4.6, −2.1]) with exenatide 2 mg LAR, +0.06%, (95% CI, −0.06, 0.18; I2 = 86.1%) (+0.7 mmol/L [95% CI, −0.7, 2.0]) with liraglutide 1.8 mg and −0.39% (95% CI, −0.49, −0.29; I2 = 88.6%) (−0.43 mmol/L [95% CI, −5.4, −3.2]) with dulaglutide 0.75 mg (Figure 2A).
The RCT conducted by Bunck et al.20 included 5 different doses of exenatide: 10 µg BID (62.1% of participants), 15 µg BID (3.4%), 10 µg 3 times daily (TID) (6.9%), 15 µg TID (3.4%) and 20 µg TID (17.2%). This RCT was included in the systematic literature review because the majority of participants (62.1%) received a licensed dose of exenatide (10 µg BID). However, because of the different doses of exenatide administered to patients as compared to other exenatide 10 µg BID trials, sensitivity analyses for HbA1c and weight were conducted to understand the impact of this trial on the overall pooled estimate and heterogeneity. This sensitivity analysis showed no impact on the overall effect estimate or heterogeneity measure for exenatide 10 µg BID vs insulin glargine with a pooled mean HbA1c change of +0.01% (95% CI, −0.11, 0.13; I2 = 0%), (+3.2 mmol/L [95% CI, −1.2, 1.4]), which is the same as the pooled estimates when including the Bunck et al. trial.
3.2.2. Body weight
A summary of all evidence for bodyweight, as well as the pairwise meta‐analyses at 26 weeks, are presented in Figure 2B. Compared with insulin glargine, the mean difference in bodyweight was −4.31 kg (95% CI, −4.71, −3.90; I2 = 76%) with exenatide 10 µg, −2.85 kg (95% CI, −3.20, −2.49; I2 = 96.5%) with exenatide 2 mg LAR, −4.65 kg (95% CI, −5.08, −4.22; I2 = 89.1%) with liraglutide 1.8 mg, and −1.98 kg (95% CI, −2.32, −1.64, I2 = 91%) with dulaglutide 0.75 mg. Removing the RCT conducted by Bunck et al.20 minimally changed the overall effect estimate for exenatide 10 µg to −4.35 kg (95% CI, −4.77, −3.93; I2 = 81.5%). Mean bodyweight and BMI change from baseline are presented in Table S3, Supporting Information.
3.3. Safety outcomes
3.3.1. Hypoglycaemia
A summary of all evidence for hypoglycaemia as well as the pairwise meta‐analyses at 26 weeks are presented in Figure 3A. Compared to insulin glargine, the odds ratio for a hypoglycaemic episode was 0.32 (95% CI, 0.22, 0.47; I2 = 33.2%) with exenatide 2 mg LAR and 0.40 (95% CI, 0.32, 0.51; I2 = 96%) with liraglutide 1.8 mg. A summary of the evidence related to the proportion of patients experiencing a hypoglycaemic episode and the background therapy is presented in Figure 3B.
Figure 3.
