Abstract
Objective
To systematically review studies addressing prediction of successful dose reduction or discontinuation of a biologic agent in rheumatoid arthritis (RA).
Methods
PubMed, Embase, and Cochrane Library databases were searched for studies that examined the predictive value of biomarkers for successful dose reduction or discontinuation of a biologic agent in RA. Two reviewers independently selected studies, and extracted data and assessed the risk of bias. A biomarker was classified as a “potential predictor” if the univariate association was either strong (odds ratio or hazard ratio >2.0 or <0.5) or statistically significant. For biomarkers that were studied multiple times, qualitative best‐evidence synthesis was performed separately for the prediction of successful dose reduction and discontinuation. Biomarkers that were defined in ≥75% of the studies as potential predictors were regarded as “predictor” for the purposes of our study.
Results
Of 3,029 nonduplicate articles initially searched, 16 articles regarding 15 cohorts were included in the present study. Overall, 17 biomarkers were studied multiple times for the prediction of successful dose reduction, and 33 for the prediction of successful discontinuation of a biologic agent. Three predictors were identified: higher adalimumab trough level for successful dose reduction and lower Sharp/van der Heijde erosion score and shorter symptom duration at the start of a biologic agent for successful discontinuation.
Conclusion
The predictive value of a wide variety of biomarkers for successful dose reduction or discontinuation of biologic treatment in RA has been investigated. We identified only 3 biomarkers as predictors, in just 2 studies. The strength of the evidence is limited by the low quality of the included studies and the likelihood of reporting bias and multiple testing.
Treatment of rheumatoid arthritis (RA) is based on the “hit hard, hit early” strategy. Starting treatment early and achieving low disease activity as soon as possible by using a combination of disease‐modifying antirheumatic drugs (DMARDs) (including glucocorticoids) and rapid escalation to biologics, if necessary, are pivotal in this strategy 1.
However, a disadvantage of such a strategy is that it leads to overtreatment with biologic agents in a considerable number of patients 2. Overtreatment is associated with an increased risk of adverse effects such as dose‐dependent serious infections, as well as higher medication costs 3. In order to reduce overtreatment, the start of intensive treatment should be followed by attempts to find the lowest individual effective dose. This can be done in patients with low disease activity by discontinuing the biologic agent all at once, or tapering the dosage. In general, discontinuation all at once of a biologic agent has proven to be inferior to continuing biologic treatment with respect to disease activity and radiologic outcomes and function 4. Alternatively, tapering of a biologic agent guided by disease activity (dose reduction until either disease activity increases or the biologic agent can be stopped) appears to be feasible, safe, and effective in RA patients with low disease activity or whose disease is in remission 4.
The ability to accurately predict the success of dose reduction or discontinuation of a biologic agent is likely to constitute a major improvement over the current trial‐and‐error, disease activity–guided tapering. When it can be predicted that dose reduction will be unsuccessful, dose reduction should not even be attempted. Such predictions would prevent disease flares, minimize physician efforts, and ease uncertainty in patients. Additionally, when it can be predicted that discontinuation will be successful, the dose tapering phase can be skipped and the biologic can be stopped directly, saving time and medication cost.
A biomarker is defined as a characteristic objectively measured and evaluated as an indicator of normal biologic processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention 5. Patient characteristics, biochemical tests, and imaging measurements can all serve as biomarkers. If there is a biomarker that can accurately predict the success of dose reduction or discontinuation prior to the tapering of a biologic, it could be used for optimizing treatment in daily clinical care.
As previous narrative reviews have demonstrated, it remains challenging to identify those patients whose treatment with biologic agents can be tapered without risk of a flare 6, 7, 8, 9. In the past few years, several studies have investigated various biomarkers for predicting successful tapering of different biologic agents. To our knowledge, these results have not yet been systematically summarized. Therefore, we conducted analysis of all prospective studies with a predefined tapering protocol, in order to provide an overview of the investigated biomarkers for predicting successful dose reduction or discontinuation of biologic treatment in RA.
MATERIALS AND METHODS
Search strategy
In November 2015, a search was conducted using PubMed, Embase, and Cochrane Library databases for studies that examined the predictive value of biomarkers for the success of dose reduction or discontinuation of biologic treatment in patients with RA. The search strategy (see Supplementary Table 1, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.39946/abstract) consisted of the Haynes broad filter (recommended for finding predictive research) 10, keywords regarding the patient group, as well as the outcome of interest (successful dose reduction or discontinuation). The patient group consisted of those who were treated with any registered biologic agent for RA (i.e., abatacept, adalimumab, certolizumab, etanercept, golimumab, infliximab, rituximab, or tocilizumab) at a standard dosing regimen for >24 weeks, who had low disease activity, and in whom the dosage of a biologic agent was subsequently reduced or discontinued. Anakinra was excluded because it is now rarely used due to lack of efficacy. The search was not limited by language, nor by year or type of publication. Included articles and excluded reviews were used for cross checking of references.
