Abstract
Aim
To evaluate the pharmacological characteristics of faster‐acting insulin aspart (faster aspart) compared with insulin aspart (IAsp) during continuous subcutaneous insulin infusion (CSII).
Methods
In this randomized, double‐blind, crossover trial, 48 men and women aged 18 to 64 years with type 1 diabetes mellitus (T1DM) received faster aspart and IAsp as a 0.15 U/kg bolus dose via CSII, on top of a basal rate (0.02 U/kg/h), in a glucose clamp setting (target 5.5 mmol/L).
Results
After a CSII bolus dose, the pharmacokinetic/pharmacodynamic profiles for faster aspart were left‐shifted compared with those for IAsp. For faster aspart vs IAsp, the early glucose‐lowering effect (area under the curve for glucose infusion rate [GIR]0‐30min) was approximately 2‐fold higher (least squares means 24.9 vs 11.4 mg/kg; estimated ratio faster aspart/IAsp 2.18, 95% confidence interval [CI] [1.33; 5.04]; P = .002), onset of glucose‐lowering effect (time to early 50% of maximum GIR) occurred 11.1 minutes earlier (41.1 vs 52.3 minutes; 95% CI faster aspart – IAsp [−15.4; −6.9]; P<.001), and offset of glucose‐lowering effect (time to late 50% of maximum GIR) occurred 24.0 minutes earlier (214.7 vs 238.7 minutes; 95% CI [−38.9; −9.1]; P=.002). Likewise, significantly greater early exposure and significantly earlier onset and offset of exposure were observed for faster aspart vs IAsp. Faster aspart and IAsp were both well tolerated.
Conclusions
In patients with T1DM using CSII, faster aspart better mimics the endogenous prandial insulin secretion and action than does IAsp. Faster aspart therefore has the potential to provide clinical benefits over current rapid‐acting insulins in the insulin pump setting.
Keywords: insulin pump therapy, pharmacodynamics, pharmacokinetics, type 1 diabetes
1. INTRODUCTION
Postprandial glycaemic control plays a substantial role in reaching recommended glycated haemoglobin (HbA1c) goals in diabetes.1 Compared with regular human insulin (RHI), rapid‐acting insulin analogues (insulin aspart, insulin lispro and insulin glulisine) have provided better postprandial glucose control through an earlier and greater peak glucose‐lowering effect.2, 3 Nevertheless, absorption of current rapid‐acting insulins occurs too slowly to adequately replicate endogenous prandial insulin action.4, 5 Consequently, optimum postprandial glucose control remains a challenge in patients with diabetes.
Continuous subcutaneous insulin infusion (CSII) is increasingly used in people with diabetes.6 CSII therapy is associated with improved glycaemic control, lower risk of hypoglycaemia and greater patient convenience compared with multiple daily injection therapy.6, 7, 8 An insulin with a fast onset and fast offset of glucose‐lowering effect might be particularly important in a CSII setting to further improve postprandial glucose control without the risk of late post‐meal hypoglycaemia, and could also be a key factor in improving the performance of closed‐loop, artificial pancreas systems.9
Faster‐acting insulin aspart (faster aspart) is insulin aspart in a new formulation that contains 2 well‐known excipients, niacinamide and L‐arginine. These are both listed in the US Food and Drug Administration inactive ingredient database, in products for injection, at higher concentrations than used in faster aspart.10 With faster aspart, niacinamide is responsible for faster initial absorption after subcutaneous administration and L‐arginine serves as a stabilizing agent. In subjects with type 1 diabetes mellitus (T1DM), faster aspart administered by subcutaneous injection had a twice‐as‐fast onset of appearance, a 2‐fold higher early exposure, and >50% greater early glucose‐lowering effect compared with insulin aspart.11
Current rapid‐acting insulin analogues showed significant improvements over RHI in the pump setting with respect to glycaemic control and reduced hypoglycaemia.12, 13 Likewise, faster aspart may provide benefits over current rapid‐acting insulin analogues when used in a CSII regimen. In the present trial we evaluated, for the first time, the pharmacokinetic and pharmacodynamic properties of faster aspart compared with insulin aspart, using CSII in a euglycaemic clamp setting in subjects with T1DM.
2. MATERIALS AND METHODS
2.1. Study design
This was a randomized, single‐centre (Profil, Neuss, Germany), double‐blind, two‐period, crossover trial in people with T1DM. The trial protocol was reviewed and approved by the local health authority (Bundesinstitut für Arzneimittel und Medizinprodukte) and by an independent ethics committee (Ärztekammer Nordrhein). The trial was performed in accordance with the Declaration of Helsinki and Good Clinical Practice, and registered at ClinicalTrials.gov (NCT01992588).
2.2. Participants
Eligible participants were men and women aged 18 to 64 years, who had been diagnosed with T1DM for ≥12 months and treated with multiple daily insulin injections or CSII for ≥12 months (total daily insulin dose <1.2 (I)U/kg/d and total daily bolus insulin dose <0.7 (I)U/kg/d), with HbA1c ≤8.5%, body mass index (BMI) 18.5 to 28.0 kg/m2, and fasting C‐peptide ≤0.3 nmol/L. Individuals were excluded if they had clinically significant concomitant diseases, abnormal values in clinical laboratory screening tests, were smokers, or were currently treated with drug(s) that may interfere with glucose metabolism. Written informed consent was obtained before initiation of any trial‐related activity.
