Summary
Ibrutinib is effective in patients with chronic lymphocytic leukaemia (CLL); however, treatment resistance remains a problem. Ublituximab is a novel, glycoengineered anti‐CD20 monoclonal antibody with single‐agent activity in relapsed CLL. We report the results of a phase 2 study evaluating combination therapy with ibrutinib and ublituximab in patients with relapsed or refractory CLL. Patients received ibrutinib 420 mg once daily. Ublituximab was administered on days 1, 8 and 15 of cycle 1 followed by day 1 of cycles 2–6. Response assessments were completed at cycles 3 and 6; patients then continued on ibrutinib monotherapy per standard of care. Forty‐one of 45 enrolled patients were evaluable for efficacy. Safety was consistent with prior experience for each drug, with infusion reactions the most prevalent adverse event. Combination therapy resulted in an overall response rate (ORR) of 88% at 6 months. In the 20 patients with high‐risk features (17p or 11q deletions or TP53 mutation) and evaluable for efficacy, the ORR was 95%, with three patients (15%) achieving negative minimal residual disease. Median time to response was 8 weeks. Ublituximab in combination with ibrutinib resulted in rapid and high response rates. The long‐term clinical benefit of ublituximab will be defined by an ongoing phase 3 trial (NCT 02301156).
Keywords: ublituximab, ibrutinib, chronic lymphocytic leukaemia, high‐risk, 17‐p deletion
Management of chronic lymphocytic leukaemia (CLL) has evolved from single‐agent chemotherapy, such as chlorambucil or fludarabine, to combination regimens, such as fludarabine and cyclophosphamide. The addition of the anti‐CD20 monoclonal antibody (mAb) rituximab to fludarabine and cyclophosphamide improved overall survival (OS) in CLL (Hallek et al, 2010), and long‐term follow‐up of this regimen has even raised the question of whether some patients with CLL can be cured of their disease with intensive chemoimmunotherapy (Fischer et al, 2016; Thompson et al, 2016).
More recently, novel B‐cell receptor (BCR)‐signalling inhibitors, such as the Bruton tyrosine kinase (BTK) inhibitor ibrutinib and the phosphoinositide‐3‐kinase (PI3K) delta inhibitor idelalisib, have transformed the management of patients with CLL and demonstrated further improvements in OS (Byrd et al, 2013, 2014; Furman et al, 2014; O'Brien et al, 2014; Burger et al, 2015). Ibrutinib has demonstrated remarkable single‐agent activity in both the relapsed and refractory settings (Byrd et al, 2013), as well as in treatment‐naïve CLL (O'Brien et al, 2014). In randomized studies, ibrutinib has improved OS compared to ofatumumab (Byrd et al, 2014) and chlorambucil (Burger et al, 2015), and has improved progression‐free survival (PFS) when administered in combination with bendamustine and rituximab (Chanan‐Khan et al, 2015).
Recent studies have suggested that ibrutinib monotherapy may result in incomplete inhibition of BCR signalling, even at approved dose levels (Poggesi et al, 2015), and that dose reductions or interruptions are associated with higher rates of progression (Barr et al, 2015). The depth of disease control in patients treated with ibrutinib monotherapy is not yet fully understood, with complete responses (CR) and minimal residual disease (MRD) negativity rare (Byrd et al, 2014). In addition, patients with adverse cytogenetic features may experience less durable disease control with ibrutinib compared with patients who lack such adverse features (Byrd et al, 2013). Further, mutations in BTK or the downstream‐signalling protein phospholipase C gamma (PLCG2) have been shown to confer ibrutinib resistance (Woyach et al, 2014a), and survival following discontinuation of ibrutinib can be very short (Jain et al, 2015; Maddocks et al, 2015). For these reasons, combination regimens that include ibrutinib have the potential to enhance patient outcomes versus those seen with ibrutinib monotherapy. Both rituximab and ofatumumab have been added to ibrutinib in separate studies, with both showing more rapid response rates and higher overall response rates (ORRs) compared to historic controls (Burger et al, 2014; Jaglowski et al, 2015). Multiple studies are also evaluating the addition of the glycoengineered anti‐CD20 mAb, obinutuzumab, to ibrutinib, including a randomized, multicentre, open‐label phase 3 study of ibrutinib in combination with obinutuzumab versus chlorambucil in combination with obinutuzumab in patients with treatment‐naïve CLL (Flinn et al, 2015).
Ublituximab is a novel, type 1, anti‐CD20 mAb that binds to a unique epitope on the CD20 antigen, distinct from rituximab, ofatumumab and obinutuzumab, and has been glycoengineered to exhibit a low‐fucose fragment crystallizable (Fc) region, thereby demonstrating enhanced antibody‐dependent cellular cytotoxicity (ADCC). In in vitro studies, ublituximab demonstrated 100 times greater natural killer (NK)‐cell‐mediated ADCC than rituximab in patient‐donor CLL cells (Le Garff‐Tavernier et al, 2011). In a first‐in‐human study in patients with relapsed CLL, ublituximab demonstrated marked and durable B‐cell depletion when administered as a single agent, resulting in a 45% ORR with a favourable toxicity profile (Cazin et al, 2011). We hypothesized that the addition of ublituximab to ibrutinib would result in quicker time to response and a greater depth of response for patients with relapsed and refractory CLL compared with ibrutinib alone.
Methods
Subjects
This was a multicentre, phase 2 study evaluating the efficacy and safety of ublituximab in combination with ibrutinib in patients with select B‐cell malignancies. Adult subjects ≥18 years of age with a confirmed diagnosis of mantle cell lymphoma, CLL, or small lymphocytic lymphoma (SLL) were enrolled in the trial. The demographics and outcomes for the CLL cohort only are reported herein. CLL subjects were required to have an indication for treatment according to the 2008 International Workshop on CLL (iwCLL) criteria (Hallek et al, 2008) and to have received at least one prior standard treatment regimen. Subjects were required to have an Eastern Cooperative Oncology Group (ECOG) performance status ≤2 with adequate organ function, defined as an absolute neutrophil count (ANC) ≥1 × 109/l and platelet count ≥50 × 109/l for the dose confirmation period and an ANC ≥0·75 × 109/l and platelet count ≥30 × 109/l for phase 2. Prior treatment with a BTK inhibitor and/or a PI3K inhibitor was permitted.