Study selection
This review was conducted and reported according to the procedures outlined in the PRISMA Statement 11. Two reviewers (EB, FB) independently selected articles, primarily by title and abstract and subsequently by review of the full text (when necessary). Any disagreement was resolved by consensus meetings with another reviewer (LT). During full‐text analyses, studies were excluded if 1) biomarkers were not determined or data on them were not collected prior to tapering of biologic agents, 2) follow‐up periods were <3 months or >24 months, 3) <20 participants were included in the cohort or a retrospective design was utilized, or 4) they were only reviews or abstracts from a conference. Additionally, studies that analyzed the data in a way that could not provide answers to our primary question (e.g., pooling the outcomes of conventional DMARD and biologic agent tapering or the outcomes of the continuing arm and tapering arm) were also excluded.
Data extraction
Data on study designs, patient characteristics, tapering strategies, biomarkers, outcomes (clinical response criteria), and analysis (association measure between biomarker and outcome) were independently extracted from each article by 2 reviewers (FB, LT) using a data extraction form. The form was pilot tested on 3 randomly selected articles (which were not included in the review) and was refined accordingly. Any doubts were resolved by consultation with a third reviewer (CvdE or AdB).
Quality assessment
Two reviewers (FB, LT) independently assessed the methodologic quality of the included studies using the Quality In Prognosis Studies (QUIPS) tool 12. The tool was operationalized a priori and pilot tested on 3 randomly selected articles that were not included in the review. One of the 6 domains of the tool, study confounding, was excluded because all of the studies included in our review investigated multiple biomarkers in an exploratory manner, making it impossible to summarize all potential confounding factors.
Each of the remaining domains was judged as having low, moderate, or high risk of bias. Disagreements were resolved by discussion and, when necessary, a third reviewer (CvdE) made final decisions. Since the use of a summed score for overall study quality is not recommended, we decided a priori to consider a study of high quality if the 2 domains that we consider to be most important (study participation and statistical analysis) were either both assessed as having a low risk of bias, or one as low risk and the other as moderate 13. Studies not meeting these criteria were considered of low quality.
Statistical analysis and data synthesis
The heterogeneity of the biomarkers, outcome measures, and statistics precluded a quantitative meta‐analysis. Therefore, results regarding the predictive value of biomarkers for the success of dose reduction and discontinuation of biologic treatment were qualitatively synthesized in 3 steps.
In step 1, we assessed whether each investigated biomarker was a potential predictor. A biomarker was defined as a potential predictor if the univariate association between the biomarker and the success of dose reduction or discontinuation was either strong (odds ratio [OR] or hazard ratio [HR] >2.0 or <0.5) or statistically significant (P < 0.05) 14. If no univariate OR or HR was provided, results of other univariate association measures, multivariate results, or textual conclusions on the statistical significance of findings were used.
In step 2, biomarkers were divided into 5 different categories (patient, treatment, disease activity, laboratory, and imaging measurements) and an overview of biomarkers that were studied multiple times (i.e., in >1 separate study) and those that were studied only once 15 was completed.
In step 3, qualitative best‐evidence synthesis of biomarkers (studied multiple times) was performed separately for predicting the success of dose reduction and discontinuation of biologic agents. A biomarker was regarded as a predictor if it was defined as a potential predictor in ≥75% of the studies in which the biomarker was investigated. Subsequently, we defined 4 levels of evidence as used in previous systematic reviews 16, 17: 1) strong evidence (consistent findings [≥75% of findings in same direction] in at least 2 high‐quality studies), 2) moderate evidence (consistent findings in 1 high‐quality study and at least 2 low‐quality studies), 3) limited evidence (findings in 1 high‐quality study or consistent findings in at least 2 low‐quality studies), and 4) conflicting evidence (inconsistent findings irrespective of study quality [<75% of findings in same direction]). Of note, the level of conflicting evidence was checked first before assigning a strong, moderate, or limited evidence level to a biomarker.
RESULTS
Study selection
Through the database search in November 2015, we retrieved 3,029 nonduplicate articles. The cross‐checking of references yielded no relevant articles. In total, 21 articles met our selection criteria. However, 5 articles were publications of the BeSt study (Behandel Strategieën voor Reumatoïde Artritis) 18, 19, 20, 21, 22 and 2 of the HONOR study (Humira Discontinuation Without Functional and Radiographic Damage Progression Following Sustained Remission) 23, 24. Of the BeSt study publications, we only analyzed the article that explicitly answered our primary question 22, as the other articles contained no additional information. Of the two HONOR study publications, we selected the article reporting the study with the longest follow‐up time 24, since the outcome measures between these publications were similar. Eventually 16 articles regarding 15 cohorts were included in this review. A flow diagram of the study selection is depicted in Figure 1.
Figure 1.
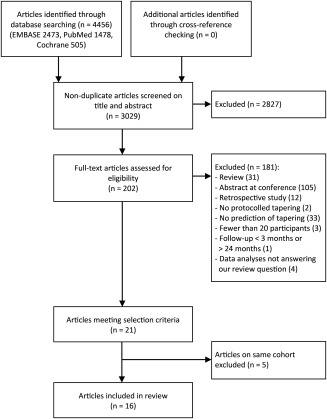
Flow diagram of study selection.