2.3. Procedures
The trial consisted of a screening visit, 2 dosing visits separated by 3 to 12 days wash‐out, and a follow‐up visit.
Participants were randomized (1:1) to receive faster aspart (100 U/mL; 3 mL Penfill; Novo Nordisk, Bagsværd, Denmark) followed by insulin aspart (NovoRapid; 100 U/mL; 3 mL Penfill; Novo Nordisk) or vice versa. The trial was double‐blind and the computer‐generated randomization scheme was prepared by Clinical Supplies Coordination, Novo Nordisk A/S (Måløv, Denmark). Based on the randomization scheme, trial products were packed, in a double‐blind manner, in boxes specific for each randomization number before delivery to the clinical site.
The trial products were administered by CSII using a MiniMed Paradigm Veo™754 insulin pump with a Paradigm Reservoir 3.0 mL and a Quick‐set infusion set with a 6‐mm cannula and a 23‐inch tube (Medtronic MiniMed, Northridge, California). The cannula was inserted subcutaneously in the region of the lower abdominal wall above the inguinal area. One infusion site was used throughout the first dosing visit, while another infusion site within the same region was used throughout the second dosing visit.
At the dosing visits, participants came to the clinical site at 5:00 pm, after fasting since 10:00 am (water and ≤20 g of rapidly absorbable carbohydrate to prevent hypoglycaemia were allowed). Participants using multiple daily insulin injections were not allowed to use insulin degludec ≤72 hours pre‐dose and insulin detemir or insulin glargine ≤48 hours pre‐dose (NPH insulin could be used instead). The last injection of NPH insulin or other intermediate‐acting insulin had to occur at least 22 hours pre‐dose, the last injection or bolus administration by CSII of insulin aspart had to occur at least 12 hours pre‐dose (RHI could be used instead) and the last injection of RHI or other short‐acting insulin (other than insulin aspart) had to occur at least 6 hours pre‐dose. Participants using CSII had to discontinue the basal rate at least 3 hours pre‐dose (or at least 8 hours pre‐dose if using insulin aspart).
Participants were excluded from the dosing visit if they had experienced hypoglycaemia (plasma glucose ≤ 3.9 mmol/L) ≤24 hours pre‐dose.
At 7:00 pm, participants received faster aspart or insulin aspart at an initial priming dose of 0.08 U/kg, followed by a basal rate of 0.02 U/kg/h for 27 hours, on top of which a single bolus dose of 0.15 U/kg was given after 13 hours (i.e. after 22 hours of fasting; Figure S1, Appendix S1). The duration of bolus infusion administered by the insulin pump was 3.7 to 8.3 minutes, depending on absolute dose.
A euglycaemic clamp procedure was initiated 30 minutes before the priming dose using ClampArt (Profil). Participants received a variable intravenous infusion of RHI (15 IU Actrapid [100 IU/mL; Novo Nordisk] in 49 mL saline and 1 mL of the participant's blood) or 20% glucose to achieve the blood glucose clamp target level of 5.5 mmol/L. The intravenous RHI infusion (if any) was only allowed until 8 hours before the bolus dose of trial product. The clamp continued for 14 hours after the bolus dose. The quality of the conducted clamps is shown in Figure S2, Appendix S1.14
Blood samples for pharmacokinetic assessment were drawn frequently at prespecified time points from before the insulin priming dose until the end of the CSII (27 hours after the priming dose).
2.4. Assessments and endpoints
Free serum insulin aspart concentrations (polyethylene glycol‐precipitated) were measured using a validated insulin aspart specific enzyme‐linked immunosorbent assay. The intravenous glucose infusion rate (GIR) needed to keep blood glucose at the clamp target level was recorded every minute during the glucose clamp. Safety assessments included adverse events, local tolerability at the infusion site, hypoglycaemic episodes (defined as “confirmed” when they were either “severe” as according to the American Diabetes Association, i.e. requiring third party assistance,15 or verified by a plasma glucose level of <3.1 mmol/L), laboratory safety variables, physical examination, vital signs and ECG.
Endpoints to evaluate onset of exposure and glucose‐lowering effect included: time to early 50% of maximum insulin concentration (tEarly 50% Cmax); time to maximum insulin concentration (tmax); time to early 50% of maximum GIR (tEarly 50% GIRmax); and time to maximum GIR (tGIRmax). Endpoints to evaluate early exposure and glucose‐lowering effect included the early partial areas under the curve (AUCs) for serum insulin aspart [AUCIAsp,0‐15min, AUCIAsp,0‐30min (primary endpoint), AUCIAsp,0‐1h, AUCIAsp,0‐1.5h, and AUCIAsp,0‐2h] and the early partial AUCs for GIR [AUCGIR,0‐30min, AUCGIR,0‐1h, AUCGIR,0‐1.5h, and AUCGIR,0‐2h]. Endpoints to evaluate offset of exposure and glucose‐lowering effect were time to late 50% of maximum insulin concentration (tLate 50% Cmax) and time to late 50% of maximum GIR (tLate 50% GIRmax; both derived post hoc). Endpoints to evaluate overall exposure and glucose‐lowering effect were total insulin exposure (AUCIAsp,0‐t), maximum insulin concentration (Cmax), total glucose‐lowering effect (AUCGIR,0‐t), and maximum GIR (GIRmax). All endpoints were corrected for the basal insulin infusion in order to obtain values related solely to the bolus insulin dose. AUCinsulin aspart,0‐t and AUCGIR,0‐t were derived from time zero until the first non‐positive baseline infusion corrected insulin aspart concentration or smoothed GIR, respectively, in the terminal part of the profile (however, no longer than 12 hours). Endpoints were derived from the raw profiles except for tEarly 50% GIRmax, GIRmax, tGIRmax and tLate 50% GIRmax, which were derived from LOESS smoothed GIR profiles (using a smoothing factor of 0.1) to ensure robust calculation.