Subjects were excluded if they had received cancer therapy within 3 weeks of cycle 1/day 1; had received an autologous haematological stem cell transplant within 3 months of study entry or any prior allogeneic haematological stem‐cell transplant; or had Richter transformation, prolymphocytic leukaemia, primary central nervous system lymphoma or the presence of any other active cancers. All subjects gave written informed consent according to International Review Board guidelines. This study was conducted in accordance with the Declaration of Helsinki and was registered at Clinicaltrials.gov [National Clinical Trial (NCT) Identifier: 02013128].
Study design
This study consisted of a dose‐confirmation safety run‐in period followed by an open enrolment into phase 2. The dose‐confirmation safety assessment enrolled six patients with CLL into each of two cohorts: Cohort 1 received ibrutinib 420 mg daily and ublituximab 600 mg on days 1, 8 and 15 of cycle 1. Safety data were reviewed after all subjects had completed Cohort 1 therapy. If ≤1 dose‐limiting toxicity (DLT) occurred in Cohort 1, the dose escalation proceeded to Cohort 2 (ibrutinib 420 mg daily; ublituximab dose increased to 900 mg on days 1, 8 and 15 of cycle 1). If ≥2 or more DLTs occurred in Cohort 1, Cohort −1 was initiated (ibrutinib 420 mg daily; ublituximab dose decreased to 450 mg on days 1, 8 and 15 of cycle 1). If ≤1 DLT was reported in Cohort 2, the dose was considered safe for phase 2.
Once the dose was confirmed, subjects were enrolled into the open phase 2 part of the study, in which they received six cycles of ublituximab 900 mg and ibrutinib according to the schedule shown in Fig 1. After cycle 6, ibrutinib was administered off study per standard of care.
Figure 1.
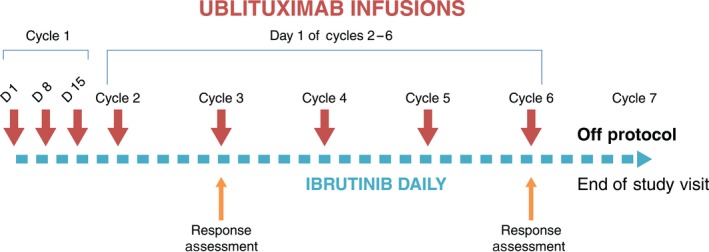
Study design and treatment schema. Patients received ibrutinib daily beginning on day 1 of cycle 1. Ublituximab infusions were given on days 1, 8, and 15 of cycle 1 and on day 1 of cycles 2 through 6. Patients were assessed for response after months 2 and 5. Each cycle is 28 days.
Ublituximab was administered as an intravenous infusion. Subjects received premedication with an antihistamine (diphenhydramine 50 mg or equivalent) and a corticosteroid (dexamethasone 12 mg or equivalent) approximately 30 min prior to each ublituximab infusion. Oral acetaminophen 650 mg was permitted in subjects who experienced fever or pyrexia after the first‐week dose or as clinically indicated. Concurrent glucocorticoid therapy was permitted if started at least 7 days prior to study entry (≤10 mg/day prednisone or equivalent) if clinically warranted. The day 1 infusion of ublituximab in cycle 1 was split between day 1 and day 2, with up to 150 mg administered on day 1 and the remainder of the dosage on day 2 to minimize infusion‐related reactions (IRRs). Prophylactic allopurinol was permitted for subjects at risk for tumour lysis syndrome.
Assessments and endpoints
The primary objective of the safety run‐in was to evaluate the safety of ublituximab in combination with ibrutinib for the doses given. The primary objective of the phase 2 study was to determine the ORR, defined as the rate of CR plus the rate of partial response (PR) of the combination. Patients who met the criteria for a CR but lacked bone marrow confirmation were considered to have a PR. Secondary objectives included safety, CR rate and the rate of MRD negativity.
Efficacy was assessed by a computed tomography (CT) scan at the start of cycle 3 and then again at approximately cycle 6. All efficacy assessments had a ±7‐day window. Responses were assessed per iwCLL criteria (Hallek et al, 2008), and PR with lymphocytosis (PR‐L) was also assessed per the suggested modification of the iwCLL guidelines (Cheson et al, 2012). Physical examinations, vital signs, haematology and serum chemistry were performed on all ublituximab dosing days, as well as an additional assessment on day 22 of cycle 1. Adverse events (AEs) were assessed by the Common Terminology Criteria for AEs (CTCAE), version 4.0 (http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf).
Statistical analysis
All statistical analyses were performed using a one‐sided hypothesis test at the overall 5% level of significance. Descriptive statistics were used for all variables. The primary efficacy variable (ORR) and the 90% one‐sided confidence interval of the rate were estimated. Per the statistical design, up to 50 patients with CLL were to be enrolled, assuming a 10% screen fail or non‐evaluable rate. If the response rate was no more than 60%, the study would be determined a failure; if a >20% increase in ORR versus the 58% ORR reported for ibrutinib (https://www.janssenmd.com/pdf/imbruvica/PI-Imbruvica.pdf) was achieved, the study would be considered to have a positive result. MRD was evaluated from peripheral blood samples in local laboratories using a minimum of four‐colour flow cytometry with a lower limit of detection of 0·01% (one malignant cell in 10 000 white blood cells). MRD analysis was completed at the start of cycles 3 and 6 following the patients' CT scan results.
This study employed a modified intent‐to‐treat (ITT) design. The modified ITT population consisted of all enrolled patients who had at least one post‐baseline efficacy measurement, and the primary efficacy analyses were performed based on the modified ITT population. The safety population included all enrolled patients who received at least one dose of the study drug. All safety assessments including toxicity were performed on the safety population.