Study characteristics
The characteristics of the included studies are listed in Supplementary Table 2 (available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.39946/abstract). In 7 studies, the dosage of the biologic agent was reduced 25, 26, 27, 28, 29, 30, 31 and in 9 studies the biologic agent was discontinued all at once 22, 24, 32, 33, 34, 35, 36, 37, 38. There were 4 randomized controlled trials 22, 27, 28, 30 and 12 observational cohort studies 24, 25, 26, 29, 31, 32, 33, 34, 35, 36, 37, 38. Overall, 1,093 participants were included in these studies. They were all in remission or had low disease activity prior to dose reduction or discontinuation of the biologic agent, according to different disease activity criteria. In 11 of the 16 studies 22, 24, 25, 26, 27, 30, 34, 35, 36, 37, 38, a minimum duration of 6 months of low disease activity was part of the inclusion criteria. Studies varied with respect to sample size (range 21–187), type of biologic agent (abatacept, adalimumab, certolizumab, etanercept, golimumab, infliximab, or tocilizumab), follow‐up period (range 24 weeks–24 months), and outcome measure (meeting criteria for low disease activity, remission, or flare).
The included studies examined a wide variety of biomarkers. Fifty‐two biomarkers were studied for their predictive value for the success of dose reduction (17 of which were studied multiple times), and 64 biomarkers for the success of discontinuation of a biologic agent (33 of which were studied multiple times).
The predictive value of biomarkers for the prediction of the success of dose reduction and discontinuation of a biologic agent were analyzed separately. The DRESS study (Dose Reduction Strategy of Subcutaneous TNF Inhibitors) examined the predictive value of biomarkers for both the success of dose reduction and the success of discontinuation of biologic treatment; data on these biomarkers were therefore included in both analyses 27. For the Spacing of TNF‐Blocker Injections in Rheumatoid Arthritis Study (STRASS) 30, only those biomarkers that were investigated in the multivariate analysis and that included the strategy of spacing TNF blockers could be analyzed in our review, since the univariate analysis with maintenance and spacing arms combined was not suitable. The results for the biomarkers that were studied multiple times for prediction of the success of dose reduction and the success of discontinuation are depicted in Supplementary Table 3. In summary, 3 predictors were identified: higher adalimumab trough level for the success of dose reduction, and lower Sharp/van der Heijde erosion score and shorter symptom duration at the start of biologic treatment for the success of discontinuation.
The results for biomarkers that were studied once for the prediction of the success of dose reduction and the success of treatment discontinuation are depicted in Supplementary Table 4 (http://onlinelibrary.wiley.com/doi/10.1002/art.39946/abstract). Ten of 35 biomarkers were classified as potential predictors for the success of dose reduction, and 8 of 31 for discontinuation.
Quality assessment and best‐evidence synthesis
A total of 80 domains (5 domains in 16 studies) were judged by two reviewers (FB and LT) who agreed on 65 of 80 domains, representing good interrater agreement (κ = 0.71) 39. Disagreements were caused by different interpretation of missing data and different judgment of the overall risk of bias of the domain study attrition. According to our predefined criteria, 5 studies were classified as high‐quality study and 11 studies as low‐quality study (see Supplementary Table 5, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.39946/abstract). Limitations of the studies mostly concerned insufficient data presentation and selective reporting of results. Qualitative best‐evidence synthesis for the prediction of the success of dose reduction and the success of discontinuation of a biologic agent is depicted in Tables 1 and 2, respectively.
Table 1.
Best‐evidence synthesis for prediction of successful dose reduction of a biologic agenta
| Biomarker studied | No. of studies | Potential predictor (refs.)b | Quality (refs.)c | Predictord | Level of evidencee | ||
|---|---|---|---|---|---|---|---|
| Yes | No | High | Low | ||||
| Age | 5 | 0 | 5 (25–27,29,31) | 1 (25) | 4 (26,27,29,31) | No | Moderate |
| Sex | 5 | 0 | 5 (25–27,29,31) | 1 (25) | 4 (26,27,29,31) | No | Moderate |
| Disease duration | 4 | 1 (31) | 3 (25,27,29) | 1 (25) | 3 (27,29,31) | No | Moderate |
| Smoking | 4 | 1 (25) | 3 (26,27,31) | 1 (25) | 3 (26,27,31) | No | Limited |
| No. of previous csDMARDs | 3 | 1 (31) | 2 (26,27) | 0 | 3 (26,27,31) | No | Conflicting |
| No. of previous biologics | 2 | 0 | 2 (27,31) | 0 | 2 (27,31) | No | Limited |
| Time from symptom onset to biologic agent | 2 | 0 | 2 (26,31) | 0 | 2 (26,31) | No | Limited |
| Duration of current biologic treatment before tapering | 3 | 1 (29) | 2 (25,27) | 1 (25) | 2 (27,29) | No | Conflicting |
| Concomitant DMARD | 4 | 0 | 4 (25–27,31) | 1 (25) | 3 (26,27,31) | No | Moderate |
| Methotrexate | 3 | 0 | 3 (25,27,29) | 1 (25) | 2 (27,29) | No | Moderate |
| Prednisone | 3 | 0 | 3 (27,29,31) | 0 | 3 (27,29,31) | No | Limited |
| DAS28‐ESR at tapering | 4 | 2 (29,31) | 2 (25,27) | 1 (25) | 3 (27,29,31) | No | Conflicting |
| Rheumatoid factor | 6 | 2 (26,30) | 4 (25,27,29,31) | 1 (25) | 5 (26,27,29–31) | No | Conflicting |
| ACPA | 5 | 0 | 5 (25,27–29,31) | 1 (25) | 4 (27–29,31) | No | Moderate |
| ESR | 2 | 0 | 2 (27,29) | 0 | 2 (27,29) | No | Limited |
| CRP | 2 | 1 (29) | 1 (27) | 0 | 2 (27,29) | No | Conflicting |
| Adalimumab trough level | 2 | 2 (28,29) | 0 | 0 | 2 (28,29) | Yes | Limited |
csDMARDs = conventional synthetic disease‐modifying antirheumatic drugs; DAS28‐ESR = Disease Activity Score in 28 joints using the erythrocyte sedimentation rate; ACPA = anti–citrullinated peptide antibodies; CRP = C‐reactive protein.