2.5. Statistical analysis
Statistical analyses of pharmacokinetic and pharmacodynamic endpoints were conducted using sas version 9.3 (SAS Institute, Cary, North Carolina) based on all randomized participants receiving at least 1 dose of trial product. Safety endpoints were summarized using descriptive statistics based on all participants receiving at least 1 dose of trial product.
To ensure sufficient power to evaluate treatment differences, both for pharmacokinetic and pharmacodynamic endpoints, the sample size calculation was performed not only for the primary pharmacokinetic endpoint, AUCIAsp,0‐30min, but also for the secondary pharmacodynamic endpoint, AUCGIR,0‐1h. The number of completers required was 31 in order to obtain 80% power for detecting a geometric mean treatment ratio of 1.5 for AUCIAsp,0‐30min. This was based on an assumed within‐subject standard deviation (on log‐scale) of 0.54 (derived from a previous trial with faster aspart11 and taking into account that the baseline correction of AUCs in this trial contributed further to the variation) and a significance level of 5%; however, in order to also obtain sufficient power for pharmacodynamic endpoints, the number of completers required in this trial was set to 44 participants. This yielded 80% power for detecting a geometric mean treatment ratio of 1.3 for AUCGIR,0‐1h, based on an assumed within‐subject standard deviation (on log‐scale) of 0.3 and a significance level of 5%.11
Pharmacokinetic and pharmacodynamic endpoints were analysed by means of a linear mixed model, with treatment and period as fixed effects and participant as random effect. For the AUCs, a multiplicative linear mixed model was used if all individual AUC values were strictly positive. If there were non‐positive individual AUC values, an additive model was used. For Cmax and GIRmax, a multiplicative model was used, while for tEarly 50% Cmax, tmax, tLate 50% Cmax, tEarly 50% GIRmax, tGIRmax and tLate 50% GIRmax, an additive model was used. For endpoints analysed using an additive model, treatment ratios and 95% confidence intervals (CIs) were calculated using Fieller's method.16
3. RESULTS
3.1. Participant disposition and baseline characteristics
A total of 58 individuals were screened, 48 were randomized and treated with trial product, and 46 completed the trial. Two participants were withdrawn (1 as a result of an adverse event of vomiting after insulin aspart treatment and 1 because of a technical problem with a catheter). Participant disposition is presented in Figure S3, Appendix S1.
The mean (± standard deviation [s.d.]) age of the participants was 46.3 (±8.6) years. The majority of participants were men (66.7%), and all participants were white. The mean body weight was 76.5 (±11.8) kg, mean BMI was 24.5 (±2.3) kg/m2, mean duration of diabetes was 24.1 (±12.2) years, and mean HbA1c at baseline was 7.4% (±0.6%). At entry into the study, 34 participants were receiving multiple daily injection therapy and 14 participants were using CSII.
3.2. Onset of exposure and glucose‐lowering effect
After a bolus dose administered by CSII, both the serum insulin concentration–time profile (Figure 1) and the glucose‐lowering effect profile (Figure 2) were shifted to the left for faster aspart vs insulin aspart. With faster aspart, tEarly 50% Cmax was 11.8 minutes earlier, and tmax was 25.7 minutes earlier, compared with insulin aspart (Table 1 and Figure 1). Likewise, tEarly 50% GIRmax was 11.1 minutes earlier, and tGIRmax was 18.7 minutes earlier for faster aspart vs insulin aspart (Table 2).
Figure 1.