Results
Patients
A total of 45 subjects with CLL were enrolled in the study, including patients in the safety run‐in and open enrolment components; all subjects were evaluable for safety, while 41 were evaluable for efficacy. Of the four subjects not evaluable for efficacy, two subjects were lost to follow‐up (one patient withdrew consent after one infusion, and one patient entered hospice after the second infusion), and two subjects discontinued the study prior to the first disease assessment due to an AE. Of the two subjects who discontinued, one was due to diarrhoea, assessed by the treating investigator as related to ibrutinib; and one was due to AEs of pneumonia and pleural effusion, which were not attributed to study drug treatment by the treating investigator (Table 1). Of the 45 patients, 47% (21 of 45) were classified as ‘high risk’, exhibiting one or more of the following cytogenetic abnormalities: del 17p (n = 12), del 11q (n = 12), and/or a TP53 (n = 2) mutation, with five having both del 17p and 11q. Prior treatment included purine analogues (22/44), bendamustine (21/44), idelalisib (2/44), a spleen‐tyrosine kinase inhibitor (2/44) and the BTK inhibitor CC‐292 (1/44). Of the evaluable patients, 29% were considered anti‐CD20‐refractory, progressing on or within 6 months of an anti‐CD20‐based regimen where prior anti‐CD20 therapy included rituximab, ofatumumab and/or obinutuzumab.
Table 1.
Demographics
| N = 45 | |
| Median age, years (range) | 71 (39–86) |
| Male/female | 23/22 |
| ECOG score, median | 1 |
| Prior regimens, median (range) | 2 (1–7) |
| ≥3 prior regimens | 16 (36%) |
| Refractory to prior therapy | 12 (27%) |
| Prior anti‐CD20 (rituximab, ofatumumab, obinutuzumab) | 42 (93%) |
| Refractory to anti‐CD20 | 13 (29%) |
| Prior alkylating agent | 29 (64%) |
| Prior purine analogue | 22 (49%) |
| High‐risk (17p or 11q deletion, TP53 mutation) | 21 (47%) |
ECOG, Eastern Cooperative Oncology Group performance status.
Safety outcomes
Overall, the combination of ublituximab and ibrutinib was well tolerated. No DLTs were seen in the six patients treated in the safety run‐in cohort. The most common AEs were IRRs, diarrhoea, fatigue, nausea and rash (Table 2). The most common (≥5%) grades 3/4 AEs were anaemia, neutropenia, IRRs and thrombocytopenia. All rash and grades 3/4 diarrhoea events were deemed related to ibrutinib per investigator assessment. All IRRs were related to ublituximab, with dose interruptions as the most common intervention; 21 of 45 patients (47%) had dose interruptions due to IRR, and one patient was dose‐reduced to 600 mg due to IRR. Other ublituximab‐related dose interruptions were due to neutropenia (two patients) and elevated aspartate aminotransferase (two patients). For ibrutinib, two patients had their dose reduced (one for diarrhoea, one for dizziness) and 10 of 45 (22%) had their dose interrupted (three for rash, two for neutropenia, one for anaemia, one for thrombocytopenia, one for nausea, one for hypercalcaemia and one for dehydration).
Table 2.
All causality adverse events in >10% of patients (n = 45)
| Adverse event | All grades n (%) | Grade 3/4 n (%) |
|---|---|---|
| Infusion‐related reaction | 24 (53) | 3 (7) |
| Diarrhoea | 18 (40) | 2 (4) |
| Fatigue | 15 (33) | |
| Cough | 12 (27) | |
| Rash | 12 (27) | |
| Nausea | 11 (24) | |
| Arthralgia | 8 (18) | 1 (2) |
| Upper respiratory tract infection | 8 (18) | |
| Anaemia | 7 (16) | 5 (11) |
| Thrombocytopenia | 7 (16) | 3 (7) |
| Constipation | 7 (16) | |
| Muscle spasms | 7 (16) | |
| Pyrexia | 7 (16) | |
| Abdominal pain | 6 (13) | |
| Chills | 6 (13) | |
| Contusion | 6 (13) | |
| Dizziness | 6 (13) | |
| Insomnia | 6 (13) | |
| Myalgia | 6 (13) | |
| Oedema, peripheral | 6 (13) | |
| Stomatitis | 6 (13) | |
| Neutropenia | 5 (11) | 5 (11) |
| Headache | 5 (11) |
Four subjects (9%) discontinued study participation due to an AE: one was due to grade 3 diarrhoea, and three were discontinued for AEs considered unrelated to study treatment per the treating investigator. Aside from IRRs, the addition of ublituximab to ibrutinib did not appear to alter the safety and tolerability profile historically seen with ibrutinib monotherapy.
Efficacy outcomes
All evaluable patients (n = 41) achieved some reduction in disease burden (Fig 2). The primary endpoint of ORR within 6 months by iwCLL criteria (Hallek et al, 2008) was met at 88%; two subjects (5%) achieved a CR and 34 subjects (83%) had a PR. One subject (2%) achieved a PR‐L; inclusion of this subject results in an ORR of 90%, according to the 2012 suggested modification of the iwCLL guidelines (Cheson et al, 2012). The four subjects who did not achieve a response included three who had stable disease and one who, although exhibiting a >70% nodal reduction, met the iwCLL criteria for disease progression with the appearance of a new lesion on CT scan. Notably, the high‐risk subgroup of CLL subjects exhibited an ORR of 95% (10% CR and 85% PR) with 15% of the high‐risk patients (one CR and two PR) achieving MRD negativity by four‐colour flow cytometry within 6 months of therapy. Of the subjects who achieved a CR or PR, all but two of the responses occurred by week 8.
Figure 2.