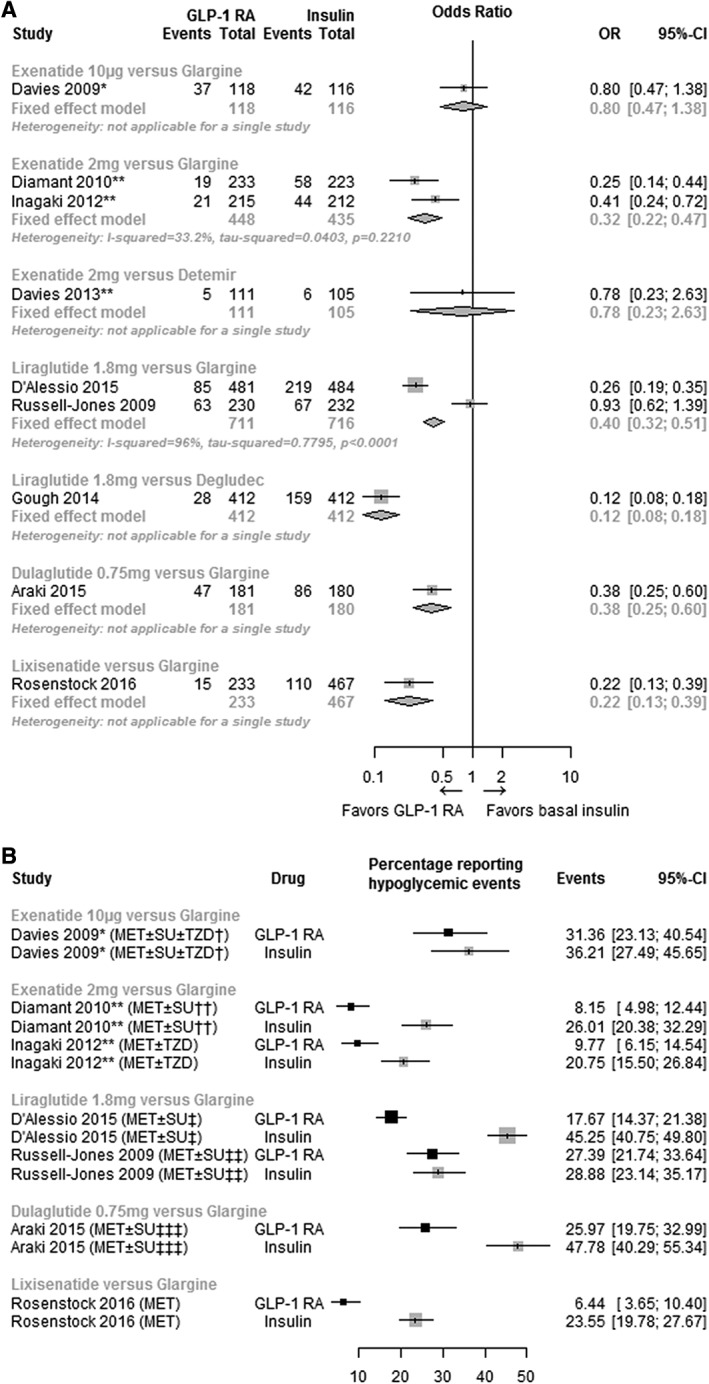
Effect of GLP‐1 RA compared to basal insulin at 26 weeks (±10 weeks) on hypoglycemia, odds ratio (A), and proportion (%) (B). Abbreviations: CI, confidence interval; GLP‐1 RA, glucagon‐like peptide‐1 receptor agonist; OR, odds ratio; prop, proportion. *Twice daily; **once weekly. †At baseline, the majority of patients in each treatment group (exenatide 84.7%; insulin glargine 86.2%) were taking SUs. ††At baseline, 70.0% and 30.0% of patients in both arms were receiving MET and MET + SU, respectively. One in four patients had a reduction in SU dose. ‡SU dosage was reduced or discontinued at the physician's discretion. SUs were initially taken by 60.0% of those receiving insulin glargine and by 63.0% receiving liraglutide. At 24 weeks, 49.0% and 48.0%, respectively, were still taking them. ‡‡At baseline, 94.0% in the liraglutide arm and 95.0% in the insulin glargine arm were receiving SU. ‡‡‡The majority of the patients in the dulaglutide group (65.0%) and insulin glargine group (63.0%) received SU at baseline. In the dulaglutide and insulin glargine groups, 13/117 (11.1%) and 11/114 (9.6%) patients, respectively, decreased their concomitant SU dose from baseline as a result of hypoglycemia. §Figure includes only insulin glargine trials to highlight the differences in proportions of patients experiencing hypoglycemic events between trials, which may be due to variations in insulin titration and background therapies.
Out of the 15 studies that reported data at week 26 (±10 weeks), 11 RCTs reported data for severe hypoglycaemia. Meta‐analyses of severe hypoglycaemia were not conducted because of the limited events observed; 3 studies reported no events in both arms28, 29, 30 and 5 studies reported no events in one arm.19, 31, 35, 36, 39 The numbers and proportions of patients experiencing an episode of hypoglycaemia or an episode of severe hypoglycaemia, and the definitions of hypoglycaemia and severe hypoglycaemia as described by the authors are presented in Table S5, Supporting Information.