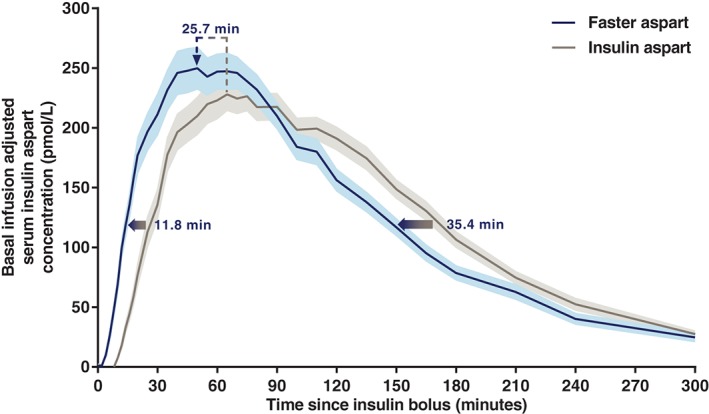
Mean serum insulin aspart concentration after a bolus dose of 0.15 U/kg faster aspart or insulin aspart administered by CSII. Variability bands show the s.e.m. The full blue/grey arrows indicate that the estimated onset and offset of exposure occurred earlier for faster aspart vs insulin aspart as reflected by the endpoints time to 50% of maximum insulin aspart concentration in the early part of the pharmacokinetic profile (tEarly 50% Cmax; estimated difference [95% CI] faster aspart – insulin aspart −11.8 minutes [−14.4; −9.2]; P < .001) and time to 50% of maximum insulin aspart concentration in the late part of the pharmacokinetic profile (tLate 50% Cmax; −35.4 minutes [−47.0; −23.8]; P < .001). Moreover, as indicated by the dashed arrow, a left shift of the time of maximum insulin aspart concentration was also observed for faster aspart vs insulin aspart (tmax; −25.7 minutes [−34.3; −17.1]; P < .001). For graphical reasons, there are some discrepancies between the length of the arrows and the actual estimated mean treatment differences. This is attributable to the fact that the estimated mean treatment differences are derived from all participants’ individual treatment differences, while the mean serum insulin aspart concentration profiles are derived as the mean of all individual serum insulin aspart concentrations at each time point. Faster aspart, n = 44; insulin aspart, n = 46.
Figure 2.
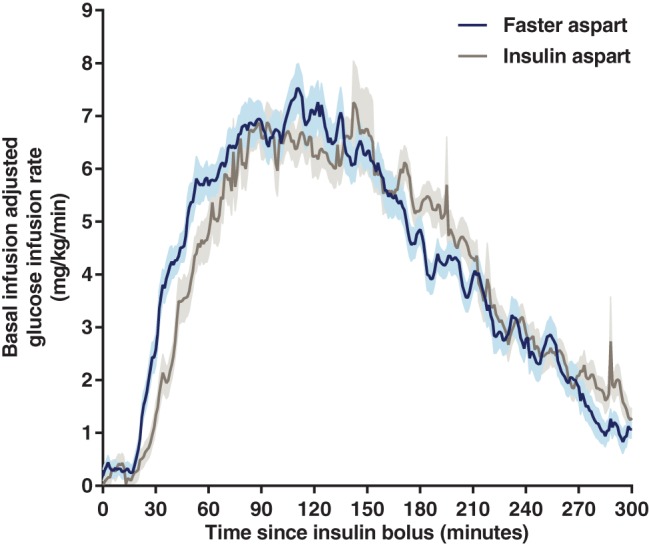
Mean glucose‐lowering effect after a bolus dose of 0.15 U/kg faster aspart or insulin aspart administered by CSII. Variability bands show the s.e.m. Faster aspart, n = 44; insulin aspart, n = 46.
Table 1.
Onset, offset and overall exposure for faster aspart (n = 44) and insulin aspart (n = 46) after a bolus dose of 0.15 U/kg administered by CSII
| Faster aspart 1 | Insulin aspart 1 | Treatment ratio 2 (95% CI) | Treatment difference 3 (95% CI) | P | |
|---|---|---|---|---|---|
| Onset of exposure, min | |||||
| tEarly 50% Cmax | 20.7 | 32.5 | 0.64 (0.57; 0.71) | −11.8 (−14.4; −9.2) | <.001 |
| tmax | 56.6 | 82.3 | 0.69 (0.60; 0.78) | −25.7 (−34.3; −17.1) | <.001 |
| Offset of exposure, min | |||||
| tLate 50% Cmax | 137.4 | 172.9 | 0.80 (0.73; 0.86) | −35.4 (−47.0; −23.8) | <.001 |
| Overall exposure | |||||
| AUCIAsp,0‐t, pmol*h/L | 606.2 | 622.8 | 0.97 (0.90; 1.05) | NA | .477 |
| Cmax, pmol/L | 278.9 | 252.1 | 1.11 (1.03; 1.19) | NA | .010 |
NA, not applicable; t, time of first non‐positive baseline infusion corrected insulin aspart concentration in the terminal part of the profile (however, no longer than 12 hours).
Data are least squares means.
Faster aspart/insulin aspart (for onset and offset of exposure endpoints, the treatment ratio was calculated using Fieller's method).
Faster aspart – insulin aspart.
Table 2.
Onset, offset and overall glucose‐lowering effect for faster aspart (n = 44) and insulin aspart (n = 45) after a bolus dose of 0.15 U/kg administered by CSII
| Faster aspart 1 | Insulin aspart 1 | Treatment ratio 2 (95% CI) | Treatment difference 3 (95% CI) | P | |
|---|---|---|---|---|---|
| Onset of glucose‐lowering effect, min | |||||
| tEarly 50% GIRmax | 41.1 | 52.3 | 0.79 (0.72; 0.86) | −11.1 (−15.4; −6.9) | <.001 |
| tGIRmax | 111.9 | 130.6 | 0.86 (0.75; 0.97) | −18.7 (−34.4; −2.9) | .021 |
| Offset of glucose‐lowering effect, min | |||||
| tLate 50% GIRmax | 214.7 | 238.7 | 0.90 (0.84; 0.96) | −24.0 (−38.9; −9.1) | .002 |
| Overall glucose‐lowering effect | |||||
| AUCGIR,0‐t, mg/kg | 1341.5 | 1295.7 | 1.04 (0.95; 1.13) | NA | .427 |
| GIRmax, mg/kg/min | 7.1 | 6.8 | 1.04 (0.94; 1.14) | NA | .439 |
NA, not applicable; t, time of first non‐positive baseline infusion corrected GIR in the terminal part of the smoothed GIR profile (however, no longer than 12 hours).