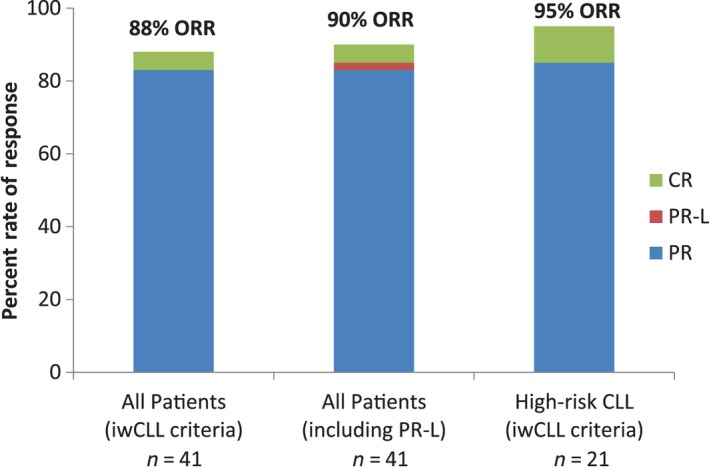
Best ORR. Forty‐one patients were evaluable for efficacy. Per iwCLL criteria (Hallek et al, 2008), the ORR within 6 months was 88% with 5% CR and 83% PR. When PR‐L is included, the ORR is 90%. In the high‐risk CLL population (n = 20), the ORR per iwCLL criteria was 95%, with 10% CR and 85% PR. CLL, chronic lymphocytic leukaemia; CR, complete response; iwCLL, International Workshop for Chronic Lymphocytic Leukaemia; ORR, overall response rate; PR, partial response; PR‐L, partial response with lymphocytosis.
All 41 subjects had nodal reduction ranging from 20% to 100%, with 93% (38 of 41) of subjects achieving a >50% nodal reduction by month 6 (Fig 3). A median nodal reduction of 62% was observed at week 8 and 77% at week 20 for all CLL subjects; while among the high‐risk subset, median nodal reductions of 64% and 85% were observed at weeks 8 and 20, respectively.
Figure 3.
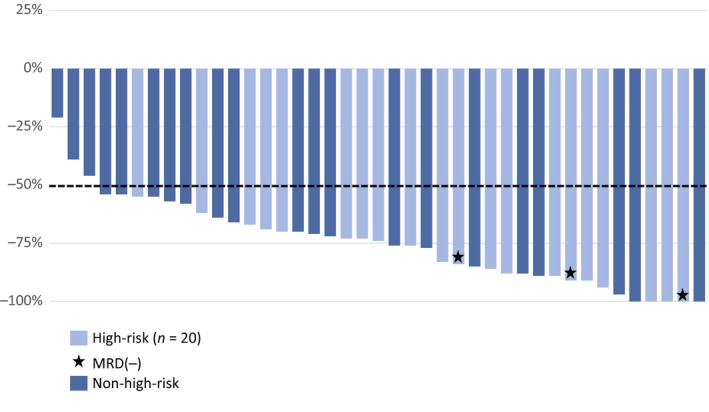
Best per cent change from baseline in nodal size: efficacy assessed at weeks 8 and 20. Thirty‐eight of the 41 evaluable subjects, including all subjects with high‐risk CLL (n = 41), achieved a ≥50% reduction in nodal size from baseline. Three subjects with high‐risk CLL achieved MRD(−) status. CLL, chronic lymphocytic leukaemia; MRD(−), negative status for minimal residual disease.
Absolute lymphocyte count (ALC) decreased significantly in the first month of treatment in most patients in the CLL group and continued to decrease over time (Fig 4). Addition of ublituximab to the treatment regimen appeared to reduce ibrutinib‐related lymphocytosis, with a median 75% decrease in ALC from baseline by the end of cycle 3. More than 70% of CLL subjects had lymphocyte counts in the normal range (<4 × 109/l) within 6 cycles of therapy. Marked B‐cell depletion and abrogation of lymphocytosis was also observed in the subset of patients who were refractory to prior anti‐CD20 therapy (Fig S1).
Figure 4.
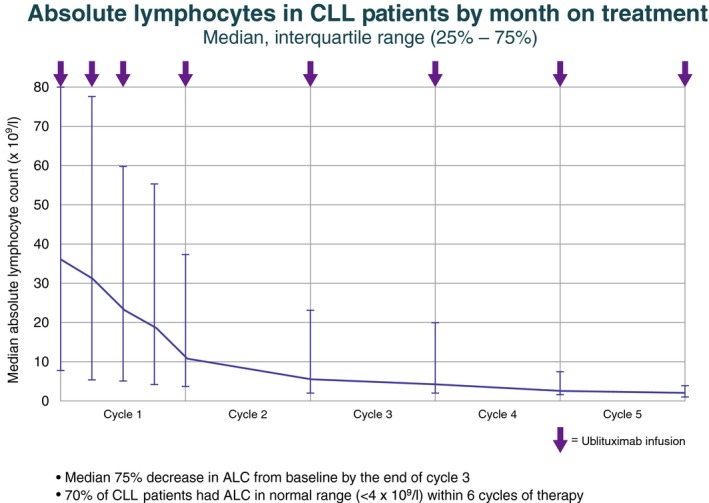
ALC by month on treatment. ALC decreased by a median of 75% from baseline by the end of cycle 3. Within 6 cycles of therapy, 70% of subjects had an ALC in the normal range (<4 × 109/l). Vertical bars indicate the interquartile range. ALC, absolute lymphocyte count; CLL, chronic lymphocytic leukaemia.
Discussion
It is not yet known whether the kinetics of ibrutinib responses impact the durability of disease control. Early analyses of studies conducted with ibrutinib to date suggest that patients achieving PR‐L on ibrutinib monotherapy exhibit similar outcomes to patients who achieved a true PR on ibrutinib (Woyach et al, 2014b), although long‐term follow‐up is not available in this selected clinical trial population. In the single‐arm, phase 2 study that led to accelerated approval of ibrutinib (Byrd et al, 2013), the ORR by iwCLL 2008 criteria (Hallek et al, 2008) at 2 and 5 months were reported as 21% and 39%, respectively; however, an additional 52% and 46% of patients at 2 and 5 months, respectively, achieved PR‐Ls, which, if included in the ORR assessment as per the suggested updates to the iwCLL criteria for response (Cheson et al, 2012), bring response rates at 2 and 5 months to 73% and 85%, respectively (Byrd et al, 2013). In a randomized, phase 3 study comparing ibrutinib to ofatumumab, the best response to ibrutinib was 43% PR, with an additional 20% demonstrating PR‐L; CR was not observed (Byrd et al, 2014).