3.3.2. Gastrointestinal events
Meta‐analyses of gastrointestinal events were not conducted as reporting across studies was insufficient to allow meaningful analyses (data not shown).
4. DISCUSSION
The meta‐analyses show statistically significant reductions in HbA1c with once‐weekly exenatide 2 mg LAR and once‐weekly dulaglutide 0.75 mg, compared to insulin glargine at 6 months, a reduction in HbA1c of 0.3% (3.3 mmol/L) and 0.4% (4.4 mmol/L), respectively. In contrast, once‐daily liraglutide and twice‐daily exenatide 10 µg did not show a statistically significant difference from insulin glargine. This difference between weekly and daily GLP‐1 RAs may be attributed to the potential impact of the weekly agents on both FPG and postprandial plasma glucose (PPG), compared to the daily agents that may predominantly regulate PPG.
The systematic review identified 15 trials reporting results at 26 weeks (±10 weeks). Eleven trials were included in pair‐wise meta‐analyses: 5 trials of exenatide 10 µg vs insulin glargine19, 20, 34, 37, 38; 2 trials of exenatide 2 mg LAR vs insulin glargine29, 33; 2 trials of liraglutide 1.8 mg vs insulin glargine36, 39; and 2 trials of dulaglutide 0.75 mg vs insulin glargine.21, 28 Although it was not possible to incorporate data for dulaglutide 1.5 mg, lixisenatide or albiglutide into the analyses, as only 1 study for each met the inclusion criteria and at least 2 studies are required for meta‐analysis, it should be considered that the 3‐armed trial identified by the systematic review that included the 1.5 mg dulaglutide dose (vs dulaglutide 0.75 mg and insulin glargine) indicated that the higher dose led to a greater reduction in HbA1c and bodyweight compared to both dulaglutide 0.75 mg and insulin glargine (Figure 2).21 With lixisenatide, a weight reduction was observed compared to insulin glarine, but HbA1c reduction was greater with glargine (Figure 2).35 With albiglutide, although there was weight reduction when compared with insulin glargine, HbA1c reduction was not different from insulin glargine (Figure 2).22 This finding is not unexpected, however, as albiglutide was inferior in glycaemic lowering efficacy when compared to liraglutide, which in this meta‐analysis also did not have a statistically significant HbA1c reduction when compared to insulin glargine.18 Analyses conducted for weight change from baseline demonstrated statistically significant weight loss with all GLP‐1 RAs compared to weight gain with basal insulins: −4.65 kg (95% CI −5.08, −4.22) for liraglutide 1.8 mg, −4.31 kg (95% CI −4.71, −3.90) for exenatide 10 µg, −2.85 kg (95% CI −3.20 to −2.49) for exenatide 2 mg LAR, and −1.98 kg (95% CI −2.32, −1.64) for dulaglutide 0.75 mg.
The results of the 2 pairwise meta‐analyses indicated that there is a lower risk of hypoglycaemia with liraglutide 1.8 mg once daily compared to insulin glargine and also with once‐weekly exenatide 2 mg LAR compared to insulin glargine. However, these results should be interpreted with caution because of the variability in definitions of hypoglycaemia in different studies, a challenge that has been identified by the American Diabetes Association.40 Moreover, there were only limited data available for severe hypoglycaemia, and where data were present, the event rates were too low to yield clinically meaningful interpretation; as such, a meta‐analysis for severe hypoglycaemia was not conducted. However, it is important to note that the outcome “hypoglycaemia” reported in this article also included “severe hypoglycaemia”.