Data are least squares means.
Faster aspart/insulin aspart (for onset and offset of glucose‐lowering effect endpoints, the treatment ratio was calculated using Fieller's method).
Faster aspart – insulin aspart.
3.3. Early exposure and glucose‐lowering effect
Early insulin exposure and glucose‐lowering effect were both greater for faster aspart than for insulin aspart, as shown by the significantly greater partial AUCs and GIR AUCs for faster aspart vs insulin aspart within the first 2 hours of the bolus insulin dose (Figure 3). Early exposure during the first 15 minutes after the bolus dose (AUCIAsp,0‐15min) was significantly greater for faster aspart vs insulin aspart (least squares means of 12.5 and 1.8 pmol*h/L, respectively; P < .001). Within the first 30 minutes after the bolus dose, a ~3‐fold higher insulin exposure (AUCIAsp,0‐30min; primary endpoint) and a ~2‐fold greater glucose‐lowering effect (AUCGIR,0‐30min) were seen with faster aspart than with insulin aspart (Figure 3).
Figure 3.
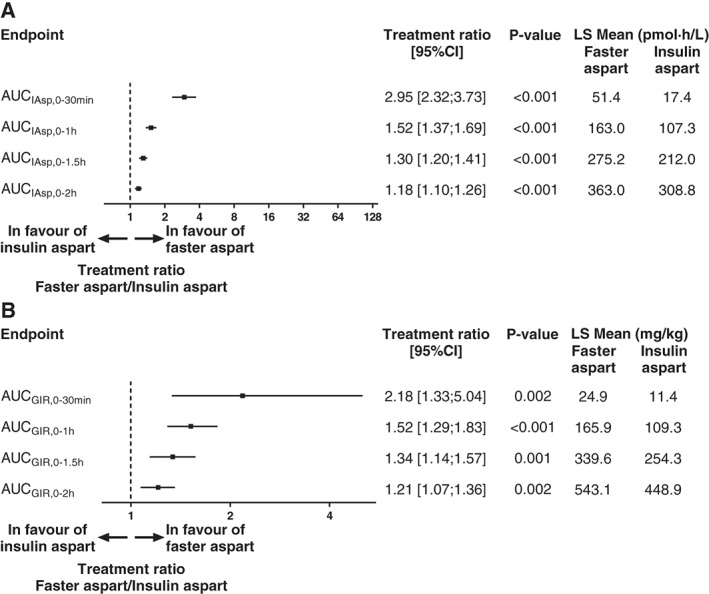
Early exposure (A) and early glucose‐lowering effect (B) for faster aspart vs insulin aspart after a bolus dose of 0.15 U/kg administered by CSII. n = 44 for faster aspart and n = 45 (AUCGIR ,0‐1.5h and AUCGIR ,0‐2h) or 46 (all other endpoints) for insulin aspart. The treatment ratio for AUCGIR ,0‐30min and AUCGIR ,0‐1h was calculated using Fieller's method. LS, least squares.
3.4. Offset of exposure and glucose‐lowering effect
The offset of insulin exposure after a bolus dose administered by CSII occurred earlier for faster aspart than for insulin aspart, as shown by a 35.4 minutes earlier tLate 50% Cmax for faster aspart vs insulin aspart (Table 1 and Figure 1). Likewise, the offset of glucose‐lowering effect occurred faster for faster aspart than for insulin aspart, as shown by a 24.0 minutes earlier tLate 50% GIRmax for faster aspart vs insulin aspart (Table 2).
3.5. Overall exposure and glucose‐lowering effect
The total insulin exposure (AUCIAsp,0‐t) was similar for faster aspart and insulin aspart, while the Cmax was slightly greater for faster aspart than for insulin aspart (Table 1). The total glucose‐lowering effect (AUCGIR,0‐t) and the GIRmax were both similar for faster aspart and insulin aspart (Table 2).
3.6. Safety
Both faster aspart and insulin aspart were well tolerated, and no safety issues were identified during the trial. No infusion site reactions were reported when administering faster aspart or insulin aspart by CSII. No confirmed hypoglycaemic episodes occurred during the trial.