Initiation of ibrutinib often causes a transient lymphocytosis. Much of the initial clinical activity of ibrutinib may result from a demargination phenomenon, whereby disruption of the supportive microenvironment results in CLL cell migration from the lymph nodes into the peripheral circulation, rather than from direct cytotoxicity (de Rooij et al, 2012).
In this study, we observed that ublituximab abrogated the lymphocytosis commonly associated with ibrutinib. The ORRs by iwCLL 2008 criteria at 2 and 6 months were 83% and 88%, respectively, excluding those patients with PR‐L. Further, within 6 months of initiating therapy, MRD‐negative status was achieved in 7% of patients, including 15% (3 of 20) with high‐risk markers (17p or 11q deletions or a TP53 mutation). In light of the recent observations that ibrutinib does not completely suppress BCR signalling in many patients and resistance is partly mediated through acquisition of BTK/PLCG2 mutations (Jain et al, 2015; Maddocks et al, 2015; Poggesi et al, 2015), we hypothesize that the addition of anti‐CD20 antibodies may protect against acquisition of resistance by providing alternative mechanisms of cytotoxicity. This study, however, does not provide confirmation of that hypothesis and will be addressed in multiple ongoing phase three studies, including: (i) ibrutinib monotherapy versus ublituximab plus ibrutinib in patients with previously treated high‐risk CLL (NCT02301156); (ii) ibrutinib monotherapy, ibrutinib plus rituximab, or bendamustine plus rituximab in older patients with previously untreated CLL (NCT01886872); and (iii) ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in patients with previously untreated CLL (NCT02264574).
Two previous reports have characterized the addition of a CD20 antibody to ibrutinib (Burger et al, 2014; Jaglowski et al, 2015). The addition of rituximab to ibrutinib in high‐risk CLL (either relapsed/refractory or treatment‐naïve) resulted in an ORR of 95% by iwCLL 2008 criteria, including 8% CR as best overall response (Burger et al, 2014). In a separate study in which ofatumumab was added to ibrutinib, the ORR in CLL/SLL patients was 83·3%, with a single patient (1·5%) achieving a CR (Jaglowski et al, 2015). These two reports approximate the experience reported herein – however, several significant differences between trials are notable. The rituximab‐plus‐ibrutinib study included treatment‐naïve patients (10% of study subjects) (Burger et al, 2014). Differences in response kinetics were also observed. In our study, the addition of ublituximab to ibrutinib caused the median lymphocyte count at 1 week to decrease. In contrast, neither rituximab nor ofatumumab therapy abrogated the lymphocytosis to the degree seen with ublituximab. In addition, 29% of enrolled subjects treated with ublituximab were deemed anti‐CD20 refractory by standard definitions (progressing on or within 6 months of an anti‐CD20‐containing regimen). Additionally, age has emerged as an important predictor of ibrutinib tolerance, with older patients less able to tolerate ibrutinib therapy (Maddocks et al, 2015). Both the rituximab and ofatumumab studies included younger patient populations (median age 63 vs. 64 vs. 71 years for rituximab, ofatumumab and ublituximab, respectively). Whether rituximab, ofatumumab, obinutuzumab or ublituximab represents the optimal partner with ibrutinib cannot be determined from these comparisons; rather, these comparisons reinforce the faster and higher response rates observed when an anti‐CD20 antibody is added.
There are concerns that ibrutinib could interfere with the activity of anti‐CD20 antibodies. Ibrutinib binds interleukin‐2 inducible tyrosine kinase (ITK) (Dubovsky et al, 2013). In NK cells stimulated by Fc receptor, ITK expression leads to calcium mobilization, granule release, and cytotoxicity (Khurana et al, 2007). Blocking of ITK by ibrutinib could, in theory, reduce the efficacy of anti‐CD20 antibodies, and such antagonism has indeed been demonstrated in vitro (Kohrt et al, 2014; Da Roit et al, 2015). However, based on the robust lymphocyte depletion demonstrated by ublituximab in the presence of ibrutinib co‐administration, no clinically meaningful evidence of such antagonism was observed in this study. In this context, the enhanced ADCC properties of ublituximab could provide an explanation for overcoming the antagonism demonstrated in vitro between rituximab and ibrutinib.
One important limitation of the current study is that it was not designed to evaluate PFS and OS. At the time this study was initiated, there were no reports of the clinical efficacy of BTK inhibitors given in combination with anti‐CD20 antibodies. This study was designed primarily to evaluate whether response rates, and particularly depth of response, were qualitatively different from those seen in the historic controls of ibrutinib monotherapy. An ongoing study using a randomized phase 3 design will determine the impact of the addition of ublituximab to ibrutinib on PFS and ORR in patients with high‐risk CLL (NCT Identifier 02301156).
Funding sources
This work was supported by TG Therapeutics, Inc.
Author contributions
Jeff Sharman, Peter Sportelli and Hari P. Miskin: Conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript.
Charles M. Farber: Collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript.
Daruka Mahadevan: Conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript, provision of study material or patients.
Marshall T. Schreeder, Kathryn S. Kolibaba, Suzanne Fanning and Daniel R. Greenwald: Collection and assembly of data, manuscript writing, final approval of manuscript.
Heather D. Brooks: Data analysis and interpretation, manuscript writing, final approval of manuscript, provision of study material or patients.
Leonard Klein: Conception and design, manuscript writing, final approval of manuscript.
Daniel R. Greenwald: Collection and assembly of data, manuscript writing, final approval of manuscript.
Michael S. Weiss: Conception and design, data analysis and interpretation, final approval of manuscript.
John M. Burke: Data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosures of conflicts of interest
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self‐held unless noted. I_Immediate Family Member, Inst_My Institution. Relationships may not relate to the subject matter of this manuscript.
Jeff Sharman
Leadership: US Oncology.
Consulting or Advisory Role: Pharmacyclics, Celgene, TG Therapeutics, Genentech, Gilead Sciences.
Speakers' Bureau: Gilead Sciences.
Research Funding: Pharmacyclics, Genentech, Celgene, Acerta Pharma, Gilead Sciences, Seattle Genetics, TG Therapeutics, Merck, Takeda.