It is pertinent that we acknowledge the effect that insulin titration may have had on the results for hypoglycaemia. The impact of insulin titration was most apparent in the RCT conducted by D'Alessio et al.36 which compared once‐daily liraglutide 1.8 mg and insulin glargine with an aggressive up‐titration of insulin glargine that resulted in a mean insulin dose of 52 units/d at study end (Table S1, Supporting Information) coupled with the down‐titration of background SU. The result of the meta‐analyses for this comparison show glycaemic control in favour of insulin glargine, a greater increase in weight from baseline and an increased risk of hypoglycaemia which may be a result of aggressive insulin titration. In contrast, the mean doses of insulin glargine in the once‐weekly exenatide 2 mg LAR studies used in the pairwise meta‐analysis for hypoglycaemia were only 16 to 31 insulin units (Table S1, Supporting Information), which may have been responsible for, not only the lower risk of hypoglycaemia, but also the lesser weight gain and lower HbA1c drop with insulin glargine.33 It is important to point out that the titration of basal insulin, even in the setting of clinical trials designed around insulin titration, is often suboptimal. For example, in the treat‐to‐target trial comparing once‐daily insulin glargine with NPH insulin, the average dose of insulin was 47.2 IU (SD = 1.3) for glargine and 41.8 IU (SD = 1.3) for NPH.41 Inadequate insulin dosing could also have implications for a real‐world setting, where basal insulin titration might not always occur as it should. As such, GLP‐1RAs, some of which do not require any dose titration, could offer a reasonable alternative to basal insulin.
Although our analysis attempted to minimize heterogeneity among studies by ensuring that treatment regimens and outcome time points were consistent, and the systematic review eligibility criteria specifying that background therapy must be received concurrently with study medication, any OAD was allowed as background therapy. However, because of the limited evidence base, heterogeneity was observed among studies, leading to uncertainty concerning the estimates. Table 1 clearly indicates that background therapy varied among the included studies, which could also have impacted the results. Whilst MET is largely weight neutral and has a low risk of hypoglycaemia associated with it, both SU and TZD treatments are associated with weight gain, which should be considered when interpreting results. In addition, SUs carry a moderate risk of hypoglycaemia, which is of particular importance because basal insulin is also associated with weight gain and an increased risk of hypoglycaemia.42 Although sensitivity analyses can be conducted to assess the impact of differing background therapies, it was not possible in this study because most meta‐analyses included only 2 trials, a number insufficient for a meaningful sensitivity analysis.
As the analyses for the present study were being conducted, a systematic review and meta‐analysis of once‐weekly GLP‐1 RAs was published.10 However, the current study has several important differences from that study and other previously published analyses. Our research question was more specific than that posed by previous analyses. First, we considered only head‐to‐head comparisons with basal insulins rather than with any antihyperglycemic treatment, which offers more comprehensive evidence for clinicians regarding choice between the two injectable options in the treatment pathway. Second, rather than being restricted to once‐weekly GLP‐1 Ras, our analysis considered all licensed dosages of all GLP‐1 RAs currently being used in clinical practice. And finally, our analysis did not pool drugs according to class; thus, each drug and dosage was considered independently and we pooled only outcome data from studies within the time frame of 16 to 36 weeks of treatment. Although these restrictions meant that some treatments were not included in the meta‐analysis, notably albiglutide 30 mg (uptitrated to 50 mg as needed), dulaglutide 1.5 mg (where only one study comparing it to basal insulin at this dose was available) and lixisenatide 20 µg, it was not thought appropriate to conduct an analysis pooling the drugs, dosages or different time frames because head‐to‐head trials of GLP‐1 RAs have demonstrated differences within the class.43 Another recently published pairwise and network meta‐analysis sought to understand the benefits and harms of once‐weekly GLP‐1 RAs.16 However, that analysis considered only once‐weekly GLP‐1 RAs and was conducted primarily to assess the cardiometabolic efficacy and adverse effects of GLP‐1 RAs and not the comparison to basal insulin in the treatment paradigm,16 as we did in our study.