Number of studies with a strong and/or significant association between biomarker and successful dose reduction.
Number of studies with a high/low quality according to the Quality in Prognosis Studies tool.
Biomarker defined as potential predictor in ≥75% of the studies in which the biomarker was investigated.
Composite outcome of predictive value and study quality.
Table 2.
Best‐evidence synthesis for the prediction of successful discontinuation of a biologic agenta
| Biomarker studied | No. of studies | Potential predictor (refs.)b | Quality (refs.)c | Predictord | Level of evidencee | ||
|---|---|---|---|---|---|---|---|
| Yes | No | High | Low | ||||
| Age | 7 | 1 (37) | 6 (22,24,27,32,33,38) | 3 (22,24,37) | 4 (27,32,33,38) | No | Strong |
| Sex | 7 | 0 | 7 (22,27,32–34,37,38) | 3 (22,34,37) | 4 (27,32,33,38) | No | Strong |
| Disease duration | 9 | 4 (22,24,35,37) | 5 (27,32–34,38) | 4 (22,24,34,37) | 5 (27,32,33,35,38) | No | Conflicting |
| Remission duration | 3 | 1 (38) | 2 (33,35) | 0 | 3 (33,35,38) | No | Conflicting |
| Smoking | 2 | 1 (22) | 1 (27) | 1 (22) | 1 (27) | No | Conflicting |
| BMI | 2 | 0 | 2 (22,27) | 1 (22) | 1 (27) | No | Limited |
| No. of previous csDMARDs | 2 | 0 | 2 (27,38) | 0 | 2 (27,38) | No | Limited |
| No. of previous biologic agents | 2 | 0 | 2 (27,38) | 0 | 2 (27,38) | No | Limited |
| Time from symptom onset to biologic agent | 2 | 2 (22,35) | 0 | 1 (22) | 1 (35) | Yes | Limited |
| Duration of current biologic treatment before tapering | 4 | 3 (22,24,38) | 1 (27) | 2 (22,24) | 2 (27,38) | No | Conflicting |
| Concomitant DMARD | 2 | 0 | 2 (27,38) | 0 | 2 (27,38) | No | Limited |
| Methotrexate | 4 | 0 | 4 (24,27,33,37) | 2 (24,37) | 2 (27,33) | No | Strong |
| Prednisone | 4 | 1 (34) | 3 (27,33,37) | 2 (34,37) | 2 (27,33) | No | Moderate |
| HAQ | 8 | 3 (34–36) | 5 (22,24,33,37,38) | 4 (22,24,34,37) | 4 (32,33,35,38) | No | Conflicting |
| DAS28‐ESR at discontinuation | 5 | 3 (24,34,37) | 2 (27,33) | 3 (24,34,37) | 2 (27,33) | No | Conflicting |
| DAS28‐CRP at discontinuation | 3 | 1 (37) | 2 (27,33) | 1 (37) | 2 (27,33) | No | Conflicting |
| DAS28‐ESR at start of biologic agent | 2 | 0 | 2 (37,38) | 1 (37) | 1 (38) | No | Limited |
| TJC | 3 | 0 | 3 (22,27,37) | 2 (22,37) | 1 (27) | No | Strong |
| SJC | 3 | 0 | 3 (22,27,37) | 2 (22,37) | 1 (27) | No | Strong |
| Disease activity VAS | 2 | 0 | 2 (22,37) | 2 (22,37) | 0 | No | Strong |
| SDAI | 2 | 0 | 2 (24,33) | 1 (24) | 1 (33) | No | Limited |
| CDAI | 2 | 0 | 2 (24,33) | 1 (24) | 1 (33) | No | Limited |
| Rheumatoid factor | 7 | 1 (34) | 6 (22,24,27,33,37,38) | 4 (22,24,34,37) | 3 (27,33,38) | No | Strong |
| ACPA | 4 | 0 | 4 (22,27,33,38) | 1 (22) | 3 (27,33,38) | No | Moderate |
| ESR | 4 | 1 (24) | 3 (22,27,37) | 3 (22,24,37) | 1 (27) | No | Strong |
| CRP | 5 | 1 (36) | 4 (22,24,27,37) | 3 (22,24,37) | 2 (27,36) | No | Strong |
| MMP‐3 concentration | 2 | 1 (34) | 1 (24) | 2 (24,34) | 0 | No | Conflicting |
| SHS total score | 4 | 1 (37) | 3 (22,24,27) | 3 (22,24,37) | 1 (27) | No | Strong |
| SHS erosion score | 2 | 2 (22,37) | 0 | 2 (22,37) | 0 | Yes | Strong |
| SHS joint space narrowing score | 2 | 0 | 2 (22,37) | 2 (22,37) | 0 | No | Strong |
| Yearly SHS progression at discontinuation | 2 | 1 (22) | 1 (37) | 2 (22,37) | 0 | No | Conflicting |
| Gray‐scale ultrasound | 2 | 1 (33) | 1 (35) | 0 | 2 (33,35) | No | Conflicting |
| Power Doppler ultrasound | 2 | 1 (33) | 1 (35) | 0 | 2 (33,35) | No | Conflicting |
BMI = body mass index; HAQ = Health Assessment Questionnaire; TJC = tender joint count; SJC = swollen joint count; VAS = visual analog scale; SDAI = Simplified Disease Activity Index; CDAI = Clinical Disease Activity Index; MMP‐3 = matrix metalloproteinase 3; SHS = Sharp/van der Heijde score (see Table 1 for other definitions).