Expert Testimony: Gilead Sciences.
Charles M. Farber
None.
Daruka Mahadevan
Speakers' Bureau: Janssen, Alexion.
Travel, Accommodations, Expenses: Janssen, Alexion.
Marshall T. Schreeder
Research Funding: TG Therapeutics.
Heather D. Brooks
None.
Kathryn S. Kolibaba
Employment: McKesson Specialty Health, Northwest Cancer Specialists.
Consulting or Advisory Role: Gilead.
Research Funding: Acerta, Amgen, Cell Therapeutics, Genentech, Gilead, GSK, Janssen, Novartis, Pharmacyclics, Seattle Genetics, Celgene, TG Therapeutics.
Travel, Accommodations, Expenses: McKesson Specialty Health.
Suzanne Fanning
Stock or Other Ownership: Celgene, Gilead, TG Pharma, Genmab.
Speakers' Bureau: Celgene, Takeda.
Leonard Klein
None.
Daniel R. Greenwald
Consulting or Advisory Role: Celgene, Phmacyclics, Genentech.
Speakers' Bureau: Genentech.
Peter Sportelli
Employment: TG Therapeutics.
Leadership: TG Therapeutics.
Stock or Other Ownership: TG Therapeutics.
Hari P. Miskin
Employment: TG Therapeutics.
Leadership: TG Therapeutics.
Stock or Other Ownership: TG Therapeutics.
Michael S. Weiss
Employment: TG Therapeutics, Fortress Biotech.
Leadership: TG Therapeutics, Fortress Biotech.
Stock or Other Ownership: TG Therapeutics, Fortress Biotech.
Other Relationship: Opus Point Partners.
John M. Burke
Consulting or Advisory Role: Gilead, Incyte, Millenium, Janssen, Pfizer.
Travel, Accommodations, Expenses: TG Therapeutics.
Supporting information
Fig S1. ALC by month in rituximab‐refractory patients on treatment.
Acknowledgements
The authors would like to acknowledge Michael Chen, PhD (TCM Groups Inc., Berkeley Heights, NJ), for statistical analysis; and Impact Communication Partners (New York, NY) for editorial assistance in the preparation of the manuscript.
Previously presented in part at the 13th International Congress on Malignant Lymphoma, Lugano, Switzerland, 2015.
References
- Barr, P.M. , Brown, J.R. , Hillmen, P. , O'Brien, S. , Barrientos, J.C. , Reddy, N.M. , Coutre, S. , Mulligan, S.P. , Jäger, U. , Furman, R.R. , Cymbalista, F. , Montillo, M. , Dearden, C. , Robak, T. , Moreno, C. , Pagel, J. , Burger, J.A. , Suzuki, S. , James, D.F. & Byrd, J.C. (2015) Dose adherence and baseline exposure analysis of the ibrutinib 420 mg dose administered to patients with previously treated chronic lymphocytic leukemia. Lancet Oncology, 33(Suppl. 15), abstr 7012. [Google Scholar]
- Burger, J.A. , Keating, M.J. , Wierda, W.G. , Hartmann, E. , Hoellenriegel, J. , Rosin, N.Y. , de Weerdt, I. , Jeyakumar, G. , Ferrajoli, A. , Cardenas‐Turanzas, M. , Lerner, S. , Jorgensen, J.L. , Nogueras‐González, G.M. , Zacharian, G. , Huang, X. , Kantarjian, H. , Garg, N. , Rosenwald, A. & O'Brien, S. (2014) Ibrutinib plus rituximab for patients with high‐risk chronic lymphocytic leukaemia: a single‐arm, phase 2 study. Lancet Oncology, 15, 1090–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger, J.A. , Tedeschi, A. , Barr, P.M. , Robak, T. , Owen, C. , Ghia, P. , Bairey, O. , Hillmen, P. , Bartlett, N.L. , Li, J. , Simpson, D. , Grosicki, S. , Devereux, S. , McCarthy, H. , Coutre, S. , Quach, H. , Gaidano, G. , Maslyak, Z. , Stevens, D.A. , Janssens, A. , Offner, F. , Mayer, J. , O'Dwyer, M. , Hellman, A. , Schuh, A. , Siddiqi, T. , Polliack, A. , Tam, C.S. , Suri, D. , Cheng, M. , Clow, F. , Styles, L. , James, D.F. & Kipps, T.J. ; RESONATE‐2 Investigators . (2015) Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. The New England Journal of Medicine, 373, 2425–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd, J.C. , Furman, R.R. , Coutre, S.E. , Flinn, J.W. , Burger, J.A. , Blum, K.A. , Grant, B. , Sharman, J.P. , Coleman, M. , Wierda, W.G. , Jones, J.A. , Zhao, W. , Heerema, N.A. , Johnson, A.J. , Sukbuntherng, F. , Chang, B.Y. , Clow, F. , Hedrick, E. , Buggy, J.J. , James, D.F. & O'Brien, S. (2013) Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. The New England Journal of Medicine, 369, 32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd, J. , Brown, J. , O'Brien, S. , Barrientos, J.C. , Kay, N.E. , Reddy, N.M. , Coutre, S. , Tam, C.S. , Mulligan, S.P. , Jaeger, U. , Devereux, S. , Barr, P.M. , Furman, R.R. , Kipps, T.J. , Cymbalista, F. , Pocock, C. , Thornton, P. , Caligaris‐Cappio, F. , Robak, T. , Delgado, J. , Schuster, S.J. , Montillo, M. , Schuh, A. , de Vos, S. , Gill, D. , Bloor, A. , Dearden, C. , Moreno, C. , Jones, J.J. , Chu, A.D. , Fardis, M. , McGreivy, J. , Clow, F. , James, D.F. & Hillmen, P. ; RESONATE Investigators . (2014) Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. The New England Journal of Medicine, 371, 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazin, B. , Leprêtre, S. , Coiffier, B. , Aurran, T. , Cartron, G. , Feugier, P. , Brehar, O. , Sadoun, A. , Segaud, F. & Ribrag, V. (2011) Multicentre phase I study with an 8‐dose regimen of single agent anti‐CD20 monoclonal antibody LFB‐R603 in patients with relapsed chronic lymphocytic leukemia (CLL). Blood, 118, abstr 2862. [Google Scholar]