There are several limitations in the current systematic literature review and meta‐analysis that should be acknowledged. One limitation is that only English‐language articles were searched and included. Although we do not envision the number of included articles to substantially increase if the scope of the search was expanded to include non‐English articles, we cannot be certain of the impact of an expanded scope on the results of the meta‐analysis. In addition, we combined the results of trials at 26 weeks (±10 weeks) and therefore are unable to draw conclusions about the long‐term efficacy and safety of GLP‐1 RAs compared to basal insulin; future studies should explore this area. Further, a priori, we developed a protocol to analyse the results of the systematic review by meta‐analysis. As future studies are published, expanding the evidence base for GLP‐1 RAs compared to basal insulin, consideration of conducting an analysis including both direct and indirect data by network meta‐analysis may be warranted.
The meta‐analysis conducted for hypoglycemia should be interpreted with caution because of high heterogeneity in defining hypoglycaemic events, an inherent problem when conducting meta‐analyses in diabetes.40 Of the 15 studies identified by the systematic review, the definitions of hypoglycaemia varied considerably (Table S5, Supporting Information). With the exception of the study by Davies et al.30 which required only the presence of symptoms for a hypoglycaemic event to be reported, the remaining studies did define a hypoglycaemic event using a blood glucose target, but this target varied between 3.0 and 4.0 mmol/L depending on the study. Interpretation of findings from the meta‐analysis of hypoglycaemia was further complicated by heterogeneity among studies with regard to background therapies and insulin titration; as such, future trials with similar background therapies, insulin titration algorithms and standardized definitions of hypoglycaemia are warranted for comparability of studies.
In conclusion, the current analysis indicates that once‐weekly GLP‐1 Ras, exenatide LAR and dulaglutide, demonstrate a greater reduction in HbA1c compared to basal insulin after 26 weeks of treatment. Once‐ or twice‐daily GLP‐1 RAs, liraglutide and exenatide, also demonstrate a reduction in HbA1c, but these changes were similar to those seen with basal insulin. Treatment with all GLP‐1 RAs results in significant reduction in bodyweight as compared to basal insulins. However, clarity and consistency is required in defining hypoglycaemia in clinical trials, to allow meaningful conclusions to be inferred for this outcome when comparing GLP‐1 RAs with basal insulin.
Supporting information
Appendix S1. Medline search strategy (OVID SP).
Figure S1. Risk of bias of included studies.
Table S1. Insulin titration algorithm and FPG targets.
Table S2. HbA1c (%) change from baseline and target achievement at primary endpoints.
Table S3. Body weight and BMI change from baseline at endpoints.
Table S4. Fasting glucose change from baseline.
Table S5. Hypoglycemic episodes at study endpoints.
ACKNOWLEDGMENTS
We thank James Eaton for study planning and Sarah Goring (both of ICON Plc) for analysis validation and manuscript editing.
Conflict of interest
S. S. has served previously on the Advisory Board of Eli Lilly and was compensated for his time. E. E. W. has served previously on a speaker's bureau for Eli Lilly, Boehringer Ingelheim and Abbott Diabetes Care, on advisory boards for Eli Lilly, Boehringer Ingelheim, Abbott Diabetes Care, Amgen and Voluntis, and as a consultant for Eli Lilly, Boehringer Ingelheim, Abbott Diabetes Care and Amgen, and has received grants from Abbott Diabetes Care. A. K. and R. J. are employees of Lilly USA, LLC and are Lilly stock holders. M. Y. is an employee of Eli Lilly Canada Inc. and is a Lilly stock holder. J. T., I. S., E. K. and N. W. are employees of the consultancy group of ICON plc, which received compensation for the completion of the analysis.
Author contributions
S. S. participated in the conception, design, analysis, and interpretation of drafting the results of the manuscript. He did not receive any compensation for his participation in the study. E. E. W. participated in the original study design, analysis of the data, and review and editing of the manuscript. He did not receive any compensation for his participation in the study. A. K., and R. J. contributed to the design of the study, analysis of the data and review and editing of the paper. M. Y. contributed to the design of the study, analysis of the data and review and editing of the paper. J. T., I. S., E. K. and N. W. participated in the conception, design, interpretation and writing of the manuscript.