Number of studies with a strong and/or significant association between biomarker and successful dose reduction.
Number of studies with a high/low quality according to the Quality in Prognosis Studies tool.
Biomarker defined as potential predictor in ≥75% of the studies in which the biomarker was investigated.
Composite outcome of predictive value and study quality.
DISCUSSION
To our knowledge, this is the first systematic review summarizing the predictive value of biomarkers for the success of dose reduction or discontinuation of a biologic agent in RA. Of all the studied biomarkers, we identified 3 as predictors. Of note, each of these 3 biomarkers was only investigated in 2 studies, meaning that more frequent investigation of specific biomarkers yielded no consistent predictors. Moreover, 2 of the biomarkers (Sharp/van der Heijde erosion score and shorter symptom duration at the start of biologic treatment) showed a statistically significant but weak association. Therefore, the clinical relevance of these identified predictors could be questioned. Also, our findings regarding the predictive value of the third biomarker (adalimumab trough level) could be questioned considering extensive multiple testing in one study 28 and disputed results in another 29, 40.
In addition, of those biomarkers that were studied only once, we found 10 of 35 biomarkers and 8 of 31 biomarkers that were classified as potential predictors for the success of dose reduction and discontinuation of a biologic agent, respectively. Most of them were serum markers and imaging measurements. This may indicate that the assessment of subclinical inflammation by laboratory or imaging testing provides a useful tool to determine a patient's risk of flare 8. However, results with these biomarkers need to be replicated in other cohorts, with a predefined tapering protocol, before they can be considered predictors.
A strength of this review is that we executed a broad literature search to identify all biomarkers that have ever been investigated for their ability to predict the success of dose reduction or that of discontinuation of a biologic agent. Furthermore, we have performed a best‐evidence synthesis to provide an overview of the results, making the review process transparent and reproducible.
For the identification of potential predictors we chose criteria that can be easily met, taking into account the fact that univariate analyses could lead to an overestimation of the strength of associations. However, even with these non‐strict criteria, only 3 biomarkers could be defined as predictors.
The studies we included show substantial heterogeneity in study design and outcome definition. Several studies included >1 biologic—precluding investigation of the potential different effect for biologic agents. Regarding outcome definition, all studies used a disease activity measure but the threshold for failure and the time point of assessment differed. To collect comparable information, the use of a standardized outcome definition is essential 41. An increase in the 28‐joint Disease Activity Score (DAS28) of >1.2 (or >0.6 if the initial score is ≥3.2) appeared most discriminating and valid, although the Outcome Measures in Rheumatology RA flare group is developing a patient‐reported flare questionnaire that could also be used in the future 42, 43.
Another important limitation is the low quality of 11 of the 16 included studies, according to the operationalized QUIPS tool. Most of the studies were defined as low quality based on incomplete reporting. This classification may have been caused by the fact that finding predictive markers for successful tapering was rarely the main research question of the included studies. Also, there is no specific guideline for predictive research. In our opinion, the use of the Standards for Reporting Diagnostic Accuracy reporting guidelines 44 should be encouraged to ensure that sufficient information for editors, peer reviewers, and readers is provided to facilitate understanding of how the research was performed and to judge the credibility of the findings.
Furthermore, data analysis was complicated by the statistical methods used in the studies. For example, in some studies data were assessed in a way that could not answer our primary question (e.g., pooling the outcomes of conventional DMARD and biologic agent tapering or the outcomes of the continuing arm and tapering arm). In addition, appropriate association measures (e.g., OR or HR) were rarely reported.
However, if more studies had been of high quality, we would not have found more potential predictors, since the mean frequency of potential predictors in high‐quality studies was slightly lower (31.6%) in comparison to low‐quality studies (38.6%). This effect of higher‐quality studies being associated with lower effect estimates has been well recognized 45.
Finally, positive findings should be interpreted with caution due to reporting bias and multiple testing. It is very likely that negative results found for potential biomarkers were not mentioned by all studies. This means that there would have been stronger evidence for nonpredictive biomarkers if all data were reported. Also, there is a chance of false‐positive results due to multiple tests, because some studies simultaneously investigated more than 20 biomarkers without correction for multiple testing by lowering the required P value.