- Chanan‐Khan, A. , Cramer, P. , Demirkan, F. , Fraser, G. , Silva, R.S. , Pylypenko, H. , Grosicki, S. , Janssens, A. , Pristupa, A. , Mayer, J. , Dilhuydy, M.S. , Loscertales, J. , Bartlett, N.L. , Avigdor, A. , Rule, S. , Sun, S. , Mahler, M. , Salman, M. , Howes, A.J. & Hallek, M.J. (2015) Ibrutinib combined with bendamustine and rituximab (BR) in previously treated chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL): first results from a randomized, double‐blind, placebo‐controlled, phase III study. Journal of Clinical Oncology, 33(Suppl. 15), abstr LBA7005. [Google Scholar]
- Cheson, B.D. , Byrd, J.C. , Rai, K.R. , Kay, N.E. , O'Brien, S.M. , Flinn, J.W. , Wiestner, A. & Kipps, T.J. (2012) Novel targeted agents and the need to refine clinical end points in chronic lymphocytic leukemia. Journal of Clinical Oncology, 30, 2820–2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Roit, F. , Engelberts, P.J. , Taylor, R.P. , Breij, E.C. , Gritti, G. , Rambaldi, A. , Introna, M. , Parren, P.W. , Beurskens, F.J. & Golay, J. (2015) Ibrutinib interferes with the cell‐mediated anti‐tumour activities of therapeutic CD20 antibodies: implications for combination therapy. Haematologica, 100, 77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubovsky, J.A. , Beckwith, K.A. , Natarajan, G. , Woyach, J.A. , Jaglowski, S. , Zhong, Y. , Hessler, J.D. , Liu, T.M. , Chang, B.Y. , Larkin, K.M. , Stefanovski, M.R. , Chappell, D.L. , Frissora, F.W. , Smith, L.L. , Smucker, K.A. , Flynn, J.M. , Jones, J.A. , Andritsos, L.A. , Maddocks, K. , Lehman, A.M. , Furman, R. , Sharman, J. , Mishra, A. , Caligiuri, M.A. , Satoskar, A.R. , Buggy, J.J. , Muthusamy, N. , Johnson, A.J. & Byrd, J.C. (2013) Ibrutinib is an irreversible inhibitor of ITK driving a Th1‐selective pressure in T lymphocytes. Blood, 122, 2539–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer, K. , Bahlo, J. , Fink, A.M. , Goede, V. , Herling, C.D. , Cramer, P. , Langerbeins, P. , von Tresckow, J. , Engelke, A. , Maurer, C. , Kovacs, G. , Herling, M. , Tausch, E. , Kreuzer, K.A. , Eichhorst, B. , Böttcher, S. , Seymour, J.F. , Ghia, P. , Marlton, P. , Kneba, M. , Wendtner, C.M. , Döhner, H. , Stilgenbauer, S. & Hallek, M. (2016) Long‐term remissions after FCR chemoimmunotherapy in previously untreated patients with CLL: updated results of the CLL8 trial. Blood, 127, 208–215. [DOI] [PubMed] [Google Scholar]
- Flinn, I. , Moreno, C. , Gill, D.S. , Kipps, T.J. , Shaw, Y. , Li, Y. , Styles, L.A. , James, D.F. & Gribben, J.G. (2015) Randomized, multicenter, open‐label, phase 3 study of the BTK inhibitor ibrutinib in combination with obinutuzumab vs. chlorambucil in combination with obinutuzumab in patients with treatment‐naïve CLL/SLL (PCYC1130): iLLUMINATE. Journal of Clinical Oncology, 33(Suppl. 15), abstr TPS7095. [Google Scholar]
- Furman, R. , Sharman, J. , Coutre, S. , Cheson, B.D. , Pagel, J.M. , Hillmen, P. , Barrientos, J.C. , Zelentz, A.D. , Kipps, T.J. , Flinn, I. , Ghia, P. , Eradat, H. , Ervin, T. , Lamnna, N. , Coiffier, B. , Pettitt, A.R. , Ma, S. , Stilgenbauer, S. , Cramer, P. , Aiello, M. , Johnson, D.M. , Miller, L.L. , Li, D. , Jahn, T.M. , Dansey, R.D. , Hallek, M. & O'Brien, S.M. (2014) Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. The New England Journal of Medicine, 370, 997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallek, M. , Cheson, B.D. , Catovsky, D. , Caligaris‐Cappio, F. , Dighiero, G. , Döhner, H. , Hillmen, P. , Keating, M.J. , Montserrat, E. , Rai, K.R. & Kipps, T.J. (2008) Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute‐Working Group 1996 guidelines. Blood, 111, 5446–5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallek, M. , Fischer, K. , Fingerle‐Rowson, G. , Fink, A.M. , Busch, R. , Mayer, J. , Hensel, M. , Hopfinger, G. , Hess, G. , von Grünhagen, U. , Bergmann, M. , Catalano, J. , Zinzani, P.L. , Caligaris‐Cappio, F. , Seymour, J.F. , Berrebi, A. , Jäger, U. , Cazin, B. , Trneny, M. , Westermann, A. , Wendtner, C.M. , Eichhorst, B.F. , Staib, P. , Bühler, A. , Winkler, D. , Zenz, T. , Böttcher, S. , Ritgen, M. , Mendila, M. , Kneba, M. , Dohner, H. & Stilgenbauer, S. ; International Group of Investigators; German Chronic Lymphocytic Leukaemia Study Group . (2010) Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomized, open‐label, phase 3 trial. Lancet, 376, 1164–1174. [DOI] [PubMed] [Google Scholar]