4. DISCUSSION
The key findings of the present study, which is the first to investigate the pharmacokinetic and pharmacodynamic properties of faster aspart in a pump setting, were that in individuals with T1DM, faster aspart provided faster onset, greater early exposure and early glucose‐lowering effect and faster offset compared with insulin aspart. In addition, faster aspart was well tolerated. Faster aspart may therefore have the potential to provide improved postprandial glycaemic control over current rapid‐acting insulins in a pump setting. Indeed, in a double‐blind, randomized, crossover study in 43 individuals with T1DM using CSII for 14 days, faster aspart provided a 25% greater plasma glucose‐lowering effect during the first 2 hours of a standardized meal test compared with insulin aspart.17 Furthermore, the mean postprandial increment in interstitial glucose across all meals over 14 days, measured by blinded continuous glucose monitoring in the outpatient setting, was ~50% lower for faster aspart than for insulin aspart, both at 60 and 120 minutes post‐meal.17 Reduction of postprandial glucose excursions may play an important role in improving overall glycaemic control, especially at lower HbA1c levels as suggested by some studies.18, 19 Although a prominent contribution of postprandial glucose to HbA1c levels was not confirmed by a later randomized controlled trial,20 all studies show that postprandial glucose has some impact on HbA1c. This was also confirmed by an improvement in HbA1c of 0.15% with faster aspart compared with insulin aspart in individuals with T1DM using multiple daily injection therapy.21 It remains to be seen if further improvements can be achieved with faster aspart in a CSII setting as the current pharmacokinetic and pharmacodynamic data suggest.
In healthy individuals, early endogenous insulin secretion in response to a meal induces a prompt suppression of hepatic glucose production, which appears to play a major role in controlling postprandial glucose levels. Accordingly, the concept of CSII in patients with diabetes relies on immediate action of the mealtime insulin administered. Current rapid‐acting insulin analogues have shown some improvements over RHI in terms of accelerated insulin absorption.2, 3 There is, however, still room for further improvement in mealtime insulin absorption rate.22, 23 The results of the present trial, as well as those of previous trials, suggest that faster aspart may better reproduce the physiological insulin action profile seen in response to a meal in healthy individuals.11, 24
Another aim when optimizing insulin for use in CSII has been to increase the rate of offset, in order to reduce the risk of late postprandial hypoglycaemia.5, 9 The duration of mealtime insulin requirement depends on several individual factors, such as food composition and rate of gastric emptying, and there is a limit to the optimum rate of offset because a very fast offset of action could imply insufficient insulin action in the late postprandial period.25 It was shown in the present study that faster aspart had a faster offset of exposure and action compared with insulin aspart. In standardized meal tests following faster aspart or insulin aspart administration by subcutaneous injection or CSII, plasma glucose for faster aspart was consistently lower than or equal to insulin aspart up to 4 to 6 hours post‐meal.17, 24 This indicates that the rate of offset is not too rapid with faster aspart. Specifically designed studies are needed to further investigate the relationship between rate of offset and the risk of late postprandial hypoglycaemia with faster aspart.
When comparing the present results for faster aspart using CSII with those previously reported for subcutaneous injection, it appears that improvements in onset as well as early exposure and action with faster aspart vs insulin aspart are most pronounced in the pump setting.11 Treatment ratios of faster aspart vs insulin aspart for AUCIAsp,0‐30min and AUCGIR,0‐30min were 2.95 and 2.18 in the present study as compared with 2.05 and 1.48 after subcutaneous injection.11 It should be noted that this is a comparison between two different studies and ideally a dedicated study needs to be performed to reach firm conclusions regarding the pharmacokinetic and pharmacodynamic properties of faster aspart relative to insulin aspart after subcutaneous injection vs CSII. One of the excipients in faster aspart, niacinamide, promotes the formation of insulin aspart monomers in diluted formulations mimicking subcutaneous conditions after administration, and augments the permeation rate of insulin aspart in human dermal‐derived microvascular capillary endothelial cell monolayers.26 It may be speculated that the continuous presence of niacinamide at the infusion site in the CSII setting due to the basal infusion rate and/or the duration of the bolus infusion may further facilitate the rapid absorption of faster aspart as compared with the single injection setting where dosing occurs almost instantaneously and niacinamide might disappear relatively quickly from the injection site. Another hypothesis could be that the mode of delivery of the bolus infusion when using CSII favours a better diffusion of faster aspart in the subcutaneous tissue until reaching the capillaries. These potential mechanisms are, however, solely speculative at the moment and further investigations are needed within this area.
Differences in duration of the bolus infusion between different insulin pumps have previously been shown to affect the pharmacokinetic and pharmacodynamic characteristics of mealtime insulin.27 In the present study, the same insulin pump with the same bolus infusion mode was used for the two treatments in a crossover design. Consequently, the duration of bolus infusion was similar for faster aspart and insulin aspart within each participant. Importantly, in the study by Bode et al.,17 reduced postprandial glucose increment was observed with faster aspart compared with insulin aspart across several models of insulin pumps with different speed of bolus delivery.
As a result of the bolus being administered on top of a basal insulin infusion, it was not possible to assess onset of appearance and onset of action in any meaningful way using recognized methodology, which is a limitation of the present study.28 Furthermore, we showed a minor but nevertheless significant difference in Cmax between faster aspart and insulin aspart, which has some impact on the interpretation of tEarly 50% Cmax and tLate 50% Cmax 28; however, as Cmax was highest for faster aspart, it would be expected that tEarly 50% Cmax and tLate 50% Cmax would have been even more in favour of faster aspart if Cmax had been similar between treatments. Furthermore, GIRmax was similar between treatments, so the interpretation of the pharmacodynamic endpoints tEarly 50% GIRmax and tLate 50% GIRmax, which were also in favour of faster aspart, is straightforward.