Singh S, Wright EE Jr., Kwan AYM, Thompson JC, Syed IA, Korol EE, Waser NA, Yu MB and Juneja R. Glucagon‐like peptide‐1 receptor agonists compared with basal insulins for the treatment of type 2 diabetes mellitus: A systematic review and meta‐analysis, Diabetes Obes Metab, 2017;19(2):228–238.
References
- 1. Rhinehart A. Adding GLP‐1 receptor agonist therapy to basal insulin for postprandial glucose control. Clin Diabetes (Translating Research to Practice). 2015;33(2):73‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient‐centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38(1):140‐149. [DOI] [PubMed] [Google Scholar]
- 3. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm. Endocr Pract. 2015;21(12):1403‐1414. [DOI] [PubMed] [Google Scholar]
- 4. International Diabetes Federation . IDF treatment algorithm for people with type 2 diabetes 2016. https://www.idf.org/sites/default/files/Type%202%20treatment%20algorithm.pdf. Accessed April 2016.
- 5. Food and Drug Administration . FDA approves Tanzeum (albiglutide) to treat type 2 diabetes. FDA [Internet]. 2014. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm393289.htm. Accessed December 2015.
- 6. European Medicines Agency . Eperzan (albiglutide). EMA [Internet]. 2014. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Public_assessment_report/human/002735/WC500165119.pdf. Accessed December 2015.
- 7. Food and Drug Administration . FDA approves Trulicity (dulaglutide) to treat type 2 diabetes. FDA [Internet]. 2014. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm415180.htm. Accessed December 2015.
- 8. European Medicines Agency . Trulicity (dulaglutide). EMA [Internet]. 2014. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Public_assessment_report/human/002825/WC500179473.pdf. Accessed December 2015.
- 9. Sun F, Chai S, Yu K, et al. Gastrointestinal adverse events of glucagon‐like peptide‐1 receptor agonists in patients with type 2 diabetes: a systematic review and network meta‐analysis. Diabetes Technol Ther. 2015;17(1):35‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Karagiannis T, Liakos A, Bekiari E, et al. Efficacy and safety of once‐weekly glucagon‐like peptide 1 receptor agonists for the management of type 2 diabetes: a systematic review and meta‐analysis of randomized controlled trials. Diabetes Obes Metab. 2015;17(11):1065‐1074. [DOI] [PubMed] [Google Scholar]
- 11. Aroda VR, Henry RR, Han J, et al. Efficacy of GLP‐1 receptor agonists and DPP‐4 inhibitors: meta‐analysis and systematic review. Clin Ther. 2012;34(6):1247‐1258. [DOI] [PubMed] [Google Scholar]
- 12. Eng C, Kramer CK, Zinman B, Retnakaran R. Glucagon‐like peptide‐1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: a systematic review and meta‐analysis. Lancet. 2014;384(9961):2228‐2234. [DOI] [PubMed] [Google Scholar]
- 13. Liu FP, Dong JJ, Yang Q, et al. Glucagon‐like peptide 1 receptor agonist therapy is more efficacious than insulin glargine for poorly controlled type 2 diabetes: a systematic review and meta‐analysis. J Diabetes. 2015;7(3):322‐328. [DOI] [PubMed] [Google Scholar]