There are currently several studies investigating predictive markers for successful tapering of biologic agents in RA (e.g., STARA [Stopping Anti–Tumor Necrosis Factor Agents in Rheumatoid Arthritis], RA‐BioStop [Ultrasound and Withdrawal of Biological DMARDs in Rheumatoid Arthritis], BioRRA [Biomarkers of Remission in Rheumatoid Arthritis]) 46. We would recommend that the various research groups validate the prognostic value of potential predictors and report the predictive value of all their investigated biomarkers with the appropriate association measures.
In conclusion, we investigated the predictive value of a wide variety of biomarkers for the success of dose reduction or discontinuation of a biologic agent in RA. We identified only 3 biomarkers as predictors, in just 2 studies. The strength of the evidence is limited by the low quality of included studies and the likelihood of reporting bias and multiple testing.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published.
Dr. Tweehuysen had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Tweehuysen, van den Ende, van den Hoogen, den Broeder.
Acquisition of data
Tweehuysen, Beeren, Been.
Analysis and interpretation of data
Tweehuysen, van den Ende, van den Hoogen, den Broeder.
Supporting information
SUPPLEMENTARY TABLE S1. Search strategy
SUPPLEMENTARY TABLE S2. Study characteristics of 16 included studies
SUPPLEMENTARY TABLE S3a. Overview of biomarkers studied multiple times for successful dose reduction of a biologic
SUPPLEMENTARY TABLE S3b. Overview of biomarkers studied multiple times for successful discontinuation of a biologic
SUPPLEMENTARY TABLE S4a. Overview of biomarkers studied once for successful dose reduction of a biologic
SUPPLEMENTARY TABLE S4b. Overview of biomarkers studied once for successful discontinuation of a biologic
SUPPLEMENTARY TABLE S5. The Quality In Prognosis studies (QUIPS) tool: risk of bias ratings for each domain of bias
ACKNOWLEDGMENT
We would like to thank J. E. Vriezekolk for her contribution in compiling the search strategy.
Dr. van den Hoogen has received personal fees from Biogen, Celltrion, Janssen, and Sandoz (less than $10,000 each).
REFERENCES
- 1. Combe B, Landewe R, Lukas C, Bolosiu HD, Breedveld F, Dougados M, et al. EULAR recommendations for the management of early arthritis: report of a task force of the European Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis 2007;66:34–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Den Broeder AA, van der Maas A, van den Bemt BJ. Dose de‐escalation strategies and role of therapeutic drug monitoring of biologics in RA. Rheumatology (Oxford) 2010;49:1801–3. [DOI] [PubMed] [Google Scholar]
- 3. Singh JA, Cameron C, Noorbaloochi S, Cullis T, Tucker M, Christensen R, et al. Risk of serious infection in biological treatment of patients with rheumatoid arthritis: a systematic review and meta‐analysis [review]. Lancet 2015;386:258–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Van Herwaarden N, den Broeder AA, Jacobs W, van der Maas A, Bijlsma JW, van Vollenhoven RF, et al. Down‐titration and discontinuation strategies of tumor necrosis factor‐blocking agents for rheumatoid arthritis in patients with low disease activity [review]. Cochrane Database Syst Rev 2014;9:CD010455. [DOI] [PubMed] [Google Scholar]
- 5. Biomarkers Definitions Working Group . Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 2001;69:89–95. [DOI] [PubMed] [Google Scholar]
- 6. Van den Broek M, Visser K, Allaart CF, Huizinga TW. Personalized medicine: predicting responses to therapy in patients with RA [review]. Curr Opin Pharmacol 2013;13:463–9. [DOI] [PubMed] [Google Scholar]
- 7. Nagy G, van Vollenhoven RF. Sustained biologic‐free and drug‐free remission in rheumatoid arthritis, where are we now? [review]. Arthritis Res Ther 2015;17:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schett G, Emery P, Tanaka Y, Burmester G, Pisetsky DS, Naredo E, et al. Tapering biologic and conventional DMARD therapy in rheumatoid arthritis: current evidence and future directions [review]. Ann Rheum Dis 2016;75:1428–37. [DOI] [PubMed] [Google Scholar]
- 9. Tanaka Y, Hirata S, Saleem B, Emery P. Discontinuation of biologics in patients with rheumatoid arthritis [review]. Clin Exp Rheumatol 2013;31 Suppl 78:S22–7. [PubMed] [Google Scholar]
- 10. Geersing GJ, Bouwmeester W, Zuithoff P, Spijker R, Leeflang M, Moons KG. Search filters for finding prognostic and diagnostic prediction studies in Medline to enhance systematic reviews. PLoS One 2012;7:e32844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009;62:e1–34. [DOI] [PubMed] [Google Scholar]
- 12. Hayden JA, Cote P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med 2006;144:427–37. [DOI] [PubMed] [Google Scholar]
- 13. Hayden JA, van der Windt DA, Cartwright JL, Cote P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med 2013;158:280–6. [DOI] [PubMed] [Google Scholar]