A strength of the present study is that both pharmacokinetic and pharmacodynamic measures were obtained after a bolus insulin dose on top of a basal infusion, thus resembling a clinically relevant dosing scheme during CSII. Furthermore, the study included individuals with T1DM, which is considered the optimum population in glucose clamp studies, as this allows comparison of exogenous insulins with respect to glucose‐lowering effect without interference from endogenous insulin.29 Nevertheless, the strictly controlled conditions of the glucose clamp method, eg, the long fasting period and the wash‐out of current insulin, differ from clinical practice and may imply certain challenges in translating to clinical outcomes.
In conclusion, in people with T1DM using CSII, faster aspart provides faster onset and greater early exposure and glucose‐lowering effect, as well as faster offset compared with insulin aspart, thereby better mimicking the endogenous prandial insulin secretion and action. Accordingly, faster aspart has been shown to provide improvements in postprandial glucose control over insulin aspart in individuals using CSII. Faster aspart used in an insulin pump setting may represent an advancement in insulin therapy towards optimum postprandial glucose control in patients with diabetes and may also have potential in advanced pumps and closed‐loop systems.
Supporting information
Appendix S1. Experimental design of each dosing visit, glucose clamp quality and participant disposition.
ACKNOWLEDGMENTS
The authors would like to thank Henrik Jarlov, MD, and Marek Demissie, MD, Novo Nordisk, for their review and input to the manuscript and Carsten Roepstorff, PhD, CR Pharma Consult, Copenhagen, Denmark for providing medical writing support, which was funded by Novo Nordisk.
Conflict of interest
T. H.’s institution has received research grants from Adocia, AstraZeneca, Becton Dickinson, Biocon, Boehringer Ingelheim, Dance Biopharm, Eli Lilly, Grünenthal, Gulf Pharmaceutical Industries, Johnson & Johnson, Marvel, MedImmune, Medtronic, Novartis, Novo Nordisk, Roche Diagnostics, Sanofi, Senseonics and Zealand Pharma. In addition, T. H. is a member of advisory boards for Novo Nordisk and has received speakers' honoraria and travel grants from Eli Lilly, Mylan and Novo Nordisk. E. Z. has received travel grants from Novo Nordisk and Dance Biopharm and speakers’ honoraria from Novo Nordisk and Roche Diabetes Care. T. R. and H. H. are employees and shareholders of Novo Nordisk. L. N. has no conflict of interest to declare.
Author contributions
T. H. contributed to the study design, conduct/data collection, analysis and writing of the manuscript. E. Z. contributed to the study design and writing of the manuscript. L. N. contributed to the study conduct/data collection and writing of the manuscript. T. R. contributed to the data analysis and writing of the manuscript. H. H. contributed to the study design and writing of the manuscript.
Heise T, Zijlstra E, Nosek L, Rikte T and Haahr H. Pharmacological properties of faster‐acting insulin aspart vs insulin aspart in patients with type 1 diabetes receiving continuous subcutaneous insulin infusion: A randomized, double‐blind, crossover trial, Diabetes Obes Metab, 2017;19(2):208–215.
Funding information This study was funded by Novo Nordisk.
REFERENCES
- 1. Woerle HJ, Neumann C, Zschau S, et al. Impact of fasting and postprandial glycemia on overall glycemic control in type 2 diabetes. Importance of postprandial glycemia to achieve target HbA1c levels. Diabetes Res Clin Pract. 2007;77:280‐285. [DOI] [PubMed] [Google Scholar]
- 2. Home PD. The pharmacokinetics and pharmacodynamics of rapid‐acting insulin analogues and their clinical consequences. Diabetes Obes Metab. 2012;14:780‐788. [DOI] [PubMed] [Google Scholar]
- 3. Hermansen K, Bohl M, Schioldan AG. Insulin aspart in the management of diabetes mellitus: 15 years of clinical experience. Drugs. 2016;76:41‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kalra S, Gupta Y. Ultra‐fast acting insulin analogues. Recent Pat Endocr Metab Immune Drug Discov. 2014;8:117‐123. [DOI] [PubMed] [Google Scholar]
- 5. Heinemann L, Muchmore DB. Ultrafast‐acting insulins: state of the art. J Diabetes Sci Technol. 2012;6:728‐742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pozzilli P, Battelino T, Danne T, Hovorka R, Jarosz‐Chobot P, Renard E. Continuous subcutaneous insulin infusion in diabetes: patient populations, safety, efficacy, and pharmacoeconomics. Diabetes Metab Res Rev. 2016;32:21‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thabit H, Hovorka R. Continuous subcutaneous insulin infusion therapy and multiple daily insulin injections in type 1 diabetes mellitus: a comparative overview and future horizons. Expert Opin Drug Deliv. 2016;13:389‐400. [DOI] [PubMed] [Google Scholar]
- 8. Yeh HC, Brown TT, Maruthur N, et al. Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: a systematic review and meta‐analysis. Ann Intern Med. 2012;157:336‐347. [DOI] [PubMed] [Google Scholar]
- 9. Cengiz E, Bode B, Van Name M, Tamborlane WV. Moving toward the ideal insulin for insulin pumps. Expert Rev Med Devices. 2016;13:57‐69. [DOI] [PubMed] [Google Scholar]
- 10. Food and Drug Administration . Inactive ingredient search for approved drug products. http://www.accessdata.fda.gov/scripts/cder/iig/index.cfm. Accessed October 21, 2016.