- Jaglowski, S.M. , Jones, J.A. , Nagar, V. , Flynn, J.M. , Andritsos, L.A. , Maddocks, K.J. , Woyach, J.A. , Blum, K.A. , Grever, M.R. , Smucker, K. , Ruppert, A.S. , Heerema, N.A. , Lozanski, G. , Stefanos, M. , Munneke, B. , West, J.S. , Neuenburg, J.K. , James, D.F. , Jall, N. , Johnson, A.J. & Byrd, J.C. (2015) Safety and activity of BTK inhibitor ibrutinib combined with ofatumumab in chronic lymphocytic leukemia: a phase 1b/2 study. Blood, 126, 842–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain, P. , Keating, M. , Wierda, W. , Estrov, Z. , Ferrajoli, A. , Jain, N. , George, B. , James, D. , Kantarjian, H. , Burger, J. & O'Brien, S. (2015) Outcomes of patients with chronic lymphocytic leukemia after discontinuing ibrutinib. Blood, 125, 2062–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana, D. , Arneson, L.N. , Schoon, R.A. , Dick, C.J. & Leibson, P.J. (2007) Differential regulation of human NK cell‐mediated cytotoxicity by the tyrosine kinase Itk. The Journal of Immunology, 178, 3575–3582. [DOI] [PubMed] [Google Scholar]
- Kohrt, H.E. , Sagiv‐Barfi, I. , Rafiq, S. , Herman, S.E. , Butchar, J.P. , Cheney, C. , Zhang, X. , Buggy, J.J. , Muthusamy, N. , Levy, R. , Johnson, A.J. & Byrd, J.C. (2014) Ibrutinib antagonizes rituximab‐dependent NK cell‐mediated cytotoxicity. Blood, 123, 1957–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Garff‐Tavernier, M. , Decocq, J. , de Romeuf, C. , Parizot, C. , Dutertre, C.A. , Chapiro, E. , Davi, F. , Debré, P. , Prost, J.F. , Teillaud, J.L. , Merle‐Beral, H. & Vieillard, V. (2011) Analysis of CD16+ CD56dim NK cells from CLL patients: evidence supporting a therapeutic strategy with optimized anti‐CD20 monoclonal antibodies. Leukemia, 25, 101–109. [DOI] [PubMed] [Google Scholar]
- Maddocks, K. , Ruppert, A. , Lozanski, G. , Heerema, N.A. , Zhao, W. , Abruzzo, L. , Lozanski, A. , Davis, M. , Gordon, A. , Smith, L.L. , Mantel, R. , Jones, J.A. , Flynn, J.M. , Jaglowski, S.M. , Andritsos, L.A. , Awan, F. , Blum, K.A. , Grever, M.R. , Johnson, A.J. , Byrd, J.C. & Woyach, J.A. (2015) Etiology of ibrutinib therapy discontinuation and outcomes in patients with chronic lymphocytic leukemia. JAMA Oncology, 1, 80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien, S. , Furman, R. , Coutre, S. , Sharman, J.P. , Burger, J.A. , Blum, K.A. , Grant, B. , Richards, D.A. , Coleman, M. , Wierda, W.G. , Jones, J.A. , Zhao, W. , Heerema, N.A. , Johnson, A.J. , Izumi, R. , Hamdy, A. , Chang, B.Y. , Graef, T. , Clow, F. , Buggy, J.J. , James, D.F. & Byrd, J.C. (2014) Ibrutinib as initial therapy for elderly patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: an open‐label, multicentre, phase 1b/2 trial. Lancet Oncology, 15, 48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggesi, I. , Sardu, M.L. , Marostica, E. , Sukbuntherng, J. , Chang, B.Y. , de Jong, J. , de Trixhe, X.W. , Vermeulen, A. , De Nicolao, G. , O'Brien, S.M. , Byrd, J.C. , Advani, R.H. , James, D.F. , Deraedt, W. , Beaupre, D. & Wang, M. (2015) Population pharmacokinetic‐pharmacodynamic (PKPD) modeling of ibrutinib in patients with B‐cell malignancies. Clinical Cancer Research, 21(Suppl. 17), abstr B19. [Google Scholar]
- de Rooij, M.F.M. , Kuil, A. , Geest, C.R. , Eldering, E. , Chang, B.Y. , Buggy, J.J. , Pals, S.T. & Spaargaren, M. (2012) The clinically active BTK inhibitor PCI‐32765 targets B‐cell receptor‐ and chemokine‐controlled adhesion and migration in chronic lymphocytic leukemia. Blood, 119, 2590–2594. [DOI] [PubMed] [Google Scholar]
- Thompson, P.A. , Tam, C.S. , O'Brien, S.M. , Wierda, W.G. , Stingo, F. , Plunkett, W. , Smith, S.C. , Kantarjian, H.M. , Freireich, E.J. & Keating, M.J. (2016) Fludarabine, cyclophosphamide and rituximab achieves long‐term disease‐free survival in IGHV‐mutated chronic lymphocytic leukemia. Blood, 127, 303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woyach, J.A. , Furman, R.R. , Liu, T.M. , Ozer, H.G. , Zapatka, M. , Ruppert, A.S. , Xue, L. , Li, D.H. , Steggerda, S.M. , Versele, M. , Dave, S.S. , Zhang, J. , Yilmaz, A.S. , Jaglowski, S.M. , Blum, K.A. , Lozanski, A. , Lozanski, G. , James, D.F. , Barrientos, J.C. , Lichter, P. , Stilgenbauer, S. , Buggy, J.J. , Chang, B.Y. , Johnson, A.J. & Byrd, J.C. (2014a) Resistance mechanisms for the Bruton's tyrosine kinase inhibitor ibrutinib. The New England Journal of Medicine, 370, 2286–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woyach, J.A. , Smucker, K. , Smith, L.L. , Lozanski, A. , Zhong, Y. , Ruppert, A.S. , Lucas, D. , Williams, K. , Zhao, W. , Rassenti, L. , Ghia, E. , Kipps, T.J. , Mantel, R. , Jones, J. , Flynn, J. , Maddocks, K. , O'Brien, S. , Furman, R.R. , James, D.F. , Clow, F. , Lozanski, G. , Johnson, A.J. & Byrd, J.C. (2014b) Prolonged lymphocytosis during ibrutinib therapy is associated with distinct molecular characteristics and does not indicate a suboptimal response to therapy. Blood, 123, 1810–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. ALC by month in rituximab‐refractory patients on treatment.