- 14. Van der Windt DA, Thomas E, Pope DP, de Winter AF, Macfarlane GJ, Bouter LM, et al. Occupational risk factors for shoulder pain: a systematic review [review]. Occup Environ Med 2000;57:433–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cuppen BV, Welsing PM, Sprengers JJ, Bijlsma JW, Marijnissen AC, van Laar JM, et al. Personalized biological treatment for rheumatoid arthritis: a systematic review with a focus on clinical applicability [review]. Rheumatology (Oxford) 2016;55:826–39. [DOI] [PubMed] [Google Scholar]
- 16. Vriezekolk JE, van Lankveld WG, Geenen R, van den Ende CH. Longitudinal association between coping and psychological distress in rheumatoid arthritis: a systematic review [review]. Ann Rheum Dis 2011;70:1243–50. [DOI] [PubMed] [Google Scholar]
- 17. Zwikker HE, van den Bemt BJ, Vriezekolk JE, van den Ende CH, van Dulmen S. Psychosocial predictors of non‐adherence to chronic medication: systematic review of longitudinal studies [review]. Patient Prefer Adherence 2014;8:519–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Allaart CF, Lems WF, Huizinga TW. The BeSt way of withdrawing biologic agents. Clin Exp Rheumatol 2013;31 Suppl 78:S14–8. [PubMed] [Google Scholar]
- 19. Klarenbeek NB, van der Kooij SM, Guler‐Yuksel M, van Groenendael JH, Han KH, Kerstens PJ, et al. Discontinuing treatment in patients with rheumatoid arthritis in sustained clinical remission: exploratory analyses from the BeSt study. Ann Rheum Dis 2011;70:315–9. [DOI] [PubMed] [Google Scholar]
- 20. Van den Broek M, Lems WF, Allaart CF. BeSt practice: the success of early‐targeted treatment in rheumatoid arthritis. Clin Exp Rheumatol 2012;30 Suppl 73:S35–8. [PubMed] [Google Scholar]
- 21. Van der Kooij SM, Goekoop‐Ruiterman YP, de Vries‐Bouwstra JK, Guler‐Yuksel M, Zwinderman AH, Kerstens PJ, et al. Drug‐free remission, functioning and radiographic damage after 4 years of response‐driven treatment in patients with recent‐onset rheumatoid arthritis. Ann Rheum Dis 2009;68:914–21. [DOI] [PubMed] [Google Scholar]
- 22. Van den Broek M, Klarenbeek NB, Dirven L, van Schaardenburg D, Hulsmans HM, Kerstens PJ, et al. Discontinuation of infliximab and potential predictors of persistent low disease activity in patients with early rheumatoid arthritis and disease activity score‐steered therapy: subanalysis of the BeSt study. Ann Rheum Dis 2011;70:1389–94. [DOI] [PubMed] [Google Scholar]
- 23. Hirata S, Saito K, Kubo S, Fukuyo S, Mizuno Y, Iwata S, et al. Discontinuation of adalimumab after attaining disease activity score 28‐erythrocyte sedimentation rate remission in patients with rheumatoid arthritis (HONOR study): an observational study. Arthritis Res Ther 2013;15:R135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tanaka Y, Hirata S, Kubo S, Fukuyo S, Hanami K, Sawamukai N, et al. Discontinuation of adalimumab after achieving remission in patients with established rheumatoid arthritis: 1‐year outcome of the HONOR study [published erratum appears in Ann Rheum Dis 2016;75:e46]. Ann Rheum Dis 2015;74:389–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Van der Maas A, Kievit W, van den Bemt BJ, van den Hoogen FH, van Riel PL, den Broeder AA. Down‐titration and discontinuation of infliximab in rheumatoid arthritis patients with stable low disease activity and stable treatment: an observational cohort study. Ann Rheum Dis 2012;71:1849–54. [DOI] [PubMed] [Google Scholar]
- 26. Marks JL, Holroyd CR, Dimitrov BD, Armstrong RD, Calogeras A, Cooper C, et al. Does combined clinical and ultrasound assessment allow selection of individuals with rheumatoid arthritis for sustained reduction of anti–tumor necrosis factor therapy? Arthritis Care Res (Hoboken) 2015;67:746–53. [DOI] [PubMed] [Google Scholar]
- 27. Van Herwaarden N, van der Maas A, Minten MJ, van den Hoogen FH, Kievit W, van Vollenhoven RF, et al. Disease activity guided dose reduction and withdrawal of adalimumab or etanercept compared with usual care in rheumatoid arthritis: open label, randomised controlled, non‐inferiority trial. BMJ 2015;350:h1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Van Herwaarden N, Bouman CA, van der Maas A, van Vollenhoven RF, Bijlsma JW, van den Hoogen FH, et al. Adalimumab and etanercept serum (anti)drug levels are not predictive for successful dose reduction or discontinuation in rheumatoid arthritis. Ann Rheum Dis 2015;74:2260–1. [DOI] [PubMed] [Google Scholar]
- 29. Chen DY, Chen YM, Hsieh TY, Hung WT, Hsieh CW, Chen HH, et al. Drug trough levels predict therapeutic responses to dose reduction of adalimumab for rheumatoid arthritis patients during 24 weeks of follow‐up. Rheumatology (Oxford) 2016;55:143–8. [DOI] [PubMed] [Google Scholar]