- 14. Li WX, Gou JF, Tian JH, Yan X, Yang L. Glucagon‐like peptide‐1 receptor agonists versus insulin glargine for type 2 diabetes mellitus: a systematic review and meta‐analysis of randomized controlled trials. Curr Ther Res Clin Exp. 2010;71(4):211‐238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang Y, Li L, Yang M, Liu H, Boden G, Yang G. Glucagon‐like peptide‐1 receptor agonists versus insulin in inadequately controlled patients with type 2 diabetes mellitus: a meta‐analysis of clinical trials. Diabetes Obes Metab. 2011;13(11):972‐981. [DOI] [PubMed] [Google Scholar]
- 16. Zaccardi F, Htike ZZ, Webb DR, Khunti K, Davies MJ. Benefits and harms of once‐weekly glucagon‐like peptide‐1 receptor agonist treatments: a systematic review and network meta‐analysis. Ann Intern Med. 2016;164(2):102‐113. [DOI] [PubMed] [Google Scholar]
- 17. Buse JB, Nauck M, Forst T, et al. Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION‐6): a randomised, open‐label study. Lancet. 2013;381(9861):117‐124. [DOI] [PubMed] [Google Scholar]
- 18. Pratley RE, Nauck MA, Barnett AHI, et al. Once‐weekly albiglutide versus once‐daily liraglutide in patients with type 2 diabetes inadequately controlled on oral drugs (HARMONY 7): a randomised, open‐label, multicentre, non‐inferiority phase 3 study. Lancet Diabetes Endocrinol. 2014;2(4):289‐297. [DOI] [PubMed] [Google Scholar]
- 19. Barnett AH, Burger J, Johns D, et al. Tolerability and efficacy of exenatide and titrated insulin glargine in adult patients with type 2 diabetes previously uncontrolled with metformin or a sulfonylurea: a multinational, randomized, open‐label, two‐period, crossover noninferiority trial. Clin Ther. 2007;29(11):2333‐2348. [DOI] [PubMed] [Google Scholar]
- 20. Bunck MC, Diamant M, Corner A, et al. One‐year treatment with exenatide improves beta‐cell function, compared with insulin glargine, in metformin‐treated type 2 diabetic patients: a randomized, controlled trial. Diabetes Care. 2009;32(5):762‐768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Giorgino F, Benroubi M, Sun JH, Zimmermann AG, Pechtner V. Efficacy and safety of once‐weekly dulaglutide versus insulin glargine in patients with type 2 diabetes on metformin and glimepiride (AWARD‐2). Diabetes Care. 2015;38(12):2241‐2249. [DOI] [PubMed] [Google Scholar]
- 22. Weissman PN, Carr MC, Ye J, et al. HARMONY 4: randomised clinical trial comparing once‐weekly albiglutide and insulin glargine in patients with type 2 diabetes inadequately controlled with metformin with or without sulfonylurea. Diabetologia. 2014;57(12):2475‐2484. [DOI] [PubMed] [Google Scholar]
- 23. Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ma J, Liu W, Hunter A, Zhang W. Performing meta‐analysis with incomplete statistical information in clinical trials clinical trials. BMC Med Res Methodol. 2008;8:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schwarzer G. General package for meta‐analysis. Package ‘meta’ [Internet]. 2015. https://cran.r‐project.org/web/packages/meta/meta.pdf. Accessed October 2015.
- 26. Borenstein M, Hedges L, Higgins J, Rothstein H. Introduction to Meta‐Analysis. 1st ed. Chichester, UK: John Wiley & Sons, Ltd; 2009. [Google Scholar]
- 27. Higgins JP, Green S. Cochrane Handbook for systematic reviews of interventions version 5.1.0. The Cochrane Collaboration, 2011 [Internet]. 2011. http://handbook.cochrane.org/front_page.htm. Accessed October 2015.