- 11. Heise T, Hövelmann U, Brøndsted L, Adrian CL, Nosek L, Haahr H. Faster‐acting insulin aspart: earlier onset of appearance and greater early pharmacokinetic and pharmacodynamic effects than insulin aspart. Diabetes Obes Metab. 2015;17:682‐688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Radermecker RP, Scheen AJ. Continuous subcutaneous insulin infusion with short‐acting insulin analogues or human regular insulin: efficacy, safety, quality of life, and cost‐effectiveness. Diabetes Metab Res Rev. 2004;20:178‐188. [DOI] [PubMed] [Google Scholar]
- 13. Bode BW. Comparison of pharmacokinetic properties, physicochemical stability, and pump compatibility of 3 rapid‐acting insulin analogues‐aspart, lispro and glulisine. Endocr Pract. 2011;17:271‐280. [DOI] [PubMed] [Google Scholar]
- 14. Benesch C, Heise T, Klein O, Heinemann L, Arnolds S. How to assess the quality of glucose clamps? Evaluation of clamps performed with ClampArt, a novel automated clamp device. J Diabetes Sci Technol. 2015;9:792‐800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. American Diabetes Association . Defining and reporting hypoglycaemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycaemia. Diabetes Care. 2005;28:1245‐1249. [DOI] [PubMed] [Google Scholar]
- 16. Fieller EC. Some problems in interval estimation. J R Stat Soc Series B Stat Methodol. 1954;16:175‐185. [Google Scholar]
- 17. Bode B, Hyveled L, Tamer SC, Ybanez P, Demissie M. Improved postprandial glycemic control with faster‐acting insulin aspart in subjects with type 1 diabetes using CSII. Diabetes. 2015;64(suppl 1):A253 (Poster 994‐P). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c). Diabetes Care. 2003;26:881‐885. [DOI] [PubMed] [Google Scholar]
- 19. Wang JS, Tu ST, Lee IT, et al. Contribution of postprandial glucose to excess hyperglycaemia in Asian type 2 diabetic patients using continuous glucose monitoring. Diabetes Metab Res Rev. 2011;27:79‐84. [DOI] [PubMed] [Google Scholar]
- 20. Riddle M, Umpierrez G, DiGenio A, Zhou R, Rosenstock J. Contributions of basal and postprandial hyperglycemia over a wide range of A1C levels before and after treatment intensification in type 2 diabetes. Diabetes Care. 2011;34:2508‐2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Russell‐Jones D, Bode B, de Block C, et al. Double‐blind mealtime faster‐acting insulin aspart vs insulin aspart in basal–bolus improves glycemic control in T1D: the onset® 1 trial. Diabetes. 2016;65(suppl 1):A77. [Google Scholar]
- 22. Cengiz E. Closer to ideal insulin action: ultra fast acting insulins. Panminerva Med. 2013;55:269‐275. [PubMed] [Google Scholar]
- 23. Maahs DM, Horton LA, Chase HP. The use of insulin pumps in youth with type 1 diabetes. Diabetes Technol Ther. 2010;12:S59‐S65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Heise T, Haahr H, Jensen L, Erichsen L, Hompesch M. Faster‐acting insulin aspart improves postprandial glycemia vs insulin aspart in patients with type 1 diabetes mellitus (T1DM). Diabetes. 2014;63(suppl 1):A34. [Google Scholar]
- 25. Bell KJ, Smart CE, Steil GM, Brand‐Miller JC, King B, Wolpert HA. Impact of fat, protein, and glycemic index on postprandial glucose control in type 1 diabetes: implications for intensive diabetes management in the continuous glucose monitoring era. Diabetes Care. 2015;38:1008‐1015. [DOI] [PubMed] [Google Scholar]
- 26. Buckley ST, Kildegaard J, Høiberg‐Nielsen R, et al. Mechanistic analysis into the mode(s) of action of niacinamide in faster‐acting insulin aspart. Diabetes Technol Ther. 2016;18(suppl 1):A116‐A117. [Google Scholar]
- 27. Regittnig W, Urschitz M, Lehki B, et al. Absorption kinetics of insulin following subcutaneous bolus administration with different bolus durations. Diabetes. 2013;62(suppl 1):A247 (Poster 966‐P). [Google Scholar]
- 28. Jain L, Parks MH, Sahajwalla C. Determination of time to onset and rate of action of insulin products: importance and new approaches. J Pharm Sci. 2013;102:271‐279. [DOI] [PubMed] [Google Scholar]
- 29. Swinnen SG, Holleman F, DeVries JH. The interpretation of glucose clamp studies of long‐acting insulin analogues: from physiology to marketing and back. Diabetologia. 2008;51:1790‐1795. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Experimental design of each dosing visit, glucose clamp quality and participant disposition.