- 30. Fautrel B, Pham T, Alfaiate T, Gandjbakhch F, Foltz V, Morel J, et al. Step‐down strategy of spacing TNF‐blocker injections for established rheumatoid arthritis in remission: results of the multicentre non‐inferiority randomised open‐label controlled trial (STRASS: Spacing of TNF‐blocker injections in Rheumatoid ArthritiS Study). Ann Rheum Dis 2016;75:59–67. [DOI] [PubMed] [Google Scholar]
- 31. Naredo E, Valor L, de la Torre I, Montoro M, Bello N, Martinez‐Barrio J, et al. Predictive value of Doppler ultrasound‐detected synovitis in relation to failed tapering of biologic therapy in patients with rheumatoid arthritis. Rheumatology (Oxford) 2015;54:1408–14. [DOI] [PubMed] [Google Scholar]
- 32. Aguilar‐Lozano L, Castillo‐Ortiz JD, Vargas‐Serafin C, Morales‐Torres J, Sanchez‐Ortiz A, Sandoval‐Castro C, et al. Sustained clinical remission and rate of relapse after tocilizumab withdrawal in patients with rheumatoid arthritis. J Rheumatol 2013;40:1069–73. [DOI] [PubMed] [Google Scholar]
- 33. Iwamoto T, Ikeda K, Hosokawa J, Yamagata M, Tanaka S, Norimoto A, et al. Prediction of relapse after discontinuation of biologic agents by ultrasonographic assessment in patients with rheumatoid arthritis in clinical remission: high predictive values of total gray‐scale and power Doppler scores that represent residual synovial inflammation before discontinuation. Arthritis Care Res (Hoboken) 2014;66:1576–81. [DOI] [PubMed] [Google Scholar]
- 34. Nishimoto N, Amano K, Hirabayashi Y, Horiuchi T, Ishii T, Iwahashi M, et al. Drug free REmission/low disease activity after cessation of tocilizumab (Actemra) Monotherapy (DREAM) study. Mod Rheumatol 2014;24:17–25. [DOI] [PubMed] [Google Scholar]
- 35. Saleem B, Keen H, Goeb V, Parmar R, Nizam S, Hensor EM, et al. Patients with RA in remission on TNF blockers: when and in whom can TNF blocker therapy be stopped? Ann Rheum Dis 2010;69:1636–42. [DOI] [PubMed] [Google Scholar]
- 36. Takeuchi T, Matsubara T, Ohta S, Mukai M, Amano K, Tohma S, et al. Biologic‐free remission of established rheumatoid arthritis after discontinuation of abatacept: a prospective, multicentre, observational study in Japan. Rheumatology (Oxford) 2015;54:683–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tanaka Y, Takeuchi T, Mimori T, Saito K, Nawata M, Kameda H, et al. Discontinuation of infliximab after attaining low disease activity in patients with rheumatoid arthritis: RRR (remission induction by Remicade in RA) study. Ann Rheum Dis 2010;69:1286–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brocq O, Millasseau E, Albert C, Grisot C, Flory P, Roux CH, et al. Effect of discontinuing TNFα antagonist therapy in patients with remission of rheumatoid arthritis. Joint Bone Spine 2009;76:350–5. [DOI] [PubMed] [Google Scholar]
- 39. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–74. [PubMed] [Google Scholar]
- 40. Bouman CA, den Broeder AA. Letter in response to the article of Chen et al: Drug trough levels predict therapeutic responses to dose reduction of adalimumab for rheumatoid arthritis patients during 24 weeks of follow‐up [eLetter]. Rheumatology (Oxford) January 21, 2016. URL: http://rheumatology.oxfordjournals.org/content/55/1/143/reply#rheumatology_el_218. [DOI] [PubMed] [Google Scholar]
- 41. Yoshida K, Sung YK, Kavanaugh A, Bae SC, Weinblatt ME, Kishimoto M, et al. Biologic discontinuation studies: a systematic review of methods [review]. Ann Rheum Dis 2014;73:595–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Van der Maas A, Lie E, Christensen R, Choy E, de Man YA, van Riel P, et al. Construct and criterion validity of several proposed DAS28‐based rheumatoid arthritis flare criteria: an OMERACT cohort validation study. Ann Rheum Dis 2013;72:1800–5. [DOI] [PubMed] [Google Scholar]
- 43. Bartlett SJ, Bykerk VP, Cooksey R, Choy EH, Alten R, Christensen R, et al. Feasibility and domain validation of rheumatoid arthritis (RA) flare core domain set: report of the OMERACT 2014 RA Flare Group Plenary. J Rheumatol 2015;42:2185–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig L, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ 2015;351:h5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ioannidis JP. Why most published research findings are false. PLoS Medicine 2005;2:e214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.ClinicalTrials.gov web site. URL: www.clinicaltrials.gov.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTARY TABLE S1. Search strategy
SUPPLEMENTARY TABLE S2. Study characteristics of 16 included studies
SUPPLEMENTARY TABLE S3a. Overview of biomarkers studied multiple times for successful dose reduction of a biologic
SUPPLEMENTARY TABLE S3b. Overview of biomarkers studied multiple times for successful discontinuation of a biologic
SUPPLEMENTARY TABLE S4a. Overview of biomarkers studied once for successful dose reduction of a biologic
SUPPLEMENTARY TABLE S4b. Overview of biomarkers studied once for successful discontinuation of a biologic
SUPPLEMENTARY TABLE S5. The Quality In Prognosis studies (QUIPS) tool: risk of bias ratings for each domain of bias