- 28. Araki E, Inagaki N, Tanizawa Y, Oura T, Takeuchi M, Imaoka T. Efficacy and safety of once‐weekly dulaglutide in combination with sulphonylurea and/or biguanide compared with once‐daily insulin glargine in Japanese patients with type 2 diabetes: a randomized, open‐label, phase III, non‐inferiority study. Diabetes Obes Metab. 2015;17(10):994‐1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Inagaki N, Atsumi Y, Oura T, Saito H, Imaoka T. Efficacy and safety profile of exenatide once weekly compared with insulin once daily in Japanese patients with type 2 diabetes treated with oral antidiabetes drug(s): results from a 26‐week, randomized, open‐label, parallel‐group, multicenter, noninferiority study. Clin Ther. 2012;34(9):1892‐1908. [DOI] [PubMed] [Google Scholar]
- 30. Davies M, Heller S, Sreenan S, et al. Once‐weekly exenatide versus once‐ or twice‐daily insulin detemir: randomized, open‐label, clinical trial of efficacy and safety in patients with type 2 diabetes treated with metformin alone or in combination with sulfonylureas. Diabetes Care. 2013;36(5):1368‐1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gough SC, Bode B, Woo V, et al. Efficacy and safety of a fixed‐ratio combination of insulin degludec and liraglutide (IDegLira) compared with its components given alone: results of a phase 3, open‐label, randomised, 26‐week, treat‐to‐target trial in insulin‐naive patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2014;2(11):885‐893. [DOI] [PubMed] [Google Scholar]
- 32. Gough SC, Bode BW, Woo VC, et al. One‐year efficacy and safety of a fixed combination of insulin degludec and liraglutide in patients with type 2 diabetes: results of a 26‐week extension to a 26‐week main trial. Diabetes Obes Metab. 2015;17(10):965‐973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Diamant M, Van GL, Stranks S, et al. Once weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes (DURATION‐3): an open‐label randomised trial. Lancet. 2010;375(9733):2234‐2243. [DOI] [PubMed] [Google Scholar]
- 34. Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med. 2005;143(8):559‐569. [DOI] [PubMed] [Google Scholar]
- 35. Rosenstock J, Aronson R, Grunberger G, et al. Benefits of lixiLan, a titratable fixed‐ratio combination of insulin glargine plus lixisenatide, versus insulin glargine and lixisenatide monocomponents in type 2 diabetes inadequately controlled with oral agents: the lixiLan‐O randomized trial. Diabetes Care. 2016;15:15. [DOI] [PubMed] [Google Scholar]
- 36. D'Alessio D, Haring HU, Charbonnel B, et al. Comparison of insulin glargine and liraglutide added to oral agents in patients with poorly controlled type 2 diabetes. Diabetes Obes Metab. 2015;17(2):170‐178. [DOI] [PubMed] [Google Scholar]
- 37. Gurkan E, Tarkun I, Sahin T, Cetinarslan B, Canturk Z. Evaluation of exenatide versus insulin glargine for the impact on endothelial functions and cardiovascular risk markers. Diabetes Res Clin Pract. 2014;106(3):567‐575. [DOI] [PubMed] [Google Scholar]
- 38. Davies MJ, Donnelly R, Barnett AH, Jones S, Nicolay C, Kilcoyne A. Exenatide compared with long‐acting insulin to achieve glycaemic control with minimal weight gain in patients with type 2 diabetes: results of the Helping Evaluate Exenatide in patients with diabetes compared with Long‐Acting insulin (HEELA) study. Diabetes Obes Metab. 2009;11(12):1153‐1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Russell‐Jones D, Vaag A, Schmitz O, et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD‐5 met + SU): a randomised controlled trial. Diabetologia. 2009;52(10):2046‐2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. American Diabetes Association. Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care. 2005;28(5):1245‐1249. [DOI] [PubMed] [Google Scholar]
- 41. Riddle MC, Rosenstock J, Gerich J. The treat‐to‐target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26(11):3080‐3086. [DOI] [PubMed] [Google Scholar]
- 42. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient‐centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2012;55(6):1577‐1596. [DOI] [PubMed] [Google Scholar]
- 43. Trujillo JM, Nuffer W, Ellis SL. GLP‐1 receptor agonists: a review of head‐to‐head clinical studies. Ther Adv Endocrinol Metab. 2015;6(1):19‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Holman RR, Farmer AJ, Davies MJ, et al. Three‐year efficacy of complex insulin regimens in type 2 diabetes. N Engl J Med. 2009;361(18):1736‐1747. [DOI] [PubMed] [Google Scholar]
- 45. Holman RR, Thorne KI, Farmer AJ, et al. Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes. N Engl J Med. 2007;357(17):1716‐1730. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Medline search strategy (OVID SP).
Figure S1. Risk of bias of included studies.
Table S1. Insulin titration algorithm and FPG targets.
Table S2. HbA1c (%) change from baseline and target achievement at primary endpoints.
Table S3. Body weight and BMI change from baseline at endpoints.
Table S4. Fasting glucose change from baseline.
Table S5. Hypoglycemic episodes at study endpoints.


